Maghemite in Brazilian Iron Ores: Quantification of the Magnetite-Maghemite Isomorphic Series by Χ-ray Diffraction and the Rietveld Method, and Confirmation by Independent Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- (i)
- 3 soil samples (Guaíra, Ilha Grande, Mato Grosso) and 3 samples from the Quadrilátero Ferrífero (Guanhães, Guanhães 2, Espinhaço);
- (ii)
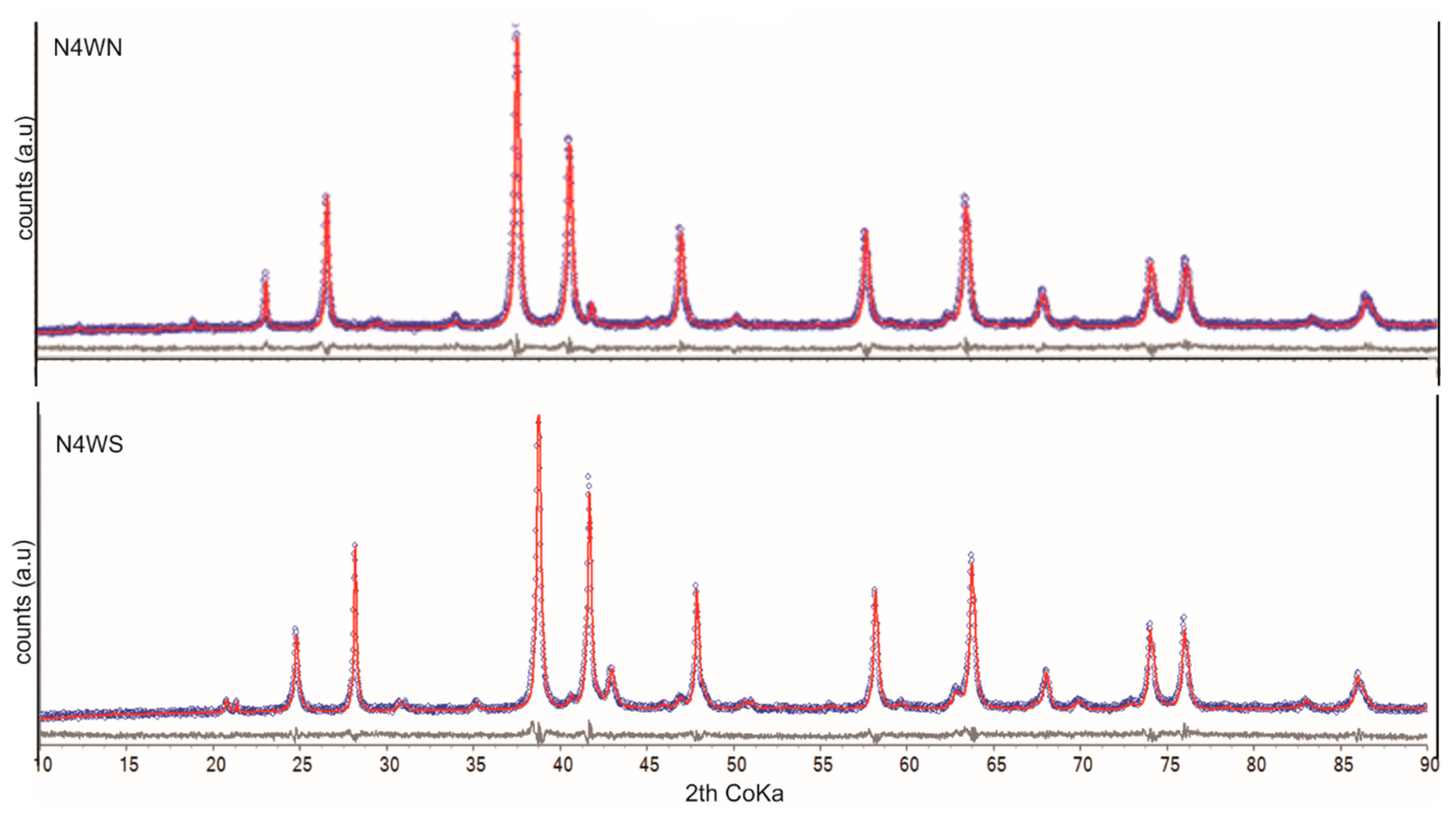
- 10 iron ore samples from each, the northern (N4WN) and southern (N4WS) segments of the N4W iron ore mine in Carajás. Although all were analyzed, only three for each segment are presented, as the results are similar for each segment.
2.2. Methods
3. Results and Discussions
3.1. First Stage
3.1.1. Iron Ore Samples from Quadrilátero Ferrífero
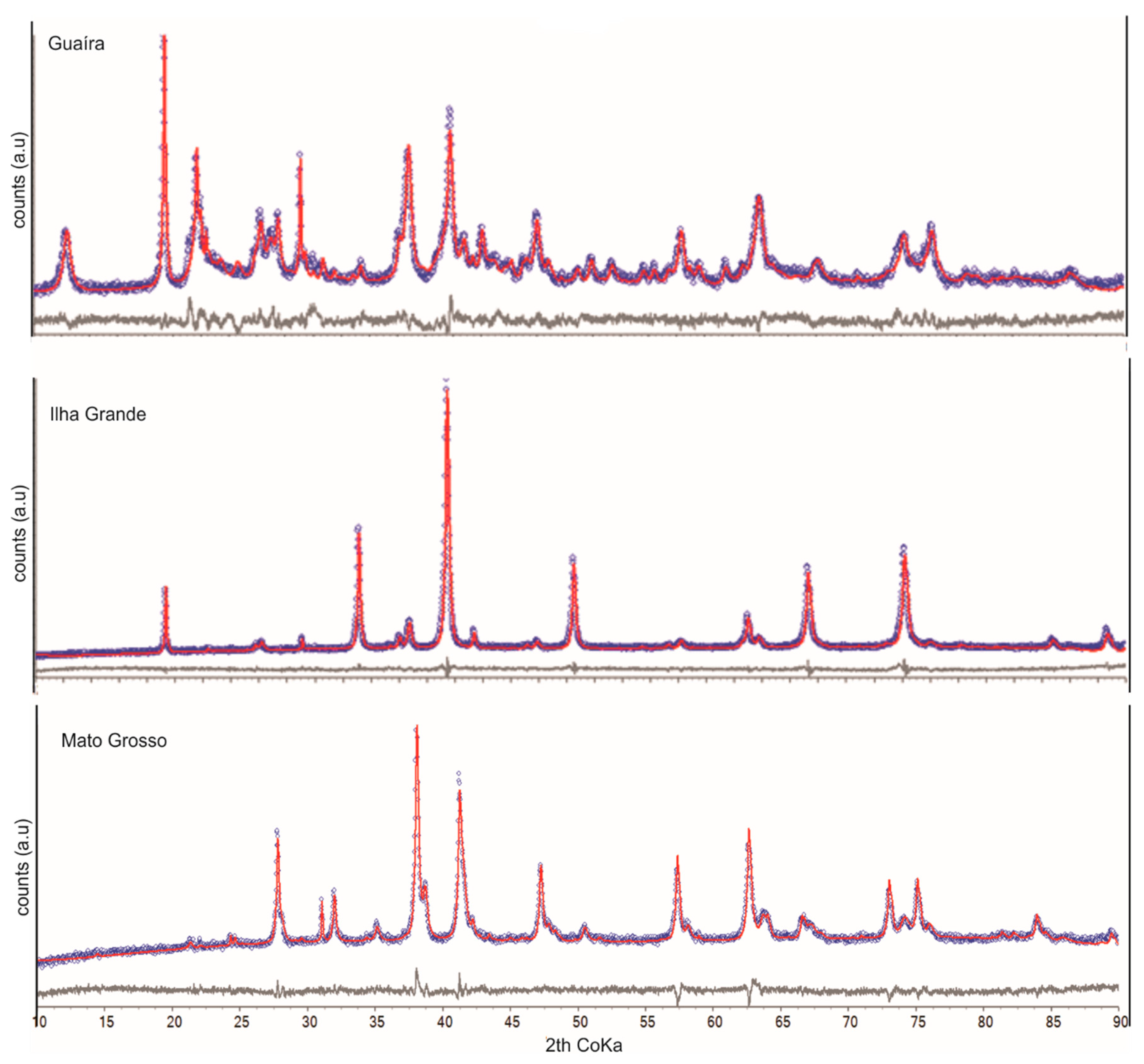
3.1.2. Soil Samples
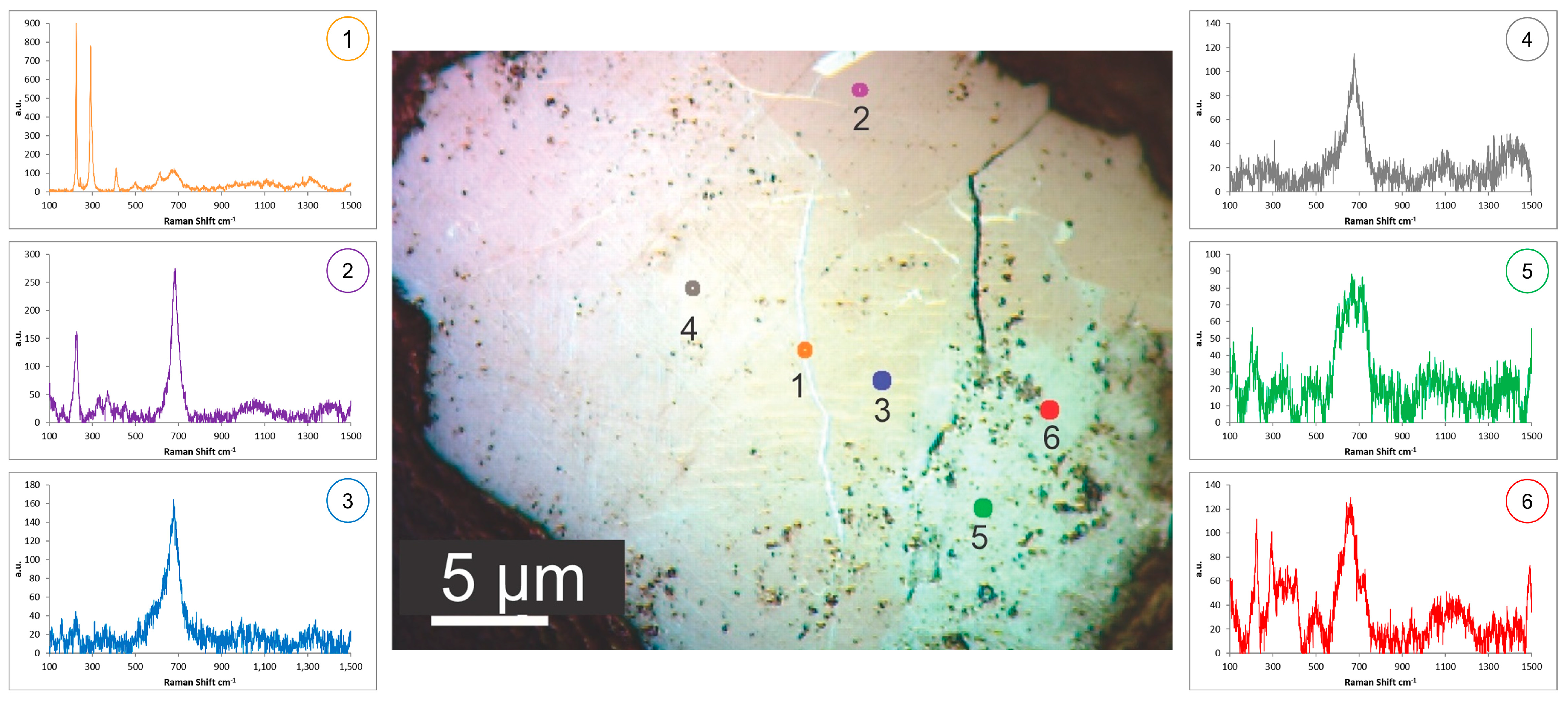
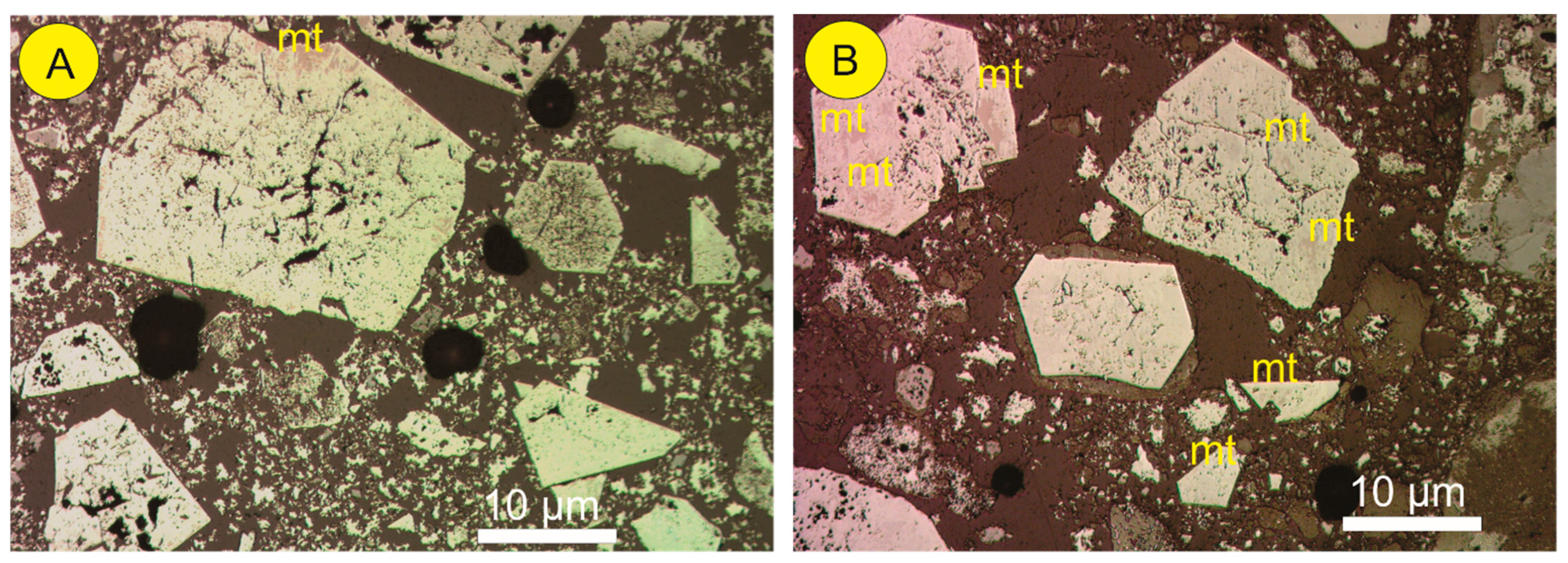
3.2. Second Stage—Iron Ore from Carajás
3.3. Solid Solution Compared to Separate Phases
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rosière, C.A.; Baars, F.J.; Seoane, J.C.S.; Lobato, L.M.; Da Silva, L.L.; De Souza, S.R.C.; Mendes, G.E. Structure and iron mineralisation of the Carajás Province. Appl. Earth Sci. 2006, 115, 126–133. [Google Scholar] [CrossRef]
- Clout, J.M.F.; Manuel, J.R. Mineralogical, chemical, and physical characteristics of iron ore. In Iron Ore: Mineralogy, Processing and Environmental Issues; Liu, L., Ed.; Woodhead Publishing: Sawston, Cambridge, UK, 2015; pp. 45–84. [Google Scholar]
- Gallagher, K.J.; Feitknecht, W.; Mannweiler, U. Mechanism of Oxidation of Magnetite to γ-Fe2O3. Nat. Cell Biol. 1968, 217, 1118–1121. [Google Scholar] [CrossRef]
- Walter, A.-V.; Nahon, D.; Flicoteaux, R.; Girard, J.; Melfi, A. Behaviour of major and trace elements and fractionation of REE under tropical weathering of a typical apatite-rich carbonatite from Brazil. Earth Planet. Sci. Lett. 1995, 136, 591–602. [Google Scholar] [CrossRef]
- Neumann, R.; Medeiros, E.B. Comprehensive mineralogical and technological characterisation of the Araxá (SE Brazil) complex REE (Nb-P) ore, and the fate of its processing. Int. J. Miner. Process. 2015, 144, 1–10. [Google Scholar] [CrossRef]
- Gorski, C.A.; Scherer, M.M. Determination of nanoparticulate magnetite stoichiometry by Mossbauer spectroscopy, acidic dissolution, and powder X-ray diffraction: A critical review. Am. Miner. 2010, 95, 1017–1026. [Google Scholar] [CrossRef]
- Bosi, F.; Biagioni, C.; Pasero, M. Nomenclature and classification of the spinel supergroup. Eur. J. Miner. 2019, 31, 183–192. [Google Scholar] [CrossRef]
- Greaves, C. A powder neutron diffraction investigation of vacancy ordering and covalence in γ-Fe2O3. J. Solid State Chem. 1983, 49, 325–333. [Google Scholar] [CrossRef]
- Grau-Crespo, R.; Al-Baitai, A.Y.; Saadoune, I.; De Leeuw, N.H. Vacancy ordering and electronic structure of γ-Fe2O3(maghemite): A theoretical investigation. J. Physics Condens. Matter 2010, 22, 255401. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2003; pp. 9–94. [Google Scholar] [CrossRef]
- D’Abreu, J.C.; Nunes, E.A.; Antonello, L.L. Maghemite occurence in LD steel mill residues. In Proceedings of the 55th Anual Congress of ABM, Rio de Janeiro, Brazil, 24–28 July 2000. (In Portuguese). [Google Scholar]
- Montes, S.; Montes Atenas, G.; Valero, E. How fine particles on hematite mineral ultimately define the mineral surface charge and the overall floatability behaviour. J. South. Afr. Inst. Min. Metall. 2007, 107, 689–695. [Google Scholar]
- De Villiers, J.P.R.; Lu, L. ΧRD analysis and evaluation of iron ores and sinters. In Iron Ore: Mineralogy, Processing and Environmental Issues; Lu, L., Ed.; Woodhead Publishing: Sawston, Cambridge, UK, 2015; pp. 85–100. [Google Scholar]
- Morris, R.C. Supergene Alteration of Banded Iron-Formation. In Iron-Formation Facts and Problems; Trendall, A.F., Morris, R.C., Eds.; Developments in Precambrian Geology; Elsevier: Amsterdam, The Netherlands, 1983; Volume 6, pp. 513–534. [Google Scholar]
- Colombo, U.; Gazzarrini, F.; Lanzavecchia, G.; Sironi, G.; Brewer, J.H.; Allgeier, D.L. Magnetite Oxidation: A Proposed Mechanism. Science 1965, 147, 1033. [Google Scholar] [CrossRef] [PubMed]
- Lagoeiro, L.E. Transformation of magnetite to hematite and its influence on the dissolution of iron oxide minerals. J. Metamorph. Geol. 2004, 16, 415–423. [Google Scholar] [CrossRef]
- Brown, G. Associated minerals. In Crystal Structures of Clay Minerals and their Χ-ray Identification; Brindley, G.W., Brown, G., Eds.; Mineralogical Society of Great Britain and Ireland: London, UK, 1980; Chapter 6; Volume 5, pp. 361–407. [Google Scholar] [CrossRef]
- Cullity, B.D.; Graham, C.D. Introduction to Magnetic Materials; John Wiley & Son: Hoboken, NJ, USA, 2009. [Google Scholar]
- Vandenberghe, R.; Barrero, C.; Da Costa, G.; Van San, E.; De Grave, E. Mössbauer characterization of iron oxides and (oxy)hydroxides: The present state of the art. Hyperfine Interact. 2000, 126, 247–259. [Google Scholar] [CrossRef]
- Roca, A.G.; Marco, J.F.; Morales, M.D.P.; Serna, C.J. Effect of Nature and Particle Size on Properties of Uniform Magnetite and Maghemite Nanoparticles. J. Phys. Chem. C 2007, 111, 18577–18584. [Google Scholar] [CrossRef]
- Doriguetto, A.C.; Fernandes, N.G.; Persiano, A.I.C.; Filho, E.N.; Grenèche, J.M.; Fabris, J.D. Characterization of a natural magnetite. Phys. Chem. Miner. 2003, 30, 249–255. [Google Scholar] [CrossRef]
- Barbosa, P.F.; Lagoeiro, L. Crystallographic texture of the magnetite-hematite transformation: Evidence for topotactic relationships in natural samples from Quadrilatero Ferrifero, Brazil. Am. Miner. 2009, 95, 118–125. [Google Scholar] [CrossRef]
- Espinosa, A.; Serrano, A.; Llavona, A.; De La Morena, J.J.; Abuin, M.; Figuerola, A.; Pellegrino, T.; Fernández, J.F.; Garcia-Hernandez, M.; Castro, G.R.; et al. On the discrimination between magnetite and maghemite by XANES measurements in fluorescence mode. Meas. Sci. Technol. 2011, 23, 015602. [Google Scholar] [CrossRef]
- Ramanaidou, B.; Wells, M.; Belton, D.; Verrall, M.; Ryan, C. Mineralogical and Microchemical Methods for the Characterization of High-Grade Banded Iron Formation-Derived Iron Ore. Soc. Econ. Geol. Rev. 2008, 15, 129–156. [Google Scholar] [CrossRef]
- Donskoi, E.; Poliakov, A.; Manuel, J.R. Automated optical image analysis of natural and sintered iron ore. In Iron Ore: Mineralogy, Processing and Environmental Issues; Lu, L., Ed.; Woodhead Publishing: Sawston, Cambridge, UK, 2015; pp. 101–159. [Google Scholar]
- Donskoi, E.; Manuel, J.R.; Hapugoda, S.; Poliakov, A.; Raynlyn, T.; Austin, P.; Peterson, M. Automated optical image analysis of goethitic iron ores. Miner. Process. Extr. Met. 2020, 1–11. [Google Scholar] [CrossRef]
- Iglesias, J.C.Á.; Augusto, K.S.; Gomes, O.D.F.M.; Domingues, A.L.A.; Vieira, M.B.; Casagrande, C.; Paciornik, S. Automatic characterization of iron ore by digital microscopy and image analysis. J. Mater. Res. Technol. 2018, 7, 376–380. [Google Scholar] [CrossRef]
- Kerr, J.C. Latossolos do Brasil: Uma revisão. Geonomos 1997, 5, 17–40. [Google Scholar] [CrossRef]
- Rosiere, C.A.; Chemale, F., Jr. Itabiritos e minérios de ferro de alto teor do Quadrilátero Ferrífero—Uma visão geral e discussão. Geonomos 2000, 8, 27–42. [Google Scholar] [CrossRef]
- Varajão, C.A.C.; Salgado, A.A.R.; Varajão, A.F.D.C.; Braucher, R.; Colin, F.; Árias, N.H., Jr. Estudo da evolução da paisagem do quadrilátero ferrífero (Minas Gerais, Brasil) por meio da mensuração das taxas de erosão (10be) e da pedogênese. Rev. Bras. Ciência Solo 2009, 33, 1409–1425. [Google Scholar] [CrossRef]
- Cheary, R.W.; Coelho, A. A fundamental parameters approach to X-ray line-profile fitting. J. Appl. Crystallogr. 1992, 25, 109–121. [Google Scholar] [CrossRef]
- Hölzer, G.; Fritsch, M.; Deutsch, M.; Härtwig, J.; Förster, E. Kα1,2andKβ1,3x-ray emission lines of the3dtransition metals. Phys. Rev. A 1997, 56, 4554–4568. [Google Scholar] [CrossRef]
- Gražulis, S.; Chateigner, D.; Downs, R.T.; Yokochi, A.T.; Quiros, M.; Lutterotti, L.; Manakova, E.; Butkus, J.; Moeck, P.; Le Bail, A. Crystallography Open Database—An open-access collection of crystal structures. J. Appl. Crystallogr. 2009, 42, 726–729. [Google Scholar] [CrossRef]
- Neder, R.B.; Burghammer, M.; Grasl, T.; Schulz, H.; Bram, A.; Fiedler, S. Refinement of the Kaolinite Structure from Single-Crystal Synchrotron Data. Clays Clay Miner. 1999, 47, 487–494. [Google Scholar] [CrossRef]
- Morris, M.C.; McMurdie, H.F.; Evans, E.H.; Paretzkin, B.; Parker, H.S.; Panagiotopoulos, N.C. Standard Χ-ray Diffraction Powder Patterns; NBS: Washington, DC, USA, 1981; Section 18; p. 106. [Google Scholar]
- Antao, S.M.; Hassan, I.; Wang, J.; Lee, P.L.; Toby, B.H. State-of-the-art high-resolution powder x-ray diffraction (HRPXRD) illustrated with Rietveld structure refinement of quartz, sodalite, tremolite, and meionite. Can. Mineral. 2008, 46, 1501–1509. [Google Scholar] [CrossRef]
- Harrison, R.K.; Aitkenhead, N.; Young, B.R.; Dagger, P.F. Goethite from Hindlow, Derbyshire. Bull. Geol. Surv. Great Britain 1975, 52, 51–54. [Google Scholar]
- Knorr, K.; Neumann, R. Advances in Quantitative Χ-Ray Mineralogy: Miχed Crystals in Bauχite. In Proceedings of the 10th International Congress for Applied Mineralogy; Broekmans, M.A.T.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 377–384. [Google Scholar]
- Sadykov, V.A.; Isupova, L.A.; Tsybulya, S.V.; Cherepanova, S.V.; Litvak, G.S.; Burgina, E.B.; Kustova, G.N.; Kolomiichuk, V.N.; Ivanov, V.P.; Paukshtis, E.A.; et al. Effect of Mechanical Activation on the Real Structure and Reactivity of Iron (III) Oχide with Corundum-Type Structure. J. Solid State Chem. 1996, 123, 191–202. [Google Scholar] [CrossRef]
- Neumann, R.; Avelar, A.N.; Da Costa, G.M. Refinement of the isomorphic substitutions in goethite and hematite by the Rietveld method, and relevance to bauxite characterisation and processing. Miner. Eng. 2014, 55, 80–86. [Google Scholar] [CrossRef]
- Della Giusta, A.; Princivalle, F.; Carbonin, S. Crystal structure and cation distribution in some natural magnetites. Miner. Pet. 1987, 37, 315–321. [Google Scholar] [CrossRef]
- Gatta, G.D.; Kantor, I.; Ballaran, T.B.; Dubrovinsky, L.; McCammon, C. Effect of non-hydrostatic conditions on the elastic behaviour of magnetite: An in situ single-crystal X-ray diffraction study. Phys. Chem. Miner. 2007, 34, 627–635. [Google Scholar] [CrossRef]
- Cisar, A.; Poulsen, K. Grant-in-Aid, JCPDS-ICDD. In ICDD; Dow Chemical Company: Freeport, TX, USA, 1979; pp. 18–33. [Google Scholar]
- Saalfeld, H.; Wedde, M. Refinement of the crystal structure of gibbsite, Al(OH)3. Z. Krist. 1974, 139, 129–135. [Google Scholar] [CrossRef]
- Morris, M.C.; McMurdie, H.F.; Evans, E.H.; Paretzkin, B.; de Groot, J.H.; Weeks, B.S.; Newberry, R.J. Standard Χ-ray Diffraction Powder Patterns; NBS: Washington, DC, USA, 1978; Section 15; p. 199. [Google Scholar]
- Wechsler, B.A.; Prewitt, C.T. Crystal structure of ilmenite (FeTiO3) at high temperature and high pressure room temperature after heating. Am. Mineral. 1984, 69, 176–185. [Google Scholar] [CrossRef]
- Perdikatsis, B.; Burzlaff, H. Strukturverfeinerung am Talk Mg3[(OH)2Si4O10]. Z. Krist. Cryst. Mater. 1981, 156, 177–186. [Google Scholar] [CrossRef]
- Baur, W.H.; Khan, A.A. Rutile-type compounds. IV. SiO2, GeO2 and a comparison with other rutile-type structures. Acta Crystallographica Section B 1971, 27, 2133–2139. [Google Scholar] [CrossRef]
- Restori, R.; Schwarzenbach, D.; Schneider, J.R. Charge density in rutile, TiO2. Acta Crystallogr. Sect. B 1987, 43, 251–257. [Google Scholar] [CrossRef]
- Howard, C.J.; Sabine, T.M.; Dickson, F. Structural and thermal parameters for rutile and anatase. Acta Crystallogr. Sect. B 1991, 47, 462–468. [Google Scholar] [CrossRef]
- Wyckoff, R.W.G. Crystal Structures, 2nd ed.; Interscience Publishers: New York, NY, USA, 1963; pp. 239–444. [Google Scholar]
- Taylor, M.; Brown, G.E. High-temperature structural study of the P21/a →A2/a phase transition in synthetic titanite, CaTiSiO5. Am. Mineral. 1976, 61, 435–447. [Google Scholar]
- Pecharroman, C.; Gonzalez-Carreno, T.; Iglesias, J.E. The infrared dielectric properties of maghemite, gamma-Fe2O3, from reflectance measurement on pressed powders. Phys. Chem. Miner. 1995, 22, 21–29. [Google Scholar] [CrossRef]
- De Faria, D.L.; Venâncio Silva, S.; De Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Gomes, O.F.M.; Paciornik, S. Multimodal Microscopy for Ore Characterization. In Scanning Electron Microscopy; Kazmiruk, V., Ed.; IntechOpen: London, UK, 2012; pp. 313–334. [Google Scholar]
- Toby, B.H. R factors in Rietveld analysis: How good is good enough? Powder Diffr. 2006, 21, 67–70. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Kubo, K.; Sato, N. Raman Spectroscopy of Thin Corrosion Films on Iron at 100 to 150 C in Air. Corrosion 1986, 42, 476–481. [Google Scholar] [CrossRef]
- Legodi, M.; De Waal, D. The preparation of magnetite, goethite, hematite and maghemite of pigment quality from mill scale iron waste. Dye. Pigment. 2007, 74, 161–168. [Google Scholar] [CrossRef]
- Van der Weerd, J.; Rehren, T.; Firth, S.; Clark, R.J.H. Identification of iron oxide impurities in earliest industrial-scale processed platinum. Mat. Charact. 2004, 53, 63–70. [Google Scholar] [CrossRef]
- Muralha, V.S.F.; Rehren, T.; Clark, R.J.H. Characterization of an iron smelting slag from Zimbabwe by Raman microscopy and electron beam analysis. J. Raman Spectrosc. 2011, 42, 2077–2084. [Google Scholar] [CrossRef]
- Anthony, J.W.; Bideaux, R.A.; Bladh, K.W.; Nichols, M.C. (Eds.) Handbook of Mineralogy [Homepage on the Internet]; Mineralogical Society of America: Chantilly, VA, USA, 2004; Available online: http://www.handbookofmineralogy.org/ (accessed on 7 December 2020).
- Pracejus, B. (Ed.) The Ore Minerals Under the Microscope: An Optical Guide; Elsevier: Amsterdam, The Netherlands, 2008; pp. 648–650. [Google Scholar]
- Neumann, U. Guide for the Microscopical Identification of Ore and Gangue Minerals; Tübingen University Press: Tübingen, Germany, 2019. [Google Scholar]
- Meurant, G. Regional Studies and Specific Deposits; Elsevier: Amsterdam, The Netherlands, 2012; pp. 102–115. [Google Scholar]
- Santos, L.D.; Brandão, P.R.G. LM, SEM and EDS study of microstructure of Brazilian iron ores. Microsc. Anal. 2005, 19, 17–19. [Google Scholar]
- Demortière, A.; Panissod, P.; Pichon, B.P.; Pourroy, G.; Guillon, D.; Donnio, B.; Bégin-Colin, S. Size-dependent properties of magnetic iron oxidenanocrystals. Nanoscale 2011, 3, 225–232. [Google Scholar] [CrossRef]
- Morales, M.P.; Veintemillas-Verdaguer, S.; Montero, M.I.; Serna, C.J.; Roig, A.; Casas, L.; Martínez, B.; Sandiumenge, F. Surface and Internal Spin Canting in γ-Fe2O3Nanoparticles. Chem. Mater. 1999, 11, 3058–3064. [Google Scholar] [CrossRef]
- Hunt, C.P.; Moskowitz, B.M.; Banerjee, S.K. Magnetic Properties of Rocks and Minerals. In Rock Physics & Phase Relations: A Handbook of Physical Constants; Ahrens, T.J., Ed.; American Geophysical Union: Washington, DC, USA, 1995; pp. 189–204. [Google Scholar]
- Da Costa, G.; De Grave, E.; Vandenberghe, R. Mössbauer studies of magnetite and Al-substituted maghemites. Hyperfine Interactions 1998, 117, 207–243. [Google Scholar] [CrossRef]
- Lobato, L.M.; Rosière, C.A.; Silva, R.C.F.; Zucchetti, M.; Baars, F.J.; Seoane, J.C.S.; Rios, F.J.; Pimentel, M.; Mendes, G.E.; Monteiro, A.M. A mineralização hidrotermal de ferro da Província Mineral de Carajás—Controle estrutural e contexto na evolução metalogenética da província. In Caracterização de Depósitos Minerais em Distritos Mineiros da Amazônia; Marini, O.J., Queiroz, E.T.D., Ramos, B.W., Eds.; DNPM/CTMineral/FINEP/ADIMB: Brasília, Brazil, 2005; pp. 25–92. [Google Scholar]




| Mineral | Database and Code Used | Reference | Cell Parameters (Å) and Range |
|---|---|---|---|
| Kaolinite | ICSD, kaolinite 87771 | Neder et al. (1999) [34] | a-5.1560 (5.1200–5.2000) b-8.9450 (8.9000–8.9900) c-7.4050 (7.3800–7.4500) |
| Quartz | Bruker, quartz ICDD 331161 COD 2011 9013321 | Morris et al. (1981) [35] Antao et al. (2008) [36] | a-4.9120 (4.9000–4.9350) b = a c-5.4040 (5.3800–5.4500) |
| Goethite, Al | Modified from Bruker goethite ICDD 290713 | Harrison et al. (1975) [37], modified by [38] | a-9.9600 (9.9000–10.0000) b-3.0230 (2.9800–3.0700) c-4.6050 (4.5500–4.6500) |
| Hematite, Al, OH | Modified from Bruker hematite ICDD 330664 | Sadykov et al. (1996) [39], modified by [40] | a-5.0370 (5.0100–5.0700) b = a c-13.7710 (13.7000–13.8300) |
| Magnetite, nonstoich. | Modified from Bruker magnetite ICDD 190629 COD 2011 9010939 | Della Giusta et al. (1987) [41], modified by [5] Gatta et al. [42] | a-8.3950 (8.3200–8.4700) b = a c = a |
| Gibbsite | Bruker, gibbsite ICDD 330018 COD 2011 9008237 | Cisar & Paulsen (1979) [43] Saafeld et al. [44] | a-8.6850 (8.6000–8.7500) b-5.0770 (5.0000–5.2000) c-9.7360 (9.6000–9.8000) |
| Ilmenite | Bruker, ilmenite ICDD 290733 COD 2011 9000911 | Morris et al. (1978) [45] Wechsler et al. [46] | a-5.0870 (5.0370–5.1390) b = a c-14.0840 (13.9440–14.2260) |
| Talc | Bruker, talc ICDD 190770 | Perdikatsis & Burzlaff (1981) [47] | a-5.2930 (5.2400–5.3460) b-9.1790 (9.0870–9.2710) c-9.4690 (9.3740–9.5640) |
| Rutile | Bruker, rutile ICDD 211276 COD 2011 9007531 | Restori et al. (1987) [48] Baur et al. (1971) [49] | a-4.5940 (4.5600–4.6400) b = a c-2.9600 (2.9500–2.9700) |
| Anatase | Bruker, anatase ICDD 211272 COD 2011 9009086 | Howard et al. (1991) [50] Wyckoff et al. (1963) [51] | a-3.7820 (3.6800–3.8800) b = a c-9.5140 (9.3000–9.6000) |
| Titanite | Bruker, titanite ICDD 250177 | Taylor & Brown (1976) [52] | a-7.4550 (7.3810–7.5300) b-8.7140 (8.6270–8.8010) c-7.0680 (6.9970–7.1390) |
| Cubic maghemite | Bruker maghemite ICDD 391346 | Bruker | a = 8.3460 (8.2800–8.4100) b = a c = a |
| Tetragonal maghemite | ICSD 172906 | Jorgensen, J.E et al. [53] | a = 8.3320 (8.2800–8.4100) b = a c = 25.113 (24.8400–25.2300) |
| Mineral (wt%) | Quadrilátero Ferrífero | ||
|---|---|---|---|
| Guanhães | Guanhães 2 | Espinhaço | |
| Hematite(-Al) | 70.1 | 11.7 | 93.7 |
| Ilmenite | 14.6 | - | - |
| Quartz | 3.8 | 0.7 | 1.6 |
| Nonstoichiometric magnetite | 0.4 | 87.6 | 4.8 |
| Talc | 2.1 | - | - |
| Substitutions (mol) | |||
| Fe3+ hematite | 0.980 | 0.988 | 0.981 |
| Al3+ hematite | 0.009 | 0.000 | 0.002 |
| OH− hematite | 0.031 | 0.054 | 0.051 |
| χ magnetite | 0.091 | 0.500 | 0.372 |
| a magnetite (Å) | 8.3523 | 8.3971 | 8.3831 |
| RWP | 2.21 | 2.69 | 3.06 |
| GOF | 2.20 | 2.62 | 3.01 |
| Mineral (wt%) | Soil | ||
|---|---|---|---|
| Guaíra | Ilha Grande | Mato Grosso | |
| Hematite(-Al) | 38.7 | 9.5 | 18.4 |
| Kaolinite | 10.1 | - | - |
| Gibbsite | 32.6 | - | - |
| Ilmenite | 4.6 | 3.5 | 61.7 |
| Anatase | 3.8 | - | 0.4 |
| Quartz | 3.0 | 0.7 | 2.1 |
| Nonstoichiometric magnetite | 4.5 | 86.3 | 9.9 |
| Titanite | 0.9 | - | - |
| Goethite-(Al) | 1.9 | - | 1.2 |
| Rutile | - | - | 6.4 |
| Substitutions (mol) | |||
| Fe3+ hematite | 0.868 | 0.977 | 0.895 |
| Al3+ hematite | 0.115 | 0.008 | 0.046 |
| OH− hematite | 0.049 | 0.04 | 0.176 |
| Al goethite | 0.236 | - | 0.358 |
| χ magnetite | 0.000 | 0.500 | 0.458 |
| a magnetite (Å) | 8.3424 | 8.3971 | 8.3925 |
| RWP | 2.57 | 2.20 | 2.43 |
| GOF | 1.85 | 2.06 | 1.70 |
| Mineral (wt%) | N4WN | N4WS | ||||
|---|---|---|---|---|---|---|
| AM-01A | AM-01B | AM-01H | AM-02A | AM-02B | AM-02E | |
| Kaolinite | 0.6 | 0.2 | 0.4 | 0.3 | 0.4 | 0.3 |
| Quartz | 0.7 | 0.7 | 0.7 | 0.5 | 0.3 | 0.5 |
| Goethite-Al | 6.1 | 6.3 | 6.0 | 17.9 | 18.0 | 18.6 |
| Hematite-Al | 88.2 | 88.3 | 88.2 | 77.5 | 77.7 | 77.0 |
| Nonstoichiometric magnetite | 4.5 | 4.5 | 4.6 | 3.4 | 3.2 | 3.2 |
| Gibbsite | 0.0 | 0.0 | 0.0 | 0.4 | 0.4 | 0.4 |
| Substitutions (mol) | ||||||
| Fe3+ hematite | 0.973 | 0.973 | 0.971 | 0.964 | 0.962 | 0.963 |
| Al3+ hematite | 0.005 | 0.005 | 0.006 | 0.005 | 0.009 | 0.007 |
| OH− hematite | 0.068 | 0.066 | 0.069 | 0.094 | 0.087 | 0.089 |
| Al goethite | 0.007 | 0.008 | 0.012 | 0.032 | 0.035 | 0.034 |
| χ magnetite | 0.049 | 0.049 | 0.062 | 0.431 | 0.373 | 0.404 |
| a magnetite (Å) | 8.3477 | 8.3478 | 8.3492 | 8.3895 | 8.3832 | 8.3866 |
| RWP | 1.84 | 1.84 | 1.83 | 1.75 | 1.86 | 1.83 |
| GOF | 1.77 | 1.78 | 1.78 | 1.68 | 1.80 | 1.76 |
| Mineral (wt%) | Guaíra | Espinhaço | ||
|---|---|---|---|---|
| Stoichiometric Magnetite and Maghemite | Magnetite-Maghemite Solid Solution | Stoichiometric Magnetite and Maghemite | Magnetite-Maghemite Solid Solution | |
| Kaolinite | 10.5 | 10.1 | ||
| Quartz | 3.0 | 3.0 | 1.6 | 1.6 |
| Goethite(-Al) | 2.3 | 1.9 | ||
| Hematite(-Al) | 37.5 | 38.7 | 93.4 | 93.7 |
| Nonstoichiometric Magnetite | 4.5 | 4.8 | ||
| Gibbsite | 32.5 | 32.6 | ||
| Magnetite | 0.5 | 3.6 | ||
| Titanite | 0.6 | 0.9 | ||
| Anatase | 3.7 | 3.8 | ||
| Maghemite (tetragonal) | 2.4 | 0.1 | ||
| Ilmenite | 4.1 | 4.6 | ||
| Maghemite (cubic) | 3.0 | 0.8 | ||
| Substitutions (mol) | ||||
| Fe3+ hematite | 0.888 | 0.868 | 0.981 | 0.981 |
| Al3+ hematite | 0.106 | 0.115 | 0.002 | 0.002 |
| OH− hematite | 0.018 | 0.049 | 0.050 | 0.051 |
| Al goethite | 0.239 | 0.236 | ||
| χ magnetite | 0.000 | 0.372 | ||
| a nonstoichiometric magnetite (Å) | 8.3424 | 8.3831 | ||
| a maghemite cubic (Å) | 8.331 | 8.353 | ||
| a magnetite (Å) | 8.437 | 8.395 | ||
| a maghemite_tetr. (Å) | 8.280 | 8.280 | ||
| c maghemite_tetr. (Å) | 25.076 | 24.843 | ||
| RWP | 2.54 | 2.57 | 3.06 | 3.06 |
| GOF | 1.82 | 1.85 | 3.01 | 3.01 |
| Mineral (wt%) | N4WN-A | N4WS-E | ||
|---|---|---|---|---|
| Stoichiometric Magnetite and Maghemite | Magnetite-Maghemite Solid Solution | Stoichiometric Magnetite and Maghemite | Magnetite-Maghemite Solid Solution | |
| Kaolinite | 0.5 | 0.6 | 0.3 | 0.3 |
| Quartz | 0.7 | 0.7 | 0.5 | 0.5 |
| Goethite(-Al) | 6.1 | 6.1 | 18.7 | 18.6 |
| Hematite(-Al) | 87.3 | 88.2 | 76.9 | 77.0 |
| Nonstoichiometric Magnetite | 4.5 | 3.2 | ||
| Gibbsite | 0.1 | 0.4 | ||
| Magnetite | 0.2 | 2.2 | ||
| Maghemite (tetragonal) | 1.1 | 0.1 | ||
| Maghemite (cubic) | 4.0 | 1.1 | ||
| Substitutions (mol) | ||||
| Fe3+ hematite | 0.972 | 0.973 | 0.964 | 0.963 |
| Al3+ hematite | 0.005 | 0.005 | 0.007 | 0.007 |
| OH− hematite | 0.073 | 0.067 | 0.088 | 0.089 |
| Al goethite | 0.068 | 0.008 | 0.034 | 0.034 |
| χ magnetite | 0.049 | 0.404 | ||
| a nonstoichiometric magnetite (Å) | 8.3477 | 8.3866 | ||
| a maghemite cubic (Å) | 8.347 | 8.356 | ||
| a magnetite (Å) | 8.418 | 8.401 | ||
| a maghemite_tetr. (Å) | 8.293 | 8.280 | ||
| c maghemite_tetr. (Å) | 25.035 | 24.840 | ||
| RWP | 1.81 | 1.84 | 1.81 | 1.83 |
| GOF | 1.75 | 1.77 | 1.74 | 1.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hiraga, R.; Gomes, O.d.F.M.; Neumann, R. Maghemite in Brazilian Iron Ores: Quantification of the Magnetite-Maghemite Isomorphic Series by Χ-ray Diffraction and the Rietveld Method, and Confirmation by Independent Methods. Minerals 2021, 11, 346. https://doi.org/10.3390/min11040346
Hiraga R, Gomes OdFM, Neumann R. Maghemite in Brazilian Iron Ores: Quantification of the Magnetite-Maghemite Isomorphic Series by Χ-ray Diffraction and the Rietveld Method, and Confirmation by Independent Methods. Minerals. 2021; 11(4):346. https://doi.org/10.3390/min11040346
Chicago/Turabian StyleHiraga, Renata, Otávio da Fonseca Martins Gomes, and Reiner Neumann. 2021. "Maghemite in Brazilian Iron Ores: Quantification of the Magnetite-Maghemite Isomorphic Series by Χ-ray Diffraction and the Rietveld Method, and Confirmation by Independent Methods" Minerals 11, no. 4: 346. https://doi.org/10.3390/min11040346
APA StyleHiraga, R., Gomes, O. d. F. M., & Neumann, R. (2021). Maghemite in Brazilian Iron Ores: Quantification of the Magnetite-Maghemite Isomorphic Series by Χ-ray Diffraction and the Rietveld Method, and Confirmation by Independent Methods. Minerals, 11(4), 346. https://doi.org/10.3390/min11040346







