Gel Formation at the Front of Expanding Calcium Bentonites
Abstract
1. Introduction
2. Materials and Methods
3. Results
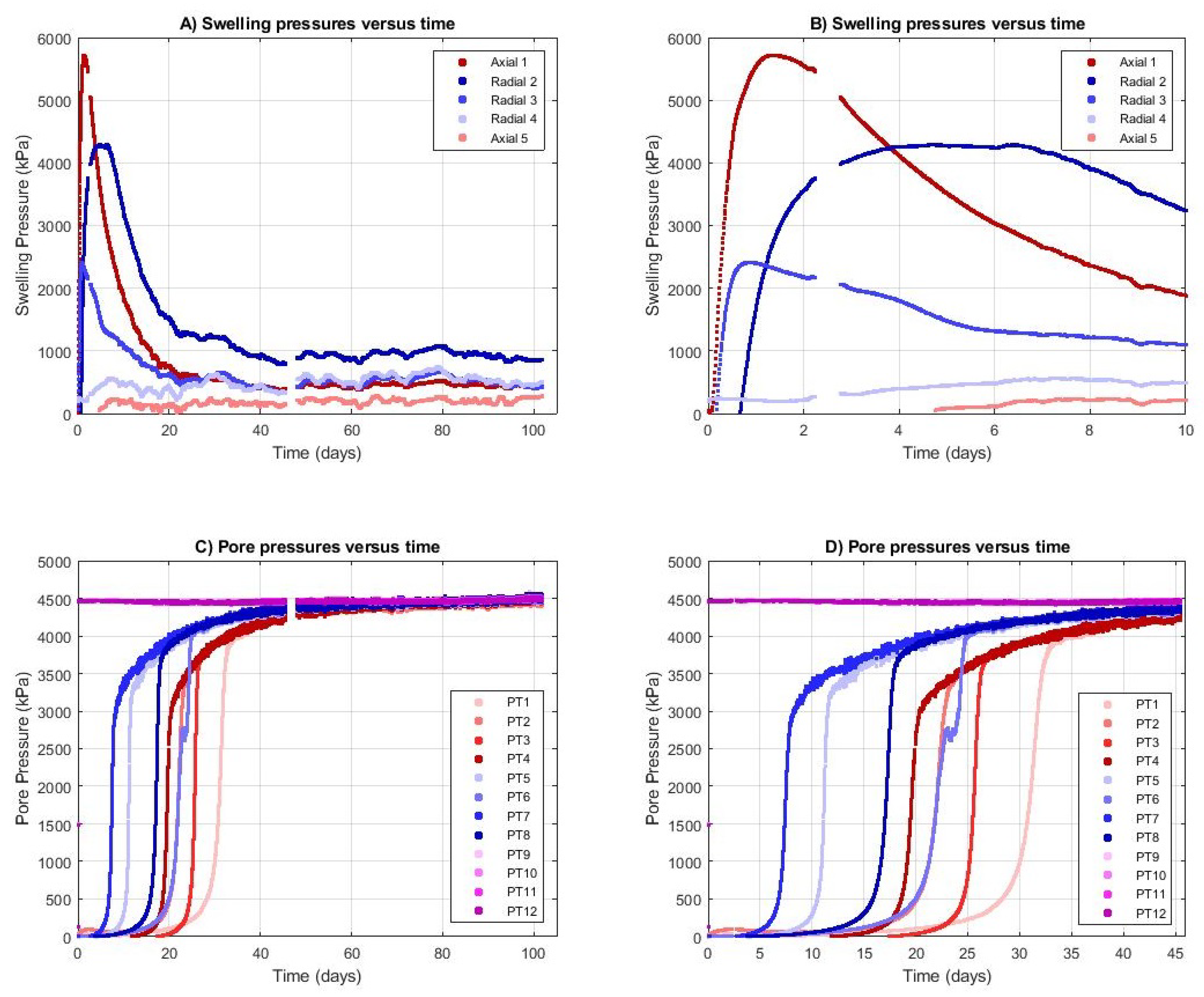
3.1. Development of Swelling and Pore Pressure
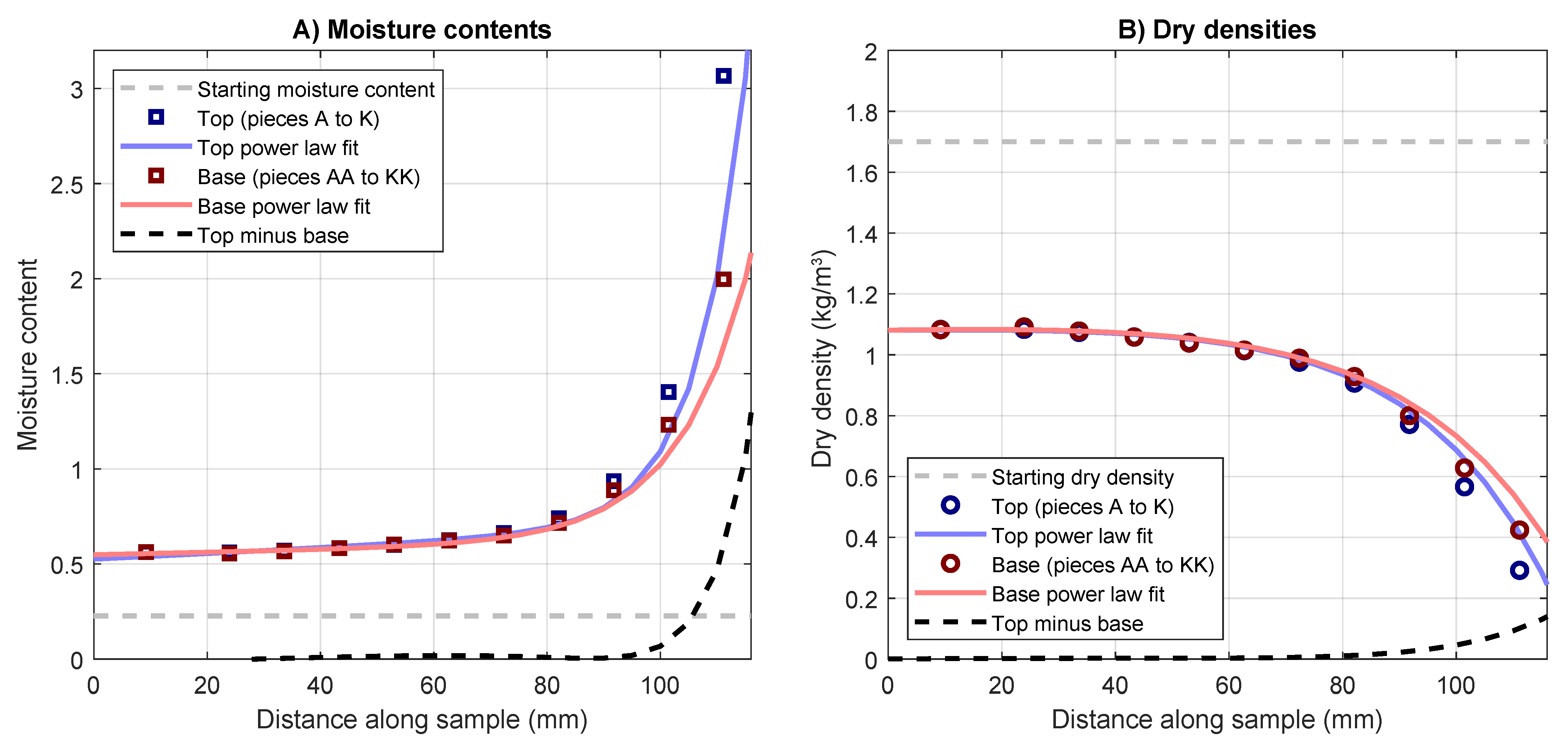
3.2. Post-Test Moisture Contents
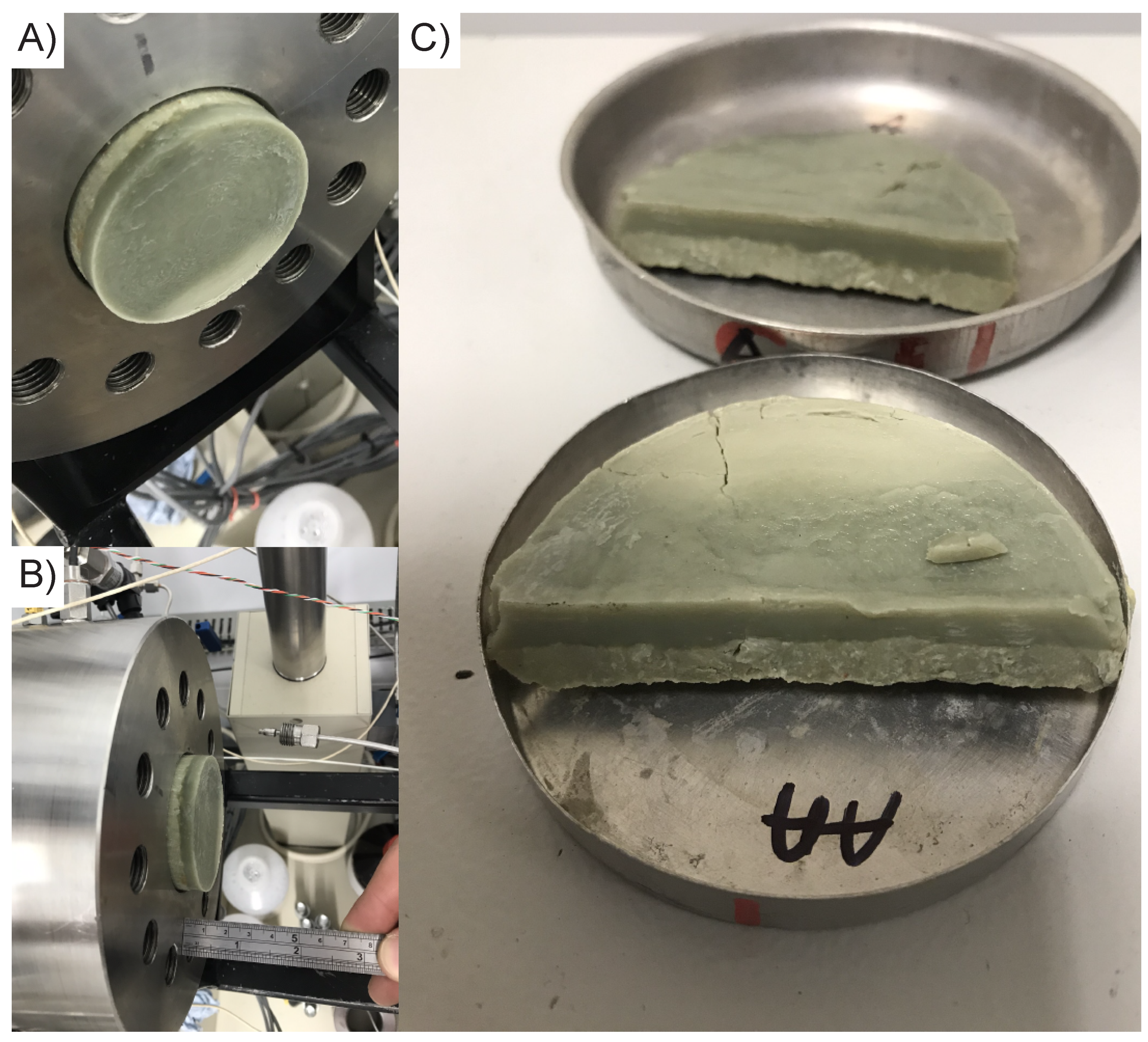
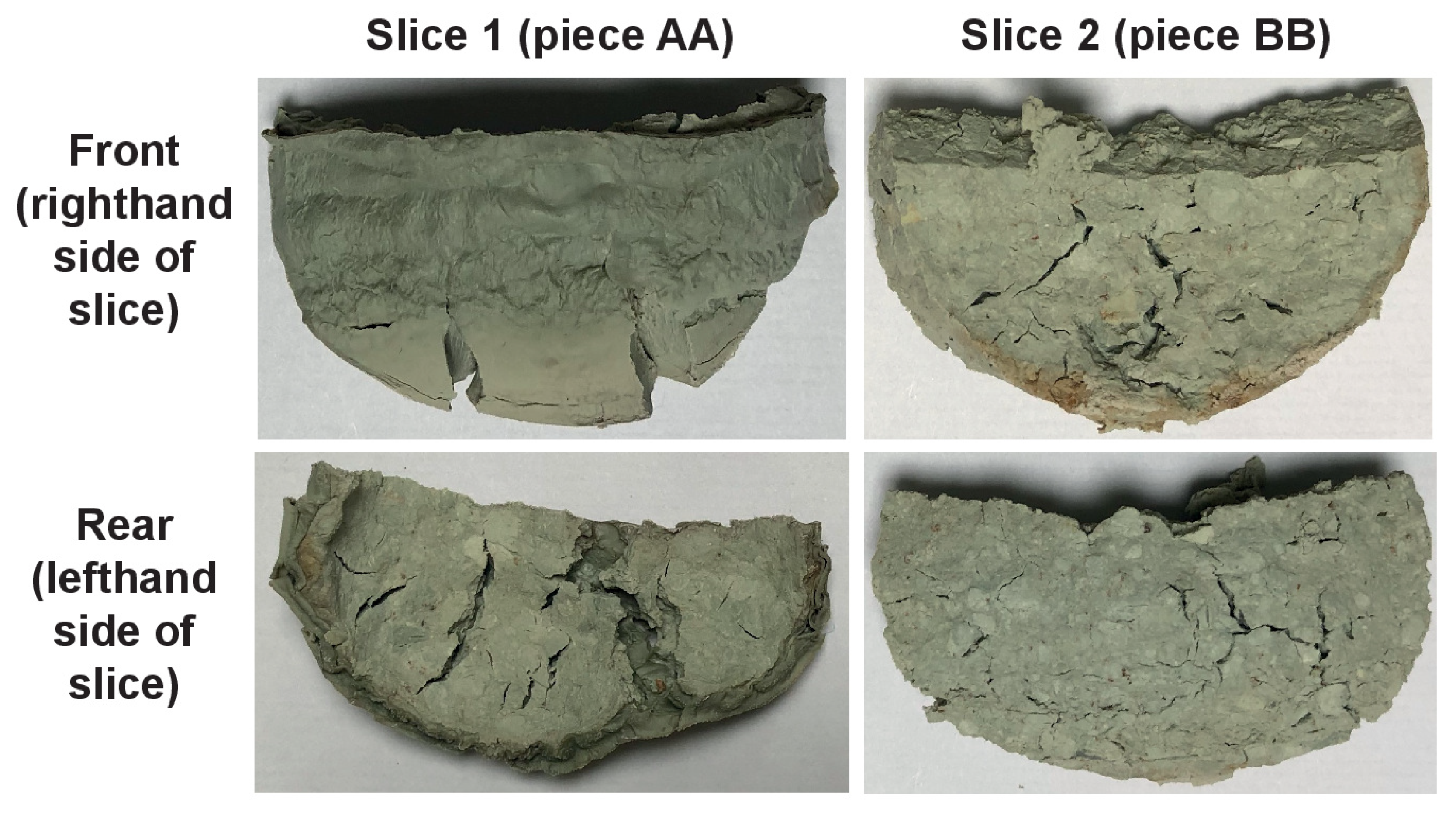
3.3. Gel Formation
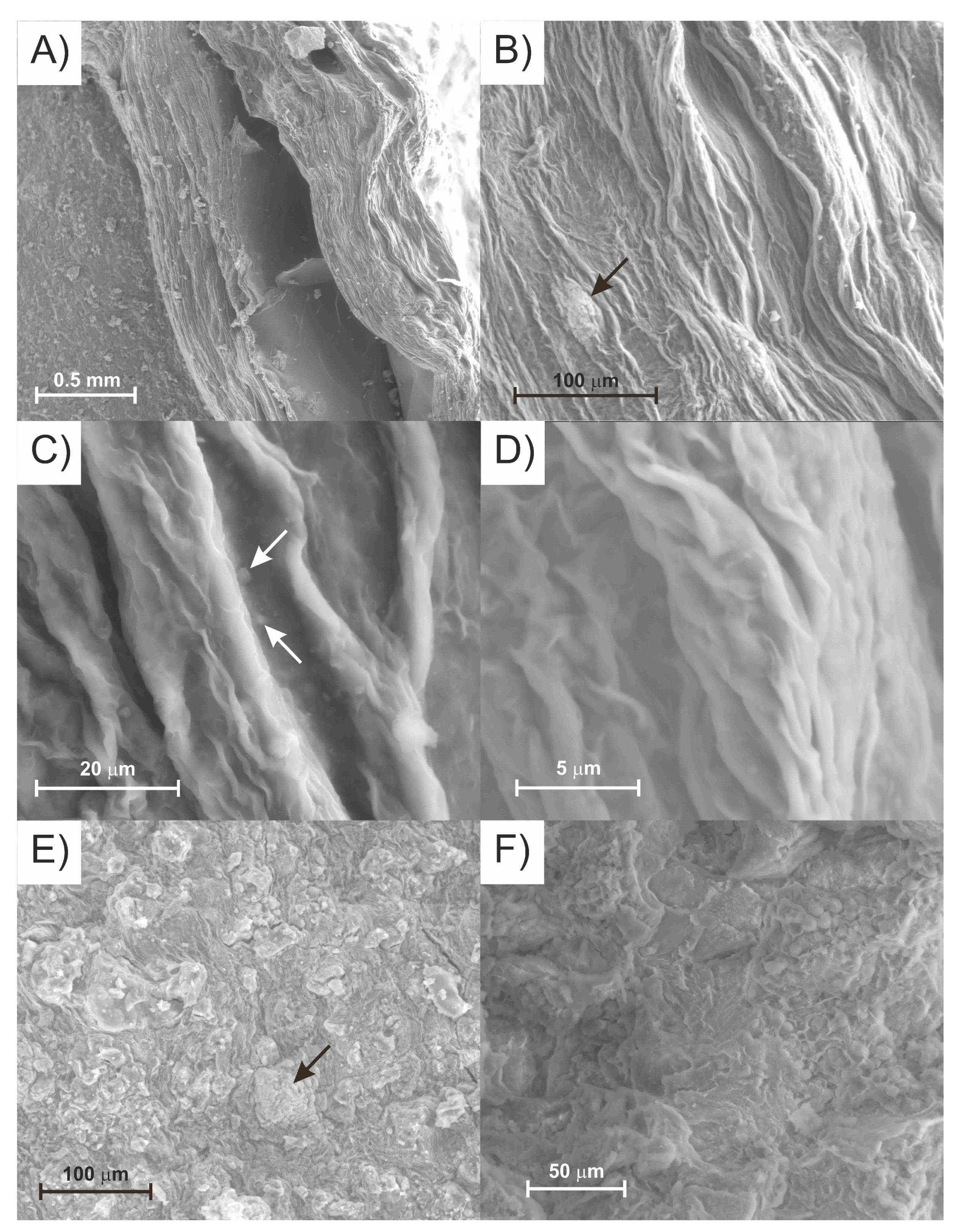
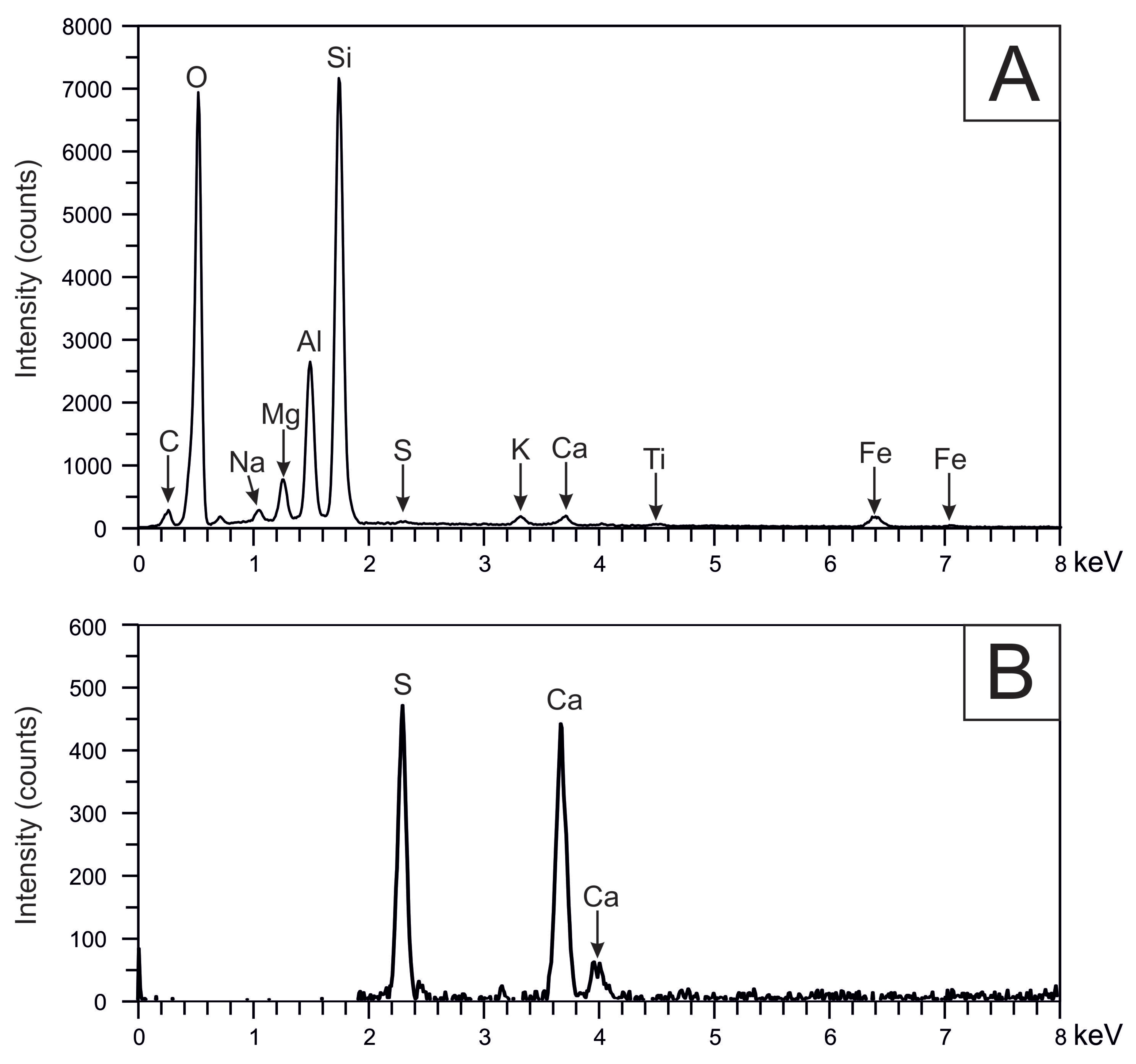
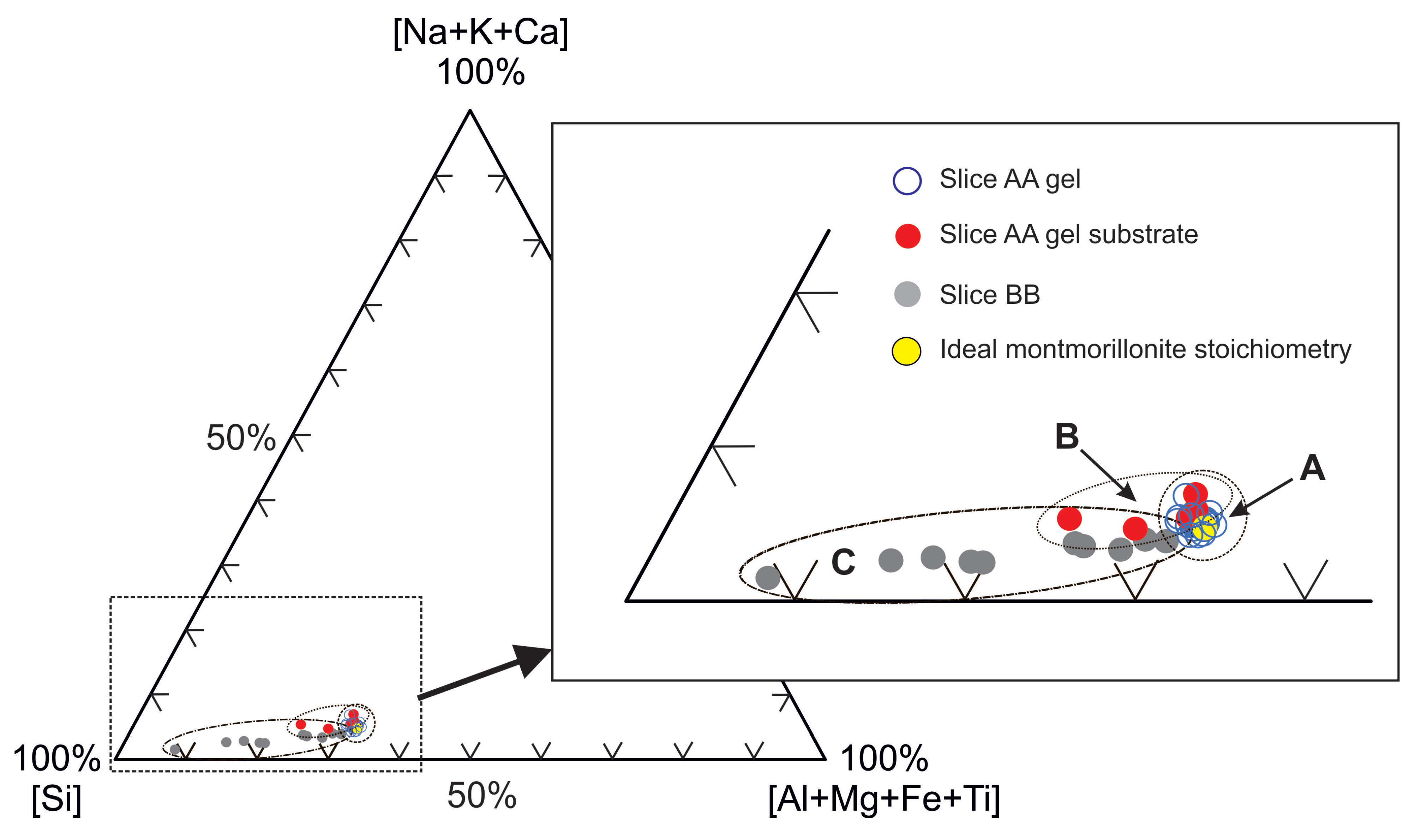
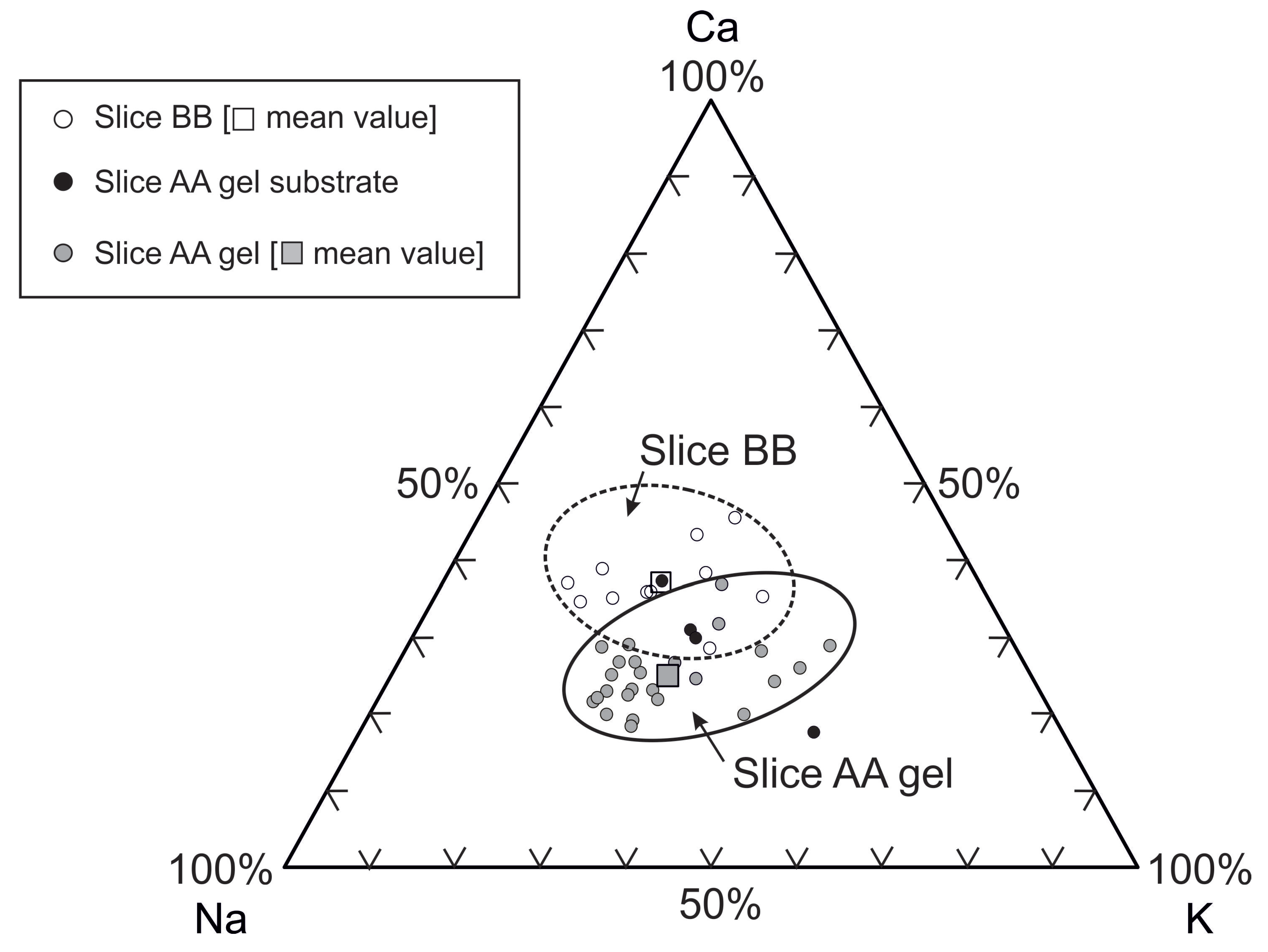
3.3.1. Petrographic and Microchemical Analysis
3.3.2. Surface Area Determinations
3.3.3. <2 m and Whole-Rock XRD Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sellin, P.; Leupin, O.X. The use of clay as an engineered barrier in radioactive waste management—A review. Clays Clay Mineral. 2013, 61, 477–498. [Google Scholar] [CrossRef]
- Johansson, E.; Siren, T.; Kemppainen, K. Onkalo—Underground Rock Characterization Facility for In-Situ Testing for Nuclear Waste Disposal. In Proceedings of the 13th ISRM International Congress of Rock Mechanics, Montreal, QC, Canada, 10–13 May 2015. [Google Scholar]
- WIPP. Waste Isolation Pilot Plant; U.S. Department of Energy; 2019. Available online: https://wipp.energy.gov/wipp-site.asp (accessed on 15 February 2021).
- Andra. Référentiel des Matériaux d’un Stockage de Déchets à Haute Activité et à Vie Longue—Tome 4: Les Matériaux à Base D’argilites Excavées et Remaniées; Technical Report CRPASCM040015B; Agence nationale pour la gestion des déchets radioactifs (ANDRA): Châtenay-Malabry, France, 2005. [Google Scholar]
- Liu, L. Permeability and expansibility of sodium bentonite in dilute solutions. Colloids Surfaces A Physicochem. Eng. Asp. 2010, 358, 68–78. [Google Scholar] [CrossRef]
- Pusch, R. The Buffer and Backfill Handbook, Part 1: Definitions, Basic Relationships, and Laboratory Methods; Technical Report TR-02-20; Svensk Kärnbränslehantering AB (SKB): Stockholm, Sweden, 2002. [Google Scholar]
- Wersin, P.; Johnson, L.H.; McKinley, I.G. Performance of the bentonite barrier at temperatures beyond 100: A critical review. Phys. Chem. Earth 2007, 32, 780–788. [Google Scholar] [CrossRef]
- Gens, A.; Valleján, B.; Zandarín, M.T.; Sánchez, M. Homogenization in clay barriers and seals: Two case studies. J. Rock Mech. Geotech. Eng. 2013, 5, 191–199. [Google Scholar] [CrossRef]
- Deniau, I.; Devol-Brown, I.; Derenne, S.; Behar, F.; Largeau, C. Comparison of the bulk geochemical features and thermal reactivity of kerogens from Mol (Boom Clay), Bure (Callovo-Oxfordian argillite) and Tournemire (Toarcian shales) underground research laboratories. Sci. Total Environ. 2008, 389, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Villar, M.V.; Lloret, A. Influence of dry density and water content on the swelling of a compacted bentonite. Appl. Clay Sci. 2008, 39, 38–49. [Google Scholar] [CrossRef]
- Zheng, L.; Rutqvist, J.; Birkholzer, J.T.; Liu, H.H. On the impact of temperatures up to 200 ∘C in clay repositories with bentonite engineer barrier systems: A study with coupled thermal, hydrological, chemical, and mechanical modelling. Eng. Geol. 2015, 197, 278–295. [Google Scholar] [CrossRef]
- Daniels, K.A.; Harrington, J.F.; Zihms, S.G.; Wiseall, A.C. Bentonite Permeability at Elevated Temperature. Geosciences 2017, 7. [Google Scholar] [CrossRef]
- SKB. Final Storage of Spent Nuclear Fuel—KBS-3; Technical Report Art716-1; Svensk Kärnbränslehantering AB (SKB): Stockholm, Sweden, 1983. [Google Scholar]
- NAGRA. Project Gewahr 1985, Nuclear Waste Management in Switzerland: Feasibility Studies and Safety Analysis; Technical Report NAGRA-NTB-85-09; Nationale Genossenschaft für die Lagerung Radioaktiver Abfälle (NAGRA): Baden, Switzerland, 1985. [Google Scholar]
- Coulon, H.; Lajudie, A.; Debrabant, P.; Atabek, R.; Jordia, M.; Jehan, R. Choice of French clays as engineered barrier components for waste disposal. Sci. Basis Nucl. Waste Manag. 1987, 10, 813–824. [Google Scholar] [CrossRef]
- Linares, J. Spanish Research Activities in Thefield of Backfilling and Sealing: A Pre-Liminary Study of Some Spanish Sedimentary (Madrid Basin) and Hydrothermal (Almeria) Bentonite; Technical Report. In Proceedings of the NEA/CEC Workshop on Sealing of RadioactiveWaste Repositories, Paris, France, 9–19 June 1989. [Google Scholar]
- Vieno, T.; Hautojarvi, A.; Koskinen, L.; Nordman, H. TVO-92 Safety Analysis of Spent Fuel Disposal; Technical Report VTJ-92-33E; VTT Technical Research Centre of Finland: Espoo, Finland, 1992. [Google Scholar]
- Villar, M.V. Thermo-Hydro-Mechanical Characterisation of a Bentonite from Cabo de Gata: A Study Applied to the Use of Bentonite as Sealing Material in High Level Radioactive Waste Repositories; Technical Report 01/2002; ENRESA: Madrid, Spain, 2002. [Google Scholar]
- Villar, M.V.; Iglesias, R.J.; García-Siñeriz, J.L. State of the in situ Febex test (GTS, Switzerland) after 18 years: A heterogeneous bentonite barrier. Environ. Geotech. 2020, 7, 147–159. [Google Scholar] [CrossRef]
- Pusch, R. The Microstructure of MX-80 Clay with Respect to Its Bulk Physical Properties under Different Environmental Conditions; Technical Report TR-01-08; Svensk Kärnbränslehantering AB (SKB): Stockholm, Sweden, 2001. [Google Scholar]
- Tabak, A.; Afsin, B.; Caglar, B.; Koksal, E. Characterization and pillaring of a Turkish bentonite (Resadiye). J. Colloid Interface Sci. 2007, 313, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Coulon, H. Proprietes Physico-Chimiques des Sediments Argileux Francais: Application au Stockage des Dechets Radioactifs. Ph.D. Thesis, University of Lille, Lille, France, 1987. [Google Scholar]
- Sun, H.; Mašín, D.; Boháč, J. Experimental characterization of retention properties and microstructure of the Czech bentonite B75. In Proceedings of the 19th International Conference on Soil Mechanics and Geotechnical Engineering, Seoul, Korea, 17–21 September 2017. [Google Scholar]
- Tessier, D.; Dardaine, M.; Beaumont, A.; Jaumet, A.M. Swelling pressure and microstructure of an activated swelling clay with temperature. Clay Miner. 1998, 33, 255–267. [Google Scholar] [CrossRef]
- Marcial, D.; Delage, P.; Cui, Y.J. On the high stress compression of bentonites. Can. Geotech. J. 2002, 39, 812–820. [Google Scholar] [CrossRef]
- Ben Rhaïem, H.; Pons, C.H.; Tessier, D. Factors Affecting the Microstructure of Smectites. Role of Cations and History of Applied Stresses; Technical Report; The Clay Mineral Society: Denver, CO, USA, 1987; pp. 292–297. [Google Scholar]
- Tessier, D. Etude Expérimentale de L’organisation des Matériaux Argileux. Ph.D. Thesis, Institut National de la Recherche Agronomique (INRA), Versailles, France, 1984. [Google Scholar]
- Chafe, N.P.; de Bruyn, J.R. Drag and relaxation in a bentonite clay suspension. J. Non Newton. Fluid Mech. 2005, 131, 44–52. [Google Scholar] [CrossRef]
- Whorlow, R.W. Rheological Techniques; Ellis Horwood: New York, NY, USA, 1992. [Google Scholar]
- Martin, P.L.; Barcala, J.M.; Huertas, F. Large-scale and long-term coupled thermo-hydro-mechanic experiments with bentonite: The febex mock-up test. J. Iber. Geol. 2006, 32, 259–282. [Google Scholar]
- Juvankoski, M. Description of Basic Design for Buffer; Posiva Technical Report 2009-131; Posiva: Eurajoki, Finland, 2010. [Google Scholar]
- Wang, Q.; Tang, A.M.; Cui, Y.J.; Delage, P.; Barnichon, J.D.; Ye, W.M. The effects of technological voids on the hydro-mechanical behaviour of compacted bentonite–sand mixture. Soils Found. 2013, 53, 232–245. [Google Scholar] [CrossRef]
- Komine, H. Simplified evaluation for swelling characteristics of bentonites. Eng. Geol. 2004, 71, 265–279. [Google Scholar] [CrossRef]
- Cuevas, J.; Villar, M.V.; Martın, M.; Cobena, J.; Leguey, S. Thermo-hydraulic gradients on bentonite: Distribution of soluble salts, microstructure and modification of the hydraulic and mechanical behaviour. Appl. Clay Sci. 2002, 22, 25–38. [Google Scholar] [CrossRef]
- Thomson, S.; Ali, P. A Laboratory Study of the Swelling Properties of Sodium and Calcium Modifications of Lake Edmon Clay. In Proceedings of the 2nd International Conference on Expansive Soils; Texas A and M University: College Station, TX, USA, 1969; pp. 256–262. [Google Scholar]
- Westsik, J.H.; Bray, L.A.; Hodges, F.N.; Wheelwright, E.J. Permeability, swelling and radionuclide retardation properties of candidate backfill materials. In Scientific Basis for Nuclear Waste Management IV, Symposium D; Cambridge University Press: Cambridge, UK, 1981; Volume 6, p. 329. [Google Scholar] [CrossRef]
- Börgesson, L.; Pusch, R. Rheological Properties of a Calcium Smectite; Technical Report TR-87-31; Svensk Kärnbränslehantering AB (SKB): Stockholm, Sweden, 1987. [Google Scholar]
- Börgesson, L.; Hokmark, H.; Karnland, O. Rheological Properties of a Sodium Smectite Clay; Technical Report TR-88-30; Svensk Kärnbränslehantering AB (SKB): Stockholm, Sweden, 1988. [Google Scholar]
- Gens, A.; Alonso, E. A framework for the behaviour of unsaturated clay. Can. Geotech. J. 1992, 29, 1013–1032. [Google Scholar] [CrossRef]
- Komine, H.; Ogata, N. Simplified evaluation for swelling characteristics of bentonites. Can. Geotech. J. 1994, 31, 478–490. [Google Scholar] [CrossRef]
- Delage, P.; Howat, M.D.; Cui, Y.J. The relationship between suction and swelling properties in a heavily compacted unsaturated clay. Eng. Geol. 1998, 50, 31–48. [Google Scholar] [CrossRef]
- Cui, Y.J.; Yahia-Aissa, M.; Delage, P. A model for the volume change behaviour of heavily compacted swelling clays. Eng. Geol. 2002, 64, 233–250. [Google Scholar] [CrossRef]
- Karnland, O.; Muurinen, A.; Karlsson, F. Bentonite Swelling Pressure in NaCl Solutions: Experimentally Determined Data and Model Calculations. In Advances in Understanding Engineered Clay Barriers, Proceedings of the International Symposium on Large Scale Field Tests in Granite, Sitges, Barcelona, Spain, 12–14 November 2003; Eduardo, E.A., Alberto, L., Eds.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Xie, M.; Moog, H.C.; Kolditz, O. Geochemical Effects on Swelling Pressure of Highly Compacted Bentonite: Experiments and Model Analysis. In Theoretical and Numerical Unsaturated Soil Mechanics; Springer Proceedings in Physics 113 Series; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Jönsson, B.; Kesson, T.; Jönsson, B.; Meehdi, S.; Janiak, J.; Wallenberg, R. Structure and Forces in Bentonite MX-80; Technical Report TR-09-06; Svensk Kärnbränslehantering AB (SKB): Stockholm, Sweden, 2009. [Google Scholar]
- Lee, J.O.; Lim, J.G.; Kang, I.M.; Kwon, S. Swelling pressures of compacted Ca-bentonite. Eng. Geol. 2012, 129–130, 20–26. [Google Scholar] [CrossRef]
- Harrington, J.F.; Daniels, K.A.; Wiseall, A.C.; Sellin, P. Bentonite homogenisation during the closure of repository voids. Int. J. Rock Mech. Min. Sci. 2020, 136, 104535. [Google Scholar] [CrossRef]
- Daniels, K.A.; Harrington, J.F.; Sellin, P.; Norris, S. Closing repository void spaces using bentonite: Does heat make a difference? Appl. Clay Sci. 2021. [Google Scholar]
- Tripathy, S.; Thomas, H.R.; Stratos, P. Response of Compacted Bentonites to Thermal and Thermo-Hydraulic Loadings at High Temperatures. Geosciences 2017, 7. [Google Scholar] [CrossRef]
- Missana, T.; Alonso, U.; Albarran, N.; Garcia-Gutierrez, M.; Cormenzana, J.L. Analysis of colloids erosion from the bentonite barrier of a high level radioactive waste repository and implications in safety assessment. Phys. Chem. Earth 2011, 36, 1607–1615. [Google Scholar] [CrossRef]
- Alonso, U.; Missana, T.; Fernandez, A.M.; Garcia-Gutierrez, M. Erosion behaviour of raw bentonites under compacted and confined conditions: Relevance of smectite content and clay/water interactions. Appl. Geochem. 2018, 94, 11–20. [Google Scholar] [CrossRef]
- Svensson, D.; Eriksson, P.; Johannesson, L.E.; Lundgren, C.; Bladström, T. Developing Strategies and Methods for Material Control of Bentonite for a High Level Radioactive Waste Repository; Technical Report TR-19-25; Svensk Kärnbränslehantering AB (SKB): Stockholm, Sweden, 2019. [Google Scholar]
- Snyder, R.L.; Bish, D.L. Quantitative analysis. In Modern Powder Diffraction, Reviews in Mineralogy; Bish, D.L., Post, J.E., Eds.; Mineralogical Society of America: Chantilly, VA, USA, 1989; Volume 20, Chapter 5; pp. 101–144. [Google Scholar]
- Kemp, S.; Smith, F.; Wagner, D.; Mounteney, I.; Bell, C.; Milne, C.; Gowing, C.; Pottas, T. An improved approach to characterise potash-bearing evaporite deposits, evidenced in North Yorkshire, U.K. Eng. Geol. 2016, 111, 719–742. [Google Scholar] [CrossRef]
- Carter, D.L.; Heilman, M.D.; Gonzalez, F.L. Ethylene glycol monoethyl ether for determining surface area of silicate minerals. Soil Sci. 1965, 100, 356–360. [Google Scholar] [CrossRef]
- Missana, T.; Alonso, U.; Fernandez, A.M.; García-Gutiérrez, M. Colloidal properties of different smectite clays: Significance for the bentonite barrier erosion and radionuclide transport in radioactive waste repositories. Appl. Geochem. 2018, 97, 157–166. [Google Scholar] [CrossRef]
- Sen, T.; Khilar, K. Review on subsurface colloids and colloid-associated contaminant transport in saturated porous media. Adv. Colloid Interface Sci. 2006, 119, 71–96. [Google Scholar] [CrossRef]
- Neretnieks, I.; Liu, L.; Moreno, L. Mechanisms and Models for Bentonite Erosion; Technical Report TR-09-35; Svensk Kärnbränslehantering AB (SKB): Stockholm, Sweden, 2009. [Google Scholar]
- Birgersson, M.; Hedström, M.; Karnland, O. Sol formation ability of Ca/Na-montmorillonite at low ionic strength. Phys. Chem. Earth 2011, 36, 1572–1579. [Google Scholar] [CrossRef]
- Kallay, N.; Barouch, E.; Matijević, E. Diffusional detachment of colloidal particles from solid/solution interfaces. Adv. Colloid Interface Sci. 1987, 27, 1–42. [Google Scholar] [CrossRef]
- Inglethorpe, S.D.J.; Morgan, D.J.; Highley, D.E.; Bloodworth, A.J. Industrial Minerals Laboratory Manual: Bentonite; Technical Report WG-93-20; British Geological Survey: Nottingham, UK, 1993. [Google Scholar]
- Svensson, D.; Lundgren, C.; Johannesson, L.E.; Norrfors, K. Developing Strategies for Acquisition and Control of Bentonite for a High Level Radioactive Waste Repository; Technical Report TR-16-14; Svensk Kärnbränslehantering AB (SKB): Stockholm, Sweden, 2017. [Google Scholar]
- Ryan, J.N.; Elimelech, M. Review: Colloid mobilization and transport in groundwater. Colloids Surf. A Physicochem. Eng. Asp. 1996, 107, 1–56. [Google Scholar] [CrossRef]
- Missana, T.; Alonso, U.; García-Gutiérrez, M.; Mingarro, M. Role of bentonite colloids on europium and plutonium migration in a granite fracture. Appl. Geochem. 2008, 23, 1484–1497. [Google Scholar] [CrossRef]
- Schaefer, T.; Huber, F.; Seher, H.; Missana, T.; Alonso, U.; Kumke, M.; Eidner, S.; Claret, F.; Enzmann, F. Nanoparticles and their influence on radionuclide mobility in deep geological formations. Appl. Geochem. 2012, 27, 390–403. [Google Scholar] [CrossRef]
- Baik, M.H.; Cho, W.J.; Hahn, P.S. Erosion of bentonite particles at the interface of a compacted bentonite and a fractured granite. Eng. Geol. 2007, 91, 229–239. [Google Scholar] [CrossRef]
- Edmunds, W.M.; Shand, P.; Hart, P.; Ward, R.S. The natural (baseline) quality of groundwater: A UK pilot study. Sci. Total Environ. 2003, 310, 25–35. [Google Scholar] [CrossRef]
- Kurosawa, S.; Kato, H.; Ueta, S.; Yokoyama, K.; Fujihara, H. Erosion Properties and Dispersion-Flocculation Behavior of Bentonite Particles; (Symposium QQ—Scientific Basis for Nuclear Waste Management XXII); Cambridge University Press: Cambridge, UK, 1999; Volume 556, p. 679. [Google Scholar] [CrossRef]
- Jansson, M. Bentonite Erosion Laboratory Studies; Technical Report TR-09-33; Svensk Kärnbränslehantering AB (SKB): Stockholm, Sweden, 2009. [Google Scholar]
- Albarran, N.; Degueldre, C.; Missana, T.; Alonso, U.; Garcia-Gutierrez, M.; Lopez, T. Size distribution analysis of colloid generated from compacted bentonite in low ionic strength aqueous solutions. Appl. Clay Sci. 2014, 95, 284–293. [Google Scholar] [CrossRef]
- Birgersson, M.; Börgesson, L.; Hedström, M.; Karnland, O.; Nilsson, U. Bentonite Erosion Final Report; Technical Report TR-09-34; Svensk Kärnbränslehantering AB (SKB): Stockholm, Sweden, 2009. [Google Scholar]
- Fernández, A.M.; Missana, T.; Alonso, U.; Rey, J.J.; Sánchez-Ledesma, D.M.; Melón, A.; Robredo, L.M. Characterisation of Different Bentonites in the Context of BELBAR Project (Bentonite Erosion: Effects on the Long Term Performance of the Engineered Barrier and Radionuclide Transport); Technical Report; CIEMAT: Madrid, Spain, 2017. [Google Scholar]
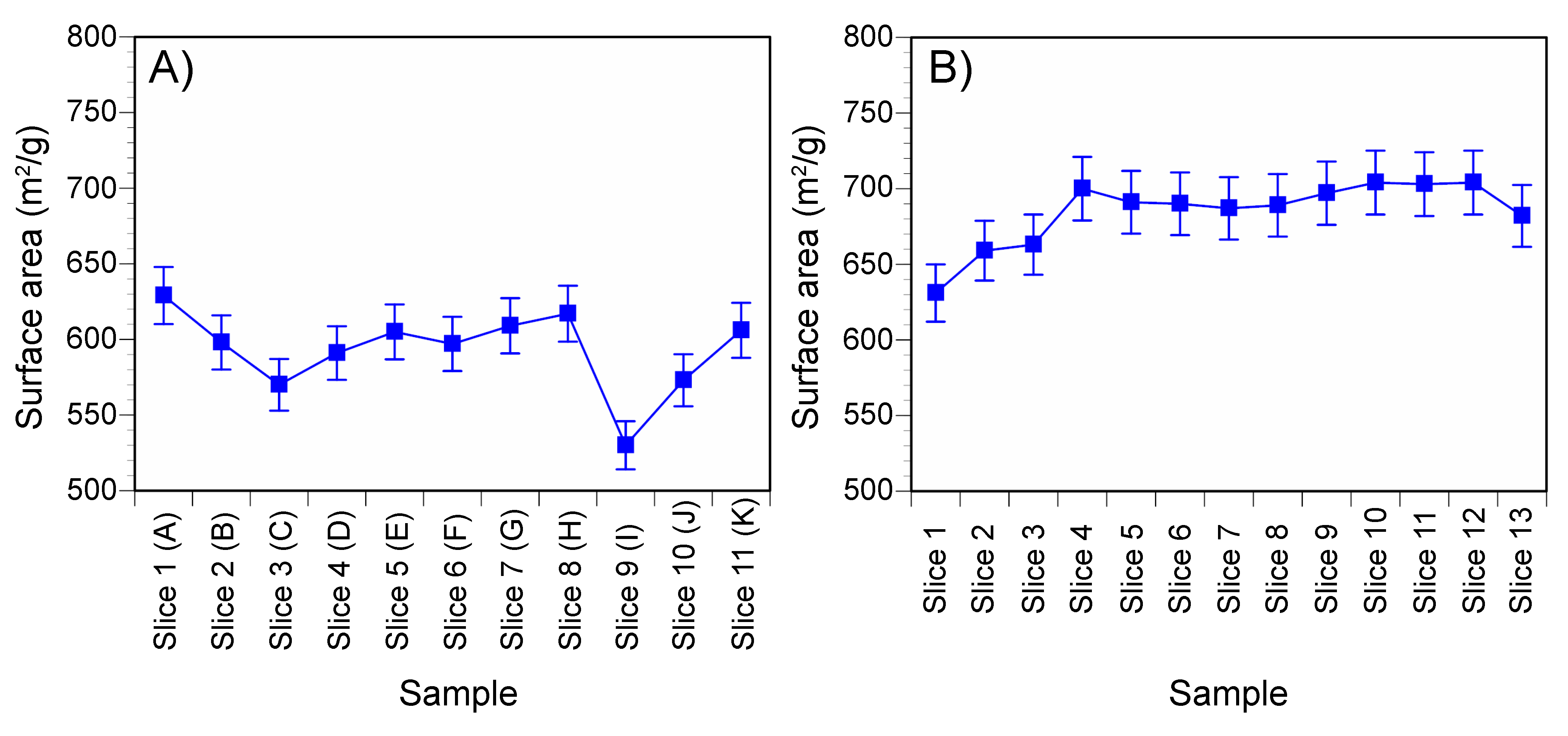
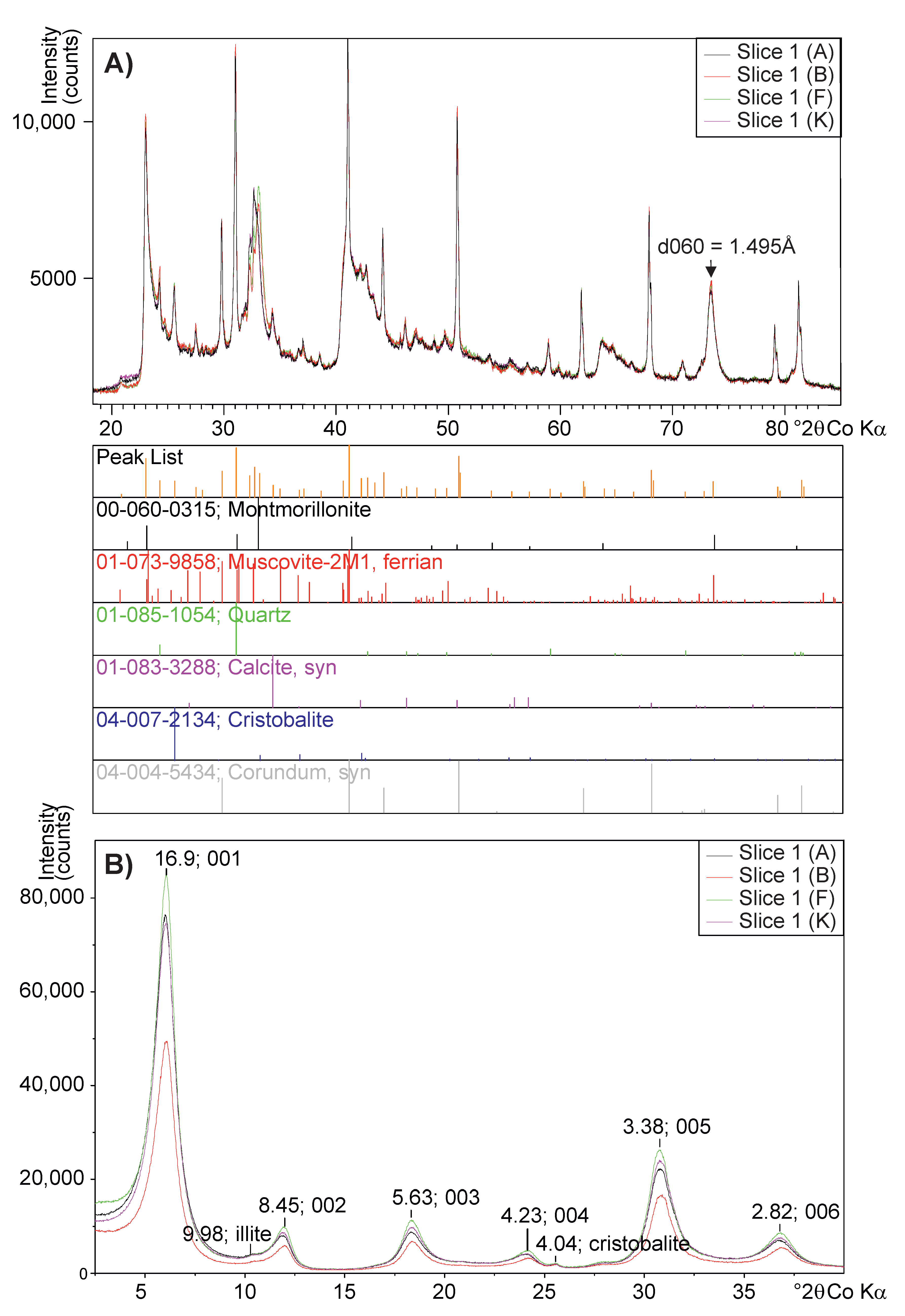
| Composition | Sample Length (mm) | Void Length (mm) | Sample Diameter (mm) | Sample Weight (g) | Bulk Density (kg/m) | Moisture Content | Fluid Pressure (kPa) | Test Duration (Days) |
|---|---|---|---|---|---|---|---|---|
| Bulgarian Ca bentonite | 64.97 | 51.03 | 59.76 | 368.32 | 2021.16 | 0.1525 | 4500 | 101.7 |
| <2 m | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Surface | Oriented XRD | Whole-Rock Powder XRD | ||||||||
| Bentonite | Slice | Area | d001 | Smectite | ’Mica’ | Quartz | Calcite | Cristo- | Total | |
| Type | No. | (m/g) | (Å) | FWHM | Balite | |||||
| Ca | 1 (A) | 629 | 13.28 | 2.95 | 77.8 | 15.1 | 1.7 | 4.1 | 1.2 | 100.0 |
| 2 (B) | 598 | 14.50 | 2.35 | 77.2 | 15.4 | 0.8 | 4.8 | 1.8 | 100.0 | |
| 3 (C) | 570 | — | — | — | — | — | — | — | — | |
| 4 (D) | 591 | — | — | — | — | — | — | — | — | |
| 5 (E) | 605 | — | — | — | — | — | — | — | — | |
| 6 (F) | 597 | 15.38 | 1.98 | 77.4 | 14.7 | <0.5 | 5.8 | 1.7 | 100.0 | |
| 7 (G) | 609 | — | — | — | — | — | — | — | — | |
| 8 (H) | 617 | — | — | — | — | — | — | — | — | |
| 9 (I) | 530 | — | — | — | — | — | — | — | — | |
| 10 (J) | 573 | — | — | — | — | — | — | — | — | |
| 11 (K) | 606 | 14.47 | 2.36 | 77.9 | 15.0 | <0.5 | 5.0 | 1.7 | 100.0 | |
| MX80 | 1 | 631 | 12.61 | 2.13 | 77.0 | 17.2 | 4.0 | 1.5 | <0.5 | 100.0 |
| 2 | 659 | 12.62 | 2.29 | 78.4 | 16.8 | 3.6 | 1.3 | <0.5 | 100.0 | |
| 3 | 663 | — | — | — | — | — | — | — | — | |
| 4 | 700 | — | — | — | — | — | — | — | — | |
| 5 | 691 | — | — | — | — | — | — | — | — | |
| 6 | 690 | — | — | — | — | — | — | — | — | |
| 7 | 687 | 12.91 | 2.26 | 77.7 | 15.8 | 4.8 | 1.2 | <0.5 | 100.0 | |
| 8 | 689 | — | — | — | — | — | — | — | — | |
| 9 | 697 | — | — | — | — | — | — | — | — | |
| 10 | 704 | — | — | — | — | — | — | — | — | |
| 11 | 703 | — | — | — | — | — | — | — | — | |
| 12 | 704 | — | — | — | — | — | — | — | — | |
| 13 | 682 | 12.87 | 2.27 | 77.4 | 16.5 | 4.5 | 1.1 | 0.5 | 100.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniels, K.A.; Harrington, J.F.; Milodowski, A.E.; Kemp, S.J.; Mounteney, I.; Sellin, P. Gel Formation at the Front of Expanding Calcium Bentonites. Minerals 2021, 11, 215. https://doi.org/10.3390/min11020215
Daniels KA, Harrington JF, Milodowski AE, Kemp SJ, Mounteney I, Sellin P. Gel Formation at the Front of Expanding Calcium Bentonites. Minerals. 2021; 11(2):215. https://doi.org/10.3390/min11020215
Chicago/Turabian StyleDaniels, Katherine A., Jon F. Harrington, Antoni E. Milodowski, Simon J. Kemp, Ian Mounteney, and Patrik Sellin. 2021. "Gel Formation at the Front of Expanding Calcium Bentonites" Minerals 11, no. 2: 215. https://doi.org/10.3390/min11020215
APA StyleDaniels, K. A., Harrington, J. F., Milodowski, A. E., Kemp, S. J., Mounteney, I., & Sellin, P. (2021). Gel Formation at the Front of Expanding Calcium Bentonites. Minerals, 11(2), 215. https://doi.org/10.3390/min11020215






