Confirmation of Siderazot, Fe3N1.33, the Only Terrestrial Nitride Mineral
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Origin
2.2. Chemical Analysis
2.3. Reflectance Measurements
2.4. Raman Spectroscopy
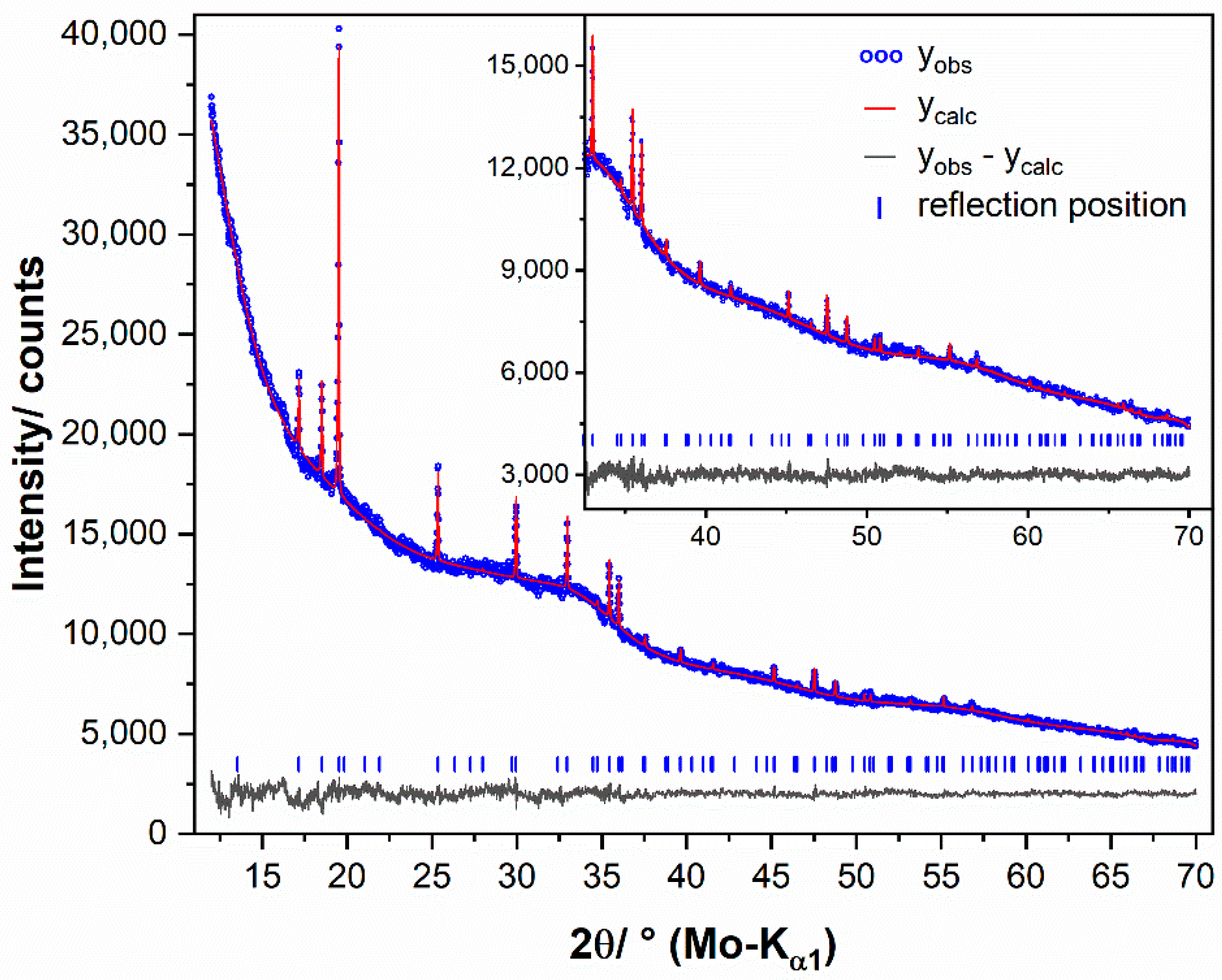
2.5. Powder X-ray Diffraction
2.6. Crystal Structure Refinements
3. Results and Discussion
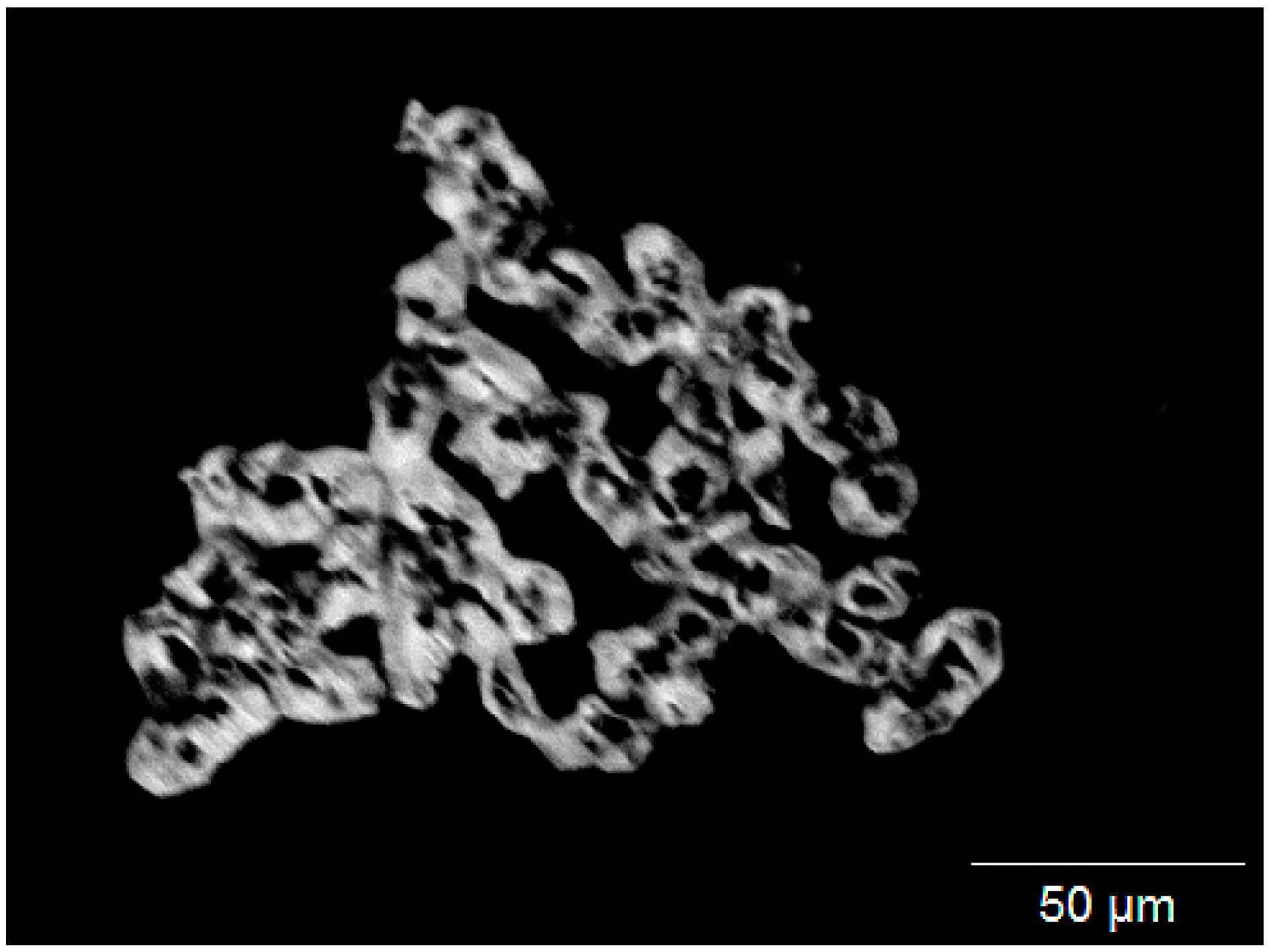
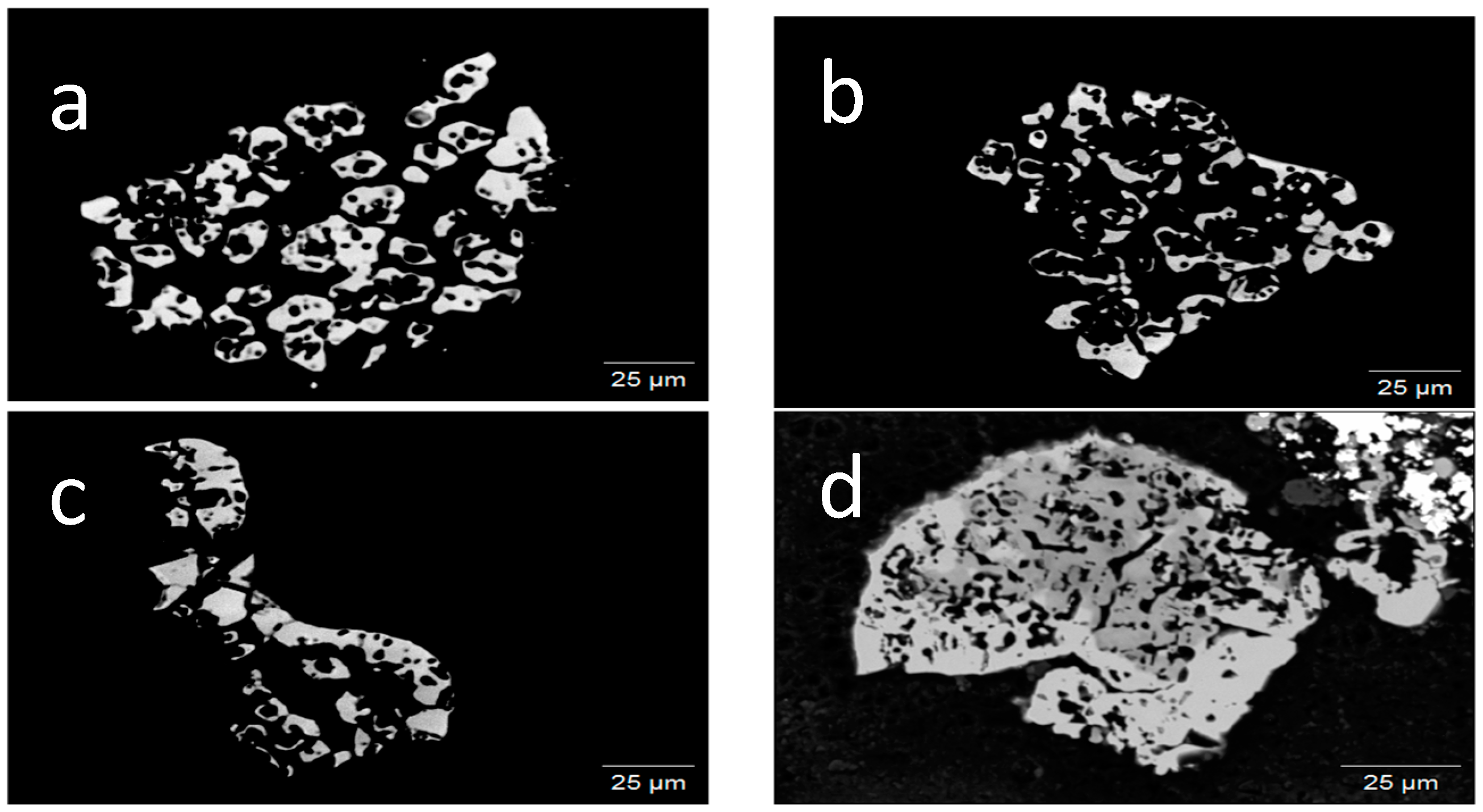
3.1. Sample Origin and Appearance
3.2. Appearance in Reflected Light
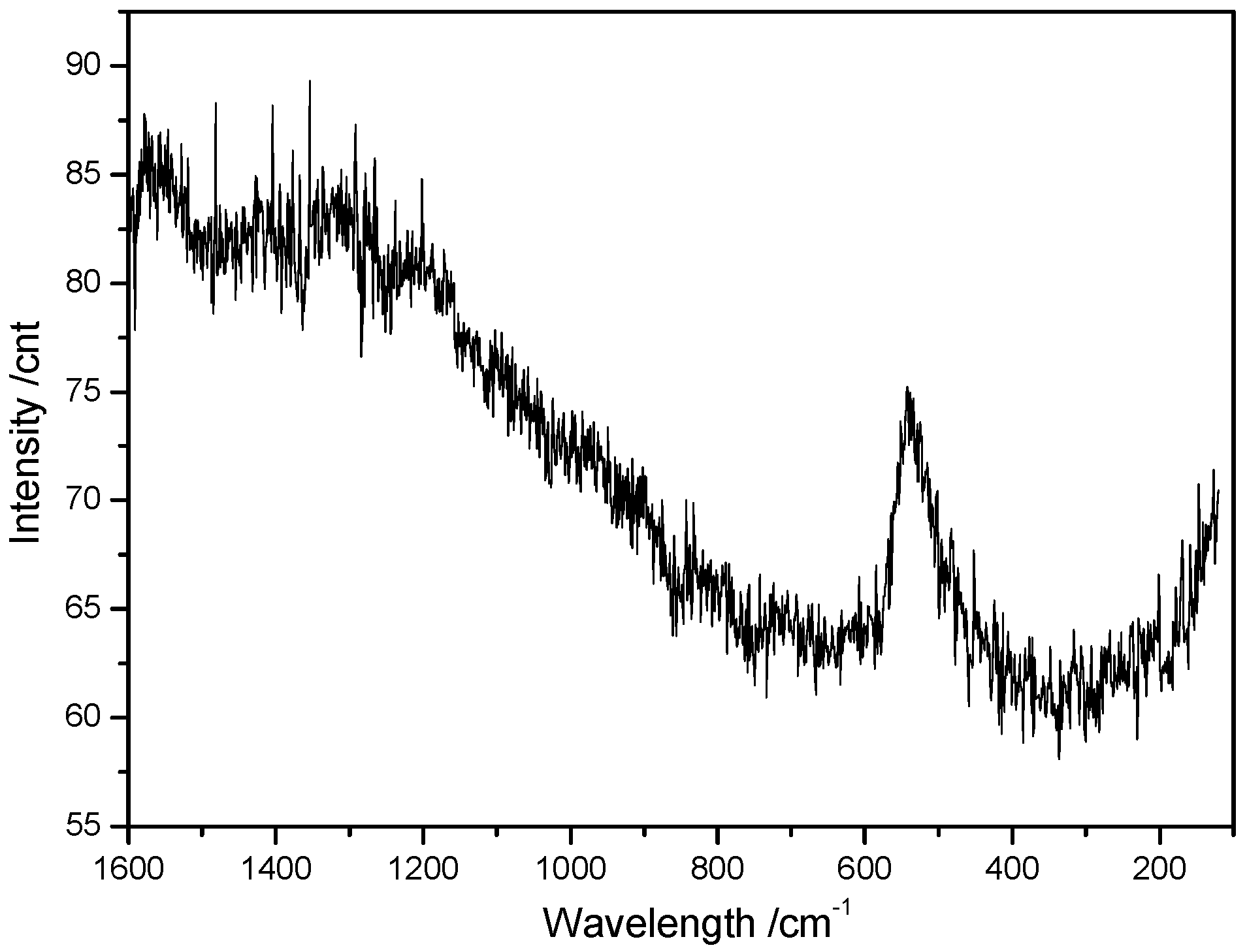
3.3. Raman Spectroscopy
3.4. Chemical Analysis
3.5. Crystal Structure Refinements
3.6. Possible Formation Conditions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anthony, J.; Bideaux, R.; Bladh, K.; Nichols, M. Halides, Hydroxides, Oxides. Handbook of Mineralogy; Mineral Data Publishing: Tuscon, AZ, USA, 1997; Volume 3. [Google Scholar]
- Lee, M.R.; Russel, S.S.; Arden, J.W.; Pillinger, C.T. Nierite (Si3N4), a new mineral from ordinary and enstatite chondrites. Meteorit. Planet. Sci. 1995, 30, 387–398. [Google Scholar] [CrossRef]
- Nittler, L.N.; Hoppe, P.; Alexander, C.M.O.’D.; Amari, S.; Eberhardt, P.; Gao, X.; Lewis, R.S.; Strebel, R.; Walker, R.M.; Zinner, E. Silicon nitride from supernovae. Astrophys. J. Lett. 1995, 453, L25–L28. [Google Scholar] [CrossRef]
- Nittler, L.R. Presolar stardust in meteroites: Recent advances and scientific frontiers. Earth Planet. Sci. Lett. 2003, 209, 259–273. [Google Scholar] [CrossRef]
- Alexander, C.M.O.’D. Presolar SiC in chondrites: How variable and how many sources? Geochim. Cosmochim. Acta 1993, 57, 2869–2888. [Google Scholar] [CrossRef]
- Alexander, C.M.O.’D.; Swan, P.D.; Prombo, P.A. Occurrence and implications of silican nitride in enstantite chondrites. Meteoritics 1994, 29, 79–85. [Google Scholar] [CrossRef]
- Stone, J.; Hutcheon, I.D.; Epstein, S.; Wasserburg, G.J. Correlated Si isotope anomalies and large 13C enrichments in a family of exotic SiC grains. Earth Planet. Sci. Lett. 1991, 107, 570–581. [Google Scholar] [CrossRef]
- Stone, J.; Hutcheon, I.D.; Epstein, S.; Wasserburg, G.J. Silicon, carbon and nitrogen isotopic studies of silicon carbide in carbonaceous and entstantite chondrites. In Stable Isotope Geochemistry: A Tribute to Samuel Epstein; Taylor, H.P., O’Neil, J.R., Kaplan, I.R., Eds.; Special Publication 3; The Geochemical Society: San Antonio, TX, USA, 1991; pp. 487–504. [Google Scholar]
- Lacroix, A. Matériaux sur les météorites pierreuses; 1. Identitéde composition des météorites de Pillistfer (1863) et de Hvittis (1901). Bull. Soc. Fr. Miner. 1905, 28, 70–76. [Google Scholar]
- Keil, K.; Andersen, C.A. Occurrences of sinoite, Si2N2O, in meteorites. Nature 1965, 207, 745. [Google Scholar] [CrossRef]
- Rubin, A.E. Sinoite (Si2N2O): Crystallization from EL chondrite impact melts. Am. Miner. 1997, 82, 1001–1006. [Google Scholar] [CrossRef]
- Andersen, C.A.; Keil, K.; Mason, B. Silicon oxynitride: A meteoritic mineral. Science 1964, 146, 256–257. [Google Scholar] [CrossRef]
- Bannister, F.A. Osbornite, meteoritic titanium nitride. Miner. Mag. 1941, 36, 36–44. [Google Scholar] [CrossRef][Green Version]
- Grady, M.M.; Wrigth, I.P.; Carr, L.; Pillinger, C.T. Compositional differences in enstatite chondrites based on carbon and nitrogen stable isotope measurements. Geochim. Cosmochim. Acta 1986, 50, 2799–2813. [Google Scholar] [CrossRef]
- Fleischer, M. New Mineral Names. Am. Miner. 1972, 57, 1311. [Google Scholar]
- Buchwald, V.F.; Scott, E.R.D. First nitride (CrN) in iron meteorites. Nat. Phys. Sci. 1971, 233, 113–114. [Google Scholar] [CrossRef]
- Cabri, L.J.; Fleischer, M.; Pabst, A. New Mineral Names. Am. Miner. 1981, 66, 1003–1099. [Google Scholar]
- Buchwald, V.F.; Nielsen, H.P. Roaldite, a new nitride in iron meteorites. Lunar Planet. Sci. 1981, 12, 112–114. [Google Scholar]
- Tunell, G.; Fahey, J.J.; Daugherty, F.W.; Gibbs, G.V. Gianellaite, a new mercury mineral. Neues Jahrb. Miner. Monatsh. 1977, 119–131. [Google Scholar]
- Fleischer, M.; Pabst, A.; Mandarino, J.A.; Chao, G.Y. New Mineral Names. Am. Miner. 1977, 62, 1057–1061. [Google Scholar]
- Airoldi, R.; Magnano, G. Sulla struttura del sulfato (di)mercurioammonico. Rass. Chim. 1967, 5, 181–189. [Google Scholar]
- Cooper, M.A.; Abdu, Y.A.; Hawthorne, F.C.; Kampf, A.R. The crystal structure of gianellaite, [(NHg2)2](SO4)(H2O)x, a framework of (NHg4) tetrahedra with ordered (SO4) groups in the interstices. Miner. Mag. 2016, 80, 869–875. [Google Scholar] [CrossRef]
- Foord, E.E.; Mills, B.A. Biaxiality in ‘isomeric’ and ‘dimeric’ crystals. Am. Miner. 1978, 63, 316–325. [Google Scholar]
- Giester, G.; Mikenda, W.; Pertlik, F. Kleinite from Terlingua, Brewster County, Texas: Investigations by single crystal X-ray diffraction, and vibrational spectroscopy. Neues Jahrb. Miner. Monatsh. 1996, 2, 49–56. [Google Scholar]
- Sachs, A. Der Kleinit, ein hexagonales Quecksilberoxychlorid von Terlingua in Texas. In Sitzungsberichte der Königlich Preussischen Akademie der Wissenschaften; Verlag der königlichen Akademie der Wissenschaften: Berlin, Germany, 1905; Zweiter Halbband; pp. 1091–1094. [Google Scholar]
- Cooper, M.A.; Abdu, Y.A.; Hawthorne, F.C.; Kampf, A.R. The crystal structure of comancheite, Hg2+55N3–24(OH,NH2)4(Cl,Br)34, and crystal-chemical and spectroscopic discrimination of N3– and O2– anions in Hg2+ compounds. Miner. Mag. 2013, 77, 3217–3237. [Google Scholar] [CrossRef]
- Silvestri, O. Das Vorkommen des Stickstoffeisens unter den Fumarolen-Producten des Aetna und künstliche Darstellung dieser Verbindung. Poggendorfs Ann. Phys. Chem. 1876, 157, 165–172. [Google Scholar] [CrossRef]
- Silvestri, O. La scombinazione chimica (dissociazione) applicata alla interpetrazione di alcuni fenomeni vulcanici; sintesi e analisi di un nuovo minerale trovato sull’Etna e di origine comune nei vulcani. Atti Accad. Gioenia Sci. Nat. 1876, 10, 17–27. [Google Scholar]
- Huggins, M.L.; Sakamoto, Y. Lattice Energies and Other Properties of Crystals of Alkaline Earth Chalcogenides. J. Phys. Soc. Jpn. 1957, 12, 241–251. [Google Scholar] [CrossRef]
- Ladd, M.F.C.; Lee, W.H. Lattice energies and related topics. Prog. Solid State Chem. 1967, 3, 265–288. [Google Scholar] [CrossRef]
- Baughan, E.C. The Repulsion Energies in Ionic Compounds. Trans. Faraday Soc. 1959, 55, 736–752. [Google Scholar] [CrossRef]
- Jack, K.H. The iron-nitrogen system: The preparation and the crystal structures of nitrogen-austenite (γ) and nitrogen-martensite (α′). Proc. R. Soc. Lond. Ser. A 1951, 208, 200–215. [Google Scholar] [CrossRef]
- Jack, K.H. The occurrence and the crystal structure of α″-iron nitride; a new type of interstitial alloy formed during the tempering of nitrogen-martensite. Proc. R. Soc. Lond. Ser. A 1951, 208, 216–224. [Google Scholar] [CrossRef]
- Widenmeyer, M.; Hansen, T.C.; Niewa, R. Formation and Decomposition of Metastable α″-Fe16N2 from in situ Powder Neutron Diffraction and Thermal Analysis. Z. Anorg. Allg. Chem. 2013, 639, 2851–2859. [Google Scholar] [CrossRef]
- Suzuki, K.; Morita, H.; Kaneko, T.; Yoshida, H.; Fujimori, H. Crystal structure and magnetic properties of the compound FeN. J. Alloys Compds. 1993, 201, 11–16. [Google Scholar] [CrossRef]
- Suzuki, K.; Yamaguchi, Y.; Kaneko, T.; Yoshida, H.; Obi, Y.; Fujimori, H.; Morita, H. Neutron Diffraction Studies of the Compounds MnN and FeN. J. Phys. Soc. Jpn. 2001, 70, 1084–1089. [Google Scholar] [CrossRef]
- Clark, W.P.; Steinberg, S.; Dronskowski, R.; McCammon, C.; Kupenko, I.; Bykov, M.; Dubrovinsky, L.; Akselrud, L.G.; Schwarz, U.; Niewa, R. High-pressure NiAs-Type Modification of FeN. Angew. Chem. Int. Ed. 2017, 56, 7302–7306. [Google Scholar] [CrossRef] [PubMed]
- Fry, A. Stickstoff im Eisen, Stahl und Sonderstahl. Ein neues Oberflächenhärtungsverfahren. Stahl Eisen 1923, 43, 1271–1279. [Google Scholar]
- Prenosil, B. Einige neue Erkenntnisse über das Gefüge von um 600 °C in der Gasatmosphäre carbonitridierten Schichten. Härt.-Technol. Mitt. 1973, 28, 157–164. [Google Scholar]
- Andriamandroso, D.; Fefilatiev, L.; Demazeau, G.; Fournès, L.; Purchard, M. Mossbauer resonance studies on Sn substituted Fe4N. Mater. Res. Bull. 1984, 19, 1187–1194. [Google Scholar] [CrossRef]
- Kim, T.K.; Takahashi, M. New magnetic materials having ultrahigh magnetic moment. Appl. Phys. Lett. 1972, 20, 492–494. [Google Scholar] [CrossRef]
- Dirba, I.; Komissinskiy, P.; Gutfleisch, O.; Alff, L. Increased magnetic moment induced by lattice expansion from α-Fe to α′-Fe8N. J. Appl. Phys. 2015, 117, 173911. [Google Scholar] [CrossRef]
- Guo, K.; Rau, D.; Toffoletti, L.; Müller, C.; Burkhardt, U.; Schnelle, W.; Niewa, R.; Schwarz, U. The ternary metastable nitrides Fe2TMN (TM = Co, Ni): High-pressure high-temperature synthesis, crystal structure, thermal stability and magnetic properties. Chem. Mater. 2012, 24, 4600–4606. [Google Scholar] [CrossRef]
- Guo, K.; Rau, D.; Schnelle, W.; Burkhardt, U.; Niewa, R.; Schwarz, U. High-Pressure–High-Temperature Synthesis of ε-Fe2IrN0.24. Z. Anorg. Allg. Chem. 2014, 640, 814–818. [Google Scholar] [CrossRef]
- Schwarz, U.; Guo, K.; Clark, W.P.; Burkhardt, U.; Bobnar, M.; Castillo, R.; Akselrud, L.; Niewa, R. Ferromagnetic ε-Fe2MnN: High-pressure synthesis, hardness and magnetic properties. Materials 2019, 12, 1993. [Google Scholar] [CrossRef] [PubMed]
- Bykov, M.; Bykova, E.; Aprilis, G.; Glazyrin, K.; Koemets, E.; Chuvashova, I.; Kupenko, I.; McCammon, C.; Mezouar, M.; Prakapenka, V.; et al. Fe–N system at high pressure reveals a compound featuring polymeric nitrogen chains. Nat. Commun. 2018, 9, 2756. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Yagi, T. Synthesis of Metal Nitride Using Nitrogen Fluid under High Pressure and High Temperature—Synthesis and Physical Property. Rev. High Press. Sci. Technol. 2004, 14, 253–259. [Google Scholar] [CrossRef][Green Version]
- Mysen, B. Nitrogen in the Earth: Abundance and transport. Prog. Earth Planetary Sci. 2019, 6, 38. [Google Scholar] [CrossRef]
- Bajgain, S.K.; Mookherjee, M.; Dasgupta, R.; Ghosh, D.B.; Karki, B.B. Nitrogen Content in the Earth’s Outer Core. Geophys. Res. Lett. 2019, 46, 89–98. [Google Scholar] [CrossRef]
- Liu, J.; Dorfman, S.M.; Lv, M.; Li, J.; Zhu, F.; Kono, Y. Loss of immiscible nitrogen from metallic melt explains Earth’s missing nitrogen. Geochem. Perp. Lett. 2019, 11, 18–22. [Google Scholar] [CrossRef]
- Adler, J.F.; Williams, Q. A high-pressure X-ray diffraction studies of iron nitrides: Implication for Earth’s core. J. Geophys. Res. 2005, 110, B011203. [Google Scholar] [CrossRef]
- Roskosz, M.; Bouhifd, M.A.; Jephcoat, A.P.; Marty, B.; Mysen, B.O. Nitrogen solubility in molten metal and silicate at high pressure and temperature. Geochim. Cosmochim. Acta 2013, 121, 15–28. [Google Scholar] [CrossRef]
- Litasov, K.D.; Shatskiy, A.; Ponomarev, D.S.; Gavryushkin, P.N. Equations of state of iron nitrides ε-Fe3Nx and γ-Fe4Ny to 30 GPa and 1200 K and implication for nitrogen in the Earth’s core. J. Geophys. Res. Solid Earth 2017, 122, 3574–3584. [Google Scholar] [CrossRef]
- Minobe, S.; Nakajima, Y.; Hirose, K.; Ohishi, Y. Stability and compressibility of a new iron-nitride β-Fe7N3 to core pressures. Geophys. Res. Lett. 2015, 42, 5206–5211. [Google Scholar] [CrossRef]
- Kaminsky, F.; Wirth, R. Nitrides and carbonitrides from the lowermost mantle and their importance in the search for Earth’s “lost” nitrogen. Amer. Miner. 2017, 102, 1667. [Google Scholar] [CrossRef]
- Miyazaki, A.; Hiyagon, H.; Sugiura, N.; Hirose, K.; Takahashi, E. Solubilities of nitrogen and noble gases in silicate melts under various oxygen fugacities: Implications for the origin and degassing history of nitrogen and noble gases in the Earth. Geochim. Cosmochim. Acta 2004, 68, 387–401. [Google Scholar] [CrossRef]
- Dobrzhinetskaya, L.F.; Wirth, R.; Yang, J.; Hutcheon, I.D.; Weber, P.K.; Green, H.W. High-pressure highly reduced nitrides and oxidesfrom chromitite of a Tibetan ophiolite. Proc. Natl. Acad. Sci. USA 2009, 106, 19233–19238. [Google Scholar] [CrossRef]
- Rodwell, G.F. Etna: A History of the Mountain and Its Eruptions; Cambridge University Press: London, UK, 1878; p. 134. [Google Scholar]
- Coelho, A.A. TOPAS and TOPAS-Academic: An optimization program integrating computer algebra and crystallographic objects written in C plus. J. Appl. Crystallogr. 2018, 51, 210–218. [Google Scholar] [CrossRef]
- Cheary, R.W.; Coelho, A.A.; Cline, J.P. Fundamental parameters line profile fitting in laboratory diffractometers. J. Res. Natl. Inst. Stand. Technol. 2004, 109, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Jacobs, H.; Rechenbach, D.; Zachwieja, U. Structure determination of γ′-Fe4N and ε-Fe3N. J. Alloys Compd. 1995, 227, 10–17. [Google Scholar] [CrossRef]
- Liapina, T.; Leineweber, A.; Mittemeijer, E.J.; Kockelmann, W. The lattice parameters of epsilon-iron nitrides: Lattice strains due to a varying degree of nitrogen ordering. Acta Mater. 2004, 52, 173–180. [Google Scholar] [CrossRef]
- Niewa, R.; Rau, D.; Wosylus, A.; Meier, K.; Hanfland, M.; Wessel, M.; Dronskowski, R.; Dzivenko, D.A.; Riedel, R.; Schwarz, U. High-Pressure, High-Temperature Single-Crystal Growth, Ab initio Electronic Structure Calculations, and Equation of State of ε-Fe3N1+x. Chem. Mater. 2009, 21, 392–398. [Google Scholar] [CrossRef]
- Middendorf, C.; Mader, W. Growth and Microstructure of Iron Nitride Layers and Pore Formation in ε-Fe3N. Z. Metallkd. 2003, 94, 333–340. [Google Scholar] [CrossRef]
- Piller, H. Colour measurements in ore-microscopy. Miner. Depos. 1966, 1, 175–192. [Google Scholar] [CrossRef]
- Atkin, B.P.; Harvey, P.K. The use of quantitative Colour Values for Opaque-Mineral Identification. Can. Miner. 1979, 17, 639–647. [Google Scholar]
- Lei, L.; Yin, W.; Jiang, X.; Lin, S.; He, D. Synthetic Route to Metal Nitrides: High-Pressure Solid-State Metathesis Reaction. Inorg. Chem. 2013, 52, 13356–13362. [Google Scholar] [CrossRef]
- Wriedt, H.A.; Gokcen, N.A.; Nafziger, R.H. The Fe-N (Iron-Nitrogen) system. Bull. Alloy Phase Diagr. 1987, 8, 355–377. [Google Scholar] [CrossRef]
- Caliro, S.; Chiodini, G.; Avino, R.; Minopoli, C.; Bocchino, B. Long time-series of chemical and isotopic compositions of Vesuvius fumaroles: Evidence for deep and shallow processes. Ann. Geophys. 2011, 54, 137–149. [Google Scholar] [CrossRef]
- Balić-Žunić, T.; Garavelli, A.; Jakobsson, S.P.; Jonasson, K.; Katerinopoulos, A.; Kyriakopoulos, K.; Acquafredda, P. Fumarolic Minerals: An Overview of Active European Volcanoes. In Updates in Volcanology—From Volcano Modelling to Volcano Geology; Nemeth, K., Ed.; IntechOpen: London, UK, 2016; pp. 267–322. [Google Scholar] [CrossRef]
- Chiodini, G.; Marini, L.; Russo, M. Geochemical evidence for the existence of high-temperature hydrothermal brines at Vesuvio volcano, Italy. Geochim. Cosmochim. Acta 2001, 65, 2129–2147. [Google Scholar] [CrossRef]
- Corsaro, R.A.; Cristofolini, R. Origin and differentiation of recent basaltic magmas from Mount Etna. Miner. Petrol. 1996, 57, 1–21. [Google Scholar] [CrossRef]
- Nicotra, E.; Ferlito, C.; Viccaro, M.; Cristofolini, R. Volcanic geology and petrology of the Val Calanna succession (Mt. Etna, Southern Italy): Discovery of a new eruptive center. Period. Miner. 2011, 2, 287–307. [Google Scholar] [CrossRef]

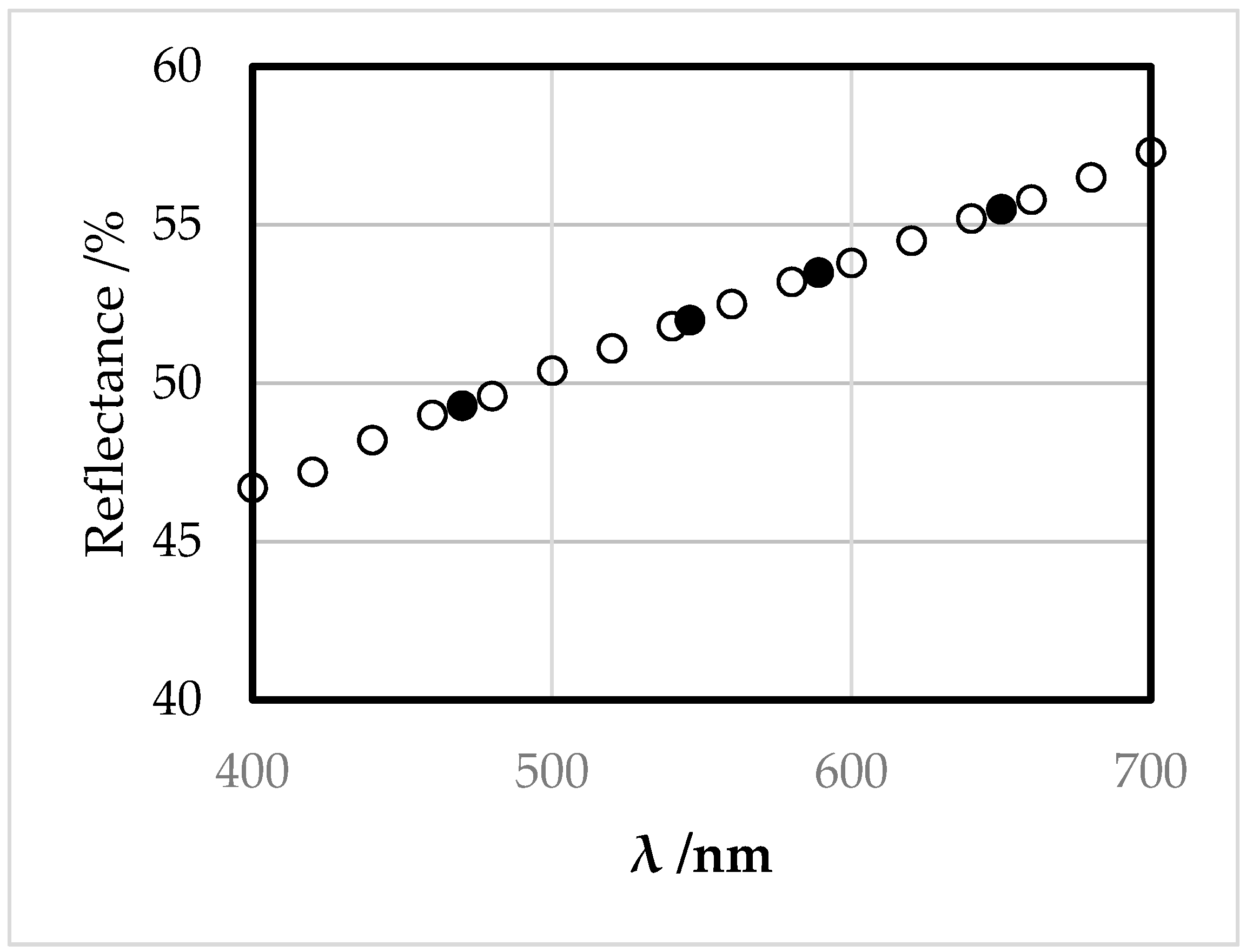
| λ/nm | R/% |
|---|---|
| 400 | 46.7 |
| 420 | 47.2 |
| 440 | 48.2 |
| 460 | 49.0 |
| 470 | 49.3 |
| 480 | 49.6 |
| 500 | 50.4 |
| 520 | 51.1 |
| 540 | 51.8 |
| 546 | 52.0 |
| 560 | 52.5 |
| 580 | 53.2 |
| 589 | 53.5 |
| 600 | 53.8 |
| 620 | 54.5 |
| 640 | 55.2 |
| 650 | 55.5 |
| 660 | 55.8 |
| 680 | 56.5 |
| 700 | 57.3 |
| Illuminate | x | y | Y/% | λd/nm | Pe/% |
|---|---|---|---|---|---|
| C | 0.3197 | 0.3245 | 52.3 | 579 | 4.8 |
| A | 0.4563 | 0.4091 | 52.8 | 588 | 7.2 |
| wt.% | n = 25 | 1σ | Min. | Max. |
|---|---|---|---|---|
| Fe | 88.71 | 0.39 | 87.33 | 89.22 |
| N | 10.37 | 0.19 | 9.85 | 10.62 |
| Na | <0.07 | 0.01 | 0.00 | 0.03 |
| O | 0.1 | 0.07 | 0.08 | 0.40 |
| Total | 99.30 | 0.40 | 98.12 | 99.87 |
| n = 25 | 1σ | Min. | Max. | |
|---|---|---|---|---|
| Fe | 3.000 | |||
| N | 1.399 | 0.026 | 1.327 | 1.434 |
| Na | 0.025 | 0.009 | 0.009 | 0.047 |
| O | 0.000 | 0.001 | 0.000 | 0.002 |
| wt.% | n = 4 | 1σ | Min. | Max. |
|---|---|---|---|---|
| Fe | 85.00 | 0.82 | 84.02 | 86.00 |
| N | 6.58 | 0.46 | 5.94 | 6.95 |
| Na | 1.99 | 0.76 | 1.05 | 2.90 |
| O | 1.68 | 0.37 | 1.18 | 2.05 |
| Mineral Name | Siderazot |
|---|---|
| Sum formula | Fe3N1+x with x = 1.33 |
| Molecular weight, g/mol | 186.22 |
| Temperature, K | 295 |
| Space group | P6322 |
| Z | 2 |
| a, Å | 4.7527(1) |
| c, Å | 4.4077(2) |
| V, Å3 | 86.22(1) |
| ρcalc, g·cm−3 | 7.17 |
| Wavelength, Å | 0.7093 |
| R-p, % 1 | 1.28 |
| R-wp, % 1 | 1.60 |
| R-F2, % 1 | 0.79 |
| R-exp, % 1 | 0.96 |
| G.O.F. 1 | 1.67 |
| No. of variables | 29 |
| Atom | Wyck. | Site | S.O.F. 1 | x | y | z | Beq, Å2 |
|---|---|---|---|---|---|---|---|
| Fe | 6g | .2. | 1.00 | 0.322(1) | 0 | 0 | 1.16(6) |
| N(1) | 2c | 3.2 | 1.00 | 1/3 | 2/3 | 1/4 | Beq(Fe) 2 |
| N(2) | 2b | 3.2 | 0.33(7) | 0 | 0 | 1/4 | Beq(Fe) 2 |
| Atoms | Distance, Å | Atoms | Angle, deg |
|---|---|---|---|
| N(1)–Fe | 6 × 1.951(8) | N(1)–Fe–N(1) | 128.6(1) |
| N(2)–Fe | 6 × 1.888(8) | N(2)–Fe–N(2) | 71.5(1) |
| Fe–Fe | 2 × 2.655(16) 4 × 2.684(5) 2 × 2.776(11) 4 × 2.790(8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bette, S.; Theye, T.; Bernhardt, H.-J.; Clark, W.P.; Niewa, R. Confirmation of Siderazot, Fe3N1.33, the Only Terrestrial Nitride Mineral. Minerals 2021, 11, 290. https://doi.org/10.3390/min11030290
Bette S, Theye T, Bernhardt H-J, Clark WP, Niewa R. Confirmation of Siderazot, Fe3N1.33, the Only Terrestrial Nitride Mineral. Minerals. 2021; 11(3):290. https://doi.org/10.3390/min11030290
Chicago/Turabian StyleBette, Sebastian, Thomas Theye, Heinz-Jürgen Bernhardt, William P. Clark, and Rainer Niewa. 2021. "Confirmation of Siderazot, Fe3N1.33, the Only Terrestrial Nitride Mineral" Minerals 11, no. 3: 290. https://doi.org/10.3390/min11030290
APA StyleBette, S., Theye, T., Bernhardt, H.-J., Clark, W. P., & Niewa, R. (2021). Confirmation of Siderazot, Fe3N1.33, the Only Terrestrial Nitride Mineral. Minerals, 11(3), 290. https://doi.org/10.3390/min11030290







