Bromine Isotope Variations in Magmatic and Hydrothermal Sodalite and Tugtupite and the Estimation of Br Isotope Fractionation between Melt and Sodalite
Abstract
1. Introduction
2. Material
3. Methods
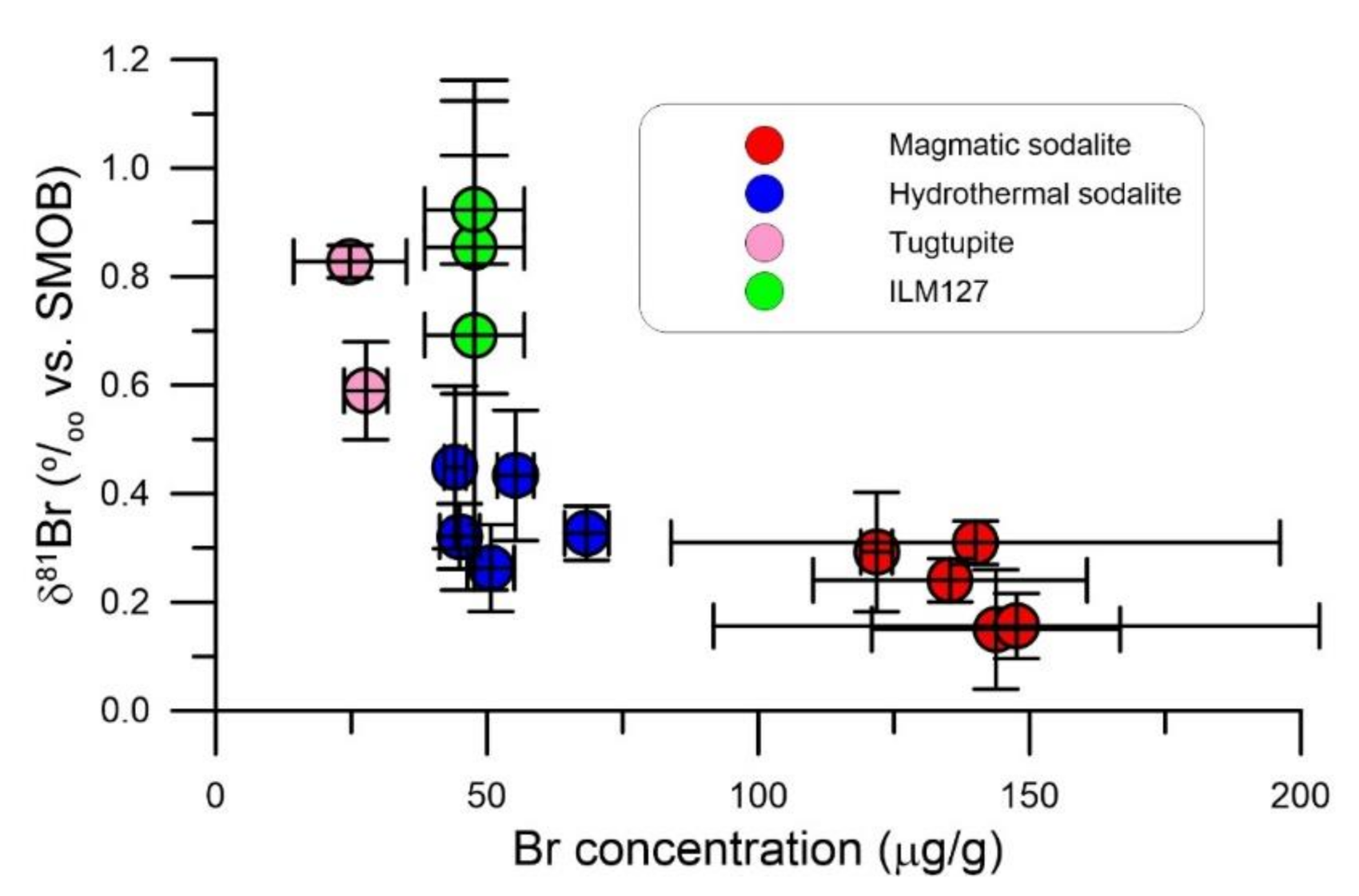
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eggenkamp, H.G.M.; Coleman, M.L. Rediscovery of classical methods and their application to the measurement of stable bromine isotopes in natural samples. Chem. Geol. 2000, 167, 393–402. [Google Scholar] [CrossRef]
- Eggenkamp, H.G.M. The Geochemistry of Stable Chlorine and Bromine Isotopes; Springer: Berlin/Heidelberg, Germany, 2014; 172p. [Google Scholar]
- Shouakar-Stash, O.; Alexeev, S.V.; Frape, S.K.; Alexeeva, L.P.; Drimmie, R.J. Geochemistry and stable isotopic signatures, including chlorine and bromine isotopes, of the deep groundwaters of the Siberian Platform, Russia. Appl. Geochem. 2007, 22, 589–605. [Google Scholar] [CrossRef]
- Louvat, P.; Bonifacie, M.; Giunta, T.; Michel, A.; Coleman, M. Determination of Bromine stable isotope ratios from saline solutions by “wet plasma” MC-ICPMS including a comparison between high- and low-resolution modes, and three introduction systems. Anal. Chem. 2016, 88, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Eggenkamp, H.G.M.; Louvat, P. A simple distillation technique to extract bromine for MC-ICP-MS isotope analyses. Rapid Comm. Mass Spectrom. 2018, 32, 612–618. [Google Scholar] [CrossRef]
- McKinney, C.R.; McCrea, J.M.; Epstein, S.; Allen, H.A.; Urey, H.C. Improvements in mass spectrometers for the measurement of small differences in isotope abundance ratios. Rev. Sci. Instr. 1950, 21, 724–730. [Google Scholar] [CrossRef]
- Du, Y.; Ma, T.; Yang, J.; Liu, L.; Shan, H.M.; Cai, H.S.; Liu, C.F.; Chen, L.Z. A precise analytical method for bromine stable isotopes in natural waters by GasBench II-IRMS. Int. J. Mass Spectrom. 2013, 338, 50–56. [Google Scholar] [CrossRef]
- Godon, A.; Jendrzejewski, N.; Eggenkamp, H.G.M.; Banks, D.A.; Ader, M.; Coleman, M.L.; Pineau, F. A cross calibration of chlorine isotopic measurements and suitability of seawater as the international reference material. Chem. Geol. 2004, 207, 1–12. [Google Scholar] [CrossRef]
- Kaufmann, R.S. Chlorine in Groundwater. Stable Isotope Distribution. Ph.D. Thesis, University of Arizona, Tucson, AZ, USA, 1984. [Google Scholar]
- Gao, C.H.; Liu, Y. First-principles calculations of equilibrium bromine isotope fractionations. Geochim. Cosmochim. Acta 2021, 297, 65–81. [Google Scholar] [CrossRef]
- Pinti, D.L.; Shouakar-Stash, O.; Castro, M.C.; Lopez-Hernandez, A.; Hall, C.M.; Rocher, O.; Shibata, T.; Ramirez-Montez, M. The bromine and chlorine isotopic composition of the mantle as revealed by deep geothermal fluids. Geochim. Comochim. Acta 2020, 276, 14–30. [Google Scholar] [CrossRef]
- Sharp, Z.D.; Barnes, J.D.; Brearley, A.J.; Chaussidon, M.; Fischer, T.P.; Kamenetsky, V.S. Chlorine isotope homogeneity of the mantle, crust and carbonaceous chondrites. Nature 2007, 446, 1062–1065. [Google Scholar] [CrossRef]
- Sharp, Z.D.; Mercer, J.A.; Jones, R.H.; Brearley, A.J.; Selverstone, J.; Bekker, A.; Stachel, T. The chlorine isotope composition of chondrites and Earth. Geochim. Cosmochim. Acta 2013, 107, 189–204. [Google Scholar] [CrossRef]
- Bonifacie, M.; Jendrzejewski, N.; Agrinier, P.; Humler, E.; Coleman, M.; Javoy, M. The chlorine isotope composition of Earth’s mantle. Science 2008, 319, 1518–1520. [Google Scholar] [CrossRef]
- Layne, G.D.; Kent, A.J.R.; Bach, W. δ37Cl systematics of a back-arc spreading system: The Lau Basin. Geology 2009, 37, 427–430. [Google Scholar] [CrossRef]
- John, T.; Layne, G.D.; Haase, K.M.; Barnes, J.D. Chlorine isotope evidence for crustal recycling into the mantle. Earth Planet. Sci. Lett. 2010, 298, 175–182. [Google Scholar] [CrossRef]
- Hoare, B.C.; Tomlinson, E.L.; Barnes, J.D.; Tappe, S.; Marks, M.A.W.; Epp, T.; Caufield, J.; Riegler, T. Tracking halogen recycling and volatile loss in kimberlite magmatism from Greenland: Evidence from combined F-Cl-Br and δ37Cl systematics. Lithos 2021, 384–385, 105976. [Google Scholar] [CrossRef]
- Carreira, P.M.; Marques, J.M.; Carvalho, M.R.; Capasso, G.; Grassa, F. Mantle-derived carbon in Hercynian granites. Stable isotopes signatures and C/He associations in the thermomineral waters, N-Portugal. J. Volcanol. Geotherm. Res. 2010, 189, 49–56. [Google Scholar] [CrossRef]
- Marques, J.M.; Eggenkamp, H.G.M.; Carreira, P.M.; da Silva, M.A. Origin and evolution of Cl in the CO2-rich thermal and mineral waters from Northern Portugal, based on a reinterpretation of its chemical and isotope characteristics. Appl. Geochem. 2020, 116, 104569. [Google Scholar] [CrossRef]
- Eggenkamp, H.G.M.; Louvat, P.; Agrinier, P.; Bonifacie, M.; Bekker, A.; Krupenik, V.; Griffioen, J.; Horita, J.; Brocks, J.J.; Bagheri, R. The bromine and chlorine isotope composition of primary halite deposits and their significance for the secular isotope composition of seawater. Geochim. Cosmochim. Acta 2019, 264, 13–29. [Google Scholar] [CrossRef]
- Eggenkamp, H.G.M.; Marks, M.A.W.; Atanasova, P.; Wenzel, T.; Markl, G. Changes in halogen (F, Cl, Br, and I) and S ratios in rock-forming minerals as monitors for magmatic differentiation, volatile loss, and hydrothermal overprint: The case for peralkaline systems. Minerals 2020, 10, 995. [Google Scholar] [CrossRef]
- Marks, M.A.W.; Markl, G. The Ilímaussaq alkaline complex, South Greenland. In Layered Intrusions; Charlier, B., Namur, O., Latypov, R., Tegner, C., Eds.; Springer Geology; Springer: Dordrecht, The Netherlands, 2015; pp. 649–691. [Google Scholar]
- Marks, M.A.W.; Markl, G. A global review on agpaitic rocks. Earth Sci. Rev. 2017, 173, 229–258. [Google Scholar] [CrossRef]
- Bureau, H.; Métrich, N. An experimental study of bromine behaviour in water-saturated silicic melts. Geochim. Cosmochim. Acta 2003, 67, 1689–1697. [Google Scholar] [CrossRef]
- Bureau, H.; Foy, E.; Raepsaet, C.; Somogyi, A.; Munsch, P.; Simon, G.; Kubsky, S. Bromine cycle in subduction zones through in situ Br monitoring in diamond anvil cells. Geochim. Cosmochim. Acta 2010, 74, 3839–3850. [Google Scholar] [CrossRef]
- Cochain, B.; Sanloup, S.; de Grouchy, C.; Crépisson, C.; Bureau, H.; Leroy, C.; Kantor, I.; Irifune, T. Bromine speciation in hydrous silicate melts at high pressure. Chem. Geol. 2015, 404, 18–26. [Google Scholar] [CrossRef]
- Cadoux, A.; Iacono-Marziano, G.; Scaillet, B.; Aiuppa, A.; Mather, T.A.; Pyle, D.M.; Deloule, E.; Gennaro, E.; Paonita, A. The role of melt composition on aqueous fluid vs. silicate melt partitioning of bromine in magmas. Earth Planet. Sci. Lett. 2018, 498, 450–463. [Google Scholar] [CrossRef]
- Schauble, E.; Sharp, Z.D. Modeling isotopic signatures of nebular chlorine condensation. Min. Mag. 2011, 75, 1810. [Google Scholar]
- Balan, E.; Créon, L.; Sanloup, C.; Aléon, J.; Blanchard, M.; Paulatto, L.; Bureau, H. First-principles modeling of chlorine isotope fractionation between chloride bearing molecules and minerals. Chem. Geol. 2019, 525, 424–434. [Google Scholar] [CrossRef]
- Czarnacki, M.; Hałas, S. Isotope fractionation in aqua–gas systems: Cl2–HCl–Cl−, Br2–HBr–Br− and H2S–S2−. Isot. Environ. Health Stud. 2012, 48, 55–64. [Google Scholar] [CrossRef]
- Eggenkamp, H.G.M.; Bonifacie, M.; Ader, M.; Agrinier, P. Experimental determination of stable chlorine and bromine isotope fractionation during precipitation of salt from a saturated solution. Chem. Geol. 2016, 433, 46–56. [Google Scholar] [CrossRef]
- Eggenkamp, H.G.M.; Louvat, P.; Agrinier, P. Halogen stable isotope evolution during experimental evaporation of seawater type brines. In Proceedings of the ESIR Workshop XV, Lublin, Poland, 23–27 June 2019. [Google Scholar]
- Eggenkamp, H.G.M.; Louvat, P.; Griffioen, J.; Agrinier, P. Chlorine and bromine isotope evolution within a fully developed Upper Permian natural salt sequence. Geochim. Cosmochim. Acta 2019, 245, 316–326. [Google Scholar] [CrossRef]
- Giehl, C.; Marks, M.A.W.; Nowak, M. An experimental study on the influence of fluorine and chlorine on phase relations in peralkaline phonolitic melts. Contrib. Mineral. Petrol. 2014, 167, 977. [Google Scholar] [CrossRef]
- Markl, G. Stability of Na-Be minerals in late-magmatic fluids of the Ilímaussaq alkaline complex, South Greenland. Geol. Greenl. Surv. Bull. 2001, 190, 145–158. [Google Scholar] [CrossRef]
- Markl, G.; Marks, M.; Schwinn, G.; Sommer, H. Phase equilibrium constraints on intensive crystallization parameters of the Ilímaussaq Complex, South Greenland. J. Petrol. 2001, 42, 2231–2258. [Google Scholar] [CrossRef]
- Graser, G.; Potter, J.; Köhler, J.; Markl, G. Isotope, major, minor and trace element geochemistry of late-magmatic fluids in the peralkaline Ilímaussaq intrusion, South Greenland. Lithos 2008, 106, 207–221. [Google Scholar] [CrossRef]

| Sample | Br− (µg/g) [21] | Br− (µg/g), This Study | δ81Br (‰ vs. SMOB) |
|---|---|---|---|
| Magmatic sodalite | Avg = 138 ± 10 | Avg = 172 ± 21 | Avg = 0.23 ± 0.07 |
| 2016-73 | 122 | 159 | 0.29 ± 0.11 |
| 2016-78 | 146 | 178 | 0.15 ± 0.11 |
| 2016-85 | 135 | 148 | 0.24 ± 0.04 |
| 2016-87 | 148 | 173 | 0.16 ± 0.06 |
| 2016-96 | 140 | 204 | 0.31 ± 0.04 |
| Hydrothermal sodalite | Avg = 53 ± 10 | Avg = 49 ± 8 | Avg = 0.36 ± 0.08 |
| ILM123 | 55.3 | 53.4 | 0.32 ± 0.12 |
| ILM138 | 45.0 | 57.2 | 0.45 ± 0.06 |
| ILM158 | 44.1 | 36.2 | 0.26 ± 0.15 |
| ILM162 | 50.8 | 48.8 | 0.33 ± 0.08 |
| GM1246 | 68.3 | 49.4 | 0.43 ± 0.05 |
| Tugtupite (hydrothermal) | Avg = 26 ± 2 | Avg = 34 ± 8 | Avg = 0.71 ± 0.17 |
| KK1 | 27.7 | 39.7 | 0.59 ± 0.09 |
| KV1 | 24.8 | 28.6 | 0.83 ± 0.03 |
| ILM127 (hydrothermal sod.) | Avg = 24 ± 2 | Avg = 0.82 ± 0.12 | |
| (1) | 47.7 ± 9.2 | 25.8 | 0.69 ± 0.47 |
| (2) | 22.2 | 0.85 ± 0.27 | |
| (3) | 24.1 | 0.92 ± 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eggenkamp, H.G.M.; Marks, M.A.W.; Louvat, P.; Markl, G. Bromine Isotope Variations in Magmatic and Hydrothermal Sodalite and Tugtupite and the Estimation of Br Isotope Fractionation between Melt and Sodalite. Minerals 2021, 11, 370. https://doi.org/10.3390/min11040370
Eggenkamp HGM, Marks MAW, Louvat P, Markl G. Bromine Isotope Variations in Magmatic and Hydrothermal Sodalite and Tugtupite and the Estimation of Br Isotope Fractionation between Melt and Sodalite. Minerals. 2021; 11(4):370. https://doi.org/10.3390/min11040370
Chicago/Turabian StyleEggenkamp, Hans G. M., Michael A. W. Marks, Pascale Louvat, and Gregor Markl. 2021. "Bromine Isotope Variations in Magmatic and Hydrothermal Sodalite and Tugtupite and the Estimation of Br Isotope Fractionation between Melt and Sodalite" Minerals 11, no. 4: 370. https://doi.org/10.3390/min11040370
APA StyleEggenkamp, H. G. M., Marks, M. A. W., Louvat, P., & Markl, G. (2021). Bromine Isotope Variations in Magmatic and Hydrothermal Sodalite and Tugtupite and the Estimation of Br Isotope Fractionation between Melt and Sodalite. Minerals, 11(4), 370. https://doi.org/10.3390/min11040370







