Preliminary Data on Geochemical Characteristics of Major and Trace Elements in Typical Biominerals: From the Perspective of Human Kidney Stones
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
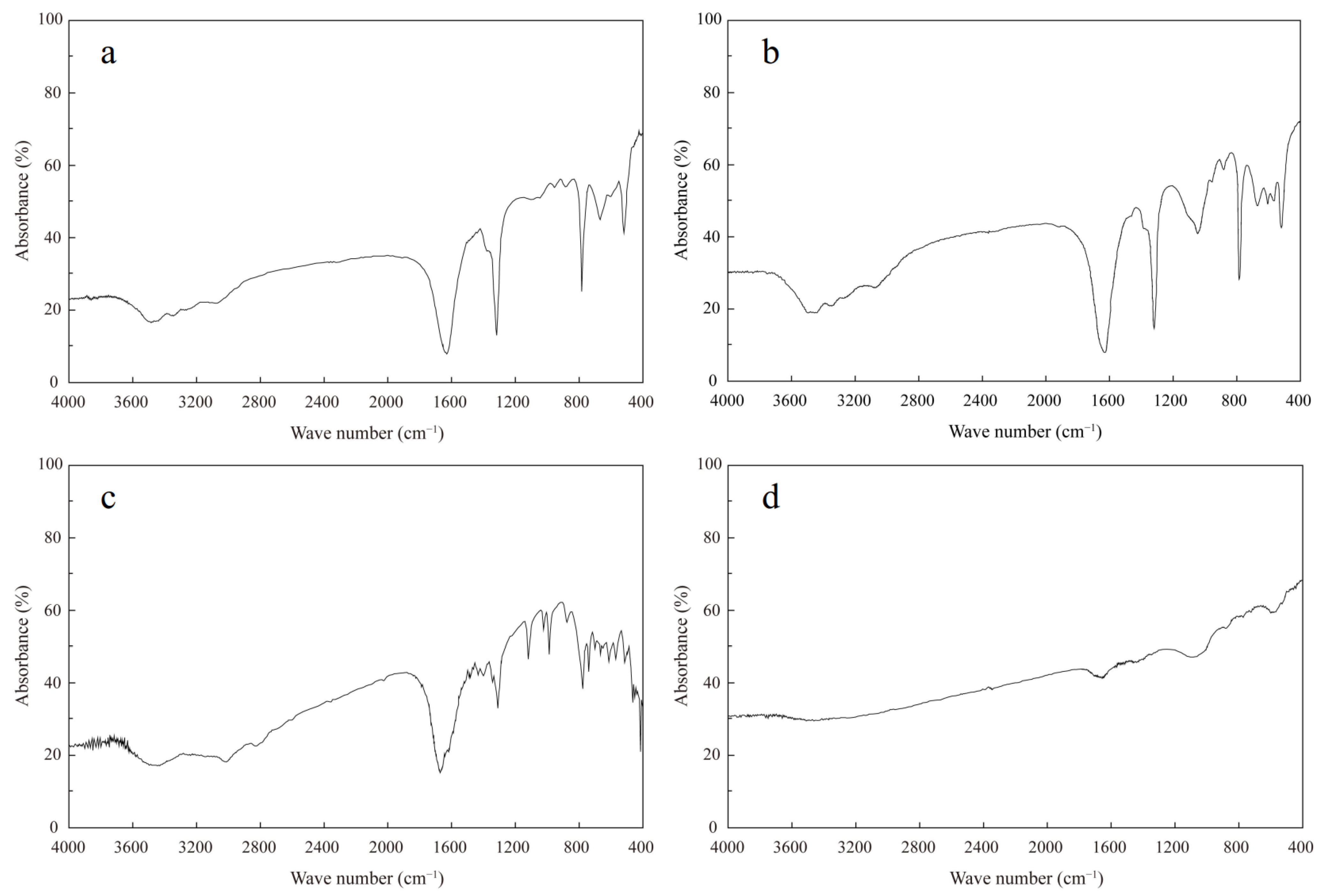
3.1. Mineralogical Compositions of Kidney Stones
3.2. Chemical Compositions of Kidney Stones
3.3. Statistical Analysis and Source Identification
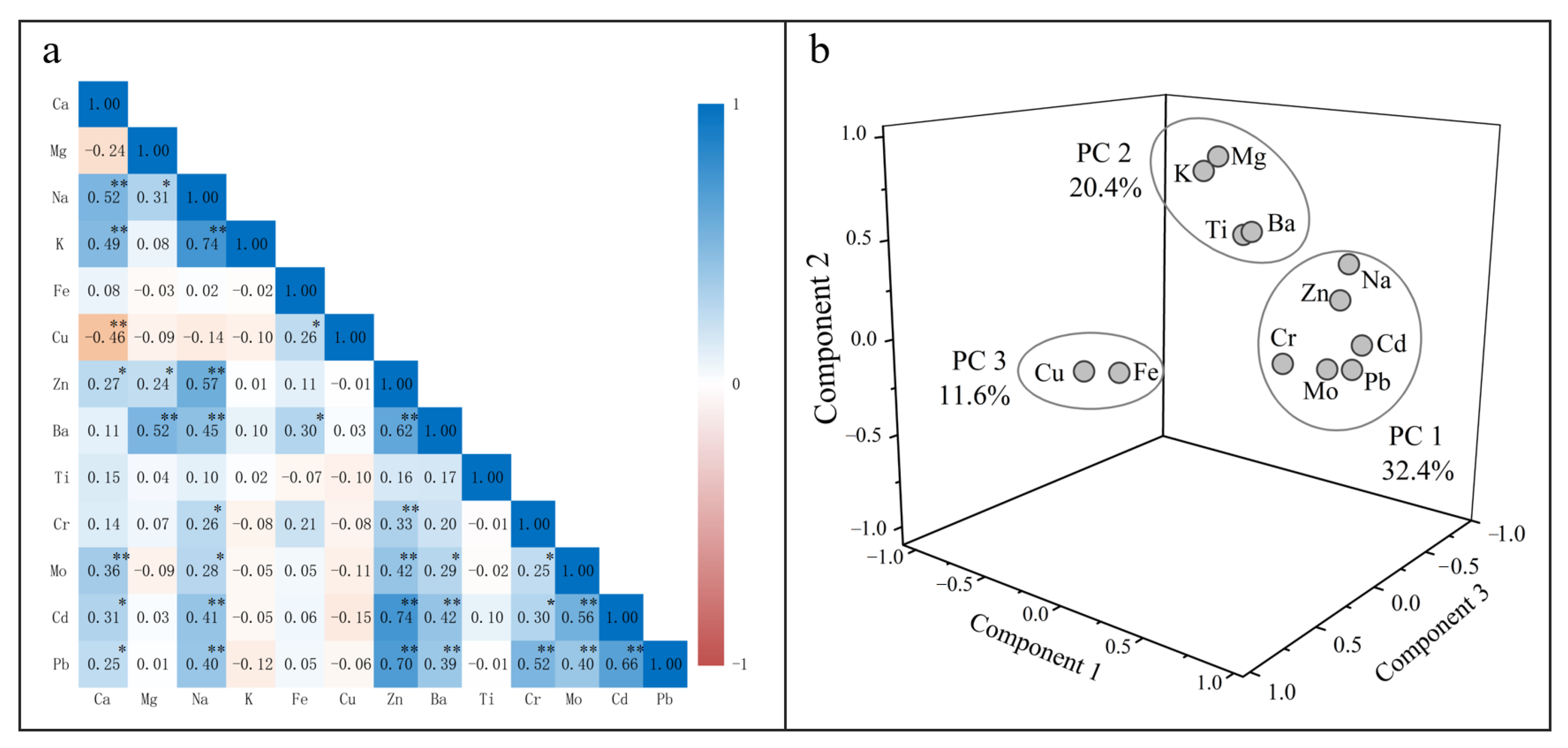
3.3.1. Correlation Analysis
3.3.2. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gebauer, D.; Jansson, K.; Oliveberg, M.; Hedin, N. Indications that Amorphous Calcium Carbonates Occur in Pathological Mineralisation—A Urinary Stone from a Guinea Pig. Minerals 2018, 8, 84. [Google Scholar] [CrossRef] [Green Version]
- Chandrajith, R.; Weerasingha, A.; Premaratne, K.M.; Gamage, D.; Abeygunasekera, A.M.; Joachimski, M.M.; Senaratne, A. Mineralogical, compositional and isotope characterization of human kidney stones (urolithiasis) in a Sri Lankan population. Environ. Geochem. Health 2019, 41, 1881–1894. [Google Scholar] [CrossRef] [PubMed]
- Izatulina, A.R.; Gurzhiy, V.V.; Krzhizhanovskaya, M.G.; Chukanov, N.V.; Panikorovskii, T.L. Thermal Behavior and Phase Transition of Uric Acid and Its Dihydrate Form, the Common Biominerals Uricite and Tinnunculite. Minerals 2019, 9, 373. [Google Scholar] [CrossRef] [Green Version]
- Chandrajith, R.; Wijewardana, G.; Dissanayake, C.B.; Abeygunasekara, A. Biomineralogy of human urinary calculi (kidney stones) from some geographic regions of Sri Lanka. Environ. Geochem. Health 2006, 28, 393–399. [Google Scholar] [CrossRef]
- Wrobel, A.; Rokita, E.; Taton, G.; Thor, P. Chemical composition and morphology of renal stones. Folia Med. Crac. 2013, 53, 5–15. [Google Scholar]
- Heilberg, I.P.; Schor, N. Renal stone disease: Causes, evaluation and medical treatment. Arq. Bras. Endocrinol. Metabol. 2006, 50, 823–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krouse, H.R.; Levinson, A.A.; Piggott, D.; Ueda, A. Further stable isotope investigations of human urinary stones: Comparison with other body components. Appl. Geochem. 1987, 2, 205–211. [Google Scholar] [CrossRef]
- Reynolds, T.M. ACP Best Practice No 181: Chemical pathology clinical investigation and management of nephrolithiasis. J. Clin. Pathol. 2005, 58, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Parks, J.H.; Worcester, E.M.; Coe, F.L.; Evan, A.P.; Lingeman, J.E. Clinical implications of abundant calcium phosphatein routinely analyzed kidney stones. Kidney Int. 2004, 66, 777–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaschko, S.D.; Miller, J.; Chi, T.; Flechner, L.; Fakra, S.; Kahn, A.; Kapahi, P.; Stoller, M.L. Microcomposition of Human Urinary Calculi Using Advanced Imaging Techniques. J. Urol. 2013, 189, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Tang, Y.; Liu, M.; Van Zwieten, L.; Yang, X.; Yu, C.; Wang, H.; Song, Z. Carbon-nitrogen isotope coupling of soil organic matter in a karst region under land use change, Southwest China. Agric. Ecosyst. Environ. 2020, 301, 107027. [Google Scholar] [CrossRef]
- Abeywickarama, B.; Ralapanawa, U.; Chandrajith, R. Geoenvironmental factors related to high incidence of human urinary calculi (kidney stones) in Central Highlands of Sri Lanka. Environ. Geochem. Health 2016, 38, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Kustov, A.V.; Strelnikov, A.I. Quantitative Mineralogical Composition of Calculi and Urine Abnormalities for Calcium Oxalate Stone Formers: A Single-Center Results. Urol. J. 2018, 15, 87–91. [Google Scholar] [CrossRef]
- Cloutier, J.; Villa, L.; Traxer, O.; Daudon, M. Kidney stone analysis: “Give me your stone, I will tell you who you are!”. World J. Urol. 2015, 33, 157–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kustov, A.V.; Berezin, B.D.; Strel’nikov, A.I.; Shevyrin, A.A.; Trostin, V.N. Interaction of a complexing agent with urolith as the basis for efficient little-invasive therapy of phosphaturia. Dokl. Phys. Chem. 2009, 428, 175–177. [Google Scholar] [CrossRef]
- Kustov, A.V.; Berezin, B.D.; Trostin, V.N. The Complexon−Renal Stone Interaction: Solubility and Electronic Microscopy Studies. J. Phys. Chem. B 2009, 113, 9547–9550. [Google Scholar] [CrossRef]
- Kustov, A.V.; Shevyrin, A.A.; Strel’nikov, A.I.; Smirnov, P.R.; Trostin, V.N. Chemolysis of calcium oxalate stones: Study in vitro and possible clinical application. Urol. Res. 2012, 40, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Fraser, I.; Meier-Augenstein, W.; Kalin, R.M. The role of stable isotopes in human identification: A longitudinal study into the variability of isotopic signals in human hair and nails. Rapid Commun. Mass Spectrom. 2006, 20, 1109–1116. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; Beiyuan, J.; Guo, X.; Wu, H.; Fang, L. Environmental and health risk assessment of potentially toxic trace elements in soils near uranium (U) mines: A global meta-analysis. Sci. Total Environ. 2021, 151556, in press. [Google Scholar] [CrossRef]
- Hareendra, P.P.G.K.; Hunais, M.M.; Suvendiran, S.; Palihakkara, S.D.; Abeygunasekera, A.M. Chemical composition of kidney stones obtained from a cohort of Sri Lankan patients. Sri Lanka J. Surg. 2015, 33, 14–19. [Google Scholar] [CrossRef]
- Li, X.; Han, G. One-step chromatographic purification of K, Ca, and Sr from geological samples for high precision stable and radiogenic isotope analysis by MC-ICP-MS. J. Anal. At. Spectrom. 2021, 36, 676–684. [Google Scholar] [CrossRef]
- Zeng, J.; Han, G.; Yang, K. Assessment and sources of heavy metals in suspended particulate matter in a tropical catchment, northeast Thailand. J. Clean. Prod. 2020, 265, 121898. [Google Scholar] [CrossRef]
- Li, X.; Han, G.; Liu, M.; Liu, J.; Zhang, Q.; Qu, R. Potassium and its isotope behaviour during chemical weathering in a tropical catchment affected by evaporite dissolution. Geochim. Cosmochim. Acta 2022, 316, 105–121. [Google Scholar] [CrossRef]
- Zeng, J.; Han, G. Preliminary copper isotope study on particulate matter in Zhujiang River, southwest China: Application for source identification. Ecotoxicol. Environ. Saf. 2020, 198, 110663. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Han, G. Tracing zinc sources with Zn isotope of fluvial suspended particulate matter in Zhujiang River, southwest China. Ecol. Indic. 2020, 118, 106723. [Google Scholar] [CrossRef]
- Chatterjee, P.; Chakraborty, A.; Mukherjee, A.K. Phase composition and morphological characterization of human kidney stones using IR spectroscopy, scanning electron microscopy and X-ray Rietveld analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 200, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.A.; Paul, P. Analysis of urinary stone constituents using powder X-ray diffraction and FT-IR. J. Chem. Sci. 2008, 120, 267–273. [Google Scholar] [CrossRef]
- Afaj, A.H.; Sultan, M.A. Mineralogical Composition of the Urinary Stones from Different Provinces in Iraq. Sci. World J. 2005, 5, 792841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuzaki, S.; Matsushita, K.; Tanikawa, K.; Masuda, A.; Matsunaga, J. Sequential analysis of recurrent calcium calculi by infrared spectroscopy. Int. J. Urol. 1995, 2, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Sekkoum, K.; Cheriti, A.; Taleb, S.; Belboukhari, N. FTIR spectroscopic study of human urinary stones from El Bayadh district (Algeria). Arab. J. Chem. 2016, 9, 330–334. [Google Scholar] [CrossRef] [Green Version]
- Levinson, A.A.; Mino, M.P.Y.; Stams, U.K.; Hariharan, A. The mineralogy of human urinary stones from Calgary, Quito and Honolulu. Am. Mineral. 1985, 70, 630–635. [Google Scholar]
- Ries, J.B. Review: Geological and experimental evidence for secular variation in seawater Mg/Ca (calcite-aragonite seas) and its effects on marine biological calcification. Biogeosciences 2010, 7, 2795–2849. [Google Scholar] [CrossRef] [Green Version]
- Wandt, M.A.E.; Underhill, L.G. Covariance Biplot Analysis of Trace Element Concentrations in Urinary Stones. J. Urol. 1989, 141, 1275. [Google Scholar] [CrossRef]
- Pal’chik, N.A.; Moroz, T.N.; Maksimova, N.V.; Dar’in, A.V. Mineral and microelement compositions of urinary stones. Russ. J. Inorg. Chem. 2006, 51, 1098–1105. [Google Scholar] [CrossRef]
- Abboud, I.A. Mineralogy and chemistry of urinary stones: Patients from North Jordan. Environ. Geochem. Health 2008, 30, 445–463. [Google Scholar] [CrossRef]
- Xu, S.; Li, S.-L.; Yue, F.; Udeshani, C.; Chandrajith, R. Natural and Anthropogenic Controls of Groundwater Quality in Sri Lanka: Implications for Chronic Kidney Disease of Unknown Etiology (CKDu). Water 2021, 13, 2724. [Google Scholar] [CrossRef]
- Muñoz, J.A.; Valiente, M. Effects of trace metals on the inhibition of calcium oxalate crystallization. Urol. Res. 2005, 33, 267–272. [Google Scholar] [CrossRef]
- Bazin, D.; Chevallier, P.; Matzen, G.; Jungers, P.; Daudon, M. Heavy elements in urinary stones. Urol. Res. 2007, 35, 179–184. [Google Scholar] [CrossRef]
- Jayatilake, N.; Mendis, S.; Maheepala, P.; Mehta, F.R. Chronic kidney disease of uncertain aetiology: Prevalence and causative factors in a developing country. BMC Nephrol. 2013, 14, 180. [Google Scholar] [CrossRef] [Green Version]
- Vlahos, P.; Schensul, S.L.; Nanayakkara, N.; Chandrajith, R.; Haider, L.; Anand, S.; Silva, K.T.; Schensul, J.J. Kidney progression project (KiPP): Protocol for a longitudinal cohort study of progression in chronic kidney disease of unknown etiology in Sri Lanka. Glob. Public Health 2019, 14, 214–226. [Google Scholar] [CrossRef]
- Rango, T.; Jeuland, M.; Manthrithilake, H.; McCornick, P. Nephrotoxic contaminants in drinking water and urine, and chronic kidney disease in rural Sri Lanka. Sci. Total Environ. 2015, 518–519, 574–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrajith, R.; Nanayakkara, S.; Itai, K.; Aturaliya, T.N.C.; Dissanayake, C.B.; Abeysekera, T.; Harada, K.; Watanabe, T.; Koizumi, A. Chronic kidney diseases of uncertain etiology (CKDue) in Sri Lanka: Geographic distribution and environmental implications. Environ. Geochem. Health 2011, 33, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Balasooriya, S.; Munasinghe, H.; Herath, A.T.; Diyabalanage, S.; Ileperuma, O.A.; Manthrithilake, H.; Daniel, C.; Amann, K.; Zwiener, C.; Barth, J.A.C.; et al. Possible links between groundwater geochemistry and chronic kidney disease of unknown etiology (CKDu): An investigation from the Ginnoruwa region in Sri Lanka. Expo. Health 2020, 12, 823–834. [Google Scholar] [CrossRef]
- Wickramarathna, S.; Balasooriya, S.; Diyabalanage, S.; Chandrajith, R. Tracing environmental aetiological factors of chronic kidney diseases in the dry zone of Sri Lanka—A hydrogeochemical and isotope approach. J. Trace Elem. Med. Biol. 2017, 44, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Nikagolla, C.; Meredith, K.T.; Dawes, L.A.; Banati, R.B.; Millar, G.J. Using water quality and isotope studies to inform research in chronic kidney disease of unknown aetiology endemic areas in Sri Lanka. Sci. Total Environ. 2020, 745, 140896. [Google Scholar] [CrossRef]
- Wasana, H.M.S.; Aluthpatabendi, D.; Kularatne, W.M.T.D.; Wijekoon, P.; Weerasooriya, R.; Bandara, J. Drinking water quality and chronic kidney disease of unknown etiology (CKDu): Synergic effects of fluoride, cadmium and hardness of water. Environ. Geochem. Health 2016, 38, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Jayasumana, C.; Gunatilake, S.; Senanayake, P. Glyphosate, Hard Water and Nephrotoxic Metals: Are They the Culprits behind the Epidemic of Chronic Kidney Disease of Unknown Etiology in Sri Lanka? Int. J. Environ. Res. Public Health 2014, 11, 2125–2147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Kidney Stone Type | Parameters | Ca | Mg | Na | K | Fe | Cu | Zn | Ba | Ti | Cr | Mo | Cd | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | ng/g | ng/g | ng/g | ng/g | μg/g | ||
| COM (n = 26) | Min | 20.5 | 98.2 | 29.9 | 88.8 | 0.0 | 0.0 | 19.9 | 0.2 | 0.0 | 0.0 | 68.1 | 22.0 | 0.0 |
| Max | 54.4 | 13,499.9 | 16,666.7 | 25,475.6 | 271.0 | 5.5 | 2678.0 | 13.5 | 20,694.4 | 215.1 | 1391.1 | 1524.9 | 25.8 | |
| Mean | 28.0 | 1880.4 | 3168.1 | 1563.2 | 28.0 | 0.7 | 506.2 | 2.8 | 1192.1 | 18.1 | 461.5 | 384.8 | 6.6 | |
| Median | 27.3 | 1147.6 | 2462.8 | 400.3 | 14.8 | 0.3 | 345.0 | 1.5 | 306.2 | 0.0 | 371.8 | 273.1 | 6.2 | |
| COM + COD (n = 23) | Min | 24.2 | 135.1 | 578.1 | 64.0 | 4.4 | 0.1 | 12.5 | 0.1 | 0.0 | 0.0 | 112.5 | 58.4 | 0.0 |
| Max | 28.3 | 4469.2 | 4765.7 | 830.2 | 191.4 | 1.9 | 873.1 | 15.3 | 845.1 | 4247.4 | 1264.5 | 780.1 | 24.0 | |
| Mean | 26.4 | 831.2 | 1817.7 | 373.3 | 23.3 | 0.5 | 269.9 | 2.3 | 221.8 | 359.1 | 502.0 | 307.6 | 6.1 | |
| Median | 26.1 | 355.2 | 1405.7 | 362.3 | 13.9 | 0.4 | 79.7 | 0.5 | 87.3 | 0.0 | 494.1 | 176.8 | 2.7 | |
| COD + Mix (n = 13) | Min | 0.3 | 8.1 | 108.8 | 21.9 | 5.2 | 0.1 | 1.4 | 0.1 | 0.0 | 0.0 | 85.5 | 11.4 | 0.0 |
| Max | 28.0 | 2753.5 | 3251.6 | 768.6 | 79.3 | 2.3 | 508.1 | 2.2 | 1625.9 | 1043.4 | 515.8 | 477.8 | 4.8 | |
| Mean | 18.9 | 743.8 | 1436.1 | 254.4 | 17.0 | 1.0 | 149.3 | 0.8 | 347.3 | 91.9 | 315.8 | 164.7 | 1.6 | |
| Median | 23.7 | 228.8 | 1158.4 | 221.3 | 10.8 | 0.6 | 24.0 | 0.5 | 55.0 | 0.0 | 297.6 | 98.0 | 1.0 | |
| AAU (n = 1) | 9.0 | 55,480.8 | 4417.0 | 2576.3 | 1.8 | 0.51 | 466.1 | 15.41 | 969.6 | 0.0 | 93.2 | 96.9 | 1.63 | |
| CA-1 (n = 1) | 30.7 | 3568.5 | 6685.1 | 1697.6 | 15.3 | 0.13 | 578.0 | 8.12 | 1576.9 | 0.0 | 1043.3 | 566.3 | 12.59 | |
| CA-2 (n = 1) | 14.8 | 40,219.1 | 6010.6 | 915.6 | 30.8 | 0.13 | 461.2 | 4.91 | 1283.8 | 2883.1 | 376.0 | 80.3 | 0.64 | |
| UA (n = 1) | 0.3 | 481.2 | 813.2 | 351.7 | 3.3 | 5.75 | 2.3 | 0.04 | 0.0 | 0.0 | 143.5 | 9.5 | 0.00 |
| Variable | PC1 | PC2 | PC3 |
|---|---|---|---|
| Eigenvalues | 3.89 | 2.44 | 1.39 |
| Variance (%) | 32.4 | 20.4 | 11.6 |
| Mg | 0.02 | 0.84 | 0.06 |
| Na | 0.80 | 0.47 | −0.04 |
| K | 0.01 | 0.89 | −0.07 |
| Fe | 0.13 | 0.01 | 0.79 |
| Cu | −0.15 | −0.06 | 0.72 |
| Zn | 0.84 | 0.32 | 0.07 |
| Ba | 0.50 | 0.63 | 0.31 |
| Ti | 0.00 | 0.46 | −0.30 |
| Cr | 0.57 | −0.03 | 0.14 |
| Mo | 0.67 | −0.09 | −0.10 |
| Cd | 0.85 | 0.06 | −0.11 |
| Pb | 0.87 | −0.03 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Han, G.; Zeng, J.; Zhang, Q.; Xu, L.; Liu, K.; Xiao, C.; Ma, L.; Zhao, Y. Preliminary Data on Geochemical Characteristics of Major and Trace Elements in Typical Biominerals: From the Perspective of Human Kidney Stones. Minerals 2021, 11, 1396. https://doi.org/10.3390/min11121396
Tian Y, Han G, Zeng J, Zhang Q, Xu L, Liu K, Xiao C, Ma L, Zhao Y. Preliminary Data on Geochemical Characteristics of Major and Trace Elements in Typical Biominerals: From the Perspective of Human Kidney Stones. Minerals. 2021; 11(12):1396. https://doi.org/10.3390/min11121396
Chicago/Turabian StyleTian, Yu, Guilin Han, Jie Zeng, Qian Zhang, Lifang Xu, Ke Liu, Chunlei Xiao, Lulin Ma, and Ye Zhao. 2021. "Preliminary Data on Geochemical Characteristics of Major and Trace Elements in Typical Biominerals: From the Perspective of Human Kidney Stones" Minerals 11, no. 12: 1396. https://doi.org/10.3390/min11121396
APA StyleTian, Y., Han, G., Zeng, J., Zhang, Q., Xu, L., Liu, K., Xiao, C., Ma, L., & Zhao, Y. (2021). Preliminary Data on Geochemical Characteristics of Major and Trace Elements in Typical Biominerals: From the Perspective of Human Kidney Stones. Minerals, 11(12), 1396. https://doi.org/10.3390/min11121396









