Abstract
Digenite fine particles are easily oxidized and ferric ions (Fe3+) commonly exist in the flotation pulp of digenite. This study investigated the effect of Fe3+ on the sulfidization flotation of oxidized digenite fine particles using sodium butyl xanthate (SBX) as a collector. The results of microflotation experiments show that the flotation rate and recovery of oxidized digenite fine particles can be improved by adding Na2S and SBX, whereas the existence of large amounts of Fe3+ is not beneficial for the sulfidization flotation of digenite. The results of Fe3+ adsorption, zeta potential, and contact angle measurements indicate that Fe3+ can be adsorbed on the digenite surface mainly in the form of Fe(OH)3, which hinders the adsorption of SBX and significantly reduces the surface hydrophobicity of digenite. X-ray photoelectron spectroscopy analysis further suggests that the poor surface hydrophobicity of digenite in the presence of Fe3+ is due to the production of large amounts of hydrophilic iron and copper oxides/hydroxides on the surface. Furthermore, optical microscopy analysis shows that these hydrophilic species effectively disperse digenite fine particles in the pulp, which eventually leads to the poor floatability of digenite. Therefore, it is necessary to reduce the amount of Fe3+ present in the pulp and adsorbed on digenite surface before sulfidization to realize effective separation of oxidized digenite fine particles and iron sulfide minerals.
1. Introduction
Copper is an important nonferrous metal and has extensive applications in the fields of medicine, machine manufacturing, and electrical and national defense industries [1,2]. Copper sulfide minerals are the main source of copper, and they are usually recovered by flotation methods. For coarse particles, copper sulfide minerals are easily floated by the addition of sulfhydryl collectors. However, once the particles become fine, it is extremely challenging to effectively recover the minerals by flotation. This is because the fine particles have the characteristics of low collision probability with the bubble, high surface energy, and large specific surface, which require a high consumption of collectors [3,4,5,6]. In addition, the fine particles are more easily oxidized when exposed to oxygen and water during mineral processing. After heavy oxidation, large amounts of hydrophilic copper oxides/hydroxides cover the surface and prevent the effective recovery of minerals. Therefore, some valuable fine particles are usually discarded in the tailings, which leads to a loss of copper.
In flotation practice, various metal ions, known as unavoidable ions, always exist in the pulp. These metal ions can enhance or inhibit the adsorption of collectors on mineral surfaces, thus influencing the flotation of the target minerals. In the flotation of base metal sulfide minerals, ferric ions (Fe3+) are common unavoidable ions, mainly originating from the surface oxidation and dissolution of iron sulfide minerals, electrochemical corrosion of the grinding media, and release of fluid inclusions [7,8,9]. Previous studies have demonstrated that Fe3+ species can be adsorbed onto the surface of unoxidized copper sulfide minerals, thus preventing the flotation of minerals. Peng et al. [10] suggested that iron oxidation species from a mild steel grinding medium could result in the low flotation recovery of chalcopyrite. Ma et al. [11] showed that Fe3+ can significantly suppress the flotation of digenite at pH 3–11 when ammonium dibutyl dithiophosphate is used as the collector; this can be attributed to the adsorption of Fe(OH)3 precipitate on the surface. However, when copper sulfide minerals are heavily oxidized, the adsorption of Fe3+ and its effect on the following flotation of minerals is still indefinite.
It is well known that presulfidization before the addition of sulfhydryl collectors is necessary for the effective recovery of base metal oxide minerals, such as malachite [12,13], azurite [14], and cerussite [15]. During sulfidization, HS- reacts with the base metal ions on or dissolved from the surface to produce the base metal copper sulfide-like minerals, thus increasing the effectiveness of the collectors and the floatability of the base metal oxide minerals. Based on this principle, sulfidization has also been widely used to treat oxidized sulfide minerals, such as digenite, chalcopyrite, and pyrite [16,17,18,19]. Therefore, in this study, the sulfidization technology was applied to try to restore the floatability of oxidized digenite fine particles, and then the effect of Fe3+ on its sulfidization flotation was investigated. Flotation was performed in batch flotation tests to determine the effect of Fe3+ on the flotation rate and recovery of minerals. The underlying mechanism was studied in detail through Fe3+ adsorption experiments, zeta potential measurements, contact angle measurements, X-ray photoelectron spectroscopy (XPS), and optical microscopy analysis. This study is expected to provide further clarification regarding the effective sulfidization flotation of fine particles of oxidized copper sulfide mineral.
2. Materials and Methods
2.1. Mineral Samples and Reagents
Digenite samples are from Fujian Province in China. Lump samples were crushed, hand-selected, dry ground in a porcelain ball mill, and then screened to obtain fine particles (−20 μm) for oxidation and microflotation experiments. The samples were oxidized by the natural oxidation method, in which the samples were kept flat on glass dishes and exposed to air for 120 days at a temperature of approximately 25 °C and relative humidity of 60%.
Sodium normal butyl xanthate (SBX) with a purity of 95% was used as the collector of digenite. Ferric chloride hexahydrate (FeCl3·6H2O) and sodium sulfide nonahydrate (Na2S·9H2O) were used as sources of Fe3+ and S2−, respectively. Hydrochloric acid (HCl) and sodium hydroxide (NaOH) were used as pH regulators. Terpenic oil was used as the frother. FeCl3·6H2O, Na2S·9H2O, HCl, and NaOH were all of analytical grade. SBX and terpenic oil were of industrial grade. Deionized (DI) water with a resistivity of 18.3 MΩ·cm was used in all the experiments.
2.2. Microflotation Experiments
The microflotation experiments were conducted using an XFD laboratory flotation machine with an impeller speed of 1752 r/min. During flotation, digenite (5.0 g) was placed in the flotation cell, and then 50 mL of DI water was added to the flotation cell for 3 min. Then, HCl or NaOH was added to regulate the pulp pH to the required value. Subsequently, FeCl3 (if needed), Na2S, SBX, and terpenic oil were sequentially added to the pulp and conditioned for 3 min each, except terpenic oil, which was conditioned for 1 min. The dosage of terpenic oil was fixed at 40 mg/L throughout the experiments. Finally, the froth products were collected at 0.2, 0.4, 0.6, 0.8, 1.0, 1.5, 2.0, and 3.0 min. After flotation, the froth products and tailings were filtered, dried, weighed, and analyzed. The obtained data were fitted using the classical first-order flotation kinetic model and expressed as follows:
where R(t) represents the floatability at a certain flotation time t (min), R∞ represents the infinite recovery, and k represents the flotation rate constant (min−1).
R(t) = R∞ × [1 − exp(−k × t)]
In many cases, the adjusted flotation rate constant (K) considering both the parameters of R∞ and k is more suitable for comparison with the flotation rate under different conditions [20,21,22]. In this study, the flotation rate was evaluated using the K value, and the formula is expressed as follows:
K = k × R∞/100
2.3. Adsorption Experiments
The amount of Fe3+ adsorbed on the surface of digenite was measured using inductively coupled plasma-optical emission spectroscopy (Agilent 5110, Agilent Technologies Co., Ltd., Santa Clara, CA, USA). During sample preparation, 5 g of digenite samples was mixed with 50 mL of DI water and magnetically stirred in a beaker for 3 min. Then, the desired pH regulator and FeCl3 were added to the solution and stirred for 10 min. The samples and solutions were separated via centrifugation. The residual Fe concentration in the solutions was determined by referring to a standard curve for absorbance as a function of the Fe concentration. The amount of Fe3+ adsorbed was calculated by the difference from the initial concentration [23].
2.4. Zeta Potential Measurements
The zeta potential values of digenite under different conditions were obtained using a Malvern Instruments Nano-ZS90 (Malvern Instruments, Malvern, UK) zeta potential analyzer at approximately 25 °C. Potassium nitrate was used to maintain the ionic strength at 1 × 10−3 mol/L [24]. During the measurements, 20 mg of digenite samples was mixed with 50 mL of DI water with and without the addition of FeCl3, Na2S, and SBX, the solutions of which were magnetically stirred for 5 min. Subsequently, the pH was adjusted using 0.1 mol/L HCl and 0.1 mol/L NaOH. After stirring for 5 min, the pulp was allowed to settle for an additional 10 min. Finally, the suspension was collected and used for the measurements. Each measurement was repeated at least five times, and the mean values and standard deviations were recorded.
2.5. Contact Angle Measurements
The contact angles of the digenite samples with different treatments (with DI water; with Na2S; with Na2S and SBX; with Fe3+; with Fe3+ and Na2S; with Fe3+, Na2S and SBX) were measured using a JC2000A (Powereach Instruments, Shanghai, China) contact angle apparatus at approximately 25 °C. The samples were prepared similarly to those for the microflotation experiments and then dried in a vacuum oven at 40 °C. Subsequently, the samples were processed using a tablet press machine to obtain laminar samples with highly smooth surfaces. Finally, the samples were placed in a rectangular glass chamber, and a stable liquid drop was introduced onto their surfaces using a microsyringe. Particular care was taken during the measurements to avoid vibration of the needle and distortion of the drop shape. Each measurement was repeated at least five times, and the mean values and standard deviations were considered.
2.6. XPS Analyses
XPS measurements were conducted using a Thermo Scientific ESCALAB 250 XI spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). The X-ray source was Al Kα (1253.6 eV), operated at 200 W, and the scanned area was 2 mm × 2 mm. The samples used for the XPS measurements were prepared according to microflotation experiments. After being conditioned in the desired solutions, the samples were rinsed with DI water, filtered, and dried in a stream of high-purity nitrogen for XPS measurements. During the tests, the vacuum pressure in the analysis chamber was 8.0 × 10−7 Pa, and the electron take-off angle was 90°. After scanning all the elements in the samples, high-resolution spectra were collected to obtain information about the elemental valence and oxidation states. The obtained XPS data were processed using Thermo Scientific Avantage 5.52 (Thermo Fisher Scientific, Waltham, MA, USA), and the C 1s peak at 284.8 eV was used as the reference to calibrate the measured binding energies [25].
2.7. Optical Microscopy Analysis
The agglomeration and dispersion behaviors of digenite fine particles with different treatments were observed using an ML 31 biological microscope (Guangzhou Micro-shot Optical Technology Co. Ltd., Guangzhou, China). During sample preparation, 5 g of digenite was mixed with 50 mL of DI water and stirred for 3 min. Then, the desired flotation reagents were added, corresponding to the microflotation experiments. Subsequently, 2 mL of the pulp was extracted using a syringe, transferred to a beaker, and diluted ten times with DI water. Following stirring, the dilute solution was dropped onto a glass slide, which was followed by optical microscopy.
3. Results
3.1. Microflotation Tests
The results of X-ray diffraction (shown in Figure 1) and chemical element analysis showed that the purity of digenite was 98.52%, meeting the testing requirements of this study.
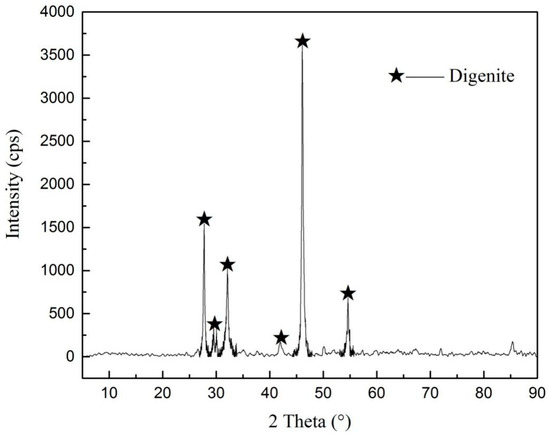
Figure 1.
X-ray diffraction pattern of digenite.
The results of the microflotation tests are displayed in terms of the recovery as a function of flotation time under different conditions. The values of the correlation index R2 for all the microflotation tests were above 0.98 (not shown here), indicating that the flotation results almost exactly matched those of the first-order kinetic model.
Figure 2 shows the effect of Na2S dosage on the flotation rate and recovery of fine digenite particles at different flotation times. As can be seen, when the digenite fine particles were heavily oxidized, the floatability was extremely low, because of which the recovery at 3 min and the flotation rate constant were only 5.66% and 0.15 min−1, respectively. However, once Na2S was introduced into the solution, the floatability of the oxidized digenite fine particles increased. When the Na2S dosage was increased from 0.21 × 10−3 mol/L to 1.25 × 10−3 mol/L, the recovery at 3 min increased from 40.35% to 88.73%, and the flotation rate constant increased from 0.34 to 2.34 min−1. With further addition of Na2S, the flotation rate constant increased, while the recovery at 3 min reached a plateau. It can be concluded that a high dosage of Na2S is beneficial for restoring the floatability of heavily oxidized digenite fine particles.
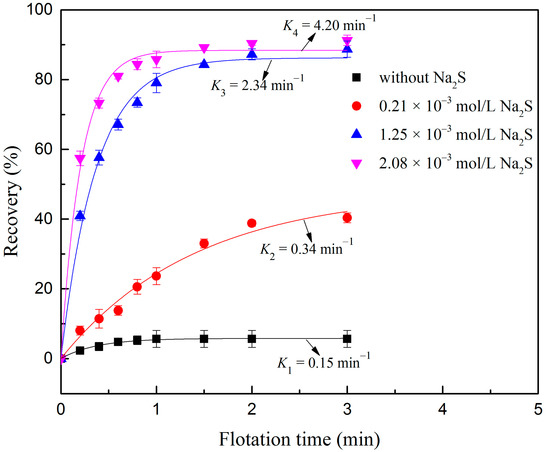
Figure 2.
Flotation recovery of digenite as a function of flotation time with respect to Na2S dosage (pH: 7.0; sodium butyl xanthate (SBX) dosage: 1 × 10−4 mol/L).
The effect of Fe3+ dosage on the sulfidization flotation of oxidized digenite fine particles at different flotation times is shown in Figure 3. As can be seen, when Fe3+ dosage was 3 × 10−4 mol/L, the recovery at 3 min and the flotation rate constant was 91.26% and 2.46 min−1, respectively. This result was nearly the same as that of oxidized digenite fine particles after sulfidization without the addition of Fe3+, as shown in Figure 2. However, once the Fe3+ dosage exceeded 5 × 10−4 mol/L, both the recovery and flotation rate constants significantly decreased. When the Fe3+ dosage was 1 × 10-3 mol/L, the recovery and flotation rate constants were only 4.81% and 0.25 min−1, respectively. This indicated that a high dosage of Fe3+ was not conducive to the sulfidization flotation of oxidized digenite fine particles. In solutions, Fe3+ species can react with xanthate anions to decrease the amount of the effective components of SBX adsorbed on the surface, thus preventing the flotation of sulfide minerals. To clarify whether the depressing effect of Fe3+ is relevant to the consumption of SBX, the sulfidization flotation of digenite after adding 1 × 10−3 mol/L Fe3+ with decantation treatment was carried out. However, compared with the corresponding values without the decantation treatment, it can be seen that both the recovery and flotation rate constants of digenite only increased to 7.38% and 0.25 min−1, respectively. This indicates that the depressing effect of Fe3+ is mainly attributed to the adsorption of Fe3+ on the surface of the oxidized digenite fine particles.
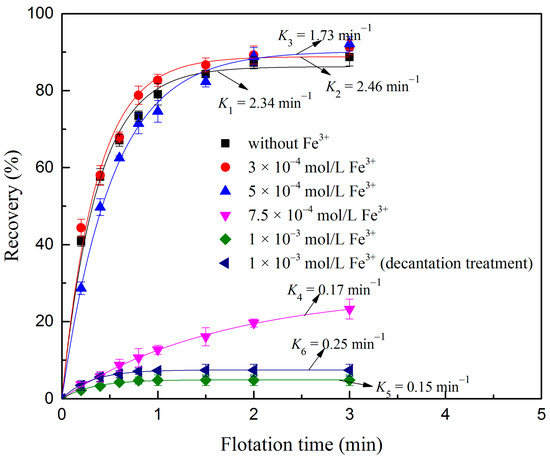
Figure 3.
Flotation recovery of digenite as a function of flotation time with respect to Fe3+ dosage (pH: 7.0; Na2S dosage: 1.25 × 10−3 mol/L; SBX dosage: 1 × 10−4 mol/L).
3.2. Fe3+ Adsorption Experiments
To verify that Fe3+ can be adsorbed on the surface of oxidized digenite fine particles, the adsorption amount of Fe3+ was measured; the results are shown in Figure 4. As seen in the figure, the adsorption amount and initial Fe3+ concentration were linearly correlated. When the initial Fe3+ concentration was increased from 1 × 10−4 to 1 × 10−3 mol/L, the adsorption amount significantly increased from 1.85 × 10−6 to 17.95 × 10−6 mol/g. Therefore, when the Fe3+ concentration was 1 × 10−3 mol/L during the flotation, the residual Fe3+ concentration in the solution was negligible. The suppressing effect of Fe3+ can be attributed mainly to the adsorption of Fe3+ on the surface of oxidized digenite fine particles.
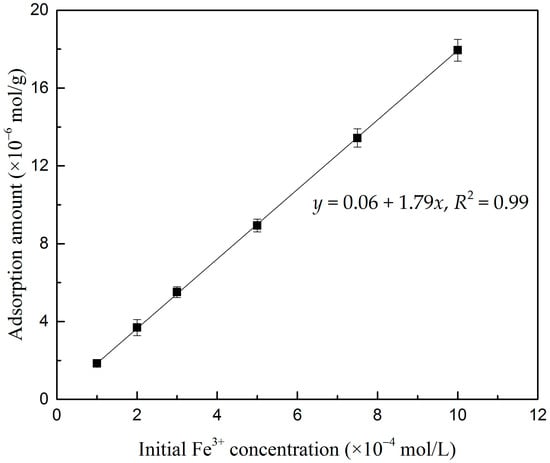
Figure 4.
Adsorption amount of Fe3+ on digenite surface as a function of the initial Fe3+ concentration at pH 7.0.
3.3. Zeta Potential Measurements
Zeta potential measurements reflect the interaction mode and intensity between the reagents and the mineral surface [26,27]. As shown in Figure 5, as the pH increased, the zeta potential values of the oxidized digenite first increased and then decreased. The isoelectric point (IEP) was found at pH 7.4 and 9.30; these values are similar to those for copper oxides/hydroxides [28]. However, when digenite was treated with Na2S, the zeta potential values in the measured pH range significantly decreased to more negative values, which was mainly because of the chemisorption of HS− hydrolyzed from Na2S on the digenite surface. With the further addition of SBX, the zeta potential values shifted to more negative values, indicating that negatively charged xanthate ions were adsorbed on the digenite surface after treatment with Na2S.
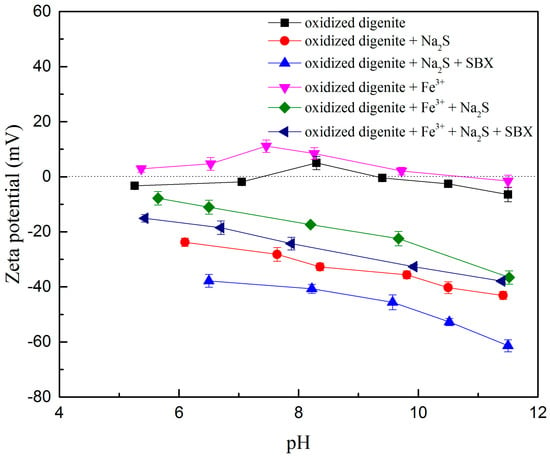
Figure 5.
Zeta potentials of oxidized digenite as a function of pH with different treatments (Fe3+ dosage: 1 × 10−3 mol/L; Na2S dosage: 1.25 × 10−3 mol/L; SBX dosage: 1 × 10−4 mol/L).
However, once Fe3+ was added to the solution, the zeta potential of oxidized digenite shifted to more positive values, and the IEP became pH 10.8. Based on the distribution diagram of the 1 × 10−3 mol/L Fe3+ shown in Figure 6, Fe3+ exists in the solutions in various forms at different pH values. When the pH is less than 2.06, the dissociated Fe3+ is the main species; the amounts of other species gradually increase as the pH increases. When the pH was greater than 2.06, the amount of positively charged species (especially Fe3+) gradually decreased as the pH increased, and Fe(OH)3 particles became the dominant species. Therefore, Fe3+ was mainly absorbed on the digenite surface in the form of Fe(OH)3 particles at pH 7.0, and the zeta potential increased at this pH because of the electrostatic adsorption of Fe(OH)2+ and Fe(OH)2+ on the surface. After the addition of Na2S, the zeta potential values shifted to between −7.8 and −36.6 mV at pH 5.6–11.5, indicating intense chemisorption of HS- on the surface. However, with further addition of SBX, the declining trend of the zeta potential values in the measured pH range was not evident, indicating that weak chemisorption of xanthate ions had occurred on the surface. This also demonstrated that the presence of Fe3+ hinders effective adsorption of SBX on the surface of oxidized digenite fine particles after sulfidization, thus reducing the recovery and flotation rate of the mineral.
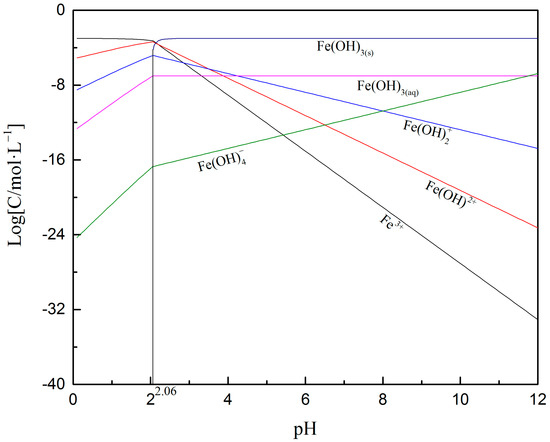
Figure 6.
Species distribution diagram of 1 × 10−3 mol/L Fe3+ in the solution as a function of pH [29].
3.4. Contact Angle Measurements
The contact angles of the oxidized digenite fine particles with different treatments in the absence and presence of Fe3+ are shown in Figure 7. For the samples without the addition of Fe3+, after the digenite fine particles were heavily oxidized, the contact angle was 8.6°; this indicates that the surface of digenite was nearly completely hydrophilic. After Na2S was added, the contact angle increased to 53.3°. With further addition of SBX, the surface hydrophobicity significantly increased, and the contact angle became 92.4°. This was the main reason that the addition of Na2S and SBX could restore the floatability of heavily oxidized digenite fine particles. However, once Fe3+ was introduced into the solution, the contact angles of digenite with different treatments decreased. When Na2S and SBX were added, the contact angle was 31.7°, which is far less than that of the sample without the addition of Fe3+. This indicates that the adsorption of Fe3+ reduced the surface hydrophobicity of digenite with different treatments, which further decreased in its floatability.
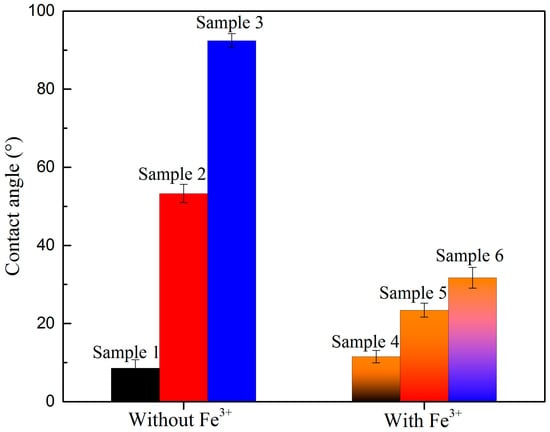
Figure 7.
Contact angle of oxidized digenite fine particles with different treatments (Sample 1: with DI water; Sample 2: with 1.25 × 10−3 mol/L Na2S; Sample 3: with 1.25 × 10−3 mol/L Na2S + 1 × 10−4 mol/L SBX; Sample 4: with 1 × 10−3 mol/L Fe3+; Sample 5: with 1 × 10−3 mol/L Fe3+ + 1.25 × 10−3 mol/L Na2S; Sample 6: with 1 × 10−3 mol/L Fe3+ + 1.25 × 10−3 mol/L Na2S + 1 × 10−4 mol/L SBX).
3.5. XPS Analysis
To further investigate the reasons for the changes in the surface hydrophobicity and floatability of digenite with different treatments, the chemical species and state on the surface of digenite were analyzed via XPS; the results are displayed in Figure 8 and Figure 9 and Table 1, Table 2, Table 3, Table 4 and Table 5.
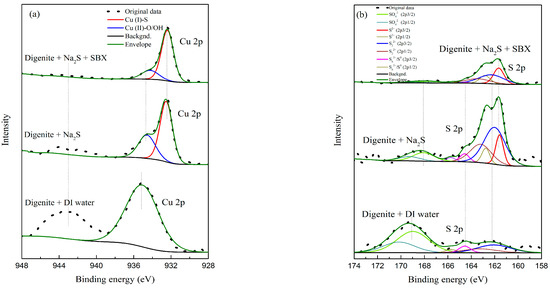
Figure 8.
Fitting peaks of Cu 2p (a) and S 2p (b) for digenite with different treatments in the absence of Fe3+ (pH: 7.0; Na2S dosage: 1.25 × 10−3 mol/L; SBX dosage: 1 × 10−4 mol/L).
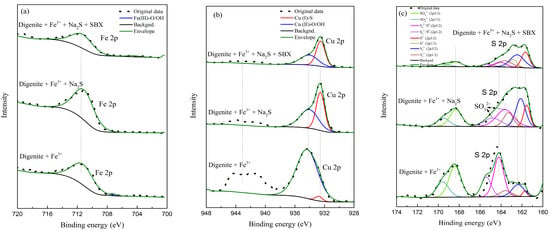
Figure 9.
Fitting peaks of Fe 2p (a), Cu 2p (b), and S 2p (c) for digenite with different treatments in the presence of Fe3+ (pH: 7.0; Fe3+ dosage: 1 × 10−3 mol/L; Na2S dosage: 1.25 × 10−3 mol/L; SBX dosage: 1 × 10−4 mol/L).

Table 1.
Cu 2p quantification of digenite with different treatments in the absence of Fe3+.

Table 2.
S 2p quantification of digenite with different treatments in the absence of Fe3+.

Table 3.
Fe 2p quantification of digenite with different treatments in the presence of Fe3+.

Table 4.
Cu 2p quantification of digenite with different treatments in the presence of Fe3+.

Table 5.
S 2p quantification of digenite with different treatments in the presence of Fe3+.
Figure 8a and Table 1 display the high-resolution spectra of Cu 2p3/2 and the corresponding species amounts for digenite samples in the absence of Fe3+. The peaks centered at 932.51 and 934.59 eV are assigned to Cu(I)-S and Cu(II)-O/OH species, respectively [30,31]. The low-intensity broad satellite peak centered at 943.01 eV indicates that the surfaces of sulfide minerals were heavily oxidized [32,33]. After digenite was heavily oxidized, only hydrophilic Cu(II)-O/OH species were detected on the surface, which was the main reason that both the recovery and flotation rate constant were extremely low. However, as Na2S was added to the solution, the amount of Cu(II)-O/OH species significantly decreased to 34.39%, and the Cu(I)-S species became the major component on the surface. Further addition of SBX reduced the amount of Cu(II)-O/OH species to 10.99%, and the satellite peak at 943.01 eV almost disappeared; this indicates that almost all hydrophilic species had been converted to Cu(I)-S species because of the oxidation of xanthate anions (X−). Thus, the surface hydrophobicity of digenite increased, and the samples could be effectively recovered, which is consistent with the microflotation results shown in Figure 2.
Figure 8b and Table 2 show the high-resolution spectra of S 2p and corresponding species amounts for digenite samples in the absence of Fe3+, which were fitted using the 2p1/2 and 2p3/2 peaks with a 1:2 area ratio and 1.18 eV splitting energy [34]. The S 2p3/2 XPS spectra can be mainly divided into four peaks at 161.51, 162.07, 164.55, and 168.98 eV, which can be assigned to monosulfides (S2−), disulfides (S22−), polysulfides/elemental sulfur (Sn2−/S0), and sulfates (2−), respectively [35]. After treatment with Na2S and SBX, S22− became the main species on the surface of digenite, which could be attributed to the adsorption of S2- and the dissolution of hydrophilic SO42− species. Previous studies have shown that hydrophobic Sn2−/S0 species are beneficial for the flotation of sulfide minerals [36]. As shown in Table 2, the amount of Sn2−/S0 species on the digenite surface treated with DI water was 7.47%, which was higher than that of the samples treated with Na2S and SBX. Combined with the analysis result of Cu 2p3/2, the optimal floatability of digenite after treatment with Na2S and SBX could mainly be attributed to the minimum amount of hydrophilic Cu(II)-O species on the surface.
Figure 9 and Table 3, Table 4 and Table 5 illustrate the high-resolution spectra and corresponding species amounts based on the decoupling of Fe 2p, Cu 2p, and S 2p for digenite samples in the presence of Fe3+. Based on the previous literature, the Fe 2p3/2 spectrum can be divided into two spectral peaks at approximately 707 and 711 eV, which are assigned to Fe(II)-S and Fe(III)-O/OH species, respectively [37,38]. However, as shown in Figure 9a, only the peaks at 711.10 eV were detected on the surface with different treatments. Combined with the species distribution diagram of Fe3+ shown in Figure 6, Fe3+ was adsorbed on the surface of digenite mainly in the form of Fe(OH)3. From the decoupling results of Cu 2p, it can be seen that after digenite was treated with Fe3+, only 2.94% Cu(I)-S species were detected, and the hydrophilic Cu(II)-O/OH species were still the main components on the surface. In addition, the satellite peak centered at 943.01 eV could also be clearly observed. After the digenite was treated with Fe3+ and Na2S, the Cu(II)-O/OH species significantly decreased to 50.98%, which is significantly higher than that after treatment with Na2S alone, as shown in Table 1. This indicates that the adsorption of Fe3+ is not beneficial for the effective sulfidization of digenite. Subsequently, with the further addition of SBX, the amount of Cu(II)-O/OH species slightly decreased to 41.02%. This is because the Fe(III)-O/OH species on the surface have a weak interaction with X−, preventing the effective adsorption of X− on the digenite surface [39]. From the decoupling results of S 2p, it was found that the amount of Sn2−/S0 species was 13.61% after treatment with Fe3+, Na2S, and SBX, which was higher than the amounts found in the absence of Fe3+. Therefore, the poor floatability of digenite in the presence of Fe3+ was attributed to the production of large amounts of hydrophilic iron and copper oxides/hydroxides.
3.6. Optical Microscopy Analysis
It has been confirmed that the effective recovery of fine metal sulfide mineral particles (such as galena, sphalerite, and pyrite) is directly related to the formation of hydrophobic agglomerates, which can be induced by adding xanthates [40,41,42]. The agglomeration and dispersion behaviors of fine mineral particles are usually assessed by optical microscopy analysis.
As shown in Figure 10, oxidized digenite fine particles without any treatment are well dispersed in DI water. It is well known that the hydrophobic agglomeration of fine particles is mainly dependent on the surface hydrophobicity of minerals. When digenite fine particles are heavily oxidized, the surface of digenite is strongly hydrophilic, and the interactions between fine particles are determined by the electric double layer force. As the zeta potential value on the surface of digenite was negative at pH 7.0, the electrostatic repulsive forces resulted in good dispersion of fine particles in the solution. However, when digenite was treated with Na2S and SBX, it can be seen that large sized agglomerates formed. Based on the results shown in Figure 5, the zeta potential value on the surface of digenite after treatment with Na2S and SBX was strongly negative at pH 7.0, indicating that the electrical interactions between particles were repulsive. However, as the addition of Na2S and SBX decreased the amount of hydrophilic species and significantly increased the surface hydrophobicity of digenite, the formation of agglomerates could mainly be attributed to the strong hydrophobic attractive forces. Moreover, instead of individual fine particles, the large hydrophobic agglomerates interacted with the bubbles, thus increasing the collision probability between the particles and bubbles and promoting effective recovery of digenite fine particles. However, after Fe3+ was introduced into the pulp, the particles were well dispersed, which was also due to the repulsive electrostatic forces between the digenite fine particles.
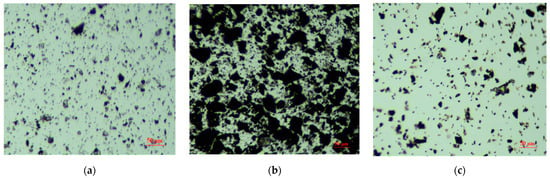
Figure 10.
Microscopy images of oxidized digenite fine particles with different treatments (a) with deionized (DI) water; (b) with 1.25 × 10−3 mol/L Na2S + 1 × 10−4 mol/L SBX; (c) with 1 × 10−3 mol/L Fe3+ + 1.25 × 10−3 mol/L Na2S + 1 × 10−4 mol/L SBX).
4. Conclusions
This study investigated the effect of Fe3+ on the sulfidization flotation performance of oxidized digenite fine particles and the underlying mechanism. The main conclusions and research significance are summarized as follows:
- A low dosage of Fe3+ had no significant effect on the sulfidization flotation of oxidized digenite fine particles. However, as the Fe3+ dosage exceeded 5 × 10−4 mol/L, both the flotation rate and recovery of digenite significantly decreased.
- Fe3+ can adsorb on the surface of oxidized digenite fine particles mainly in the form of hydrophilic Fe(OH)3 species, hindering the effective sulfidization of digenite, which decreases the surface hydrophobicity, and further prevents the flotation recovery of digenite.
- With the addition of Na2S and SBX, a remarkable agglomeration performance of oxidized digenite fine particles was observed, which was the main reason for the good floatability of digenite. However, the presence of Fe3+ prevents the formation of hydrophobic agglomerates, resulting in a low flotation rate and recovery of digenite.
- This study is of great significance for realizing the effective separation of oxidized digenite fine particles and iron sulfide minerals. When the amount of Fe3+ dissolved from oxidized iron sulfide minerals is less than a critical value, digenite can be recovered using sulfidization technology. However, once the dissolved Fe3+ exceeds the critical value, measurements must be taken before sulfidization to desorb the Fe3+ species adsorbed on the surface and decrease the amount of Fe3+ in the pulp.
Author Contributions
Methodology, J.X., S.W. and X.B.; validation, J.X. and S.W.; investigation, D.R. and T.C.; resources, J.X., X.B., Z.S. and C.Z.; data curation, J.X. and X.B.; writing—original draft preparation, J.X.; writing—review and editing, S.W. and Z.S.; supervision, X.B.; funding acquisition, J.X. and X.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Open Foundation of State Key Laboratory of Mineral Processing (BGRIMM-KJSKL-2021-19), the Talent Science and Technology Fund of Xi’an University of Architecture and Technology (ZR19062), and the National Natural Science Foundation of China (52074206).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schipper, B.W.; Lin, H.C.; Meloni, M.A.; Wansleeben, K.; Heijungs, R.; van der Voet, E. Estimating global copper demand until 2100 with regression and stock dynamics. Resour. Conserv. Recycl. 2018, 132, 28–36. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Su, Z.; Lu, M.; Gu, F.; Liu, J.; Jiang, T. Recycling the domestic copper scrap to address the China’s copper sustainability. J. Mater. Res. Technol. 2020, 9, 2846–2855. [Google Scholar] [CrossRef]
- Bilal, M.; Ito, M.; Koike, K.; Hornn, V.; Ul Hassan, F.; Jeon, S.; Park, I.; Hiroyoshi, N. Effects of coarse chalcopyrite on flotation behavior of fine chalcopyrite. Miner. Eng. 2021, 163, 106776. [Google Scholar] [CrossRef]
- Yin, W.; Tang, Y. Interactive effect of minerals on complex ore flotation: A brief review. Int. J. Miner. Metall. Mater. 2020, 27, 571–583. [Google Scholar] [CrossRef]
- Sivamohan, R. The problem of recovering very fine particles in mineral processing—A review. Int. J. Miner. Process. 1990, 28, 247–288. [Google Scholar] [CrossRef]
- Subrahmanyam, T.V.; Forssberg, K.S.E. Fine particles processing: Shear-flocculation and carrier flotation—A review. Int. J. Miner. Process. 1990, 30, 265–286. [Google Scholar] [CrossRef]
- Finkelstein, N.P. Addendum to: The activation of sulphide minerals for flotation: A review. Int. J. Miner. Process. 1999, 55, 283–286. [Google Scholar] [CrossRef]
- Deng, J.; Wen, S.; Xian, Y.; Liu, J.; Bai, S. New discovery of unavoidable ions source in chalcopyrite flotation pulp: Fluid inclusions. Miner. Eng. 2013, 42, 22–28. [Google Scholar] [CrossRef]
- Bai, S.; Wen, S.; Xian, Y.; Liu, J.; Deng, J. New source of unavoidable ions in galena flotation pulp: Components released from fluid inclusions. Miner. Eng. 2013, 45, 94–99. [Google Scholar] [CrossRef]
- Peng, Y.; Grano, S.; Fornasiero, D.; Ralston, J. Control of grinding conditions in the flotation of galena and its separation from pyrite. Int. J. Miner. Process. 2003, 70, 67–82. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, F.; Yin, W.; Tang, Y.; Zhang, S. Influence and mechanism of Fe3+ on flotation of digenite. Chin. J. Nonferrous Met. 2018, 28, 817–822. (In Chinese) [Google Scholar]
- Liu, R.; Liu, D.; Li, J.; Li, J.; Liu, Z.; Jia, X.; Yang, S.; Li, J.; Ning, S. Sulfidization mechanism in malachite flotation: A heterogeneous solid-liquid reaction that yields CuxSy phases grown on malachite. Miner. Eng. 2020, 154, 106420. [Google Scholar] [CrossRef]
- Wang, H.; Wen, S.; Han, G.; Feng, Q. Applied Surface Science Modification of malachite surfaces with lead ions and its contribution to the sulfidization flotation. Appl. Surf. Sci. 2021, 550, 149350. [Google Scholar] [CrossRef]
- Zhang, Q.; Wen, S.; Feng, Q.; Liu, J. Surface modification of azurite with lead ions and its effects on the adsorption of sulfide ions and xanthate species. Appl. Surf. Sci. 2021, 543, 148795. [Google Scholar] [CrossRef]
- Liu, R.; Liu, D.; Li, J.; Liu, S.; Liu, Z.; Gao, L.; Jia, X.; Ao, S. Improved understanding of the sulfidization mechanism in cerussite flotation: An XPS, ToF-SIMS and FESEM investigation. Colloids Surf. A 2020, 595, 124508. [Google Scholar] [CrossRef]
- Orwe, D.; Grano, S.R.; Lauder, D.W. Increasing fine copper recovery at the Ok Tedi concentrator, Papua New Guinea. Miner. Eng. 1998, 11, 171–187. [Google Scholar] [CrossRef]
- Newell, A.J.H.; Skinner, W.M.; Bradshaw, D.J. Restoring the floatability of oxidised sulfides using sulfidisation. Int. J. Miner. Process. 2007, 84, 108–117. [Google Scholar] [CrossRef]
- Moimane, T.; Huai, Y.; Peng, Y. Evaluating the sulphidisation and flotation of oxidised chalcopyrite. Miner. Eng. 2021, 164, 106816. [Google Scholar] [CrossRef]
- Cao, Z.; Chen, X.; Peng, Y. The role of sodium sulfide in the flotation of pyrite depressed in chalcopyrite flotation. Miner. Eng. 2018, 119, 93–98. [Google Scholar] [CrossRef]
- Xu, M. Modified flotation rate constant and selectivity index. Miner. Eng. 1998, 11, 271–278. [Google Scholar] [CrossRef]
- Zhang, N.; Zhou, C.; Liu, C.; Pan, J.; Tang, M.; Cao, S.; Ouyang, C.; Peng, C. Bin Effects of particle size on flotation parameters in the separation of diaspore and kaolinite. Powder Technol. 2017, 317, 253–263. [Google Scholar] [CrossRef]
- Natarajan, R.; Nirdosh, I. Effect of molecular structure on the kinetics of flotation of a Canadian nickel ore by N-arylhydroxamic acids. Int. J. Miner. Process. 2009, 93, 284–288. [Google Scholar] [CrossRef]
- Yao, J.; Sun, H.; Han, F.; Yin, W.; Hong, J.; Wang, Y.; Won, C.; Du, L. Enhancing selectivity of modifier on magnesite and dolomite surfaces by pH control. Powder Technol. 2020, 362, 698–706. [Google Scholar] [CrossRef]
- Li, Z.; Han, Y.; Gao, P.; Wang, H.; Liu, J. The interaction among multiple charged particles induced by cations and direct force measurements by AFM. Colloids Surf. A 2020, 589, 124440. [Google Scholar] [CrossRef]
- Yang, B.; Yin, W.; Zhu, Z.; Sun, H.; Sheng, Q.; Fu, Y.; Yao, J.; Zhao, K. Differential adsorption of hydrolytic polymaleic anhydride as an eco-friendly depressant for the selective flotation of apatite from dolomite. Sep. Purif. Technol. 2021, 256, 117803. [Google Scholar] [CrossRef]
- Liu, W.; Liu, W.; Wang, B.; Duan, H.; Peng, X.; Chen, X.; Zhao, Q. Novel hydroxy polyamine surfactant N-(2-hydroxyethyl)-N-dodecyl-ethanediamine: Its synthesis and flotation performance study to quartz. Miner. Eng. 2019, 142, 105894. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, D.; Yang, B.; Yin, W.; Ardakani, M.S.; Yao, J.; Drelich, J.W. Effect of nano-sized roughness on the flotation of magnesite particles and particle-bubble interactions. Miner. Eng. 2020, 151, 106340. [Google Scholar] [CrossRef]
- Fullston, D.; Fornasiero, D.; Ralston, J. Zeta potential study of the oxidation of copper sulfide minerals. Colloids Surf. A 1999, 146, 113–121. [Google Scholar] [CrossRef]
- Wang, D.; Hu, Y. Solution Chemistry of Flotation; Hunan Science and Technology Press: Changsha, China, 1988; pp. 132–134. [Google Scholar]
- Dong, J.; Liu, Q.; Yu, L.; Subhonqulov, S.H. The interaction mechanism of Fe3+ and NH4+ on chalcopyrite surface and its response to flotation separation of chalcopyrite from arsenopyrite. Sep. Purif. Technol. 2021, 256, 117778. [Google Scholar] [CrossRef]
- Moimane, T.; Huai, Y.; Peng, Y. The critical degree of bornite surface oxidation in flotation. Miner. Eng. 2020, 155, 106445. [Google Scholar] [CrossRef]
- Moimane, T.; Plackowski, C.; Peng, Y. The critical degree of mineral surface oxidation in copper sulphide flotation. Miner. Eng. 2020, 145, 106075. [Google Scholar] [CrossRef]
- Hirajima, T.; Miki, H.; Suyantara, G.P.W.; Matsuoka, H.; Elmahdy, A.M.; Sasaki, K.; Imaizumi, Y.; Kuroiwa, S. Selective flotation of chalcopyrite and molybdenite with H2O2 oxidation. Miner. Eng. 2017, 100, 83–92. [Google Scholar] [CrossRef]
- Liu, J.; Liu, G.; Yang, X.; Dong, Y.; Zhang, Z. 6-Hexyl-1,2,4,5-tetrazinane-3-thione: Flotation selectivity and mechanism to copper sulfide mineral. Miner. Eng. 2020, 152, 106345. [Google Scholar] [CrossRef]
- Yu, L.; Liu, Q.; Li, S.; Deng, J.; Luo, B.; Lai, H. Depression mechanism involving Fe3+ during arsenopyrite flotation. Sep. Purif. Technol. 2019, 222, 109–116. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, P.; Zhang, W.; Zeng, X.; Cao, Y. Mechanism of sodium sulfide on flotation of cyanide-depressed pyrite. Trans. Nonferrous Met. Soc. China 2020, 30, 484–491. [Google Scholar] [CrossRef]
- Mikhlin, Y.; Karacharov, A.; Tomashevich, Y.; Shchukarev, A. Cryogenic XPS study of fast-frozen sulfide minerals: Flotation-related adsorption of n-butyl xanthate and beyond. J. Electron Spectros. Relat. Phenom. 2016, 206, 65–73. [Google Scholar] [CrossRef]
- Tian, M.; Liu, R.; Gao, Z.; Chen, P.; Han, H.; Wang, L.; Zhang, C.; Sun, W.; Hu, Y. Activation mechanism of Fe (III) ions in cassiterite flotation with benzohydroxamic acid collector. Miner. Eng. 2018, 119, 31–37. [Google Scholar] [CrossRef]
- Niu, X.; Chen, J.; Li, Y.; Xia, L.; Li, L.; Sun, H.; Ruan, R. Correlation of surface oxidation with xanthate adsorption and pyrite flotation. Appl. Surf. Sci. 2019, 495, 143411. [Google Scholar] [CrossRef]
- Song, S.; Lopez-Valdivieso, A.; Reyes-Bahena, J.L.; Bermejo-Perez, H.I.; Trass, O. Hydrophobic flocculation of galena fines in aqueous suspensions. J. Colloid Interface Sci. 2000, 227, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Lopez-Valdivieso, A.; Reyes-Bahena, J.L.; Bermejo-Perez, H.I. Hydrophobic flocculation of sphalerite fines in aqueous suspensions induced by ethyl and amyl xanthates. Colloids Surf. A 2001, 181, 159–169. [Google Scholar] [CrossRef]
- Cheng, W.; Deng, Z.; Tong, X.; Lu, T. Hydrophobic agglomeration of fine pyrite particles induced by flotation reagents. Minerals 2020, 10, 801. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).