Primary Minerals and Age of The Hydrothermal Quartz Veins Containing U-Mo-(Pb, Bi, Te) Mineralization in the Majerská Valley near Čučma (Gemeric Unit, Spišsko-Gemerské Rudohorie Mts., Slovak Republic)
Abstract
:1. Introduction
2. Regional Geological Setting
Localization and Geology of The Studied Occurrence
3. Methods Used
4. Results
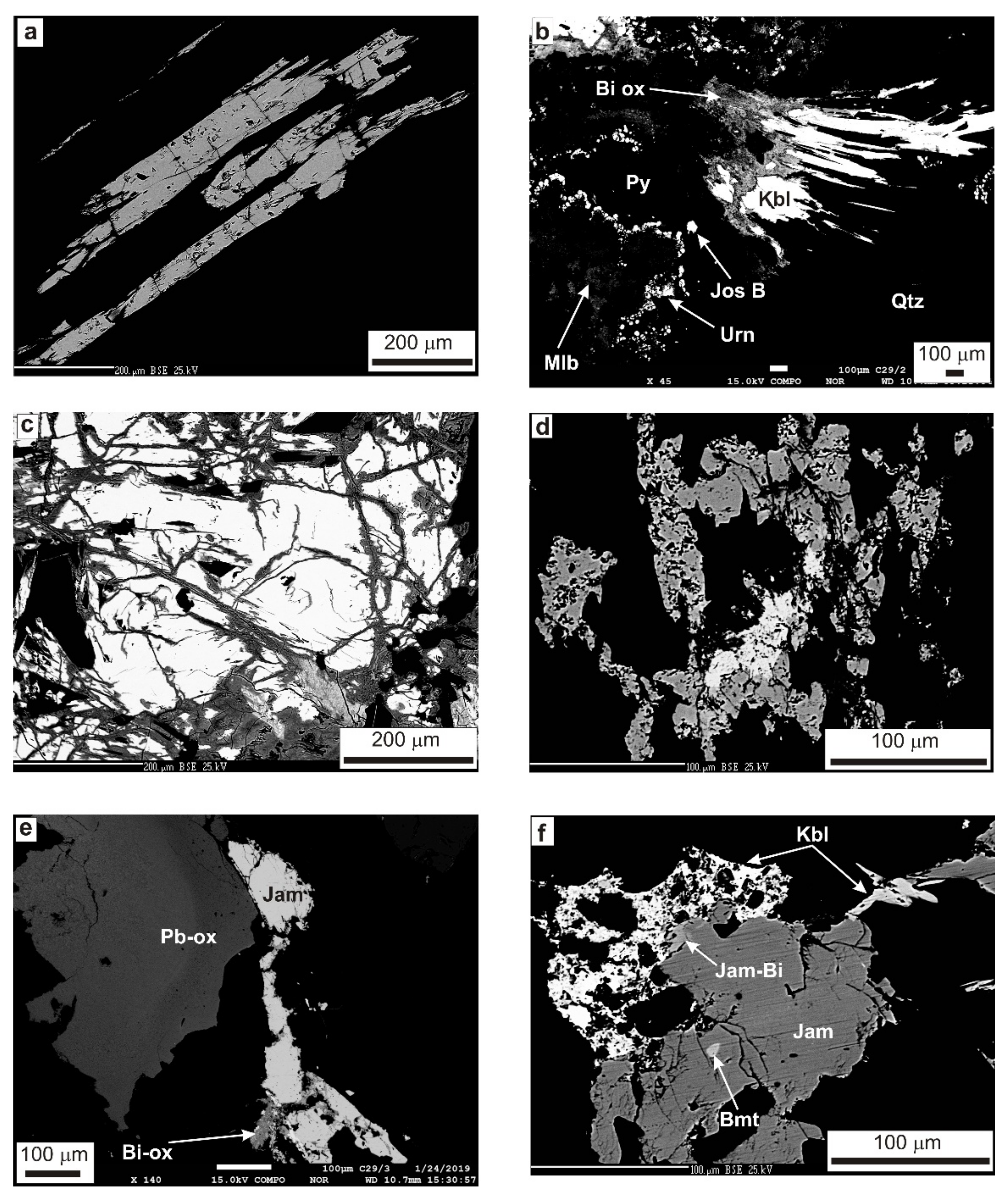
4.1. Character of Mineralization
4.2. Primary Vein Minerals
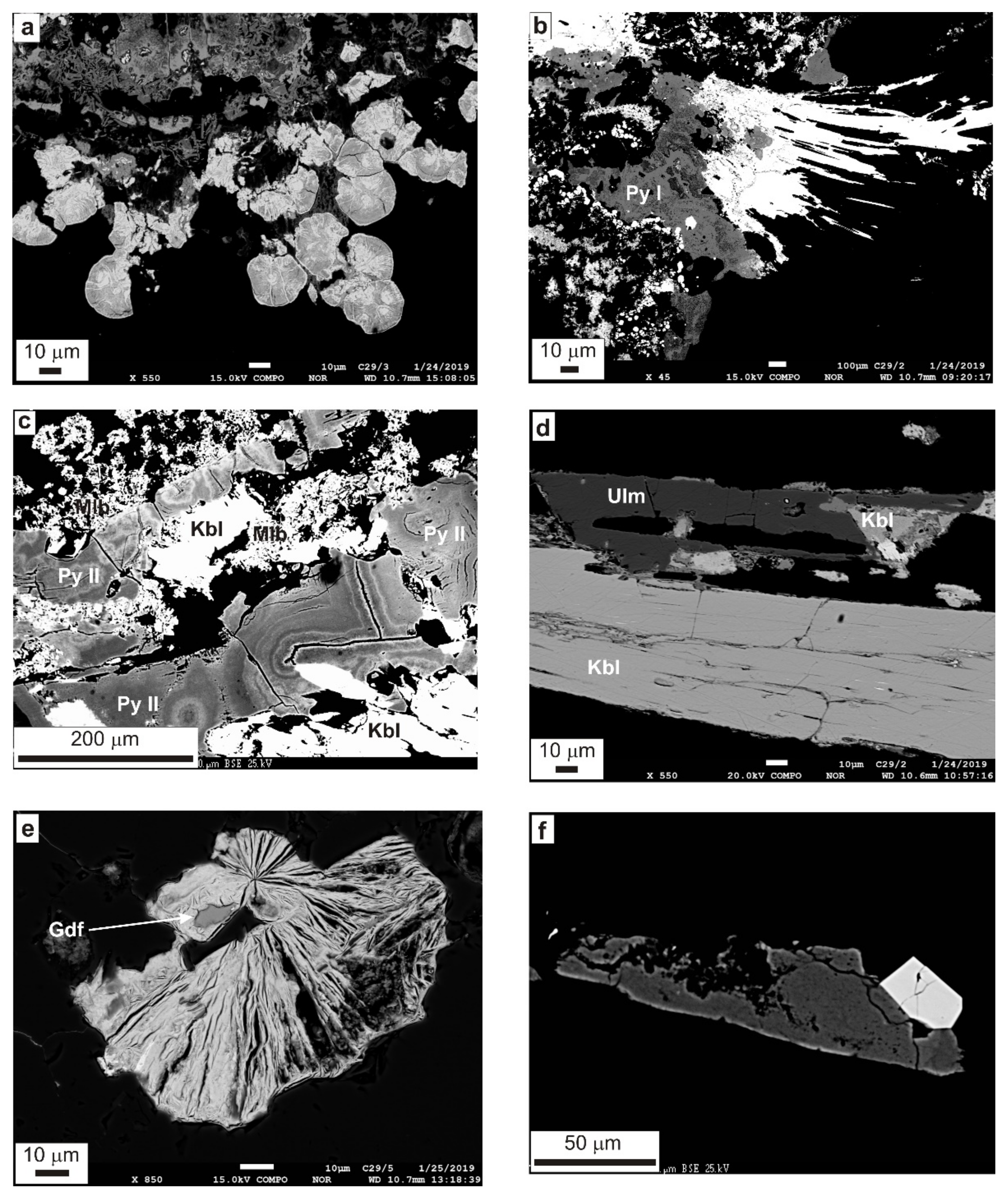
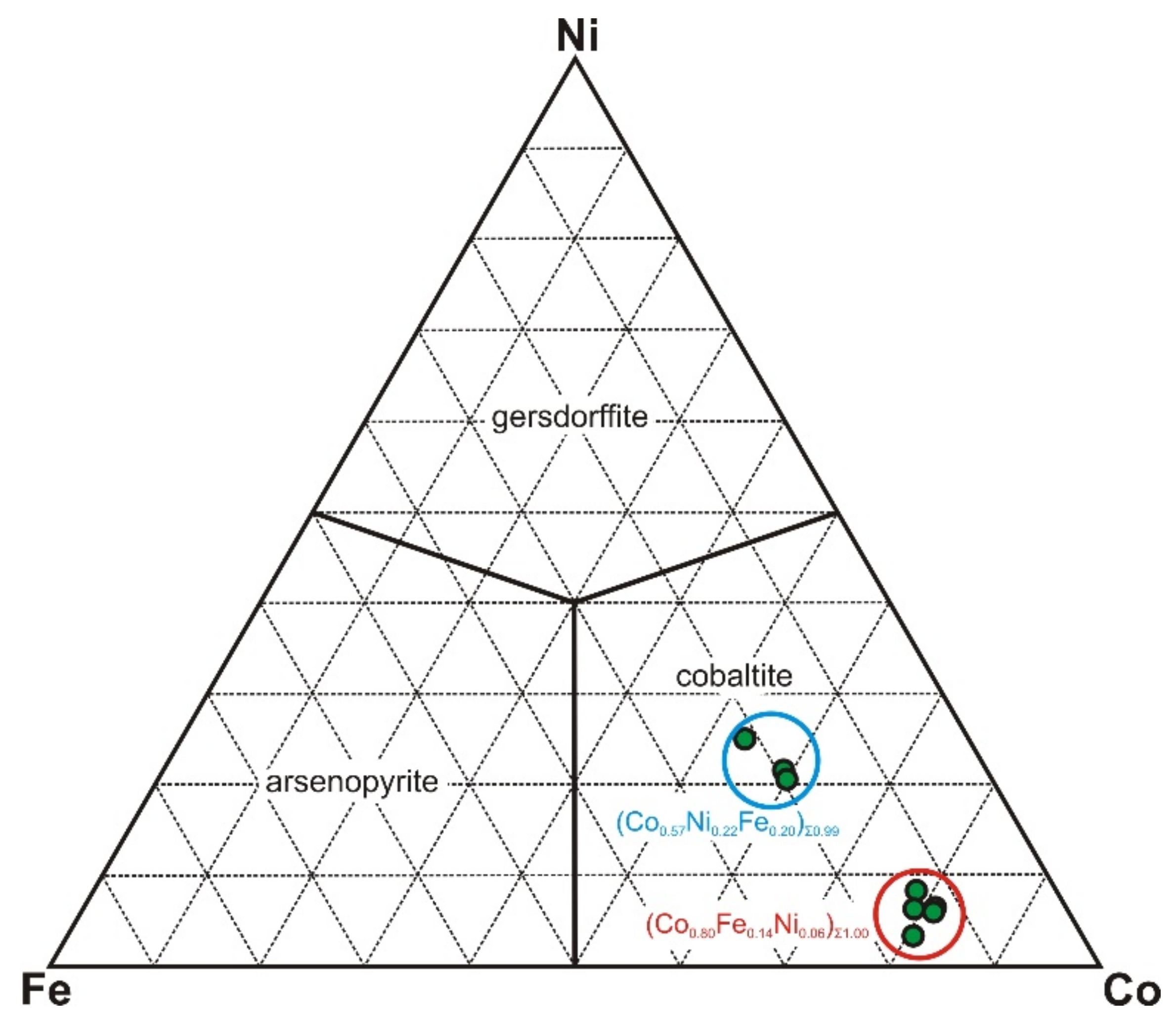
4.2.1. Ore (Opaque) Minerals
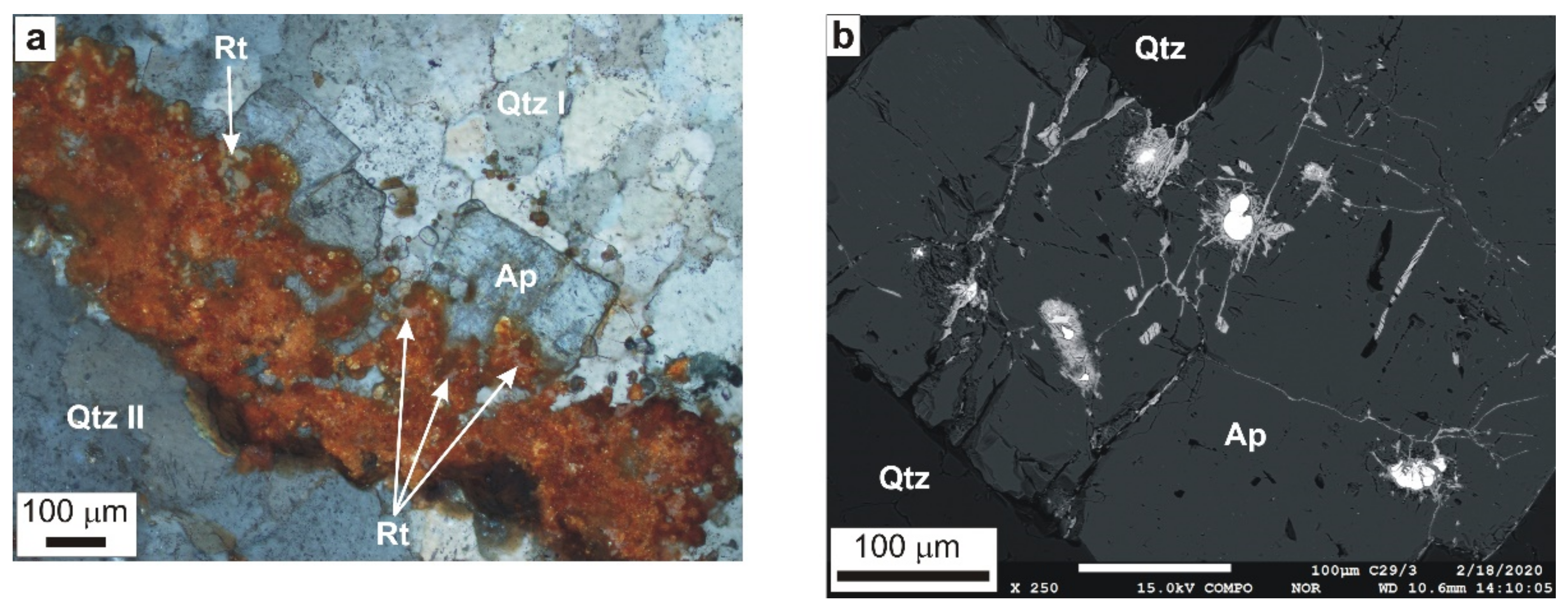
4.2.2. Non-Ore and Accessory Minerals
5. Discussion
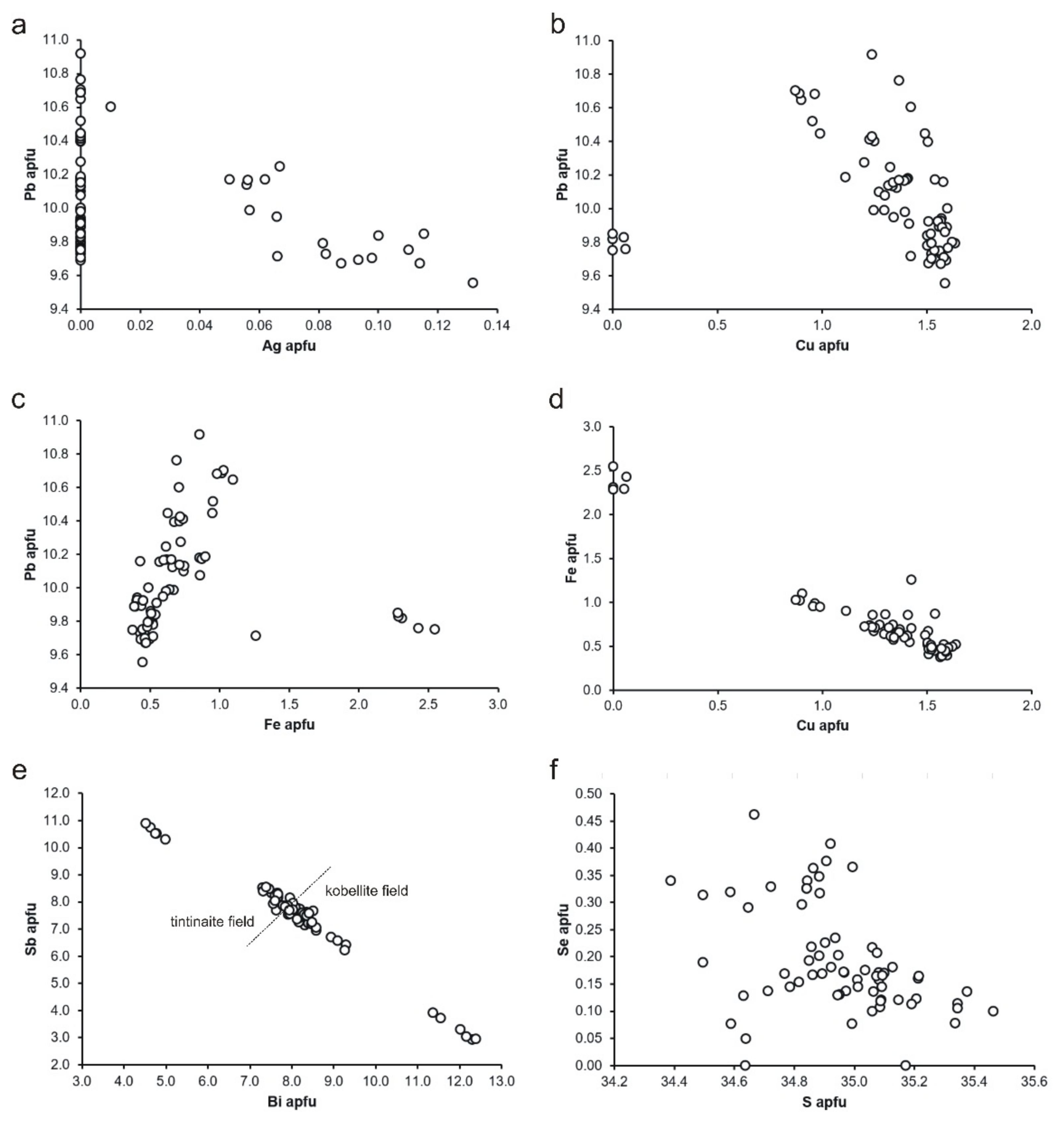
5.1. Succession of Mineralization
5.2. Mineralization Type
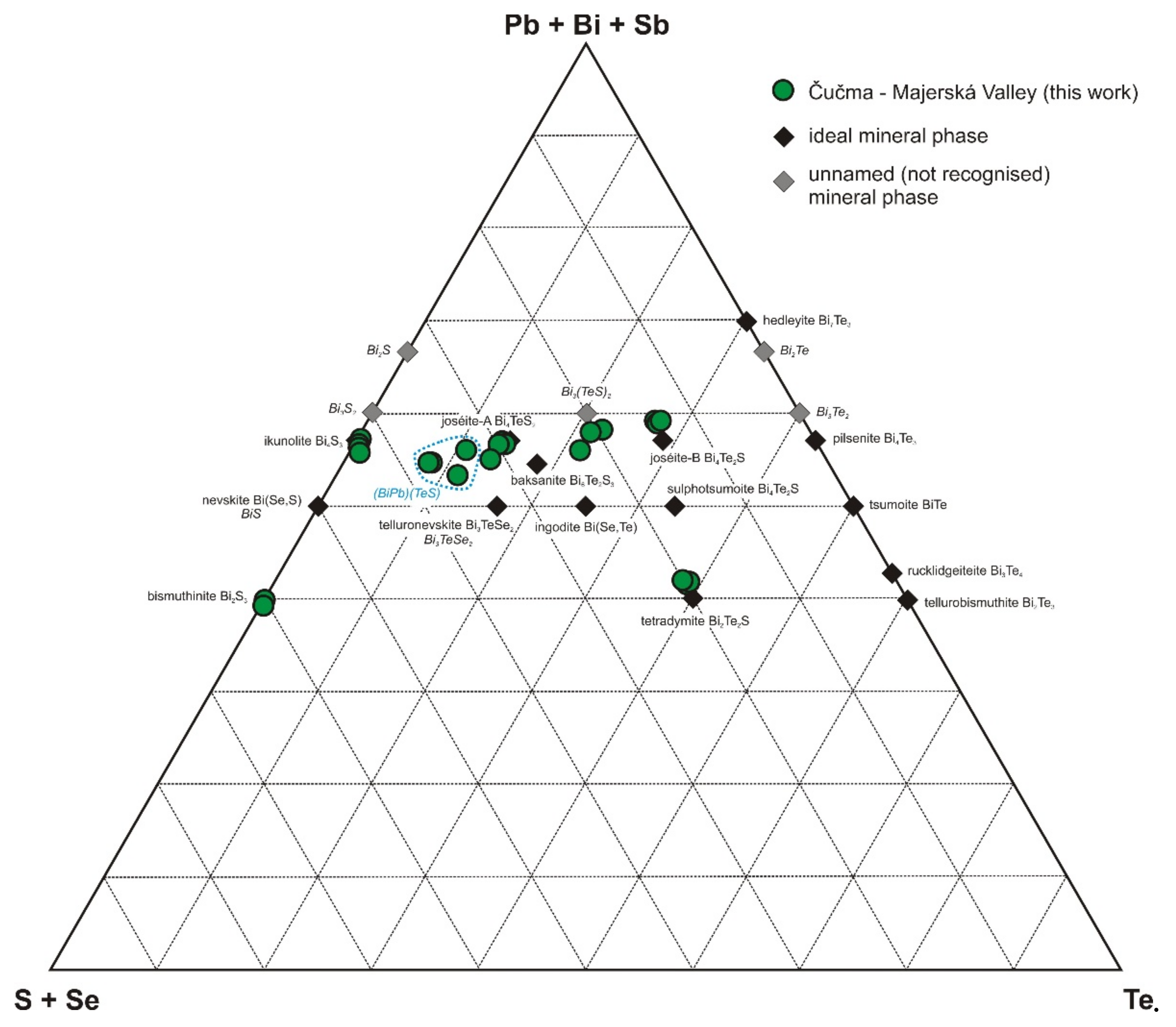
- The most abundant ore minerals are molybdenite and uraninite, accompanied by a diverse (but less represented) assemblage of sulphides (pyrite, galena), sulphosalts (mainly minerals of the kobellite–tintinaite series), sulphoarsenides (cobaltite, gersdorffite, ullmannite), Bi minerals (bismuth, bismuthinite) and sulphotellurides (tetradymite, joséite-A, joséite-B, etc.).
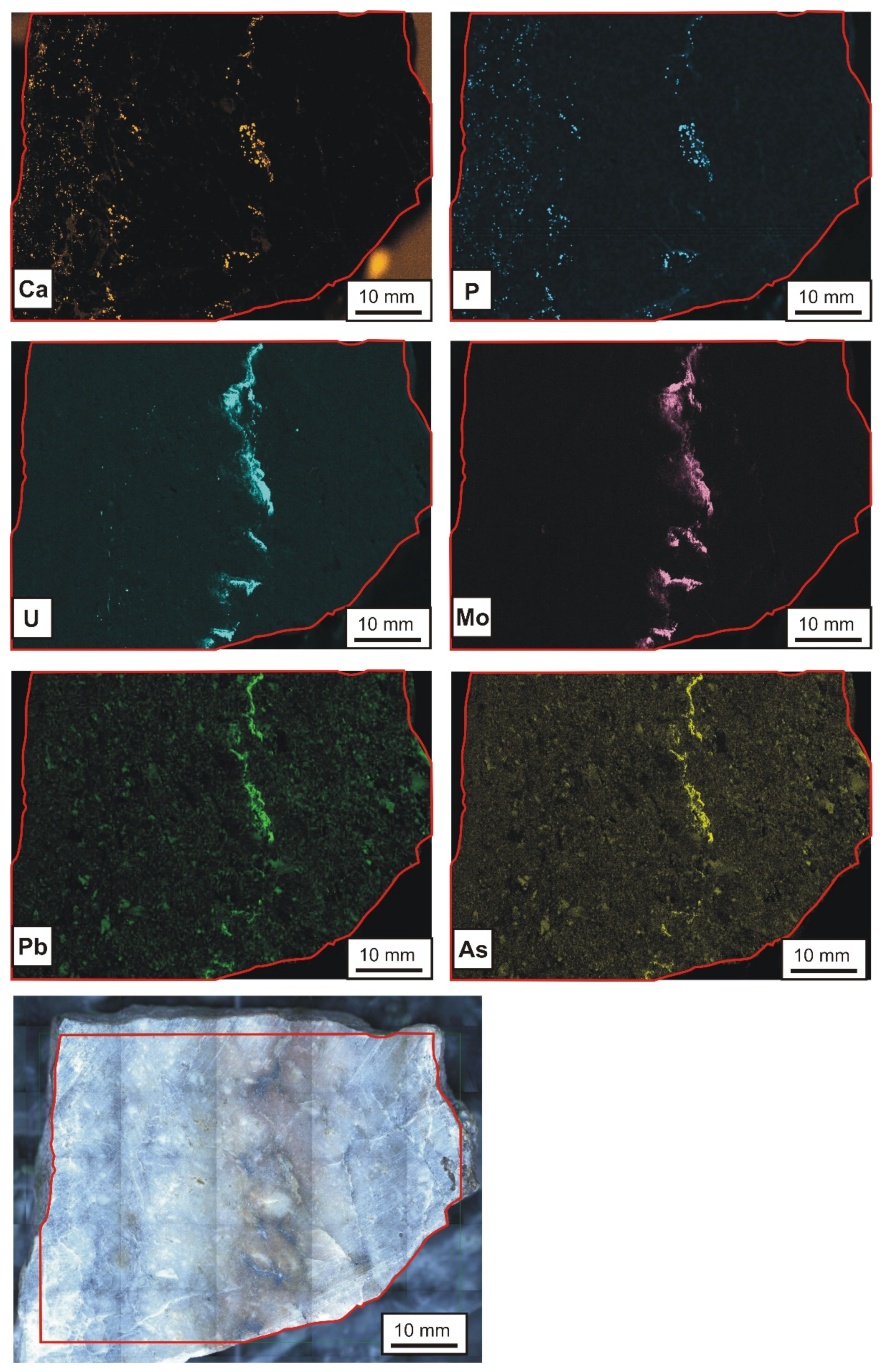
- The typical association of the elements in the studied veins is (the elements in parentheses have a less significance) as follows: Si-(P)-(Ca)-U-Mo-(Pb)-(Bi)-(Te).
5.3. Genesis and Age of U-Mo Mineralization
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Šváb, J.; Tulis, J.; Badár, J. Final Report about Results of Geological Survey in Čučma Locality; State Geological Institute of D. Štúr Archive: Bratislava, Slovakia, 1966. (In Slovak) [Google Scholar]
- Melnikova, A.M. Short Reports of Mineral Composition of Some Uranium Occurrences in Slovakia; Uranpres Archive: Spišská Nová Ves, Slovakia, 1973. (In Russian) [Google Scholar]
- Tréger, M. Occurrences of uranium-bearing phosphates in the Spišsko-gemerské rudohorie Mts. Miner. Slov. 1973, 5, 61–64. (In Slovak) [Google Scholar]
- Varček, C. Mineralogical Research of Vein Uranium Mineralization in the Central Part of the Spišsko-Gemerské Rudohorie Mts; Uranpres Archive: Spišská Nová Ves, Slovakia, 1975. (In Slovak) [Google Scholar]
- Varček, C. Some rare types of mineralization in the Spišsko-gemerské rudohorie Mts. In Deposit Forming Processes in the Western Carpathians Conference Proceedings, Bratislava, Slovakia, 29–30 May 2019; Háber, M., Ed.; FNS UK: Bratislava, Slovakia, 1977; pp. 93–99. [Google Scholar]
- Donát, A. Evaluation of REE Anomalies Occurrences in the Permian and in the Gelnica Gropup of SGRl; State Geological Institute of D. Štúr Archive: Bratislava, Slovakia, 1998. (In Slovak) [Google Scholar]
- Donát, A.; Mihál, F.; Novotný, L. Geological Survey Works on Au in the Older Paleozoic in SGR; State Geological Institute of D. Štúr Archive: Bratislava, Slovakia, 2000. (In Slovak) [Google Scholar]
- Rojkovič, I. Minerals of the crandallite series in quartz-apatite vein near Čučma. Miner. Slov. 1993, 25, 151–153. (In Slovak) [Google Scholar]
- Rojkovič, I.; Konečný, P.; Novotný, L.; Puškelová, L.; Streško, V. Quartz-apatite-REE vein mineralization in Early Paleozoic rocks of the Gemeric Superunit, Slovakia. Geol. Carpath. 1999, 50, 215–227. [Google Scholar]
- Števko, M.; Uher, P.; Ondrejka, M.; Ozdín, D.; Bačík, P. Quartz–apatite–REE phosphates–uraninite vein mineralization near Čučma (eastern Slovakia): A product of early Alpine hydrothermal activity in the Gemeric Superunit, Western Carpathians. J. Geosci. 2014, 59, 209–222. [Google Scholar] [CrossRef] [Green Version]
- Ferenc, Š.; Rojkovič, I.; Mato, L. Uranyl minerals of Western Carpathians. In Mineralogy of Bohemian Massif and Western Carpathians; Conference Proceedings; Zimák, J., Ed.; Palacký University: Olomouc, Czech Republic, 2003; pp. 17–23. (In Slovak) [Google Scholar]
- Ferenc, Š.; Biroň, A.; Sejkora, J.; Sýkorová, M. Phosphuranylite from the oxidation zone of the vein quartz-apatite-REE-U mineralization at Majerská Valley near Čučma (Slovenské Rudohorie Mts., Gemeric Unit). Bull. Miner. Petrolog. 2017, 25, 23–32. (In Slovak) [Google Scholar]
- Ferenc, Š.; Mikuš, T.; Spišiak, J.; Milovská, S. Supergene minerals in quartz fluorapatite hydrothermal veins with U-Mo and U-REE mineralization near Čučma (Gemeric Unit, Western Carpathians, eastern Slovakia): Preliminary study. In Proceedings from Mineralogical-Petrological Conference Petros; Ondrejka, M., Fridrichová, J., Eds.; FNS UK: Bratislava, Slovakia, 2019; pp. 17–19. [Google Scholar]
- Mahel, M.; Vozár, J. Contribution to knowledge of Permian and Triassic in the North-Gemeric syncline. Geol. Pr. Spr. 1971, 56, 47–66. (In Slovak) [Google Scholar]
- Snopko, L.; Ivanička, J. Considerations on the paleogeography in the Lower Paleozoic of Spišsko-Gemerské Rudohorie Mts. In Paleogeographical Evolution of the Western Carpathians, 1st ed.; Vozár, J., Ed.; Geological Institution of D. Štúr: Bratislava, Slovakia, 1978; pp. 269–280. (In Slovak) [Google Scholar]
- Ivanička, J.; Snopko, L.; Snopková, P.; Vozárová, A. Gelnica Group—Lower Unit of Spišsko-Gemerské Rudohorie Mts. (West Carpathians), Early Paleozoic. Geol. Zbor. Geol. Carpath. 1989, 40, 483–501. [Google Scholar]
- Vozárová, A. Variscan metamorphism and crustal evolution of the Gemericum. Západ Karpaty. Sér Miner. Petrogr. Geochém. Metalogen. 1993, 16, 55–117. (In Slovak) [Google Scholar]
- Putiš, M.; Sergeev, S.; Ondrejka, M.; Larionov, A.; Siman, P.; Spišiak, J.; Uher, P.; Paderin, I. Cambrian–Ordovician metaigneous rocks associated with Cadomian fragments in the West-Carpathian basement dated by SHRIMP on zircons: A record the Gondwana active margin setting. Geol. Carpath. 2008, 59, 3–18. [Google Scholar]
- Faryad, S.W. Metamorphism of the Early Paleozoic salic to intermediate volcanic rocks. Miner. Slov. 1991, 23, 325–332. (In Slovak) [Google Scholar]
- Faryad, S.W. Metamorphism of the Early Paleozoic sedimentary rocks in Gemericum. Miner. Slov. 1991, 23, 315–324. (In Slovak) [Google Scholar]
- Bajaník, Š.; Hanzel, V.; Mello, J.; Pristaš, J.; Reichwalder, P.; Snopko, L.; Vozár, J.; Vozárová, A. Explanation to Geological Map of the Slovenské Rudohorie Mts.—Eastern Part, 1:50,000, 1st ed.; State Geological Institute of D. Štúr: Bratislava, Slovakia, 1983; 223p. (In Slovak) [Google Scholar]
- Bajaník, Š.; Ivanička, J.; Mello, J.; Reichwalder, P.; Pristaš, J.; Snopko, L.; Vozár, J.; Vozárová, A. Geological Map of the Slovenské Rudohorie Mts.—Eastern Part, 1:50,000, 1st ed.; State Geological Institute of D. Štúr: Bratislava, Slovakia, 1984. (In Slovak) [Google Scholar]
- Uher, P.; Broska, I. Post-orogenic Permian granitic rocks in the Western Carpathian–Pannonian area: Geochemistry, mineralogy and evolution. Geol. Carpath. 1996, 47, 311–321. [Google Scholar]
- Šefara, J.; Bielik, M.; Vozár, J.; Katona, M.; Szalaiová, V.; Vozárová, A.; Šimonová, B.; Pánisová, J.; Schmidt, S.; Götze, H.J. 3D density modelling of Gemeric granites of the Western Carpathians. Geol. Carpath. 2017, 68, 177–192. [Google Scholar] [CrossRef] [Green Version]
- Vozárová, A.; Šarinová, K.; Sergeev, S.; Larionov, A.; Presnyakov, S. Late Cambrian/Ordovician magmatic arc type volcanism in the Southern Gemericum basement, Western Carpathians, Slovakia: U–Pb (SHRIMP) data from zircons. Int. J. Earth Sci. 2010, 99, 17–37. [Google Scholar] [CrossRef]
- Vozárová, A.; Rodionov, N.; Šarinová, K.; Presnyakov, S. New zircon ages on the Cambrian–Ordovician volcanism of the Southern Gemericum basement (Western Carpathians, Slovakia): SHRIMP dating, geochemistry and provenance. Int. J. Earth Sci. 2017, 106, 2147–2170. [Google Scholar] [CrossRef]
- Grecula, P.; Kobulský, J.; Gazdačko, L.; Németh, Z.; Hraško, L.; Novotný, L.; Maglay, J. Geological Map of the Spišsko-Gemerské Rudohorie Mts., 1:50,000, 1st ed.; State Geological Institute of D. Štúr: Bratislava, Slovakia, 1984. (In Slovak) [Google Scholar]
- Grecula, P.; Kobulský, J.; Gazdačko, L.; Németh, Z.; Hraško, L.; Novotný, L.; Maglay, J.; Pramuka, S.; Radvanec, M.; Kucharič, L.; et al. Explanations to Geological Map of the Spišsko-Gemerské Rudohorie Mts., 1:50,000, 1st ed.; State Geological Institute of D. Štúr: Bratislava, Slovakia, 2011; p. 308. (In Slovak) [Google Scholar]
- Montel, J.M.; Foret, S.; Veschambre, M.; Nicollet, C.; Provost, A. Electron microprobe dating of monazite. Chem. Geol. 1996, 131, 37–53. [Google Scholar] [CrossRef]
- Pouchou, J.L.; Pichoir, F. “PAP” (φρZ) procedure for improved quantitative microanalysis. In Microbeam Analysis; Armstrong, J.T., Ed.; San Francisco Press: San Francisco, CA, USA, 1985; pp. 104–106. [Google Scholar]
- Makovicky, E.; Makovicky, M. Representation of composition in the bismuthinite–aikinite series. Can. Miner. 1978, 16, 405–409. [Google Scholar]
- Zakrzewski, M.A.; Makovicky, E. Izoklakeite from Vena, Sweden, and the kobellite homologous series. Can. Miner. 1986, 24, 7–18. [Google Scholar]
- Rojkovič, I.; Novotný, L. Uranium mineralization in Gemericum. Miner. Slov. 1993, 25, 368–370. (In Slovak) [Google Scholar]
- Rojkovič, I. Uranium Mineralization in Slovakia, 1st ed.; Comenius University: Bratislava, Slovakia, 1997; p. 117. [Google Scholar]
- Novotný, L.; Čížek, P. New occurrence of uranium and gold, southern from Prakovce in Spišsko-gemerské Rudohoriie Mts. Miner. Slov. 1979, 11, 188–190. (In Slovak) [Google Scholar]
- Rojkovič, I.; Háber, M.; Novotný, L. U–Au–Co–Bi–REE mineralization in the Gemeric Unit (Western Carpathians, Slovakia). Geol. Carpath. 1997, 48, 303–313. [Google Scholar]
- Novotný, L.; Háber, M.; Križáni, I.; Rojkovič, I.; Mihál, F. Gold in Early Paleozoic rocks in the central part of the Spišsko-gemerské rudohorie Mts. Miner. Slov. 1999, 31, 211–216. (In Slovak) [Google Scholar]
- Rojkovič, I. U-Mo-Cu mineralization at Matejovce nad Hornádom. Miner. Slov. 1996, 28, 491–500. (In Slovak) [Google Scholar]
- Nash, J.T. Volcanic Uranium Deposits—Geology, Geochemical Processes, and Criteria for Resource Assessment; Open-File Report; U.S. Geological Survey: Reston, VA, USA, 2010.
- Breit, G.N.; Hall, S.M. Deposit Model for Volcanogenic Uranium Deposits; Open-File Report; U.S. Geological Survey: Reston, VA, USA, 2011.
- Castor, S.B.; Henry, C.D. Geology, geochemistry, and origin of volcanic rock-hosted uranium deposits in northwestern Nevada and southeastern Oregon, USA. Ore Geol. Rev. 2000, 16, 1–40. [Google Scholar] [CrossRef]
- Wilde, A.; Otto, A.; Jory, J.; MacRae, C.; Pownceby, M.; Wilson, N.; Torpy, A. Geology and mineralogy of uranium deposits from Mount Isa, Austarlia: Implications for albitite uranium deposit models. Minerals 2013, 3, 258–283. [Google Scholar] [CrossRef]
- McKay, A.D.; Miezitis, Y. Australia’s Uranium Resources, Geology and Development of Deposits; AGSO-Geoscienece: Canberra, Australia, 2001; pp. 1–184. [Google Scholar]
- Dahlkhamp, F.J. Uranium Deposits of the World; Springer: Berlin/Heidelberg, Germany, 2016; p. 792. [Google Scholar]
- Rojkovič, I.; Puškelová, L.; Khun, M.; Medved, J. U–REE–Au in veins and black shales of the Gemericum, Slovakia. In Mineral Deposits: From Their Origin to Their Environmental Impacts; Conference Proceedings; Pašava, J., Kříbek, B., Žák, K., Eds.; Balkema: Rotterdam, The Netherlands, 1995; pp. 789–792. [Google Scholar]
- Radvanec, M.; Konečný, P.; Ondrejka, M.; Putiš, M.; Uher, P.; Németh, Z. The Gemeric granites as an indicator of the crustal extension above the Late-Variscan subduction zone during the Early Alpine riftogenesis (Western Carpathians): An interpretation from the monazite and zircon ages dates by CHIME and SHRIMP methods. Miner. Slov. 2009, 41, 381–394. (In Slovak) [Google Scholar]
- Broska, I.; Uher, P. Whole-rock chemistry and genetic typology of the West-Carpathian Variscan granites. Geol. Carpath. 2001, 52, 79–90. [Google Scholar]
- Kubiš, M.; Broska, I. The role of boron and fluorine in evolved granitic rock systems (on the example of the Hnilec area, Western Carpathians). Geol. Carpath. 2005, 56, 193–204. [Google Scholar]
- Kubiš, M.; Broska, I. The granite system near Betliar village (Gemeric Superunit, Western Carpathians): Evolution of a composite silicic reservoir. J. Geosci. 2010, 55, 131–148. [Google Scholar] [CrossRef]
- Broska, I.; Kubiš, M. Accessory minerals and evolution of tin-bearing S-type granites in the western segment of the Gemeric Unit (Western Carpathians). Geol. Carpath. 2018, 59, 483–497. [Google Scholar] [CrossRef] [Green Version]
- Villaseñor, G.; Catlos, E.J.; Broska, I.; Kohút, M.; Hraško, L.; Aguilera, K.; Etzel, T.M.; Kyle, R.; Stockli, D.F. Evidence for widespread mid-Permian magmatic activity related to rifting following the Variscan orogeny (Western Carpathians). Lithos 2021, 390–391, 106083. [Google Scholar] [CrossRef]
- Finger, F.; Broska, I. The Gemeric S-type granites in southeastern Slovakia: Late Palaeozoic or Alpine intrusions? Evidence from electron-microprobe dating of monazite. Schweiz Miner. Petrogr. Mitt. 1999, 79, 439–443. [Google Scholar]
- Poller, U.; Uher, P.; Broska, I.; Plašienka, D.; Janák, M. First Permian—Early Triassic zircon ages for tin-bearing granites from the Gemeric unit (Western Carpathians, Slovakia): Connection to the post-collisional extension of the Variscan orogen and S-type granite magmatism. Terra Nova 2002, 14, 41–48. [Google Scholar] [CrossRef]
- Kohút, M.; Stein, H. Re-Os molybdenite dating of granite-related Sn-W-Mo mineralisation at Hnilec, Gemeric Superunit, Slovakia. Miner. Petrol. 2005, 85, 117–129. [Google Scholar] [CrossRef]
- Pecho, J.; Beňka, J.; Gargulák, M.; Václav, J. Geological Research of Stibnite Deposits in the Betliar-Čučma-Volovec Area; State Geological Institute of D. Štúr Archive: Bratislava, Slovakia, 1981. (In Slovak) [Google Scholar]
- Cohen, K.M.; Finney, S.C.; Gibbard, P.L.; Fan, J.X. The ICS International Chronostratigraphic Chart. Episodes 2013, 36, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Grandstaff, D.E. A kinetic study of the dissolution of uraninite. Econ. Geol. 1976, 71, 1493–1506. [Google Scholar] [CrossRef]
- Finch, R.J.; Ewing, R.C. The corrosion of uraninite under oxidizing conditions. J. Nuclear Mater. 1992, 190, 133–156. [Google Scholar] [CrossRef]
- Kotzer, T.G.; Kyser, T.K. O, U, and Pb isotopic and chemical variations in uraninite: Implications for determining the temporal and fluid history of ancient terrains. Am. Miner. 1993, 78, 1262–1274. [Google Scholar]
- Alexandre, P.; Kyser, T.K. Effects of cationic substitutions and alteration in uraninite, and implications for the dating of uranium deposits. Can. Miner. 2005, 43, 1005–1017. [Google Scholar] [CrossRef]
- Timón-Sánchez, S.M.; López-Moro, F.J.; Romer Elrhede, D.; Fernández-Fernández, A.; Moro-Benito, C. Late-Variscan multistage hydrothermal processes unveiled by chemical ages coupled with compositional and textural uraninite variations in W-Au deposits in the western Spanish Central System Batolith. Geol. Acta 2019, 17.1, 1–19. [Google Scholar]
- Yuan, F.; Jiang, S.Y.; Liu, J.; Zhang, S.; Xiao, Z.; Liu, G.; Hu, X. Geochronology and geochemistry of uraninite and coffinite: Insights into ore forming process in the pegmatite-hosted uraniferous province, North Qinling, Central China. Minerals 2019, 9, 552. [Google Scholar] [CrossRef] [Green Version]
- Corcoran, L.; Simonetti, A. Geochronology of uraninite revisited. Minerals 2020, 10, 205. [Google Scholar] [CrossRef] [Green Version]
- Ruzicka, V. Vein uranium deposits. Ore Geol. Rev. 1993, 8, 247–276. [Google Scholar] [CrossRef]
- Robinson, P.C. A genetic classification of Canadian uranium deposits. Can. Miner. 1958, 6, 513–521. [Google Scholar]
- Lang, A.H.; Griffith, J.W.; Steacy, H.R. Canadian Deposits of Uranium and Thorium; Geological Survey of Canada: Ottawa, ON, Canada, 1962; p. 324. [Google Scholar]
- Ruzicka, V. Geological comparison between East Europian and Canadian uranium deposists. Geol. Surv. Can. Pap. 1971, 48–70, 1–195. [Google Scholar]
- Rich, R.A.; Holland, H.D.; Petersen, U. Hydrothermal Uranium Deposits; Elsevier: Amsterdam, The Netherlands, 1977; p. 264. [Google Scholar]







| Q-Ap-U-REE | Q-U-Mo | Q-Ap-U-REE | Q-U-Mo | ||||
|---|---|---|---|---|---|---|---|
| Li | 2.3 | 3.7 | ppm | In | 0.03 | <0.01 | ppm |
| Na | 0.032 | 0.016 | wt. % | Tl | <0.05 | 0.26 | ppm |
| K | 0.2 | 0.19 | wt. % | P | 3.039 | 0.031 | wt. % |
| Rb | 10.3 | 13.3 | ppm | As | 19.9 | 1823.9 | ppm |
| Cs | 0.2 | 1.2 | ppm | Sb | 15.8 | 20.26 | ppm |
| Be | <1 | 1 | ppm | Bi | 6.92 | 3.85 | ppm |
| Mg | 0.05 | 0.1 | wt. % | Al | 0.56 | 0.47 | wt. % |
| Ca | 7.26 | 0.08 | wt. % | S | <0.04 | 0.08 | wt. % |
| Sr | 204 | 16 | ppm | Se | <0.3 | <0.3 | ppm |
| Ba | 73 | 39 | ppm | Te | 0.29 | 0.1 | ppm |
| Ti | 0.025 | 0.011 | wt. % | Sc | 13.7 | 5 | ppm |
| Zr | 0.3 | 1.9 | ppm | Y | 104.1 | 24.5 | ppm |
| Hf | <0.02 | <0.02 | ppm | La | 106.5 | 3.3 | ppm |
| V | <1 | <1 | ppm | Ce | 281.8 | 8.7 | ppm |
| Nb | 4.25 | 2.37 | ppm | Pr | 41.8 | 1.6 | ppm |
| Ta | <0.01 | <0.01 | ppm | Nd | 209.5 | 9.6 | ppm |
| Cr | 2 | 2 | ppm | Sm | 75.1 | 6.8 | ppm |
| Mo | 1.51 | 73.36 | ppm | Eu | 29.2 | 1.5 | ppm |
| W | 37 | 45.1 | ppm | Gd | 68.7 | 8.1 | ppm |
| Sn | 3.4 | 0.3 | ppm | Tb | 10.1 | 1.7 | ppm |
| Re | <0.002 | <0.002 | ppm | Dy | 48.5 | 10.4 | ppm |
| Mn | 75 | 23 | ppm | Ho | 5.7 | 1.4 | ppm |
| Fe | 0.3 | 0.45 | wt. % | Er | 15.4 | 3.7 | ppm |
| Co | 0.4 | 7.9 | ppm | Tm | 1.8 | 0.5 | ppm |
| Ni | 0.1 | 6.3 | ppm | Yb | 13 | 3.6 | ppm |
| Cu | 41.8 | 37.1 | ppm | Lu | 1.2 | 0.3 | ppm |
| Zn | 3.4 | 9.6 | ppm | Th | 2.6 | 0.3 | ppm |
| Pb | 189.93 | 93.23 | ppm | U | >4000 | 2639.1 | ppm |
| Cd | 0.03 | 0.17 | ppm | YREE | 1012.4 | 85.7 | ppm |
| Ag | 154 | 139 | ppm | LREE | 812.6 | 39.6 | ppm |
| Ga | 3.73 | 1.55 | ppm | HREE | 95.7 | 21.6 | ppm |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UO2 | 88.64 | 89.20 | 88.90 | 89.58 | 89.04 | 89.64 | 88.89 | 90.42 | 88.95 | 88.73 | 88.98 | 89.47 | 89.25 | 89.92 |
| CaO | 0.57 | 0.54 | 0.70 | 0.67 | 0.56 | 0.63 | 0.70 | 0.67 | 0.82 | 0.75 | 0.50 | 0.69 | 0.60 | 0.67 |
| FeO | 0.01 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | 0.00 | 0.00 | 0.09 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| PbO | 3.08 | 3.11 | 3.08 | 3.09 | 3.08 | 3.10 | 3.07 | 3.09 | 2.77 | 3.02 | 3.08 | 3.12 | 3.04 | 3.05 |
| Al2O3 | 0.12 | 0.08 | 0.08 | 0.08 | 0.13 | 0.10 | 0.08 | 0.11 | 0.05 | 0.12 | 0.09 | 0.07 | 0.12 | 0.05 |
| Y2O3 | 0.42 | 0.55 | 0.61 | 0.64 | 0.34 | 0.58 | 0.64 | 0.56 | 0.77 | 0.54 | 0.59 | 0.65 | 0.41 | 0.71 |
| La2O3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ce2O3 | 0.23 | 0.24 | 0.24 | 0.23 | 0.31 | 0.27 | 0.21 | 0.22 | 0.34 | 0.28 | 0.34 | 0.30 | 0.25 | 0.23 |
| Pr2O3 | 0.32 | 0.39 | 0.33 | 0.32 | 0.28 | 0.39 | 0.36 | 0.40 | 0.33 | 0.27 | 0.31 | 0.31 | 0.31 | 0.35 |
| Nd2O3 | 0.16 | 0.17 | 0.21 | 0.18 | 0.02 | 0.15 | 0.21 | 0.19 | 0.26 | 0.16 | 0.19 | 0.23 | 0.14 | 0.22 |
| Sm2O3 | 0.21 | 0.26 | 0.30 | 0.34 | 0.22 | 0.26 | 0.25 | 0.23 | 0.36 | 0.28 | 0.27 | 0.28 | 0.20 | 0.25 |
| Eu2O3 | 0.30 | 0.35 | 0.30 | 0.35 | 0.30 | 0.37 | 0.31 | 0.31 | 0.36 | 0.27 | 0.31 | 0.36 | 0.35 | 0.41 |
| Gd2O3 | 0.30 | 0.26 | 0.35 | 0.38 | 0.28 | 0.41 | 0.36 | 0.37 | 0.44 | 0.34 | 0.33 | 0.37 | 0.29 | 0.39 |
| Tb2O3 | 0.12 | 0.09 | 0.08 | 0.14 | 0.09 | 0.09 | 0.05 | 0.09 | 0.14 | 0.10 | 0.11 | 0.09 | 0.15 | 0.17 |
| Dy2O3 | 0.18 | 0.18 | 0.28 | 0.29 | 0.16 | 0.22 | 0.26 | 0.25 | 0.35 | 0.25 | 0.21 | 0.28 | 0.20 | 0.30 |
| Ho2O3 | 0.09 | 0.07 | 0.03 | 0.05 | 0.03 | 0.15 | 0.10 | 0.00 | 0.03 | 0.00 | 0.07 | 0.04 | 0.09 | 0.06 |
| Er2O3 | 0.56 | 0.70 | 0.64 | 0.59 | 0.58 | 0.65 | 0.54 | 0.57 | 0.60 | 0.63 | 0.60 | 0.61 | 0.53 | 0.59 |
| Tm2O3 | 0.12 | 0.10 | 0.13 | 0.14 | 0.12 | 0.09 | 0.12 | 0.15 | 0.10 | 0.10 | 0.12 | 0.16 | 0.13 | 0.15 |
| Yb2O3 | 0.26 | 0.34 | 0.30 | 0.37 | 0.23 | 0.33 | 0.32 | 0.34 | 0.36 | 0.28 | 0.33 | 0.28 | 0.28 | 0.30 |
| Lu2O3 | 0.10 | 0.14 | 0.15 | 0.08 | 0.04 | 0.10 | 0.13 | 0.19 | 0.12 | 0.07 | 0.17 | 0.18 | 0.19 | 0.10 |
| SiO2 | 1.43 | 1.05 | 1.09 | 0.95 | 1.54 | 1.05 | 0.98 | 1.30 | 0.73 | 1.39 | 1.21 | 0.92 | 1.35 | 0.77 |
| P2O5 | 0.04 | 0.04 | 0.04 | 0.04 | 0.07 | 0.06 | 0.04 | 0.05 | 0.04 | 0.06 | 0.04 | 0.05 | 0.06 | 0.04 |
| As2O5 | 0.16 | 0.15 | 0.15 | 0.16 | 0.18 | 0.15 | 0.16 | 0.15 | 0.16 | 0.16 | 0.15 | 0.16 | 0.16 | 0.15 |
| SO3 | 0.02 | 0.01 | 0.01 | 0.02 | 0.00 | 0.00 | 0.00 | 0.03 | 0.04 | 0.00 | 0.00 | 0.01 | 0.02 | 0.02 |
| Total wt. % | 97.44 | 98.01 | 98.00 | 98.68 | 97.61 | 98.80 | 97.78 | 99.69 | 98.19 | 97.81 | 97.99 | 98.63 | 98.10 | 98.87 |
| Y + REE wt. % | 3.36 | 3.82 | 3.95 | 4.09 | 2.99 | 4.06 | 3.86 | 3.86 | 4.54 | 3.59 | 3.94 | 4.16 | 3.51 | 4.21 |
| empirical formula (calculation on basis of 2 oxygens) | ||||||||||||||
| U | 0.855 | 0.868 | 0.861 | 0.866 | 0.855 | 0.863 | 0.867 | 0.855 | 0.866 | 0.852 | 0.861 | 0.867 | 0.857 | 0.874 |
| Ca | 0.027 | 0.026 | 0.033 | 0.031 | 0.026 | 0.029 | 0.033 | 0.030 | 0.038 | 0.035 | 0.023 | 0.032 | 0.028 | 0.031 |
| Fe | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.003 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Pb | 0.036 | 0.037 | 0.036 | 0.036 | 0.036 | 0.036 | 0.036 | 0.035 | 0.033 | 0.035 | 0.036 | 0.037 | 0.035 | 0.036 |
| Al | 0.006 | 0.004 | 0.004 | 0.004 | 0.006 | 0.005 | 0.004 | 0.006 | 0.002 | 0.006 | 0.005 | 0.004 | 0.006 | 0.003 |
| Y | 0.010 | 0.013 | 0.014 | 0.015 | 0.008 | 0.013 | 0.015 | 0.013 | 0.018 | 0.012 | 0.014 | 0.015 | 0.009 | 0.016 |
| La | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Ce | 0.004 | 0.004 | 0.004 | 0.004 | 0.005 | 0.004 | 0.003 | 0.003 | 0.005 | 0.004 | 0.005 | 0.005 | 0.004 | 0.004 |
| Pr | 0.005 | 0.006 | 0.005 | 0.005 | 0.004 | 0.006 | 0.006 | 0.006 | 0.005 | 0.004 | 0.005 | 0.005 | 0.005 | 0.006 |
| Nd | 0.002 | 0.003 | 0.003 | 0.003 | 0.000 | 0.002 | 0.003 | 0.003 | 0.004 | 0.002 | 0.003 | 0.004 | 0.002 | 0.003 |
| Sm | 0.003 | 0.004 | 0.004 | 0.005 | 0.003 | 0.004 | 0.004 | 0.003 | 0.005 | 0.004 | 0.004 | 0.004 | 0.003 | 0.004 |
| Eu | 0.004 | 0.005 | 0.004 | 0.005 | 0.004 | 0.005 | 0.005 | 0.005 | 0.005 | 0.004 | 0.005 | 0.005 | 0.005 | 0.006 |
| Gd | 0.004 | 0.004 | 0.005 | 0.005 | 0.004 | 0.006 | 0.005 | 0.005 | 0.006 | 0.005 | 0.005 | 0.005 | 0.004 | 0.006 |
| Tb | 0.002 | 0.001 | 0.001 | 0.002 | 0.001 | 0.001 | 0.001 | 0.001 | 0.002 | 0.001 | 0.002 | 0.001 | 0.002 | 0.003 |
| Dy | 0.002 | 0.002 | 0.004 | 0.004 | 0.002 | 0.003 | 0.004 | 0.003 | 0.005 | 0.004 | 0.003 | 0.004 | 0.003 | 0.004 |
| Ho | 0.001 | 0.001 | 0.000 | 0.001 | 0.000 | 0.002 | 0.001 | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 | 0.001 | 0.001 |
| Er | 0.008 | 0.010 | 0.009 | 0.008 | 0.008 | 0.009 | 0.007 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.007 | 0.008 |
| Tm | 0.002 | 0.001 | 0.002 | 0.002 | 0.002 | 0.001 | 0.002 | 0.002 | 0.001 | 0.001 | 0.002 | 0.002 | 0.002 | 0.002 |
| Yb | 0.003 | 0.005 | 0.004 | 0.005 | 0.003 | 0.004 | 0.004 | 0.004 | 0.005 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 |
| Lu | 0.001 | 0.002 | 0.002 | 0.001 | 0.001 | 0.001 | 0.002 | 0.002 | 0.002 | 0.001 | 0.002 | 0.002 | 0.002 | 0.001 |
| Si | 0.062 | 0.046 | 0.047 | 0.041 | 0.066 | 0.045 | 0.043 | 0.055 | 0.032 | 0.060 | 0.052 | 0.040 | 0.058 | 0.034 |
| P | 0.002 | 0.002 | 0.002 | 0.001 | 0.003 | 0.002 | 0.001 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.001 |
| As | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.003 | 0.004 | 0.003 | 0.004 | 0.004 | 0.003 | 0.004 | 0.004 | 0.003 |
| S | 0.001 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 |
| Y + REE | 0.052 | 0.060 | 0.062 | 0.065 | 0.046 | 0.064 | 0.062 | 0.059 | 0.073 | 0.056 | 0.062 | 0.066 | 0.054 | 0.067 |
| LREE | 0.023 | 0.026 | 0.026 | 0.027 | 0.021 | 0.028 | 0.026 | 0.026 | 0.032 | 0.024 | 0.027 | 0.028 | 0.023 | 0.028 |
| HREE | 0.019 | 0.022 | 0.022 | 0.023 | 0.017 | 0.022 | 0.021 | 0.021 | 0.023 | 0.019 | 0.022 | 0.022 | 0.021 | 0.023 |
| Sample | Pb meas. | Th wt. % | U wt. % | Pb wt. % | Y wt. % | Ucorr. | Pbcorr. | Th 2σ | U 2σ | Pb 2σ | U at. % | Pb at. % | Age (Ma) | 1σ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | C29-6 | 2.8617 | blld | 78.1362 | 2.9151 | 0.3276 | 75.7921 | 2.9058 | 0 | 0.2715 | 0.0234 | 95.9646 | 4.0354 | 268 | 2.3 |
| 2 | C29-6 | 2.8862 | blld | 78.6259 | 2.9395 | 0.4301 | 76.2671 | 2.9298 | 0 | 0.2726 | 0.0236 | 95.9568 | 4.0432 | 268 | 2.3 |
| 3 | C29-6 | 2.8628 | blld | 78.3653 | 2.9156 | 0.4793 | 76.0143 | 2.9058 | 0 | 0.2718 | 0.0233 | 95.9757 | 4.0243 | 267 | 2.3 |
| 4 | C29-6 | 2.8746 | blld | 78.9599 | 2.9277 | 0.5013 | 76.5911 | 2.9178 | 0 | 0.2735 | 0.0235 | 95.9892 | 4.0108 | 266 | 2.3 |
| 5 | C29-6 | 2.8707 | blld | 78.8515 | 2.9238 | 0.4952 | 76.4860 | 2.9138 | 0 | 0.2731 | 0.0234 | 95.9890 | 4.0110 | 266 | 2.3 |
| 6 | C29-6 | 2.8577 | blld | 78.4893 | 2.9116 | 0.2694 | 76.1346 | 2.9024 | 0 | 0.2725 | 0.0233 | 95.9870 | 4.0130 | 266 | 2.3 |
| 7 | C29-6 | 2.8826 | blld | 79.0197 | 2.9360 | 0.4603 | 76.6491 | 2.9262 | 0 | 0.2735 | 0.0236 | 95.9810 | 4.0190 | 267 | 2.3 |
| 8 | C29-6 | 2.8700 | blld | 78.5859 | 2.9231 | 0.4610 | 76.2283 | 2.9133 | 0 | 0.2725 | 0.0235 | 95.9767 | 4.0233 | 267 | 2.3 |
| 9 | C29-6 | 2.8542 | blld | 78.3536 | 2.9069 | 0.5019 | 76.0030 | 2.8969 | 0 | 0.2720 | 0.0234 | 95.9869 | 4.0131 | 266 | 2.3 |
| 10 | C29-6 | 2.8105 | blld | 78.5054 | 2.8634 | 0.4521 | 76.1502 | 2.8537 | 0 | 0.2724 | 0.0231 | 96.0530 | 3.9470 | 262 | 2.3 |
| 11 | C29-2 | 2.8083 | blld | 78.4629 | 2.8613 | 0.4380 | 76.1090 | 2.8516 | 0 | 0.2723 | 0.0231 | 96.0538 | 3.9462 | 262 | 2.3 |
| 12 | C29-2 | 2.8682 | blld | 79.7079 | 2.9221 | 0.4409 | 77.3167 | 2.9124 | 0 | 0.2757 | 0.0235 | 96.0334 | 3.9666 | 263 | 2.3 |
| 13 | C29-2 | 2.5789 | blld | 78.4052 | 2.6304 | 0.6066 | 76.0530 | 2.6202 | 0 | 0.2719 | 0.0215 | 96.3633 | 3.6367 | 241 | 2.1 |
| 14 | C29-2 | 2.8355 | blld | 78.7048 | 2.8888 | 0.4129 | 76.3437 | 2.8792 | 0 | 0.2728 | 0.0233 | 96.0285 | 3.9715 | 264 | 2.3 |
| 15 | C29-2 | 2.8035 | blld | 78.2172 | 2.8563 | 0.4273 | 75.8707 | 2.8466 | 0 | 0.2717 | 0.0231 | 96.0482 | 3.9518 | 262 | 2.3 |
| 16 | C29-2 | 2.8910 | blld | 78.8949 | 2.9444 | 0.4444 | 76.5281 | 2.9347 | 0 | 0.2734 | 0.0237 | 95.9637 | 4.0363 | 268 | 2.3 |
| 17 | C29-2 | 2.8649 | blld | 78.4324 | 2.9179 | 0.4630 | 76.0794 | 2.9081 | 0 | 0.2722 | 0.0235 | 95.9762 | 4.0238 | 267 | 2.3 |
| 18 | C29-2 | 2.8956 | blld | 78.8696 | 2.9487 | 0.5106 | 76.5035 | 2.9387 | 0 | 0.2733 | 0.0237 | 95.9565 | 4.0435 | 268 | 2.3 |
| 19 | C29-2 | 2.8202 | blld | 78.6703 | 2.8739 | 0.3232 | 76.3102 | 2.8646 | 0 | 0.2729 | 0.0231 | 96.0469 | 3.9531 | 262 | 2.3 |
| 20 | C29-2 | 2.8051 | blld | 78.7543 | 2.8580 | 0.4814 | 76.3917 | 2.8482 | 0 | 0.2731 | 0.0229 | 96.0724 | 3.9276 | 261 | 2.2 |
| 21 | C29-2 | 2.8504 | blld | 78.3925 | 2.9031 | 0.5135 | 76.0407 | 2.8931 | 0 | 0.2721 | 0.0234 | 95.9939 | 4.0061 | 266 | 2.3 |
| 22 | C29-2 | 2.8345 | blld | 79.2638 | 2.8874 | 0.5555 | 76.8859 | 2.8773 | 0 | 0.2743 | 0.0233 | 96.0579 | 3.9421 | 262 | 2.3 |
| 23 | C29-2 | 2.8850 | blld | 78.4314 | 2.9380 | 0.4641 | 76.0785 | 2.9282 | 0 | 0.2721 | 0.0235 | 95.9490 | 4.0510 | 269 | 2.3 |
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| Fe | 35.36 | 37.47 | 37.72 | 40.29 | 42.34 |
| Ni | 0.11 | 0.09 | 0.11 | 0.07 | 0.08 |
| Cu | 0.27 | 0.35 | 0.34 | 0.25 | 0.33 |
| Pb | 0.6 | 0.78 | 0.58 | 0.5 | 0.65 |
| Sb | 4.37 | 3.07 | 3.16 | 1.33 | 0.76 |
| Bi | 9.91 | 6.9 | 7.19 | 3.36 | 2.11 |
| S | 38.59 | 42.6 | 42.31 | 47.07 | 48.51 |
| Total wt. % | 89.21 | 91.26 | 91.41 | 92.87 | 94.78 |
| Empirical formula (on basis of the 3 atoms) | |||||
| Fe | 0.985 | 0.973 | 0.981 | 0.973 | 0.990 |
| Ni | 0.003 | 0.002 | 0.003 | 0.002 | 0.002 |
| Cu | 0.007 | 0.008 | 0.008 | 0.005 | 0.007 |
| Pb | 0.005 | 0.005 | 0.004 | 0.003 | 0.004 |
| Sb | 0.056 | 0.037 | 0.038 | 0.015 | 0.008 |
| Bi | 0.074 | 0.048 | 0.050 | 0.022 | 0.013 |
| Σ | 1.128 | 1.073 | 1.083 | 1.020 | 1.024 |
| S | 1.872 | 1.927 | 1.917 | 1.980 | 1.976 |
| Cat./S | 0.60 | 0.56 | 0.57 | 0.51 | 0.52 |
| 1 | 2 | 3 | |
|---|---|---|---|
| Ni | 27.21 | 27.43 | 27.46 |
| Co | 0.05 | 0.09 | 0.03 |
| Fe | 0.04 | 0.03 | 0.04 |
| As | 0.00 | 0.07 | 0.01 |
| Sb | 56.20 | 56.00 | 55.92 |
| Bi | 0.12 | 0.08 | 0.17 |
| Se | 0.05 | 0.03 | 0.12 |
| S | 14.42 | 14.48 | 14.85 |
| Total wt. % | 98.09 | 98.21 | 98.59 |
| Empirical formula (on basis of 3 atoms) | |||
| Ni | 1.009 | 1.014 | 1.007 |
| Co | 0.002 | 0.003 | 0.001 |
| Fe | 0.001 | 0.001 | 0.002 |
| As | 0.000 | 0.002 | 0.000 |
| Sb | 1.005 | 0.998 | 0.988 |
| Bi | 0.001 | 0.001 | 0.002 |
| Se | 0.001 | 0.001 | 0.003 |
| S | 0.980 | 0.980 | 0.997 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| Fe | 4.94 | 4.48 | 4.48 | 4.30 | 5.63 | 7.12 | 6.46 | 6.57 |
| Co | 28.10 | 27.69 | 29.70 | 29.39 | 29.43 | 18.95 | 20.86 | 21.21 |
| Ni | 2.19 | 2.90 | 2.20 | 2.33 | 1.21 | 8.80 | 7.55 | 7.26 |
| As | 41.96 | 42.17 | 41.43 | 41.81 | 40.13 | 44.60 | 44.33 | 44.32 |
| S | 21.85 | 21.22 | 21.38 | 21.25 | 22.28 | 19.63 | 19.61 | 19.78 |
| Total wt. % | 99.05 | 98.46 | 99.19 | 99.08 | 98.68 | 99.10 | 98.81 | 99.14 |
| Empirical formula (on basis of the 3 atoms) | ||||||||
| Fe | 0.144 | 0.132 | 0.131 | 0.126 | 0.163 | 0.212 | 0.193 | 0.195 |
| Co | 0.776 | 0.773 | 0.821 | 0.815 | 0.809 | 0.534 | 0.589 | 0.597 |
| Ni | 0.061 | 0.081 | 0.061 | 0.065 | 0.033 | 0.249 | 0.214 | 0.205 |
| Σ | 0.980 | 0.986 | 1.013 | 1.005 | 1.006 | 0.995 | 0.996 | 0.997 |
| As | 0.911 | 0.926 | 0.901 | 0.912 | 0.868 | 0.989 | 0.985 | 0.981 |
| S | 1.109 | 1.089 | 1.086 | 1.083 | 1.126 | 1.017 | 1.019 | 1.023 |
| Σ | 2.020 | 2.014 | 1.987 | 1.995 | 1.994 | 2.005 | 2.004 | 2.003 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bi | 59.64 | 59.76 | 59.07 | 77.84 | 78.66 | 78.73 | 79.51 | 79.56 | 75.01 | 74.34 | 75.10 | 71.06 | 72.41 | 72.27 |
| Sb | 0.82 | 1.04 | 1.01 | 0.53 | 0.29 | 0.29 | 0.29 | 0.29 | 0.40 | 0.43 | 0.36 | 1.96 | 0.55 | 0.65 |
| Fe | 0.00 | 0.01 | 0.00 | 0.46 | 0.70 | 0.24 | 0.05 | 0.06 | 0.19 | 0.28 | 0.22 | 0.09 | 0.00 | 0.00 |
| Cu | 0.00 | 0.00 | 0.00 | 0.08 | 0.00 | 0.00 | 0.00 | 0.07 | 0.00 | 0.00 | 0.00 | 0.23 | 0.44 | 0.47 |
| In | 0.06 | 0.00 | 0.09 | 0.08 | 0.00 | 0.00 | 0.06 | |||||||
| Pb | 0.00 | 0.19 | 0.32 | 1.60 | 1.17 | 0.93 | 1.44 | 1.45 | 0.98 | 1.04 | 1.38 | 2.28 | 2.84 | 2.80 |
| Te | 34.14 | 34.03 | 34.63 | 11.74 | 11.96 | 11.99 | 12.18 | 11.95 | 21.56 | 21.75 | 21.49 | 17.86 | 17.89 | 17.31 |
| S | 4.22 | 4.44 | 4.34 | 6.13 | 5.96 | 6.58 | 6.07 | 6.12 | 2.63 | 2.68 | 2.75 | 4.32 | 3.44 | 3.70 |
| Se | 0.26 | 0.20 | 0.30 | 1.00 | 0.91 | 1.09 | 0.90 | 0.85 | 0.09 | 0.20 | 0.19 | 1.07 | 1.13 | 1.05 |
| Total wt. % | 99.08 | 99.67 | 99.66 | 99.44 | 99.65 | 99.94 | 100.52 | 100.35 | 100.87 | 100.71 | 101.50 | 98.85 | 98.70 | 98.31 |
| Empirical formulae | ||||||||||||||
| Bi | 2.054 | 2.033 | 2.011 | 3.776 | 3.829 | 3.758 | 3.873 | 3.876 | 4.036 | 3.980 | 3.996 | 2.575 | 2.734 | 2.715 |
| Sb | 0.048 | 0.061 | 0.059 | 0.044 | 0.024 | 0.024 | 0.024 | 0.024 | 0.037 | 0.040 | 0.033 | 0.122 | 0.035 | 0.042 |
| Fe | 0.000 | 0.001 | 0.000 | 0.084 | 0.128 | 0.043 | 0.009 | 0.011 | 0.039 | 0.055 | 0.045 | 0.012 | 0.000 | 0.000 |
| Cu | 0.000 | 0.000 | 0.000 | 0.013 | 0.000 | 0.000 | 0.000 | 0.011 | 0.000 | 0.000 | 0.000 | 0.027 | 0.055 | 0.058 |
| In | 0.005 | 0.000 | 0.008 | 0.007 | 0.000 | 0.000 | 0.004 | |||||||
| Pb | 0.000 | 0.007 | 0.011 | 0.078 | 0.057 | 0.045 | 0.071 | 0.071 | 0.053 | 0.056 | 0.074 | 0.083 | 0.108 | 0.106 |
| Σ | 2.103 | 2.102 | 2.081 | 4.000 | 4.038 | 3.877 | 3.985 | 3.994 | 4.165 | 4.131 | 4.148 | 2.819 | 2.933 | 2.925 |
| Te | 1.926 | 1.896 | 1.930 | 0.933 | 0.953 | 0.937 | 0.972 | 0.953 | 1.900 | 1.907 | 1.873 | 1.060 | 1.106 | 1.065 |
| S | 0.948 | 0.984 | 0.962 | 1.939 | 1.891 | 2.047 | 1.928 | 1.943 | 0.922 | 0.934 | 0.952 | 1.019 | 0.848 | 0.906 |
| Se | 0.024 | 0.018 | 0.027 | 0.128 | 0.117 | 0.138 | 0.116 | 0.110 | 0.013 | 0.028 | 0.026 | 0.103 | 0.113 | 0.104 |
| Σ | 0.972 | 1.002 | 0.988 | 2.067 | 2.008 | 2.185 | 2.044 | 2.053 | 0.935 | 0.962 | 0.979 | 1.122 | 0.961 | 1.010 |
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Bi | 64.37 | 70.13 | 71.34 | 71.68 |
| Sb | 4.13 | 1.62 | 3.94 | 4.01 |
| Fe | 0.17 | 0.17 | 0.00 | 0.00 |
| Cu | 0.46 | 0.28 | 0.37 | 0.40 |
| In | 0.00 | 0.07 | 0.00 | 0.07 |
| Pb | 11.40 | 6.33 | 7.21 | 8.11 |
| Te | 10.71 | 9.26 | 7.73 | 7.59 |
| S | 7.95 | 6.63 | 8.31 | 8.56 |
| Se | 1.16 | 1.49 | 1.54 | 1.40 |
| Total wt. % | 100.35 | 95.98 | 100.44 | 101.82 |
| Empirical formula (calculated on the basis of 2 atoms) | ||||
| Bi | 0.817 | 0.979 | 0.906 | 0.895 |
| Sb | 0.090 | 0.039 | 0.086 | 0.086 |
| Fe | 0.008 | 0.009 | 0.000 | 0.000 |
| Cu | 0.019 | 0.013 | 0.015 | 0.016 |
| In | 0.000 | 0.002 | 0.000 | 0.002 |
| Pb | 0.146 | 0.089 | 0.092 | 0.102 |
| Σ | 1.080 | 1.130 | 1.100 | 1.101 |
| Te | 0.223 | 0.212 | 0.161 | 0.155 |
| S | 0.658 | 0.603 | 0.688 | 0.697 |
| Se | 0.039 | 0.055 | 0.052 | 0.046 |
| Σ | 0.920 | 0.870 | 0.900 | 0.899 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bi | 98.40 | 82.77 | 79.70 | 64.51 | 63.16 | 62.54 | 63.08 | 62.96 | 89.77 | 87.93 | 87.48 | 88.69 | 88.72 |
| Sb | 0.51 | 0.00 | 0.01 | 13.21 | 13.40 | 13.44 | 13.54 | 13.60 | 0.00 | 0.00 | 0.08 | 0.00 | 0.00 |
| Cu | 0.30 | 0.14 | 0.09 | 0.49 | 0.38 | 0.37 | 0.40 | 0.26 | 0.15 | 0.21 | 0.12 | 0.00 | 0.00 |
| In | 0.00 | 0.06 | 0.00 | 0.00 | 0.08 | 0.09 | 0.07 | ||||||
| Pb | 0.66 | 0.66 | 1.55 | 1.66 | 1.26 | 1.22 | 1.29 | 1.05 | 0.00 | 0.04 | 0.04 | 0.00 | 0.00 |
| S | 18.24 | 17.67 | 18.01 | 19.98 | 19.81 | 19.92 | 20.27 | 10.35 | 10.24 | 10.43 | 10.52 | 10.39 | |
| Se | 0.28 | 0.00 | 0.02 | 0.71 | 0.74 | 0.74 | 0.73 | 0.27 | 0.00 | 0.11 | 0.19 | 0.00 | 0.00 |
| Te | 0.00 | 0.00 | 0.00 | 0.00 | 0.12 | 0.43 | 0.96 | 0.44 | 0.43 | ||||
| Total wt. % | 100.15 | 101.81 | 99.04 | 98.59 | 98.92 | 98.12 | 98.97 | 98.47 | 100.27 | 98.53 | 98.42 | 99.30 | 99.18 |
| Empirical formulae | |||||||||||||
| Bi | 0.968 | 2.041 | 2.025 | 1.538 | 1.430 | 1.427 | 1.429 | 1.423 | 3.979 | 3.935 | 3.869 | 3.926 | 3.949 |
| Sb | 0.009 | 0.000 | 0.000 | 0.541 | 0.521 | 0.526 | 0.527 | 0.528 | 0.000 | 0.000 | 0.006 | 0.000 | 0.000 |
| Cu | 0.010 | 0.011 | 0.007 | 0.039 | 0.029 | 0.028 | 0.030 | 0.019 | 0.022 | 0.031 | 0.017 | 0.000 | 0.000 |
| In | 0.000 | 0.002 | 0.000 | 0.000 | 0.006 | 0.007 | 0.006 | ||||||
| Pb | 0.007 | 0.016 | 0.040 | 0.040 | 0.029 | 0.028 | 0.030 | 0.024 | 0.000 | 0.002 | 0.002 | 0.000 | 0.000 |
| Σ | 0.993 | 2.069 | 2.072 | 2.157 | 2.008 | 2.009 | 2.015 | 1.997 | 4.001 | 3.968 | 3.901 | 3.933 | 3.954 |
| S | 0.000 | 2.931 | 2.927 | 2.798 | 2.948 | 2.946 | 2.941 | 2.987 | 2.990 | 2.987 | 3.007 | 3.035 | 3.014 |
| Se | 0.007 | 0.000 | 0.001 | 0.045 | 0.044 | 0.045 | 0.044 | 0.016 | 0.000 | 0.013 | 0.022 | 0.000 | 0.000 |
| Te | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.009 | 0.032 | 0.070 | 0.032 | 0.031 | |
| Σ | 0.007 | 2.931 | 2.928 | 2.843 | 2.992 | 2.991 | 2.985 | 3.003 | 2.999 | 3.032 | 3.099 | 3.067 | 3.046 |
| Bi/(Bi+Sb) | 0.99 | 1.00 | 1.00 | 0.74 | 0.73 | 0.73 | 0.73 | 0.73 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ag | 0.00 | 0.00 | 0.00 | 0.18 | 0.24 | 0.00 | 0.11 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| Cu | 0.00 | 0.00 | 0.00 | 1.65 | 1.70 | 1.30 | 1.47 | 1.61 | 1.51 | 1.73 | 1.66 | 1.29 | 0.98 | 0.90 | 0.89 |
| Fe | 2.29 | 2.29 | 2.55 | 0.44 | 0.42 | 0.79 | 0.57 | 0.38 | 0.66 | 0.46 | 0.35 | 0.68 | 0.86 | 0.96 | 0.89 |
| Cd | 0.00 | 0.00 | 0.00 | 0.31 | 0.23 | 0.20 | 0.09 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| Pb | 36.70 | 36.09 | 36.26 | 34.28 | 33.38 | 37.23 | 34.70 | 34.56 | 36.68 | 34.12 | 33.76 | 35.74 | 35.20 | 34.66 | 34.75 |
| Bi | 17.01 | 17.16 | 17.91 | 26.57 | 27.03 | 26.24 | 26.98 | 28.39 | 27.64 | 29.15 | 29.74 | 28.28 | 38.98 | 40.37 | 40.63 |
| Sb | 23.86 | 23.18 | 22.97 | 17.58 | 17.00 | 15.42 | 15.69 | 15.96 | 15.30 | 15.43 | 15.62 | 14.62 | 7.31 | 5.61 | 5.63 |
| S | 20.38 | 20.12 | 20.33 | 19.12 | 18.97 | 18.25 | 18.38 | 18.81 | 18.46 | 18.89 | 18.58 | 18.61 | 18.19 | 17.58 | 17.54 |
| Se | 0.15 | 0.19 | 0.11 | 0.23 | 0.16 | 0.10 | 0.20 | 0.50 | 0.41 | 0.18 | 0.61 | 0.23 | 0.23 | 0.28 | 0.27 |
| Total wt. % | 100.39 | 99.03 | 100.13 | 100.36 | 99.13 | 99.32 | 98.30 | 100.21 | 100.68 | 100.05 | 100.32 | 99.45 | 101.75 | 100.36 | 100.60 |
| Empirical formula (calculated on the basis of 63 atoms) | |||||||||||||||
| Ag | 0.000 | 0.000 | 0.000 | 0.098 | 0.132 | 0.000 | 0.062 | 0.000 | 0.010 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Cu | 0.000 | 0.000 | 0.000 | 1.522 | 1.586 | 1.239 | 1.405 | 1.507 | 1.423 | 1.620 | 1.563 | 1.225 | 0.955 | 0.901 | 0.892 |
| Fe | 2.280 | 2.311 | 2.545 | 0.462 | 0.446 | 0.855 | 0.620 | 0.405 | 0.706 | 0.490 | 0.375 | 0.735 | 0.953 | 1.094 | 1.015 |
| Cd | 0.000 | 0.000 | 0.000 | 0.162 | 0.121 | 0.000 | 0.108 | 0.000 | 0.000 | 0.048 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Σ | 2.280 | 2.311 | 2.545 | 2.244 | 2.286 | 2.094 | 2.195 | 1.912 | 2.139 | 2.158 | 1.938 | 1.960 | 1.908 | 1.996 | 1.908 |
| Pb | 9.848 | 9.818 | 9.752 | 9.700 | 9.554 | 10.917 | 10.170 | 9.924 | 10.602 | 9.800 | 9.747 | 10.411 | 10.518 | 10.647 | 10.685 |
| Bi | 4.526 | 4.629 | 4.776 | 7.454 | 7.670 | 7.629 | 7.840 | 8.082 | 7.921 | 8.301 | 8.513 | 8.168 | 11.548 | 12.296 | 12.387 |
| Sb | 10.896 | 10.731 | 10.513 | 8.465 | 8.280 | 7.693 | 7.825 | 7.798 | 7.528 | 7.541 | 7.674 | 7.247 | 3.717 | 2.933 | 2.946 |
| Σ | 15.421 | 15.360 | 15.289 | 15.919 | 15.950 | 15.322 | 15.666 | 15.881 | 15.449 | 15.842 | 16.187 | 15.416 | 15.265 | 15.229 | 15.333 |
| S | 35.345 | 35.375 | 35.337 | 34.966 | 35.090 | 34.589 | 34.816 | 34.907 | 34.496 | 35.064 | 34.667 | 35.037 | 35.128 | 34.903 | 34.857 |
| Se | 0.106 | 0.136 | 0.078 | 0.171 | 0.120 | 0.077 | 0.154 | 0.377 | 0.314 | 0.136 | 0.462 | 0.176 | 0.180 | 0.226 | 0.218 |
| Σ | 35.450 | 35.511 | 35.415 | 35.137 | 35.210 | 34.666 | 34.969 | 35.283 | 34.810 | 35.200 | 35.129 | 35.213 | 35.308 | 35.128 | 35.074 |
| Bi/(Sb + Bi) | 0.29 | 0.30 | 0.31 | 0.47 | 0.48 | 0.50 | 0.50 | 0.51 | 0.51 | 0.52 | 0.53 | 0.53 | 0.76 | 0.81 | 0.81 |
| Sb/(Sb + Bi) | 0.71 | 0.70 | 0.69 | 0.53 | 0.52 | 0.50 | 0.50 | 0.49 | 0.49 | 0.48 | 0.47 | 0.47 | 0.24 | 0.19 | 0.19 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Pb | 39.91 | 39.85 | 40.50 | 40.13 | 40.04 | 39.75 | 39.75 | 40.29 | 40.06 | 40.20 |
| Mn | 0.08 | 0.07 | 0.08 | 0.09 | 0.08 | 0.10 | 0.08 | 0.10 | 0.10 | 0.07 |
| Fe | 0.15 | 0.13 | 0.16 | 0.14 | 0.15 | 0.13 | 0.11 | 0.15 | 0.14 | 0.14 |
| Cu | 0.13 | 0.15 | 0.18 | 0.16 | 0.16 | 0.51 | 0.43 | 0.16 | 0.18 | 0.16 |
| Cd | 0.44 | 0.44 | 0.48 | 0.50 | 0.47 | 0.42 | 0.39 | 0.50 | 0.49 | 0.49 |
| In | 0.06 | 0.06 | 0.06 | 0.08 | 0.00 | 0.07 | 0.00 | 0.07 | 0.00 | 0.07 |
| Sb | 4.09 | 4.07 | 4.45 | 4.52 | 4.15 | 3.84 | 3.86 | 4.15 | 4.26 | 4.38 |
| Bi | 37.89 | 38.08 | 38.62 | 38.01 | 37.88 | 38.87 | 38.65 | 37.55 | 38.16 | 38.39 |
| S | 16.87 | 16.73 | 16.88 | 16.83 | 16.38 | 16.42 | 16.49 | 16.65 | 16.64 | 16.96 |
| Se | 0.33 | 0.41 | 0.41 | 0.42 | 0.41 | 1.00 | 0.78 | 0.37 | 0.41 | 0.41 |
| Total wt. % | 99.95 | 99.99 | 101.82 | 100.88 | 99.72 | 101.11 | 100.54 | 99.99 | 100.44 | 101.27 |
| Empirical formula (calculated on the basis of 9 atoms) | ||||||||||
| Pb | 1.828 | 1.831 | 1.832 | 1.826 | 1.859 | 1.816 | 1.824 | 1.853 | 1.838 | 1.820 |
| Mn | 0.014 | 0.012 | 0.014 | 0.015 | 0.014 | 0.017 | 0.014 | 0.017 | 0.017 | 0.012 |
| Fe | 0.025 | 0.022 | 0.027 | 0.024 | 0.026 | 0.022 | 0.019 | 0.026 | 0.024 | 0.024 |
| Cu | 0.019 | 0.022 | 0.027 | 0.024 | 0.024 | 0.076 | 0.064 | 0.024 | 0.027 | 0.024 |
| Cd | 0.037 | 0.037 | 0.040 | 0.042 | 0.040 | 0.035 | 0.033 | 0.042 | 0.041 | 0.041 |
| In | 0.005 | 0.005 | 0.005 | 0.007 | 0.000 | 0.006 | 0.000 | 0.006 | 0.000 | 0.006 |
| Σ | 1.928 | 1.930 | 1.944 | 1.937 | 1.963 | 1.972 | 1.954 | 1.968 | 1.948 | 1.926 |
| Sb | 0.319 | 0.318 | 0.342 | 0.350 | 0.328 | 0.299 | 0.301 | 0.325 | 0.333 | 0.338 |
| Bi | 1.720 | 1.735 | 1.732 | 1.715 | 1.744 | 1.761 | 1.759 | 1.712 | 1.736 | 1.724 |
| Σ | 2.039 | 2.053 | 2.074 | 2.064 | 2.072 | 2.059 | 2.060 | 2.037 | 2.069 | 2.061 |
| S | 4.993 | 4.968 | 4.934 | 4.948 | 4.915 | 4.848 | 4.891 | 4.950 | 4.934 | 4.964 |
| Se | 0.040 | 0.049 | 0.049 | 0.050 | 0.050 | 0.120 | 0.094 | 0.045 | 0.049 | 0.049 |
| Σ | 5.032 | 5.017 | 4.982 | 4.999 | 4.965 | 4.968 | 4.985 | 4.994 | 4.984 | 5.013 |
| Bi/(Bi+Sb) | 0.84 | 0.84 | 0.83 | 0.83 | 0.84 | 0.86 | 0.85 | 0.84 | 0.84 | 0.84 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe | 2.38 | 2.40 | 2.36 | 2.37 | 2.39 | 2.62 | 2.46 | 2.29 | 2.32 | 2.23 | 0.48 |
| Mn | 0.05 | 0.00 | 0.07 | 0.05 | 0.06 | 0.05 | 0.05 | 0.00 | 0 | ||
| Cu | 0.00 | 0.00 | 0.00 | 0.08 | 0.07 | 0.00 | 0.00 | 0.15 | 1.55 | ||
| Pb | 36.70 | 36.70 | 36.14 | 35.69 | 36.12 | 36.33 | 36.56 | 36.43 | 35.70 | 34.72 | 33.47 |
| As | 0.02 | 0.13 | |||||||||
| Sb | 23.48 | 23.33 | 23.15 | 23.53 | 23.58 | 23.56 | 23.68 | 23.67 | 23.08 | 17.87 | 16.14 |
| Bi | 16.15 | 16.28 | 16.68 | 16.37 | 16.70 | 16.61 | 16.37 | 16.44 | 16.69 | 24.23 | 27.59 |
| S | 19.52 | 19.39 | 20.11 | 20.21 | 20.42 | 20.27 | 20.05 | 19.93 | 20.10 | 19.45 | 18.81 |
| Se | 0.17 | 0.15 | 0.15 | 0.14 | 0.19 | 0.17 | 0.14 | 0.40 | 0.24 | ||
| Total wt. % | 98.25 | 98.22 | 98.66 | 98.32 | 99.43 | 99.66 | 99.44 | 98.98 | 98.08 | 99.05 | 98.28 |
| Empirical formula (calculated on the basis of 25 atoms) | |||||||||||
| Fe | 0.970 | 0.979 | 0.946 | 0.949 | 0.946 | 1.037 | 0.980 | 0.920 | 0.933 | 0.921 | 0.205 |
| Mn | 0.020 | 0.000 | 0.028 | 0.020 | 0.024 | 0.020 | 0.020 | 0.000 | 0.000 | ||
| Cu | 0.000 | 0.000 | 0.000 | 0.028 | 0.025 | 0.000 | 0.000 | 0.054 | 0.581 | ||
| Σ | 0.970 | 0.979 | 0.966 | 0.949 | 0.974 | 1.085 | 1.029 | 0.940 | 0.954 | 0.975 | 0.786 |
| Pb | 4.029 | 4.040 | 3.904 | 3.850 | 3.854 | 3.874 | 3.927 | 3.944 | 3.870 | 3.863 | 3.850 |
| As | 0.006 | 0.038 | |||||||||
| Sb | 4.387 | 4.371 | 4.256 | 4.319 | 4.281 | 4.275 | 4.328 | 4.360 | 4.258 | 3.384 | 3.160 |
| Bi | 1.758 | 1.777 | 1.786 | 1.751 | 1.767 | 1.756 | 1.743 | 1.764 | 1.794 | 2.673 | 3.147 |
| Σ | 6.152 | 6.186 | 6.042 | 6.070 | 6.048 | 6.032 | 6.072 | 6.125 | 6.052 | 6.057 | 6.306 |
| S | 13.848 | 13.796 | 14.040 | 14.089 | 14.081 | 13.970 | 13.919 | 13.943 | 14.084 | 13.988 | 13.985 |
| Se | 0.048 | 0.042 | 0.042 | 0.039 | 0.054 | 0.048 | 0.040 | 0.117 | 0.072 | ||
| Σ | 13.848 | 13.796 | 14.088 | 14.132 | 14.123 | 14.009 | 13.972 | 13.992 | 14.124 | 14.104 | 14.057 |
| Sb/(Sb+Bi) | 0.71 | 0.71 | 0.70 | 0.71 | 0.71 | 0.71 | 0.71 | 0.71 | 0.70 | 0.56 | 0.50 |
| 1 | 2 | |
|---|---|---|
| Pb | 83.76 | 83.41 |
| Fe | 0.00 | 0.05 |
| Cu | 0.09 | 0.06 |
| In | 0.06 | 0.06 |
| Bi | 0.70 | 0.57 |
| S | 12.81 | 12.79 |
| Se | 2.11 | 2.32 |
| Total wt. % | 99.55 | 99.27 |
| Empirical formula (on 2 atoms) | ||
| Pb | 0.967 | 0.963 |
| Fe | 0.000 | 0.002 |
| Cu | 0.004 | 0.002 |
| In | 0.001 | 0.001 |
| Bi | 0.008 | 0.007 |
| Σ | 0.980 | 0.975 |
| S | 0.956 | 0.954 |
| Se | 0.064 | 0.070 |
| Σ | 1.020 | 1.025 |
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| Na2O | 0.04 | 0.03 | 0.03 | 0.04 | 0.01 | 0.03 |
| CaO | 55.03 | 55.15 | 55.17 | 55.47 | 55.33 | 55.49 |
| BaO | 0.29 | 0.00 | 0.00 | 0.12 | 0.00 | 0.00 |
| MnO | 0.01 | 0.00 | 0.04 | 0.00 | 0.00 | 0.04 |
| FeO | 0.00 | 0.00 | 0.02 | 0.10 | 0.04 | 0.01 |
| PbO | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.02 |
| Al2O3 | 0.02 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 |
| Y2O3 | 0.08 | 0.08 | 0.08 | 0.01 | 0.13 | 0.09 |
| La2O3 | 0.05 | 0.00 | 0.03 | 0.01 | 0.06 | 0.00 |
| Ce2O3 | 0.04 | 0.06 | 0.08 | 0.07 | 0.09 | 0.08 |
| Pr2O3 | 0.01 | 0.01 | 0.10 | 0.00 | 0.00 | 0.06 |
| Nd2O3 | 0.03 | 0.05 | 0.06 | 0.06 | 0.05 | 0.12 |
| Sm2O3 | 0.02 | 0.00 | 0.00 | 0.07 | 0.12 | 0.03 |
| Eu2O3 | 0.00 | 0.02 | 0.04 | 0.00 | 0.01 | 0.04 |
| Gd2O3 | 0.10 | 0.08 | 0.04 | 0.04 | 0.01 | 0.00 |
| Tb2O3 | 0.01 | 0.02 | 0.00 | 0.02 | 0.00 | 0.06 |
| Dy2O3 | 0.08 | 0.00 | 0.00 | 0.00 | 0.12 | 0.03 |
| Ho2O3 | 0.10 | 0.04 | 0.01 | 0.04 | 0.12 | 0.00 |
| Er2O3 | 0.02 | 0.02 | 0.00 | 0.00 | 0.08 | 0.00 |
| Lu2O3 | 0.00 | 0.00 | 0.04 | 0.02 | 0.09 | 0.00 |
| TiO2 | 0.06 | 0.00 | 0.09 | 0.01 | 0.09 | 0.11 |
| ZrO2 | 0.06 | 0.11 | 0.05 | 0.06 | 0.10 | 0.11 |
| ThO2 | 0.01 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 |
| UO2 | 0.00 | 0.02 | 0.02 | 0.00 | 0.00 | 0.01 |
| SiO2 | 0.04 | 0.03 | 0.00 | 0.01 | 0.00 | 0.01 |
| P2O5 | 42.82 | 43.00 | 42.42 | 43.06 | 42.63 | 42.88 |
| As2O5 | 0.01 | 0.10 | 0.02 | 0.00 | 0.03 | 0.00 |
| SO3 | 0.01 | 0.02 | 0.00 | 0.03 | 0.02 | 0.01 |
| H2O * | 0.31 | 0.27 | 0.04 | |||
| F | 4.21 | 4.20 | 3.89 | 3.47 | 3.50 | 3.77 |
| Total wt. % | 103.15 | 103.01 | 102.23 | 103.03 | 102.86 | 103.03 |
| F = O | −1.77 | −1.77 | −1.64 | −1.46 | −1.47 | −1.59 |
| Total wt. % | 101.37 | 101.25 | 100.59 | 101.57 | 101.39 | 101.45 |
| Empirical formula (calculated on the basis of 13 anions) | ||||||
| Na | 0.006 | 0.005 | 0.004 | 0.006 | 0.002 | 0.004 |
| Ca | 4.882 | 4.883 | 4.937 | 4.934 | 4.945 | 4.928 |
| Ba | 0.009 | 0.000 | 0.000 | 0.004 | 0.000 | 0.000 |
| Mn | 0.001 | 0.000 | 0.003 | 0.000 | 0.000 | 0.003 |
| Fe | 0.000 | 0.000 | 0.002 | 0.007 | 0.003 | 0.000 |
| Pb | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Al | 0.002 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 |
| Y | 0.004 | 0.004 | 0.004 | 0.000 | 0.006 | 0.004 |
| La | 0.002 | 0.000 | 0.001 | 0.000 | 0.002 | 0.000 |
| Ce | 0.001 | 0.002 | 0.002 | 0.002 | 0.003 | 0.002 |
| Pr | 0.000 | 0.000 | 0.003 | 0.000 | 0.000 | 0.002 |
| Nd | 0.001 | 0.001 | 0.002 | 0.002 | 0.001 | 0.004 |
| Sm | 0.001 | 0.000 | 0.000 | 0.002 | 0.003 | 0.001 |
| Eu | 0.000 | 0.001 | 0.001 | 0.000 | 0.000 | 0.001 |
| Gd | 0.003 | 0.002 | 0.001 | 0.001 | 0.000 | 0.000 |
| Tb | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.002 |
| Dy | 0.002 | 0.000 | 0.000 | 0.000 | 0.003 | 0.001 |
| Ho | 0.003 | 0.001 | 0.000 | 0.001 | 0.003 | 0.000 |
| Er | 0.001 | 0.000 | 0.000 | 0.000 | 0.002 | 0.000 |
| Lu | 0.000 | 0.000 | 0.001 | 0.001 | 0.002 | 0.000 |
| Ti | 0.004 | 0.000 | 0.006 | 0.001 | 0.006 | 0.007 |
| Zr | 0.003 | 0.005 | 0.002 | 0.002 | 0.004 | 0.004 |
| Th | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| U | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Σ M | 4.923 | 4.904 | 4.970 | 4.964 | 4.985 | 4.964 |
| Si | 0.003 | 0.002 | 0.000 | 0.001 | 0.000 | 0.001 |
| P | 3.001 | 3.008 | 3.000 | 3.027 | 3.010 | 3.009 |
| As | 0.000 | 0.004 | 0.001 | 0.000 | 0.001 | 0.000 |
| S | 0.001 | 0.001 | 0.000 | 0.002 | 0.001 | 0.001 |
| Σ T | 3.006 | 3.016 | 3.001 | 3.030 | 3.012 | 3.010 |
| F | 1.102 | 1.098 | 1.028 | 0.911 | 0.923 | 0.988 |
| OH | 0.089 | 0.077 | 0.012 | |||
| Σ X | 1.102 | 1.098 | 1.028 | 1.000 | 1.000 | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferenc, Š.; Števko, M.; Mikuš, T.; Milovská, S.; Kopáčik, R.; Hoppanová, E. Primary Minerals and Age of The Hydrothermal Quartz Veins Containing U-Mo-(Pb, Bi, Te) Mineralization in the Majerská Valley near Čučma (Gemeric Unit, Spišsko-Gemerské Rudohorie Mts., Slovak Republic). Minerals 2021, 11, 629. https://doi.org/10.3390/min11060629
Ferenc Š, Števko M, Mikuš T, Milovská S, Kopáčik R, Hoppanová E. Primary Minerals and Age of The Hydrothermal Quartz Veins Containing U-Mo-(Pb, Bi, Te) Mineralization in the Majerská Valley near Čučma (Gemeric Unit, Spišsko-Gemerské Rudohorie Mts., Slovak Republic). Minerals. 2021; 11(6):629. https://doi.org/10.3390/min11060629
Chicago/Turabian StyleFerenc, Štefan, Martin Števko, Tomáš Mikuš, Stanislava Milovská, Richard Kopáčik, and Eva Hoppanová. 2021. "Primary Minerals and Age of The Hydrothermal Quartz Veins Containing U-Mo-(Pb, Bi, Te) Mineralization in the Majerská Valley near Čučma (Gemeric Unit, Spišsko-Gemerské Rudohorie Mts., Slovak Republic)" Minerals 11, no. 6: 629. https://doi.org/10.3390/min11060629
APA StyleFerenc, Š., Števko, M., Mikuš, T., Milovská, S., Kopáčik, R., & Hoppanová, E. (2021). Primary Minerals and Age of The Hydrothermal Quartz Veins Containing U-Mo-(Pb, Bi, Te) Mineralization in the Majerská Valley near Čučma (Gemeric Unit, Spišsko-Gemerské Rudohorie Mts., Slovak Republic). Minerals, 11(6), 629. https://doi.org/10.3390/min11060629






