Mining Wastes of an Albite Deposit as Raw Materials for Vitrified Mullite Ceramics
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Chemical Analysis by XRF of the Raw Samples and after Wet Sieving under 63 μm Samples
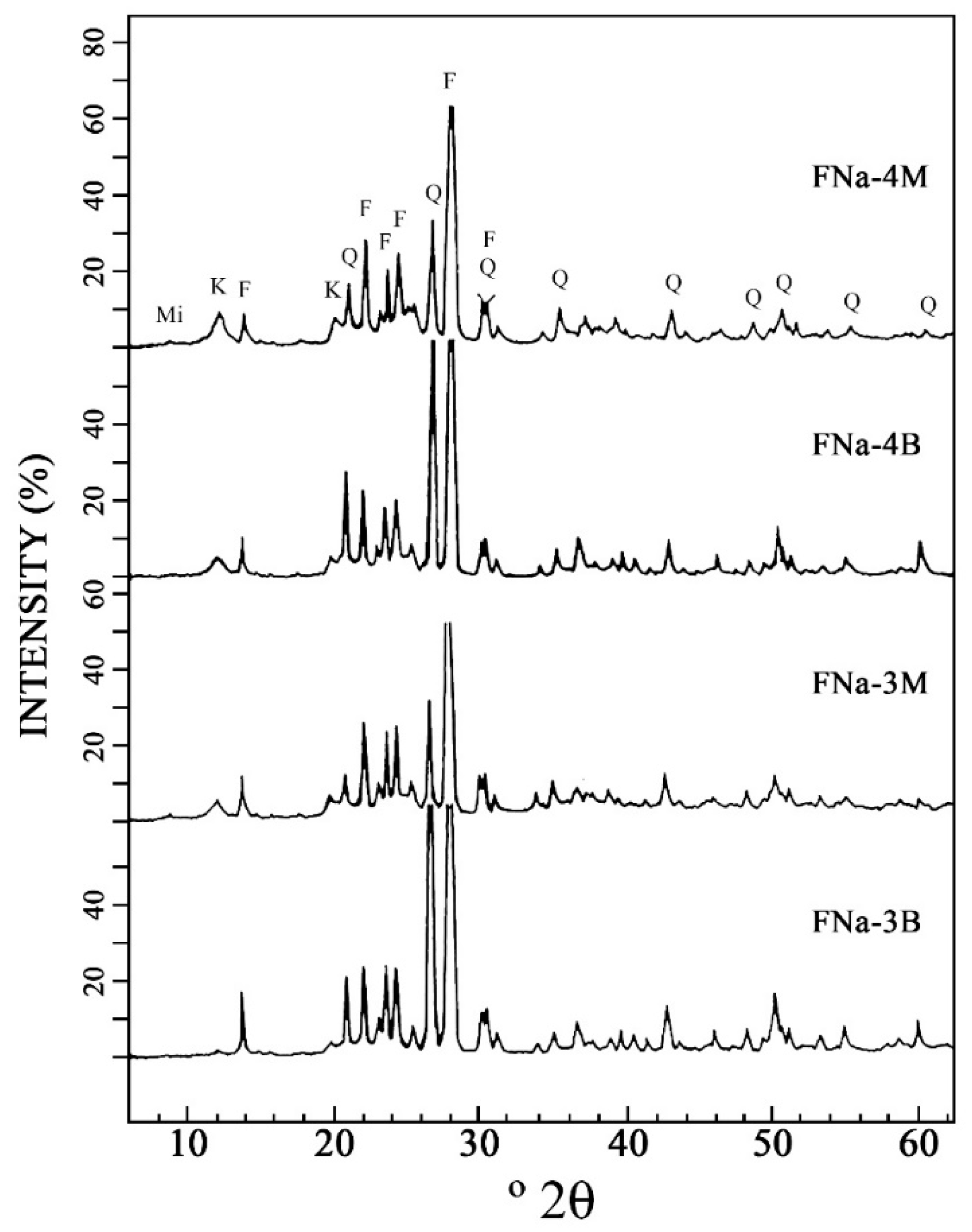
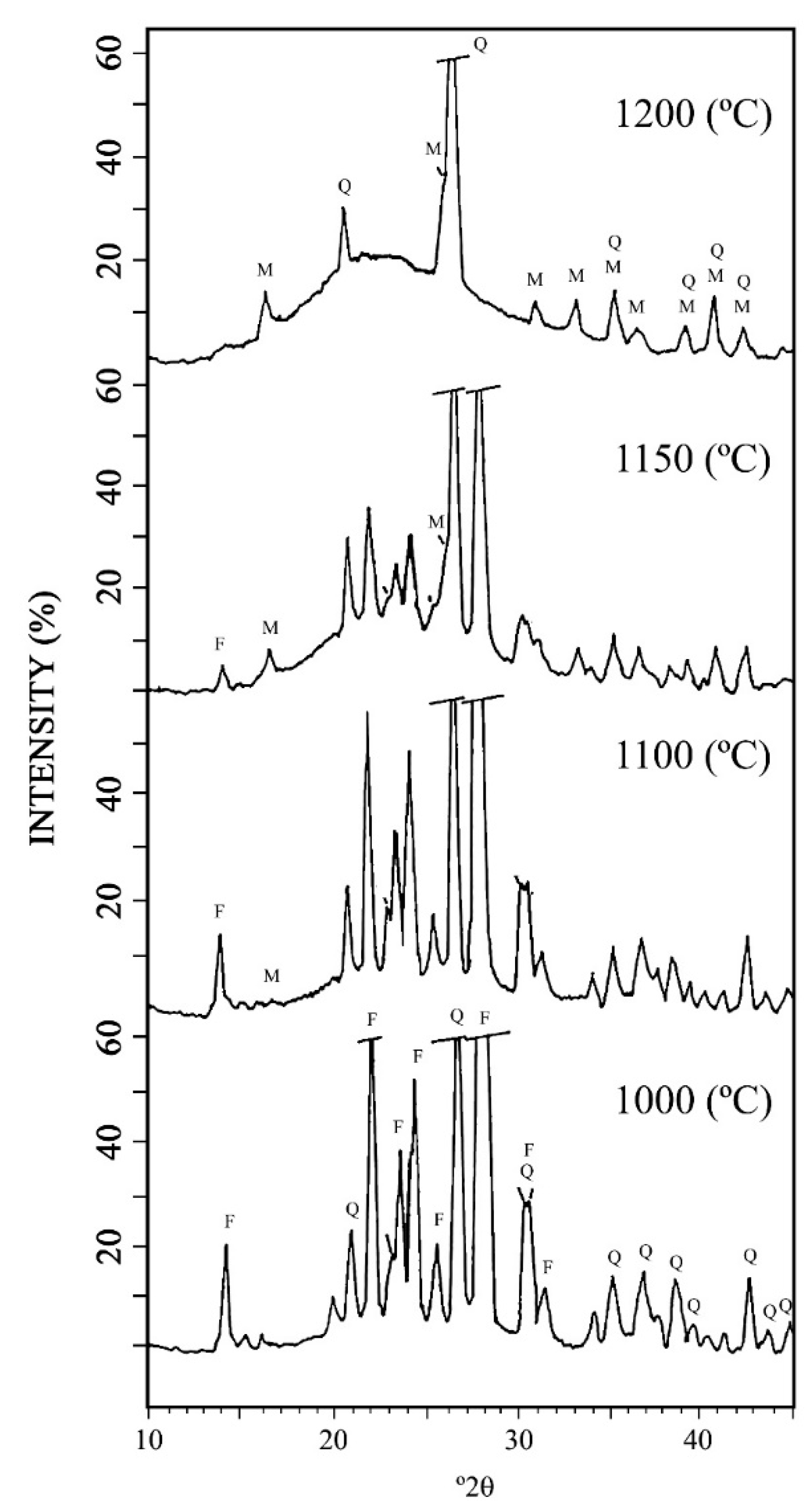
3.2. XRD of the Raw Samples and Samples after Wet Sieving under 63 μm
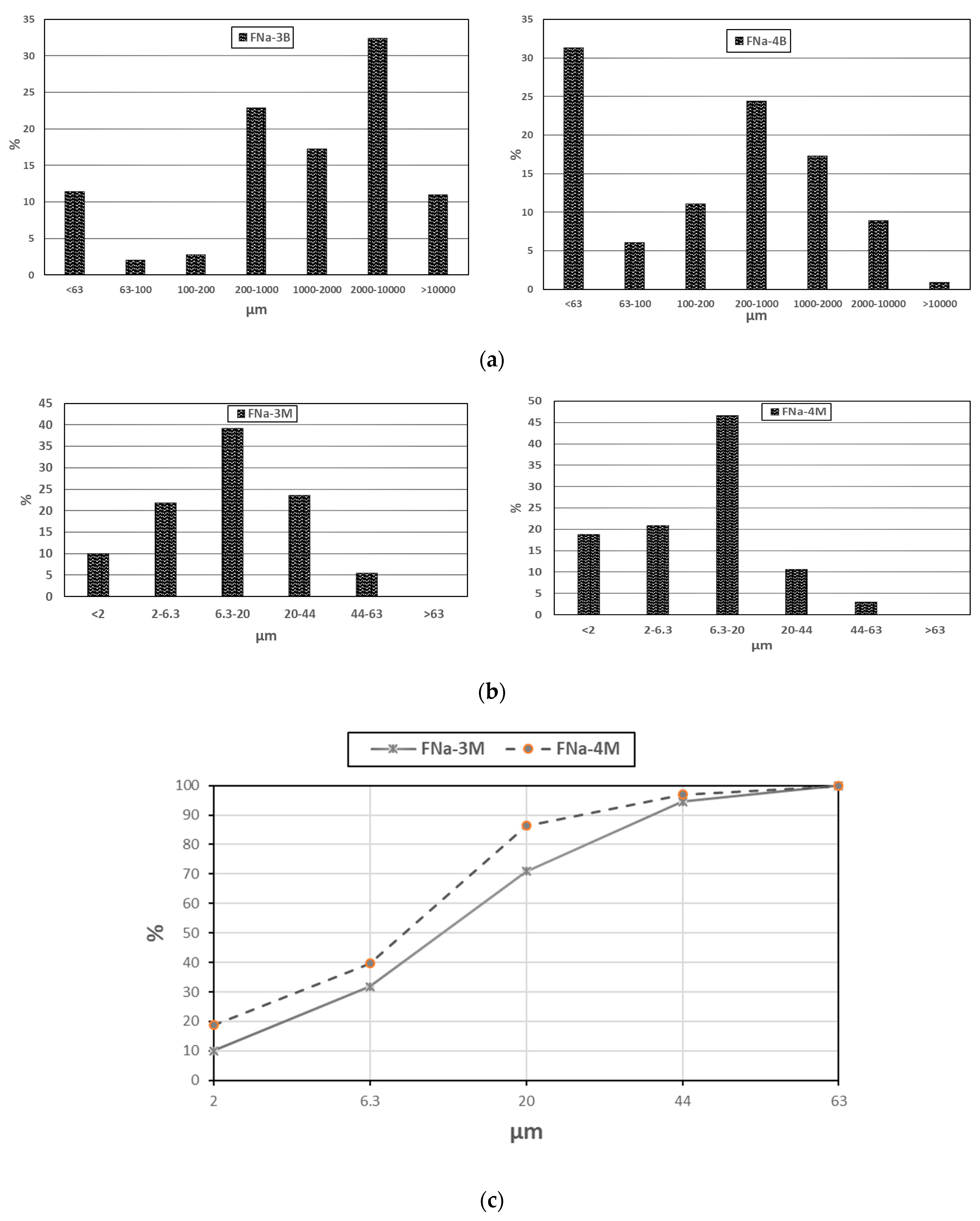
3.3. Particle Size Analysis of Samples by Wet Sieving
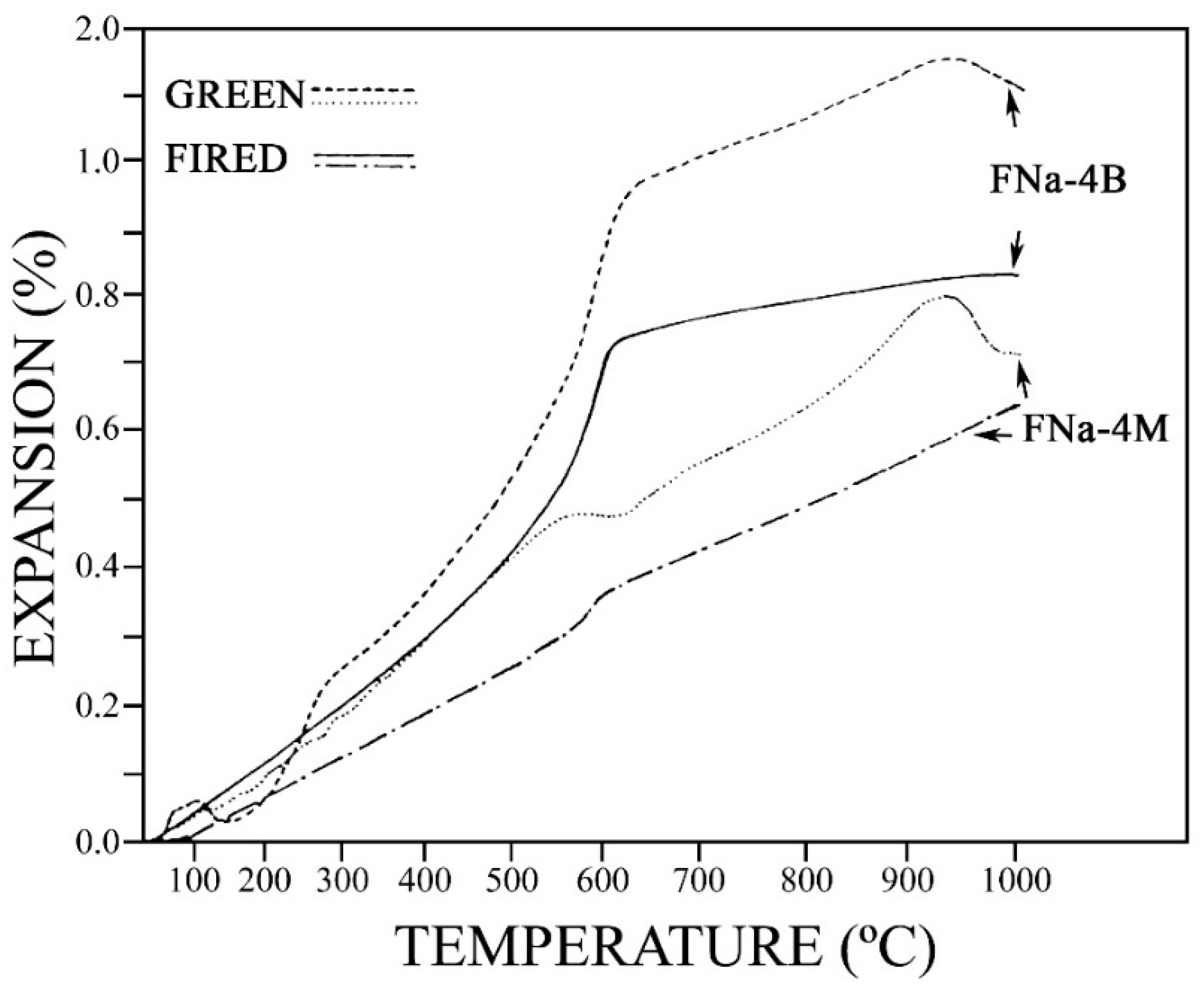
3.4. Thermal Analysis of Samples
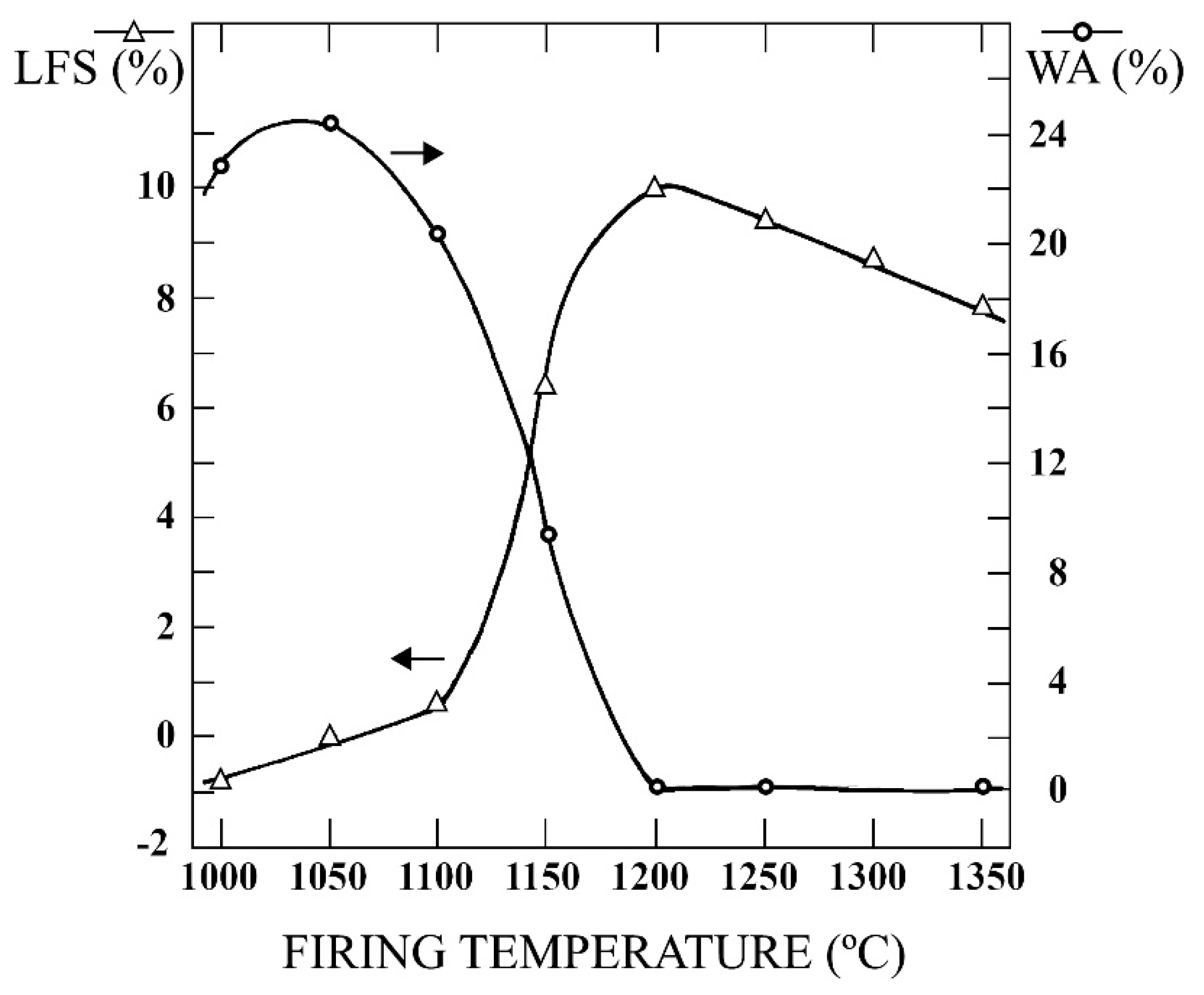
3.5. Physical Properties of Fired Samples
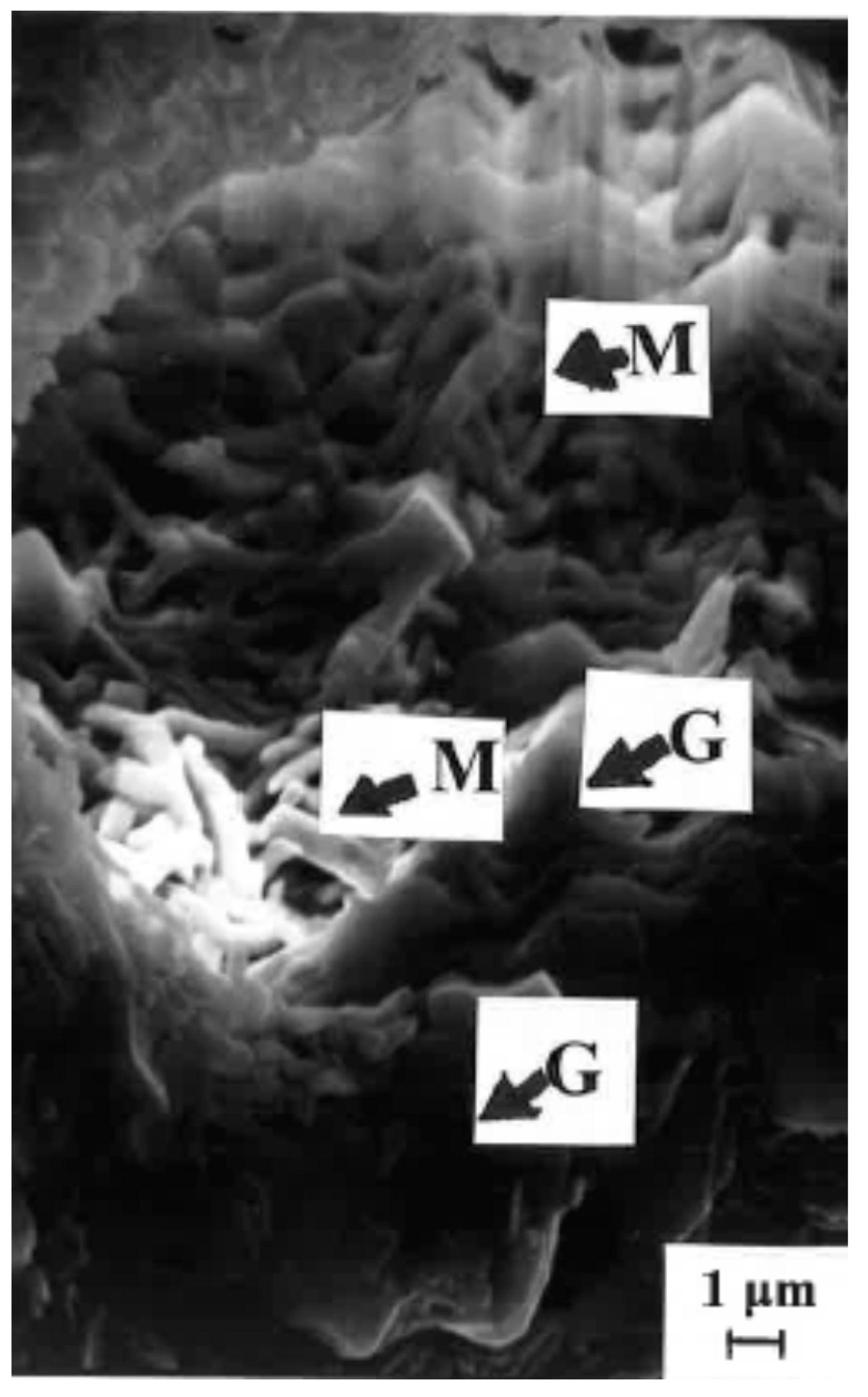
3.6. XRD and SEM of Fired FNa-4M Sample
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, R.F.; Pask, J.A. Diffusion and Reaction Studies in the System Al2O3-SiO2. J. Am. Ceram. Soc. 1972, 55, 525–531. [Google Scholar] [CrossRef]
- Aksay, I.A.; Pask, J.A. Stable and metastable equilibrium in the system SiO2-Al2O3. J. Am. Ceram. Soc. 1975, 58, 507–512. [Google Scholar] [CrossRef]
- Peretz, I.; Bradt, R.C. Linear thermal expansion coefficients of mullite-matrix aluminosilicate refractory bodies. J. Am. Ceram. Soc. 1983, 66, 823–829. [Google Scholar] [CrossRef]
- Aksay, I.A.; Dabbs, D.M.; Sarikaya, M. Mullite for structural, electronic and optical applications. J. Am. Ceram. Soc. 1991, 74, 2343–2354. [Google Scholar] [CrossRef]
- Pask, J.A.; Tomsia, A.P. Formation of mullite form sol-gel mixtures and kaolinite. J. Am. Ceram. Soc. 1991, 74, 2367–2373. [Google Scholar] [CrossRef]
- Schneider, H.; Okada, K.; Pask, J.A. Mullite and Mullite Ceramics; Wiley: Chichester, UK, 1994. [Google Scholar]
- Schneider, H.; Komarneni, S. Mullite; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2005. [Google Scholar]
- Schneider, H.; Schreuer, J.; Hildmann, B. Structure and properties of mullite-a review. J. Eur. Ceram. Soc. 2008, 28, 329–344. [Google Scholar] [CrossRef]
- Sacks, M.D.; Bozkurt, N.; Scheiffele, G.W. Fabrication of mullite and mullite-matrix composites by transient viscous sintering of composite powders. J. Am. Ceram. Soc. 1991, 74, 2428–2437. [Google Scholar] [CrossRef]
- Requena, J.; Bartolomé, J.F.; Moya, J.S.; De Aza, S.; Guitián, F.; Thomas, G. Mullite-aluminosilicate glassy matrix substrates obtained by reactive coating. J. Eur. Ceram. Soc. 1996, 16, 249–254. [Google Scholar] [CrossRef]
- Ebadzadeh, T. Formation of mullite from precursor powders: Sintering, microstructure and mechanical properties. Mater. Sci. Eng. A. 2003, 355, 56–61. [Google Scholar] [CrossRef]
- Ganesh, I.; Ferreira, J.M.F. Influence of raw material type and of the overall chemical composition of phase formation and sintered microstructure of mullite aggregates. Ceram. Int. 2009, 35, 2007–2015. [Google Scholar] [CrossRef]
- Sanz, J.; Madani, A.; Serratosa, J.M.; Moya, J.S.; De Aza, S. Aluminum-27 and Silicon-29 MAS NMR study of the kaolinite-mullite transformation. J. Am. Ceram. Soc. 1988, 70, 837–842. [Google Scholar]
- Sacks, M.D.; Lee, H.W.; Pask, J.A. A review of powder preparation methods and densification procedures for fabricating high density mullite. In Mullite and Mullite Matrix Composites; Davis, R.F., Pask, J.A., Somiya, S., Eds.; The American Ceramic Society: Westerville, OH, USA, 1990. [Google Scholar]
- Gualteri, A.; Bertolani, M. Mullite and cristobalite formation in fired products starting from halloysite clay. Appl. Clay Sci. 1992, 7, 251–262. [Google Scholar] [CrossRef]
- Carty, W.M.; Senapati, U. Porcelain-Raw Materials, Processing, Phase Evolution and Mechanical Behavior. J. Am. Ceram. Soc. 1998, 81, 3–20. [Google Scholar] [CrossRef]
- Sánchez-Soto, P.J.; Pérez-Rodríguez, J.L.; Sobrados, I.; Sanz, J. Influence of grinding in pyrophyllite-mullite thermal transformation assessed by 29Si and 27Al MAS-NMR spectroscopies. Chem. Mater. 1997, 9, 677–684. [Google Scholar] [CrossRef]
- Castelein, O.; Soulestin, B.; Bonnet, J.; Blanchart, P. The influence of heating rate on the thermal behavior and mullite formation from a kaolin raw material. Ceram. Int. 2001, 27, 517–522. [Google Scholar] [CrossRef]
- Liu, K.C.; Thomas, G.; Caballero, A.; Moya, J.S.; De Aza, S. Mullite formation in kaolinite-Al2O3. Acta Met. Mater. 1994, 42, 489–495. [Google Scholar] [CrossRef]
- Liu, K.C.; Thomas, G.; Caballero, A.; Moya, J.S.; De Aza, S. Time-temperature-transformation curves for kaolinite-α-alumina. J. Am. Ceram. Soc. 1994, 77, 1545–1552. [Google Scholar] [CrossRef]
- Wang, K.; Sacks, M.D. Mullite formation by endothermic reaction of α-alumina/silica microcomposite particles. J. Am. Ceram. Soc. 1996, 79, 12–16. [Google Scholar] [CrossRef]
- Yamuna, A.; Devanarayanan, S.; Latithambika, M. Phase-pure mullite from kaolinite. J. Am. Ceram. Soc. 2002, 85, 1409–1413. [Google Scholar] [CrossRef]
- Tripathi, H.S.; Mukherjee, B.; Das, S.K.; Ghosh, A.; Banerjee, G. Effect of sillimanite beach sand composition on mullitization and properties of Al2O3-SiO2 system. Bull. Mater. Sci. 2003, 26, 217–220. [Google Scholar] [CrossRef][Green Version]
- Panneerselvam, M.; Rao, K.J. Novel microwave method for the synthesis and sintering of mullite from kaolinite. Chem. Mater. 2003, 15, 2247–2252. [Google Scholar] [CrossRef]
- Chen, Y.F.; Wang, M.C.; Hon, M.H. Phase transformation and growth of mullite in kaolin ceramics. J. Eur. Ceram. Soc. 2004, 24, 2389–2397. [Google Scholar] [CrossRef]
- Okada, K. Activation energy of mullitization from various starting materials. J. Eur. Ceram. Soc. 2008, 28, 377–382. [Google Scholar] [CrossRef]
- González-Miranda, F.M.; Garzón, E.; Reca, J.; Pérez-Villarejo, L.; Martínez-Martínez, S.; Sánchez-Soto, P.J. Thermal behavior of sericite clays as precursors of mullite materials. J. Anal. Calorim. 2018, 132, 967–977. [Google Scholar] [CrossRef]
- Hammas, A.; Lecomte-Nana, G.; Daou, I.; Tessier-Doyen, N.; Peyratout, C.; Zibouche, F. Kaolinite-Magnesite or Kaolinite-Talc-Based Ceramics. Part II: Microstructure and the Final Properties Related Sintered Tapes. Minerals 2020, 10, 1080. [Google Scholar] [CrossRef]
- Xu, G.; Ma, Y.; Cui, H.; Ruan, G.; Zhang, Z.; Zhao, H. Preparation of porous mullite-corundum ceramics with controlled pore size using bioactive yeast as pore-forming agent. Mater. Lett. 2014, 116, 349–352. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.H.; Guan, W.M.; Fu, M.J.; Liu, L.J. Emulsion-templated high porosity mullite-corundum ceramics with sericite induced textured structures. Mater. Des. 2016, 89, 1041–1047. [Google Scholar] [CrossRef]
- Cividanes, L.S.; Campos, T.M.B.; Rodrigues, L.A.; Brunelli, D.D.; Thim, G.P. Review of mullite synthesis routes by sol-gel method. J. Sol Gel Sci. Technol. 2010, 55, 111–125. [Google Scholar] [CrossRef]
- Choo, T.F.; Salleh, M.A.M.; Kok, K.Y.; Matori, K.A. A Review on Synthesis of Mullite Ceramics from Industrial Wastes. Recycling 2019, 4, 39. [Google Scholar] [CrossRef]
- Khalil, N.M.; Algamal, Y. Recycling of ceramic wastes for the production of high performance mullite refractories. Silicon 2020, 12, 1557–1565. [Google Scholar] [CrossRef]
- Martins, I.M.; Sousa, J.B.; Catarino, L.; Vieira, M.T.; Oliveira, M. The formation of mullite from rock wastes containing alumina and silica. Key Eng. Mater. 2002, 230, 380–383. [Google Scholar] [CrossRef]
- Brasileiro, M.I.; Menezes, R.R.; Farias, M.O.; Lira, H.R.; Neves, G.A.; Santana, L.N.L. Use of kaolin processing waste for the production of mullite bodies. Mater. Sci. Forum 2008, 591–593, 799–804. [Google Scholar] [CrossRef]
- Menezes, R.R.; Farias, F.F.; Oliveira, M.F.; Santana, L.N.L.; Neves, G.A.; Lira, H.L.; Ferreira, H.C. Kaolin processing waste applied in the manufacturing of ceramic tiles and mullite bodies. Waste Manag. Res. 2009, 27, 78–86. [Google Scholar] [CrossRef]
- Alves, H.P.A.; Silva, J.B.; Campos, L.F.A.; Torres, S.M.; Dutra, R.P.S.; Macedo, D.A. Preparation of mullite based ceramics from clay-kaolin waste mixtures. Ceram. Int. 2016, 42, 19086–19090. [Google Scholar] [CrossRef]
- Choo, T.F.; Murshidi, J.A.; Saidin, N.U.; Paulus, W.; Abdullah, Y. Production of mullite ceramic bodies from kaolin processing waste and aluminum hydroxide. Mater. Sci. Forum 2017, 888, 81–85. [Google Scholar] [CrossRef]
- Cao, J.; Dong, X.; Li, L.; Dong, Y.; Hampshire, S. Recycling of waste fly ash for production of porous mullite ceramic membrane supports with increasing porosity. J. Eur. Ceram. Soc. 2014, 34, 3181–3194. [Google Scholar] [CrossRef]
- Choo, T.F.; Salleh, M.A.M.; Kok, K.Y.; Matori, K.A. Mineralogy and thermal expansion study of mullite-based ceramics synthesized from coal fly ash and aluminum dross industrial wastes. Ceram. Int. 2019, 45, 884–890. [Google Scholar]
- Pascual, J.; Zapatero, J.; Sánchez-Soto, P.J.; Jiménez, M.C.; Justo, A.; Pérez-Rodríguez, J.L. Procedimiento para la obtención de materiales cerámicos porosos compuestos de mullita en forma de fibras cortas monocristalinas. Spanish Patent P009801122 1998. [Google Scholar]
- Pascual, J.; Zapatero, J.; Jiménez de Haro, M.C.; Varona, I.; Justo, A.; Pérez-Rodríguez, J.L.; Sánchez-Soto, P.J. Porous mullite and mullite-based composites by chemical processing of kaolinite and aluminium metal wastes. J. Mater. Chem. 2000, 10, 1409–1414. [Google Scholar] [CrossRef]
- Pascual, J.; Zapatero, J.; Jiménez de Haro, M.C.; Ramírez del Valle, A.J.; Pérez-Rodríguez, J.L.; Sánchez-Soto, P.J. Preparation of mullite ceramics from coprecipitated aluminum hydroxide and kaolinite using hexamethylenediamine. J. Am. Ceram. Soc. 2000, 83, 2677–2680. [Google Scholar] [CrossRef]
- Ruiz-Conde, A.; Pascual, J.; Garzón, E.; Morales, L.; Raigón, M.; Sánchez-Soto, P.J. Processing of mullite and mullite-based ceramic composites from metal wastes and by-products of mining. In Proceedings of the 1st Spanish National Conference on Advances in Materials Recycling and Eco-Energy, Madrid, Spain, 12–13 November 2009; pp. 144–147. [Google Scholar]
- Chang, Y.K.; Ho, C.C.; Lee, K.C.; Yeap, E.B. Physicochemical characterization of tin tailings slurries. Appl. Clay Sci. 1994, 9, 83–96. [Google Scholar] [CrossRef]
- Raigón-Pichardo, M.; García-Ramos, G.; Sánchez-Soto, P.J. Characterization of a waste washing solid product of mining granitic tin-bearing sands and its application as ceramic raw material. Resour. Conserv. Recycl. 1996, 17, 109–124. [Google Scholar] [CrossRef]
- Sánchez-Soto, P.J.; Eliche-Quesada, D.; Martínez-Martínez, S.; Garzón, E.; Pérez-Villarejo, L.; Rincón, J.M. The effect of vitreous phase on mullite and mullite-based ceramic composites from kaolin wastes as by-products of mining, sericite clays and kaolinite. Mater. Lett. 2018, 223, 154–158. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock-Forming Minerals; Longman: Hong Kong, China, 1992. [Google Scholar]
- Dondi, M. Feldspathic fluxes for ceramics: Sources, production trends and technological value. Resour. Conserv. Recycl. 2018, 133, 191–205. [Google Scholar] [CrossRef]
- Das, S.K.; Dana, K. Differences in densification behaviour of K- and Na-feldspar-containing porcelain bodies. Acta 2003, 406, 199–206. [Google Scholar] [CrossRef]
- Martin-Marquez, J.; De la Torre, A.G.; Aranda, M.A.G.; Rincón, J.M.; Romero, M. Evolution with temperature of crystalline and amorphous phases in porcelain stoneware. J. Am. Ceram. Soc. 2009, 92, 229–234. [Google Scholar] [CrossRef]
- Bernasconi, A.; Diella, V.; Pagani, A.; Pavese, A.; Francescon, F.; Young, K.; Stuart, J.; Tunnicliffe, L. The role of firing temperature, firing time and quartz grain size on phase-formation, thermal dilatation and water absorption in sanitary-ware vitreous bodies. J. Eur. Ceram. Soc. 2011, 31, 1353–1360. [Google Scholar] [CrossRef]
- Feng, D.; Provis, J.L.; van Deventer, J.S.J. Thermal activation of albite for the synthesis of one-part mix geopolymers. J. Am. Ceram. Soc. 2012, 95, 565–572. [Google Scholar] [CrossRef]
- Bernasconi, A.; Marinoni, N.; Pavese, A.; Francescon, F.; Young, K. Feldspar and firing cycle effects on the evolution of sanitary-ware vitreous body. Ceram. Int. 2014, 40, 6389–6398. [Google Scholar] [CrossRef]
- Waldron, K.A.; Parsons, I. Feldspar microtextures and multistage thermal history of syenites from the Coldwell Complex, Ontario. Contrib. Miner. Pet. 1992, 111, 222–234. [Google Scholar] [CrossRef]
- Schoene, B.; Bowring, S.A. U-Pb systematics of the McClure Mountain syenite: Thermochronological constrains on the age of the 40Ar/39Ar standard MMhb. Contrib. Miner. Pet. 2006, 151, 615–630. [Google Scholar] [CrossRef]
- Poyato, J.; Pérez-Rodríguez, J.L.; García-Ramos, G.; González-García, F. Contribution to the knowledge of kaolin deposits of West Andalusia. In Proceedings of the 8th International Kaolin Symposium and Meeting on Alunite, Madrid, Roma, 7–16 September 1977; pp. 1–15. [Google Scholar]
- Schultz, L.G. Quantitative interpretation of mineralogical composition from X-ray and chemical data for the Pierre Shale. US Geol. Surv. Prof. Pap. 1964, 391C. [Google Scholar] [CrossRef]
- Biscaye, P.E. Mineralogy and sedimentation of recent deep-sea clay in the Atlantic ocean and adjacent sea and oceans. Geol. Soc. Am. Bull. 1965, 76, 803–831. [Google Scholar] [CrossRef]
- López, F.; Galán, E.; Martín, J.L. Sobre la mineralogía y génesis de dos yacimientos de caolín en la provincia de Valencia. Estud. Geol. 1971, 27, 145–152. [Google Scholar]
- Jordán, M.M.; Boix, A.; Sanfeliú, T.; De la Fuente, C. Firing transformations of Cretaceous clays used in the manufacturing of ceramic tiles. Appl. Clay Sci. 1999, 14, 225–234. [Google Scholar] [CrossRef]
- Dolinar, B.; Mišič, M.; Trauner, L. Correlation between surface area and Atterberg limits of fine-grained soils. Clays Clay Miner. 2007, 55, 519–523. [Google Scholar] [CrossRef]
- Carretero, M.I.; Pozo, M.; Legido, J.L.; Fernández-González, M.V.; Delgado, R.; Gómez, I.; Armijo, F.; Maraver, F. Assessment of three Spanish clays for their use in pelotherapy. Appl. Clay Sci. 2014, 99, 131–143. [Google Scholar] [CrossRef]
- Garzón, E.; Sánchez-Soto, P.J. An improved method for determining the external specific surface area and the plasticity of clayey samples based on a simplified method for non-swelling fine-grained soils. Appl. Clay Sci. 2015, 115, 97–107. [Google Scholar] [CrossRef]
- Galán, E.; Espinosa de los Monteros, J. El Caolín en España. Características, Identificación y Ensayos Cerámicos; SECV: Madrid, Spain, 1975. [Google Scholar]
- Ryan, W. Properties of Ceramic Raw Materials, 2nd ed.; Pergamon Press: North Staffordshire Polytechnic, UK, 1978. [Google Scholar]
- Parfitt, R.L.; Kimble, J.M. Conditions for formation of allophane in soils. Soil Sci. Am. J. 1989, 53, 971–977. [Google Scholar] [CrossRef]
- Farmer, V.C.; Russell, J.D. The structure and genesis of allophane and imogolite; their distribution in non-volcanic soils. In Soil Colloids and Their Association in Soil Aggregates; NATO Advanced Studies Workshop: Ghent, Belgium; Plenum: New York, NY, USA, 1985. [Google Scholar]
- Romero-González, P.; González, J.C.; Bustamante, A.; Ruiz-Conde, A.; Sánchez-Soto, P.J. Study in situ of the thermal transformation of limonite used as pigment coming from Perú. Bol. Soc. Esp. Ceram. Vidr. 2013, 52, 127–131. [Google Scholar]
- Norris, A.W.; Taylor, D.; Thorpe, I. Range curves: An experimental method for the study of vitreous pottery bodies. Br. Ceram. Trans. J. 1979, 78, 102–108. [Google Scholar]
- Osborn, E.F.; Muan, A. System SiO2-Al2O3-Na2O. In Phase Diagrams for Ceramists; Plate 501; The American Ceramic Society: Columbus, OH, USA, 1964; p. 181. [Google Scholar]
- Simakin, A.G.; Rincón, J.M. Structural thermodynamic model of the melt in the system albite (NaAlSi3O8)-quartz (SiO2) [Ab-Q]. Phys. Chem. Glasses 2002, 43, 184–188. [Google Scholar]
- Celik, H. Technological characterization and industrial application of two Turkish clays for the ceramic industry. Appl. Clay Sci. 2010, 50, 245–254. [Google Scholar] [CrossRef]
- Bernardo, E.; Doyle, J.; Hampshire, S. Sintered feldspar glass-ceramics and glass-ceramic matrix composites. Ceram. Int. 2008, 34, 2037–2042. [Google Scholar] [CrossRef]

| wt. % | FNa-3B | FNa-3M | FNa-4B | FNa-4M |
|---|---|---|---|---|
| SiO2 | 72.30 | 68.11 | 72.51 | 62.02 |
| Al2O3 | 18.71 | 22.62 | 18.73 | 25.12 |
| Fe2O3 | 0.75 | 0.72 | 0.50 | 0.37 |
| TiO2 | 0.19 | 0.48 | 0.12 | 0.34 |
| CaO | 0.36 | 0.29 | 0.28 | 1.74 |
| MgO | 0.07 | 0.11 | 0.05 | 0.08 |
| Na2O | 5.44 | 3.09 | 4.58 | 4.84 |
| K2O | 0.68 | 0.74 | 0.72 | 0.63 |
| LOI | 1.08 | 3.40 | 2.24 | 4.45 |
| Total | 99.58 | 99.56 | 99.73 | 99.59 |
| Kaolinite | ~8 | ~25 | ~16 | ~32 |
| T (°C) | WL (wt. %) | LFS (%) | WA (%) | BD (g/cm3) | AP (%) |
|---|---|---|---|---|---|
| 1000 | 4.20 | −0.80 | 22.80 | 1.62 | 36.93 |
| 1050 | 4.35 | 0.01 | 24.23 | 1.60 | 38.76 |
| 1100 | 4.40 | 0.60 | 20.34 | 1.70 | 34.58 |
| 1150 | 4.32 | 6.42 | 9.34 | 2.04 | 19.05 |
| 1200 | 4.28 | 10.01 | 0.07 | 2.38 | 0.17 |
| 1250 | 4.30 | 9.41 | 0.03 | 2.29 | 0.07 |
| 1300 | 4.25 | 8.73 | 0.07 | 2.18 | 0.15 |
| 1350 | 4.42 | 7.80 | 0.12 | 2.16 | 0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Soto, P.J.; Garzón, E.; Pérez-Villarejo, L.; Angelopoulos, G.N.; Eliche-Quesada, D. Mining Wastes of an Albite Deposit as Raw Materials for Vitrified Mullite Ceramics. Minerals 2021, 11, 232. https://doi.org/10.3390/min11030232
Sánchez-Soto PJ, Garzón E, Pérez-Villarejo L, Angelopoulos GN, Eliche-Quesada D. Mining Wastes of an Albite Deposit as Raw Materials for Vitrified Mullite Ceramics. Minerals. 2021; 11(3):232. https://doi.org/10.3390/min11030232
Chicago/Turabian StyleSánchez-Soto, Pedro J., Eduardo Garzón, Luis Pérez-Villarejo, George N. Angelopoulos, and Dolores Eliche-Quesada. 2021. "Mining Wastes of an Albite Deposit as Raw Materials for Vitrified Mullite Ceramics" Minerals 11, no. 3: 232. https://doi.org/10.3390/min11030232
APA StyleSánchez-Soto, P. J., Garzón, E., Pérez-Villarejo, L., Angelopoulos, G. N., & Eliche-Quesada, D. (2021). Mining Wastes of an Albite Deposit as Raw Materials for Vitrified Mullite Ceramics. Minerals, 11(3), 232. https://doi.org/10.3390/min11030232










