Novel Approach for Fine Ilmenite Flotation Using Hydrophobized Glass Bubbles as the Buoyant Carrier
Abstract
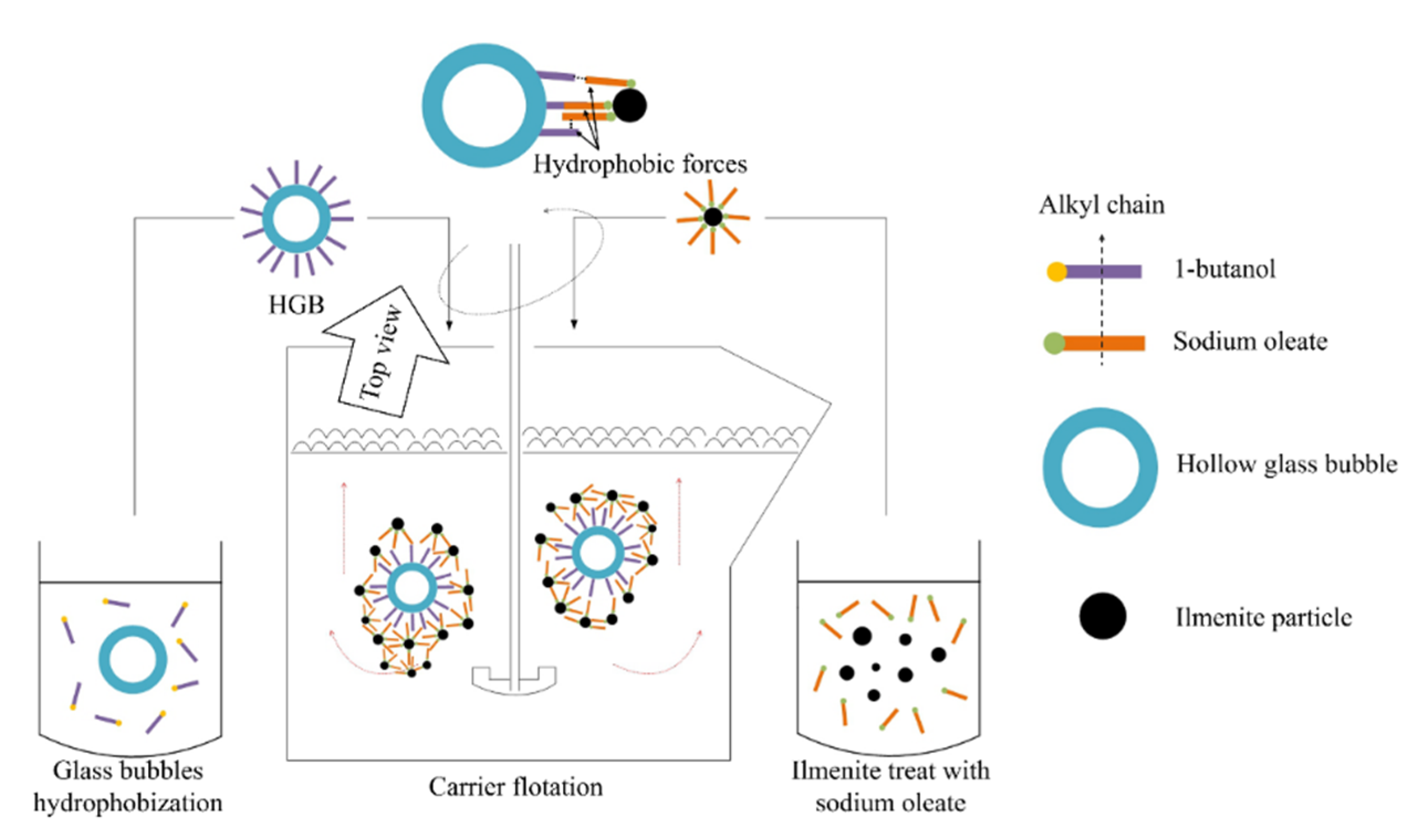
:1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Materials Characterization
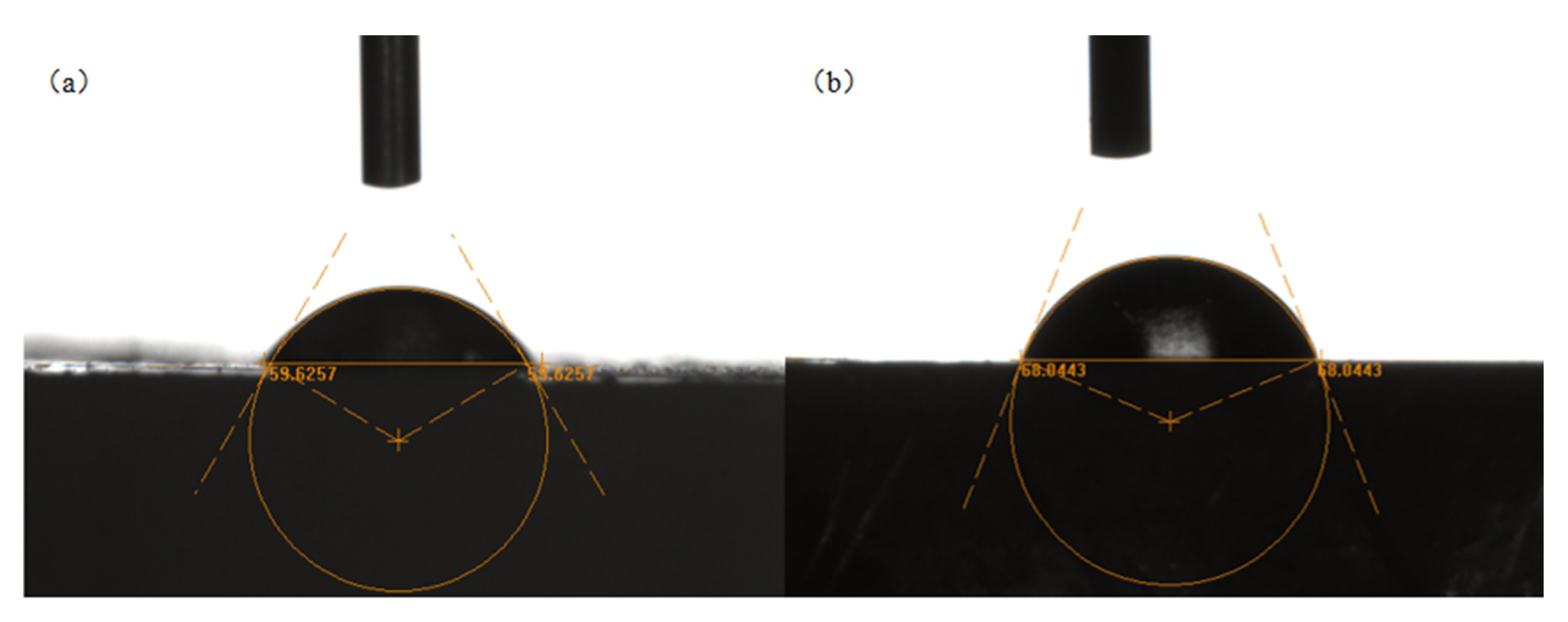
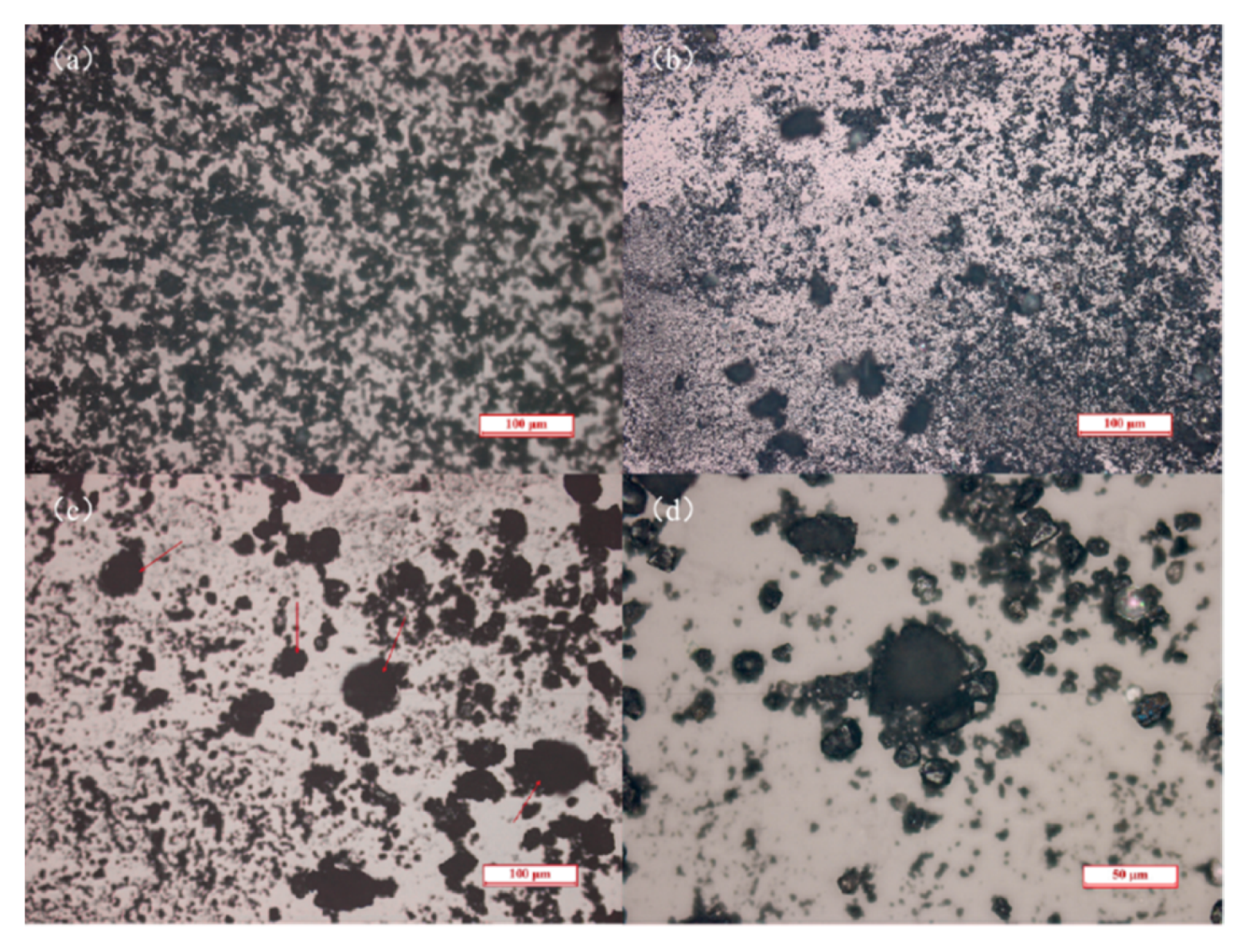
2.3. Optical Microscope Observation
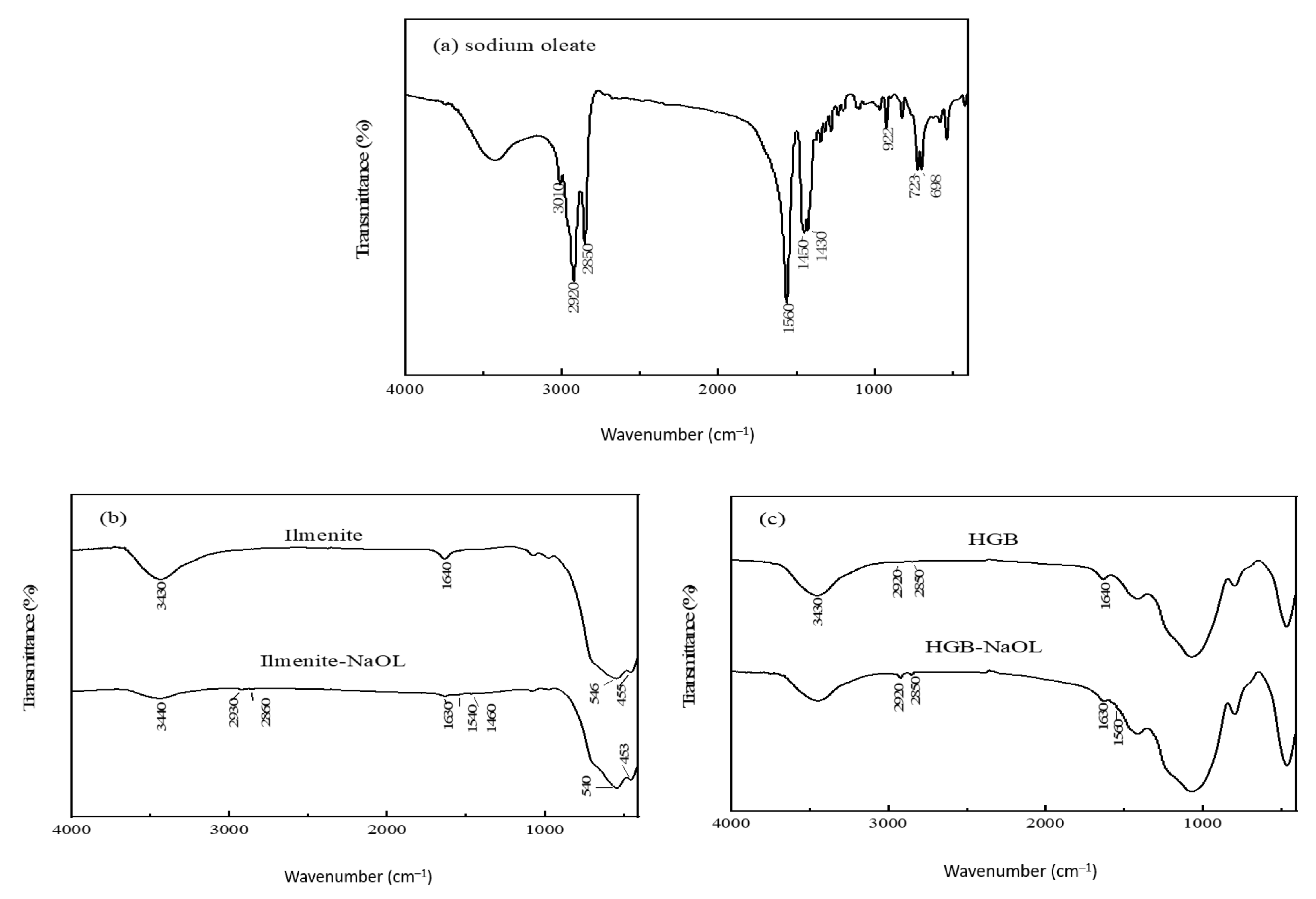
2.4. FTIR Measurements
2.5. Glass Bubbles Hydrophobization
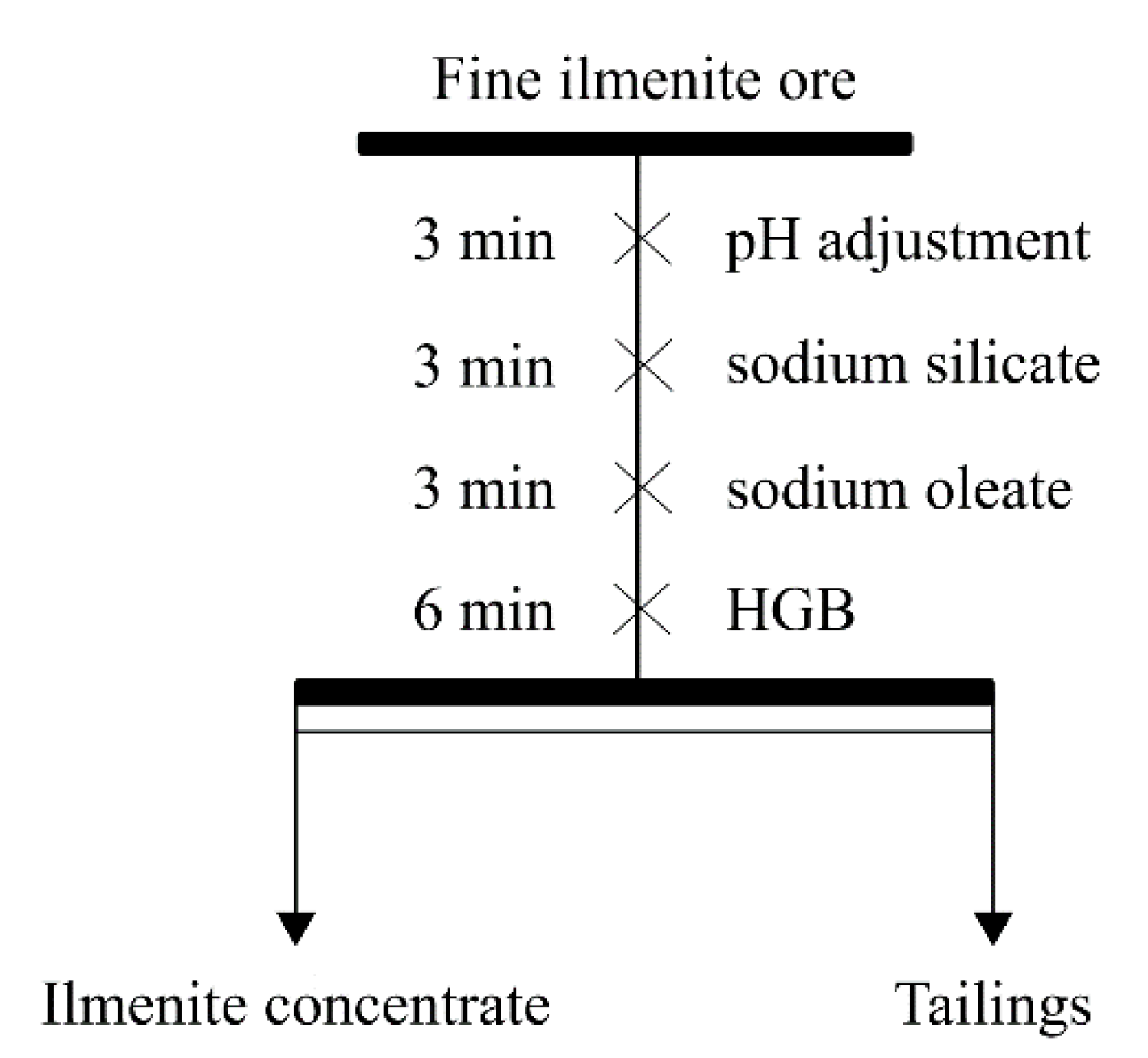
2.6. Micro-Flotation Experiment
3. Results and Discussion
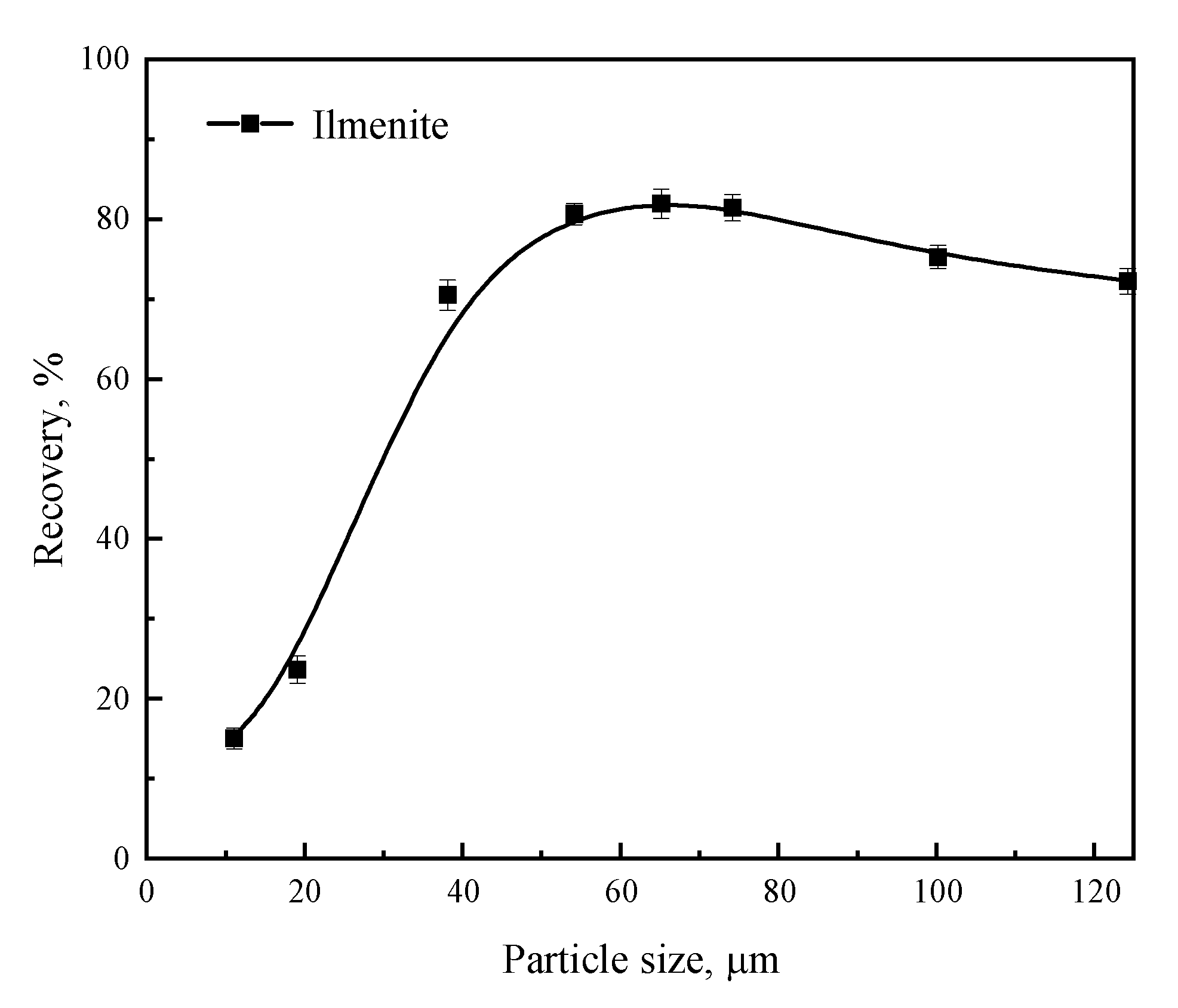
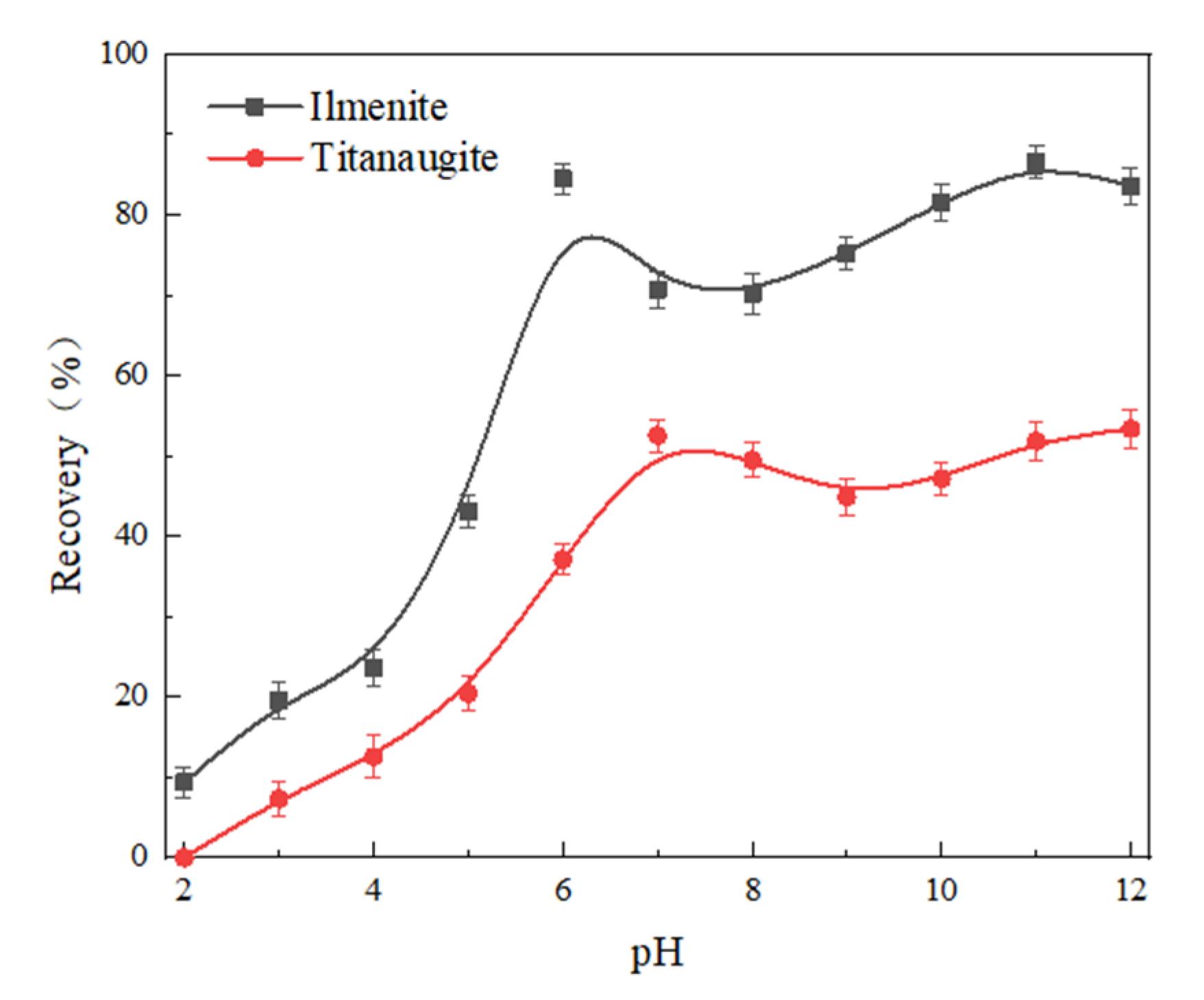
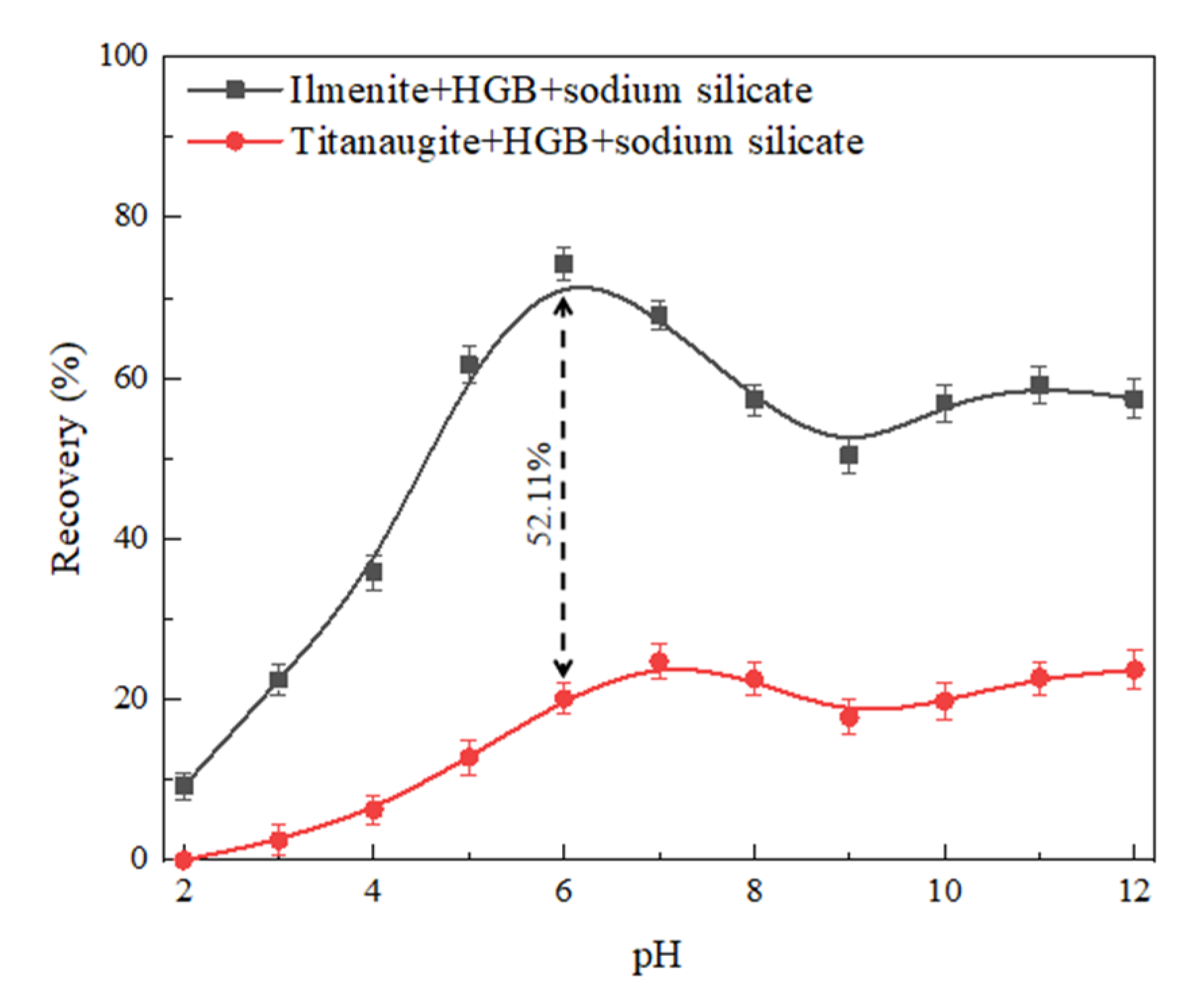
3.1. Micro-Flotation
3.2. FTIR Analysis
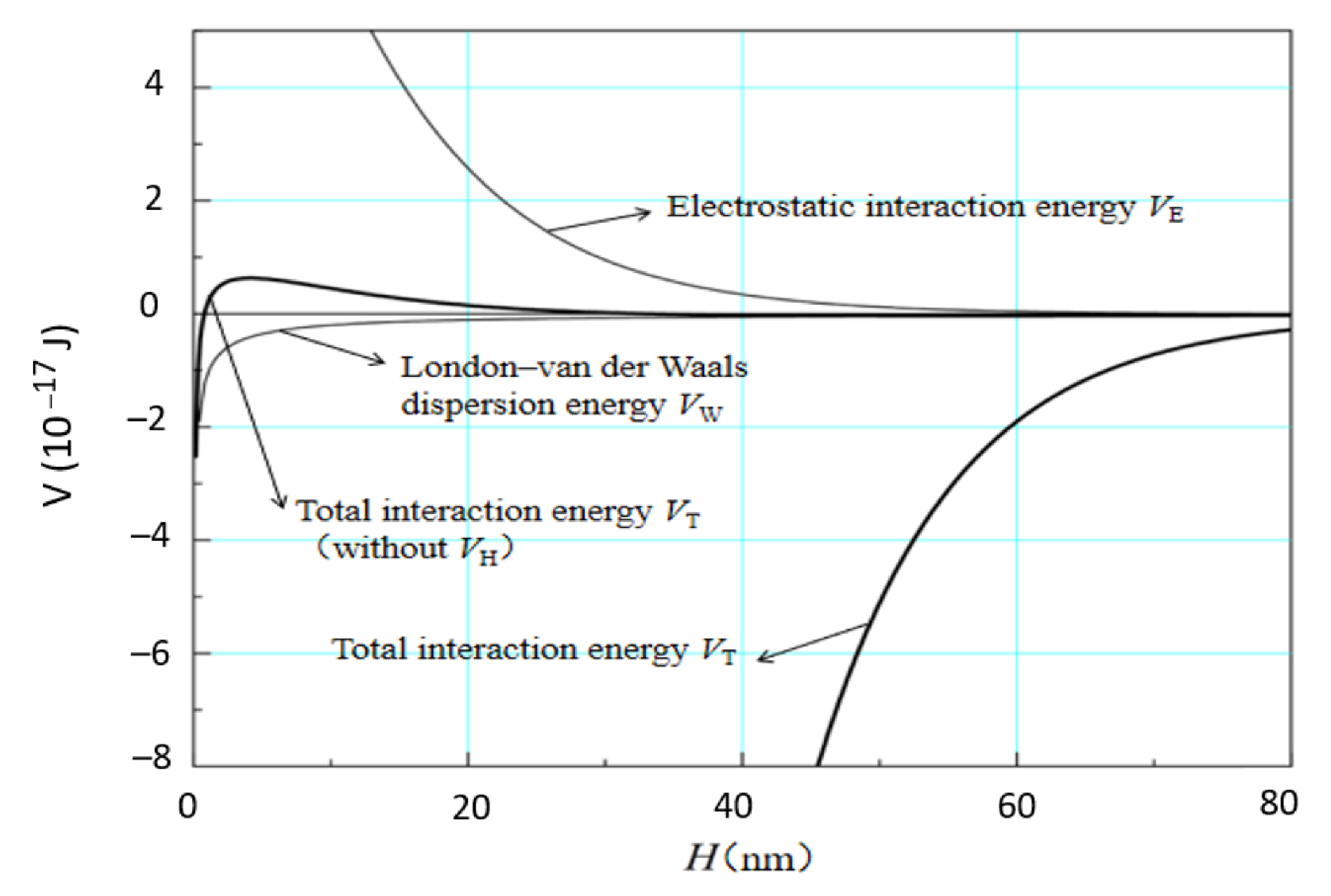
3.3. Estimation of Interaction Energies by Extended DLVO Theory
3.4. Optical Microscope Observation of Ilmenite
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bulatovic, S.; Wyslouzil, D.M. Process development for treatment of complex perovskite, ilmenite and rutile ores. Miner. Eng. 1999, 12, 1407–1417. [Google Scholar] [CrossRef]
- Chen, P.; Zhai, J.; Sun, W.; Hu, Y.; Yin, Z.; Lai, X. Adsorption mechanism of lead ions at ilmenite/water interface and its influence on ilmenite flotability. J. Ind. Eng. Chem. 2017, 53, 285–293. [Google Scholar] [CrossRef]
- Zhou, M.-F.; Chen, W.T.; Wang, C.Y.; Prevec, S.A.; Liu, P.P.; Howarth, G.H. Two stages of immiscible liquid separation in the formation of Panzhihua-type Fe-Ti-V oxide deposits, SW China. Geosci. Front. 2013, 4, 481–502. [Google Scholar] [CrossRef]
- Shellnutt, J.G.; Jahn, B.M. Formation of the Late Permian Panzhihua plutonic-hypabyssal-volcanic igneous complex: Implications for the genesis of Fe-Ti oxide deposits and A-type granites of SW China. Earth Planet. Sci. Lett. 2010, 289, 509–519. [Google Scholar] [CrossRef]
- Shahbazi, B.; Rezai, B.; Javad Koleini, S.M. Bubble–particle collision and attachment probability on fine particles flotation. Chem. Eng. Process. Process Intensif. 2010, 49, 622–627. [Google Scholar] [CrossRef]
- Ge, L.; Evans, G.M.; Moreno-Atanasio, R. CFD-DEM investigation of the interaction between a particle swarm and a stationary bubble: Particle-bubble collision efficiency. Powder Technol. 2020, 366, 641–652. [Google Scholar] [CrossRef]
- Chen, S.; Chen, X.; Wan, D.; Yi, X.; Sun, X.; Ji, L.; Wang, G. A lattice Boltzmann study of the collisions in a particle-bubble system under turbulent flows. Powder Technol. 2020, 361, 759–768. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Hassas, B.V.; Kouachi, S.; Brabcova, Z.; Çelik, M.S. Effect of bubble size and velocity on collision efficiency in chalcopyrite flotation. Colloids Surf. A Physicochem. Eng. Asp. 2016, 498, 258–267. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Ralston, J.; Schulze, H.J. On modelling of bubble–particle attachment probability in flotation. Int. J. Miner. Process. 1998, 53, 225–249. [Google Scholar] [CrossRef]
- Ireland, P.M.; Jameson, G.J. Collision of a rising bubble–particle aggregate with a gas–liquid interface. Int. J. Miner. Process. 2014, 130, 1–7. [Google Scholar] [CrossRef]
- Wang, H.; Yang, W.; Yan, X.; Wang, L.; Wang, Y.; Zhang, H. Regulation of bubble size in flotation: A review. J. Environ. Chem. Eng. 2020, 8, 104070. [Google Scholar] [CrossRef]
- Farrokhpay, S.; Filippova, I.; Filippov, L.; Picarra, A.; Rulyov, N.; Fornasiero, D. Flotation of fine particles in the presence of combined microbubbles and conventional bubbles. Miner. Eng. 2020, 155, 106439. [Google Scholar] [CrossRef]
- Jameson, G.J. Hydrophobicity and floc density in induced-air flotation for water treatment. Colloids Surf. A Physicochem. Eng. Asp. 1999, 151, 269–281. [Google Scholar] [CrossRef]
- Shamlooh, M.; Rimeh, A.; Nasser, M.S.; Al-Ghouti, M.A.; El-Naas, M.H.; Qiblawey, H. Enhancement of flocculation and shear resistivity of bentonite suspension using a hybrid system of organic coagulants and anionic polyelectrolytes. Sep. Purif. Technol. 2020, 237, 116462. [Google Scholar] [CrossRef]
- Kostoglou, M.; Karapantsios, T.D.; Evgenidis, S. On a generalized framework for turbulent collision frequency models in flotation: The road from past inconsistencies to a concise algebraic expression for fine particles. Adv. Colloid Interface Sci. 2020, 284, 102270. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Nguyen, A.V. Effect of microturbulence on bubble-particle collision during the bubble rise in a flotation cell. Miner. Eng. 2020, 155, 106418. [Google Scholar] [CrossRef]
- Loganathan, S.; Sankaran, S. Surface chemical and selective flocculation studies on iron oxide and silica suspensions in the presence of xanthan gum. Miner. Eng. 2021, 160, 106668. [Google Scholar] [CrossRef]
- Roth, C.M.; Unger, K.K.; Lenhoff, A.M. Mechanistic model of retention in protein ion-exchange chromatography. J. Chromatogr. A 1996, 726, 45–56. [Google Scholar] [CrossRef]
- Sadowski, Z.; Polowczyk, I. Agglomerate flotation of fine oxide particles. Int. J. Miner. Process. 2004, 74, 85–90. [Google Scholar] [CrossRef]
- Zou, W.; Gong, L.; Huang, J.; Zhang, Z.; Sun, C.; Zeng, H. Adsorption of hydrophobically modified polyacrylamide P(AM-NaAA-C16DMAAC) on model coal and clay surfaces and the effect on selective flocculation of fine coal. Miner. Eng. 2019, 142, 105887. [Google Scholar] [CrossRef]
- Bu, X.; Wang, X.; Zhou, S.; Li, B.; Zhan, H.; Xie, G. Discrimination of Six Flotation Kinetic Models Used in the Conventional Flotation and Carrier Flotation of-74 mu m Coal Fines. ACS Omega 2020, 5, 13813–13821. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Meng, Q.; Yuan, Z.; Zhang, Y.; Li, L. Effect of sodium silicate on the magnetic separation of ilmenite from titanaugite by magnetite selective coating. Powder Technol. 2019, 344, 233–241. [Google Scholar] [CrossRef]
- Ateşok, G.; Boylu, F.; Çelĭk, M.S. Carrier flotation for desulfurization and deashing of difficult-to-float coals. Miner. Eng. 2001, 14, 661–670. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, X.; Xi, H.; Sun, J.; Liang, X.; Wei, J.; Xiao, X.; Liu, Z.; Li, S.; Liang, Z.; et al. Interpretation of adhesion behaviors between bacteria and modified basalt fiber by surface thermodynamics and extended DLVO theory. Colloids Surf. B Biointerfaces 2019, 177, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Liu, W.; Jin, L.; Li, Y.; Li, Z.; Liu, G.; Huang, D.; Wu, Z.; Yin, H. Recovery of trace Cu2+ using a process of nano-adsorption coupled with flotation: SNP as an adsorbing carrier. Sep. Purif. Technol. 2017, 184, 257–263. [Google Scholar] [CrossRef]
- Rubio, J.; Hoberg, H. The process of separation of fine mineral particles by flotation with hydrophobic polymeric carrier. Int. J. Miner. Process. 1993, 37, 109–122. [Google Scholar] [CrossRef]
- Arriagada, S.; Acuña, C.; Vera, M. New technology to improve the recovery of fine particles in froth flotation based on using hydrophobized glass bubbles. Miner. Eng. 2020, 156, 106364. [Google Scholar] [CrossRef]
- Hunter, T.N.; Wanless, E.J.; Jameson, G.J. Effect of esterically bonded agents on the monolayer structure and foamability of nano-silica. Colloids Surf. A Physicochem. Eng. Asp. 2009, 334, 181–190. [Google Scholar] [CrossRef]
- Utsugi, H.; Horikoshi, H.; Matsuzawa, T. Mechanism of esterification of alcohols with surface silanols and hydrolysis of surface esters on silica gels. J. Colloid Interface Sci. 1975, 50, 154–161. [Google Scholar] [CrossRef]
- Salmani Nuri, O.; Irannajad, M.; Mehdilo, A. Reagent adsorption on modified mineral surfaces: Isotherm, kinetic and thermodynamic aspects. J. Mol. Liq. 2019, 291, 111311. [Google Scholar] [CrossRef]
- Du, Y.; Meng, Q.; Yuan, Z.; Ma, L.; Xu, Y. Study on the flotation behavior and mechanism of ilmenite and titanaugite with sodium oleate. Miner. Eng. 2020, 152, 106366. [Google Scholar] [CrossRef]
- Meng, Q.; Yuan, Z.; Yu, L.; Xu, Y.; Du, Y.; Zhang, C. Selective depression of titanaugite in the ilmenite flotation with carboxymethyl starch. Appl. Surf. Sci. 2018, 440, 955–962. [Google Scholar] [CrossRef]
- Xiao, W.; Ren, Y.-x.; Yang, J.; Cao, P.; Wang, J.; Qin, W.-q.; Qiu, G.-z. Adsorption mechanism of sodium oleate and styryl phosphonic acid on rutile and amphibole surfaces. Trans. Nonferrous Met. Soc. China 2019, 29, 1939–1947. [Google Scholar] [CrossRef]
- Chen, P.; Lu, X.; Chai, X.; Mulenga, H.; Gao, J.; Liu, H.; Meng, Q.; Sun, W.; Gao, Y. Influence of Fe–BHA complexes on the flotation behavior of ilmenite. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 125964. [Google Scholar] [CrossRef]
- Chen, P.; Zhai, J.; Sun, W.; Hu, Y.; Yin, Z. The activation mechanism of lead ions in the flotation of ilmenite using sodium oleate as a collector. Miner. Eng. 2017, 111, 100–107. [Google Scholar] [CrossRef]
- Fan, G.; Zhang, C.; Wang, T.; Deng, J.; Cao, Y.; Chang, L. New insight into surface adsorption thermodynamic, kinetic properties and adsorption mechanisms of sodium oleate on ilmenite and titanaugite. Adv. Powder Technol. 2020, 31, 3628–3639. [Google Scholar] [CrossRef]
- Van Oss, C.J. Chapter Three—The Extended DLVO Theory. Interface Sci. Technol. 2008, 16, 31–48. [Google Scholar]
- Adamczyk, Z.; Weroński, P. Application of the DLVO theory for particle deposition problems. Adv. Colloid Interface Sci. 1999, 83, 137–226. [Google Scholar] [CrossRef]
- Piñeres, J.; Barraza, J. Energy barrier of aggregates coal particle–bubble through the extended DLVO theory. Int. J. Miner. Process. 2011, 100, 14–20. [Google Scholar] [CrossRef]
- Mei, W.; Li, X.; Cao, Y. Chemical Formulas Manual; Transl. Scienmce Press: Beijing, China, 1987. [Google Scholar]
- Oss, C.J.V.; Giese, R.F.; Costanzo, P.M. DLVO and non-DLVO interactions in hectorite. Clays Clay Miner. 1990, 38, 151–159. [Google Scholar]
- Farahat, M.; Hirajima, T.; Sasaki, K.; Doi, K. Adhesion of Escherichia coli onto quartz, hematite and corundum: Extended DLVO theory and flotation behavior. Colloids Surf. B Biointerfaces 2009, 74, 140–149. [Google Scholar] [CrossRef] [PubMed]



| Sample | TiO2 | Fe2O3 | SiO2 | CaO | MgO | Al2O3 | Others |
|---|---|---|---|---|---|---|---|
| Ilmenite | 48.51 | 35.39 | 4.67 | 1.35 | 4.84 | 1.61 | 3.63 |
| Titanaugite | 3.50 | 14.38 | 15.60 | 40.34 | 10.56 | 6.72 | 8.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.; Chen, Y.; Liu, H.; Li, H.; Chai, X.; Lu, X.; Sun, W.; Wang, H.; Luo, Y.; Wang, X. Novel Approach for Fine Ilmenite Flotation Using Hydrophobized Glass Bubbles as the Buoyant Carrier. Minerals 2021, 11, 231. https://doi.org/10.3390/min11030231
Chen P, Chen Y, Liu H, Li H, Chai X, Lu X, Sun W, Wang H, Luo Y, Wang X. Novel Approach for Fine Ilmenite Flotation Using Hydrophobized Glass Bubbles as the Buoyant Carrier. Minerals. 2021; 11(3):231. https://doi.org/10.3390/min11030231
Chicago/Turabian StyleChen, Pan, Youchuan Chen, Hang Liu, Haoyu Li, Xujian Chai, Xiaolong Lu, Wei Sun, Hongbin Wang, Yangyong Luo, and Xianyun Wang. 2021. "Novel Approach for Fine Ilmenite Flotation Using Hydrophobized Glass Bubbles as the Buoyant Carrier" Minerals 11, no. 3: 231. https://doi.org/10.3390/min11030231
APA StyleChen, P., Chen, Y., Liu, H., Li, H., Chai, X., Lu, X., Sun, W., Wang, H., Luo, Y., & Wang, X. (2021). Novel Approach for Fine Ilmenite Flotation Using Hydrophobized Glass Bubbles as the Buoyant Carrier. Minerals, 11(3), 231. https://doi.org/10.3390/min11030231







