Optical and Luminescence Spectroscopy of Varicolored Gem Spinel from Mogok, Myanmar and Lục Yên, Vietnam
Abstract
1. Introduction
2. Geological Settings
3. Materials and Methods
4. Results
4.1. Chemical Composition
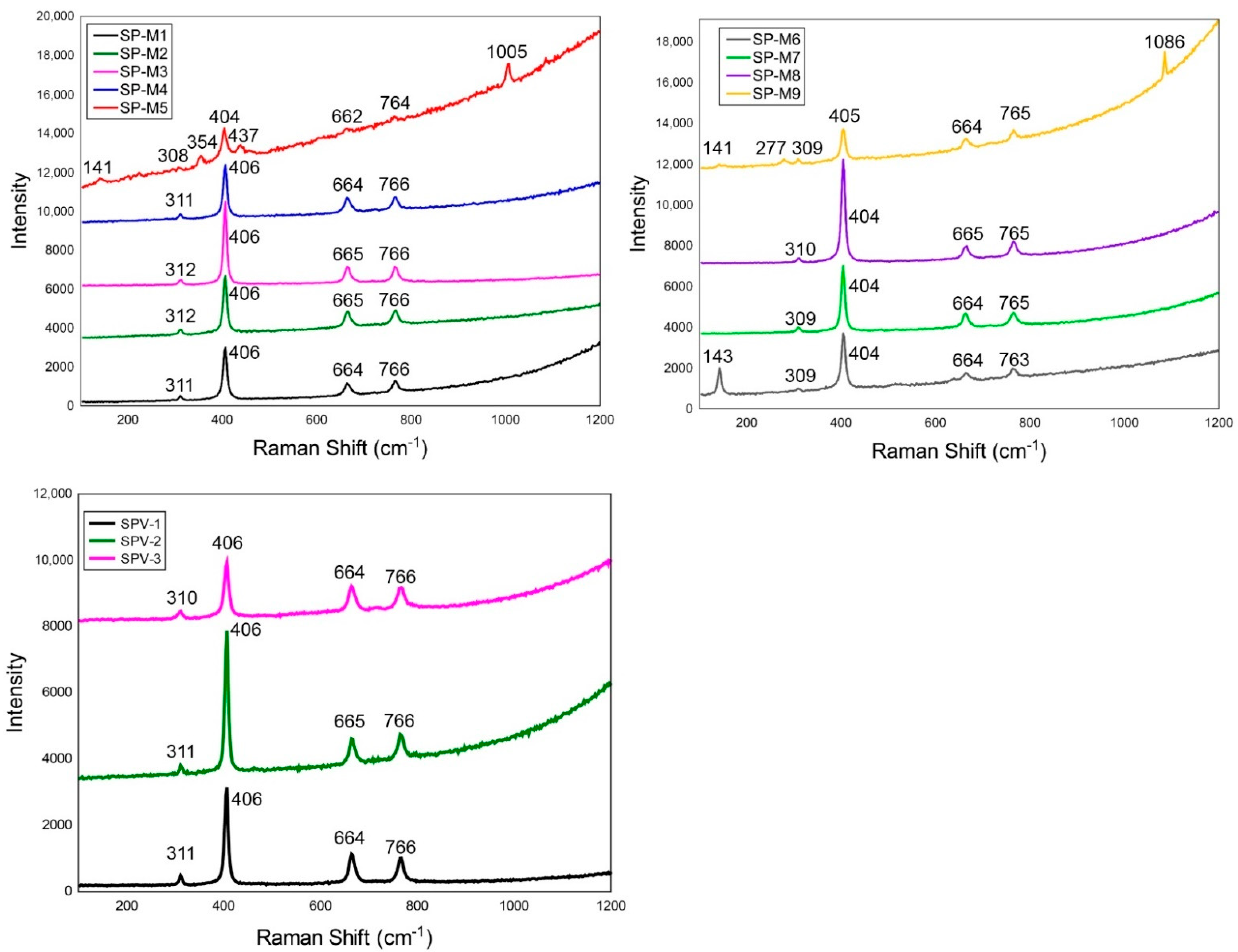
4.2. Raman Spectra
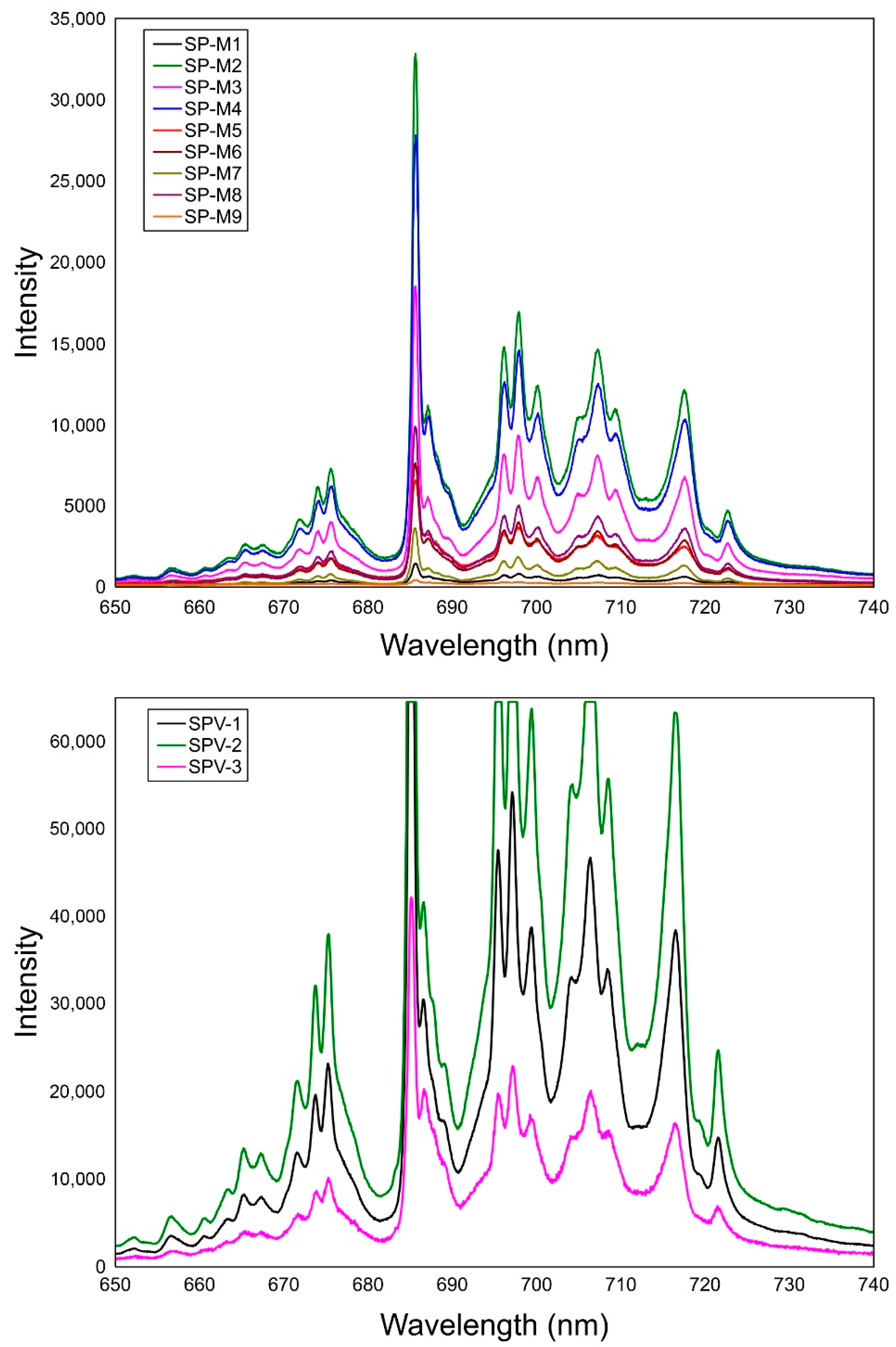
4.3. Luminescence Spectra
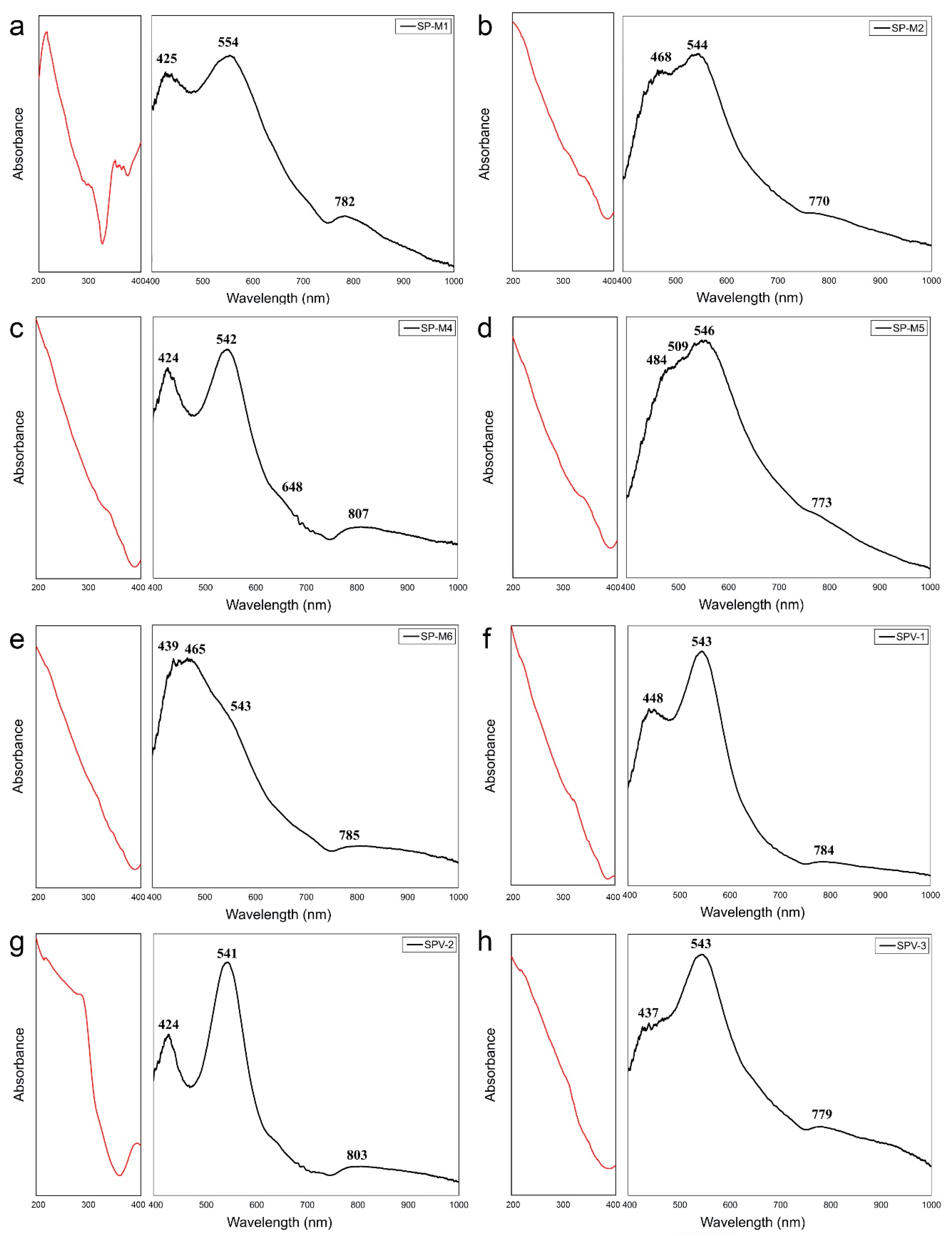
4.4. Optical Absorption Spectroscopy
5. Discussion
5.1. Raman Spectroscopy
5.2. Luminescence Spectroscopy
5.3. Optical Absorption Spectroscopy
5.3.1. V and Cr Spinels
5.3.2. Fe Spinels
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- D’Ippolito, V.; Andreozzi, G.B.; Hålenius, U.; Skogby, H.; Hametner, K.; Günther, D. Color mechanisms in spinel: Cobalt and iron interplay for the blue color. Phys. Chem. Miner. 2015, 42, 431–439. [Google Scholar] [CrossRef]
- Anthony, J.W.; Bideaux, R.A.; Bladh, W.; Nichols, M.C. Handbook of Mineralogy; Mineralogical Society of America: Chantilly, VA, USA, 2017; Available online: http://www.handbookofmineralogy.org/ (accessed on 19 February 2020).
- Bosi, F.; Biagioni, C.; Pasero, M. Nomenclature and classification of the spinel supergroup. Eur. J. Mineral. 2019, 31, 183–192. [Google Scholar] [CrossRef]
- Uchida, H.; Lavina, B.; Downs, R.T.; Chesley, J. Single–crystal X–ray diffraction of spinels from the San Carlos Volcanic Field, Arizona: Spinel as a geothermometer. Am. Mineral. 2005, 90, 1900–1908. [Google Scholar] [CrossRef]
- Cynn, H.; Sharma, S.K.; Cooney, T.F.; Nicol, M. High–temperature Raman investigation of order–disorder behavior in the MgAl2O4 spinel. Phys. Rev. B 1992, 45, 500–502. [Google Scholar] [CrossRef]
- Busca, G.; Lorenzelli, V.; Ramis, G.; Willeyi, R.J. Surface Sites on Spinel-Type and Corundum-Type Metal Oxide Powders. Am. Chem. Soc. 1993, 9, 1492–1499. [Google Scholar] [CrossRef]
- Hazen, R.M.; Yang, H. Effect of cation substitution and order–disorder on P–V–T equations of state of cubic spinels. Am. Mineral. 1999, 84, 1956–1960. [Google Scholar] [CrossRef]
- Kušnír, I. Mineral resources of Vietnam. Acta Montan. Slovaca 2000, 5, 165–172. [Google Scholar]
- Garnier, V.; Ohnenstetter, D.; Giuliani, G.; Maluski, H.; Deloule, E.; Phan Trong, T.; Pham Van, L.; Hoàng Quang, V. Age and significance of ruby-bearing marble from the Red River Shear Zone, northern Vietnam. Can. Mineral. 2005, 43, 1315–1329. [Google Scholar] [CrossRef]
- Leloup, P.H.; Arnaud, N.; Lacassin, R.; Kienast, J.R.; Harrison, T.M.; Phan Trong, T.T.; Replumaz, A.; Taponnier, P. New constraints on the structure, thermochronology, and timing of the Ailo Shan-Red River shear zone, SE Asia. J. Geophys. Res. Atmos. 2001, 106, 6683–6732. [Google Scholar] [CrossRef]
- Garnier, V.; Giuliani, G.; Ohnenstetter, D.; Fallick, A.E.; Dubessy, J.; Banks, D.; Vinh, H.Q.; Lhomme, T.; Maluski, H.; Pêcher, A.; et al. Marble-hosted ruby deposits from Central and South-East Asia: Toward a new genetic model. Ore Geol. Rev. 2008, 34, 169–191. [Google Scholar] [CrossRef]
- Chauviré, B.; Rondeau, B.; Fritsch, E.; Ressigeac, P.; Devidal, J.L. Blue spinel from the Luc Yen district of Vietnam. Gems Gemmol. 2015, 51, 2–17. [Google Scholar] [CrossRef]
- Long, P.V.; Pardieu, V.; Giuliani, G. Update on gemstone mining in Luc Yen, Vietnam. Gems Gemmol. 2013, 49, 233–245. [Google Scholar] [CrossRef]
- Barley, M.E.; Pickard, A.L.; Za, K.; Rak, P.; Doyle, M.G. Jurassic to Miocene magmatism and metamorphism in the Mogok Metamorphic Belt and the India-Eurasia collision in Myanmar. Tectonics 2003, 22, 1–11. [Google Scholar] [CrossRef]
- Searle, M.P.; Noble, S.R.; Cottle, J.M.; Waters, D.J.; Mitchell, A.H.G.; Hlaing, T.; Horstwood, M.S.A. Tectonic evolution of the Mogok Metamorphic Belt, Burma (Myanmar) constrained by U-Th-Pb dating of metamorphic and magmatic rocks. Tectonics 2007, 26, 1–24. [Google Scholar] [CrossRef]
- Thu, K. The Igneous Rocks of the Mogok Stone Tract: Their Distributions, Petrography, Petrochemistry, Sequence, Geochronology and Economic Geology. Ph.D. Thesis, Department of Geology, University of Yangon, Yangon, Myanmar, 2007. Available online: http://m.palaminerals.com/mogok (accessed on 17 December 2020).
- Iyer, L.A.N. The geology and gemstones of the Mogok Stone Tract, Burma. Mem. Geol. Surv. India 1953, 82, 100. [Google Scholar]
- Thein, M. Modes of occurrence and origin of precious gemstone deposits of the Mogok Stone Tract. J. Myanmar Geosci. Soc. 2008, 1, 75–84. [Google Scholar]
- Mogok Township, Pyin–Oo–Lwin District, Mandalay Region, Myanmar. Available online: https://www.mindat.org/loc-24345.html (accessed on 24 February 2019).
- Chopelas, A.; Hofmeister, A.M. Vibrational spectroscopy of aluminate spinels at 1 atm and of MgAl2O4 to over 200 kbar. Phys. Chem. Miner. 1991, 18, 279–293. [Google Scholar] [CrossRef]
- Gaft, M.; Reisfeld, R.; Panczer, G. Modern Luminescence Spectroscopy of Minerals and Materials, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2015; p. 606. [Google Scholar]
- Andreozzi, G.B.; D’Ippolito, V.; Skogby, H.; Hålenius, U.; Bosi, F. Color mechanisms in spinel: A multi-analytical investigation of natural crystals with range of coloration. Phys. Chem. Miner. 2018, 42, 431–439. [Google Scholar] [CrossRef]
- Lazzeri, M.; Thibaudeau, P. Ab initio Raman spectrum of the normal and disordered MgAl2O4 spinel. Phys. Rev. B 2006, 74, 140301. [Google Scholar] [CrossRef]
- Slotznick, S.P.; Shim, S.H. In situ Raman spectroscopy measurements of Mg Al2O4 spinel up to 1400 °C. Am. Mineral. 2008, 93, 470–476. [Google Scholar] [CrossRef]
- Widmer, R.; Malsy, A.K.; Armbruster, T. Effect of heat treatment on red gemstone spinel: Single- crystal X–ray, Raman, and photoluminescence study. Phys. Chem. Miner. 2015, 42, 251–260. [Google Scholar] [CrossRef]
- Phyo, M.M.; Bieler, E.; Franz, L.; Balmer, W.; Krzemnicki, M.S. Spinel from Mogok Myanmar–A detailed inclusion study by Raman Microspectroscopy and Scanning Electron Microscopy. J. Gemol. 2019, 36, 418–435. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Anbalagan, G.; Pandi, S. Raman and infrared spectra of carbonates of calcite structure. J. Raman Spectrosc. 2006, 37, 892–899. [Google Scholar] [CrossRef]
- Jouini, A. MgAl2O4 Spinel Laser Crystals: Pure and Ti-, Mn-, or Ni- Doped. Shaped Crystals. In Advances in Materials Research, 8; Fukuda, T., Chani, V.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 203–215. [Google Scholar]
- Mohler, R.L.; White, W.B. Influence of Structural Order on the Luminescence of Oxide Spinels: Cr3+−Activated Spinels. J. Electrochem. Soc. 1995, 11, 3923–3927. [Google Scholar] [CrossRef]
- Derkosch, J.; Mikenda, W.; Preisinger, A. N–Lines and Chromium–Pairs in the Luminescence Spectra of the Spinels ZnAl2O4: Cr3+ and MgAl2O4: Cr3+. J. Solid State Chem. 1977, 22, 127–133. [Google Scholar] [CrossRef]
- Tarashchan, A. Luminescence of Minerals; Naukova Dumka: Kiev, Ukraine, 1978. (In Russian) [Google Scholar]
- De Souza, S.S.; Ayres, F.; Blak, A.R. Simulation models of deffects in MgAl2O4:Fe2+, Fe3+ spinels. Radiat. Eff. Defects Solids 2001, 156, 311–316. [Google Scholar] [CrossRef]
- Nassau, K. The Physics and Chemistry of Color, 2nd ed.; John Wiley & Sons, Inc.: Etobicoke, ON, Canada, 1983; p. 454. [Google Scholar]
- Nassau, K. The origins of color in minerals. Am. Mineral. 1978, 63, 219–229. [Google Scholar]
- Tilley, R.J.D. Transition–Metal Ion Colors. In Encyclopedia of Color Science and Technology; Springer Science: New York, NY, USA, 2013; pp. 1–10. [Google Scholar]
- Burns, R.G. Mineralogical Applications of Crystal Field Theory, 2nd ed.; Cambridge University Press: Cambridge, UK, 1993; p. 576. [Google Scholar]
- Taran, M.N.; Koch-Müller, M.; Langer, K. Electronic absorption spectroscopy of natural (Fe2+, Fe3+)-bearing spinels of spinel s.s.-hercynite and gahnite-hercynite solid solutions at different temperatures and high-pressures. Phys. Chem. Miner. 2005, 32, 175–188. [Google Scholar] [CrossRef]
- Dickson, B.L.; Smith, G. Low temperature optical absorption and Mössbauer spectra of staurolite and spinel. Can. Mineral. 1976, 14, 206–215. [Google Scholar]
- Schmetzer, K.; Haxel, C.; Bank, H. Colour of natural spinels, gahnospinels and gahnites. Neues Jahrb. Mineral. 1989, 160, 159–180. [Google Scholar]
- Fregola, R.A.; Skogby, H.; Bosi, F.; D’Ippolito, V.; Andreozzi, G.B.; Hålenius, U. Optical absorption spectroscopy study of the causes for color variations in natural Fe-bearing gahnite: Insights from iron valency and site distribution data. Am. Mineral. 2014, 99, 2187–2195. [Google Scholar] [CrossRef]
- Hålenius, U.; Skogby, H.; Andreozzi, G.B. Influence of cation distribution on the optical absorption spectra of Fe3+-bearing spinel s.s.–hercynite crystals: Evidence for electron transitions in VIFe2+–VIFe3+ clusters. Phys. Chem. Miner. 2002, 29, 319–330. [Google Scholar]

| SP–M1 | SP–M2 | SP–M3 | SP–M4 | SP–M5 | SP–M6 | SP–M7 | SP–M8 | SP–M9 | SPV–1 | SPV–2 | SPV–3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 0.05 | 0.07 | 0.08 | 0.04 | 0.06 | 0.02 | 0.05 | 0.04 | 0.05 | 0.03 | 0.06 | 0.04 |
| TiO2 | 0.01 | 0.01 | 0.01 | 0.03 | 0.14 | 0.00 | 0.00 | 0.00 | 0.01 | 0.04 | 0.00 | 0.08 |
| Al2O3 | 71.28 | 71.64 | 71.29 | 71.48 | 71.12 | 71.06 | 70.27 | 70.36 | 70.80 | 70.87 | 70.52 | 70.86 |
| V2O3 | 0.12 | 0.12 | 0.01 | 0.08 | 0.24 | 0.11 | 0.04 | 0.05 | 0.01 | 0.19 | 0.06 | 0.07 |
| Cr2O3 | 0.00 | 0.06 | 0.07 | 0.04 | 0.07 | 0.02 | 0.04 | 0.03 | 0.02 | 0.17 | 0.31 | 0.12 |
| Fe2O3 | 0.14 | 0.03 | 0.20 | 0.11 | 0.22 | 0.15 | 0.65 | 0.36 | 0.38 | 0.22 | 0.46 | 0.35 |
| FeO | 0.12 | 0.05 | 0.05 | 0.00 | 0.11 | 0.00 | 0.85 | 0.65 | 0.11 | 0.08 | 0.00 | 0.31 |
| MnO | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.02 | 0.01 | 0.02 |
| MgO | 28.12 | 28.23 | 28.19 | 28.25 | 28.30 | 28.39 | 27.34 | 27.49 | 28.01 | 28.08 | 28.18 | 27.92 |
| ZnO | 0.11 | 0.16 | 0.06 | 0.33 | 0.00 | 0.07 | 0.28 | 0.13 | 0.03 | 0.11 | 0.10 | 0.26 |
| NiO | 0.01 | 0.01 | 0.01 | 0.03 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | 0.00 |
| CoO | 0.02 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.02 | 0.00 | 0.00 |
| CaO | 0.01 | 0.00 | 0.01 | 0.02 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 |
| Total | 99.98 | 100.44 | 99.99 | 100.40 | 100.31 | 99.82 | 99.54 | 99.12 | 99.42 | 99.83 | 99.71 | 100.02 |
| Si4+ | 0.001 | 0.002 | 0.002 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| Al3+ | 1.995 | 1.996 | 1.994 | 1.992 | 1.985 | 1.989 | 1.987 | 1.992 | 1.993 | 1.988 | 1.981 | 1.987 |
| V3+ | 0.002 | 0.002 | 0.000 | 0.002 | 0.004 | 0.002 | 0.001 | 0.001 | 0.000 | 0.004 | 0.001 | 0.001 |
| Cr3+ | 0.000 | 0.001 | 0.001 | 0.001 | 0.001 | 0.000 | 0.001 | 0.001 | 0.000 | 0.003 | 0.006 | 0.002 |
| Fe3+ | 0.003 | 0.001 | 0.004 | 0.002 | 0.006 | 0.003 | 0.012 | 0.006 | 0.007 | 0.005 | 0.008 | 0.009 |
| Ti4+ | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Mg2+ | 0.000 | 0.000 | 0.000 | 0.003 | 0.003 | 0.006 | 0.000 | 0.000 | 0.000 | 0.000 | 0.003 | 0.000 |
| B | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 |
| Ti4+ | 0.000 | 0.000 | 0.000 | 0.000 | 0.003 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.002 |
| Fe2+ | 0.002 | 0.001 | 0.001 | 0.000 | 0.000 | 0.000 | 0.017 | 0.013 | 0.002 | 0.000 | 0.000 | 0.003 |
| Mn2+ | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Mg2+ | 0.995 | 0.995 | 0.998 | 0.993 | 0.996 | 0.998 | 0.978 | 0.984 | 0.997 | 0.996 | 0.998 | 0.990 |
| Zn2+ | 0.002 | 0.003 | 0.001 | 0.006 | 0.000 | 0.001 | 0.005 | 0.002 | 0.000 | 0.002 | 0.002 | 0.005 |
| Ni2+ | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Co2+ | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Ca2+ | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| A | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| Mineral | Symmetry | SP–M1 | SP–M2 | SP–M3 | SP–M4 | SP–M5 | SP–M6 |
|---|---|---|---|---|---|---|---|
| 141 | 143 | ||||||
| Spinel | T2g | 311 | 312 | 312 | 311 | 308 | 309 |
| 354 | |||||||
| Spinel | Eg | 406 | 406 | 406 | 406 | 404 | 404 |
| 437 | |||||||
| Spinel | T2g | 664 | 665 | 665 | 664 | 662 | 664 |
| Spinel | A1g | 766 | 767 | 766 | 766 | 764 | 763 |
| 1005 | |||||||
| SP–M7 | SP–M8 | SP–M9 | SPV–1 | SPV–2 | SPV–3 | ||
| Calcite/Dolomite | 141 | ||||||
| Calcite/Dolomite | 277 | ||||||
| Spinel | T2g | 309 | 310 | 309 | 311 | 311 | 310 |
| Spinel | Eg | 404 | 404 | 405 | 406 | 406 | 406 |
| Spinel | T2g | 664 | 665 | 664 | 664 | 665 | 664 |
| Spinel | A1g | 765 | 765 | 765 | 766 | 766 | 766 |
| Calcite/Dolomite | 1086 |
| Sample | Provenance | Color | Determined Chromophore |
|---|---|---|---|
| SP–M1 | Mogok, Myanmar | Light grayish pink | Vanadium–chromium |
| SP–M2 | Mogok, Myanmar | pale orange | Vanadium–chromium |
| SP–M3 | Mogok, Myanmar | very pale pink | Iron (Fe3+) |
| SP–M4 | Mogok, Myanmar | purplish pink | Vanadium–chromium |
| SP–M5 | Mogok, Myanmar | very dark red | Vanadium–chromium |
| SP–M6 | Mogok, Myanmar | very soft orange | Vanadium–chromium |
| SP–M7 | Mogok, Myanmar | very dark cyan | Iron (Fe2+) |
| SP–M8 | Mogok, Myanmar | dark blue | Iron (Fe2+) |
| SP–M9 | Mogok, Myanmar | light grayish yellow | Iron (Fe3+) |
| SPV–1 | Lục Yên, Yên Bái, Vietnam | reddish pink | Vanadium–chromium |
| SPV–2 | Lục Yên, Yên Bái, Vietnam | strong pink | Chromium–vanadium |
| SPV–3 | Lục Yên, Yên Bái, Vietnam | grayish pink | Chromium–vanadium |
| SP–M1 | SP–M2 | SP–M4 | SP–M5 | SP–M6 | SPV–1 | SPV–2 | SPV–3 | Assignment |
|---|---|---|---|---|---|---|---|---|
| 398 | 399 | 396 | 397 | 399 | 397 | V3 | ||
| 425 | 424 | 484 | 439 | 448 | 424 | 437 | V3+, Cr3+ | |
| 468 | 509 | 465 | Cr3+ | |||||
| 554 | 544 | 542 | 546 | 543 | 543 | 541 | 543 | V3+, Cr3+ |
| 648 | ||||||||
| 782 | 770 | 773 | 785 | 784 | 779 | Fe2+ | ||
| 807 | 803 | Fe2+ |
| SP–M3 | SP–M7 | SP–M8 | SP–M9 | Assignment |
|---|---|---|---|---|
| 438 | 415 | 422 | 426 | Fe2+ |
| 464 | 461 | 461 | 461 | Fe3+ |
| 541 | 557 | 553 | 557 | Fe2+ |
| 641 | 647 | 639 | 641 | IVCT Fe2+↔Fe3+ |
| 778 | 775 | 770 | 795 | Fe2+ |
| 914 | 908 | 932 | Fe2+ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malíčková, I.; Bačík, P.; Fridrichová, J.; Hanus, R.; Illášová, Ľ.; Štubňa, J.; Furka, D.; Furka, S.; Škoda, R. Optical and Luminescence Spectroscopy of Varicolored Gem Spinel from Mogok, Myanmar and Lục Yên, Vietnam. Minerals 2021, 11, 169. https://doi.org/10.3390/min11020169
Malíčková I, Bačík P, Fridrichová J, Hanus R, Illášová Ľ, Štubňa J, Furka D, Furka S, Škoda R. Optical and Luminescence Spectroscopy of Varicolored Gem Spinel from Mogok, Myanmar and Lục Yên, Vietnam. Minerals. 2021; 11(2):169. https://doi.org/10.3390/min11020169
Chicago/Turabian StyleMalíčková, Iveta, Peter Bačík, Jana Fridrichová, Radek Hanus, Ľudmila Illášová, Ján Štubňa, Daniel Furka, Samuel Furka, and Radek Škoda. 2021. "Optical and Luminescence Spectroscopy of Varicolored Gem Spinel from Mogok, Myanmar and Lục Yên, Vietnam" Minerals 11, no. 2: 169. https://doi.org/10.3390/min11020169
APA StyleMalíčková, I., Bačík, P., Fridrichová, J., Hanus, R., Illášová, Ľ., Štubňa, J., Furka, D., Furka, S., & Škoda, R. (2021). Optical and Luminescence Spectroscopy of Varicolored Gem Spinel from Mogok, Myanmar and Lục Yên, Vietnam. Minerals, 11(2), 169. https://doi.org/10.3390/min11020169






