Enriched Co-Treatment of Pharmaceutical and Acidic Metal-Containing Wastewater with Nano Zero-Valent Iron
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Initial Experiments
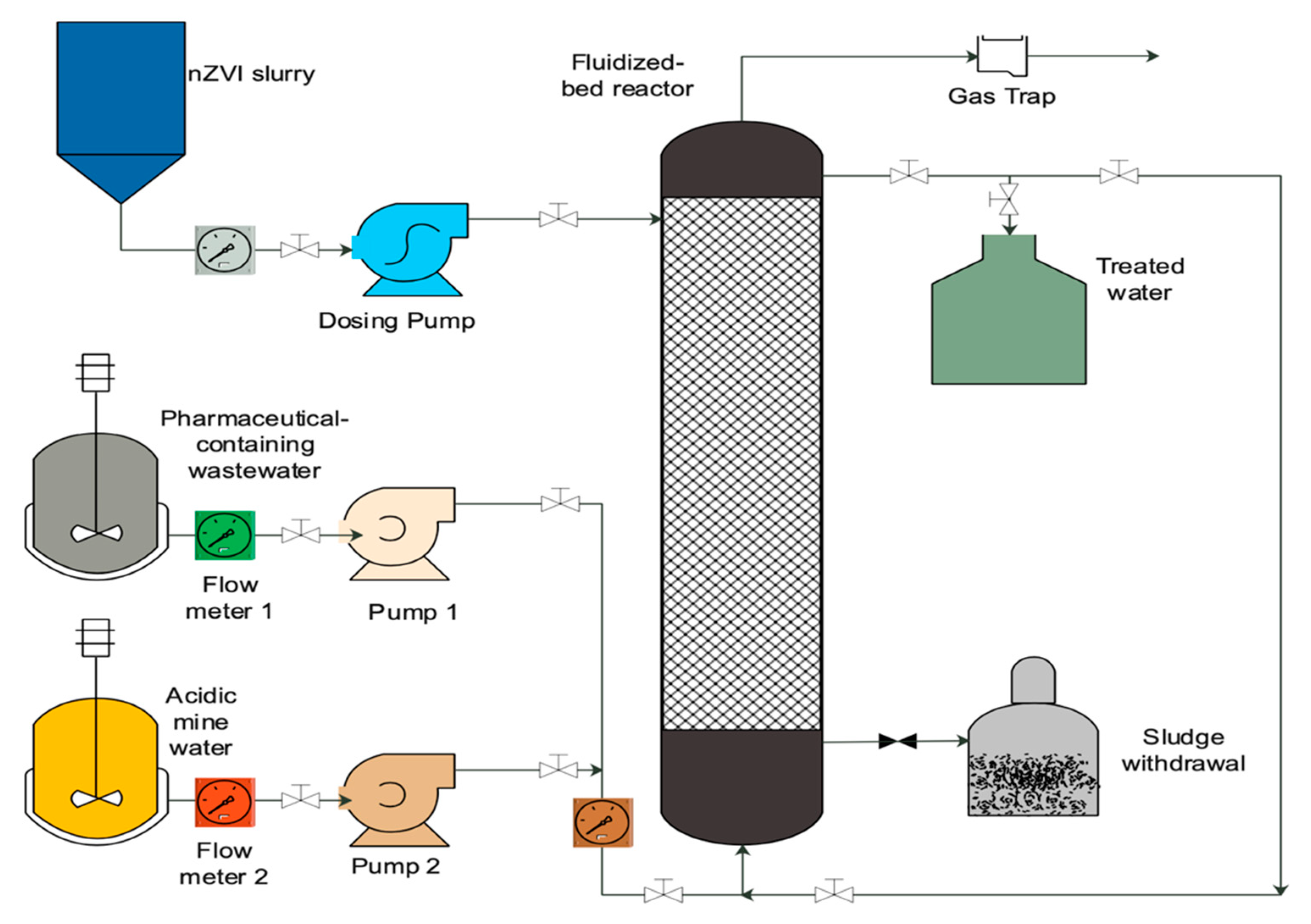
2.2. Fluidized-Bed Reactors
2.3. Analytical Procedures
2.4. Pharmaceutical Analysis Procedure
2.5. Sludge Characterization
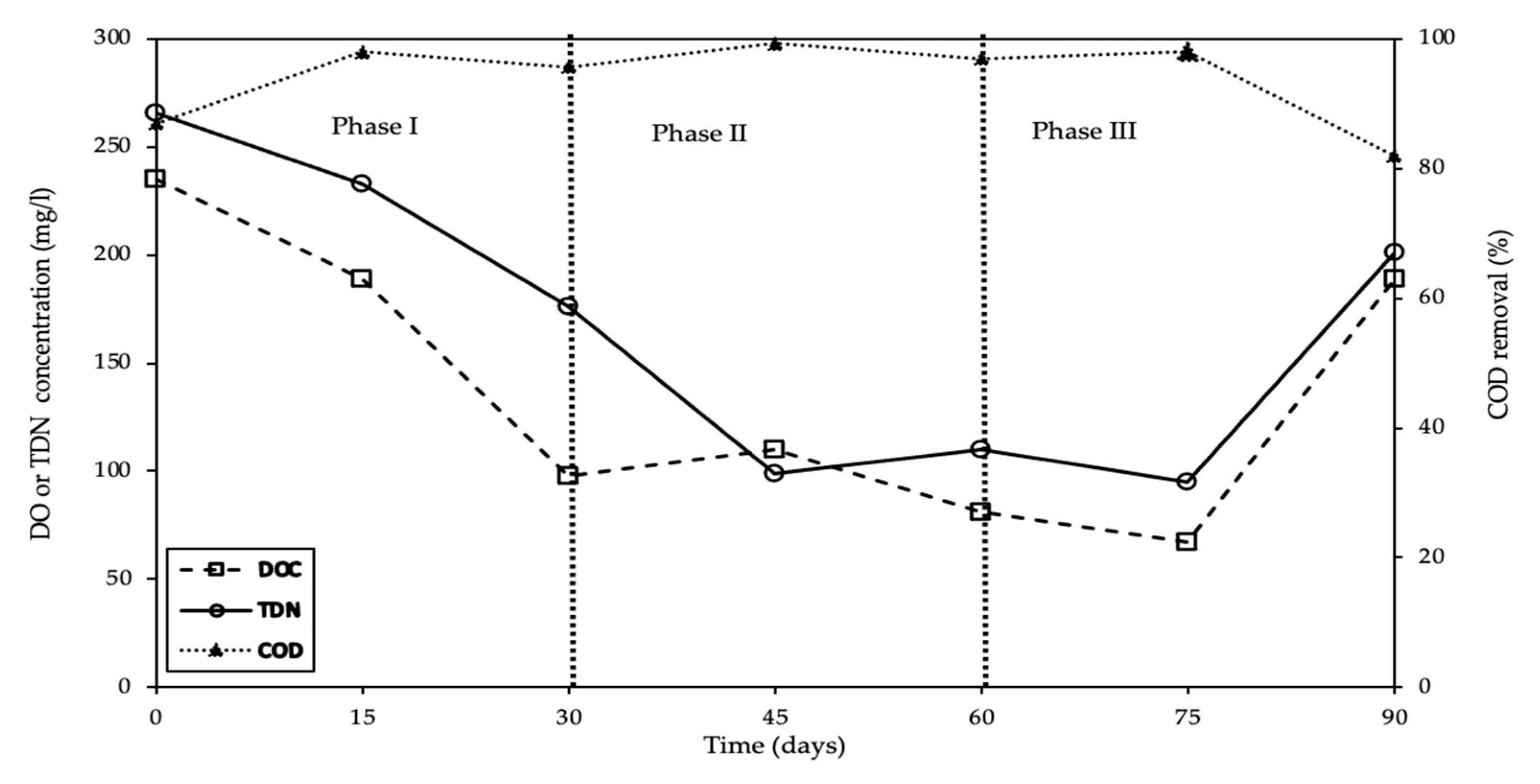
3. Results and Discussion
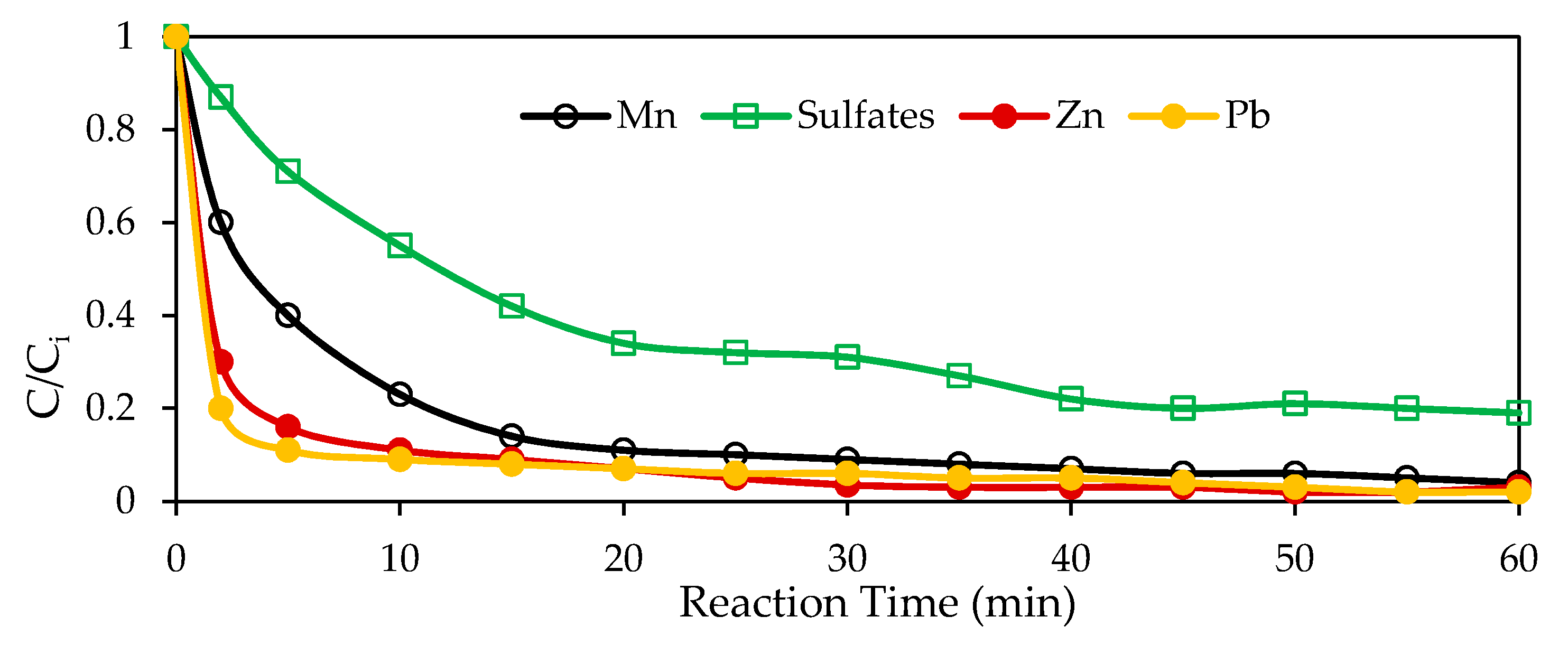
3.1. Batch Experiments
3.2. Reaction Mechanism
3.3. Influence of pH and the Source of Carbon
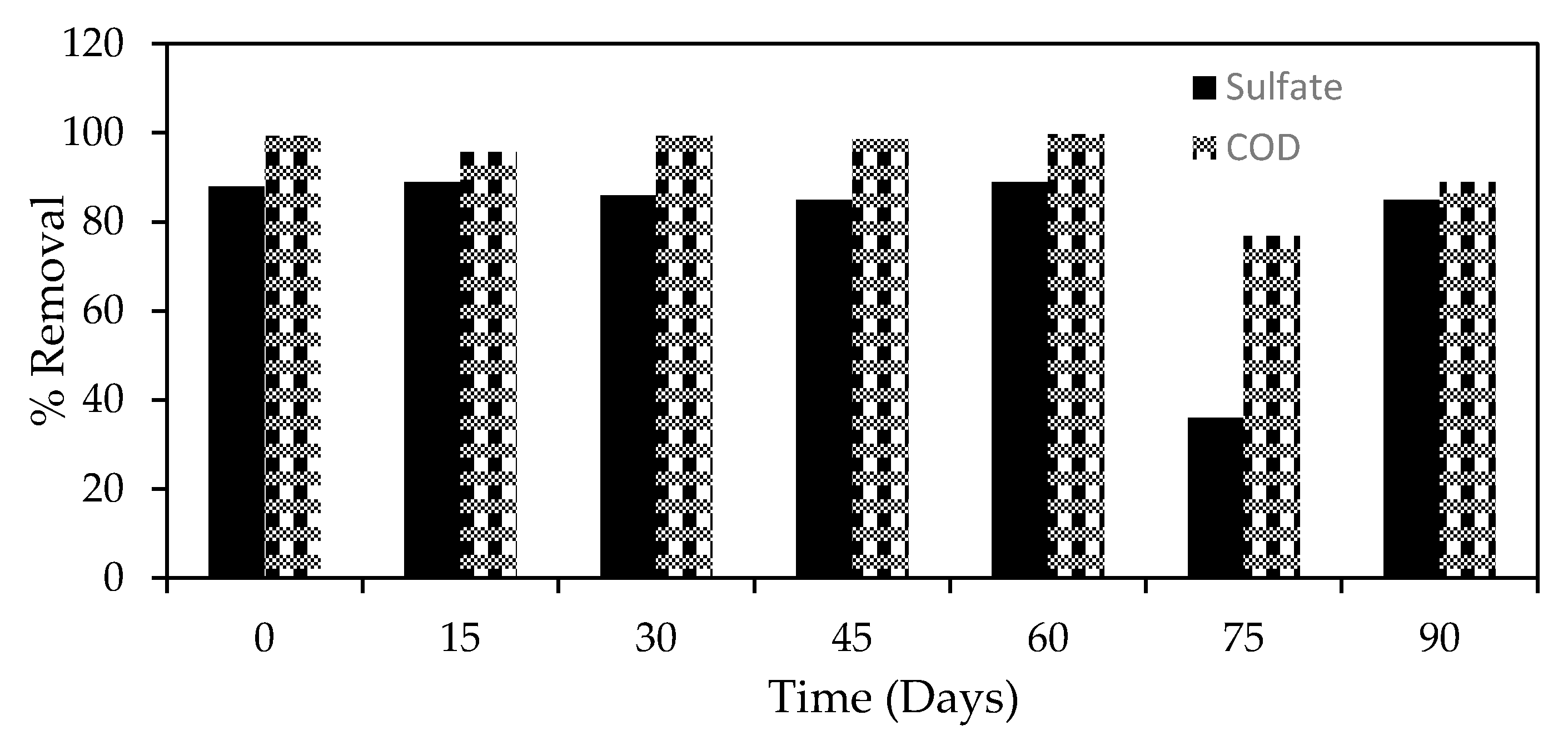
3.4. Effects of COD and Sulfates
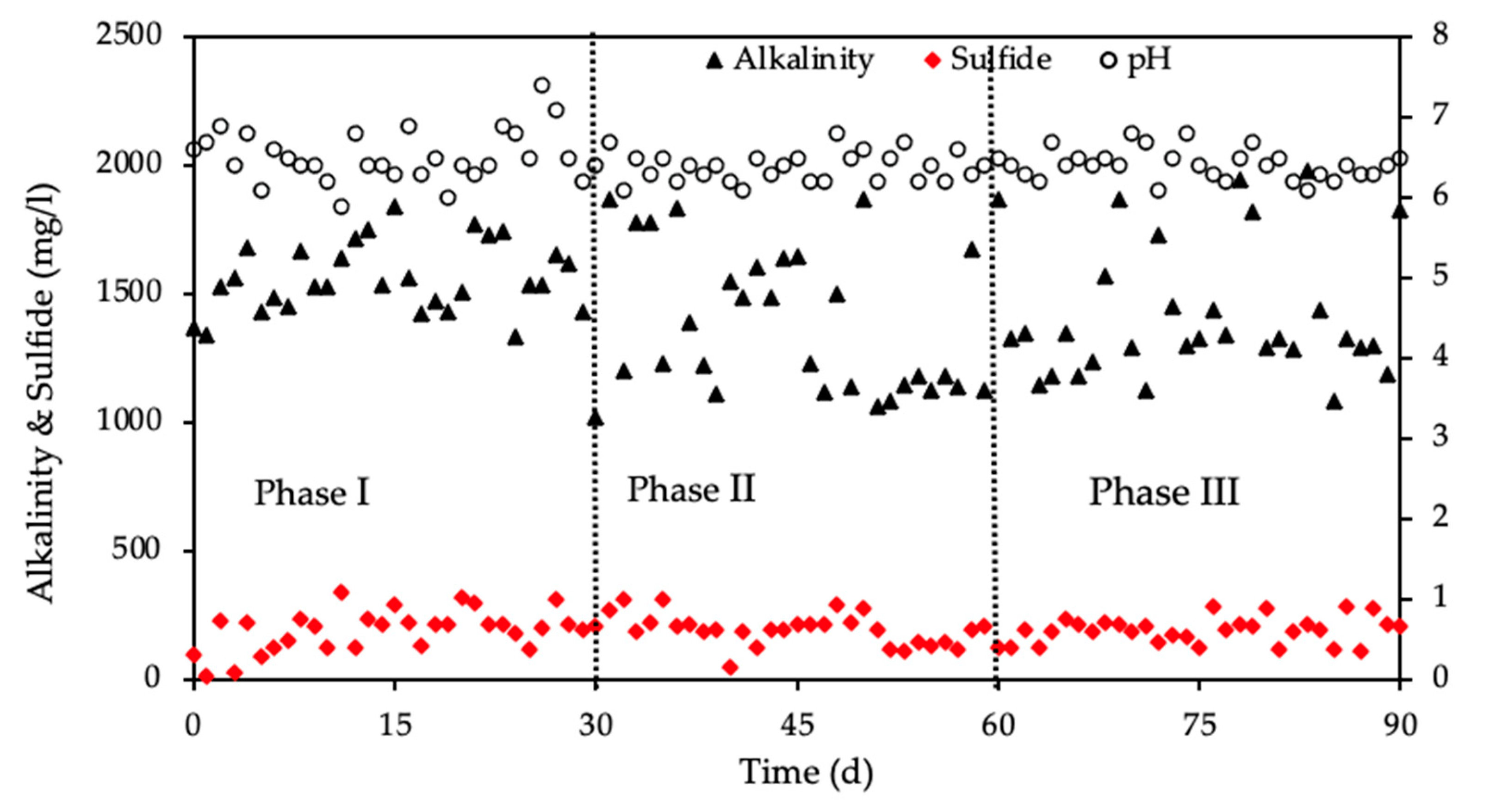
3.5. Reactor Monitoring Using Eh
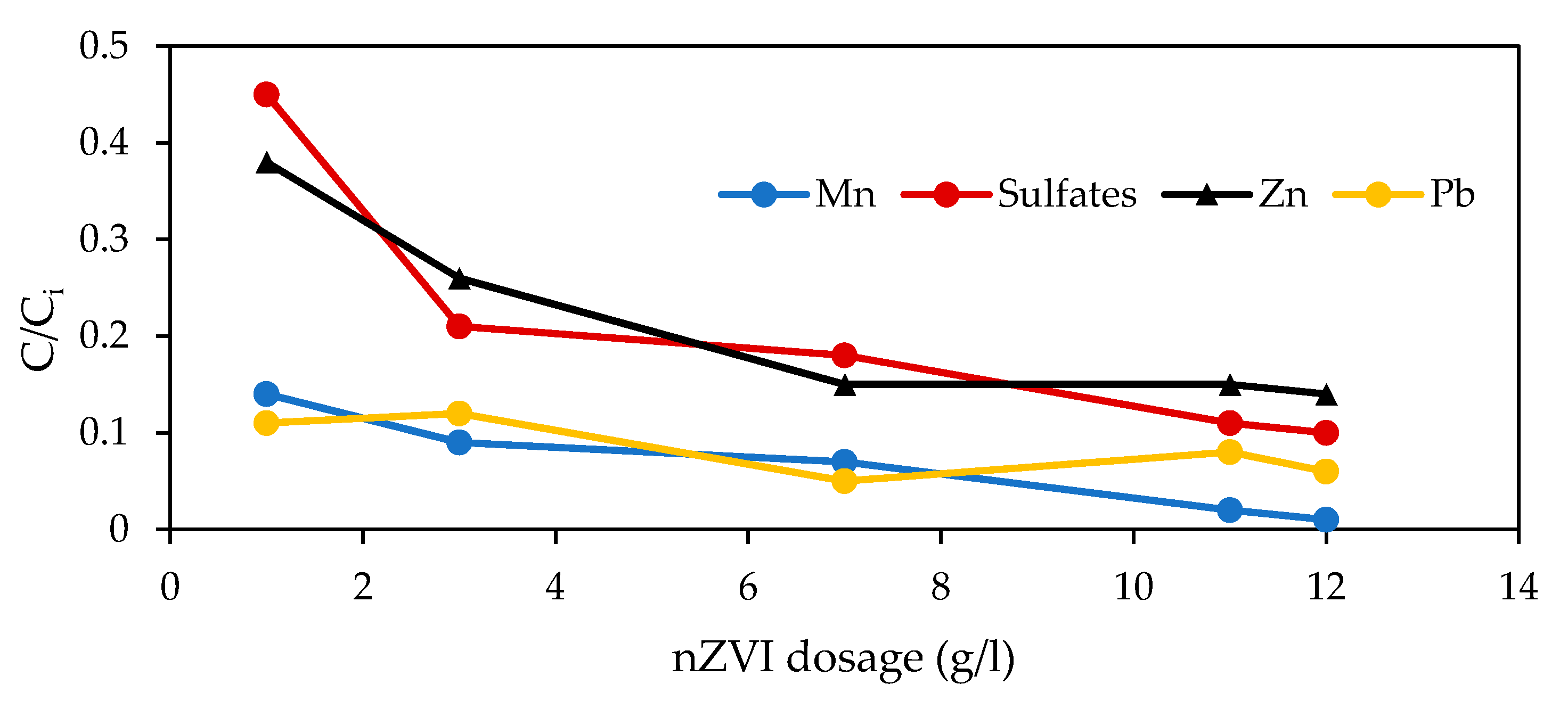
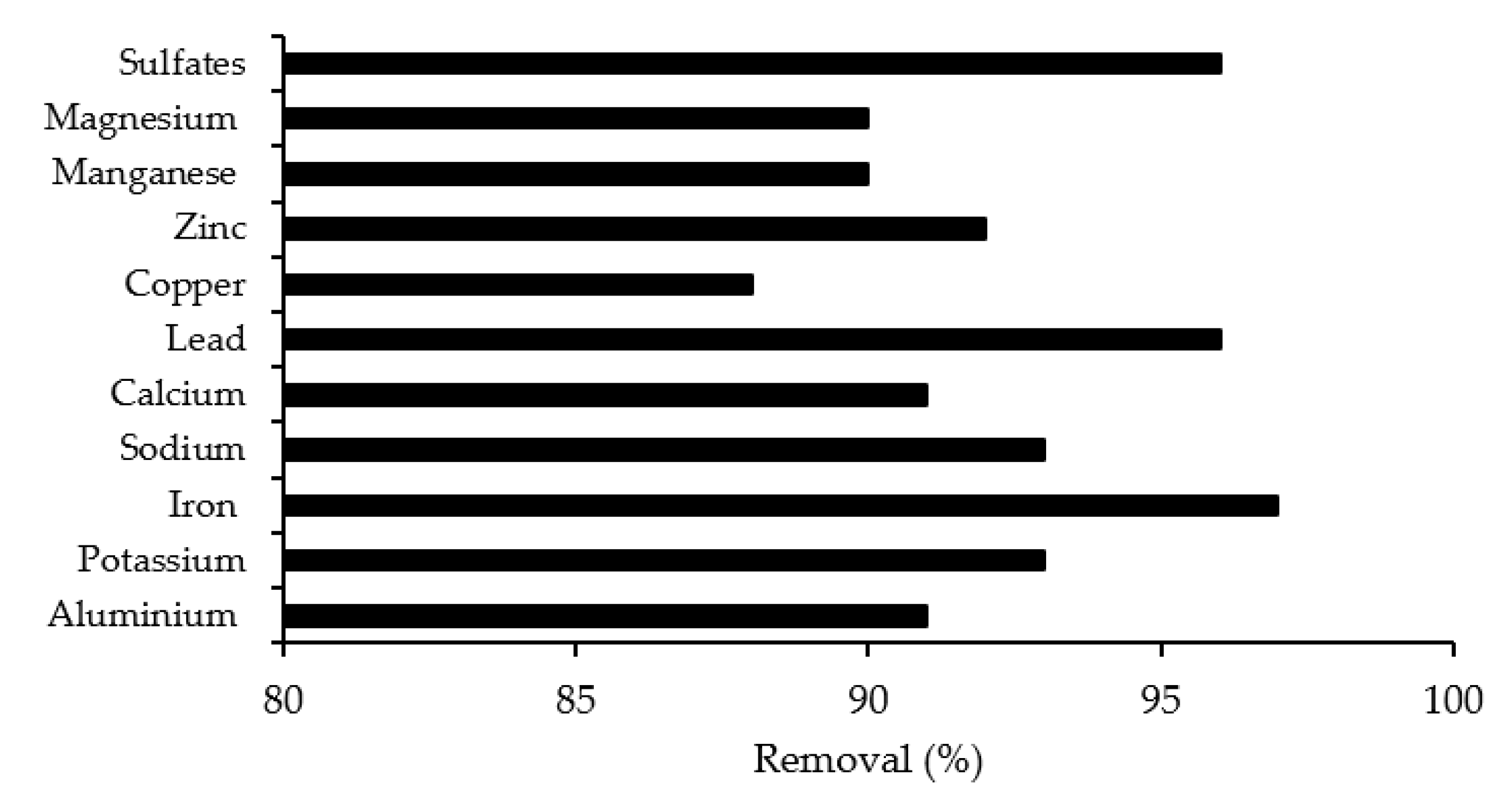
3.6. Metal Concentration Removal
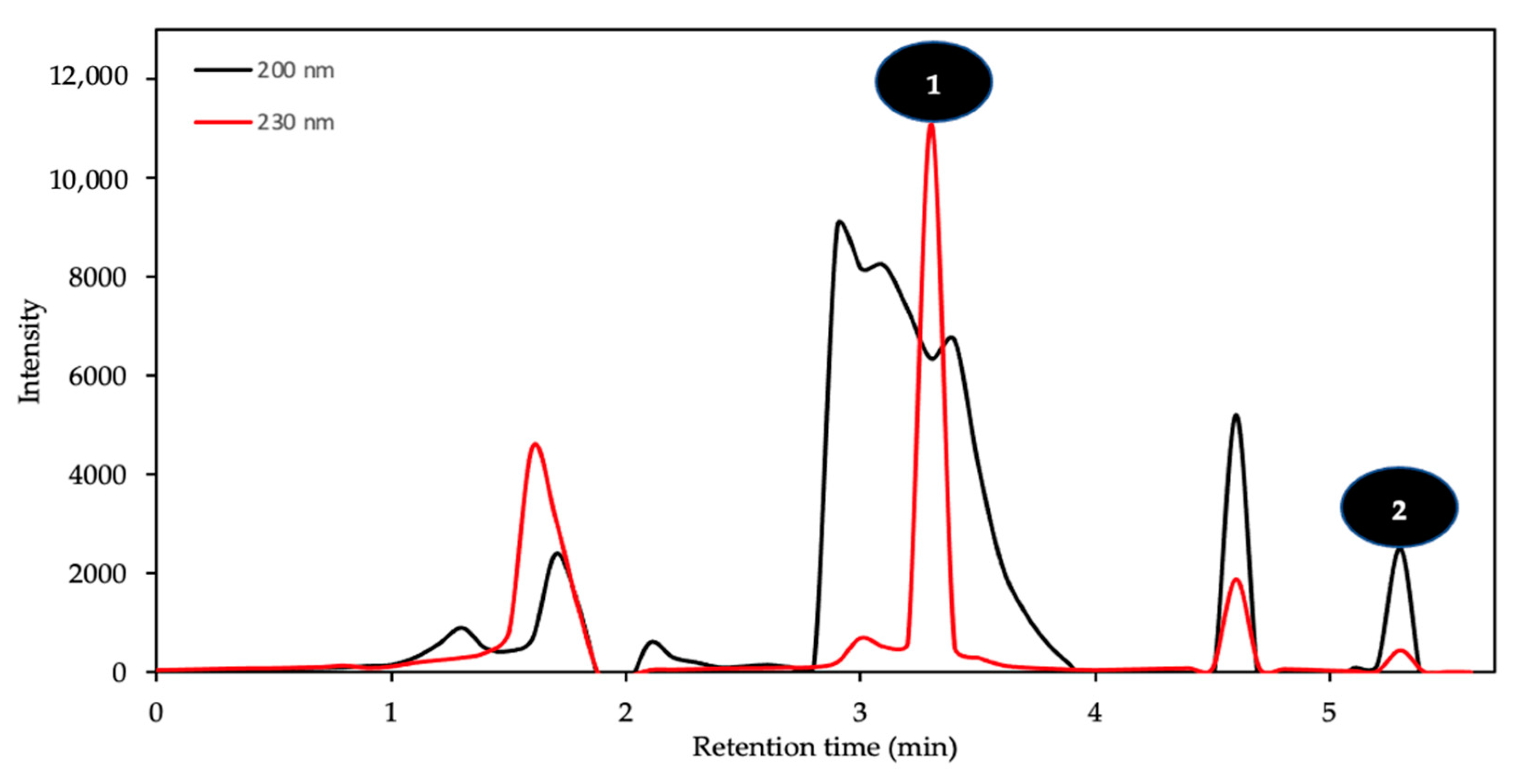
3.7. Pharmaceutical Compounds Assessment
3.8. Sludge Management with nZVI
3.9. General Overview of the nZVI Cost
3.10. The Overall Pollution Reduction in AMD
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masindi, V.; Akinwekomi, V.; Maree, J.P.; Muedi, K.L. Comparison of mine water neutralisation efficiencies of different alkaline generating agents. J. Environ. Chem. Eng. 2017, 5, 3903–3913. [Google Scholar] [CrossRef]
- Masindi, V.; Muedi, K.L. Environmental contamination by heavy metals. Heavy Met. 2018, 10, 115–132. [Google Scholar] [CrossRef]
- Naidoo, S. Acid Mine Drainage in South Africa: Development Actors, Policy Impacts, and Broader Implications; Springer International Publishing AG: Cham, Switzerland, 2017. [Google Scholar]
- Gumbi, B.P.; Moodley, B.; Birungi, G.; Ndungu, P.G. Detection and quantification of acidic drug residues in South African surface water using gas chromatography-mass spectrometry. Chemosphere 2017, 168, 1042–1050. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Chimuka, L. Simultaneous determination of naproxen, ibuprofen and diclofenac in wastewater using solid-phase extraction with high performance liquid chromatography. Water Sa 2017, 43, 264–274. [Google Scholar] [CrossRef]
- Chonova, T.; Keck, F.; Labanowski, J.; Montuelle, B.; Rimet, F.; Bouchez, A. Separate treatment of hospital and urban wastewaters: A real scale comparison of effluents and their effect on microbial communities. Sci. Total Environ. 2016, 542, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.L.; Younger, P.L. The co-treatment of sewage and mine waters in aerobic wetlands. Eng. Geol. 2006, 85, 53–61. [Google Scholar] [CrossRef]
- McCullough, C.D.; Lund, M.A.; May, J.M. Field-scale demonstration of the potential for sewage to remediate acidic mine waters. Mine Water Environ. 2008, 27, 31–39. [Google Scholar] [CrossRef]
- Strosnider, W.H.J.; Winfrey, B.K.; Nairn, R.W. Novel passive co-treatment of acid mine drainage and municipal wastewater. J. Environ. Qual. 2011, 40, 206–213. [Google Scholar] [CrossRef]
- Smyntek, P.M.; Chastel, J.; Peer, R.A.M.; Anthony, E.; McCloskey, J.; Bach, E.; Wagner, R.C.; Bandstra, J.Z.; Strosnider, W.H.J. Assessment of sulphate and iron reduction rates during reactor start-up for passive anaerobic co-treatment of acid mine drainage and sewage. Geochem. Explor. Environ. Anal. 2018, 18, 76–84. [Google Scholar] [CrossRef]
- Strosnider, W.H.J.; Winfrey, B.K.; Nairn, R.W. Alkalinity generation in a novel multi-stage high-strength acid mine drainage and municipal wastewater passive co-treatment system. Mine Water Environ. 2011, 30, 47–53. [Google Scholar] [CrossRef]
- Strosnider, W.H.J.; Winfrey, B.K.; Peer, R.A.M.; Nairn, R.W. Passive co-treatment of acid mine drainage and sewage: Anaerobic incubation reveals a regeneration technique and further treatment possibilities. Ecol. Eng. 2013, 61, 268–273. [Google Scholar] [CrossRef]
- Makhathini, T.P.; Mulopo, J.; Bakare, B.F. Effective biotreatment of acidic mine water and hospital wastewater using fluidized-bed reactors. J. Water Process Eng. 2020, 37, 101505. [Google Scholar] [CrossRef]
- Dong, H.; Li, L.; Lu, Y.; Cheng, Y.; Wang, Y.; Ning, Q.; Wang, B.; Zhang, L.; Zeng, G. Integration of nanoscale zero-valent iron and functional anaerobic bacteria for groundwater remediation: A review. Environ. Int. 2019, 124, 265–277. [Google Scholar] [CrossRef]
- Xiao, X.; Sheng, G.-P.; Mu, Y.; Yu, H.-Q. A modeling approach to describe ZVI-based anaerobic system. Water Res. 2013, 47, 6007–6013. [Google Scholar] [CrossRef]
- Kumar, N.; Chaurand, P.; Rose, J.; Diels, L.; Bastiaens, L. Synergistic effects of sulfate reducing bacteria and zero valent iron on zinc removal and stability in aquifer sediment. Chem. Eng. J. 2015, 260, 83–89. [Google Scholar] [CrossRef]
- Guo, J.; Kang, Y.; Feng, Y. Bioassessment of heavy metal toxicity and enhancement of heavy metal removal by sulfate-reducing bacteria in the presence of zero valent iron. J. Environ. Manag. 2017, 203, 278–285. [Google Scholar] [CrossRef]
- Kolmert, Å.; Johnson, D.B. Remediation of acidic waste waters using immobilised, acidophilic sulfate-reducing bacteria. J. Chem. Technol. Biotechnol. Int. Res. Process. Environ. Clean Technol. 2011, 76, 836–843. [Google Scholar] [CrossRef]
- Gallegos-Garcia, M.; Celis, L.B.; Rangel-Méndez, R.; Razo-Flores, E. Precipitation and recovery of metal sulfides from metal containing acidic wastewater in a sulfidogenic down-flow fluidized bed reactor. Biotechnol. Bioeng. 2009, 102, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Sahinkaya, E.; Dursun, N.; Ozkaya, B.; Kaksonen, A.H. Use of landfill leachate as a carbon source in a sulfidogenic fluidized-bed reactor for the treatment of synthetic acid mine drainage. Miner. Eng. 2013, 48, 56–60. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Riekkola-Vanhanen, M.-L.; Puhakka, J.A. Optimization of metal sulphide precipitation in fluidized-bed treatment of acidic wastewater. Water Res. 2003, 37, 255–266. [Google Scholar] [CrossRef]
- Makhathini, T.P.; Mulopo, J.; Bakare, B.F. Possibilities for Acid Mine Drainage Co-treatment with Other Waste Streams: A Review. Mine Water Environ. 2020, 39, 13–26. [Google Scholar] [CrossRef]
- Laber, J.; Haberl, R.; Shrestha, R. Two-stage constructed welland for treating hospital wastewater in nepal. Water Sci. Technol. 1999, 40, 317–324. [Google Scholar] [CrossRef]
- Sheoran, A.S.; Sheoran, V. Heavy metal removal mechanism of acid mine drainage in wetlands: A critical review. Miner. Eng. 2006, 19, 105–116. [Google Scholar] [CrossRef]
- Allende, K.L.; McCarthy, D.T.; Fletcher, T.D. The influence of media type on removal of arsenic, iron and boron from acidic wastewater in horizontal flow wetland microcosms planted with Phragmites australis. Chem. Eng. J. 2014, 246, 217–228. [Google Scholar] [CrossRef]
- Younger, P.L.; Henderson, R. Synergistic wetland treatment of sewage and mine water: Pollutant removal performance of the first full-scale system. Water Res. 2014, 55, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Ribas, D.; Cernik, M.; Benito, A.; Filip, J.; Marti, V. Activation process of air stable nanoscale zero-valent iron particles. Chem. Eng. J. 2017, 320, 290–299. [Google Scholar] [CrossRef]
- Liu, Y.; Majetich, S.S.; Tilton, R.D.; Sholl, D.S.; Lowry, G.V. TCE dechlorination rates, pathways, and efficiency of nanoscale iron particles with different properties. Environ. Sci. Technol. 2005, 39, 1338–1345. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Utgikar, V.P.; Harmon, S.M.; Chaudhary, N.; Tabak, H.H.; Govind, R.; Haines, J.R. Inhibition of sulfate-reducing bacteria by metal sulfide formation in bioremediation of acid mine drainage. Environ. Toxicol. 2002, 17, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Newbury, D.E.; Ritchie, N.W.M. Performing elemental microanalysis with high accuracy and high precision by scanning electron microscopy/silicon drift detector energy-dispersive X-ray spectrometry (SEM/SDD-EDS). J. Mater. Sci. 2015, 50, 493–518. [Google Scholar] [CrossRef]
- Janssen, A.J.H.; Ruitenberg, R.; Buisman, C.J.N. Industrial applications of new sulphur biotechnology. Water Sci. Technol. 2001, 44, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Velimirovic, M.; Simons, Q.; Bastiaens, L. Use of CAH-degrading bacteria as test-organisms for evaluating the impact of fine zerovalent iron particles on the anaerobic subsurface environment. Chemosphere 2015, 134, 338–345. [Google Scholar] [CrossRef]
- Kirschling, T.L.; Gregory, K.B.; Minkley, J.; Edwin G, J.; Lowry, G.V.; Tilton, R.D. Impact of nanoscale zero valent iron on geochemistry and microbial populations in trichloroethylene contaminated aquifer materials. Environ. Sci. Technol. 2010, 44, 3474–3480. [Google Scholar] [CrossRef]
- Venzlaff, H.; Enning, D.; Srinivasan, J.; Mayrhofer, K.J.J.; Hassel, A.W.; Widdel, F.; Stratmann, M. Accelerated cathodic reaction in microbial corrosion of iron due to direct electron uptake by sulfate-reducing bacteria. Corros. Sci. 2013, 66, 88–96. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, G.; Liu, S.; Fu, Z.; Chen, J.; Ma, C. Bioremoval of arsenic and antimony from wastewater by a mixed culture of sulfate-reducing bacteria using lactate and ethanol as carbon sources. Int. Biodeterior. Biodegrad. 2018, 126, 152–159. [Google Scholar] [CrossRef]
- Zhang, Z.; Moon, H.S.; Myneni, S.C.B.; Jaffé, P.R. Effect of dissimilatory iron and sulfate reduction on arsenic dynamics in the wetland rhizosphere and its bioaccumulation in wetland plants (Scirpus actus). J. Hazard. Mater. 2017, 321, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, B.; Yuan, H.; Cheng, Y.; Wang, S.; He, Z. Microbial reduction of vanadium (V) in groundwater: Interactions with coexisting common electron acceptors and analysis of microbial community. Environ. Pollut. 2017, 231, 1362–1369. [Google Scholar] [CrossRef]
- Bai, H.; Kang, Y.; Quan, H.; Han, Y.; Feng, Y. Treatment of copper wastewater by sulfate reducing bacteria in the presence of zero valent iron. Int. J. Miner. Process. 2012, 112, 71–76. [Google Scholar] [CrossRef]
- Ravikumar, K.V.G.; Shubham, A.; Shruthi, V.S.; Mrudula, P.; Natarajan, C.; Amitava, M. Nano-Bio sequential removal of hexavalent chromium using polymer-nZVI composite film and sulfate reducing bacteria under anaerobic condition. Environ. Technol. Innov. 2018, 9, 122–133. [Google Scholar] [CrossRef]
- Mulopo, J. Pilot scale assessment of the continuous biological sulphate removal from coal acid mine effluent using grass cutting as carbon and energy sources. J. Water Process Eng. 2016, 11, 104–109. [Google Scholar] [CrossRef]
- Song, H.; Yim, G.-J.; Ji, S.-W.; Nam, I.-H.; Neculita, C.M.; Lee, G. Performance of mixed organic substrates during treatment of acidic and moderate mine drainage in column bioreactors. J. Environ. Eng. 2012, 138, 1077–1084. [Google Scholar] [CrossRef]
- Marquez-Reyes, J.M.; Lopez-Chuken, U.J.; Valdez-Gonzalez, A.; Luna-Olvera, H.A. Removal of chromium and lead by a sulfate-reducing consortium using peat moss as carbon source. Bioresour. Technol. 2013, 144, 128–134. [Google Scholar] [CrossRef]
- Deng, D.; Weidhaas, J.L.; Lin, L.-S. Kinetics and microbial ecology of batch sulfidogenic bioreactors for co-treatment of municipal wastewater and acid mine drainage. J. Hazard. Mater. 2016, 305, 200–208. [Google Scholar] [CrossRef]
- Neculita, C.-M.; Zagury, G.J.; Bussière, B. Passive treatment of acid mine drainage in bioreactors using sulfate-reducing bacteria. J. Environ. Qual. 2007, 36, 1–16. [Google Scholar] [CrossRef]
- Sánchez-Andrea, I.; Sanz, J.L.; Bijmans, M.F.M.; Stams, A.J.M. Sulfate reduction at low pH to remediate acid mine drainage. J. Hazard. Mater. 2014, 269, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, W.; Liang, F.; Zhang, W. Heavy metal removal using nanoscale zero-valent iron (nZVI): Theory and application. J. Hazard. Mater. 2017, 322, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Kieu, H.T.Q.; Muller, E.; Horn, H. Heavy metal removal in anaerobic semi-continuous stirred tank reactors by a consortium of sulfate-reducing bacteria. Water Res. 2011, 45, 3863–3870. [Google Scholar] [CrossRef]
- Younger, P.L.; Banwart, S.A.; Hedin, R.S. Mine Water: Hydrology, Pollution, Remediation; Springer Science & Business Media: Berlin, Germany, 2002. [Google Scholar]
- Clyde, E.J.; Champagne, P.; Jamieson, H.E.; Gorman, C.; Sourial, J. The use of a passive treatment system for the mitigation of acid mine drainage at the Williams Brothers Mine (California): Pilot-scale study. J. Clean. Prod. 2016, 130, 116–125. [Google Scholar] [CrossRef]
- Kiran, M.G.; Pakshirajan, K.; Das, G. An overview of sulfidogenic biological reactors for the simultaneous treatment of sulfate and heavy metal rich wastewater. Chem. Eng. Sci. 2017, 158, 606–620. [Google Scholar] [CrossRef]
- Peer, R.A.M.; LaBar, J.A.; Winfrey, B.K.; Nairn, R.W.; Llanos Lopez, F.S.; Strosnider, W.H.J. Removal of less commonly addressed metals via passive cotreatment. J. Environ. Qual. 2015, 44, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Strosnider, W.H.J.; Nairn, R.W.; Peer, R.A.M.; Winfrey, B.K. Passive co-treatment of Zn-rich acid mine drainage and raw municipal wastewater. J. Geochemical Explor. 2013, 125, 110–116. [Google Scholar] [CrossRef]
- Crane, R.A.; Scott, T.B. Nanoscale zero-valent iron: Future prospects for an emerging water treatment technology. J. Hazard. Mater. 2012, 211, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Madikizela, L.M.; Chimuka, L. Determination of ibuprofen, naproxen and diclofenac in aqueous samples using a multi-template molecularly imprinted polymer as selective adsorbent for solid-phase extraction. J. Phar. Biomed. Anal. 2016, 128, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Amday, R.; Chimuka, L.; Cukrowska, E. Determination of naproxen, ibuprofen and triclosan in wastewater using the polar organic chemical integrative sampler (POCIS): A laboratory calibration and field application. Water Sa 2014, 40, 407–414. [Google Scholar] [CrossRef]
- Agunbiade, F.O.; Moodley, B. Pharmaceuticals as emerging organic contaminants in Umgeni River water system, KwaZulu-Natal, South Africa. Environ. Monit. Assess. 2014, 186, 7273–7291. [Google Scholar] [CrossRef] [PubMed]
- Dahane, S.; Gil Garcia, M.D.; Martinez Bueno, M.J.; Ucles Monreno, A.; Martinez Galera, M. Determination of drugs in river and wastewaters using solid-phase extraction by packed multi-walled carbon nanotubes and liquid chromatography–quadrupole-linear ion trap-mass spectrometry. J. Chromatogr. A. 2013, 1297, 17–28. [Google Scholar] [CrossRef]
- Fichtel, K.; Mathes, F.; Könneke, M.; Cypionka, H.; Engelen, B. Isolation of sulfate-reducing bacteria from sediments above the deep-subseafloor aquifer. Front. Microbiol. 2012, 3, 65. [Google Scholar] [CrossRef]
- Zacarías-Estrada, O.L.; Ballinas-Casarrubias, L.; Montero-Cabrera, M.E.; Loredo-Portales, R.; Orrantia-Borunda, E.; Luna-Velasco, A. Arsenic removal and activity of a sulfate reducing bacteria-enriched anaerobic sludge using zero valent iron as electron donor. J. Hazard. Mater. 2020, 384, 121392. [Google Scholar] [CrossRef]
- Redmile-Gordon, M.; Chen, L. Zinc toxicity stimulates microbial production of extracellular polymers in a copiotrophic acid soil. Int. Biodeterior. Biodegrad. 2017, 119, 413–418. [Google Scholar] [CrossRef]
- Labrenz, M.; Druschel, G.K.; Thomsen-Ebert, T.; Gilbert, B.; Welch, S.A.; Kemmer, K.M.; Logan, G.A.; Summons, R.E.; De Stasio, G.; Bond, P.L.; et al. Formation of sphalerite (ZnS) deposits in natural biofilms of sulfate-reducing bacteria. Science. 2000, 290, 1744–1747. [Google Scholar] [CrossRef]
- Kumar, N.; Couture, R.-M.; Millot, R.; Battaglia-Brunet, F.; Rose, J. Microbial sulfate reduction enhances arsenic mobility downstream of zerovalent-iron-based permeable reactive barrier. Environ. Sci. Technol. 2016, 50, 7610–7617. [Google Scholar] [CrossRef] [PubMed]
- Papirio, S.; Villa-Gomez, D.K.; Esposito, G.; Pirozzi, F.; Lens, P.N.L. Acid mine drainage treatment in fluidized-bed bioreactors by sulfate-reducing bacteria: A critical review. Crit. Rev. Environ. Sci. Technol. 2013, 43, 2545–2580. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, K.B.; Johnson, D.B. Biological manganese removal from acid mine drainage in constructed wetlands and prototype bioreactors. Sci. Total Environ. 2005, 338, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Mayes, W.M.; Batty, L.C.; Younger, P.L.; Jarvis, A.P.; Koiv, M.; Mander, U. Wetland treatment at extremes of pH: A review. Sci. Total Environ. 2009, 407, 3944–3957. [Google Scholar] [CrossRef] [PubMed]
- Pearson, F.H.; Nesbitt, J.B. Acid mine drainage as a chemical coagulant for treatment of municipal wastewater. In Proceedings of the 5th Symposium Coal Mine Drainage Research, Lousville, KY, USA, 22–24 October 1974; pp. 181–191. [Google Scholar]



| Parameters | Acidic Mine Water | Hospital Wastewater |
|---|---|---|
| pH | ±2.1–3.8 | ±6.2–7.9 |
| COD (mg/L) | ±98 | ±156–1234 |
| PO43− | ±11–31 | ±9.8–14 |
| NH4+ | ±34–87 | ±34–87 |
| Total nitrogen (mg/L) | ±21–123 | ±45–223 |
| Aluminum (mg/L) | ±275 | <2 |
| Potassium | ±34 | <1 |
| Iron (mg/L) | ±5329 | ±12 |
| Sodium (mg/L) | <0.05 | - |
| Calcium (mg/L) | ±210 | <6 |
| Copper (mg/L) | ±112 | ±56 |
| Zinc (mg/L) | ±145 | ±23 |
| Manganese (mg/L) | ±113 | - |
| Magnesium (mg/L) | ±243 | ±29 |
| Sulfate (mg/L) | ±4900 | ±134 |
| Turbidity (NTU) | ±46–55 | ±11–52 |
| Conductivity (μS/cm) | >82 | >413 |
| Naproxen (μg/L) | - | 2.39 ± 0.75 |
| Ibuprofen (μg/L) | - | 8.72 ± 0.98 |
| Parameter | HWW + AMD |
|---|---|
| Zinc (mg/L) | 138 ± 11.2 |
| Manganese (mg/L) | 102 ± 7.8 |
| Lead (mg/L) | 114 ± 10.1 |
| SO42− (mg/L) | 5210 ± 12.3 |
| COD (mg/L) | 1523 ± 20.9 |
| pH | ±6.8–8.1 |
| Period | Days | Pb | Cu | Zn | Fe | Ca | Mn | |
|---|---|---|---|---|---|---|---|---|
| Total metals in influent (mg/L) | ||||||||
| I | 0–30 | Concentration | 33.12 | 112 | 421 | 3112 | 212 | 355 |
| SD | 0.11 | 1.1 | 3.4 | 17 | 1.3 | 0.1 | ||
| II | 31–60 | Concentration | 18.1 | 59 | 81 | 589 | 119 | 117 |
| SD | 9 | 0.2 | 2.1 | 7 | 1.2 | 0.2 | ||
| III | 61–90 | Concentration | 12 | 21 | 44 | 187 | 211 | 45 |
| SD | 4 | 1.1 | 1.1 | 12 | 1 | 2.1 | ||
| Total metals in effluent (mg/L) | ||||||||
| I | 0–30 | Concentration | 12.1 | 1.02 | 0.2 | 11.9 | 2.1 | <0.02 |
| SD | 1.9 | 0.1 | 0.01 | 1.3 | 0.09 | <0.02 | ||
| II | 31–60 | Concentration | 9.9 | 1.12 | 0.7 | 17 | 1.8 | <0.05 |
| SD | 2.3 | 0.4 | 0.3 | 1.4 | 0.01 | <0.05 | ||
| III | 61–90 | Concentration | - | <0.05 | <0.05 | 19 | <0.05 | <0.05 |
| SD | - | - | - | 1.1 | - | - | ||
| Pharmaceutical Matrix | Effluent | Deionized | ||
|---|---|---|---|---|
| Ibuprofen | Naproxen | Ibuprofen | Naproxen | |
| SPE efficiency (%) | 82 ± 3.4 | 81 ± 8.7 | 83 | 84 |
| Detection limit | 1.2–1.9 | 0.2–1.1 | 0.3 | 0.2 |
| Quantification limit | 0.5–1.4 | 2.3–3.7 | 0.6 | 1.2 |
| r2 | 0.98 | 0.99 | 0.99 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makhathini, T.P.; Mulopo, J.; Bakare, B.F. Enriched Co-Treatment of Pharmaceutical and Acidic Metal-Containing Wastewater with Nano Zero-Valent Iron. Minerals 2021, 11, 220. https://doi.org/10.3390/min11020220
Makhathini TP, Mulopo J, Bakare BF. Enriched Co-Treatment of Pharmaceutical and Acidic Metal-Containing Wastewater with Nano Zero-Valent Iron. Minerals. 2021; 11(2):220. https://doi.org/10.3390/min11020220
Chicago/Turabian StyleMakhathini, Thobeka Pearl, Jean Mulopo, and Babatunde Femi Bakare. 2021. "Enriched Co-Treatment of Pharmaceutical and Acidic Metal-Containing Wastewater with Nano Zero-Valent Iron" Minerals 11, no. 2: 220. https://doi.org/10.3390/min11020220
APA StyleMakhathini, T. P., Mulopo, J., & Bakare, B. F. (2021). Enriched Co-Treatment of Pharmaceutical and Acidic Metal-Containing Wastewater with Nano Zero-Valent Iron. Minerals, 11(2), 220. https://doi.org/10.3390/min11020220








