Quantitative in Situ X-ray Diffraction Analysis of Early Hydration of Belite-Calcium Sulfoaluminate Cement at Various Defined Temperatures
Abstract
1. Introduction
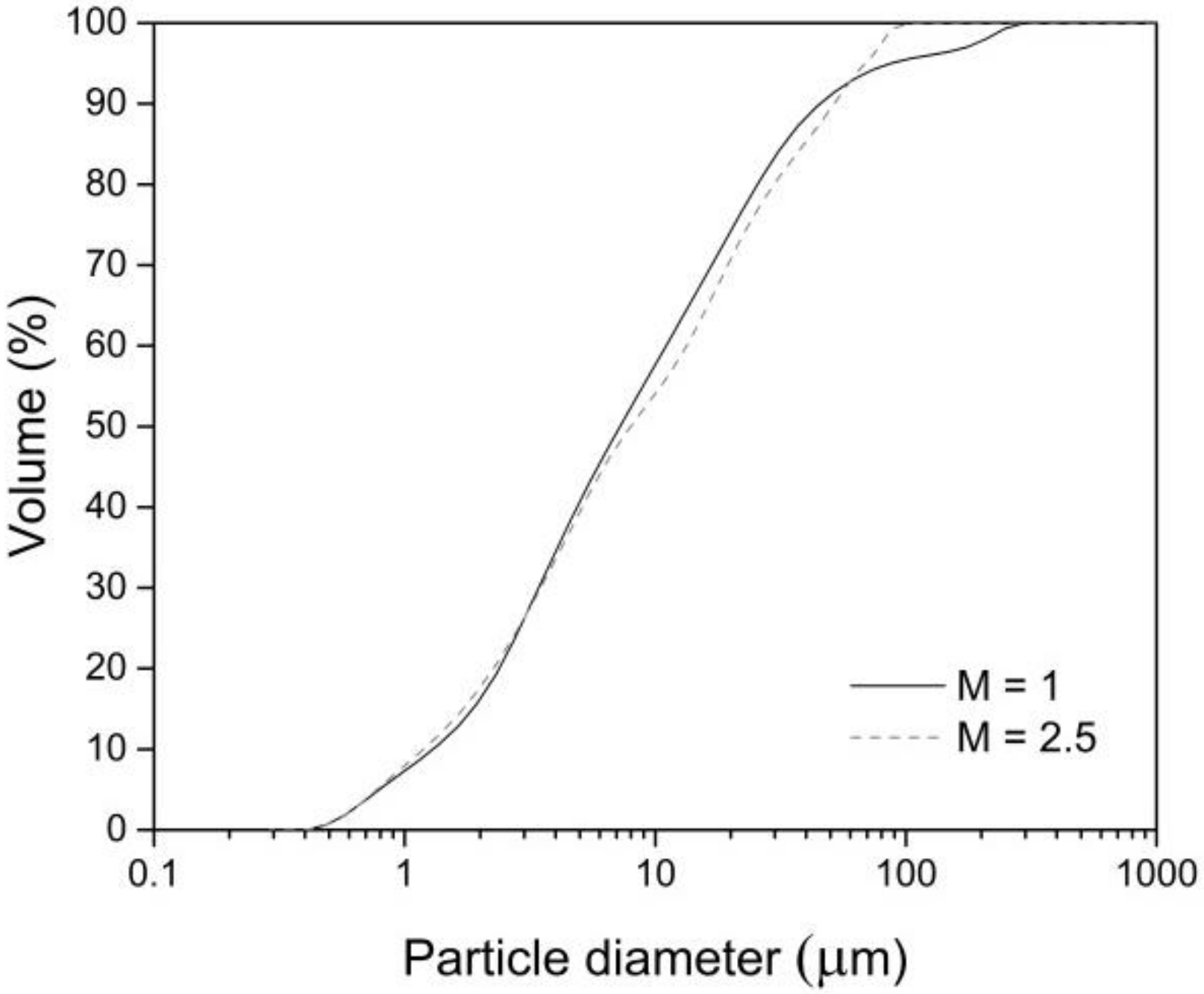
2. Materials and Methods
3. Results
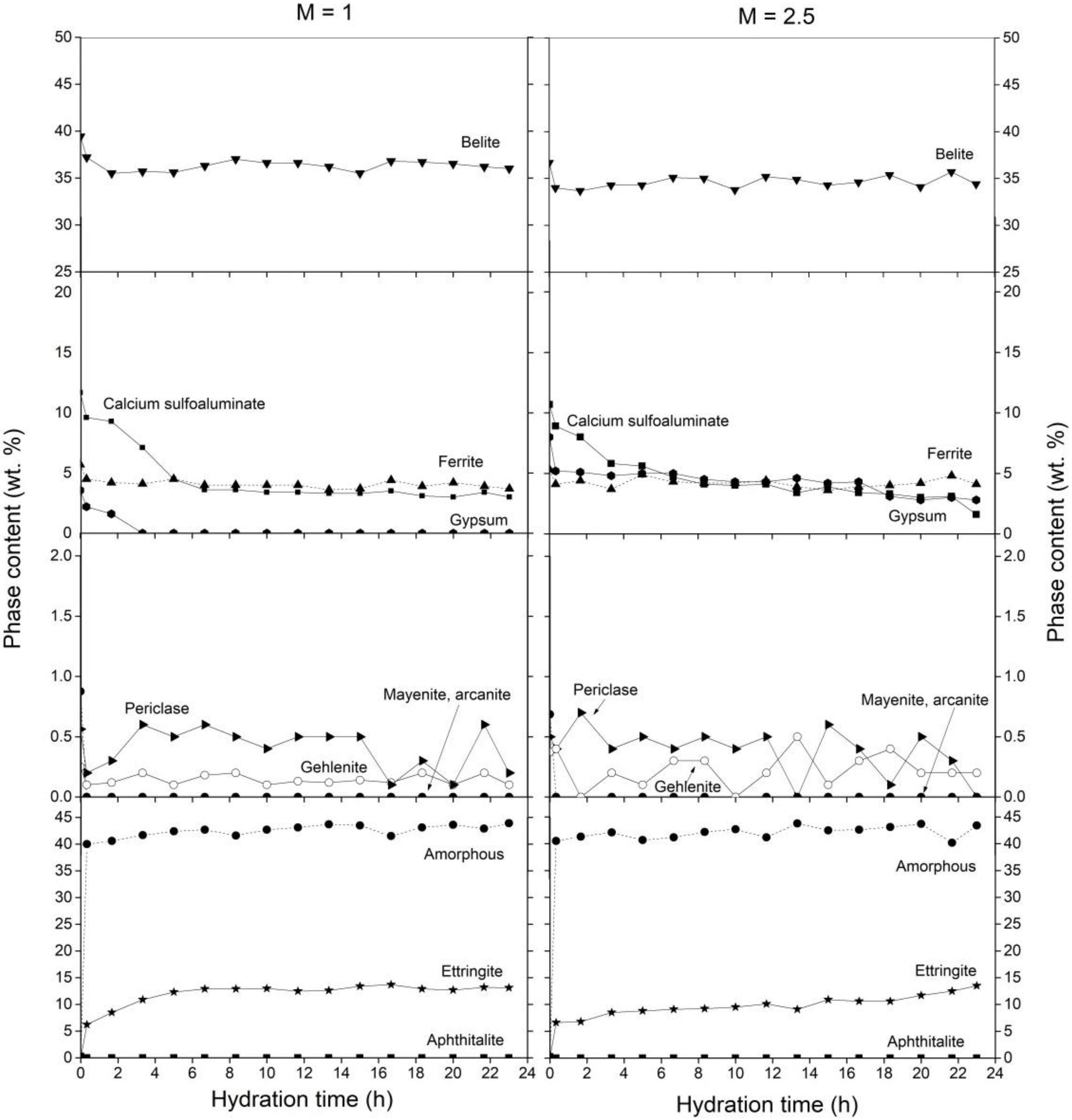
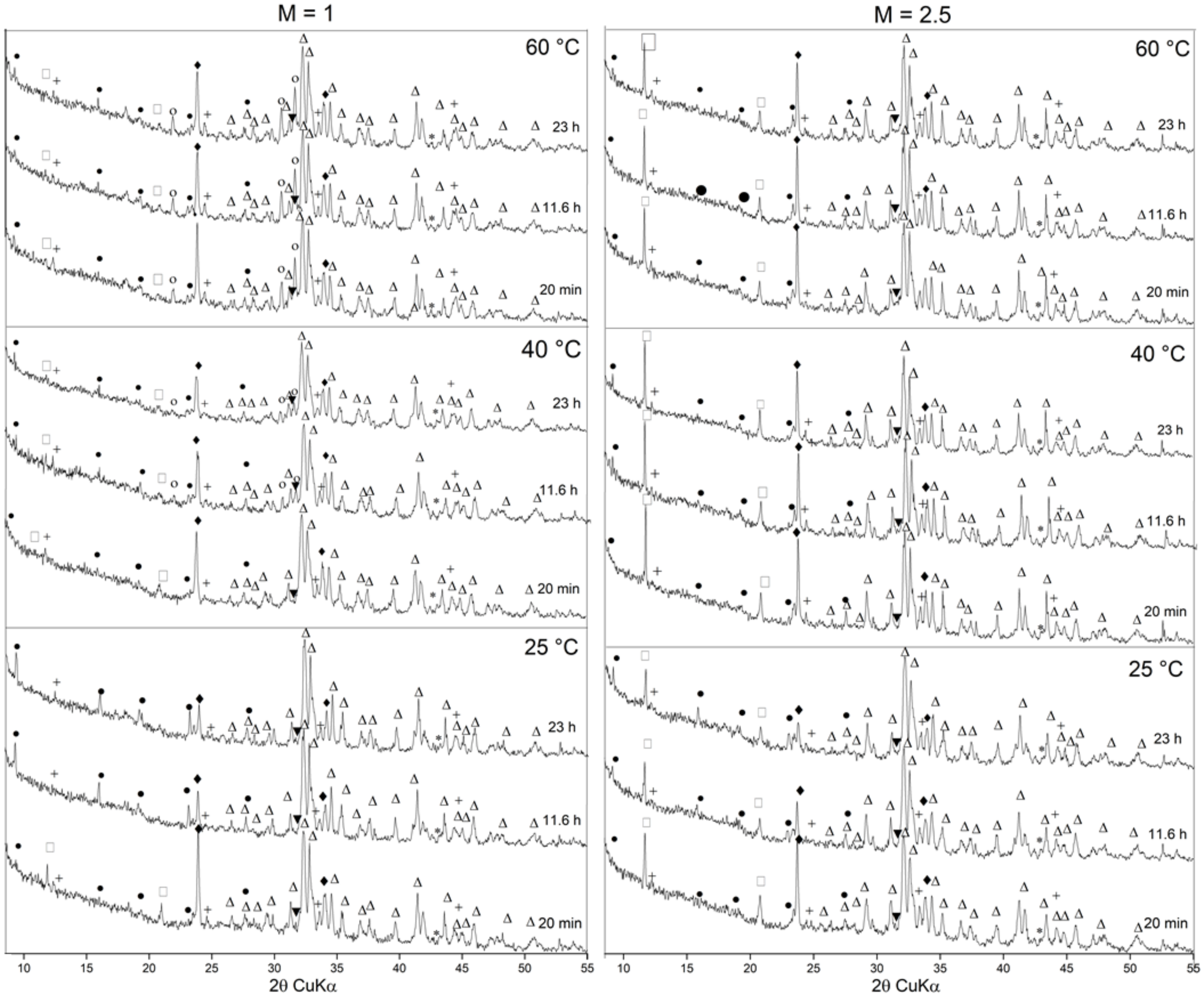
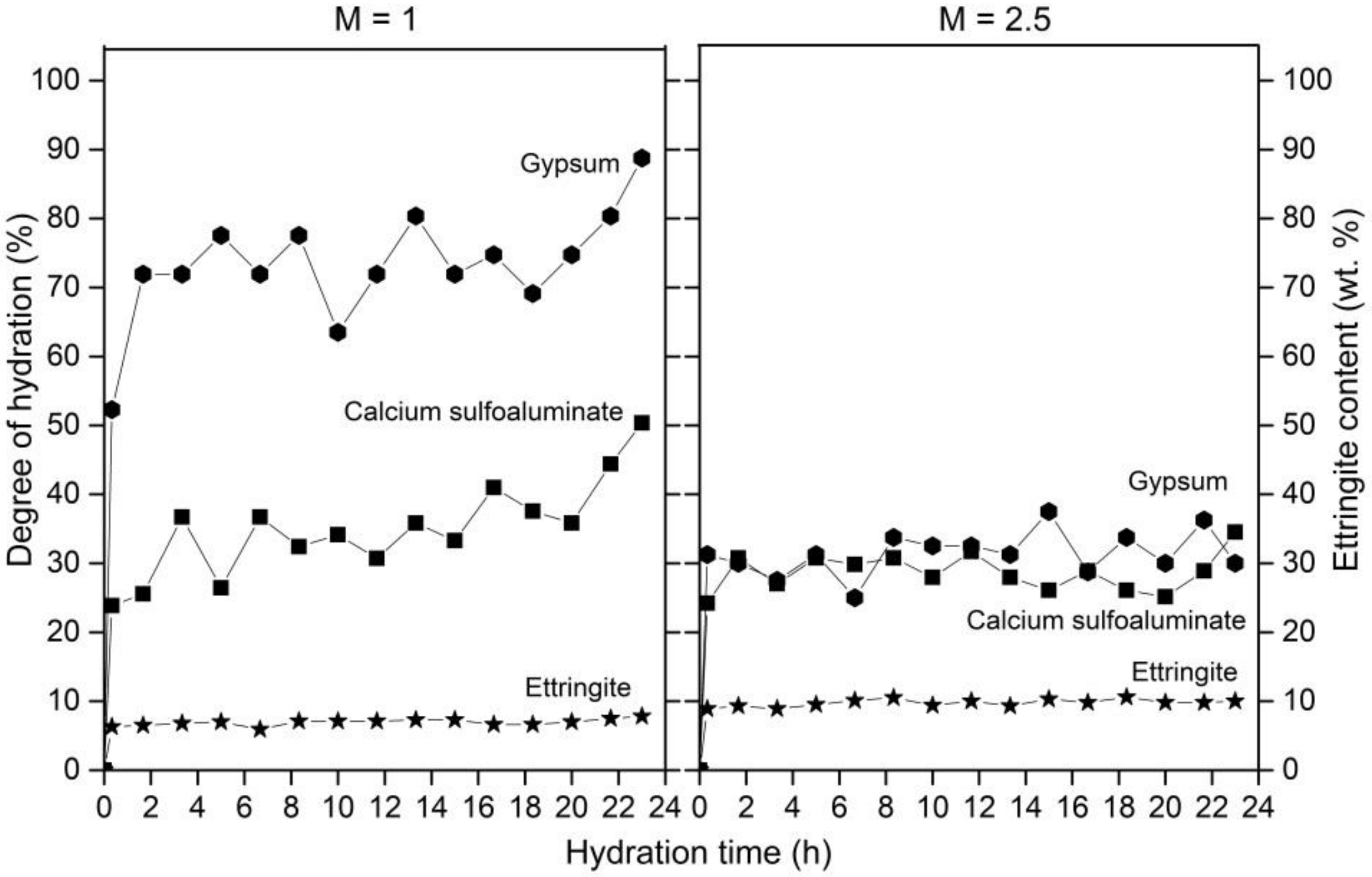
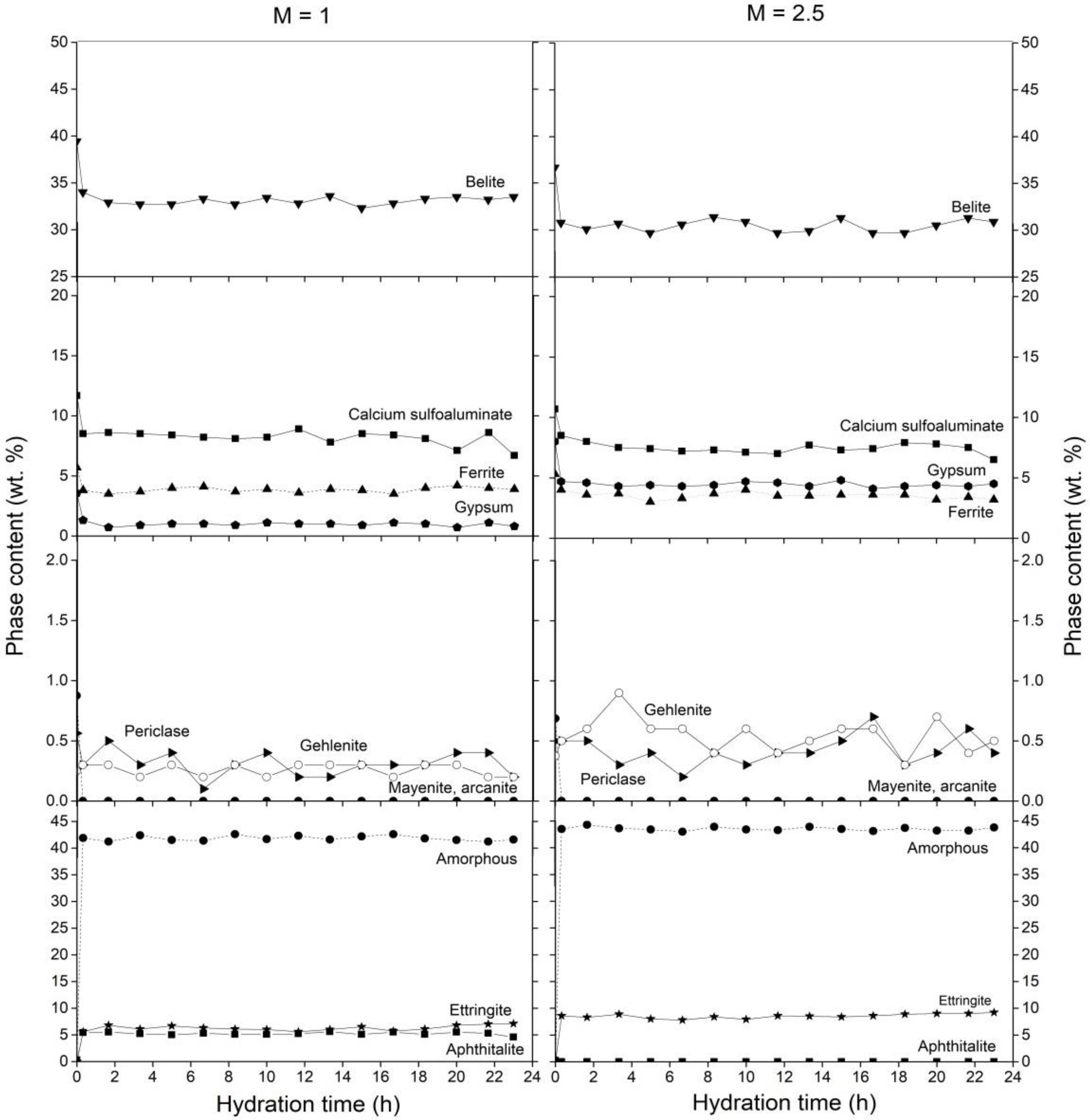
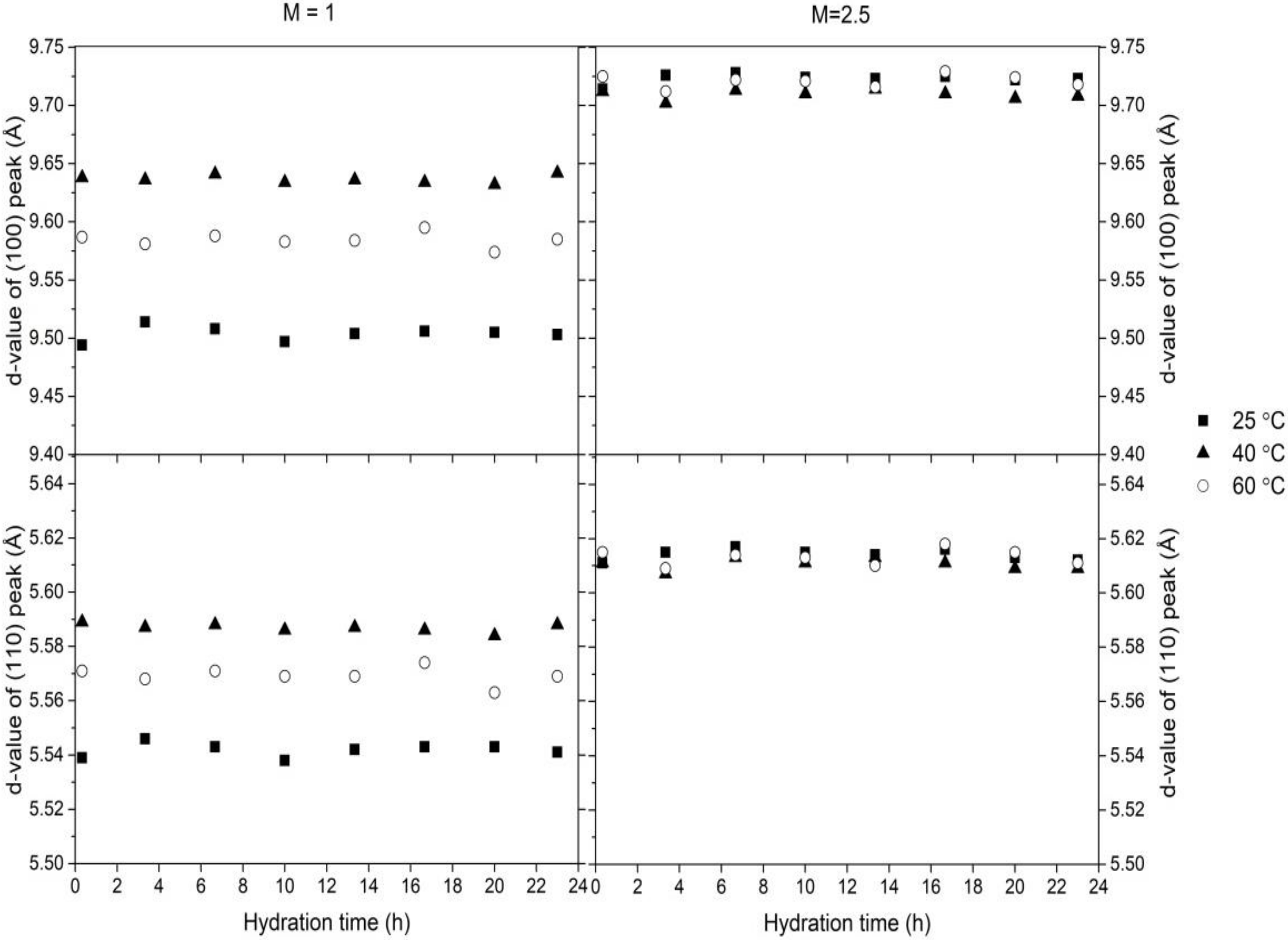
3.1. Cement Hydration at 25 °C
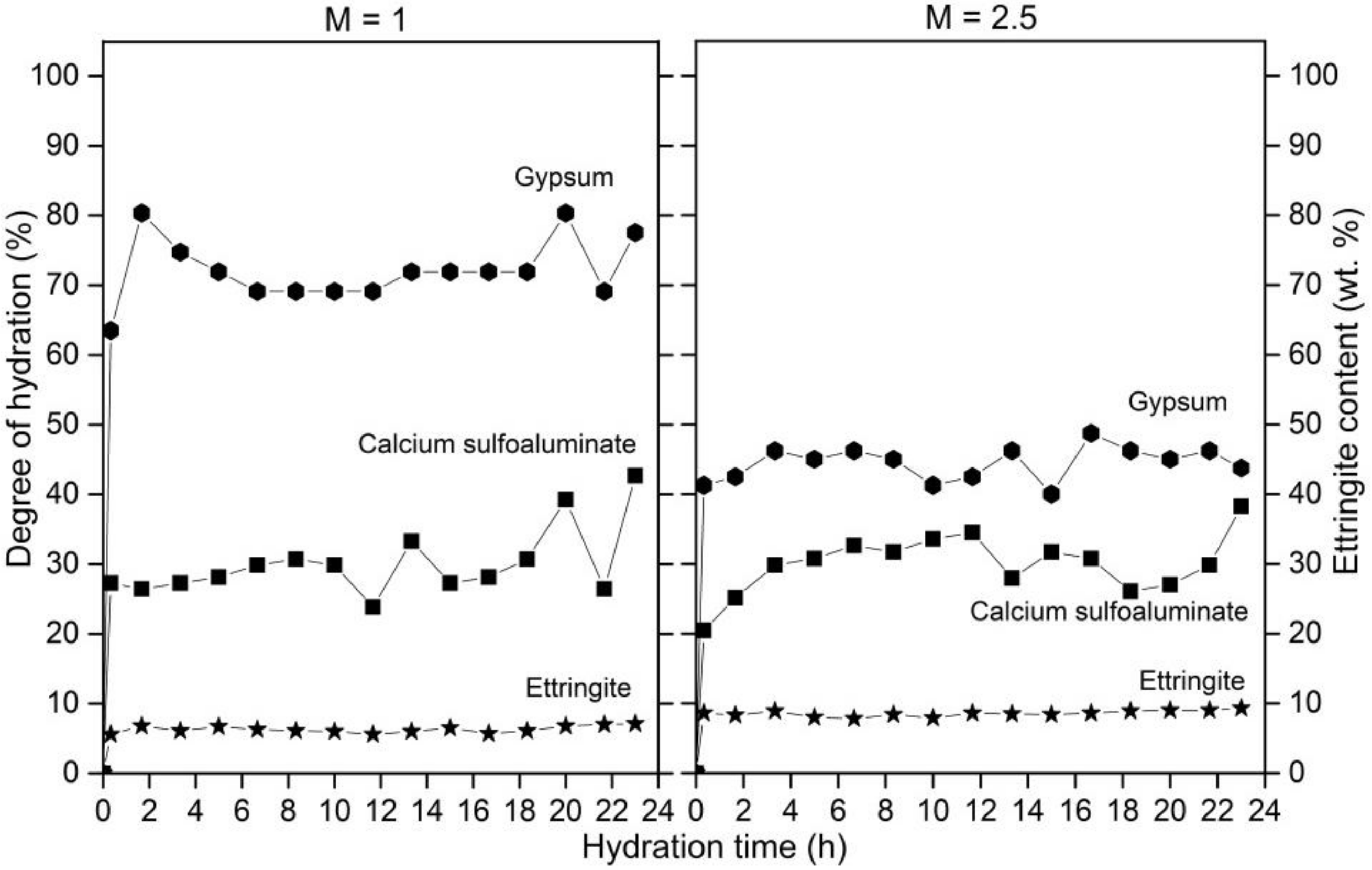
3.2. Cement Hydration at 40 °C
3.3. Cement Hydration at 60 °C
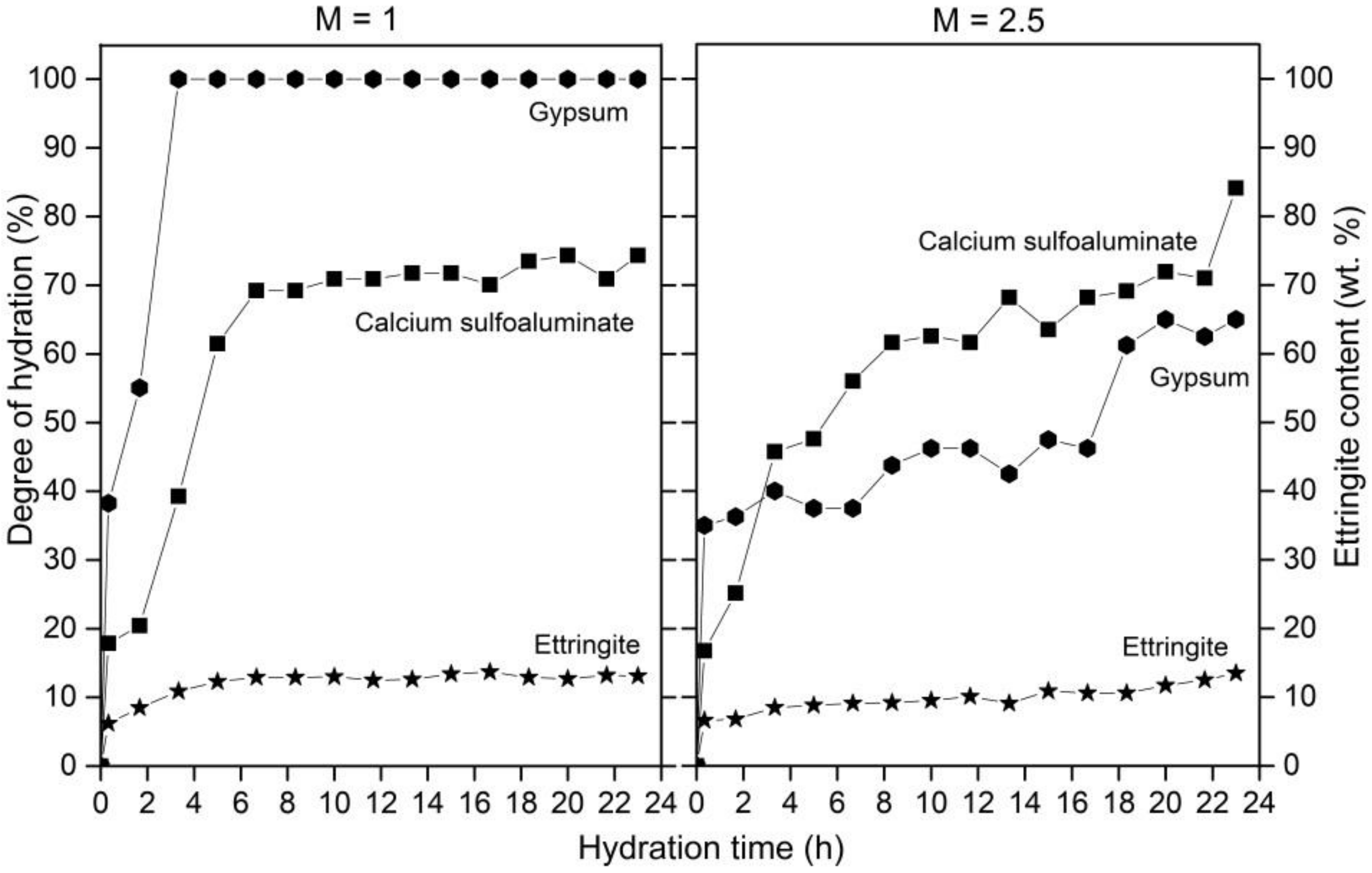
3.4. Ettringite during Cement Hydration
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Álvarez-Pinazo, G.; Cuesta, A.; García-Maté, M.; Santacruz, I.; Losilla, E.R.; Sanfélix, S.G.; Fauth, F.; Aranda, M.A.G.; De la Torre, A.G. In-Situ Early-Age Hydration Study of Sulfobelite Cements by Synchrotron Powder Diffraction. Cem. Concr. Res. 2014, 56, 12–19. [Google Scholar] [CrossRef]
- Senff, L.; Castela, A.; Hajjaji, W.; Hotza, D.; Labrincha, J.A. Formulations of Sulfobelite Cement through Design of Experiments. Constr. Build. Mater. 2011, 25, 3410–3416. [Google Scholar] [CrossRef]
- Álvarez-Pinazo, G.; Santacruz, I.; León-Reina, L.; Aranda, M.A.G.; De la Torre, A.G. Hydration Reactions and Mechanical Strength Developments of Iron-Rich Sulfobelite Eco-Cements. Ind. Eng. Chem. Res. 2013, 52, 16606–16614. [Google Scholar] [CrossRef]
- Álvarez-Pinazo, G.; Santacruz, I.; Aranda, M.A.G.; De la Torre, Á.G. Hydration of Belite–Ye’elimite–Ferrite Cements with Different Calcium Sulfate Sources. Adv. Cem. Res. 2016, 28, 529–543. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Zajac, M.; Skocek, J.; Ben Haha, M. The Role of Boron during the Early Hydration of Belite Ye’elimite Ferrite Cements. Constr. Build. Mater. 2019, 215, 252–263. [Google Scholar] [CrossRef]
- Morin, V.; Termkhajornkit, P.; Huet, B.; Pham, G. Impact of Quantity of Anhydrite, Water to Binder Ratio, Fineness on Kinetics and Phase Assemblage of Belite-Ye’elimite-Ferrite Cement. Cem. Concr. Res. 2017, 99, 8–17. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Schmitt, D.; Ben Haha, M. Effect of Raw Mix Design and of Clinkering Process on the Formation and Mineralogical Composition of (Ternesite) Belite Calcium Sulphoaluminate Ferrite Clinker. Cem. Concr. Res. 2014, 59, 87–95. [Google Scholar] [CrossRef]
- Cuberos, A.J.M.; De la Torre, Á.G.; Álvarez-Pinazo, G.; Martín-Sedeño, M.C.; Schollbach, K.; Pöllmann, H.; Aranda, M.A.G. Active Iron-Rich Belite Sulfoaluminate Cements: Clinkering and Hydration. Environ. Sci. Technol. 2010, 44, 6855–6862. [Google Scholar] [CrossRef]
- Jeong, Y.; Hargis, C.W.; Chun, S.-C.; Moon, J. The Effect of Water and Gypsum Content on Strätlingite Formation in Calcium Sulfoaluminate-Belite Cement Pastes. Constr. Build. Mater. 2018, 166, 712–722. [Google Scholar] [CrossRef]
- Rossetto, C.M.; Ichikawa, R.U.; Martinez, L.G.; Carezzato, G.L.; Carvalho, A.M.G.; Turrillas, X. In Situ Hydration of Sulfoaluminate Cement Mixtures Monitored by Synchrotron X-Ray Diffraction. MSF 2018, 930, 153–157. [Google Scholar] [CrossRef]
- Liao, Y.; Wei, X.; Li, G. Early Hydration of Calcium Sulfoaluminate Cement through Electrical Resistivity Measurement and Microstructure Investigations. Constr. Build. Mater. 2011, 25, 1572–1579. [Google Scholar] [CrossRef]
- Martín-Sedeño, M.C.; Cuberos, A.J.M.; De la Torre, Á.G.; Álvarez-Pinazo, G.; Ordónez, L.M.; Gateshki, M.; Aranda, M.A.G. Aluminum-Rich Belite Sulfoaluminate Cements: Clinkering and Early Age Hydration. Cem. Concr. Res. 2010, 40, 359–369. [Google Scholar] [CrossRef]
- Morin, V.; Walenta, G.; Gartner, E.; Termkhajornkit, P.; Baco, I.; Casabonne, J.M. Hydration of a Belite-Calcium Sulfoaluminate-Ferrite cement: AetherTM. In Proceedings of the 13th International Congress on the Chemistry of Cement, Madrid, Spain, 3–8 July 2011. [Google Scholar]
- Winnefeld, F.; Martin, L.H.J.; Müller, C.J.; Lothenbach, B. Using Gypsum to Control Hydration Kinetics of CSA Cements. Constr. Build. Mater. 2017, 155, 154–163. [Google Scholar] [CrossRef]
- Winnefeld, F.; Barlag, S. Calorimetric and Thermogravimetric Study on the Influence of Calcium Sulfate on the Hydration of Ye’elimite. J. Therm. Anal. Calorim. 2010, 101, 949–957. [Google Scholar] [CrossRef]
- Glasser, F.P.; Zhang, L. High-Performance Cement Matrices Based on Calcium Sulfoaluminate–Belite Compositions. Cem. Concr. Res. 2001, 31, 1881–1886. [Google Scholar] [CrossRef]
- Sahu, S.; Havlica, J.; Tomkova, V.; Majling, J. Hydration Behaviour of Sulphoaluminate Belite Cement in the Presence of Various Calcium Sulphates. Thermochim. Acta 1991, 175, 45–52. [Google Scholar] [CrossRef]
- Zhang, L.; Glasser, F.P. Hydration of Calcium Sulfoaluminate Cement at Less than 24 h. Adv. Cem. Res. 2002, 14, 15. [Google Scholar] [CrossRef]
- García-Maté, M.; De la Torre, A.G.; León-Reina, L.; Losilla, E.R.; Aranda, M.A.G.; Santacruz, I. Effect of Calcium Sulfate Source on the Hydration of Calcium Sulfoaluminate Eco-Cement. Cem. Concr. Compos. 2015, 55, 53–61. [Google Scholar] [CrossRef]
- Zajac, M.; Skocek, J.; Bullerjahn, F.; Ben Haha, M. Effect of Retarders on the Early Hydration of Calcium-Sulpho-Aluminate (CSA) Type Cements. Cem. Concr. Res. 2016, 84, 62–75. [Google Scholar] [CrossRef]
- Beltagui, H.; Jen, G.; Whittaker, M.; Imbabi, M.S. The Influence of Variable Gypsum and Water Content on the Strength and Hydration of a Belite-Calcium Sulphoaluminate Cement. Adv. Appl. Ceram. 2017, 116, 199–206. [Google Scholar] [CrossRef]
- Chen, I.A.; Juenger, M.C.G. Synthesis and Hydration of Calcium Sulfoaluminate-Belite Cements with Varied Phase Compositions. J. Mater. Sci. 2011, 46, 2568–2577. [Google Scholar] [CrossRef]
- Kaufmann, J.; Winnefeld, F.; Lothenbach, B. Stability of Ettringite in CSA Cement at Elevated Temperatures. Adv. Cem. Res. 2016, 28, 251–261. [Google Scholar] [CrossRef]
- Borštnar, M.; Daneu, N.; Dolenec, S. Phase Development and Hydration Kinetics of Belite-Calcium Sulfoaluminate Cements at Different Curing Temperatures. Ceram. Int. 2020, 46, 29421–29428. [Google Scholar] [CrossRef]
- Jakob, C.; Jansen, D.; Ukrainczyk, N.; Koenders, E.; Pott, U.; Stephan, D.; Neubauer, J. Relating Ettringite Formation and Rheological Changes during the Initial Cement Hydration: A Comparative Study Applying XRD Analysis, Rheological Measurements and Modeling. Materials 2019, 12, 2957. [Google Scholar] [CrossRef] [PubMed]
- Hesse, C.; Goetz-Neunhoeffer, F.; Neubauer, J.; Braeu, M.; Gaeberlein, P. Quantitative in Situ X-Ray Diffraction Analysis of Early Hydration of Portland Cement at Defined Temperatures. Powder Diffr. 2009, 24, 112–115. [Google Scholar] [CrossRef]
- Jansen, D.; Spies, A.; Neubauer, J.; Ectors, D.; Goetz-Neunhoeffer, F. Studies on the Early Hydration of Two Modifications of Ye’elimite with Gypsum. Cem. Concr. Res. 2017, 91, 106–116. [Google Scholar] [CrossRef]
- Zajac, M.; Skocek, J.; Bullerjahn, F.; Lothenbach, B.; Scrivener, K.; Ben Haha, M. Early Hydration of Ye’elimite: Insights from Thermodynamic Modelling. Cem. Concr. Res. 2019, 120, 152–163. [Google Scholar] [CrossRef]
- Merlini, M.; Artioli, G.; Meneghini, C.; Cerulli, T.; Bravo, A.; Cella, F. The Early Hydration and the Set of Portland Cements: In Situ X-Ray Powder Diffraction Studies. Powder Diffr. 2007, 22, 201–208. [Google Scholar] [CrossRef]
- Gastaldi, D.; Paul, G.; Marchese, L.; Irico, S.; Boccaleri, E.; Mutke, S.; Buzzi, L.; Canonico, F. Hydration Products in Sulfoaluminate Cements: Evaluation of Amorphous Phases by XRD/Solid-State NMR. Cem. Concr. Res. 2016, 90, 162–173. [Google Scholar] [CrossRef]
- Jeong, Y.; Hargis, C.W.; Kang, H.; Chun, S.-C.; Moon, J. The Effect of Elevated Curing Temperatures on High Ye’elimite Calcium Sulfoaluminate Cement Mortars. Materials 2019, 12, 1072. [Google Scholar] [CrossRef]
- Valentini, L.; Dalconi, M.C.; Favero, M.; Artioli, G.; Ferrari, G. In-Situ XRD Measurement and Quantitative Analysis of Hydrating Cement: Implications for Sulfate Incorporation in C-S-H. J. Am. Ceram. Soc. 2015, 98, 1259–1264. [Google Scholar] [CrossRef]
- Hesse, C.; Goetz-Neunhoeffer, F.; Neubauer, J. A New Approach in Quantitative In-Situ XRD of Cement Pastes: Correlation of Heat Flow Curves with Early Hydration Reactions. Cem. Concr. Res. 2011, 41, 123–128. [Google Scholar] [CrossRef]
- Rietveld, H.M. A Profile Refinement Method for Nuclear and Magnetic Structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Žibret, L.; Ipavec, A.; Kramar, S. Microstructure of Belite Sulfoaluminate Clinker and Its Influence on Clinker Reactivity. In Proceedings of the International Workshop on Calcium Sulfoaluminate Cements, Murten, Switzerland, 4 June 2018; Volume 2018, p. 1. [Google Scholar]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; T. Telford: London, UK, 1997; ISBN 978-0-7277-2592-9. [Google Scholar]
- Dolenec, S.; Šter, K.; Borštnar, M.; Nagode, K.; Ipavec, A.; Žibret, L. Effect of the Cooling Regime on the Mineralogy and Reactivity of Belite-Sulfoaluminate Clinkers. Minerals 2020, 10, 910. [Google Scholar] [CrossRef]
- NSAI. EN 196-6:2018. Methods of Testing Cement-Part 6: Determination of Fineness; NSAI: Dublin, Ireland, 2010. [Google Scholar]
- Snellings, R. X-ray powder diffraction applied to cement. In A Practical Guide to Microstructural Analysis of Cementitious Materials; CRC Press: Boca Raton, FL, USA, 2016; pp. 126–195. [Google Scholar]
- Cuesta, A.; De la Torre, A.G.; Losilla, E.R.; Peterson, V.K.; Rejmak, P.; Ayuela, A.; Frontera, C.; Aranda, M.A.G. Structure, Atomistic Simulations, and Phase Transition of Stoichiometric Yeelimite. Chem. Mater. 2013, 25, 1680–1687. [Google Scholar] [CrossRef]
- Cuesta, A.; De la Torre, Á.G.; Losilla, E.R.; Santacruz, I.; Aranda, M.A.G. Pseudocubic Crystal Structure and Phase Transition in Doped Ye’elimite. Cryst. Growth Des. 2014, 14, 5158–5163. [Google Scholar] [CrossRef]
- Mumme, W.G.; Hill, R.J.; Bushnell-Wye, G.; Segnit, E.R. Rietveld Crystal Structure Refinements, Crystal Chemistry and Calculated Powder Diffraction Data for the Polymorphs of Dicalcium Silicate and Related Phases. Miner. Geochem. 1995, 1, 35–68. [Google Scholar]
- Redhammer, G.J.; Tippelt, G.; Roth, G.; Amthauer, G. Structural Variations in the Brownmillerite Series Ca2(Fe2−xAlx)O5: Single-Crystal X-Ray Diffraction at 25 °C and High-Temperature X-Ray Powder Diffraction (25 °C ≤ T ≤ 1000 °C). Am. Mineral. 2004, 89, 405–420. [Google Scholar] [CrossRef]
- Boeyens, J.; Ichharam, V. Redetermination of the Crystal Structure of Calcium Sulphate Dihydrate, CaSO4·2H2O. Zeitschrift für Kristallographie. New Cryst. Struct. 2002, 217, 9–10. [Google Scholar]
- Sasaki, S.; Fujino, K.; Takéuchi, Y. X-Ray Determination of Electron-Density Distributions in Oxides, MgO, MnO, CoO, and NiO, and Atomic Scattering Factors of Their Constituent Atoms. Proc. Jpn. Acad. Ser. B 1979, 55, 43–48. [Google Scholar] [CrossRef]
- Sakakura, T.; Tanaka, K.; Takenaka, Y.; Matsuishi, S.; Hosono, H.; Kishimoto, S. Determination of the Local Structure of a Cage with an Oxygen Ion in Ca12Al14O33. Acta Cryst. 2011, 67, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Gemmi, M.; Merlini, M.; Cruciani, G.; Artioli, G. Non-Ideality and Defectivity of the Akermanite-Gehlenite Solid Solution: An X-Ray Diffraction and TEM Study. Am. Mineral. 2007, 92, 1685–1694. [Google Scholar] [CrossRef]
- Ojima, K.; Nishihata, Y.; Sawada, A. Structure of Potassium Sulfate at Temperatures from 296 K down to 15 K. Acta Cryst. B 1995, 51, 287–293. [Google Scholar] [CrossRef]
- Okada, K.; Ossaka, J. Structures of Potassium Sodium Sulphate and Tripotassium Sodium Disulphate. Acta Cryst. B 1980, 36, 919–921. [Google Scholar] [CrossRef]
- Goetz-Neunhoeffer, F.; Neubauer, J. Refined Ettringite (Ca6Al2(SO4)3(OH)12·26H2O) Structure for Quantitative X-Ray Diffraction Analysis. Powder Diffr. 2006, 21, 4–11. [Google Scholar] [CrossRef]
- Champenois, J.-B.; Dhoury, M.; Cau Dit Coumes, C.; Mercier, C.; Revel, B.; Le Bescop, P.; Damidot, D. Influence of Sodium Borate on the Early Age Hydration of Calcium Sulfoaluminate Cement. Cem. Concr. Res. 2015, 70, 83–93. [Google Scholar] [CrossRef]
- Cuesta, A.; Zea-Garcia, J.D.; Londono-Zuluaga, D.; De la Torre, A.G.; Santacruz, I.; Vallcorba, O.; Dapiaggi, M.; Sanfélix, S.G.; Aranda, M.A.G. Multiscale Understanding of Tricalcium Silicate Hydration Reactions. Sci. Rep. 2018, 8, 8544. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Füllmann, T.; Gallucci, E.; Walenta, G.; Bermejo, E. Quantitative Study of Portland Cement Hydration by X-Ray Diffraction/Rietveld Analysis and Independent Methods. Cem. Concr. Res. 2004, 34, 1541–1547. [Google Scholar] [CrossRef]
- Cuberos, A.J.M.; De la Torre, Á.G.; Martín-Sedeño, M.C.; Moreno-Real, L.; Merlini, M.; Ordónez, L.M.; Aranda, M.A.G. Phase Development in Conventional and Active Belite Cement Pastes by Rietveld Analysis and Chemical Constraints. Cem. Concr. Res. 2009, 39, 833–842. [Google Scholar] [CrossRef]
- Zajac, M.; Skocek, J.; Stabler, C.; Bullerjahn, F.; Ben Haha, M. Hydration and Performance Evolution of Belite-Ye’elimite-Ferrite Cement. Adv. Cem. Res. 2019, 31, 124–137. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Zajac, M.; Ben Haha, M.; Scrivener, K.L. Factors Influencing the Hydration Kinetics of Ye’elimite; Effect of Mayenite. Cem. Concr. Res. 2019, 116, 113–119. [Google Scholar] [CrossRef]
- Jeong, Y.; Hargis, C.; Chun, S.; Moon, J. Effect of Calcium Carbonate Fineness on Calcium Sulfoaluminate-Belite Cement. Materials 2017, 10, 900. [Google Scholar] [CrossRef] [PubMed]
- Halaweh, M. Effect of Alkalis and Sulfates on Portland Cement Systems. Ph.D. Thesis, University of South Florida, Tampa, FL, USA, 2006. [Google Scholar]
- Ye, H.; Radli, A. Effect of Alkalis on Cementitious Materials: Understanding the Relationship between Composition, Structure, and Volume Change Mechanism. J. Adv. Concr. Technol. 2017, 15, 14. [Google Scholar] [CrossRef]
- Tambara, L.U.D.; Cheriaf, M.; Rocha, J.C.; Palomo, A.; Fernández-Jiménez, A. Effect of Alkalis Content on Calcium Sulfoaluminate (CSA) Cement Hydration. Cem. Concr. Res. 2020, 128, 105953. [Google Scholar] [CrossRef]
- López-Acevedo, V.; Viedma, C.; Gonzalez, V.; La Iglesia, A. Salt Crystallization in Porous Construction Materials. II. Mass Transport and Crystallization Processes. J. Cryst. Growth 1997, 182, 103–110. [Google Scholar] [CrossRef]
- Escalante-Garcia, J.I.; Sharp, J.H. The Effect of Temperature on the Early Hydration of Portland Cement and Blended Cements. Adv. Cem. Res. 2000, 12, 121–130. [Google Scholar] [CrossRef]
- Richardson, I.G.; Wilding, C.R.; Dickson, M.J. The Hydration of Blastfurnace Slag Cements. Adv. Cem. Res. 1989, 2, 147–157. [Google Scholar] [CrossRef]
- Goergens, J.; Manninger, T.; Goetz-Neunhoeffer, F. In-Situ XRD Study of the Temperature-Dependent Early Hydration of Calcium Aluminate Cement in a Mix with Calcite. Cem. Concr. Res. 2020, 136, 106160. [Google Scholar] [CrossRef]
- Lothenbach, B.; Winnefeld, F.; Alder, C.; Wieland, E.; Lunk, P. Effect of Temperature on the Pore Solution, Microstructure and Hydration Products of Portland Cement Pastes. Cem. Concr. Res. 2007, 37, 483–491. [Google Scholar] [CrossRef]
- Kjellsen, K.O.; Detwiler, R.J.; Gjørv, O.E. Development of Microstructures in Plain Cement Pastes Hydrated at Different Temperatures. Cem. Concr. Res. 1991, 21, 179–189. [Google Scholar] [CrossRef]
- Kjellsen, K.O.; Detwiler, R.J.; Gjørv, O.E. Backscattered Electron Imaging of Cement Pastes Hydrated at Different Temperatures. Cem. Concr. Res. 1990, 20, 308–311. [Google Scholar] [CrossRef]
- Thomas, S.; Thomas, R.; Zachariah, A. Microscopy Methods in Nanomaterials Characterization; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Clark, S.M.; Colas, B.; Kunz, M.; Speziale, S.; Monteiro, P.J.M. Effect of Pressure on the Crystal Structure of Ettringite. Cem. Concr. Res. 2008, 38, 19–26. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Tyrer, M.; Hills, C.D.; Yang, X.M.; Carey, P. Immobilisation of Heavy Metal in Cement-Based Solidification/Stabilisation: A Review. Waste Manag. 2009, 29, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Kuznik, F.; Horgnies, M.; Johannes, K.; Morin, V.; Gengembre, E. Physicochemical Properties of Ettringite/Meta-Ettringite for Thermal Energy Storage: Review. Sol. Energy Mater. Sol. Cells 2019, 193, 320–334. [Google Scholar] [CrossRef]
- Suyolcu, Y.E.; Wang, Y.; Baiutti, F.; Al-Temimy, A.; Gregori, G.; Cristiani, G.; Sigle, W.; Maier, J.; van Aken, P.A.; Logvenov, G. Dopant Size Effects on Novel Functionalities: High-Temperature Interfacial Superconductivity. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]


| Σ Belite | Σ Calcium Sulfoaluminate | Ferrite | Mayenite | Periclase | Gehlenite | Arcanite | Aphthitalite | |
|---|---|---|---|---|---|---|---|---|
| Targeted phase composition (wt. %) | 65.0 | 20.0 | 10.0 | - | - | - | - | - |
| Observed phase composition (wt. %) | 65.7 | 17.7 | 11.6 | 2.5 | 1.1 | 0.4 | 0.4 | 0.6 |
| M = 1 | M = 2.5 | |
|---|---|---|
| Calcium sulfoaluminate-orthorhombic | 8.0 | 6.8 |
| Calcium sulfoaluminate-cubic | 10.7 | 10.3 |
| Σ Calcium sulfoaluminate | 18.7 | 17.1 |
| Belite-beta | 58.0 | 53.4 |
| Belite-gamma | 5.1 | 5.3 |
| Σ Belite | 63.1 | 58.7 |
| Ferrite | 9.1 | 8.5 |
| Periclase | 0.9 | 0.8 |
| Mayenite | 1.4 | 1.1 |
| Gehlenite | 0.4 | 0.6 |
| Arcanite | 0.1 | 0.1 |
| Aphthitalite | 0.5 | 0.4 |
| Gypsum | 5.7 | 12.8 |
| Phase | Cement Notation | ICSD | References |
|---|---|---|---|
| Calcium sulfoaluminate-orthorhombic | C4A3 | 237892 | [40] |
| Calcium sulfoaluminate-cubic | C4A3 | 194482 | [41] |
| Belite-beta | β-C2S | 81096 | [42] |
| Belite-gamma | γ-C2S | 81095 | [42] |
| Ferrite | C4AF | 98836 | [43] |
| Gypsum | CH2 | 409581 | [44] |
| Periclase | M | 9863 | [45] |
| Mayenite | C12A7 | 261586 | [46] |
| Gehlenite | C2AS | 158171 | [47] |
| Arcanite | K2 | 79777 | [48] |
| Aphthitalite | K3N2 | 26818 | [49] |
| Ettringite | C63H32 | 155395 | [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borštnar, M.; Lengauer, C.L.; Dolenec, S. Quantitative in Situ X-ray Diffraction Analysis of Early Hydration of Belite-Calcium Sulfoaluminate Cement at Various Defined Temperatures. Minerals 2021, 11, 297. https://doi.org/10.3390/min11030297
Borštnar M, Lengauer CL, Dolenec S. Quantitative in Situ X-ray Diffraction Analysis of Early Hydration of Belite-Calcium Sulfoaluminate Cement at Various Defined Temperatures. Minerals. 2021; 11(3):297. https://doi.org/10.3390/min11030297
Chicago/Turabian StyleBorštnar, Maruša, Christian L. Lengauer, and Sabina Dolenec. 2021. "Quantitative in Situ X-ray Diffraction Analysis of Early Hydration of Belite-Calcium Sulfoaluminate Cement at Various Defined Temperatures" Minerals 11, no. 3: 297. https://doi.org/10.3390/min11030297
APA StyleBorštnar, M., Lengauer, C. L., & Dolenec, S. (2021). Quantitative in Situ X-ray Diffraction Analysis of Early Hydration of Belite-Calcium Sulfoaluminate Cement at Various Defined Temperatures. Minerals, 11(3), 297. https://doi.org/10.3390/min11030297






