Fabrication of Adsorbed Fe(III) and Structurally Doped Fe(III) in Montmorillonite/TiO2 Composite for Photocatalytic Degradation of Phenol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Iron-Modified Mt and Clay/TiO2 Composites
2.3. Solid Characterization
2.4. Photocatalytic Activity Test
3. Results
3.1. Characterizations
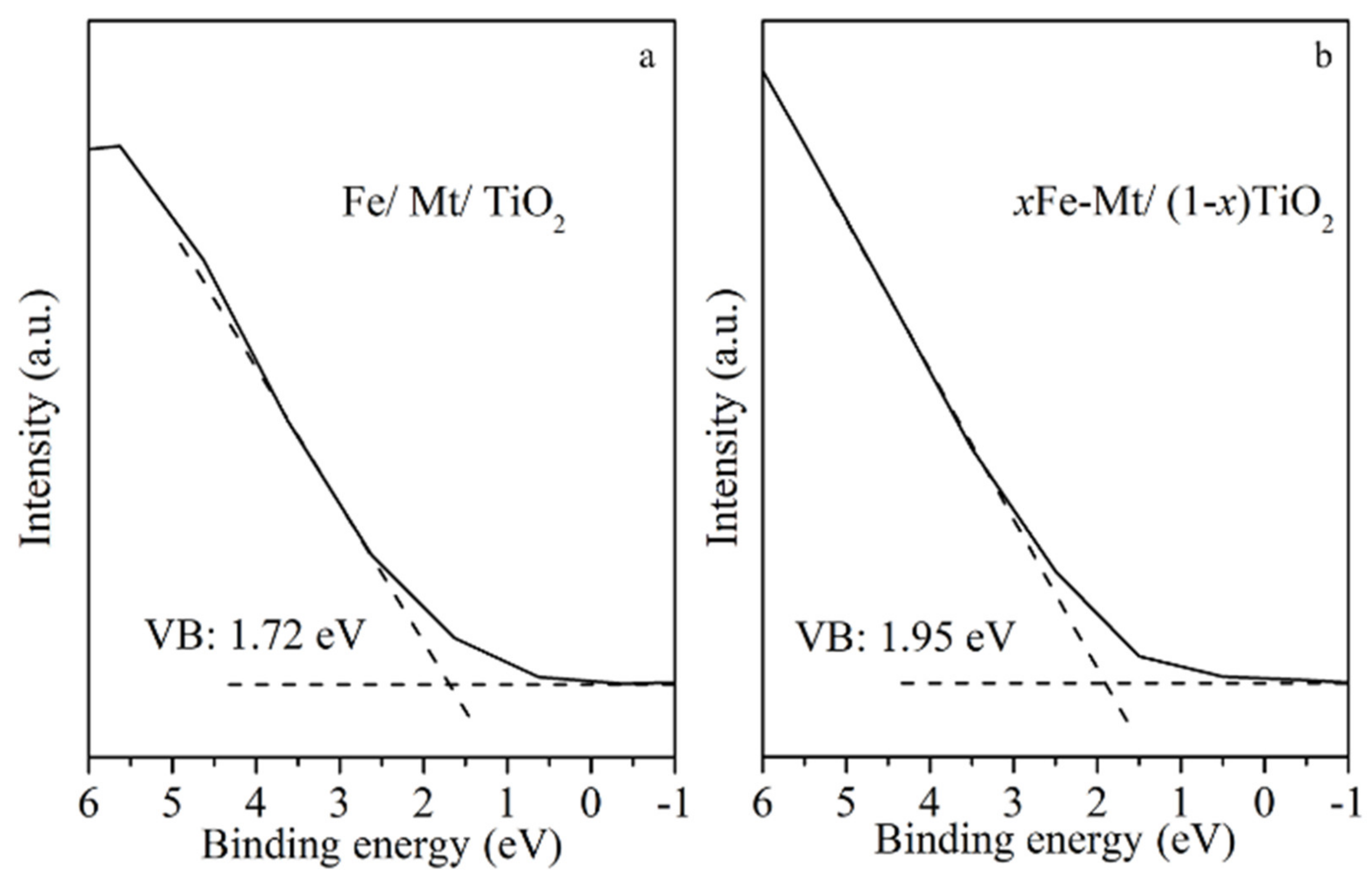
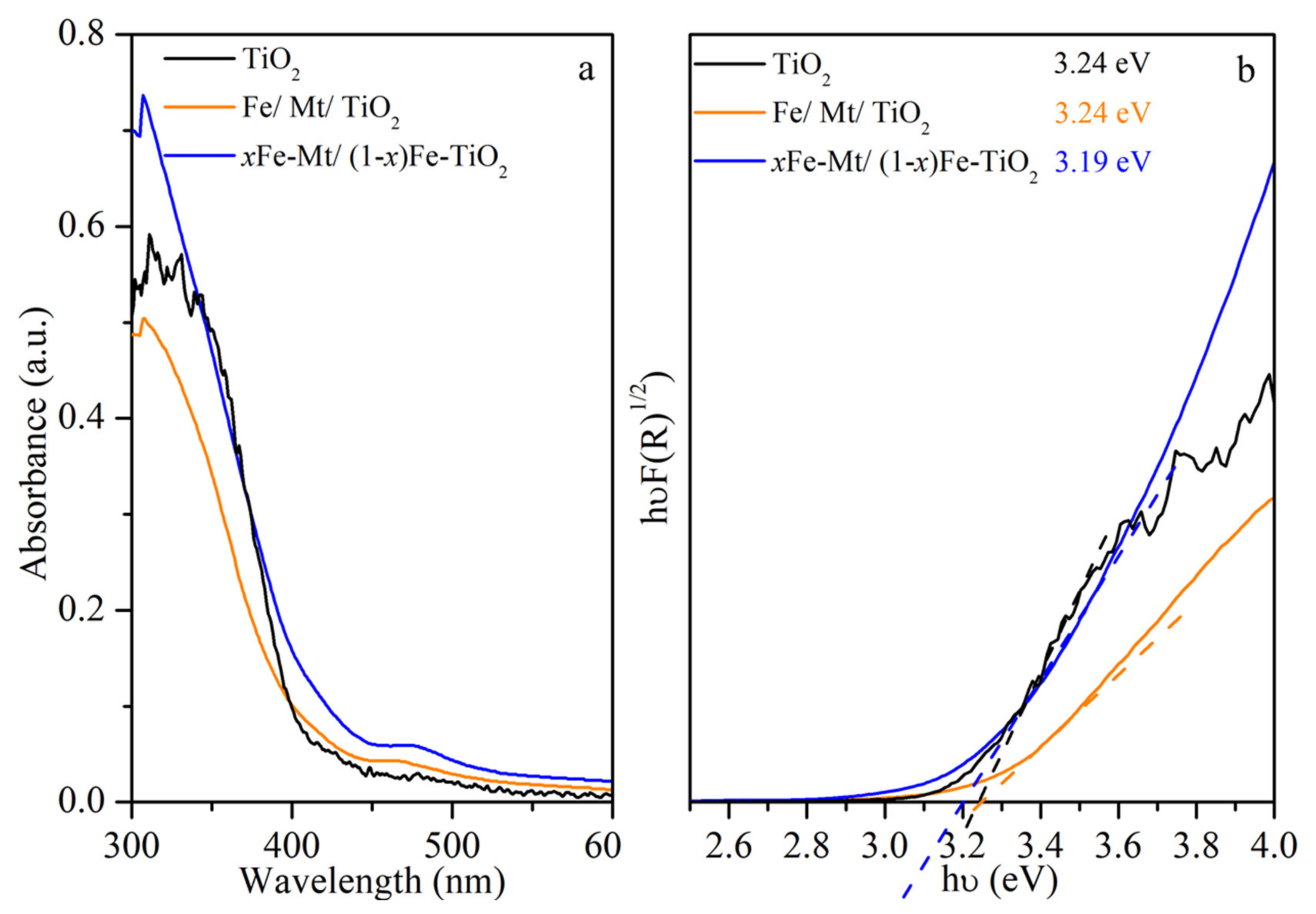
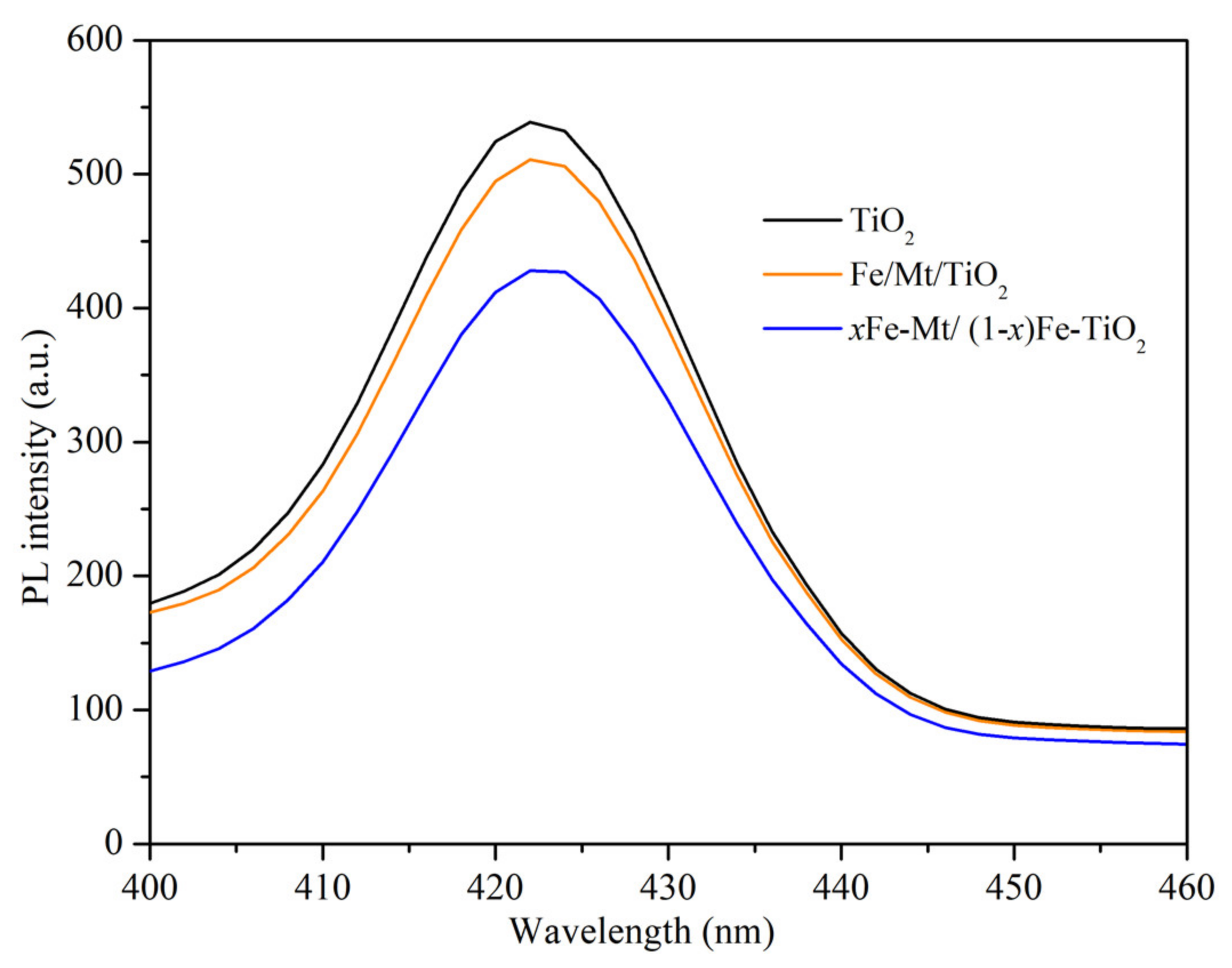
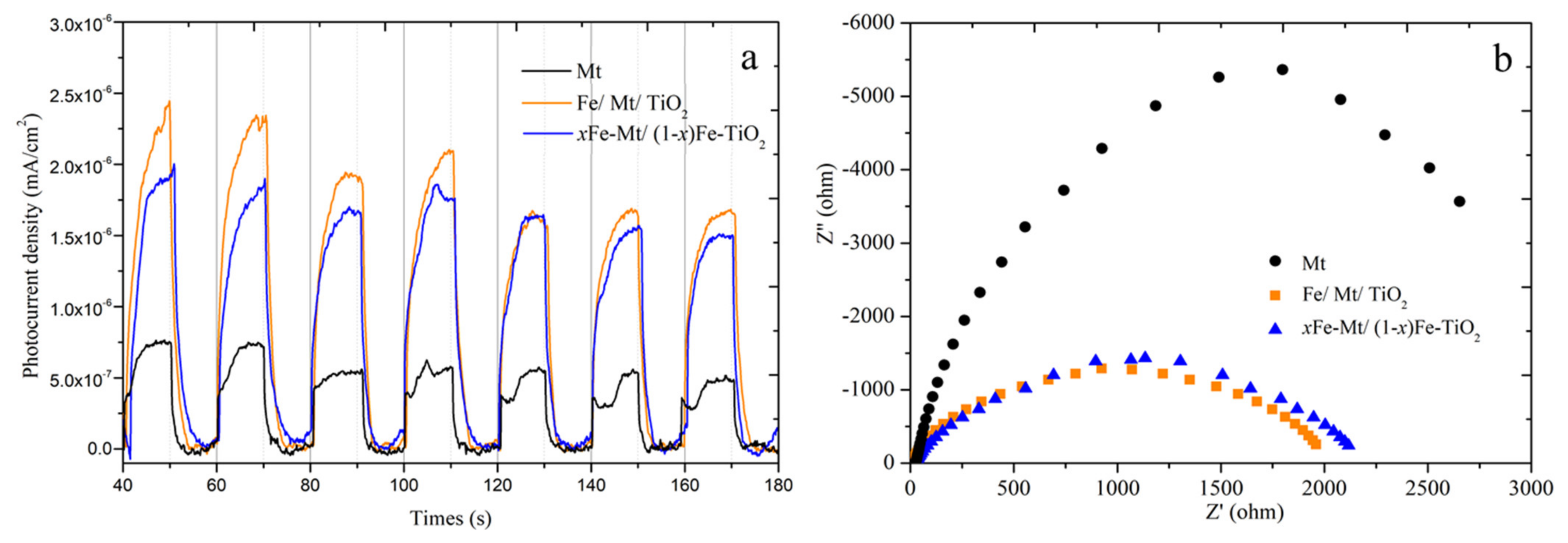
3.2. Investigation of Optical and Charge Separation Properties
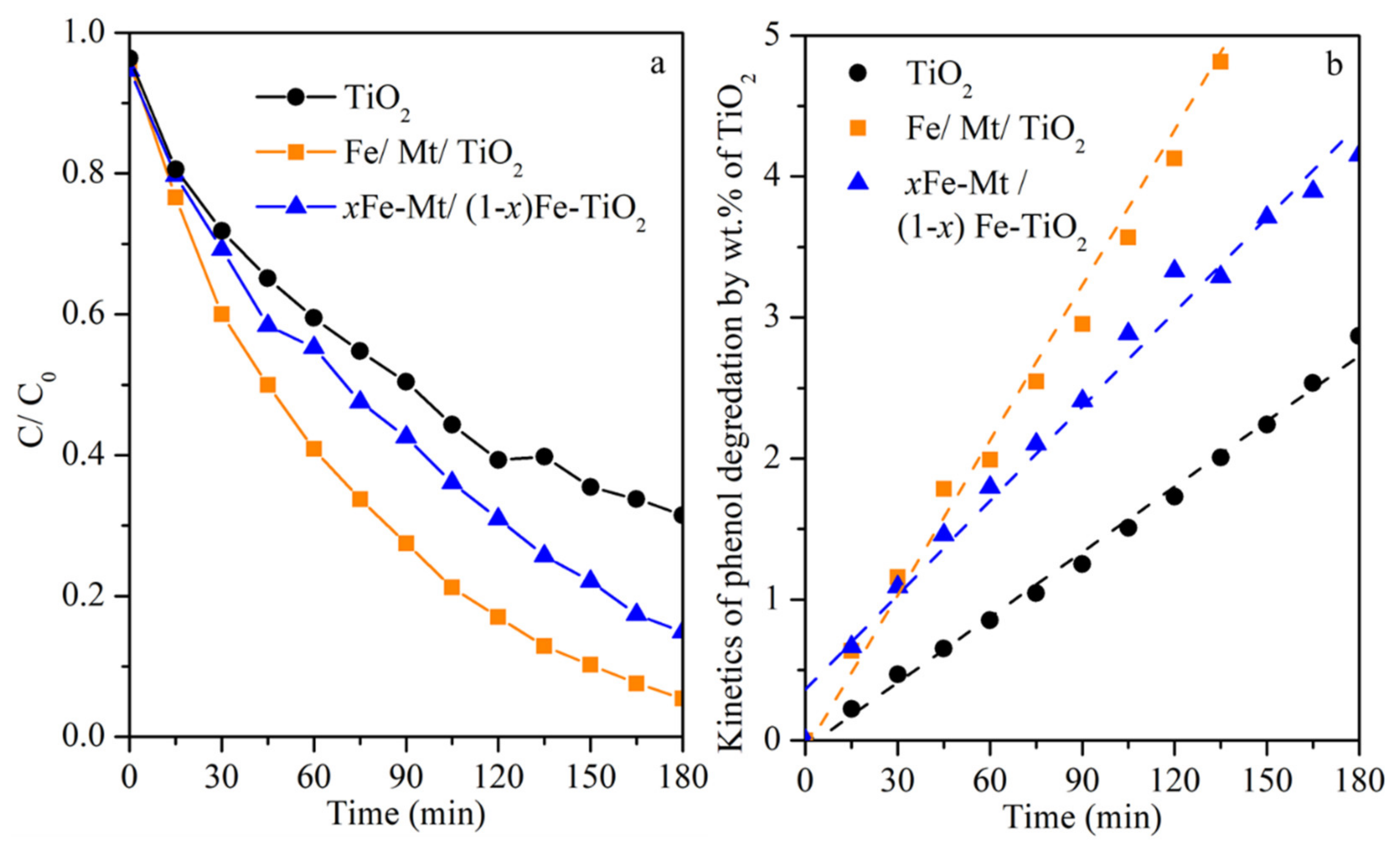
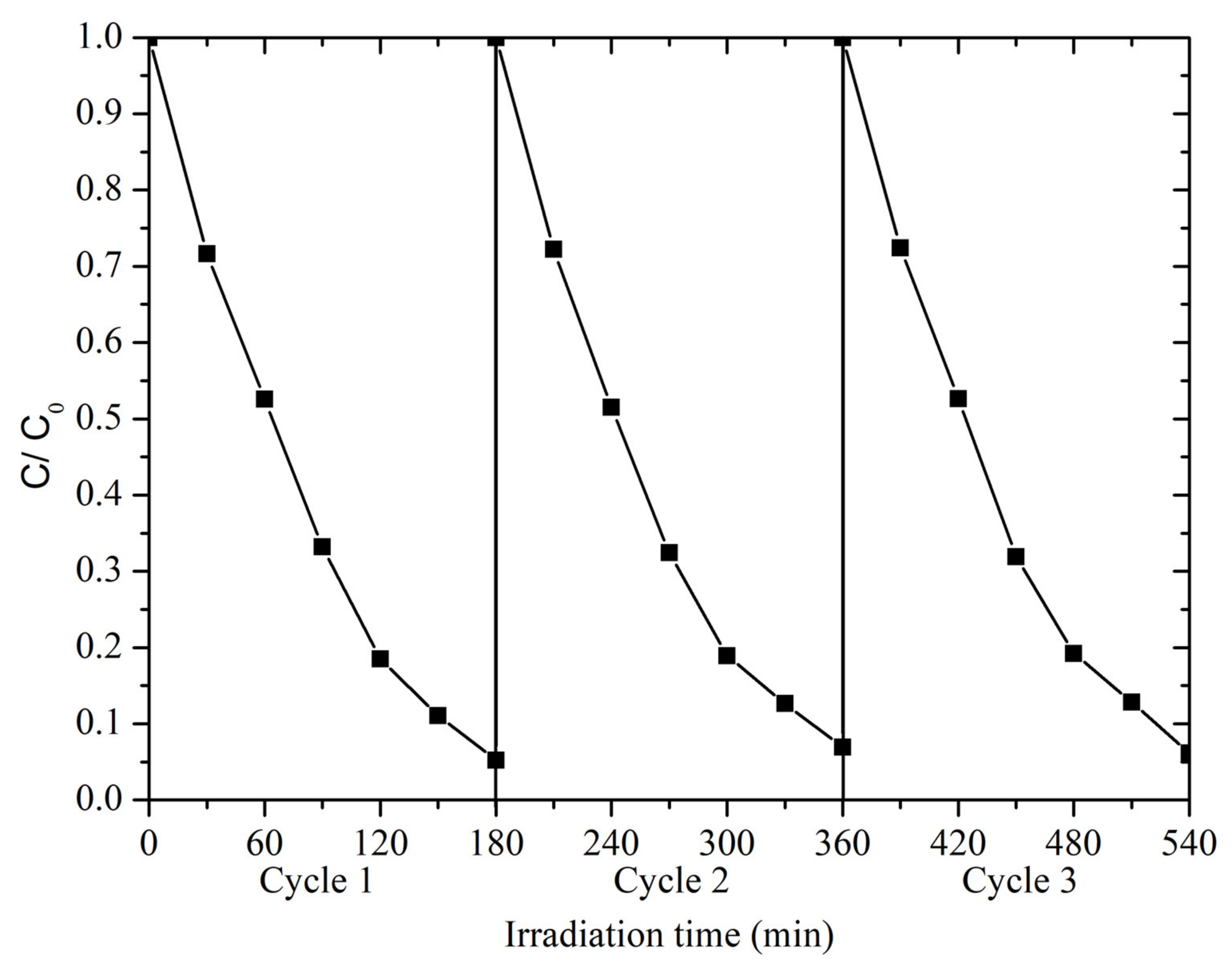
3.3. Photocatalytic Activity
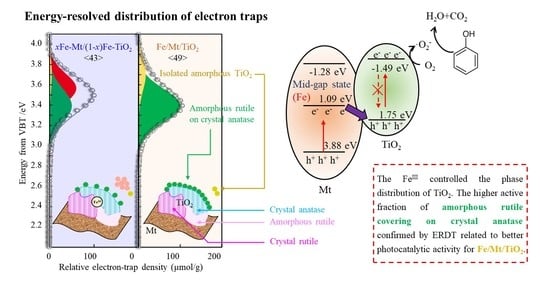
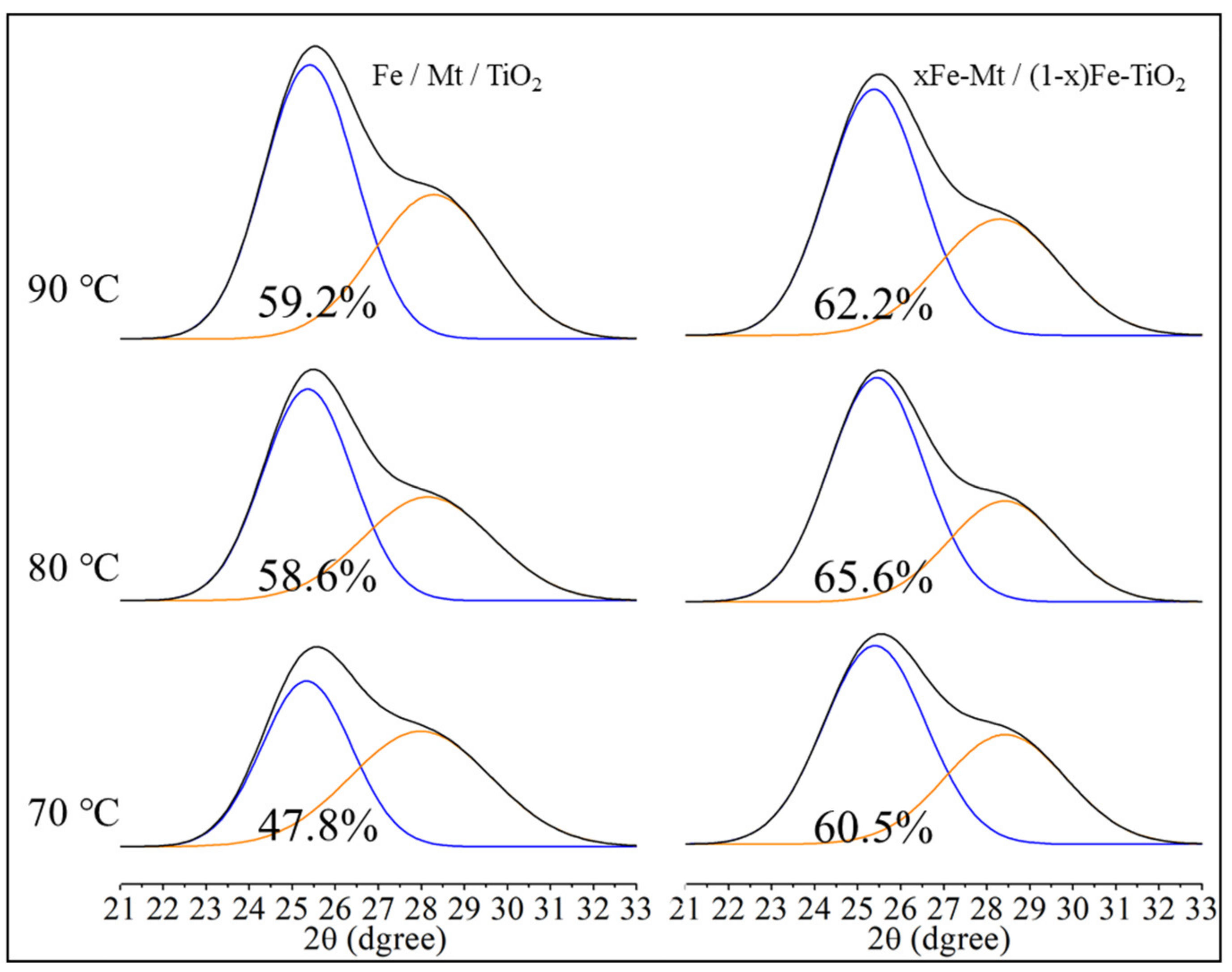
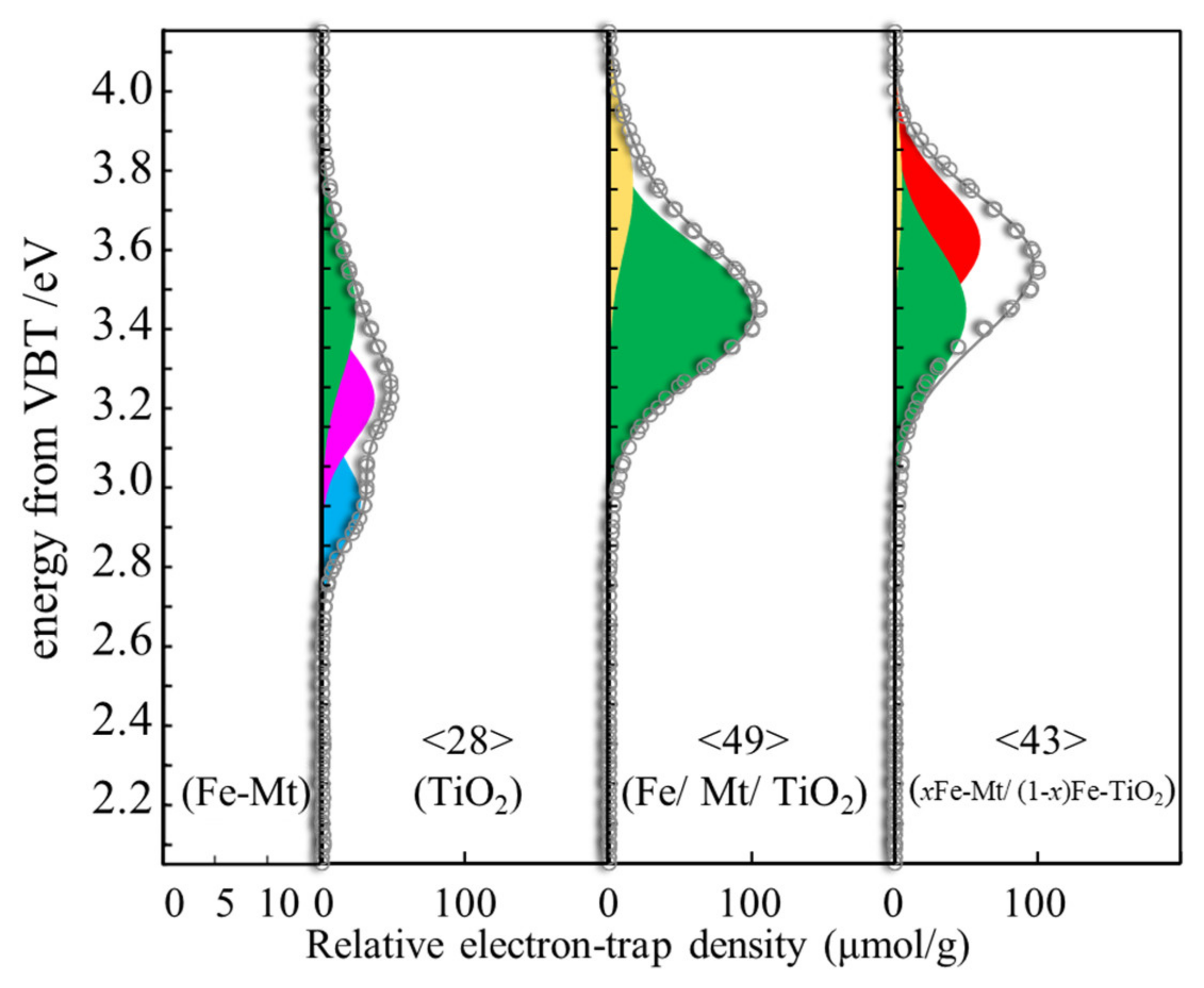
3.4. ERDT Patterns of TiO2, Fe/Mt/TiO2, and xFe-Mt/(1 − x)Fe-TiO2
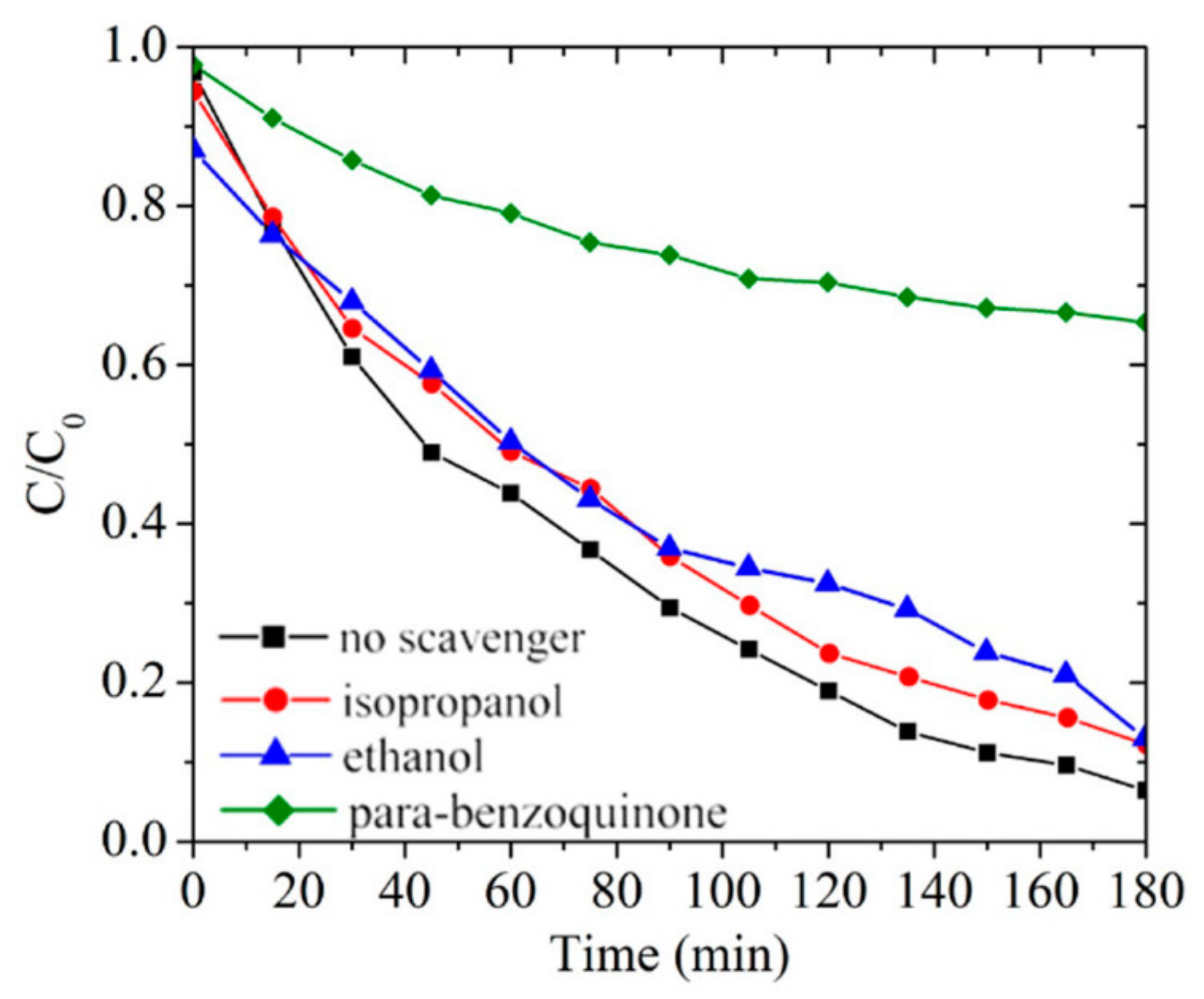
3.5. Radical Scavenger Tests
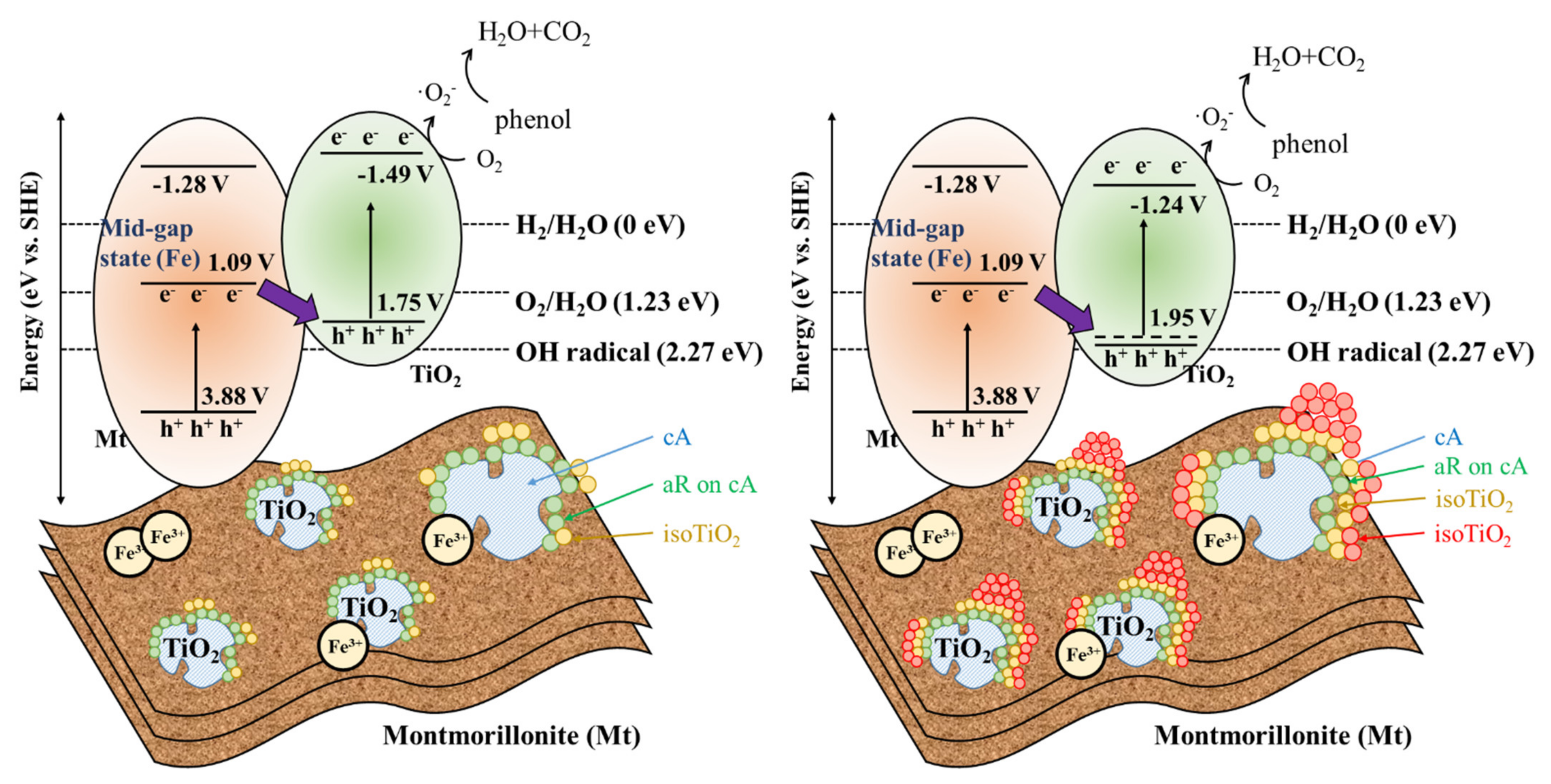
3.6. Photocatalytic Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cerrato, G.; Marchese, L.; Morterra, C. Structural and morphological modifications of sintering microcrystalline TiO2: An XRD, HRTEM and FTIR study. Appl. Surf. Sci. 1993, 70–71, 200–205. [Google Scholar] [CrossRef]
- Zhaobin, W.; Qin, X.; Xiexian, G.; Sham, E.; Grange, P.; Delmon, B. Titania-modified hydrodesulphurization catalysts: I. Effect of preparation techniques on morphology and properties of TiO2—Al2O3 carrier. Appl. Catal. 1990, 63, 305–317. [Google Scholar] [CrossRef]
- Wen, J.; Li, X.; Liu, W.; Fang, Y.; Xie, J.; Xu, Y. Photocatalysis fundamentals and surface modification of TiO2 nanomaterials. Chin. J. Catal. 2015, 36, 2049–2070. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Z. Dye-Sensitized TiO2 Nanotube Solar Cells with Markedly Enhanced Performance via Rational Surface Engineering. Chem. Mater. 2010, 22, 579–584. [Google Scholar] [CrossRef]
- Bian, Z.; Tachikawa, T.; Kim, W.; Choi, W.; Majima, T. Superior Electron Transport and Photocatalytic Abilities of Metal-Nanoparticle-Loaded TiO2 Superstructures. J. Phys. Chem. C 2012, 116, 25444–25453. [Google Scholar] [CrossRef]
- Cozzoli, P.D.; Fanizza, E.; Comparelli, R.; Curri, M.L.; Agostiano, A.; Laub, D. Role of metal nanoparticles in TiO2/Ag nanocomposite-based microheterogeneous photocatalysis. J. Phys. Chem. B 2004, 108, 9623–9630. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Selli, E. Doping TiO2 with p-block elements: Effects on photocatalytic activity. J. Photochem. Photobiol. C Photochem. Rev. 2013, 14, 13–28. [Google Scholar] [CrossRef]
- Zaleska, A. Doped-TiO2: A review. Recent Pat. Eng. 2008, 2, 157–164. [Google Scholar] [CrossRef]
- Fukahori, S.; Ichiura, H.; Kitaoka, T.; Tanaka, H. Capturing of bisphenol A photodecomposition intermediates by composite TiO2–zeolite sheets. Appl. Catal. B Environ. 2003, 46, 453–462. [Google Scholar] [CrossRef]
- Todorova, N.; Giannakopoulou, T.; Karapati, S.; Petridis, D.; Vaimakis, T.; Trapalis, C. Composite TiO2/clays materials for photocatalytic NOx oxidation. Appl. Surf. Sci. 2014, 319, 113–120. [Google Scholar] [CrossRef]
- Mohammad, A.; Khan, M.E.; Cho, M.H.; Yoon, T. Fabrication of binary SnO2/TiO2 nanocomposites under a sonication-assisted approach: Tuning of band-gap and water depollution applications under visible light irradiation. Ceram. Int. 2021, 47, 15073–15081. [Google Scholar] [CrossRef]
- Mohammad, A.; Khan, M.E.; Cho, M.H.; Yoon, T. Adsorption promoted visible-light-induced photocatalytic degradation of antibiotic tetracycline by tin oxide/cerium oxide nanocomposite. Appl. Surf. Sci. 2021, 565, 150337. [Google Scholar] [CrossRef]
- Khan, M.M.; Min, B.-K.; Cho, M.H. Microbial fuel cell assisted band gap narrowed TiO2 for visible light-induced photocatalytic activities and power generation. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damian, G.; Damian, F.; Szakács, Z.; Iepure, G.; Aştefanei, D. Mineralogical and Physico-Chemical Characterization of the Oraşu-Nou (Romania) Bentonite Resources. Minerals 2021, 11, 938. [Google Scholar] [CrossRef]
- Laufek, F.; Hanusová, I.; Svoboda, J.; Vašíček, R.; Najser, J.; Koubová, M.; Čurda, M.; Pticen, F.; Vaculíková, L.; Sun, H.; et al. Mineralogical, Geochemical and Geotechnical Study of BCV 2017 Bentonite—The Initial State and the State following Thermal Treatment at 200 °C. Minerals 2021, 11, 871. [Google Scholar] [CrossRef]
- DeWitt, J.; Chirico, P.; Alessi, M.; Boston, K. Remote Sensing Inventory and Geospatial Analysis of Brick Kilns and Clay Quarrying in Kabul, Afghanistan. Minerals 2021, 11, 296. [Google Scholar] [CrossRef]
- Li, C.; Li, Z.; Wu, T.; Luo, Y.; Zhao, J.; Li, X.; Yang, W.; Chen, X. Metallogenic Characteristics and Formation Mechanism of Naomugeng Clay-Type Lithium Deposit in Central Inner Mongolia, China. Minerals 2021, 11, 238. [Google Scholar] [CrossRef]
- Belver, C.; Bedia, J.; Rodriguez, J. Titania–clay heterostructures with solar photocatalytic applications. Appl. Catal. B Environ. 2015, 176-177, 278–287. [Google Scholar] [CrossRef]
- Papoulis, D.; Komarneni, S.; Panagiotaras, D.; Stathatos, E.; Toli, D.; Christoforidis, K.C.; Fernández-García, M.; Li, H.; Yin, S.; Sato, T.; et al. Halloysite–TiO2 nanocomposites: Synthesis, characterization and photocatalytic activity. Appl. Catal. B Environ. 2013, 132-133, 416–422. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, G. Recent advances in synthesis and applications of clay-based photocatalysts: A review. Phys. Chem. Chem. Phys. 2014, 16, 8178–8192. [Google Scholar] [CrossRef]
- Man-Chao, H.; Zhi-Jie, F.; Ping, Z. Atomic and electronic structures of montmorillonite in soft rock. Chin. Phys. B 2009, 18, 2933–2937. [Google Scholar] [CrossRef]
- Jack, R.S.; Ayoko, G.A.; Adebajo, M.O.; Frost, R.L. A review of iron species for visible-light photocatalytic water purification. Environ. Sci. Pollut. Res. 2015, 22, 7439–7449. [Google Scholar] [CrossRef]
- Parida, K.; Mohapatra, L. Carbonate intercalated Zn/Fe layered double hydroxide: A novel photocatalyst for the enhanced photo degradation of azo dyes. Chem. Eng. J. 2012, 179, 131–139. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, Y.; Lee, D.K.; Lee, J.W.; Kang, J.K. Efficient Co–Fe layered double hydroxide photocatalysts for water oxidation under visible light. J. Mater. Chem. A 2014, 2, 4136–4139. [Google Scholar] [CrossRef]
- Chuaicham, C.; Xiong, Y.; Sekar, K.; Chen, W.; Zhang, L.; Ohtani, B.; Dabo, I.; Sasaki, K. A promising Zn-Ti layered double hydroxide/Fe-bearing montmorillonite composite as an efficient photocatalyst for Cr(VI) reduction: Insight into the role of Fe impurity in montmorillonite. Appl. Surf. Sci. 2021, 546, 148835. [Google Scholar] [CrossRef]
- Zheng, X.; Li, Y.; You, W.; Lei, G.; Cao, Y.; Zhang, Y.; Jiang, L. Construction of Fe-doped TiO2−x ultrathin nanosheets with rich oxygen vacancies for highly efficient oxidation of H2S. Chem. Eng. J. 2021, 430, 132917. [Google Scholar] [CrossRef]
- Kanjana, N.; Maiaugree, W.; Poolcharuansin, P.; Laokul, P. Synthesis and characterization of Fe-doped TiO2 hollow spheres for dye-sensitized solar cell applications. Mater. Sci. Eng. B 2021, 271, 115311. [Google Scholar] [CrossRef]
- Mancuso, A.; Sacco, O.; Vaiano, V.; Sannino, D.; Pragliola, S.; Venditto, V.; Morante, N. Visible light active Fe-Pr co-doped TiO2 for water pollutants degradation. Catal. Today 2021, 380, 93–104. [Google Scholar] [CrossRef]
- Kara, F.; Kurban, M.; Coşkun, B. Evaluation of electronic transport and optical response of two-dimensional Fe-doped TiO2 thin films for photodetector applications. Optik 2020, 210, 164605. [Google Scholar] [CrossRef]
- Pezeshkian, W.; Marrink, S.J. Simulating realistic membrane shapes. Curr. Opin. Cell Biol. 2021, 71, 103–111. [Google Scholar] [CrossRef]
- Lapworth, D.J.; Baran, N.; Stuart, M.E.; Ward, R.S. Emerging organic contaminants in groundwater: A review of sources, fate and occurrence. Environ. Pollut. 2012, 163, 287–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daly, G.L.; Wania, F. Organic Contaminants in Mountains. Environ. Sci. Technol. 2005, 39, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Alkorta, I.; Garbisu, C. Phytoremediation of organic contaminants in soils. Bioresour. Technol. 2001, 79, 273–276. [Google Scholar] [CrossRef]
- Kim, J.; Du, P.; Liu, W.; Luo, C.; Zhao, H.; Huang, C.-H. Cobalt/Peracetic Acid: Advanced Oxidation of Aromatic Organic Compounds by Acetylperoxyl Radicals. Environ. Sci. Technol. 2020, 54, 5268–5278. [Google Scholar] [CrossRef]
- Hodges, B.C.; Cates, E.L.; Kim, J.-H. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nat. Nanotechnol. 2018, 13, 642–650. [Google Scholar] [CrossRef]
- Mohammadi, S.; Kargari, A.; Sanaeepur, H.; Abbassian, K.; Najafi, A.; Mofarrah, E. Phenol removal from industrial wastewaters: A short review. Desalin. Water Treat. 2015, 53, 2215–2234. [Google Scholar] [CrossRef]
- Jover, J.; Bosque, R.; Sales, J. Neural Network Based QSPR Study for Predicting pKa of Phenols in Different Solvents. QSAR Comb. Sci. 2007, 26, 385–397. [Google Scholar] [CrossRef]
- Srikhaow, A.; Smith, S.M.; Uraisin, K.; Suttiponparnit, K.; Kongmark, C.; Chuaicham, C. Catalytic remediation of phenol contaminated wastewater using Cu–Zn hydroxide nitrate. RSC Adv. 2016, 6, 36766–36774. [Google Scholar] [CrossRef]
- Zhang, Y.; Gan, H.; Zhang, G. A novel mixed-phase TiO2/kaolinite composites and their photocatalytic activity for degradation of organic contaminants. Chem. Eng. J. 2011, 172, 936–943. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Y.; Zhang, G.; Gao, Y. Stable TiO2/rectorite: Preparation, characterization and photocatalytic activity. Appl. Clay Sci. 2011, 51, 335–340. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Deng, K.; Chen, X.; Zou, Z. Low Temperature Synthesis and Photocatalytic Activity of Rutile TiO2 Nanorod Superstructures. J. Phys. Chem. C 2007, 111, 2709–2714. [Google Scholar] [CrossRef]
- Tansel, B. Significance of thermodynamic and physical characteristics on permeation of ions during membrane separation: Hydrated radius, hydration free energy and viscous effects. Sep. Purif. Technol. 2012, 86, 119–126. [Google Scholar] [CrossRef]
- Liu, H.; Chaudhary, D.; Yusa, S.-I.; Tadé, M.O. Glycerol/starch/Na+-montmorillonite nanocomposites: A XRD, FTIR, DSC and 1H NMR study. Carbohydr. Polym. 2011, 83, 1591–1597. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Lee, H.-N.; Kumar, R. Formation of oxygen vacancies and Ti3+ state in TiO2 thin film and enhanced optical properties by air plasma treatment. Sci. Rep. 2016, 6, srep32355. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhao, H.; Cao, T.; Qian, L.; Wang, Y.; Zhao, G. Efficient degradation of high concentration azo-dye wastewater by heterogeneous Fenton process with iron-based metal-organic framework. J. Mol. Catal. A Chem. 2015, 400, 81–89. [Google Scholar] [CrossRef]
- Xiao, S.; Xu, P.; Peng, Q.; Chen, J.; Huang, J.; Wang, F.; Noor, N. Layer-by-Layer Assembly of Polyelectrolyte Multilayer onto PET Fabric for Highly Tunable Dyeing with Water Soluble Dyestuffs. Polymers 2017, 9, 735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jichao, W. On the cultivation of science college students’ innovative and entrepreneurial ability. J. China Univ. Pet. Ed. Soc. Sci. 2016, 5, 106–109. [Google Scholar]
- Wu, L.Q.; Li, Y.C.; Li, S.Q.; Li, Z.Z.; Tang, G.D.; Qi, W.H.; Xue, L.C.; Ge, X.S.; Ding, L.L. Method for estimating ionicities of oxides using O1s photoelectron spectra. AIP Adv. 2015, 5, 097210. [Google Scholar] [CrossRef]
- Chuaicham, C.; Karthikeyan, S.; Pawar, R.R.; Xiong, Y.; Dabo, I.; Ohtani, B.; Kim, Y.; Song, J.T.; Ishihara, T.; Sasaki, K. Energy-resolved distribution of electron traps for O/S-doped carbon nitrides by reversed double-beam photoacoustic spectroscopy and the photocatalytic reduction of Cr(vi). Chem. Commun. 2020, 56, 3793–3796. [Google Scholar] [CrossRef]
- Ferré, F.G.; Mairov, A.; Iadicicco, D.; Vanazzi, M.; Bassini, S.; Utili, M.; Tarantino, M.; Bragaglia, M.; Lamastra, F.; Nanni, F.; et al. Corrosion and radiation resistant nanoceramic coatings for lead fast reactors. Corros. Sci. 2017, 124, 80–92. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Lefrant, S. XPS study of SiO thin films and SiO-metal interfaces. J. Phys. Condens. Matter 1989, 1, 5197–5204. [Google Scholar] [CrossRef]
- Dong, F.; Zhao, Z.; Xiong, T.; Ni, Z.; Zhang, W.; Sun, Y.; Ho, W.-K. In Situ Construction of g-C3N4/g-C3N4 Metal-Free Heterojunction for Enhanced Visible-Light Photocatalysis. ACS Appl. Mater. Interfaces 2013, 5, 11392–11401. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Yang, J.; Yu, X.; Zhu, Y.; Zhang, Y. In Situ grown hierarchical 50%BiOCl/BiOI hollow flowerlike microspheres on reduced graphene oxide nanosheets for enhanced visible-light photocatalytic degradation of rhodamine B. Appl. Surf. Sci. 2018, 433, 502–512. [Google Scholar] [CrossRef]
- Yamada, Y.; Nakamura, T.; Endo, M.; Wakamiya, A.; Kanemitsu, Y. Near-band-edge optical responses of solution-processed organic–inorganic hybrid perovskite CH3NH3PbI3on mesoporous TiO2electrodes. Appl. Phys. Express 2014, 7, 32302. [Google Scholar] [CrossRef]
- Sekar, K.; Chuaicham, C.; Balijapalli, U.; Li, W.; Wilson, K.; Lee, A.F.; Sasaki, K. Surfactant- and template-free hydrothermal assembly of Cu2O visible light photocatalysts for trimethoprim degradation. Appl. Catal. B Environ. 2021, 284, 119741. [Google Scholar] [CrossRef]
- Sekar, K.; Chuaicham, C.; Vellaichamy, B.; Li, W.; Zhuang, W.; Lu, X.; Ohtani, B.; Sasaki, K. Cubic Cu2O nanoparticles decorated on TiO2 nanofiber heterostructure as an excellent synergistic photocatalyst for H2 production and sulfamethoxazole degradation. Appl. Catal. B Environ. 2021, 294, 120221. [Google Scholar] [CrossRef]
- Chuaicham, C.; Karthikeyan, S.; Song, J.T.; Ishihara, T.; Ohtani, B.; Sasaki, K. Importance of ZnTiO3 phase in ZnTi-mixed metal oxide photocatalysts derived from layered double hydroxide. ACS Appl. Mater. Interfaces 2020, 12, 9169–9180. [Google Scholar] [CrossRef] [PubMed]
- Sood, S.; Umar, A.; Mehta, S.K.; Kansal, S.K. Highly effective Fe-doped TiO2 nanoparticles photocatalysts for visible-light driven photocatalytic degradation of toxic organic compounds. J. Colloid Interface Sci. 2015, 450, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Tripathi, P.; Azam, A.; Raza, W.; Ahmed, A.S.; Ahmed, A.; Muneer, M. Photocatalytic performance of Fe-doped TiO2nanoparticles under visible-light irradiation. Mater. Res. Express 2017, 4, 015022. [Google Scholar] [CrossRef]
- Benotti, M.J.; Stanford, B.D.; Wert, E.C.; Snyder, S. Evaluation of a photocatalytic reactor membrane pilot system for the removal of pharmaceuticals and endocrine disrupting compounds from water. Water Res. 2009, 43, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Nitta, A.; Takashima, M.; Murakami, N.; Takase, M.; Ohtani, B. Reversed double-beam photoacoustic spectroscopy of metal-oxide powders for estimation of their energy-resolved distribution of electron traps and electronic-band structure. Electrochim. Acta 2018, 264, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Su, D.; Dou, S.; Wang, G. Anatase TiO2: Better Anode Material than Amorphous and Rutile Phases of TiO2 for Na-Ion Batteries. Chem. Mater. 2015, 27, 6022–6029. [Google Scholar] [CrossRef]
- Viana, M.; Mohallem, N.; Miquita, D.R.; Balzuweit, K.; Silva-Pinto, E. Preparation of amorphous and crystalline Ag/TiO2 nanocomposite thin films. Appl. Surf. Sci. 2013, 265, 130–136. [Google Scholar] [CrossRef]
- Lottiaux, M.; Boulesteix, C.; Nihoul, G.; Varnier, F.; Flory, F.; Galindo, R.; Pelletier, E. Morphology and structure of TiO2 thin layers vs. thickness and substrate temperature. Thin Solid Films 1989, 170, 107–126. [Google Scholar] [CrossRef]

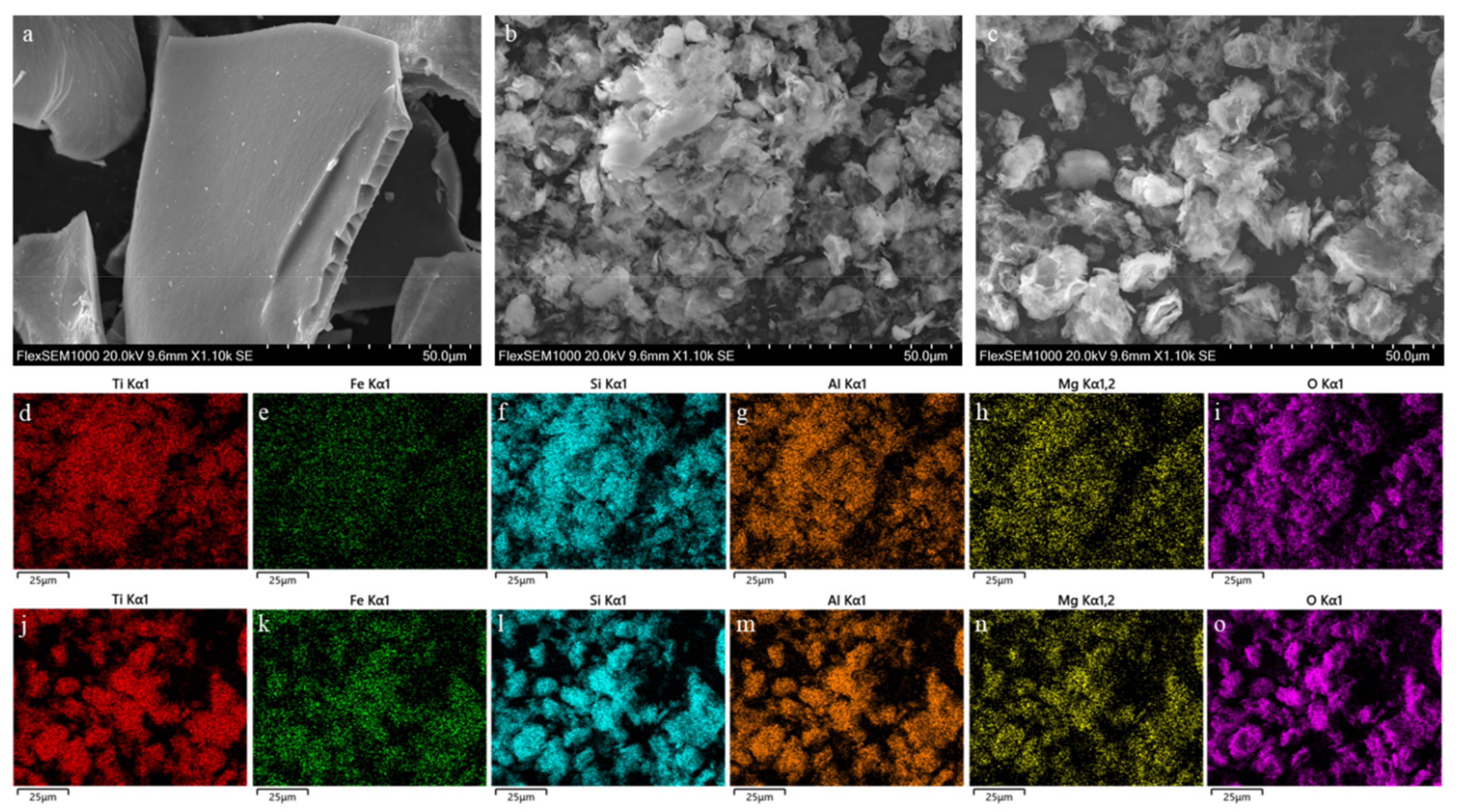

| T (°C) | Sample | Na (%) | Mg (%) | Al (%) | Si (%) | Ca (%) | Ti (%) | Fe (%) | TiO2 (%) |
|---|---|---|---|---|---|---|---|---|---|
| 90 | Fe/Mt/TiO2 | N.D. | 1.84 | 11.78 | 31.08 | 0.05 | 50.07 | 5.19 | 27.63 |
| xFe-Mt/(1 − x)Fe-TiO2 | N.D. | 1.97 | 12.28 | 32.28 | 0.05 | 47.49 | 5.92 | 26.74 | |
| 80 | Fe/Mt/TiO2 | N.D. | 1.86 | 11.65 | 30.74 | 0.04 | 49.62 | 6.10 | 27.65 |
| xFe-Mt/(1 − x)Fe-TiO2 | N.D. | 1.86 | 11.80 | 30.54 | 0.07 | 48.80 | 6.94 | 27.50 | |
| 70 | Fe/Mt/TiO2 | N.D. | 1.88 | 12.29 | 32.29 | 0.03 | 48.23 | 5.28 | 26.91 |
| xFe-Mt/(1 − x)Fe-TiO2 | N.D. | 1.88 | 12.04 | 32.14 | 0.08 | 47.19 | 6.66 | 26.77 |
| Sample | Peak Assignment | Peak Position (Ev) | Relative Intensity (%) |
|---|---|---|---|
| TiO2 | c-R | 2.96 | 31.1% |
| a-R | 3.22 | 42.0% | |
| a-R on c-A | 3.44 | 27.0% | |
| iso-TiO2 | 3.62 | N.D. | |
| iso-TiO2 | 3.76 | N.D. | |
| Fe/Mt/TiO2 | c-R | 2.96 | N.D. |
| a-R | 3.22 | N.D. | |
| a-R on c-A | 3.44 | 43.5% | |
| iso-TiO2 | 3.62 | 52.2% | |
| iso-TiO2 | 3.76 | 4.3% | |
| xFe-Mt/(1 − x)Fe-TiO2 | c-R | 2.96 | N.D. |
| a-R | 3.22 | N.D. | |
| a-R on c-A | 3.44 | 85.4% | |
| iso-TiO2 | 3.62 | N.D. | |
| iso-TiO2 | 3.76 | 14.6% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Chuaicham, C.; Balakumar, V.; Ohtani, B.; Sasaki, K. Fabrication of Adsorbed Fe(III) and Structurally Doped Fe(III) in Montmorillonite/TiO2 Composite for Photocatalytic Degradation of Phenol. Minerals 2021, 11, 1381. https://doi.org/10.3390/min11121381
Zhang L, Chuaicham C, Balakumar V, Ohtani B, Sasaki K. Fabrication of Adsorbed Fe(III) and Structurally Doped Fe(III) in Montmorillonite/TiO2 Composite for Photocatalytic Degradation of Phenol. Minerals. 2021; 11(12):1381. https://doi.org/10.3390/min11121381
Chicago/Turabian StyleZhang, Li, Chitiphon Chuaicham, Vellaichamy Balakumar, Bunsho Ohtani, and Keiko Sasaki. 2021. "Fabrication of Adsorbed Fe(III) and Structurally Doped Fe(III) in Montmorillonite/TiO2 Composite for Photocatalytic Degradation of Phenol" Minerals 11, no. 12: 1381. https://doi.org/10.3390/min11121381
APA StyleZhang, L., Chuaicham, C., Balakumar, V., Ohtani, B., & Sasaki, K. (2021). Fabrication of Adsorbed Fe(III) and Structurally Doped Fe(III) in Montmorillonite/TiO2 Composite for Photocatalytic Degradation of Phenol. Minerals, 11(12), 1381. https://doi.org/10.3390/min11121381