The Role of Microorganisms in the Formation, Dissolution, and Transformation of Secondary Minerals in Mine Rock and Drainage: A Review
Abstract
1. Introduction
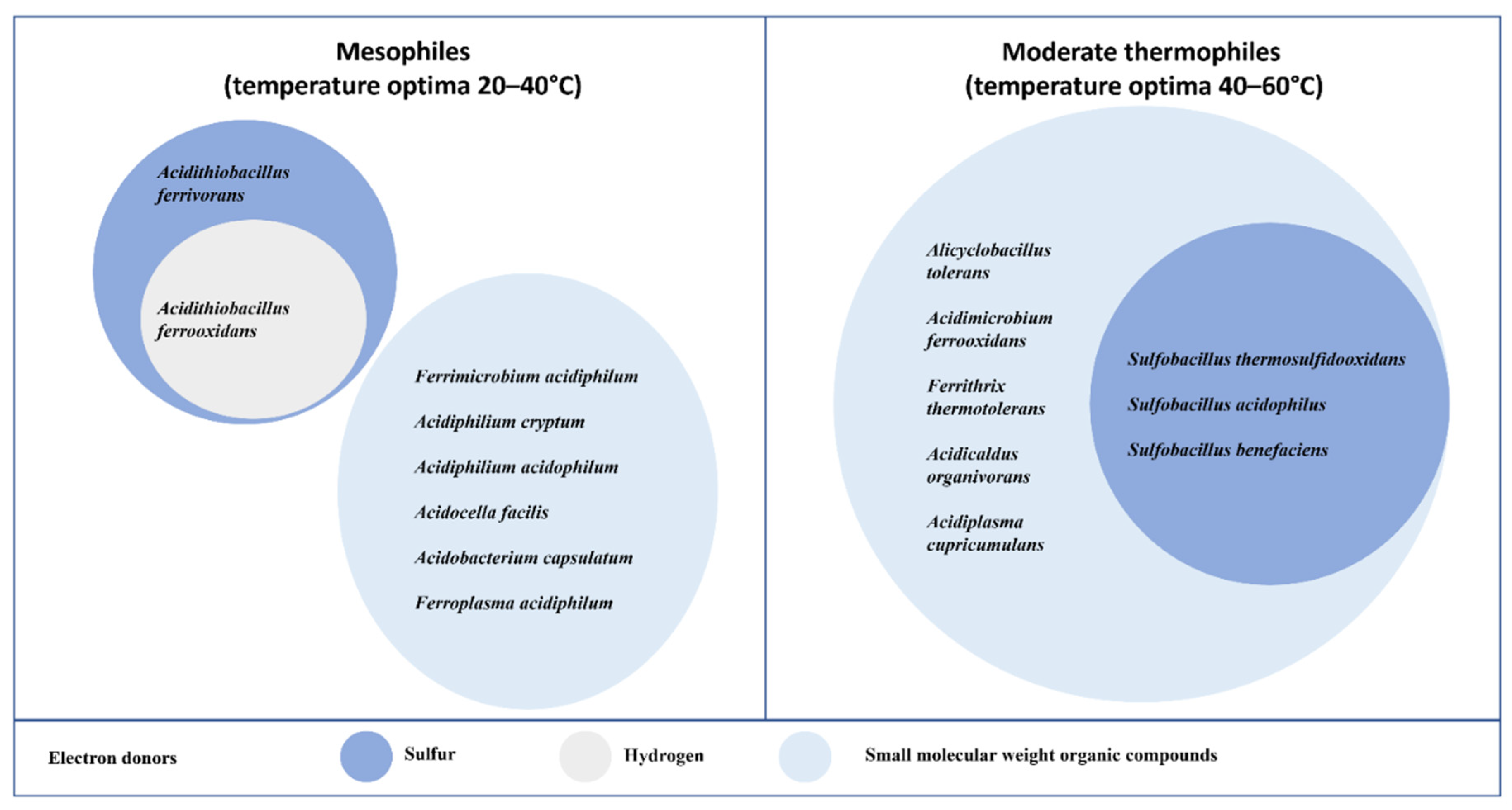
2. Microbial Communities in Mine Waste Rock Piles
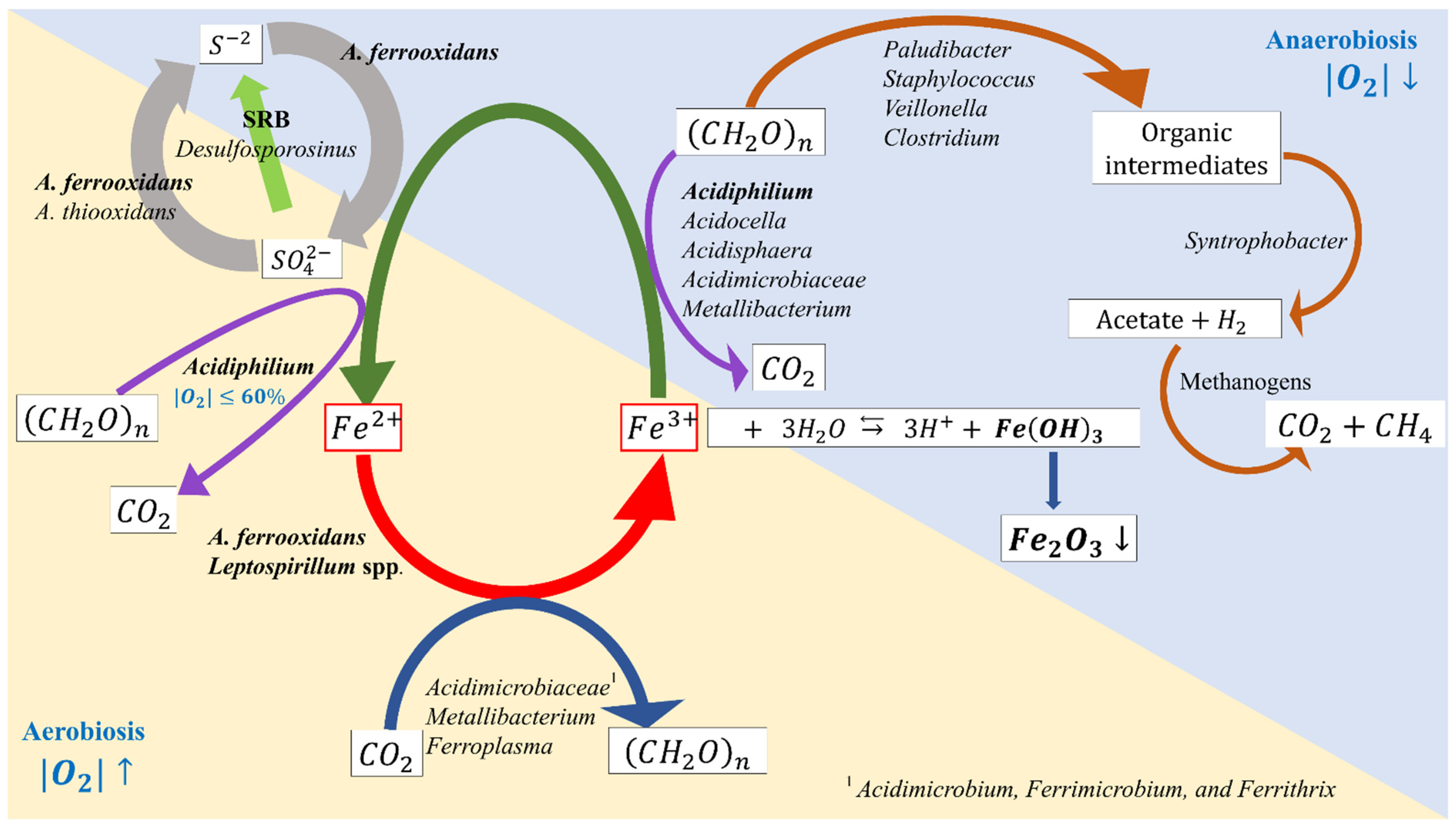
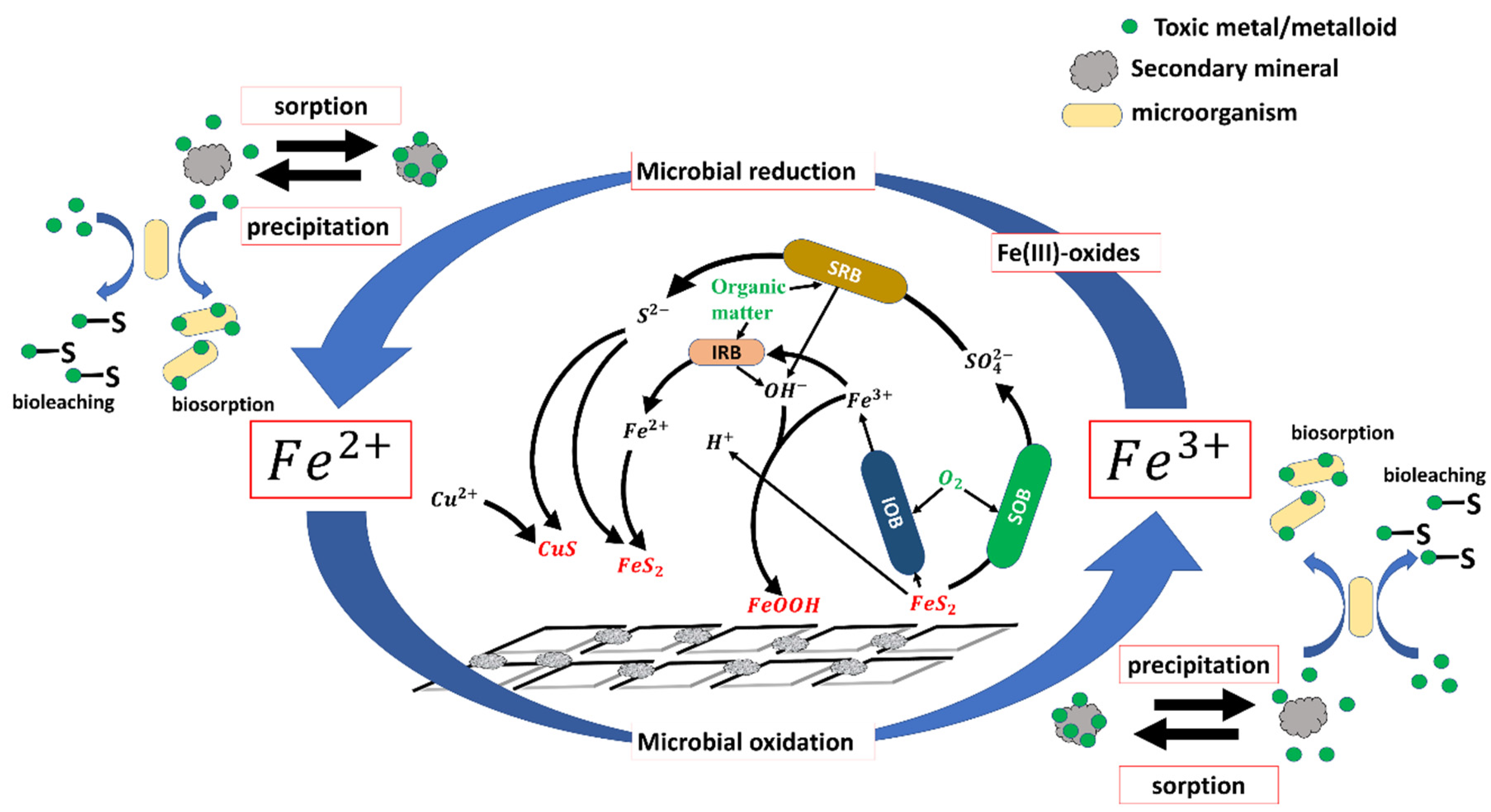
3. Microbial-Facilitated Iron and Sulfur Cycling
4. Influence of Microorganisms on Acid Neutralization by Carbonate and Silicate Minerals
4.1. Neutralization by Carbonate Minerals
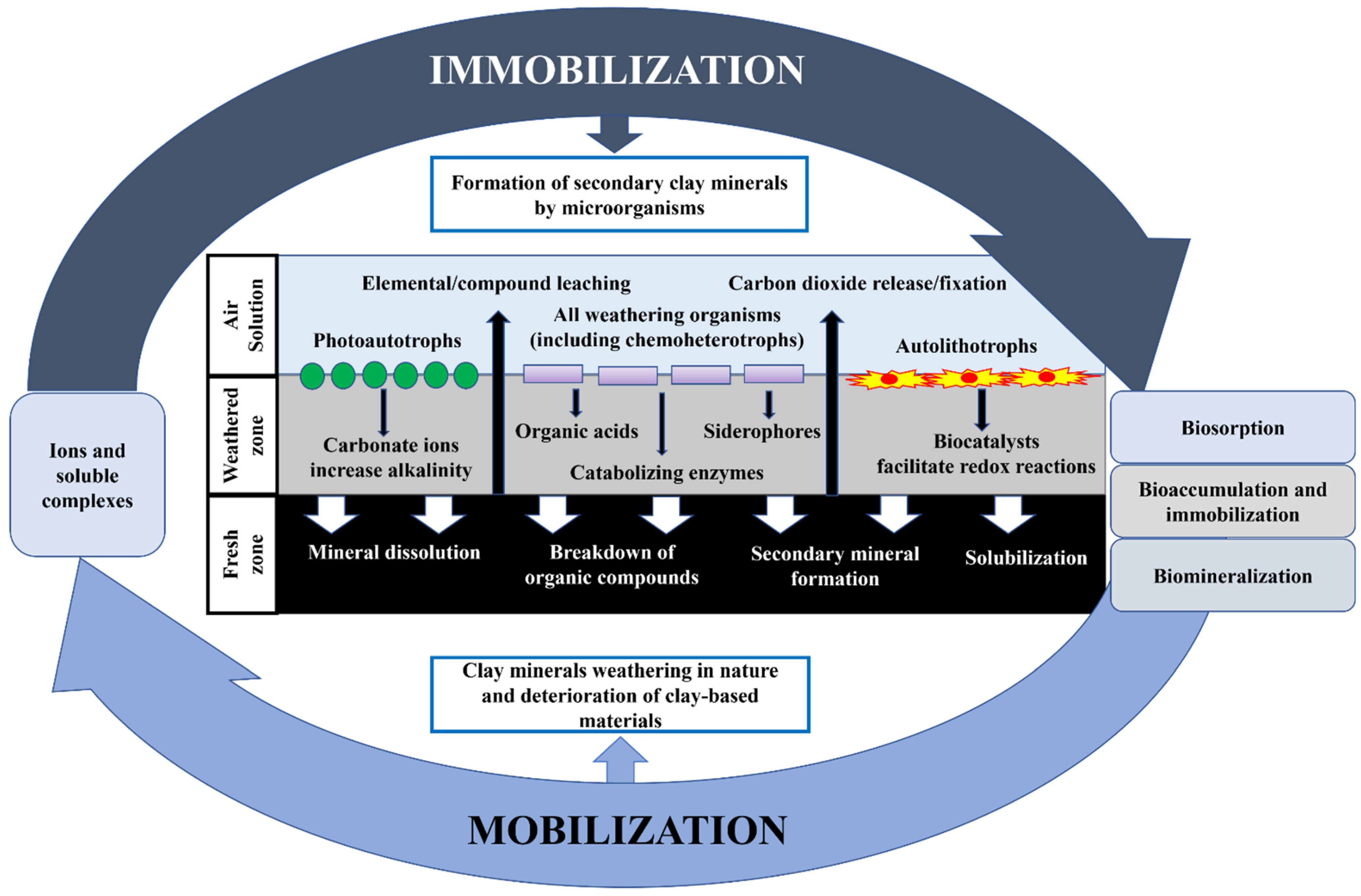
4.2. Neutralization by Silicate Minerals
5. Formation of Secondary Minerals
5.1. Soluble Metal Sulfate Salts
5.2. Relatively Insoluble, Amorphous Hydroxysulfates, and Oxyhydroxides
5.3. Well-Crystalline Minerals of the Alunite Supergroup
6. Influence of Secondary Minerals on the Mobility of Toxic Elements
6.1. Uptake of Toxic Elements by Secondary Minerals
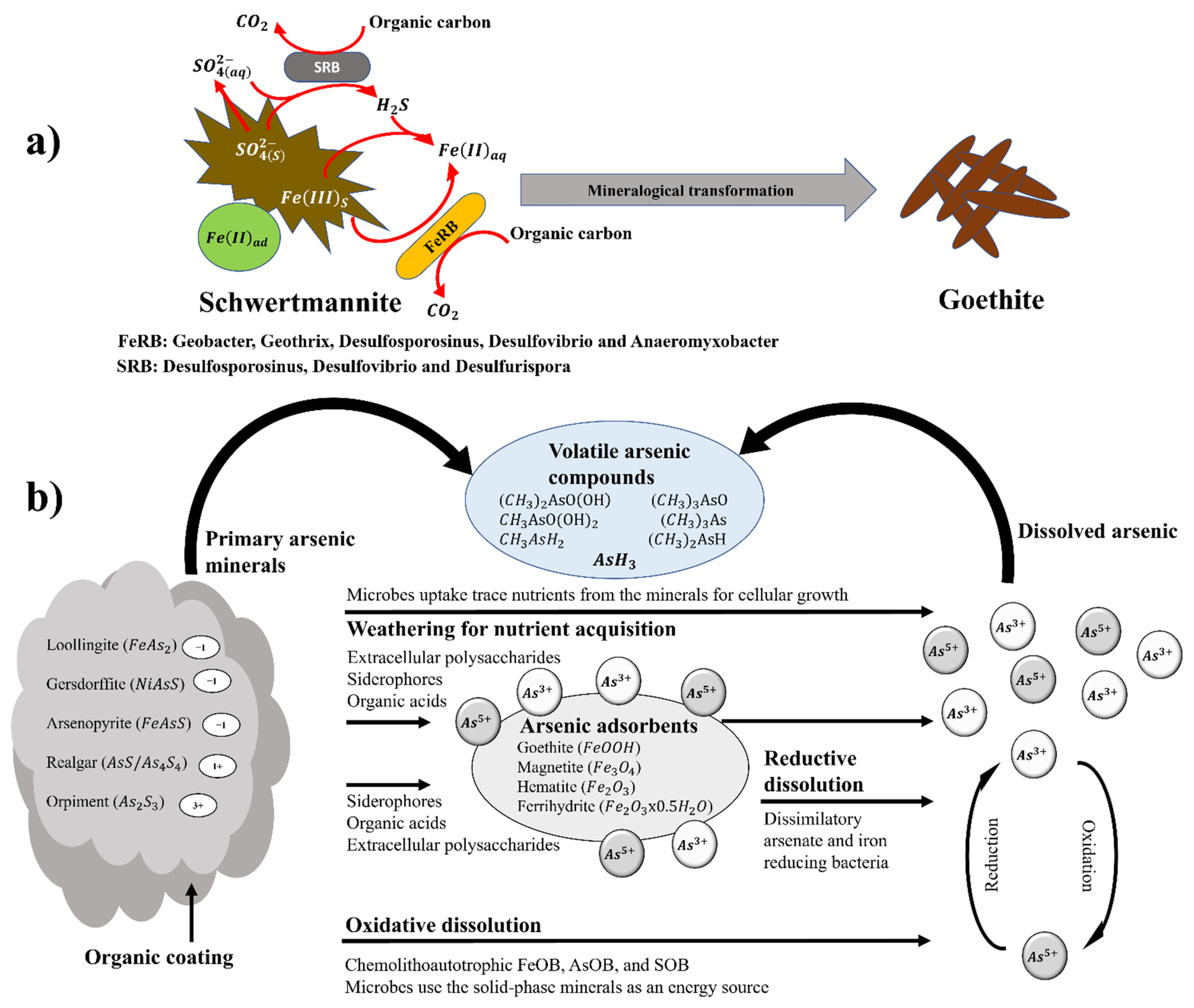
6.2. Transformation of Secondary Minerals Mediated by Microorganisms
6.3. Release of Toxic Elements during Secondary Mineral Transformation
7. Future Practical Applications of Microbial-Mineral Interactions for Remediation
Author Contributions
Funding
Conflicts of Interest
References
- Hudson-Edwards, K.A.; Jamieson, H.E.; Lottermoser, B. Mine Wastes: Past, Present, Future. Elements 2011, 7, 375–380. [Google Scholar] [CrossRef]
- Vriens, B.; Plante, B.; Seigneur, N.; Jamieson, H. Mine waste rock: Insights for sustainable hydrogeochemical management. Minerals 2020, 10, 728. [Google Scholar] [CrossRef]
- Jamieson, H.E. Geochemistry and mineralogy of solid mine waste: Essential knowledge for predicting environmental impact. Elements 2011, 7, 381–386. [Google Scholar] [CrossRef]
- Jamieson, H.E.; Walker, S.R.; Parsons, M. Mineralogical characterization of mine waste. Appl. Geochem. 2015, 57, 85–105. [Google Scholar] [CrossRef]
- Nordstrom, D.K. Hydrogeochemical processes governing the origin, transport and fate of major and trace elements from mine wastes and mineralized rock to surface waters. Appl. Geochem. 2011, 26, 1777–1791. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; Blowes, D.W.; Ptacek, C.J. Hydrogeochemistry and Microbiology of Mine Drainage: An Update; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; Volume 5, ISBN 3035413037. [Google Scholar]
- Ferguson, K.D.; Erickson, P.M. Environmental Management of Solid Waste: Dredged Material and Mine Tailings; Springer: Berlin/Heidelberg, Germany, 1988; ISBN 978-3-642-61362-3. [Google Scholar]
- Ehrlich, H.L. How microbes influence mineral growth and dissolution. Chem. Geol. 1996, 132, 5–9. [Google Scholar] [CrossRef]
- Amos, R.T.; Blowes, D.W.; Bailey, B.L.; Sego, D.C.; Smith, L.; Ritchie, A.I.M. Waste-rock hydrogeology and geochemistry. Appl. Geochem. 2015, 57, 140–156. [Google Scholar] [CrossRef]
- Jambor, J.L.; Nordstrom, D.K.; Alpers, C.N. Metal-sulfate salts from sulfide mineral oxidation. Rev. Miner. Geochem. 2000, 40, 303–350. [Google Scholar] [CrossRef]
- Antelo, J.; Fiol, S.; Gondar, D.; López, R.; Arce, F. Comparison of arsenate, chromate and molybdate binding on schwertmannite: Surface adsorption vs anion-exchange. J. Colloid Interface Sci. 2012, 386, 338–343. [Google Scholar] [CrossRef]
- Baleeiro, A.; Fiol, S.; Otero-Fariña, A.; Antelo, J. Surface chemistry of iron oxides formed by neutralization of acidic mine waters: Removal of trace metals. Appl. Geochem. 2018, 89, 129–137. [Google Scholar] [CrossRef]
- Smuda, J.; Dold, B.; Friese, K.; Morgenstern, P.; Glaesser, W. Mineralogical and geochemical study of element mobility at the sulfide-rich Excelsior waste rock dump from the polymetallic Zn–Pb–(Ag–Bi–Cu) deposit, Cerro de Pasco, Peru. J. Geochem. Explor. 2007, 92, 97–110. [Google Scholar] [CrossRef]
- Brown, G.E.; Foster, A.L.; Ostergren, J.D. Mineral surfaces and bioavailability of heavy metals: A molecular-scale perspective. Proc. Natl. Acad. Sci. USA 1999, 96, 3388–3395. [Google Scholar] [CrossRef]
- Schippers, A.; Breuker, A.; Blazejak, A.; Bosecker, K.; Kock, D.; Wright, T. The biogeochemistry and microbiology of sulfidic mine waste and bioleaching dumps and heaps, and novel Fe(II)-oxidizing bacteria. Hydrometallurgy 2010, 104, 342–350. [Google Scholar] [CrossRef]
- Dong, H. Mineral-microbe interactions: A review. Front. Earth Sci. China 2010, 4, 127–147. [Google Scholar] [CrossRef]
- Miot, J.; Benzerara, K.; Kappler, A. Investigating Microbe-Mineral Interactions: Recent Advances in X-Ray and Electron Microscopy and Redox-Sensitive Methods. Annu. Rev. Earth Planet. Sci. 2014, 42, 271–289. [Google Scholar] [CrossRef]
- Singleton, R. The sulfate-reducing bacteria: An overview. In The Sulfate-Reducing Bacteria: Contemporary Perspectives; Odom, J., Singleton, J.-R., Eds.; Springer: New York, NY, USA, 1993; pp. 1–20. ISBN 978-1-4613-9263-7. [Google Scholar]
- Nemati, M.; Harrison, S.; Hansford, G.; Webb, C. Biological oxidation of ferrous sulphate by Thiobacillus ferrooxidans: A review on the kinetic aspects. Biochem. Eng. J. 1998, 1, 171–190. [Google Scholar] [CrossRef]
- Barker, W.W.; Welch, S.; Chu, S.; Banfield, J. Experimental observations of the effects of bacteria on aluminosilicate weathering. Am. Miner. 1998, 83, 1551–1563. [Google Scholar] [CrossRef]
- Cummings, D.E.; Fendorf, S.; Singh, N.; Sani, R.K.; Peyton, B.M.; Magnuson, T.S. Reduction of Cr(VI) under acidic conditions by the facultative Fe(III)-reducing bacterium Acidiphilium cryptum. Environ. Sci. Technol. 2007, 41, 146–152. [Google Scholar] [CrossRef]
- Mesa, V.; Rodríguez-Gallego, J.L.; González-Gil, R.; Lauga, B.; Sánchez, J.; Méndez-García, C.; Peláez, A.I. Bacterial, archaeal, and eukaryotic diversity across distinct microhabitats in an acid mine drainage. Front. Microbiol. 2017, 8, 1756. [Google Scholar] [CrossRef]
- Druschel, G.K.; Baker, B.J.; Gihring, T.M.; Banfield, J. F Acid mine drainage biogeochemistry at Iron Mountain, California. Geochem. Trans. 2004, 5, 13–32. [Google Scholar] [CrossRef]
- Baker, B.; Banfield, J. Microbial communities in acid mine drainage. FEMS Microbiol. Ecol. 2003, 44, 139–152. [Google Scholar] [CrossRef]
- Schippers, A.; Hallmann, R.; Wentzien, S.; Sand, W. Microbial diversity in uranium mine waste heaps. Appl. Environ. Microbiol. 1995, 61, 2930–2935. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.J.; Bond, P.; Druschel, G.K.; McGuire, M.M.; Hamers, R.J.; Banfield, J. Geochemical and biological aspects of sulfide mineral dissolution: Lessons from Iron Mountain, California. Chem. Geol. 2000, 169, 383–397. [Google Scholar] [CrossRef]
- Baumler, D.J.; Jeong, K.-C.; Fox, B.G.; Banfield, J.F.; Kaspar, C.W. Sulfate requirement for heterotrophic growth of “Ferroplasma acidarmanus” strain fer1. Res. Microbiol. 2005, 156, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B. The biogeochemistry of biomining. In Geomicrobiology: Molecular and Environmental Perspective; Barton, L.L., Mandl, M., Loy, A., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 401–426. ISBN 9789048192038. [Google Scholar]
- Jones, A.A.; Bennett, P.C. Mineral ecology: Surface specific colonization and geochemical drivers of biofilm accumulation, composition, and phylogeny. Front. Microbiol. 2017, 8, 491. [Google Scholar] [CrossRef]
- Ullman, W.J.; Kirchman, D.L.; Welch, S.; Vandevivere, P. Laboratory evidence for microbially mediated silicate mineral dissolution in nature. Chem. Geol. 1996, 132, 11–17. [Google Scholar] [CrossRef]
- Banfield, J.; Barker, W.W.; Welch, S.; Taunton, A. Biological impact on mineral dissolution: Application of the lichen model to understanding mineral weathering in the rhizosphere. Proc. Natl. Acad. Sci. USA 1999, 96, 3404–3411. [Google Scholar] [CrossRef]
- Hersman, L.; Lloyd, T.; Sposito, G. Siderophore-promoted dissolution of hematite. Geochim. Cosmochim. Acta 1995, 59, 3327–3330. [Google Scholar] [CrossRef]
- Welch, S.A.; Vandevivere, P. Effect of microbial and other naturally occurring polymers on mineral dissolution. Geomicrobiol. J. 1994, 12, 227–238. [Google Scholar] [CrossRef]
- Valentín-Vargas, A.; Root, R.A.; Neilson, J.W.; Chorover, J.; Maier, R.M. Environmental factors influencing the structural dynamics of soil microbial communities during assisted phytostabilization of acid-generating mine tailings: A mesocosm experiment. Sci. Total Environ. 2014, 500–501, 314–324. [Google Scholar] [CrossRef]
- Mendez, M.O.; Neilson, J.W.; Maier, R.M. Characterization of a bacterial community in an abandoned semiarid lead-zinc mine tailing site. Appl. Environ. Microbiol. 2008, 74, 3899–3907. [Google Scholar] [CrossRef] [PubMed]
- Schippers, A. Biogeochemistry of metal sulfide oxidation in mining environments, sediments, and soils. In Sulfur Biogeochemistry —Past and Present; Geological Society of America: Boulder, CO, USA, 2004; pp. 49–62. ISBN 9780813723792. [Google Scholar]
- Blowes, D.W.; Ptacek, C.J.; Jambor, J.L.; Weisener, C.G. The geochemistry of acid mine drainage. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Elsevier-Pergamon: Oxford, UK, 2003; pp. 149–204. ISBN 978-0-08-043751-4. [Google Scholar]
- Singer, P.C.; Stumm, W. Acidic Mine Drainage: The Rate-Determining Step. Science 1970, 167, 1121–1123. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, R.V.; Gillham, R.W.; Reardon, E.J. Pyrite oxidation in carbonate-buffered solution: 1. Experimental kinetics. Geochim. Cosmochim. Acta 1988, 52, 1077–1085. [Google Scholar] [CrossRef]
- Williamson, M.A.; Rimstidt, J. The kinetics and electrochemical rate-determining step of aqueous pyrite oxidation. Geochim. Cosmochim. Acta 1994, 58, 5443–5454. [Google Scholar] [CrossRef]
- Frau, F. The formation-dissolution-precipitation cycle of melanterite at the abandoned pyrite mine of Genna Luas in Sardinia, Italy: Environmental implications. Miner. Mag. 2000, 64, 995–1006. [Google Scholar] [CrossRef]
- Nicholson, R.V.; Scharer, J.M. Chapter 2. Laboratory studies of pyrrhotite oxidation kinetics. In Environmental Geochemistry of Sulfide Oxidation; Alpers, C.N., Blowes, D.W., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1994; pp. 14–30. ISBN 9780841227729. [Google Scholar]
- Pugh, C.E.; Hossner, L.R.; Dixon, J.B. Oxidation rate of iron sulfides as affected by surface area, morphology, oxygen concentration, and autotrophic bacteria. Soil Sci. 1984, 137, 309–314. [Google Scholar] [CrossRef]
- Janzen, M.P.; Nicholson, R.V.; Scharer, J.M. Pyrrhotite reaction kinetics: Reaction rates for oxidation by oxygen, ferric iron, and for nonoxidative dissolution. Geochim. Cosmochim. Acta 2000, 64, 1511–1522. [Google Scholar] [CrossRef]
- Luther, G. Pyrite oxidation and reduction: Molecular orbital theory considerations. Geochim. Cosmochim. Acta 1987, 51, 3193–3199. [Google Scholar] [CrossRef]
- Meruane, G.; Vargas, T. Bacterial oxidation of ferrous iron by Acidithiobacillus ferrooxidans in the pH range 2.5–7.0. Hydrometallurgy 2003, 71, 149–158. [Google Scholar] [CrossRef]
- Nordstrom, D.K. The rate of ferrous iron oxidation in a stream receiving acid mine effluent. In Selected Papers in the Hydrologic Sciences; Subitzky, S., Ed.; United States Geological Survey Water-Supply Paper: Reston, VA, USA, 1985; Volume 2270, pp. 113–119. [Google Scholar]
- Edwards, K.J.; Bond, P.L.; Banfield, J.F. Characteristics of attachment and growth of Thiobacillus caldus on sulphide minerals: A chemotactic response to sulphur minerals? Environ. Microbiol. 2000, 2, 324–332. [Google Scholar] [CrossRef]
- Stoner, D.L.; Miller, K.S.; Fife, D.J.; Larsen, E.D.; Tolle, C.R.; Johnson, J.A. Use of an intelligent control system to evaluate multiparametric effects on iron oxidation by Thermophilic Bacteria. Appl. Environ. Microbiol. 1998, 64, 4555–4565. [Google Scholar] [CrossRef]
- Edwards, K.J.; Schrenk, M.O.; Hamers, R.; Banfield, J.F. Microbial oxidation of pyrite; experiments using microorganisms from an extreme acidic environment. Am. Miner. 1998, 83, 1444–1453. [Google Scholar] [CrossRef]
- Sand, W. Ferric iron reduction by Thiobacillus ferrooxidans at extremely low pH-values. Biogeochemistry 1989, 7, 195–201. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; Southam, G. Geomicrobiology of sulfide mineral oxidation. In Geomicrobiology: Interactions between Microbes and Minerals; Banfield, J.F., Nealson, K.H., Eds.; Reviews in Mineralogy; Mineralogical Society of America: Washington, DC, USA, 1997; Volume 35, pp. 361–390. ISBN 9781501509247. [Google Scholar]
- Silverman, M.P.; Ehrlich, H.L. Microbial formation and degradation of minerals. Adv. Appl. Microbiol. 1964, 6, 153–206. [Google Scholar] [CrossRef]
- Sand, W.; Gehrke, T.; Jozsa, P.-G.; Schippers, A. (Bio)chemistry of bacterial leaching—Direct vs. indirect bioleaching. Hydrometallurgy 2001, 59, 159–175. [Google Scholar] [CrossRef]
- Tributsch, H. Direct versus indirect bioleaching. Hydrometallurgy 2001, 59, 177–185. [Google Scholar] [CrossRef]
- Boon, M.; Heijnen, J. Chemical oxidation kinetics of pyrite in bioleaching processes. Hydrometallurgy 1998, 48, 27–41. [Google Scholar] [CrossRef]
- Fowler, T.A.; Holmes, P.R.; Crundwell, F.K. Mechanism of pyrite dissolution in the presence of Thiobacillus ferrooxidans. Appl. Environ. Microbiol. 1999, 65, 2987–2993. [Google Scholar] [CrossRef] [PubMed]
- Vera, M.; Schippers, A.; Sand, W. Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation—part A. Appl. Microbiol. Biotechnol. 2013, 97, 7529–7541. [Google Scholar] [CrossRef]
- Schippers, A.; Rohwerder, T.; Sand, W. Intermediary sulfur compounds in pyrite oxidation: Implications for bioleaching and biodepyritization of coal. Appl. Microbiol. Biotechnol. 1999, 52, 104–110. [Google Scholar] [CrossRef]
- Toniazzo, V.; Mustin, C.; Benoit, R.; Humbert, B.; Berthelin, J. Superficial compounds produced by Fe(III) mineral oxidation as essential reactants for bio-oxidation of pyrite by Thiobacillus ferrooxidans. Cultures 1999, 9, 177–186. [Google Scholar] [CrossRef]
- Liu, C.; Jia, Y.; Sun, H.; Tan, Q.; Niu, X.; Leng, X.; Ruan, R. Limited role of sessile acidophiles in pyrite oxidation below redox potential of 650 mV. Sci. Rep. 2017, 7, 5032. [Google Scholar] [CrossRef]
- Dong, B.; Jia, Y.; Tan, Q.; Sun, H.; Ruan, R. Contributions of Microbial “Contact Leaching” to Pyrite Oxidation under Different Controlled Redox Potentials. Minerals 2020, 10, 856. [Google Scholar] [CrossRef]
- Sánchez-Andrea, I.; Rodríguez, N.; Amils, R.; Sanz, J.L. Microbial diversity in anaerobic sediments at Río Tinto, a naturally acidic environment with a high heavy metal content. Appl. Environ. Microbiol. 2011, 77, 6085–6093. [Google Scholar] [CrossRef]
- Johnson, D. Biodiversity and ecology of acidophilic microorganisms. FEMS Microbiol. Ecol. 1998, 27, 307–317. [Google Scholar] [CrossRef]
- Hedrich, S.; Schippers, A. Distribution of Acidophilic microorganisms in natural and man-made acidic environments. Curr. Issues Mol. Biol. 2020, 40, 25–48. [Google Scholar] [CrossRef] [PubMed]
- Kucera, J.; Lochman, J.; Bouchal, P.; Pakostova, E.; Mikulasek, K.; Hedrich, S.; Janiczek, O.; Mandl, M.; Johnson, D.B. A model of aerobic and anaerobic metabolism of hydrogen in the extremophile Acidithiobacillus ferrooxidans. Front. Microbiol. 2020, 11, 610836. [Google Scholar] [CrossRef] [PubMed]
- Bridge, T.A.M.; Johnson, D.B. Reduction of soluble iron and reductive dissolution of ferric iron-containing minerals by moderately thermophilic iron-oxidizing bacteria. Appl. Environ. Microbiol. 1998, 64, 2181–2186. [Google Scholar] [CrossRef]
- Johnson, D. Acidophilic microbial communities: Candidates for bioremediation of acidic mine effluents. Int. Biodeterior. Biodegradation 1995, 35, 41–58. [Google Scholar] [CrossRef]
- Johnson, D.B.; McGinness, S. Ferric Iron Reduction by Acidophilic Heterotrophic Bacteria. Appl. Environ. Microbiol. 1991, 57, 207–211. [Google Scholar] [CrossRef]
- González-Toril, E.; Aguilera, Á. Microbial ecology in extreme acidic environments: Use of molecular tools. In Microbial Diversity in the Genomic Era; Elsevier: Amsterdam, The Netherlands, 2019; pp. 227–238. ISBN 9780128148501. [Google Scholar]
- Borilova, S.; Mandl, M.; Zeman, J.; Kucera, J.; Pakostova, E.; Janiczek, O.; Tuovinen, O.H. Can Sulfate Be the First Dominant Aqueous Sulfur Species Formed in the Oxidation of Pyrite by Acidithiobacillus ferrooxidans? Front. Microbiol. 2018, 9, 3134. [Google Scholar] [CrossRef]
- Osorio, H.; Mangold, S.; Denis, Y.; Ñancucheo, I.; Esparza, M.; Johnson, D.B.; Bonnefoy, V.; Dopson, M.; Holmes, D.S. Anaerobic sulfur metabolism coupled to dissimilatory iron reduction in the extremophile Acidithiobacillus ferrooxidans. Appl. Environ. Microbiol. 2013, 79, 2172–2181. [Google Scholar] [CrossRef]
- Mardanov, A.; Panova, I.; Beletsky, A.; Avakyan, M.; Kadnikov, V.V.; Antsiferov, D.; Banks, D.; Frank, Y.A.; Pimenov, N.V.; Ravin, N.; et al. Genomic insights into a new acidophilic, copper-resistantDesulfosporosinusisolate from the oxidized tailings area of an abandoned gold mine. FEMS Microbiol. Ecol. 2016, 92, 1–14. [Google Scholar] [CrossRef]
- Sela-Adler, M.; Ronen, Z.; Herut, B.; Antler, G.; Vigderovich, H.; Eckert, W.; Sivan, O. Co-existence of Methanogenesis and Sulfate Reduction with Common Substrates in Sulfate-Rich Estuarine Sediments. Front. Microbiol. 2017, 8, 766. [Google Scholar] [CrossRef] [PubMed]
- Blowes, D.W.; Ptacek, C.J.; Frind, E.O.; Johnson, R.H.; Robertson, W.D.; Molson, J.W. Acid-Neutralization reactions in inactive mine tailings impoundments and their effect on the transport of dissolved metals. J. Am. Soc. Min. Reclam. 1994, 429–438. [Google Scholar] [CrossRef]
- Sherlock, E.J.; Lawrence, R.W.; Poulin, R. On the neutralization of acid rock drainage by carbonate and silicate minerals. Environ. Earth Sci. 1995, 25, 43–54. [Google Scholar] [CrossRef]
- Strömberg, B.; Banwart, S. Weathering kinetics of waste rock from the Aitik copper mine, Sweden: Scale dependent rate factors and pH controls in large column experiments. J. Contam. Hydrol. 1999, 39, 59–89. [Google Scholar] [CrossRef]
- Karlsson, T.; Räisänen, M.L.; Lehtonen, M.; Alakangas, L. Comparison of static and mineralogical ARD prediction methods in the Nordic environment. Environ. Monit. Assess. 2018, 190, 719. [Google Scholar] [CrossRef] [PubMed]
- Maest, A.S.; Nordstrom, D.K. A geochemical examination of humidity cell tests. Appl. Geochem. 2017, 81, 109–131. [Google Scholar] [CrossRef]
- USEPA-United States Environmental Protection Agency. Acid Mine Drainage Prediction: Technical Document, US EPA, Office of Solid Waste Special Waste Branch, EPA530-R94-036, NTIS PB94-201829; USEPA: Washington, DC, USA, 1994.
- Kwong, Y.T. Prediction and Prevention of Acid Rock Drainage from a Geological and Mineralogical Perspective, MEND, Project 1.32.1.; Canmet: Ottawa, ON, Canada, 1993. [Google Scholar]
- Dold, B. Acid rock drainage prediction: A critical review. J. Geochem. Explor. 2017, 172, 120–132. [Google Scholar] [CrossRef]
- Lüttge, A.; Conrad, P.G. Direct observation of microbial inhibition of calcite dissolution. Appl. Environ. Microbiol. 2004, 70, 1627–1632. [Google Scholar] [CrossRef] [PubMed]
- Luttge, A. Mineral surfaces and their implications for microbial attachment: Results from Monte Carlo simulations and direct surface observations. Am. J. Sci. 2005, 305, 766–790. [Google Scholar] [CrossRef]
- Parbhakar-Fox, A.; Lottermoser, B. A critical review of acid rock drainage prediction methods and practices. Miner. Eng. 2015, 82, 107–124. [Google Scholar] [CrossRef]
- Becker, M.; Dyantyi, N.; Broadhurst, J.; Harrison, S.T.; Franzidis, J.-P. A mineralogical approach to evaluating laboratory scale acid rock drainage characterisation tests. Miner. Eng. 2015, 80, 33–36. [Google Scholar] [CrossRef]
- Luce, R.W.; Bartlett, R.W.; Parks, G.A. Dissolution kinetics of magnesium silicates. Geochim. Cosmochim. Acta 1972, 36, 35–50. [Google Scholar] [CrossRef]
- Miller, S.D.; Stewart, W.S.; Rusdinar, Y.; Schumann, R.E.; Ciccarelli, J.M.; Li, J.; Smart, R.S. Methods for estimation of long-term non-carbonate neutralisation of acid rock drainage. Sci. Total Environ. 2010, 408, 2129–2135. [Google Scholar] [CrossRef]
- McClelland, J.E. The effect of time, temperature, and particle size on the release of bases from some common soil-forming minerals of different crystal structure. Soil Sci. Soc. Proc. 1950, 15, 301–307. [Google Scholar] [CrossRef]
- Huang, W.H.; Keller, W.D. Dissolution of rock-forming silicate minerals in organic acids: Simulated first-stage weathering of fresh mineral surfaces. Am. Mineral. 1970, 55, 2076–2094. [Google Scholar]
- Malmström, M.; Banwart, S. Biotite dissolution at 25 °C: The pH dependence of dissolution rate and stoichiometry. Geochim. Cosmochim. Acta 1997, 61, 2779–2799. [Google Scholar] [CrossRef]
- Ye, L.; Wang, L.; Jing, C. Biotransformation of adsorbed arsenic on iron minerals by coexisting arsenate-reducing and arsenite-oxidizing bacteria. Environ. Pollut. 2020, 256, 113471. [Google Scholar] [CrossRef]
- White, A.F.; Bullen, T.D.; Schulz, M.S.; Blum, A.E.; Huntington, T.G.; Peters, N.E. Differential rates of feldspar weathering in granitic regoliths. Geochim. Cosmochim. Acta 2001, 65, 847–869. [Google Scholar] [CrossRef]
- Blackmore, S.; Vriens, B.; Sorensen, M.; Power, I.M.; Smith, L.; Hallam, S.J.; Mayer, K.U.; Beckie, R. Microbial and geochemical controls on waste rock weathering and drainage quality. Sci. Total Environ. 2018, 640–641, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Bennett, P.C.; Rogers, J.R.; Choi, W.J.; Hiebert, F.K. Silicates, silicate weathering, and microbial ecology. Geomicrobiol. J. 2001, 18, 3–19. [Google Scholar] [CrossRef]
- Ivanova, E.; Chizhikova, N. Degradative crystal–chemical transformations of clay minerals under the influence of cyanobacterium-actinomycetal symbiotic associations. Eurasian J. Soil Sci. (EJSS) 2014, 3, 116. [Google Scholar] [CrossRef][Green Version]
- Samuels, T.; Bryce, C.; Landenmark, H.; Marie-Loudon, C.; Nicholson, N.; Stevens, A.H.; Cockell, C. Microbial weathering of minerals and rocks in natural environments. In Biogeochemical Cycles: Ecological Drivers and Environmental Impact; American Geophysical Union (AGU): Washington, DC, USA, 2020; pp. 59–79. ISBN 9781119413332. [Google Scholar]
- Fomina, M.; Skorochod, I. Microbial interaction with clay minerals and its environmental and biotechnological implications. Minerals 2020, 10, 861. [Google Scholar] [CrossRef]
- Ahmed, E.; Holmström, S.J. Microbe–mineral interactions: The impact of surface attachment on mineral weathering and element selectivity by microorganisms. Chem. Geol. 2015, 403, 13–23. [Google Scholar] [CrossRef]
- Balland, C.; Poszwa, A.; Leyval, C.; Mustin, C. Dissolution rates of phyllosilicates as a function of bacterial metabolic diversity. Geochim. Cosmochim. Acta 2010, 74, 5478–5493. [Google Scholar] [CrossRef]
- Uroz, S.; Calvaruso, C.; Turpault, M.-P.; Frey-Klett, P. Mineral weathering by bacteria: Ecology, actors and mechanisms. Trends Microbiol. 2009, 17, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Stranghoener, M.; Schippers, A.; Dultz, S.; Behrens, H. Experimental microbial alteration and fe mobilization from basaltic rocks of the ICDP HSDP2 Drill Core, Hilo, Hawaii. Front. Microbiol. 2018, 9, 1252. [Google Scholar] [CrossRef]
- Kalinowski, B.E.; Liermann, L.J.; Givens, S.; Brantley, S.L. Rates of bacteria-promoted solubilization of Fe from minerals: A review of problems and approaches. Chem. Geol. 2000, 169, 357–370. [Google Scholar] [CrossRef]
- Hutchens, E. Microbial selectivity on mineral surfaces: Possible implications for weathering processes. Fungal Biol. Rev. 2009, 23, 115–121. [Google Scholar] [CrossRef]
- Vandevivere, P.; Welch, S.; Ullman, W.; Kirchman, D. Enhanced dissolution of silicate minerals by bacteria at near-neutral pH. Microb. Ecol. 1994, 27, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.R.; Bennett, P.C.; Choi, W.J. Feldspars as a source of nutrients for microorganisms. Am. Miner. 1998, 83, 1532–1540. [Google Scholar] [CrossRef]
- Bonneville, S.; Morgan, D.; Schmalenberger, A.; Bray, A.; Brown, A.; Banwart, S.; Benning, L.G. Tree-mycorrhiza symbiosis accelerate mineral weathering: Evidences from nanometer-scale elemental fluxes at the hypha–mineral interface. Geochim. Cosmochim. Acta 2011, 75, 6988–7005. [Google Scholar] [CrossRef]
- Bonneville, S.; Smits, M.M.; Brown, A.; Harrington, J.; Leake, J.; Brydson, R.; Benning, L.G. Plant-driven fungal weathering: Early stages of mineral alteration at the nanometer scale. Geology 2009, 37, 615–618. [Google Scholar] [CrossRef]
- Bennett, P.C.; Hiebert, F.K.; Choi, W.J. Microbial colonization and weathering of silicates in a petroleum-contaminated groundwater. Chem. Geol. 1996, 132, 45–53. [Google Scholar] [CrossRef]
- Roberts, J.A.; Fowle, D.; Hughes, B.T.; Kulczycki, E. Attachment Behavior ofShewanella putrefaciensonto Magnetite under Aerobic and Anaerobic Conditions. Geomicrobiol. J. 2006, 23, 631–640. [Google Scholar] [CrossRef]
- Rogers, J.R.; Bennett, P.C. Mineral stimulation of subsurface microorganisms: Release of limiting nutrients from silicates. Chem. Geol. 2004, 203, 91–108. [Google Scholar] [CrossRef]
- Liu, W.; Xu, X.; Wu, X.; Yang, Q.; Luo, Y.; Christie, P. Decomposition of silicate minerals by Bacillus mucilaginosus in liquid culture. Environ. Geochem. Heath 2006, 28, 133–140. [Google Scholar] [CrossRef]
- Hoffland, E.; Kuyper, T.W.; Wallander, H.; Plassard, C.; Gorbushina, A.A.; Haselwandter, K.; Holmström, S.; Landeweert, R.; Lundström, U.S.; Rosling, A.; et al. The role of fungi in weathering. Front. Ecol. Environ. 2004, 2, 258–264. [Google Scholar] [CrossRef]
- Welch, S.; Ullman, W. The effect of microbial glucose metabolism on bytownite feldspar dissolution rates between 5 and 35 °C. Geochim. Cosmochim. Acta 1999, 63, 3247–3259. [Google Scholar] [CrossRef]
- Bigham, J.M.; Nordstrom, D.K. Iron and aluminum hydroxysulfates from acid sulfate waters. Rev. Miner. Geochem. 2000, 40, 351–403. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; Jambor, J.L. Jarosites and their application in hydrometallurgy. Rev. Miner. Geochem. 2000, 40, 405–452. [Google Scholar] [CrossRef]
- Dold, B. Evolution of acid mine drainage formation in sulphidic mine tailings. Minerals 2014, 4, 621–641. [Google Scholar] [CrossRef]
- Kocourková, E.; Sracek, O.; Houzar, S.; Cempírek, J.; Losos, Z.; Filip, J.; Hršelová, P. Geochemical and mineralogical control on the mobility of arsenic in a waste rock pile at Dlouhá Ves, Czech Republic. J. Geochem. Explor. 2011, 110, 61–73. [Google Scholar] [CrossRef]
- Lindsay, M.; Moncur, M.; Bain, J.G.; Jambor, J.L.; Ptacek, C.J.; Blowes, D.W. Geochemical and mineralogical aspects of sulfide mine tailings. Appl. Geochem. 2015, 57, 157–177. [Google Scholar] [CrossRef]
- Cravotta, C.A.I. Secondary iron-sulfate minerals as sources of sulfate and acidity. In Environmental Geochemistry of Sulfide Oxidation; Alpers, C.N., Blowes, D.W., Eds.; American Chemical Society: Washington, DC, USA, 1994; pp. 345–364. [Google Scholar]
- Harris, D.L.; Lottermoser, B.; Duchesne, J. Ephemeral acid mine drainage at the Montalbion silver mine, north Queensland. Aust. J. Earth Sci. 2003, 50, 797–809. [Google Scholar] [CrossRef]
- Sáinz, A.; Grande, J.; DE LA Torre, M.L.; Rodasc, D. Characterisation of sequential leachate discharges of mining waste rock dumps in the Tinto and Odiel rivers. J. Environ. Manag. 2002, 64, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.; Beveridge, T.J. Mineral formation by bacteria in natural microbial communities. FEMS Microbiol. Ecol. 1998, 26, 79–88. [Google Scholar] [CrossRef]
- Bigham, J.; Schwertmann, U.; Traina, S.; Winland, R.; Wolf, M. Schwertmannite and the chemical modeling of iron in acid sulfate waters. Geochim. Cosmochim. Acta 1996, 60, 2111–2121. [Google Scholar] [CrossRef]
- Walker, S.R.; Parsons, M.; Jamieson, H.E.; Lanzirotti, A. Arsenic mineralogy of near-surface tailings and soils: Influences on arsenic mobility and bioaccessibility in the Nova Scotia Gold mining districts. Can. Miner. 2009, 47, 533–556. [Google Scholar] [CrossRef]
- Xie, Y.; Lu, G.; Yang, C.; Qu, L.; Chen, M.; Guo, C.; Dang, Z. Mineralogical characteristics of sediments and heavy metal mobilization along a river watershed affected by acid mine drainage. PLoS ONE 2018, 13, e0190010. [Google Scholar] [CrossRef]
- Yu, J.-Y.; Heo, B.; Choi, I.-K.; Cho, J.-P.; Chang, H.-W. Apparent solubilities of schwertmannite and ferrihydrite in natural stream waters polluted by mine drainage. Geochim. Cosmochim. Acta 1999, 63, 3407–3416. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; Plummer, L.N.; Langmuir, D.; Busenberg, E.; May, H.M.; Jones, B.F.; Parkhurst, D.L. Revised chemical equilibrium data for major water—mineral reactions and their limitations. In Chemical Modeling of Aqueous Systems II; Bassett, R.L., Melchior, D.C., Eds.; ACS Symposium Series 416; American Chemical Society: Washington, DC, USA, 1990; Volume 416, pp. 398–413. [Google Scholar]
- Baron, D.; Palmer, C.D. Solubility of jarosite at 4–35 °C. Geochim. Cosmochim. Acta 1996, 60, 185–195. [Google Scholar] [CrossRef]
- Acero, P.; Ayora, C.; Torrentó, C.; Nieto, J.M. The behavior of trace elements during schwertmannite precipitation and subsequent transformation into goethite and jarosite. Geochim. Cosmochim. Acta 2006, 70, 4130–4139. [Google Scholar] [CrossRef]
- Burton, E.; Bush, R.; Sullivan, L.; Mitchell, D. Schwertmannite transformation to goethite via the Fe(II) pathway: Reaction rates and implications for iron–sulfide formation. Geochim. Cosmochim. Acta 2008, 72, 4551–4564. [Google Scholar] [CrossRef]
- Liu, H.; Li, P.; Zhu, M.; Wei, Y.; Sun, Y. Fe(II)-induced transformation from ferrihydrite to lepidocrocite and goethite. J. Solid State Chem. 2007, 180, 2121–2128. [Google Scholar] [CrossRef]
- Fortin, D.; Langley, S. Formation and occurrence of biogenic iron-rich minerals. Earth-Science Rev. 2005, 72, 1–19. [Google Scholar] [CrossRef]
- Huang, S.; Zhou, L. Fe2+ oxidation rate drastically affect the formation and phase of secondary iron hydroxysulfate mineral occurred in acid mine drainage. Mater. Sci. Eng. C 2012, 32, 916–921. [Google Scholar] [CrossRef]
- Zhu, J.; Gan, M.; Zhang, D.; Hu, Y.; Chai, L. The nature of Schwertmannite and Jarosite mediated by two strains of Acidithiobacillus ferrooxidans with different ferrous oxidation ability. Mater. Sci. Eng. C 2013, 33, 2679–2685. [Google Scholar] [CrossRef]
- Hedrich, S.; Lünsdorf, H.; Kleeberg, R.; Heide, G.; Seifert, J.; Schlömann, M. schwertmannite formation adjacent to bacterial cells in a mine water treatment plant and in pure cultures of Ferrovum myxofaciens. Environ. Sci. Technol. 2011, 45, 7685–7692. [Google Scholar] [CrossRef] [PubMed]
- Tischler, J.S.; Wiacek, C.; Janneck, E.; Schlömann, M. Microbial abundance in the schwertmannite formed in a mine water treatment plant. Mine Water Environ. 2013, 32, 258–265. [Google Scholar] [CrossRef]
- Feng, K.; Wang, X.; Zhou, B.; Xu, M.; Liang, J.; Zhou, L. Hydroxyl, Fe2+, and Acidithiobacillus ferrooxidans jointly determined the crystal growth and morphology of schwertmannite in a sulfate-rich acidic environment. ACS Omega 2021, 6, 3194–3201. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, E.J.; Gorski, C.A.; Flynn, T.M.; Scherer, M.M. Electron donor utilization and secondary mineral formation during the bioreduction of lepidocrocite by Shewanella putrefaciens CN32. Minerals 2019, 9, 434. [Google Scholar] [CrossRef]
- O’Reilly, S.E. Secondary mineral formation associated with respiration of nontronite, NAu-1 by iron reducing bacteria. Geochem. Trans. 2005, 6, 67–76. [Google Scholar] [CrossRef]
- Bayliss, P.; Kolitsch, U.; Nickel, E.H.; Pring, A. Alunite supergroup: Recommended nomenclature. Miner. Mag. 2010, 74, 919–927. [Google Scholar] [CrossRef]
- Qian, G.; Fan, R.; Short, M.; Schumann, R.C.; Li, J.; Li, Y.; Smart, R.S.; Gerson, A.R. Evaluation of the rate of dissolution of secondary sulfate minerals for effective acid and metalliferous drainage mitigation. Chem. Geol. 2019, 504, 14–27. [Google Scholar] [CrossRef]
- Jones, F. Crystallization of Jarosite with variable Al3+ content: The transition to Alunite. Minerals 2017, 7, 90. [Google Scholar] [CrossRef]
- Potter-McIntyre, S.L.; McCollom, T.M. Jarosite and Alunite in ancient terrestrial sedimentary rocks: Reinterpreting martian depositional and diagenetic environmental conditions. Life 2018, 8, 32. [Google Scholar] [CrossRef]
- Wray, R.A.L. Alunite formation within Silica Stalactites from the Sydney Region, South-eastern Australia. Int. J. Speleol. 2011, 40, 109–116. [Google Scholar] [CrossRef][Green Version]
- Nazari, B.; Jorjani, E.; Hani, H.; Manafi, Z.; Riahi, A. Formation of jarosite and its effect on important ions for Acidithiobacillus ferrooxidans bacteria. Trans. Nonferrous Met. Soc. China 2014, 24, 1152–1160. [Google Scholar] [CrossRef]
- Dockrey, J.W.; Lindsay, M.B.J.; Mayer, K.U.; Beckie, R.D.; Norlund, K.L.I.; Warren, L.A.; Southam, G. Acidic Microenvironments in waste rock characterized by neutral drainage: Bacteria–Mineral interactions at sulfide surfaces. Minerals 2014, 4, 170–190. [Google Scholar] [CrossRef]
- Fortin, D.; Davis, B.; Southam, G.; Beveridge, T.J. Biogeochemical phenomena induced by bacteria within sulfidic mine tailings. J. Ind. Microbiol. 1995, 14, 178–185. [Google Scholar] [CrossRef]
- Castro, L.; Blázquez, M.L.; González, F.; Muñoz, J.A.; Ballester, A. Anaerobic bioreduction of Jarosites and Biofilm formation by a natural microbial consortium. Minerals 2019, 9, 81. [Google Scholar] [CrossRef]
- Antelo, J.; Fiol, S.; Gondar, D.; Pérez, C.; López, R.; Arce, F. Cu(II) incorporation to schwertmannite: Effect on stability and reactivity under AMD conditions. Geochim. Cosmochim. Acta 2013, 119, 149–163. [Google Scholar] [CrossRef]
- Maillot, F.; Morin, G.; Juillot, F.; Bruneel, O.; Casiot, C.; Ona-Nguema, G.; Wang, Y.; Lebrun, S.; Aubry, E.; Vlaic, G.; et al. Structure and reactivity of As(III)- and As(V)-rich schwertmannites and amorphous ferric arsenate sulfate from the Carnoulès acid mine drainage, France: Comparison with biotic and abiotic model compounds and implications for As remediation. Geochim. Cosmochim. Acta 2013, 104, 310–329. [Google Scholar] [CrossRef]
- Morin, G.; Calas, G. Arsenic in soils, mine tailings, and former industrial sites. Elements 2006, 2, 97–101. [Google Scholar] [CrossRef]
- Peterson, R.C. the relationship between Cu content and distortion in the atomic structure of melanterite from the Richmond Mine, Iron Mountain, California. Can. Miner. 2003, 41, 937–949. [Google Scholar] [CrossRef]
- Martínez, C.E.; McBride, M.B. Cd, Cu, Pb, and Zn coprecipitates in Fe oxide formed at different pH: Aging effects on metal solubility and extractability by citrate. Environ. Toxicol. Chem. 2001, 20, 122–126. [Google Scholar] [CrossRef]
- Fitzpatrick, R.; Mosley, L.; Raven, M.; Shand, P. Schwertmannite formation and properties in acidic drain environments following exposure and oxidation of acid sulfate soils in irrigation areas during extreme drought. Geoderma 2017, 308, 235–251. [Google Scholar] [CrossRef]
- Paikaray, S.; Göttlicher, J.; Peiffer, S. Removal of As(III) from acidic waters using schwertmannite: Surface speciation and effect of synthesis pathway. Chem. Geol. 2011, 283, 134–142. [Google Scholar] [CrossRef]
- Paikaray, S.; Göttlicher, J.; Peiffer, S. As(III) retention kinetics, equilibrium and redox stability on biosynthesized schwertmannite and its fate and control on schwertmannite stability on acidic (pH 3.0) aqueous exposure. Chemosphere 2012, 86, 557–564. [Google Scholar] [CrossRef]
- Peak, D.; Sparks, D.L. Mechanisms of Selenate Adsorption on Iron Oxides and Hydroxides. Environ. Sci. Technol. 2002, 36, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- Wijnja, H.; Schulthess, C.P. Vibrational spectroscopy study of selenate and sulfate adsorption mechanisms on Fe and Al (Hydr)oxide surfaces. J. Colloid Interface Sci. 2000, 229, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Lǚ, B.; Wu, J.; Zhou, L.; Lan, Y. Reduction of Cr(VI) facilitated by biogenetic jarosite and analysis of its influencing factors with response surface methodology. Mater. Sci. Eng. C 2013, 33, 3723–3729. [Google Scholar] [CrossRef]
- Zhou, P.; Li, Y.; Shen, Y.; Lan, Y.; Zhou, L. Facilitating role of biogenetic schwertmannite in the reduction of Cr(VI) by sulfide and its mechanism. J. Hazard. Mater. 2012, 237–238, 194–198. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Dopson, M.; Wexler, M.; Sawers, R.G.; Stemmler, A.; Rosen, B.P.; Bond, P.L. Extreme arsenic resistance by the acidophilic archaeon ‘Ferroplasma acidarmanus’ Fer1. Extremophiles 2007, 11, 425–434. [Google Scholar] [CrossRef]
- Banerjee, P.C. Genetics of metal resistance in acidophilic prokaryotes of acidic mine environments. Indian J. Exp. Biol. 2004, 42, 9–25. [Google Scholar]
- Navarro, C.A.; Von Bernath, D.; Jerez, C.A. Heavy metal resistance strategies of acidophilic bacteria and their acquisition: Importance for biomining and bioremediation. Biol. Res. 2013, 46, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Edopson, M.; Ossandon, F.J.; Lã¶vgren, L.; Holmes, D. Metal resistance or tolerance? Acidophiles confront high metal loads via both abiotic and biotic mechanisms. Front. Microbiol. 2014, 5, 157. [Google Scholar] [CrossRef]
- Miot, J.; Etique, M. Iron oxides from nature to applications. In Iron Oxides from Nature to Applications; Faivre, D., Ed.; WILEY-VCH: Weinheim, Germany, 2016; pp. 53–98. ISBN 978-3-527-29669-9. [Google Scholar]
- Geesey, G.G.; Neal, A.L.; Suci, P.A.; Peyton, B.M. A review of spectroscopic methods for characterizing microbial transformations of minerals. J. Microbiol. Methods 2002, 51, 125–139. [Google Scholar] [CrossRef]
- Vithana, C.L.; Sullivan, L.A.; Burton, E.; Bush, R.T. Stability of schwertmannite and jarosite in an acidic landscape: Prolonged field incubation. Geoderma 2015, 239, 47–57. [Google Scholar] [CrossRef]
- Bao, Y.; Guo, C.; Lu, G.; Yi, X.; Wang, H.; Dang, Z. Role of microbial activity in Fe(III) hydroxysulfate mineral transformations in an acid mine drainage-impacted site from the Dabaoshan Mine. Sci. Total Environ. 2018, 616–617, 647–657. [Google Scholar] [CrossRef]
- ThomasArrigo, L.K.; Bouchet, S.; Kaegi, R.; Kretzschmar, R. Organic matter influences transformation products of ferrihydrite exposed to sulfide. Environ. Sci. Nano 2020, 7, 3405–3418. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, H.; Guo, C.; Wan, J.; Fan, C.; Reinfelder, J.R.; Lu, G.; Wu, F.; Huang, W.; Dang, Z. Schwertmannite transformation via direct or indirect electron transfer by a sulfate reducing enrichment culture. Environ. Pollut. 2018, 242, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Neal, A.L.; Bank, T.L.; Hochella, M.F.; Rosso, K.M. Cell adhesion of Shewanella oneidensisto iron oxide minerals: Effect of different single crystal faces. Geochem. Trans. 2005, 6, 77. [Google Scholar] [CrossRef]
- Cutting, R.; Coker, V.; Fellowes, J.; Lloyd, J.; Vaughan, D. Mineralogical and morphological constraints on the reduction of Fe(III) minerals by Geobacter sulfurreducens. Geochim. Cosmochim. Acta 2009, 73, 4004–4022. [Google Scholar] [CrossRef]
- Bryce, C.; Blackwell, N.; Schmidt, C.; Otte, J.M.; Huang, Y.-M.; Kleindienst, S.; Tomaszewski, E.; Schad, M.; Warter, V.; Peng, C.; et al. Microbial anaerobic Fe(II) oxidation—Ecology, mechanisms and environmental implications. Environ. Microbiol. 2018, 20, 3462–3483. [Google Scholar] [CrossRef] [PubMed]
- Widdel, F.; Schnell, S.; Heising, S.; Ehrenreich, A.; Assmus, B.; Schink, B. Ferrous iron oxidation by anoxygenic phototrophic bacteria. Nature 1993, 362, 834–836. [Google Scholar] [CrossRef]
- Straub, K.L.; Benz, M.; Schink, B.; Widdel, F. Anaerobic, nitrate-dependent microbial oxidation of ferrous iron. Appl. Environ. Microbiol. 1996, 62, 1458–1460. [Google Scholar] [CrossRef]
- Camacho, A.; Walter, X.A.; Picazo, A.; Zopfi, J. Photoferrotrophy: Remains of an ancient photosynthesis in modern environments. Front. Microbiol. 2017, 8, 323. [Google Scholar] [CrossRef]
- Posth, N.R.; Huelin, S.; Konhauser, K.O.; Kappler, A. Size, density and composition of cell–mineral aggregates formed during anoxygenic phototrophic Fe(II) oxidation: Impact on modern and ancient environments. Geochim. Cosmochim. Acta 2010, 74, 3476–3493. [Google Scholar] [CrossRef]
- Oshiki, M.; Ishii, S.; Yoshida, K.; Fujii, N.; Ishiguro, M.; Satoh, H.; Okabe, S. Nitrate-dependent ferrous iron oxidation By Anaerobic Ammonium Oxidation (Anammox) bacteria. Appl. Environ. Microbiol. 2013, 79, 4087–4093. [Google Scholar] [CrossRef]
- Mejia, J.; Roden, E.E.; Ginder-Vogel, M. Influence of oxygen and nitrate on Fe (Hydr)oxide mineral transformation and soil microbial communities during Redox Cycling. Environ. Sci. Technol. 2016, 50, 3580–3588. [Google Scholar] [CrossRef] [PubMed]
- Miot, J.; Remusat, L.; Duprat, E.; Gonzalez, A.; Pont, S.; Poinsot, M. Fe biomineralization mirrors individual metabolic activity in a nitrate-dependent Fe(II)-oxidizer. Front. Microbiol. 2015, 6, 879. [Google Scholar] [CrossRef]
- Churchman, G.J.; Lowe, D.J. Alteration, formation, and occurrence of minerals in soils. In Handbook of Soil Sciences, 2nd ed.; Huang, P.M., Summer, M.E., Eds.; Taylor & Francis: Boca Ratón, FL, USA, 2012; Volume 1, pp. 1–72. [Google Scholar]
- Claridge, G.; Campbell, I. Mineral transformation during the weathering of dolerite under cold arid conditions in Antarctica. N. Z. J. Geol. Geophys. 1984, 27, 537–545. [Google Scholar] [CrossRef]
- Frierdich, A.J.; Catalano, J. Controls on Fe(II)-activated trace element release from goethite and hematite. Environ. Sci. Technol. 2012, 46, 1519–1526. [Google Scholar] [CrossRef]
- Caporale, A.G.; Violante, A. Chemical processes affecting the mobility of heavy metals and metalloids in soil environments. Curr. Pollut. Rep. 2016, 2, 15–27. [Google Scholar] [CrossRef]
- Lovley, D.R.; Holmes, D.E.; Nevin, K.P. Dissimilatory Fe(III) and Mn(IV) reduction. Adv. Microb. Physiol. 2004, 49, 219–286. [Google Scholar] [CrossRef]
- Wielinga, B.; Lucy, J.K.; Moore, J.N.; Seastone, O.F.; Gannon, J.E. Microbiological and geochemical characterization of fluvially deposited sulfidic mine tailings. Appl. Environ. Microbiol. 1999, 65, 1548–1555. [Google Scholar] [CrossRef]
- Bingjie, O.; Xiancai, L.; Huan, L.; Juan, L.; Tingting, Z.; Xiangyu, Z.; Jianjun, L.; Rucheng, W. Reduction of jarosite by Shewanella oneidensis MR-1 and secondary mineralization. Geochim. Cosmochim. Acta 2014, 124, 54–71. [Google Scholar] [CrossRef]
- Francis, A.J.; Dodge, C.J. Anaerobic microbial remobilization of toxic metals coprecipitated with iron oxide. Environ. Sci. Technol. 1990, 24, 373–378. [Google Scholar] [CrossRef]
- Paikaray, S.; Peiffer, S. Abiotic schwertmannite transformation kinetics and the role of sorbed As(III). Appl. Geochem. 2012, 27, 590–597. [Google Scholar] [CrossRef]
- Paikaray, S.; Schröder, C.; Peiffer, S. Schwertmannite stability in anoxic Fe(II)-rich aqueous solution. Geochim. Cosmochim. Acta 2017, 217, 292–305. [Google Scholar] [CrossRef]
- Burton, E.D.; Johnston, S.; Watling, K.; Bush, R.T.; Keene, A.F.; Sullivan, L.A. Arsenic effects and behavior in association with the Fe(II)-catalyzed transformation of Schwertmannite. Environ. Sci. Technol. 2010, 44, 2016–2021. [Google Scholar] [CrossRef]
- Fan, C.; Guo, C.; Zeng, Y.; Tu, Z.; Ji, Y.; Reinfelder, J.; Chen, M.; Huang, W.; Lu, G.; Yi, X.; et al. The behavior of chromium and arsenic associated with redox transformation of schwertmannite in AMD environment. Chemosphere 2019, 222, 945–953. [Google Scholar] [CrossRef]
- Karn, S.K.; Pan, X.; Jenkinson, I. Bio-transformation and stabilization of arsenic (As) in contaminated soil using arsenic oxidizing bacteria and FeCl3 amendment. 3 Biotech 2017, 7, 50. [Google Scholar] [CrossRef]
- Johnston, S.G.; Burton, E.; Keene, A.F.; Planer-Friedrich, B.; Voegelin, A.; Blackford, M.G.; Lumpkin, G.R. Arsenic mobilization and iron transformations during sulfidization of As(V)-bearing jarosite. Chem. Geol. 2012, 334, 9–24. [Google Scholar] [CrossRef]
- Drewniak, L.; Skłodowska, A. Arsenic-transforming microbes and their role in biomining processes. Environ. Sci. Pollut. Res. 2013, 20, 7728–7739. [Google Scholar] [CrossRef]
- Courchesne, B.; Schindler, M.; Mykytczuk, N.C.S. Relationships between the microbial composition and the geochemistry and mineralogy of the cobalt-bearing legacy mine tailings in Northeastern Ontario. Front. Microbiol. 2021, 12, 660190. [Google Scholar] [CrossRef]
- Huang, L.-N.; Zhou, W.-H.; Hallberg, K.B.; Wan, C.-Y.; Li, J.; Shu, W.-S. Spatial and temporal analysis of the microbial community in the tailings of a Pb-Zn Mine generating acidic drainage. Appl. Environ. Microbiol. 2011, 77, 5540–5544. [Google Scholar] [CrossRef]
- Londry, K.L.; Sherriff, B.L. Comparison of microbial biomass, biodiversity, and biogeochemistry in three contrasting Gold Mine tailings deposits. Geomicrobiol. J. 2005, 22, 237–247. [Google Scholar] [CrossRef]
- Rosario, K.; Iverson, S.L.; Henderson, D.A.; Chartrand, S.; McKeon, C.; Glenn, E.P.; Maier, R.M. Bacterial community changes during plant establishment at the San Pedro River Mine tailings site. J. Environ. Qual. 2007, 36, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Schippers, P.-G.J.A. Microbiological pyrite oxidation in a mine tailings heap and its relevance to the death of vegetation. Geomicrobiol. J. 2000, 17, 151–162. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Ayangbenro, A.S.; Olanrewaju, O.; Babalola, O.O.; Ayangbenro, A. Sulfate-reducing bacteria as an effective tool for sustainable acid mine bioremediation. Front. Microbiol. 2018, 9, 1986. [Google Scholar] [CrossRef]
- Malki, M.; González-Toril, E.; Sanz, J.; Gómez, F.; Rodríguez, N.; Amils, R. Importance of the iron cycle in biohydrometallurgy. Hydrometallurgy 2006, 83, 223–228. [Google Scholar] [CrossRef]
- Martinez, P.; Vera, M.; Bobadilla-Fazzini, R. Omics on bioleaching: Current and future impacts. Appl. Microbiol. Biotechnol. 2015, 99, 8337–8350. [Google Scholar] [CrossRef]
- Violante, A. Elucidating mechanisms of competitive sorption at the mineral/water interface. In Advances in Agronomy; Sparks, D.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 118, pp. 111–176. ISBN 9780124059429. [Google Scholar]
- Lu, Y.; Liu, H.; Huang, X.; Xu, L.; Zhou, J.; Qian, G.; Shen, J.; Chen, X. Nitrate removal during Fe(III) bio-reduction in microbial-mediated iron redox cycling systems. Water Sci. Technol. 2021, 84, 985–994. [Google Scholar] [CrossRef]
- Liu, T.; Chen, D.; Li, X.; Li, F. Microbially mediated coupling of nitrate reduction and Fe(II) oxidation under anoxic conditions. FEMS Microbiol. Ecol. 2019, 95, fiz030. [Google Scholar] [CrossRef]
- Lybrand, R.A.; Austin, J.C.; Fedenko, J.; Gallery, R.E.; Rooney, E.; Schroeder, P.A.; Zaharescu, D.G.; Qafoku, O. A coupled microscopy approach to assess the nano-landscape of weathering. Sci. Rep. 2019, 9, 5377. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Kim, M.-G.; Kim, I.Y.; Hwang, S.-J.; Hur, H.-G. Biological synthesis of free-standing uniformed goethite nanowires by Shewanella sp. HN-41. J. Mater. Chem. A 2012, 1, 1646–1650. [Google Scholar] [CrossRef]
- Logan, B.E.; Rossi, R.; Ragab, A.I.; Saikaly, P.E. Electroactive microorganisms in bioelectrochemical systems. Nat. Rev. Genet. 2019, 17, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Phyo, A.K.; Jia, Y.; Tan, Q.; Sun, H.; Liu, Y.; Dong, B.; Ruan, R. Competitive growth of sulfate-reducing bacteria with bioleaching Acidophiles for bioremediation of heap bioleaching residue. Int. J. Environ. Res. Public Health 2020, 17, 2715. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Castillo, J.E.; Mirazimi, M.; Mohammadi, M.; Dy, E.; Liu, W. The Role of Microorganisms in the Formation, Dissolution, and Transformation of Secondary Minerals in Mine Rock and Drainage: A Review. Minerals 2021, 11, 1349. https://doi.org/10.3390/min11121349
Ortiz-Castillo JE, Mirazimi M, Mohammadi M, Dy E, Liu W. The Role of Microorganisms in the Formation, Dissolution, and Transformation of Secondary Minerals in Mine Rock and Drainage: A Review. Minerals. 2021; 11(12):1349. https://doi.org/10.3390/min11121349
Chicago/Turabian StyleOrtiz-Castillo, Jose Eric, Mohamad Mirazimi, Maryam Mohammadi, Eben Dy, and Wenying Liu. 2021. "The Role of Microorganisms in the Formation, Dissolution, and Transformation of Secondary Minerals in Mine Rock and Drainage: A Review" Minerals 11, no. 12: 1349. https://doi.org/10.3390/min11121349
APA StyleOrtiz-Castillo, J. E., Mirazimi, M., Mohammadi, M., Dy, E., & Liu, W. (2021). The Role of Microorganisms in the Formation, Dissolution, and Transformation of Secondary Minerals in Mine Rock and Drainage: A Review. Minerals, 11(12), 1349. https://doi.org/10.3390/min11121349




