Preparation of Monoclinic Pyrrhotite by Thermal Decomposition of Jarosite Residues and Its Heavy Metal Removal Performance
Abstract
1. Introduction
2. Materials and Methods
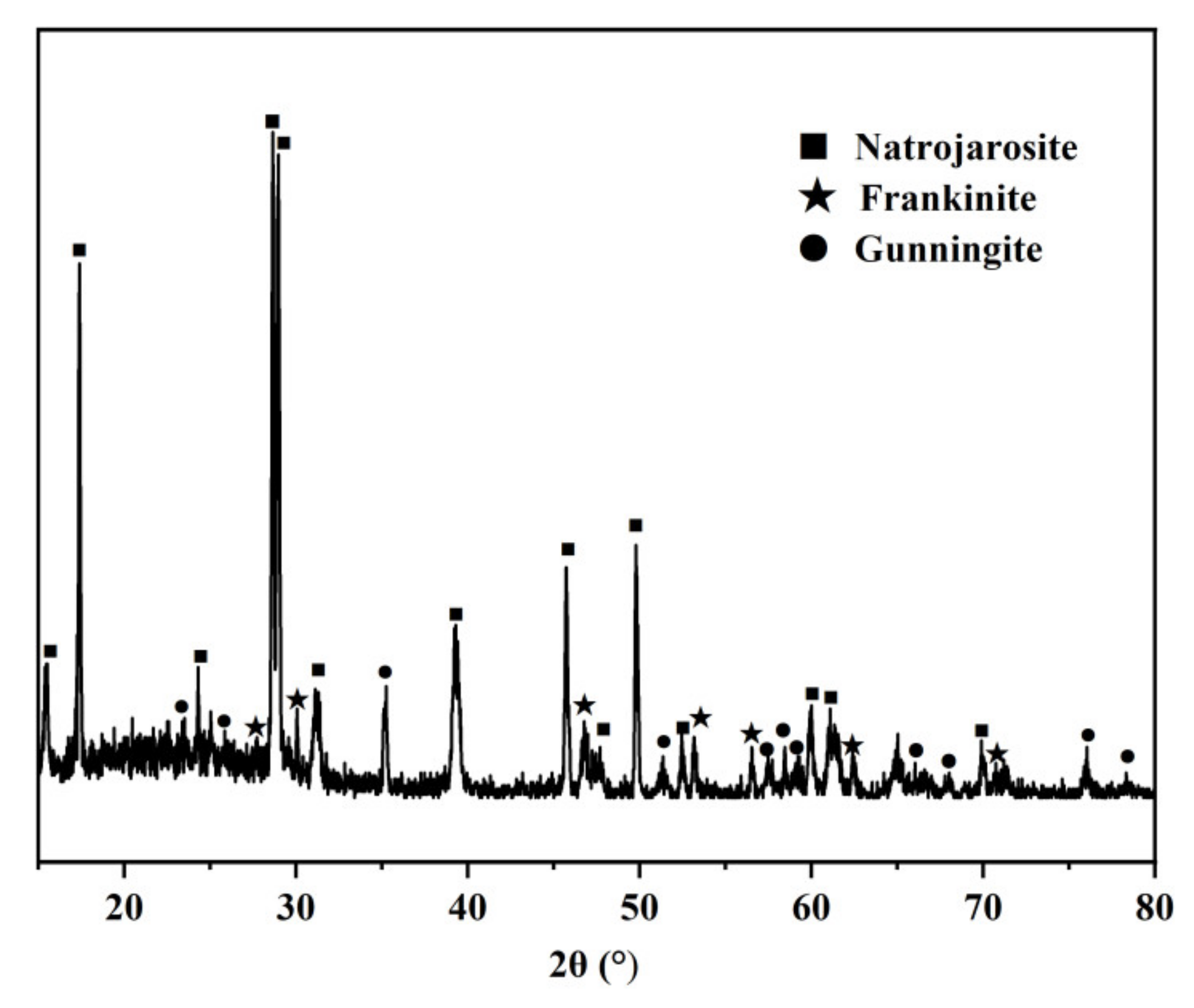
2.1. Sample Setting
2.2. Reaction Apparatus and Experiments
2.3. Analytical Methods
3. Results and Discussion
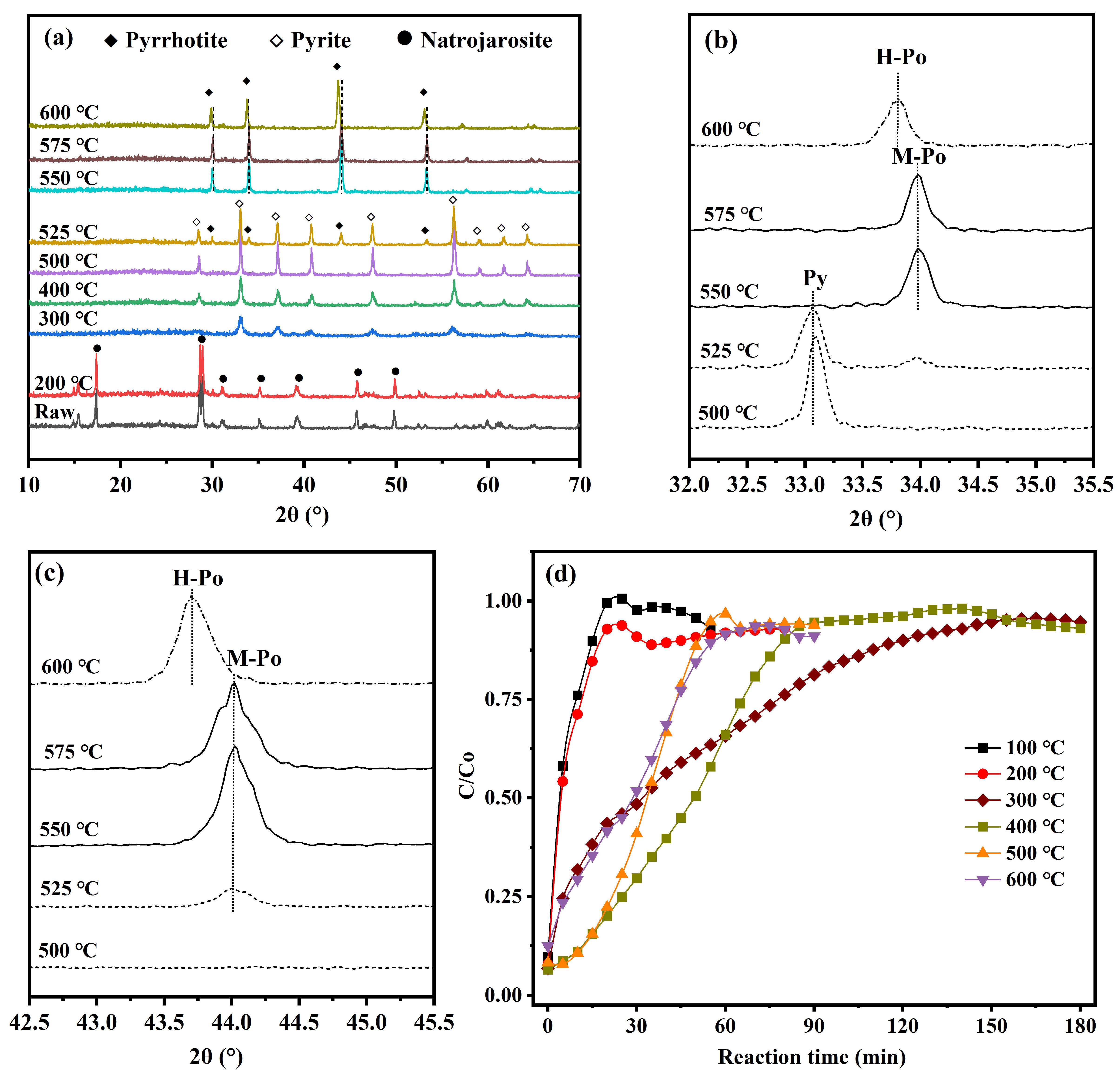
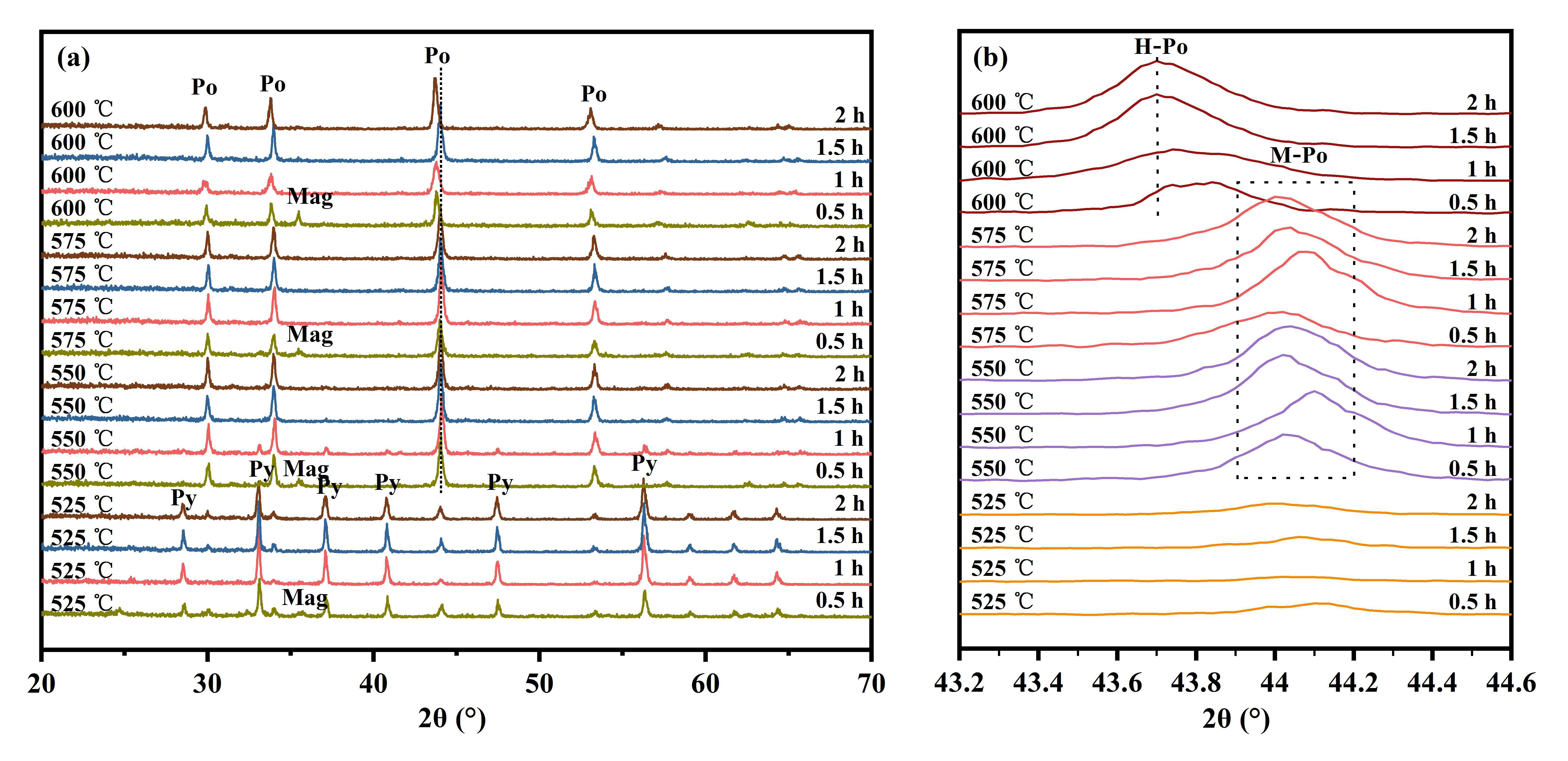
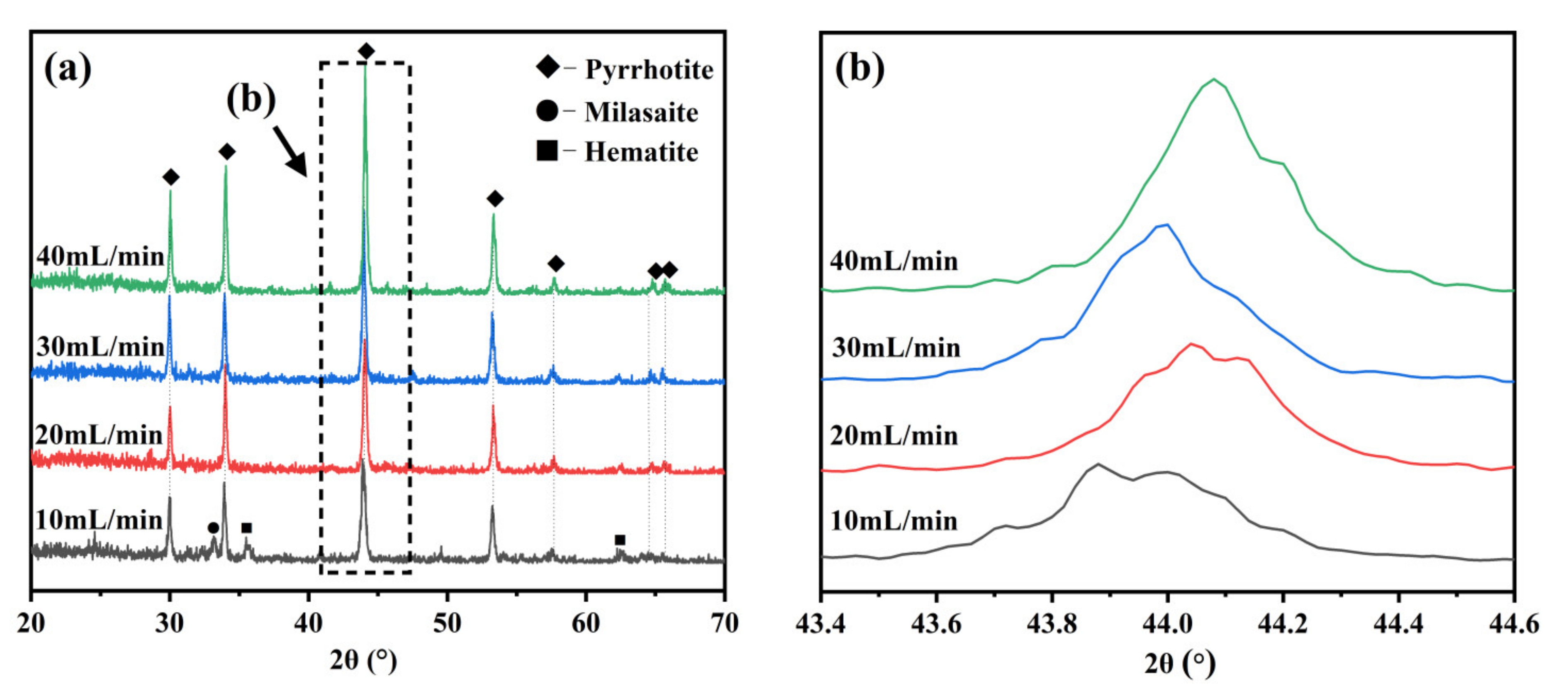
3.1. Determination of Optimal Sulfidation Conditions
3.1.1. Effect of Reaction Temperature
3.1.2. Effect of Reaction Time
3.1.3. Effect of Gas Speed
3.2. XPS
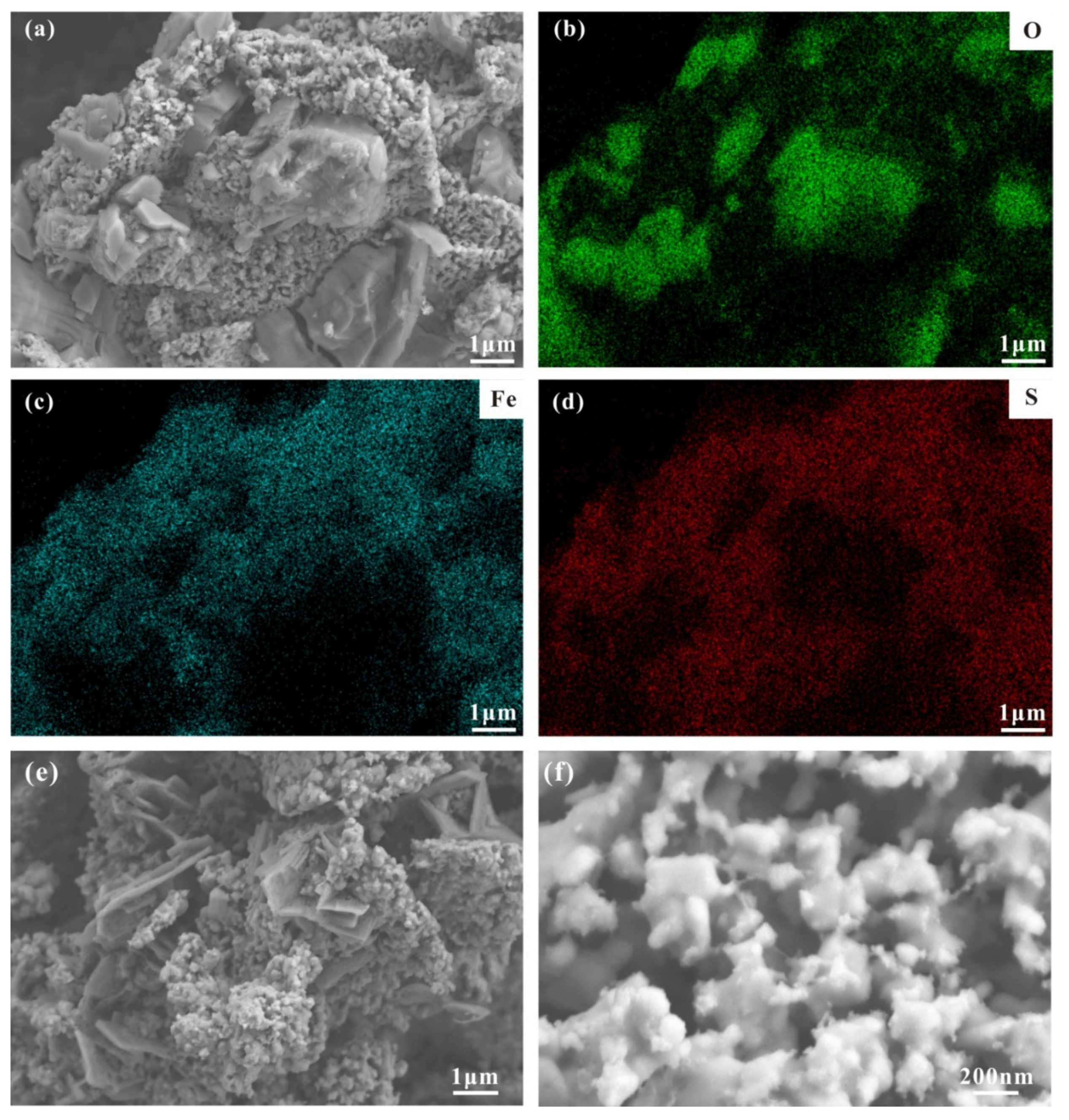
3.3. Mineral Morphology
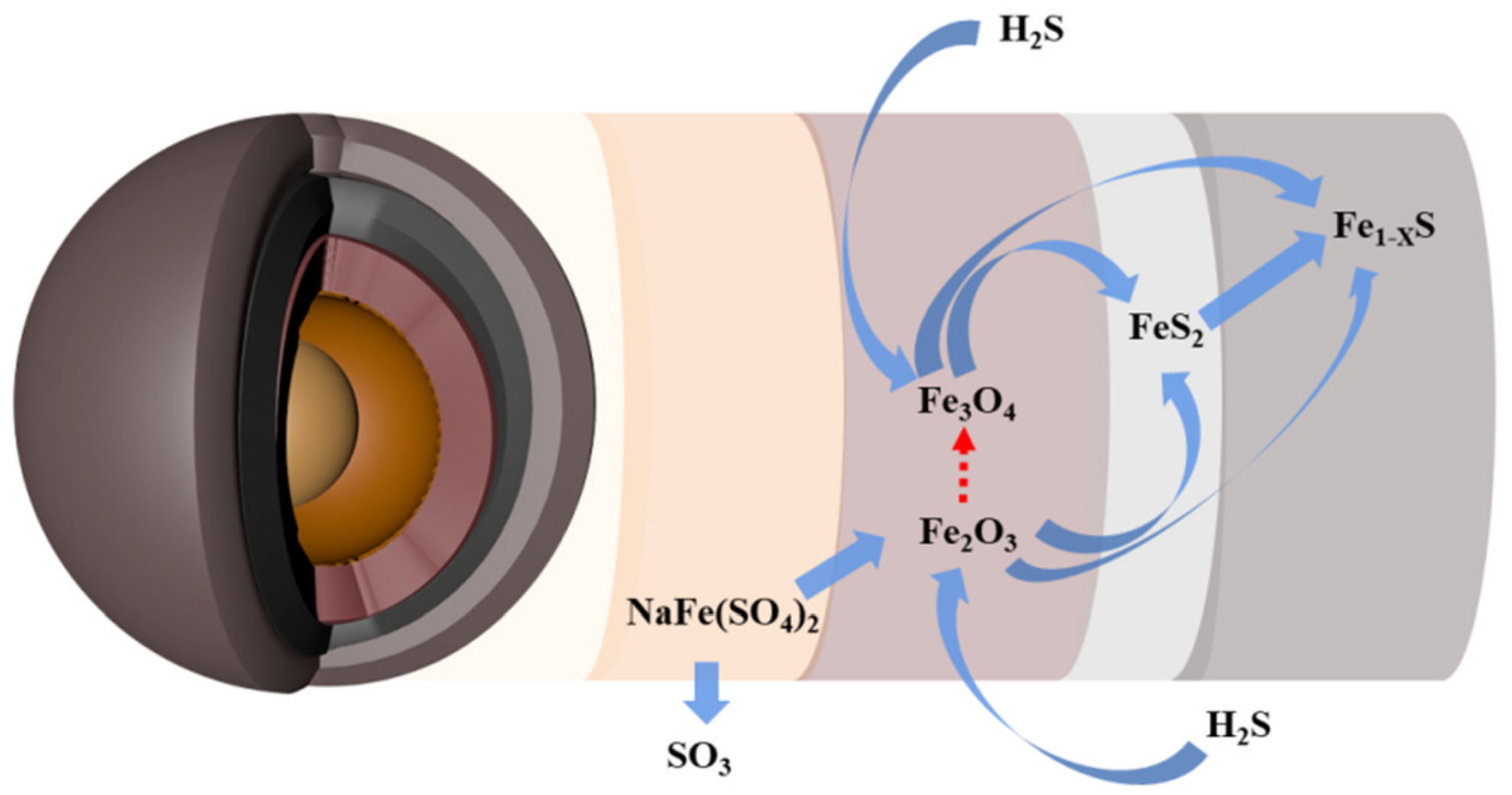
3.4. Reaction Mechanism
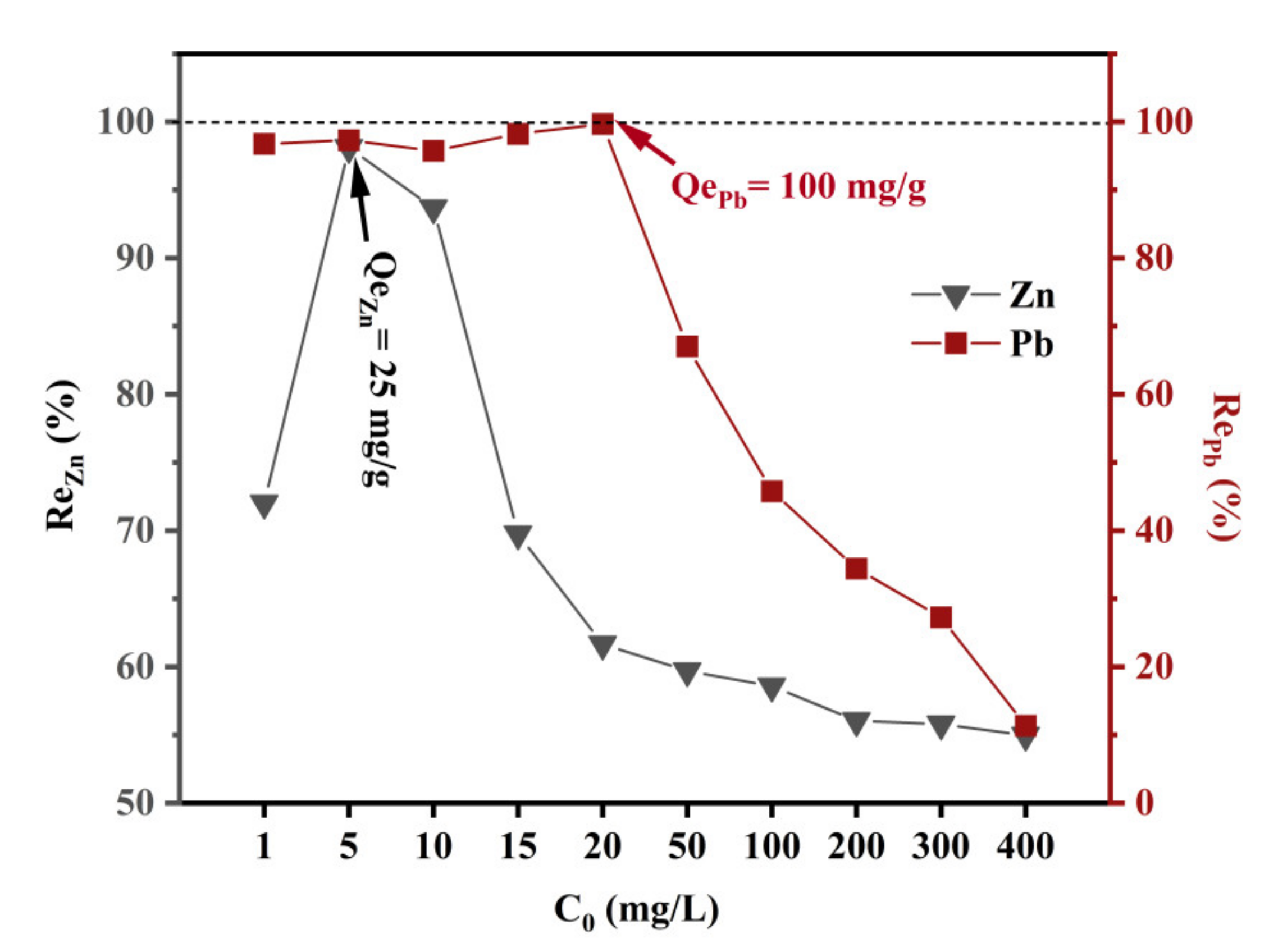
3.5. Removal Efficiencies of Zn and Pb by Sulfidation Product
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, C.; Ju, S.H.; Zhang, L.B.; Srinivasakannan, C.; Peng, J.H.; Le, T.Q.X.; Guo, Z.Y. Recovery of valuable metals from jarosite by sulphuric acid roasting using microwave and water leaching. Can. Metall. Q. 2016, 56, 1–9. [Google Scholar] [CrossRef]
- Ju, S.-H.; Zhang, L.-B.; Peng, J.-H.; Shi, Z.; Guo, S.-H.; Liu, B.-G.; Wang, Y.-J. Thermodynamics of leaching roasted jarosite residue from zinc hydrometallurgy in NH4Cl system. Trans. Nonferr. Met. Soc. China 2013, 23, 1179–1183. [Google Scholar] [CrossRef]
- Wang, F.; Yu, J.; Xiong, W.; Xu, Y.; Chi, R.-A. A two-step leaching method designed based on chemical fraction distribution of the heavy metals for selective leaching of Cd, Zn, Cu, and Pb from metallurgical sludge. Environ. Sci. Pollut. Res. 2017, 25, 1752–1765. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; Chen, T.T. A Mineralogical Study of the Jarosite Phase Formed During the Autoclave Leaching of Zinc Concentrate. Can. Metall. Q. 1984, 23, 147–157. [Google Scholar] [CrossRef]
- Elgersma, F.; Witkamp, G.; Van Rosmalen, G. Incorporation of zinc in continuous jarosite precipitation. Hydrometallurgy 1993, 33, 313–339. [Google Scholar] [CrossRef]
- Kerolli–Mustafa, M.; Fajković, H.; Rončević, S.; Ćurković, L. Assessment of metal risks from different depths of jarosite tailing waste of Trepça Zinc Industry, Kosovo based on BCR procedure. J. Geochem. Explor. 2015, 148, 161–168. [Google Scholar] [CrossRef]
- Islas, H.; Flores, M.U.; Reyes, I.A.; Juárez, J.C.; Reyes, M.; Teja, A.M.; Palacios, E.G.; Pandiyan, T.; Aguilar-Carrillo, J. Determination of the dissolution rate of hazardous jarosites in different conditions using the shrinking core kinetic model. J. Hazard. Mater. 2020, 386, 121664. [Google Scholar] [CrossRef]
- Dong, Z.; Jiang, T.; Xu, B.; Yang, J.; Chen, Y.; Li, Q.; Yang, Y. Comprehensive recoveries of selenium, copper, gold, silver and lead from a copper anode slime with a clean and economical hydrometallurgical process. Chem. Eng. J. 2020, 393, 124762. [Google Scholar] [CrossRef]
- Zhang, C.; Min, X.; Zhang, J.; Wang, M.; Li, Y.; Fei, J. Reductive clean leaching process of cadmium from hydrometallurgical zinc neutral leaching residue using sulfur dioxide. J. Clean. Prod. 2016, 113, 910–918. [Google Scholar] [CrossRef]
- Calla-Choque, D.; Lapidus, G. Acid Decomposition and silver leaching with thiourea and oxalate from an industrial jarosite sample. Hydrometallurgy 2020, 192, 105289. [Google Scholar] [CrossRef]
- Palden, T.; Regadio, M.; Onghena, B.; Binnemans, K. Selective Metal Recovery from Jarosite Residue by Leaching with Acid-Equilibrated Ionic Liquids and Precipitation-Stripping. ACS Sustain. Chem. Eng. 2019, 7, 4239–4246. [Google Scholar] [CrossRef]
- Mymrin, V.A.; Ponte, H.A.; Impinnisi, P.R. Potential application of acid jarosite wastes as the main component of construction materials. Constr. Build. Mater. 2005, 19, 141–146. [Google Scholar] [CrossRef]
- Asokan, P.; Saxena, M.; Asolekar, S. Recycling hazardous jarosite waste using coal combustion residues. Mater. Charact. 2010, 61, 1342–1355. [Google Scholar] [CrossRef]
- Asokan, P.; Saxena, M.; Asolekar, S.R. Hazardous jarosite use in developing non-hazardous product for engineering application. J. Hazard. Mater. 2006, 137, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Pappu, A.; Thakur, V.K.; Patidar, R.; Asolekar, S.R.; Saxena, M. Recycling marble wastes and Jarosite wastes into sustainable hybrid composite materials and validation through Response Surface Methodology. J. Clean. Prod. 2019, 240, 118249. [Google Scholar] [CrossRef]
- Gupta, T.; Sachdeva, S.N. Investigations on Jarosite Mixed Cement Concrete Pavements. Arab. J. Sci. Eng. 2019, 44, 8787–8797. [Google Scholar] [CrossRef]
- Gupta, T.; Sachdeva, S. Study of mechanical, micro-structural and environmental properties of concrete containing zinc industry waste for pavements. Constr. Build. Mater. 2020, 245, 118331. [Google Scholar] [CrossRef]
- Isteri, V.; Ohenoja, K.; Hanein, T.; Kinoshita, H.; Tanskanen, P.; Illikainen, M.; Fabritius, T. Production and properties of ferrite-rich CSAB cement from metallurgical industry residues. Sci. Total Environ. 2020, 712, 136208. [Google Scholar] [CrossRef] [PubMed]
- Mehra, P.; Gupta, R.C.; Thomas, B.S. Assessment of durability characteristics of cement concrete containing jarosite. J. Clean. Prod. 2016, 119, 59–65. [Google Scholar] [CrossRef]
- Belebchouche, C.; Moussaceb, K.; Aït-Mokhtar, A. Evaluation of the encapsulation of nickel, chromium and lead-rich wastes in cement matrices by TCLP test. Eur. J. Environ. Civ. Eng. 2015, 20, 1–14. [Google Scholar] [CrossRef]
- Benavente, D.; Pla, C.; Valdes-Abellan, J.; Cremades-Alted, S. Remediation by waste marble powder and lime of jarosite-rich sediments from Portman Bay (Spain). Environ. Pollut. 2020, 264, 114786. [Google Scholar] [CrossRef]
- Linsong, W.; Peng, Z.; Yu, F.; SuJun, L.; Yue, Y.; Li, W.; Wei, S. Recovery of metals from jarosite of hydrometallurgical nickel production by thermal treatment and leaching. Hydrometallurgy 2020, 198, 105493. [Google Scholar] [CrossRef]
- Rämä, M.; Nurmi, S.; Jokilaakso, A.; Klemettinen, L.; Taskinen, P.; Salminen, J. Thermal Processing of Jarosite Leach Residue for a Safe Disposable Slag and Valuable Metals Recovery. Metals 2018, 8, 744. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Yang, H.-F.; Jiang, B.; Song, R.-L.; Zhang, W.-H. Comprehensive recovery of lead, zinc, and iron from hazardous jarosite residues using direct reduction followed by magnetic separation. Int. J. Miner. Met. Mater. 2018, 25, 123–130. [Google Scholar] [CrossRef]
- Han, H.; Sun, W.; Hu, Y.; Jia, B.; Tang, H. Anglesite and silver recovery from jarosite residues through roasting and sulfidization-flotation in zinc hydrometallurgy. J. Hazard. Mater. 2014, 278, 49–54. [Google Scholar] [CrossRef]
- Calla-Choque, D.; Nava-Alonso, F.; Fuentes-Aceituno, J. Acid Decomposition and thiourea leaching of silver from hazardous jarosite residues: Effect of some cations on the stability of the thiourea system. J. Hazard. Mater. 2016, 317, 440–448. [Google Scholar] [CrossRef]
- Chang, L.; Wang, Y.; Luo, S.; Liu, H.; Wang, Q. Carbothermal reduction preparation and performance of LiFePO4/C by using ammonium jarosite extracted from vanadium slag as iron source. Ionics 2019, 25, 5725–5734. [Google Scholar] [CrossRef]
- Xu, Z.; Liang, J.; Zhou, L. Photo-Fenton-like degradation of azo dye methyl orange using synthetic ammonium and hydronium jarosite. J. Alloy. Compd. 2013, 546, 112–118. [Google Scholar] [CrossRef]
- Hu, H.; Lin, C.; Zhang, Y.; Cai, X.; Huang, Z.; Chen, C.; Qin, Y.; Liang, J. Preparation of a Stable Nanoscale Manganese Residue-Derived FeS@Starch-Derived Carbon Composite for the Adsorption of Safranine T. Nanomaterials 2019, 9, 839. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, C.; Zhuang, J.; Zheng, G.; Zhou, L. Assessment of schwertmannite, jarosite and goethite as adsorbents for efficient adsorption of phenanthrene in water and the regeneration of spent adsorbents by heterogeneous fenton-like reaction. Chemosphere 2020, 244, 125523. [Google Scholar] [CrossRef] [PubMed]
- Drouet, C.; Navrotsky, A. Synthesis, characterization, and thermochemistry of K-Na-H3O jarosites. Geochim. Cosmochim. Acta 2003, 67, 2063–2076. [Google Scholar] [CrossRef]
- Flores, M.U.; Reyes, I.A.; Palacios, E.G.; Patiño, F.; Juárez, J.C.; Reyes, M.; Teja, A.M.; Islas, H.; Gutiérrez, E.J. Kinetic Analysis of the Thermal Decomposition of a Synthetic Mercury Jarosite. Minerals 2019, 9, 200. [Google Scholar] [CrossRef]
- Wang, H.; Chen, T.; Liu, H.; Li, W.; Zou, X.; Wang, C.; Li, M. Comprehensive Application of Oolitic Hematite for H2S Removal at High Temperature: Performance and Mechanism. Energy Fuels 2019, 33, 2037–2044. [Google Scholar] [CrossRef]
- Chen, T.; Yang, Y.; Li, P.; Liu, H.; Xie, J.; Xie, Q.; Zhan, X. Performance and characterization of calcined colloidal pyrite used for copper removal from aqueous solutions in a fixed bed column. Int. J. Miner. Process. 2014, 130, 82–87. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, T.; Li, P.; Xie, Q.; Zhan, X. Cu Removal from Acid Mine Drainage by Modified Pyrite: Batch and Column Experiments. Mine Water Environ. 2016, 36, 371–378. [Google Scholar] [CrossRef]
- Lu, P.; Chen, T.; Liu, H.; Li, P.; Peng, S.; Yang, Y. Green Preparation of Nanoporous Pyrrhotite by Thermal Treatment of Pyrite as an Effective Hg(Ⅱ) Adsorbent: Performance and Mechanism. Minerals 2019, 9, 74. [Google Scholar] [CrossRef]
- Zhu, Y.; Peng, S.; Lu, P.; Chen, T.; Yang, Y. Mercury Removal from Aqueous Solutions Using Modified Pyrite: A Column Experiment. Minerals 2019, 10, 43. [Google Scholar] [CrossRef]
- Zakarina, N.A.; Volkova, L.D.; Kim, O.K.; Brodskii, A.R.; Latypov, I.F.; Yaskevich, V.I.; Komashko, L.V. Natural iron-containing materials and catalysts on their basis on use for photocatalytic Decomposition of hydrogen sulfide. Pet. Chem. 2013, 53, 181–186. [Google Scholar] [CrossRef]
- Herbert, F.; Krishnamoorthy, A.; Ma, W.; Van Vliet, K.; Yildiz, B. Dynamics of point defect formation, clustering and pit initiation on the pyrite surface. Electrochim. Acta 2014, 127, 416–426. [Google Scholar] [CrossRef]
- Jeong, H.Y.; Han, Y.-S.; Park, S.W.; Hayes, K.F. Aerobic oxidation of mackinawite (FeS) and its environmental implication for arsenic mobilization. Geochim. Cosmochim. Acta 2010, 74, 3182–3198. [Google Scholar] [CrossRef]
- Shu, D.; Chen, T.; Zou, X.; Li, M.; Wang, C.; Wang, H.; Han, Z.; Liu, H. Effect of iron minerals during coaling on the transformation of NO in the presence of NH3: Take pyrite as an example. Sci. Total Environ. 2020, 731, 138951. [Google Scholar] [CrossRef]
- Jovanović, D. Kinetics of thermal Decomposition of pyrite in an inert atmosphere. J. Therm. Anal. Calorim. 1989, 35, 1483–1492. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, P.; Cheng, H.; Liu, Q. Thermal phase transition of pyrite from coal. J. Therm. Anal. Calorim. 2018, 134, 2391–2396. [Google Scholar] [CrossRef]
- Lambert, J.M.; Simkovich, G.; Walker, P.L. The kinetics and mechanism of the pyrite-to-pyrrhotite transformation. Met. Mater. Trans. A 1998, 29, 385–396. [Google Scholar] [CrossRef]
- Gong, Y.; Tang, J.; Zhao, D. Application of iron sulfide particles for groundwater and soil remediation: A review. Water Res. 2016, 89, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Chiu, H. Adsorption of zinc(II) from water with purified carbon nanotubes. Chem. Eng. Sci. 2006, 61, 1138–1145. [Google Scholar] [CrossRef]
- Asencios, Y.J.; Sun-Kou, M.R. Synthesis of high-surface-area γ-Al2O3 from aluminum scrap and its use for the adsorption of metals: Pb(II), Cd(II) and Zn(II). Appl. Surf. Sci. 2012, 258, 10002–10011. [Google Scholar] [CrossRef]
- Grover, V.A.; Hu, J.; Engates, K.E.; Shipley, H.J. Adsorption and desorption of bivalent metals to hematite nanoparticles. Environ. Toxicol. Chem. 2011, 31, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Kalavathy, H.; Karthik, B.; Miranda, L.R. Removal and recovery of Ni and Zn from aqueous solution using activated carbon from Hevea brasiliensis: Batch and column studies. Colloids Surf. B Biointerfaces 2010, 78, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.A.K.; Ikram, S.; Ahmad, J. Adsorption of Pb(II) on a composite material prepared from polystyrene-alumina and activated carbon: Kinetic and thermodynamic studies. J. Iran. Chem. Soc. 2011, 8, 931–943. [Google Scholar] [CrossRef]
- Kim, S.A.; Kamala-Kannan, S.; Lee, K.-J.; Park, Y.-J.; Shea, P.J.; Lee, W.-H.; Kim, H.-M.; Oh, B.-T. Removal of Pb(II) from aqueous solution by a zeolite–nanoscale zero-valent iron composite. Chem. Eng. J. 2013, 217, 54–60. [Google Scholar] [CrossRef]



| Adsorbent | Adsorption Capacities of Zn (mg/g) | Reference |
|---|---|---|
| Modified Carbon Nanofibers | 1.05 | [46] |
| γAl2O3 | 7.60 | [47] |
| Hematite nanoparticles | 8.56 | [48] |
| Activated carbon | 22.03 | [49] |
| Pyrrhotite prepared by jarosite residues | 25.00 | This study |
| Adsorbent | Adsorption Capacities of Pb (mg/g) | Reference |
|---|---|---|
| Composite material: polystyrene/alumina/carbon | 10.64 | [50] |
| γAl2O3 | 13.11 | [47] |
| Zeolite | 39.10 | [51] |
| Z-nZVI composite | 96.20 | [51] |
| Pyrrhotite prepared by jarosite residues | 100 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Xie, Q.; Xu, F.; Zhou, Y.; Wang, H.; Chen, T.; Peng, S. Preparation of Monoclinic Pyrrhotite by Thermal Decomposition of Jarosite Residues and Its Heavy Metal Removal Performance. Minerals 2021, 11, 267. https://doi.org/10.3390/min11030267
Xu C, Xie Q, Xu F, Zhou Y, Wang H, Chen T, Peng S. Preparation of Monoclinic Pyrrhotite by Thermal Decomposition of Jarosite Residues and Its Heavy Metal Removal Performance. Minerals. 2021; 11(3):267. https://doi.org/10.3390/min11030267
Chicago/Turabian StyleXu, Cuimin, Qiaoqin Xie, Fan Xu, Yuefei Zhou, Hanlin Wang, Tianhu Chen, and Shuchuan Peng. 2021. "Preparation of Monoclinic Pyrrhotite by Thermal Decomposition of Jarosite Residues and Its Heavy Metal Removal Performance" Minerals 11, no. 3: 267. https://doi.org/10.3390/min11030267
APA StyleXu, C., Xie, Q., Xu, F., Zhou, Y., Wang, H., Chen, T., & Peng, S. (2021). Preparation of Monoclinic Pyrrhotite by Thermal Decomposition of Jarosite Residues and Its Heavy Metal Removal Performance. Minerals, 11(3), 267. https://doi.org/10.3390/min11030267







