3.1. Mineral and Chemical Composition of the Lancustrine Chalk Tested
Calcite (CaCO3) is the main mineral component of lacustrine chalk, including white, dark and silicified. In addition to calcite, the presence of quartz was found in the mineral composition of the dark and silicified chalk using the X-ray diffraction method. In the dark chalk samples, clay minerals represented mainly by kaolinite, illite and pyrite were also identified.
In microscopic observations, it was found that calcite occurs in the form of a microcrystalline rock background with a crystal size from 2 to 5 μm. It is a typical orthochemical component of rock, formed as a result of precipitation from lake water rich in calcium acid carbonate, due to the decrease in CO
2 content, as a result of waves, temperature changes and the development of organic life. Additionally, it plays the role of cement. A small part of the calcite micrite may exist in the form of the so-called pseudo micrite, which has clear cement features but is formed during diagenesis or may come from abrasion of limestone rocks from the edge of the Kleszczów tectonic graben [
16]. In the dark variety, the microcrystalline rock background is tinted with lignite dust, and in the silicified type, it is impregnated with silica, mainly opal and chalcedony, less often microcrystalline quartz. Within the microcrystalline rock background, there are the sparite calcite crystals, usually forming aggregates, in each variety of the lacustrine chalk. Moreover, a group of allochemical components performed function a skeleton in the rock. In addition, a number of allochemical components have been identified as a skeleton in the rock. These are mainly crushed carbonate shells of molluscs and calcitized algae remains, as well as intraclasts, oncoids and peloids. The fragments of Jurassic limestones were also identified, originating from the eroded slopes of the tectonic trench. The allochemical components are usually in a dispersed form, which allows them to be classified according to Dunham’s classification [
17] to Floatstone (>10% of allochemical components are larger than 2 mm) and Wackstone (<10% of allochemical components is greater than 2 mm). At the same time, these are the types of limestone most frequently represented by the lacustrine chalk in the Szczerców field. The Grainstone and Rudstone were rare among the tested lake chalk samples. The Bindstone, an autochthonous variety of limestone, formed by calcified algae mats, has been very rarely identified. This type of limestone was difficult to identify due to its poor state of preservation and recrystallization.
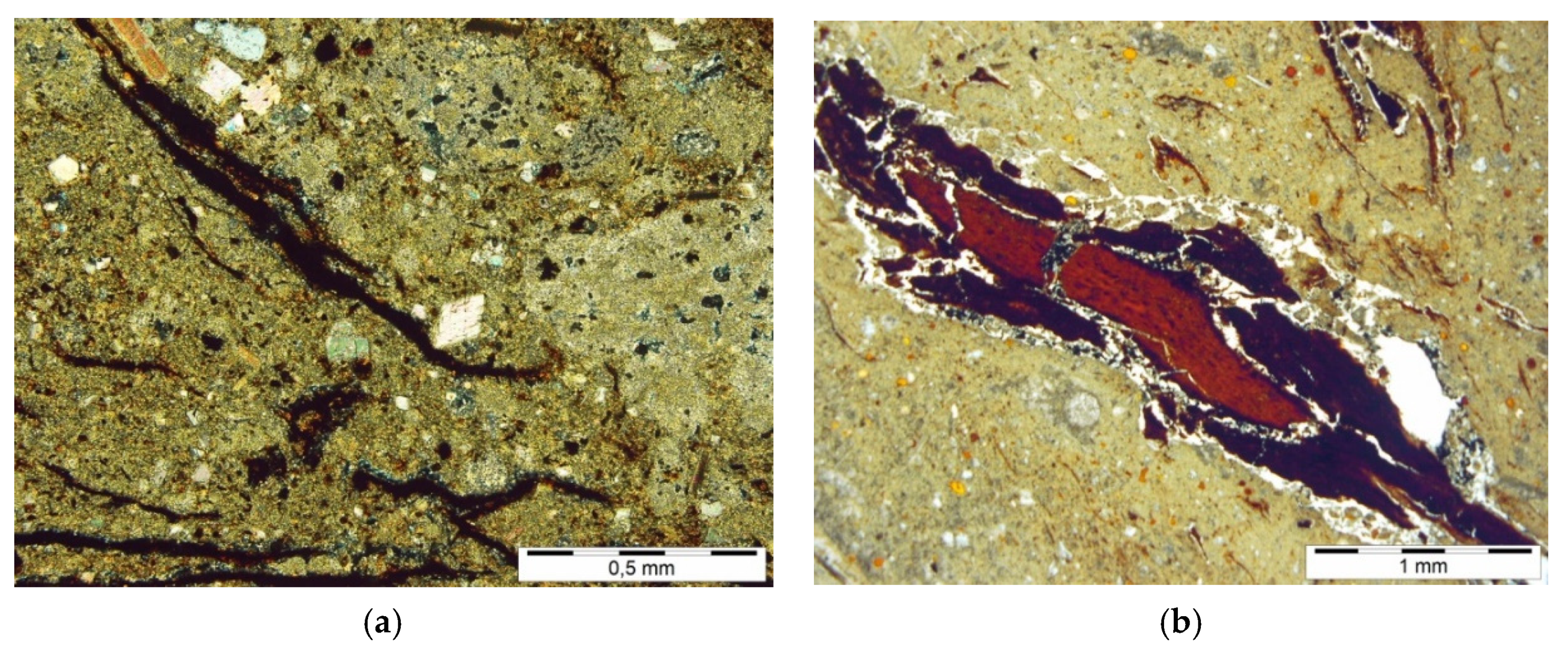
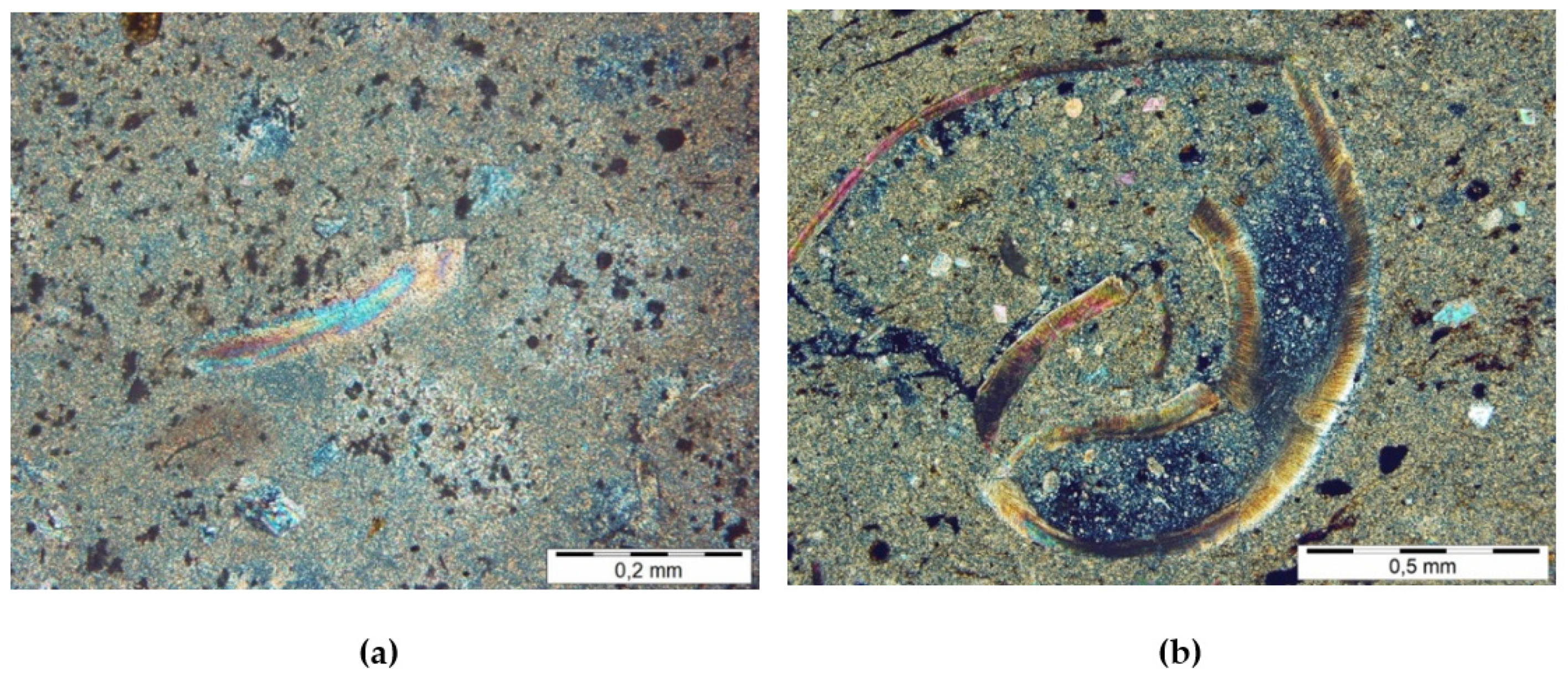
Among the noncarbonate components, mainly quartz grains, fewer feldspars were distinguished. They are poorly rounded and have different sizes, from pelitic through aleurite to psamite. A permanent allochemical component of lacrustine chalk is also organic material, represented by fragments of mainly brittle (
Figure 4a) and gelled xylites (
Figure 4b), carbonised plant detritus and components resistant to oxidation and destructive action of bacteria, such as waxes and resins, characterised by a high content of organic carbon. Among the noncarbonate components of orthochemical origin, clay minerals and pyrite, mainly framboidal, were identified.
The content of noncarbonate components in the lacustrine chalk individual lithological varieties is strongly diversified. In the white chalk, it is usually at the level of a few percent of the rock’s volume, and in the dark variety, it reaches even 45% by volume. In addition, white chalk is characterised by a very low variability of the mineral composition, while dark and silicified chalk show significant differences both in the content of calcite and noncarbonate components. The noncarbonate components are unevenly distributed in the rock. The clay substance occurs in the streaks and laminas form, where it is usually heavily masked with lignite dust.
This type of clay minerals and organic material occurrence is accompanied by pyrite, often framboidal (
Figure 5a,b), and iron compounds of oxide nature (their identification was difficult due to the presence of lignite matter). Quartz grains, in turn, are responsible for the presence of sanded chalk inserts.
The silification process is associated with the presence of opal and chalcedony and less frequently autogenous quartz. The most often manifested in the replacement of the calcite in bioclasts, impregnating the pores of the rock opal and chalcedony and rarely autogenic quartz (
Figure 6a,b). The forms of silica occurrence indicate that the process took place at the stage of diagenesis and was cement-like, but the biogenic structure of the rock did not change. The source of silica could be formation water, squeezed out of neogeneous clay sediments under the influence of mechanical compaction. The sources of silica can also be searched for in the process of calcification of volcanic glass and feldspars, which are present in the pyroclastic material from which the tonsteins were formed [
18].
The content of noncarbonate components has a decisive influence on the structural and textural diversity of the rock. The only carbonate varieties are characterised by a microcrystalline structure and a disordered and porous texture (
Figure 7). The increased content of both organic and terrigenous material makes the structure become detrital in nature, and textures are streaking, even layering. The dark and siliceous substances also reduce the porosity of the rock (
Figure 8a,b) while increasing its compactness and hardness.
The mineral composition of lacustrine chalk identified by microscopy was detailed by the X-ray examinations. In the phase composition of the clay substance, illite and kaolinite were distinguished. The presence of aragonite was found in the samples of lacustrine chalk, which was rich in calcareous shells of molluscs. The iron oxide compounds of hematite nature and trace amounts of gypsum were additionally identified in the material from the anthropogenic deposit. Hematite is an effect of pyrite oxidation under atmospheric conditions. Gypsum was formed as a result of the CaO reaction (coming from the dissolution of calcite under the influence of atmospheric factors), with SO2 resulting from the oxidation of pyrite.
Table 2 shows the chemical composition of individual lithological varieties of lacustrine chalk collected from drilling cores and the anthropogenic deposit. The content of moisture (W
tr, W
a) and organic carbon (C
org) is also included.
The chemical composition is different between the lithological varieties of chalk and the carbonised chalk samples taken from the drilling cores and the anthropogenic deposit. The differences concern mainly the content of CaO (CaCO3) as well as Corg and SiO2. The white variety is characterised by the highest (on average 53.89% wt.), and the silicified has the lowest (on average 19% wt.) content of CaO. In the dark variety, the CaO content is on average 38.26% wt. in samples taken from drill cores and 48.46% wt. is taken from an anthropogenic deposit. The organic carbon content in the white and silicified form is low and amounts to 0.14% wt. and 0.35% wt., respectively. In the dark chalk, the average Corg content is 16.13% wt. in samples taken from drill cores and 9.41% wt. in samples taken from the anthropogenic deposit. The SiO2 content is an indicator of the advancement of the silification process. It may also be evidence of the sandiness of the lacustrine chalk. In the silicified variety, the SiO2 content ranges from 19.23% to 42.11% wt. and averages 30.08% wt. The contents of the remaining chemical components are less differentiated. MgO present in the samples is in the MgCO3 form. It should be combined with the presence of magnesium calcite, as evidenced by the higher MgCO3 content in the white variety (on average 0.31% wt.) compared to other varieties of lacustrine chalk (on average 0.25%–0.27% wt.). The content of K2O and Na2O should be associated with the presence of clay substance, hence slightly higher contents in the dark chalk. In the carbonised version, higher Fe2O3 contents were also found, confirming its relationship with pyrite and iron oxide compounds, formed as a result of oxidation of this mineral, as well as TiO2 and MnO, which should be combined with both clay and terrigenous material that could transport these elements.
The moisture is an important utility feature of the lacustrine chalk, especially in the case of energetic use. Introduced into the combustion chamber, due to the high heat of water evaporation, it can reduce the calorific value of the fuel. It also has a negative effect on the temperature of the so-called exhaust gas “dew point” (exhaust gas temperature at which the process of condensation of water vapor or its sublimation at a set pressure) can begin. It is responsible for the economics of transporting this mineral and thus the territorial range of profitability of its use. In Polish climatic conditions, especially in the winter, the moisture can cause serious problems related to freezing during transport and storage. The content of deposit moisture (W
tr) and analytical moisture (W
a) is presented in
Table 2. The deposited (total) moisture is understood as the moisture that the chalk samples contain at the moment and shortly after being extracted from the deposit (drilling a hole in this case). It is the sum of transient (unstable) and hygroscopic (permanent) moisture. The analytical moisture (W
a) is close to the hygroscopic moisture content and determines the moisture content at 105 °C, in the sample in equilibrium with the environment (air-dried for 72 h). The lacustrine chalk in the deposit, similarly to lignite, is dehydrated and dried up due to the removal of the overburden. Despite this, the deposit moisture content (W
tr) is at a high level and amounts on average to: 34.27% wt. in the carbonised variety, 24.11% wt. in the white and 27.36% wt. from the anthropogenic deposit. Only in the silicified variety, it is clearly lower (6.12% wt). The analytical moisture content (W
a) is, on average: 4.48% wt. in the dark chalk, 0.95% wt. in white and 4.01% wt. in the variety from an anthropogenic deposit. In the silicified variety, it is at the level of 1.07% wt. The results of the research show that lacustrine chalk easily gives back and absorbs surface moisture in atmospheric conditions. This exchange is very clearly visible in the chalk samples taken from the anthropogenic deposit, where the total moisture content is the most diversified and is in the range of 11.11–42.78% wt. The atmospheric conditions during sampling are responsible for this diversity. The high moisture content is undoubtedly associated with the addition of carbonised organic matter (lignite) and with the structural and textural character of the rock, especially with significant porosity.
The chemical composition of the industrial sorbent is characterised by a high CaO content (55.57 wt.%) with a negligible content of other chemical components. The analytical moisture content does not exceed 0.20% wt.
3.2. Parameters of the Porous Texture Lacustrine Chalk
The parameters of the porous texture, determined by the low-temperature nitrogen sorption method, indicate the differentiation of both the specific surface area and porosity (
Table 3). It should be noted that the white 4.68 m
2/g and the silicified 3.11 m
2/g varieties are clearly lower in the surface compared value to the dark variety, both in the case of samples taken from drill cores (8.28 m
2/g) and from the anthropogenic deposit (8.01 m
2/g). The specific surface area of white chalk is expanded mainly by macropores and mesopores, whose share in the total pore volume is 56.3% and 40.6%, respectively. In the case of the silicified and dark variety, both in the case of samples from the drilling cores and the anthropogenic deposit, the specific surface area was shaped mainly by mesopores (46.2%, 45.7% and 47.4%) and micropores (30.8%, 30.4% and 44.7%). The content of macropores is clearly lower, especially in the case of dark chalk from an anthropogenic deposit (7.9%). This is confirmed by the average pore diameter. In the case of white chalk, it is characterised by a significantly higher value compared to other types of chalk. The industrial sorbent, made of Jurassic limestone, compared to chalk varieties, has a much smaller specific surface (1.22 m
2/g). In turn, the total volume of pores, as well as the share of micropores, mesopores and macropores, correspond to the sifted variety.
The other values represent the parameters of the porous texture, determined by mercury porosimetry (
Table 4). It is associated with a measuring range of pore diameters of both research methods. The mercury porosimetry method does not cover the micropore measuring range, i.e., pores below 2 nm in diameter. Additionally, in this case, the highest value of the specific surface area is a characteristic of dark chalk: 6.85 m
2/g (taken from the drill cores) and 6.36 m
2/g (coming from an anthropogenic deposit). However, the differences between the white and dark variety are much smaller. In the case of the other pore space parameters, such as the total pore volume, average pore diameter and the effective porosity, larger values are assigned white chalk (
Table 4).
The parameters of the industrial sorbent porous texture determined by the mercury porosimetry method, in particular the specific surface area and effective porosity, compared to lacustrine chalk, especially of the white and dark varieties, achieve significantly lower values (
Table 4).
3.4. The Lacustrine Chalk Parameters Responsible for the Efficiency of SO2 Sorption
The decisive influence on the efficiency of SO2 capture in fluidized bed boilers, apart from the phase and chemical composition, is:
- the structural and textural nature of the sorbent, and above all its porosity, which shapes the course of the sulphation process, both in the laboratory and industrial conditions. The process of binding SO2 takes place on the inner surface of the pores formed during the decarbonation of the sorbent;
- the course and temperature of calcite thermal dissociation [
19,
20].
An attempt was made to define the parameters of lacustrine chalk affecting its reactivity. The analysis of the rock texture parameters presented in
Table 3 and
Table 4 showed that the sorption properties of chalk could not be explained by a simple relationship related to the size of the pores in the sorbent and the size of the specific surface area. The white chalk, even though it has a smaller specific surface (S
BET—4.43 m
2/g; S
POR—4.68 m
2/g) compared to dark chalk, coming from both drilling cores (S
BET—12.28 m
2/g; S
POR—6.85 m
2/g) and the anthropogenic deposit (S
BET—11.89 m
2/g; S
POR—6.11 m
2/g), characterised by comparable RI and CI values. The porosity is also essentially unaffected by the efficiency of SO
2 sorption. The white chalk is characterised by a clearly higher effective porosity compared to the dark one (
Table 4). The observed differences should be related to the size of the rock pores. The specific surface of the white chalk was developed mainly based on mesopores and macropores, with an insignificant share of micropores. This is confirmed by the value of the average pore diameter, which is 17.4 nm and is clearly higher compared to the average pore diameter of the dark chalk—9.5 nm and 8.3 nm (
Table 4). The specific surface of dark chalk, both taken from boreholes and from the associated minerals storage site, is developed mainly by micropores and mesopores with a smaller share of macropores (
Table 3). In the light of the research [
18,
20], the texture parameters of the porous dark chalk should be considered unfavourable for SO
2 sorption, while the chalky texture parameters are highly favourable. Despite this, the efficiency of SO
2 capture in both varieties is very similar.
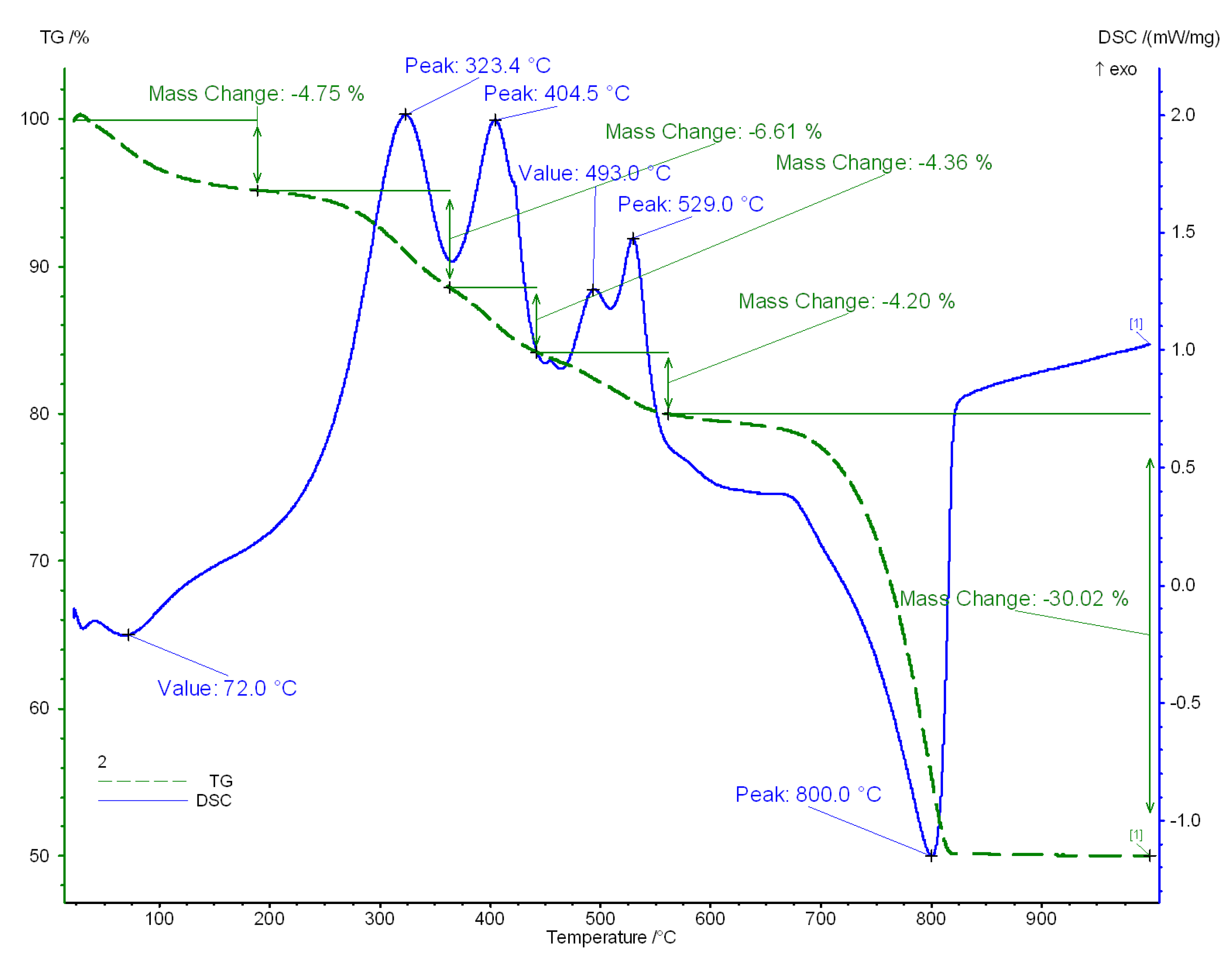
The recorded thermal effects of the individual varieties of lacustrine chalk thermal decomposition in the temperature range of 25–1000 °C have the same course in all tested samples (
Table 6 and
Figure 9). They differ only in weight loss (
Table 7), which is determined by the content of moisture, organic matter of plant origin and calcite. All recorded thermograms are dominated by a wide endothermic effect, starting at approx. 620 °C and ending at approx. 830 °C with a maximum between 800.0–807.9 °C and with a sharp collapse (almost at right angles) from the side of higher temperatures responsible for the course of the process thermal dissociation of calcite (decarbonation) (
Figure 9). In addition, there are exothermic effects associated with the decomposition of organic matter of plant origin. Four reactions of this type were recorded in each variety of the chalk. The reactions maximum in the white and silicified varieties are in the temperature ranges: 323 °C—1st maximum; 385–386 °C—2nd maximum; 408 °C—3rd maximum; 525–526 °C—4th maximum. In the dark chalk, the distribution of maximum temperatures of exothermic effects is slightly different. The first maximum was recorded in a temperature range similar to that of the white and silicified variety; 2nd—404.5–407 °C; 3rd—493–495 °C; 4th—526.1–529.0 °C. This shows the different nature of the lignite substance and its carbonisation state, which is confirmed by microscopic observations. The reactions should be associated with the oxidation of organic compounds [
21]. Moreover, in low-temperature ranges, thermal effects related to the loss of residual moisture were recorded: surface—23–31 °C (in all varieties of chalk) and 50–72 °C—hygroscopic (except for white chalk). The thermal decomposition of lacustrine chalk ends at a temperature of 825–827 °C with a total weight loss from 39.06% wt. (silicified chalk) to 50.03% wt. (dark chalk). Raising the temperature to 1000 °C does not cause any additional weight loss or thermal reactions. The decomposition temperature of calcite in lacustrine chalk is lower compared to the decomposition temperature of this mineral in limestone. In the case of industrial sorbent, the calcite decarbonization temperature begins at approx. 680 °C and ends at 921.6 °C. The calcite decomposition is accompanied by a weight loss, which at the temperature of 850 °C amounts to 49.32% wt. The decarbonation degree of the industrial sorbent at 850 °C is 85% wt.
The course of thermal decomposition of individual varieties of lacustrine chalk has a decisive influence on the development of the porosity and specific surface of the sorbent.
Table 8 and
Table 9 present the parameters of the porous texture after the decarbonation process at the temperature of 850 °C (combustion temperature in fluidized bed boilers) of individual lithological varieties of lacustrine chalk, taken from drilling cores and from an anthropogenic deposit, in relation to industrial sorbent made of limestone, determined using low-temperature sorption nitrogen (
Table 8) and mercury porosimetry (
Table 9).
The thermal decomposition process of lacustrine chalk causes the expansion of the porous texture parameters. However, the specific surface area of individual varieties of the chalk after the decarbonation process at 850 °C is diverse. According to measurements, white chalk expanded the area to the greatest extent: S
BET—9.12 m
2/g (before the S
BET decarbonation process—4.68 m
2/g); S
POR—8.72 m
2/g (before the S
POR decarbonation process—4.43 m
2/g). The chalk surface area values were determined before (S
BET—8.28 m
2/g and 8.01 m
2/g; S
POR—6.85 m
2/g and 6.36 m
2/g) and after the decarbonation process (S
BET—8.78 m
2/g and 8.01 m
2/g; S
POR—6.98 m
2/g and 6.41 m
2/g) are on the same level. This may suggest that the surface has not been developed with secondary pores. However, other texture parameters such as effective porosity (before—28.85% and 27.45%; after—68.12% and 64.87%) and the average pore diameter (before—0.18 µm and 0.12 µm; after—0.64 µm and 0.56 µm) (
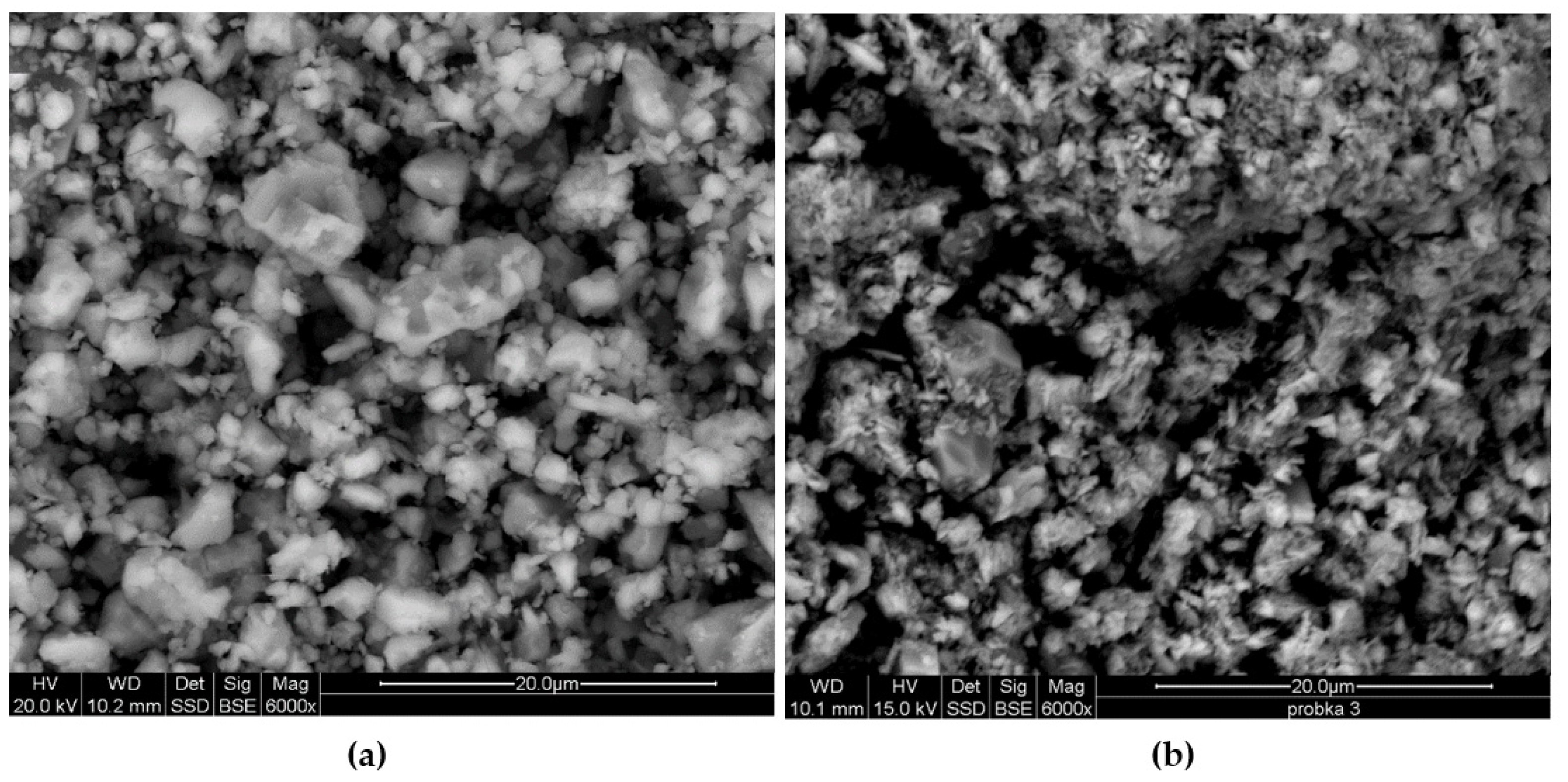
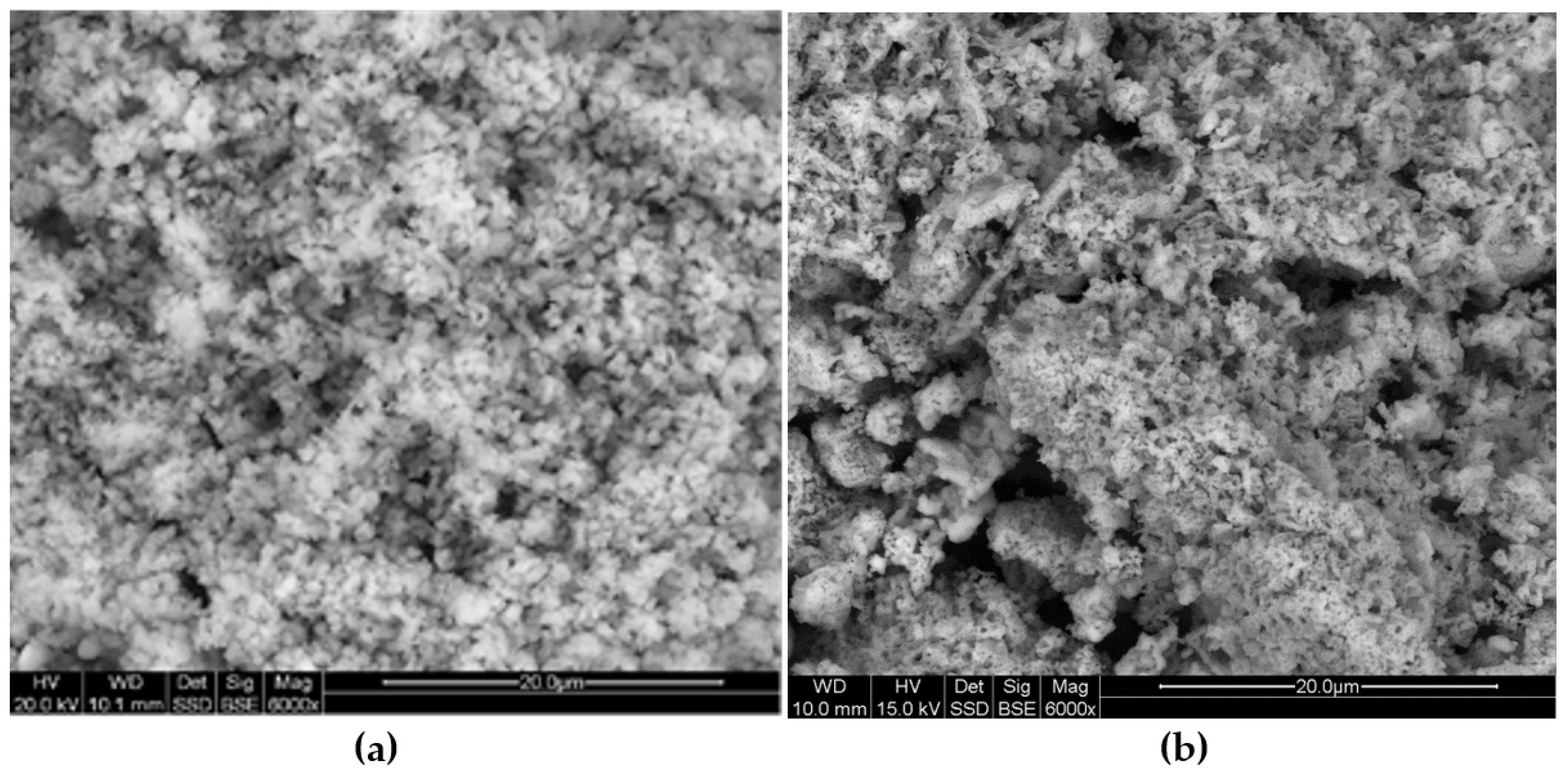
Table 9), as well as observations of the surface morphology made with the use of a scanning microscope (
Figure 10a,b), indicate the expansion of porosity and thus the development of the surface area. The differences are visible mainly in the case of the effective porosity determined for the pore diameter range: 0.01–10 μm, considered as sorptive [
19,
20]. In the case of the tested dark chalk samples, they are 12.11% and 11.56% before the decarbonation process and 66.22% and 63.52% after the decarbonation process. These values are much higher, both than white and silicified chalk, as well as industrial sorbent. The industrial sorbent, in comparison with white and dark chalk, has the lowest effective porosity value both for the full range of pore diameters (0.0005–1000 μm)—52.17% and the range of sorption pore diameters (0.01–10 μm)—38,21%. The differences in the surface formation of the white (
Figure 10a,b), dark (
Figure 11a) and silicified (
Figure 11b) chalk and industrial sorbent (
Figure 12b) are visible in microscopic observations (SEM).
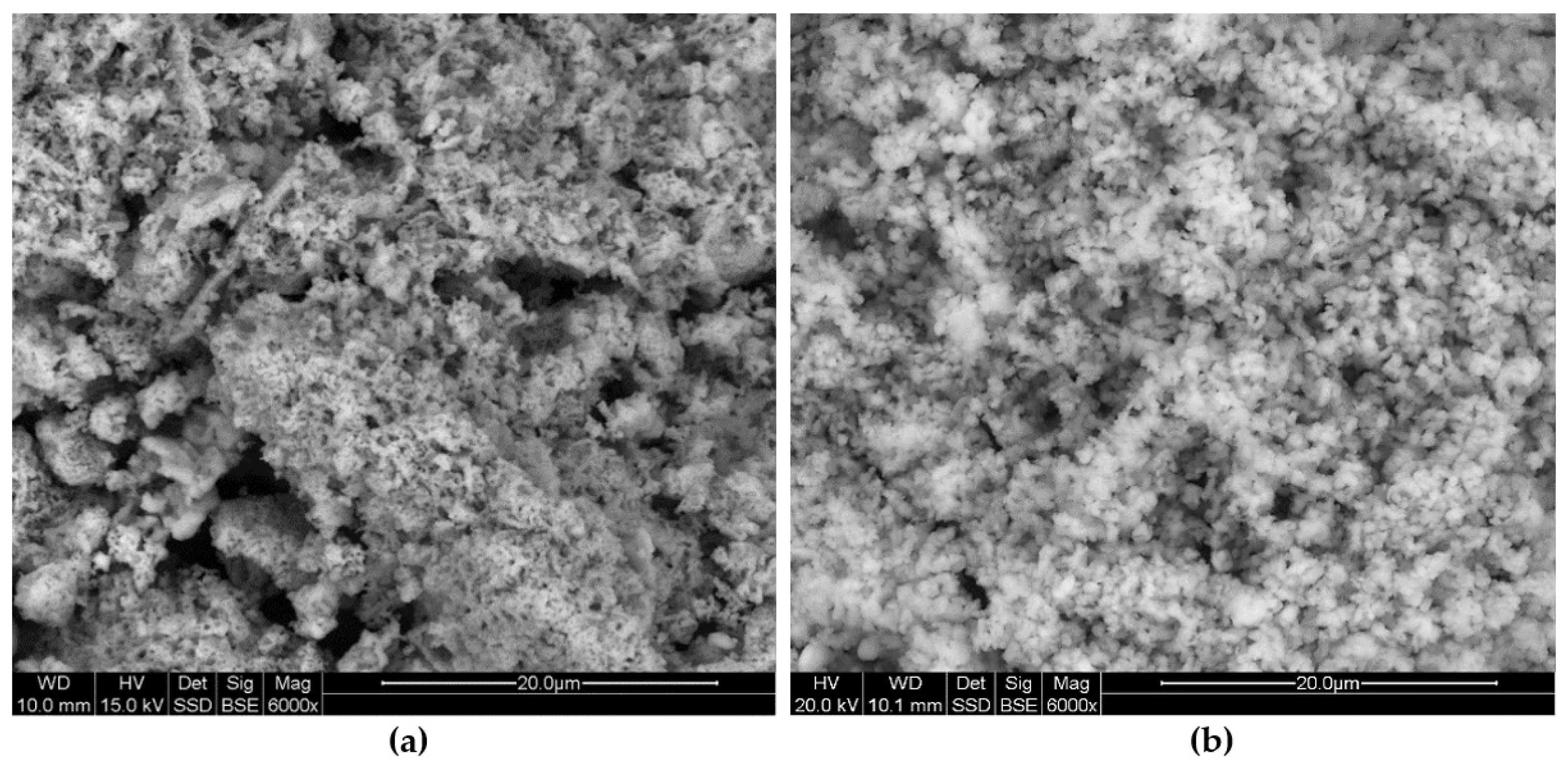
The parameters of the sorbent porous texture after the decarbonation process, such as the pore diameter and the effective porosity, have a decisive influence on the structural and textural character of the calcium sulphate formed on the sorbent grains surface. The grains of the white and dark lacustrine chalk were covered in sulphate with an extremely porous texture (
Figure 13a,b). The sulphate crusts were formed on the surfaces of the silicified chalk and industrial sorbent grains, with less numerous open pores and a much smaller diameter (
Figure 13c,d). The porous texture of the calcium sulphates formed on the sorbent grains surface (
Figure 13a,b) guarantees the free flow of SO
2, ensuring uniform sulphation of the grains [
22]. If a sulphate with a limited porosity texture forms on the sorbent grains surface (
Figure 13c,d), the diffusion of SO
2 in the initial desulphurisation stage will be stopped, which will result in a low degree of sorbent utilisation, manifested by the presence of unreacted, and under industrial conditions, undissociated sorbent grain cores [
19,
23,
24]. It should be concluded that the porosity of the sorbent, and not the CaCO
3 content in the sorbent, determines the efficiency of SO
2 sorption under the fluidized bed combustion technology. The SO
2 sorption process takes place on the inner surface of the pores formed during thermal dissociation. The reactivity and the ability to bind SO
2 increase with the expansion of porosity and an increase in the specific surface area [
25]. Due to the fact that the molar volume of the resulting desulphurisation products (CaSO
4) is greater than that of CaCO
3, the reactivity of the sorbent will be determined by the size of the specific surface area capable of reacting with SO
2. The surface capable of reacting with SO
2 should be considered one that has been developed through pores of sufficiently large diameters on the border of the mesopores and macropores (division according to IUPAC) [
19,
20].
Typically, in the case of calcareous sorbents, secondary porosity is formed during the calcite thermal dissociation process. In the case of lacustrine chalk containing carbonised plant matter, the specific surface is additionally expanded by its complete oxidation (burning). Due to the fact that the combustion of organic matter takes place at much lower temperatures (approx. 320–530 °C) than the decomposition of calcite (approx. 800 °C), the pores formed to intensify the CO
2 removal process from the calcite structure and SO
2 migration into the sorbent grains, fulfilling the function of diffusion channels. It will be important in the real conditions, where the processes of decarbonation and SO
2 sorption occur almost simultaneously. An attempt to reflect this type of condition is the simultaneous sulphation and decarbonation method (sorbent grains are sulphated without prior decarbonisation). The RI and CI indexes, determined by this method for the dark lacustrine chalk samples, correspond to the values obtained by the sulphation method, preceded by the decarbonation process. In the case of white chalk, the differences are barely noticeable. They differ significantly in the case of silicified chalk and the industrial sorbent (
Table 5).
The analysis of the pore size after the decarbonation process leads to interesting conclusions. The pore volume distributions as a function of their diameter presented in
Figure 14 show that the specific surface of white and carbonised chalk after the decarbonation process, compared to the industrial sorbent, was much more developed based on pores, on the mesopore and macropore border, the so-called sorption pores. In the lacustrine chalk, they cover a wide range of diameters, from approx. 0.05–3 µm. In the industrial sorbent, the range of diameters is narrower; it is in the range from approx. 0.2–3 µm. In both cases, the pores formed during decarbonation are built up with CaSO
4. In the case of white and dark chalk, the pores are completely covered with sulphate, and in the case of sorbent, some of the pores remain unfilled (
Figure 14). In the phase composition of the samples after the sulphation process by the simultaneous calcination and sulphation method, no unreacted portions of CaO and undissociated CaCO
3 were identified by X-ray diffraction. The efficiency of SO
2 sorption of lacustrine chalk, white and dark varieties, compared to the industrial sorbent, is higher. The micropores and smaller mesopores are responsible for the lower degree of sulphate filling the industrial sorbent pore space. At the initial stage of sulphation, their inlets are blocked sulphate particles, making it difficult to diffuse SO
2 inside the grains’ sorbent. On the sorbent grains surface, the massive sulphate crusts are formed, as shown in
Figure 13d. The SO
2 sorption process is stopped, resulting in the presence of unreacted portions of CaO and even undissociated CaCO
3, visible on the X-ray diffraction patterns of samples sulphated by simultaneous calcination and sulphation method. The sorption process is similar in the case of the silicified chalk. The pores considered to be sorptive were formed in the smallest amount, and the specific surface to a large extent was expanded based on micropores and smaller mesopores that do not participate in the binding of SO
2. Additionally, given the low CaCO
3 content, the silicified chalk cannot be used as an SO
2 sorbent.