Self-Consistent Thermodynamic Parameters of Diopside at High Temperatures and High Pressures: Implications for the Adiabatic Geotherm of an Eclogitic Upper Mantle
Abstract
1. Introduction
2. Methods
2.1. Theory
2.2. Thermoelastic Data
2.2.1. Elastic Wave Velocity under High-Temperature and High-Pressure Conditions
2.2.2. Unit-Cell Volume under High-Temperature and Ambient Pressure Conditions
2.2.3. Heat Capacity under High-Temperature and Ambient Pressure Conditions
3. Results
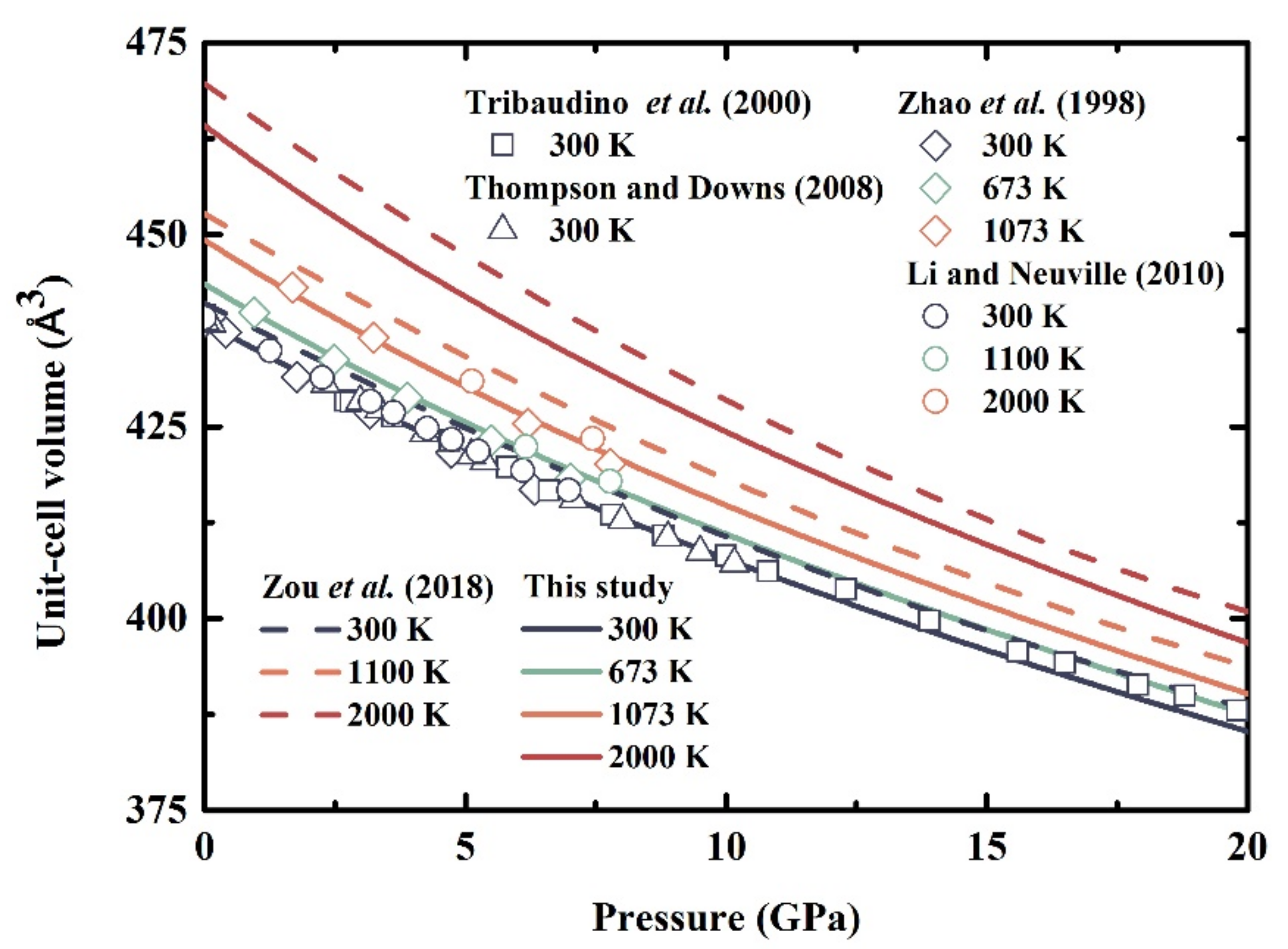
3.1. Unit-Cell Volumes at High Temperatures and High Pressures
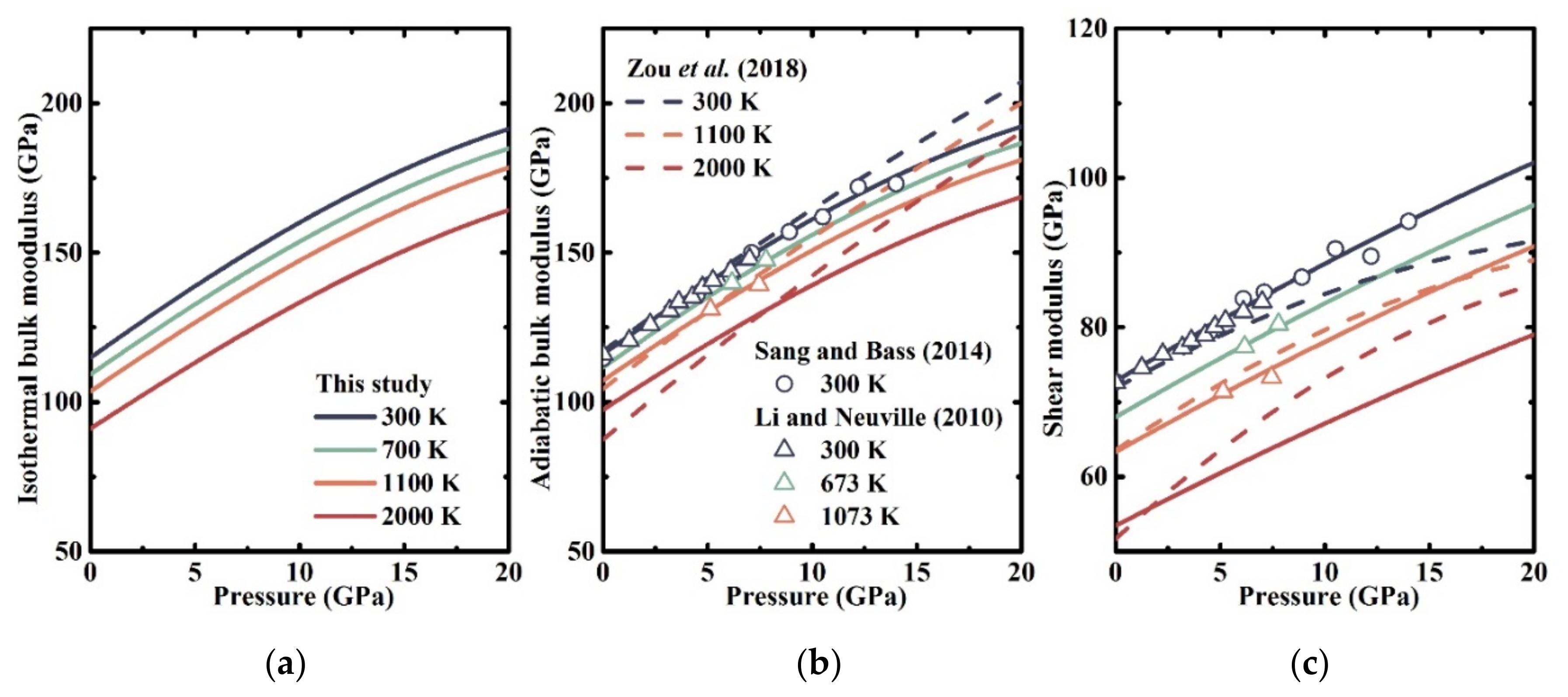
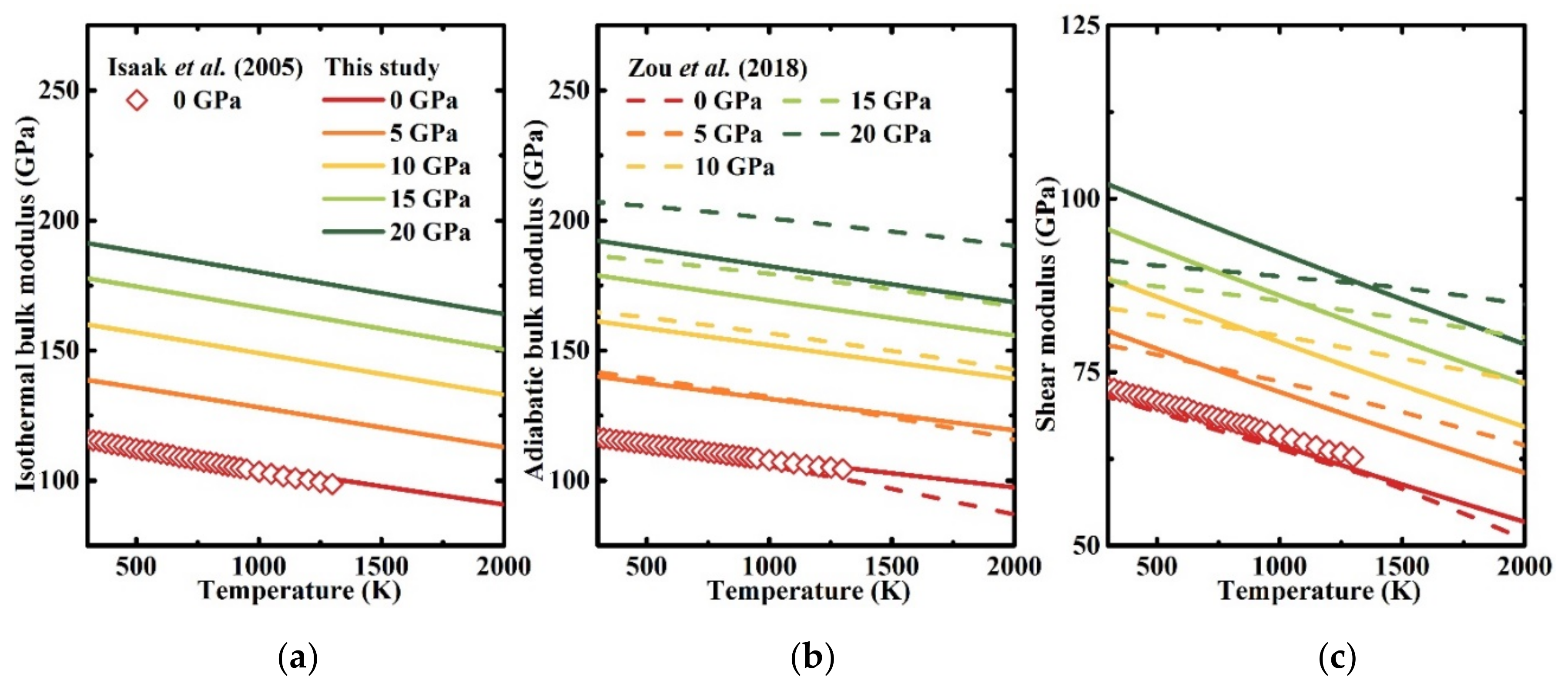
3.2. Elastic Properties at High Temperatures and High Pressures
| KT0 GPa | (∂KT/∂P)T | (∂2KT/∂P2)T /GPa | (∂KT/∂T)P 10–2 GPa/K | References |
| 114 (4) | 4.5 (1.8) | Levien and Prewitt (1981) [44] | ||
| 109.1 | 4.84 | −2.05 | Zhao et al. (1998) [25] | |
| 105.1 (9) | 6.8 (1) | – | Tribaudino et al. (2000) [43] | |
| 118 (1) | 3.8 (2) | Thompson and Downs (2008) [42] | ||
| 122 | 4.7 | Walker et al. (2008) [46] | ||
| 116.4 | 4.62 | −1.72 | Zou et al. (2018) [24] | |
| 114.8 | 5.17 | –0.066 | −1.42 | This study |
| KS0 GPa | (∂KS/∂P)T | (∂2KS/∂P2)T /GPa | (∂KS/∂T)P 10–2 GPa/K | References |
| 116.4 (7) | 4.9 (1) | −1.2 (1) | Li and Neuville (2010) [23] | |
| 114.6 (7) | Sang et al. (2011) [45] | |||
| 114.6 (7) | 4.8 (2) | Sang and Bass (2014) [37] | ||
| 117.5 | 5.0 | −0.026 | −1.5 | Zou et al. (2018) |
| 116.0 | 5.18 | −0.067 | −1.10 | This study |
| G0 GPa | (∂G/∂P)T | (∂2G/∂P2)T /GPa | (∂G/∂T)P 10–2 GPa/K | References |
| 73.0 (4) | 1.6 (1) | −1.1 (1) | Li and Neuville (2010) [23] | |
| 72.7 (4) | Sang et al. (2011) [45] | |||
| 72.7 (4) | 1.7 (1) | Sang and Bass (2014) [37] | ||
| 71.8 | 1.56 | −0.0302 | −0.871 | Zou et al. (2018) [24] |
| 72.8 | 1.67 | −0.0106 | −1.14 | This study |
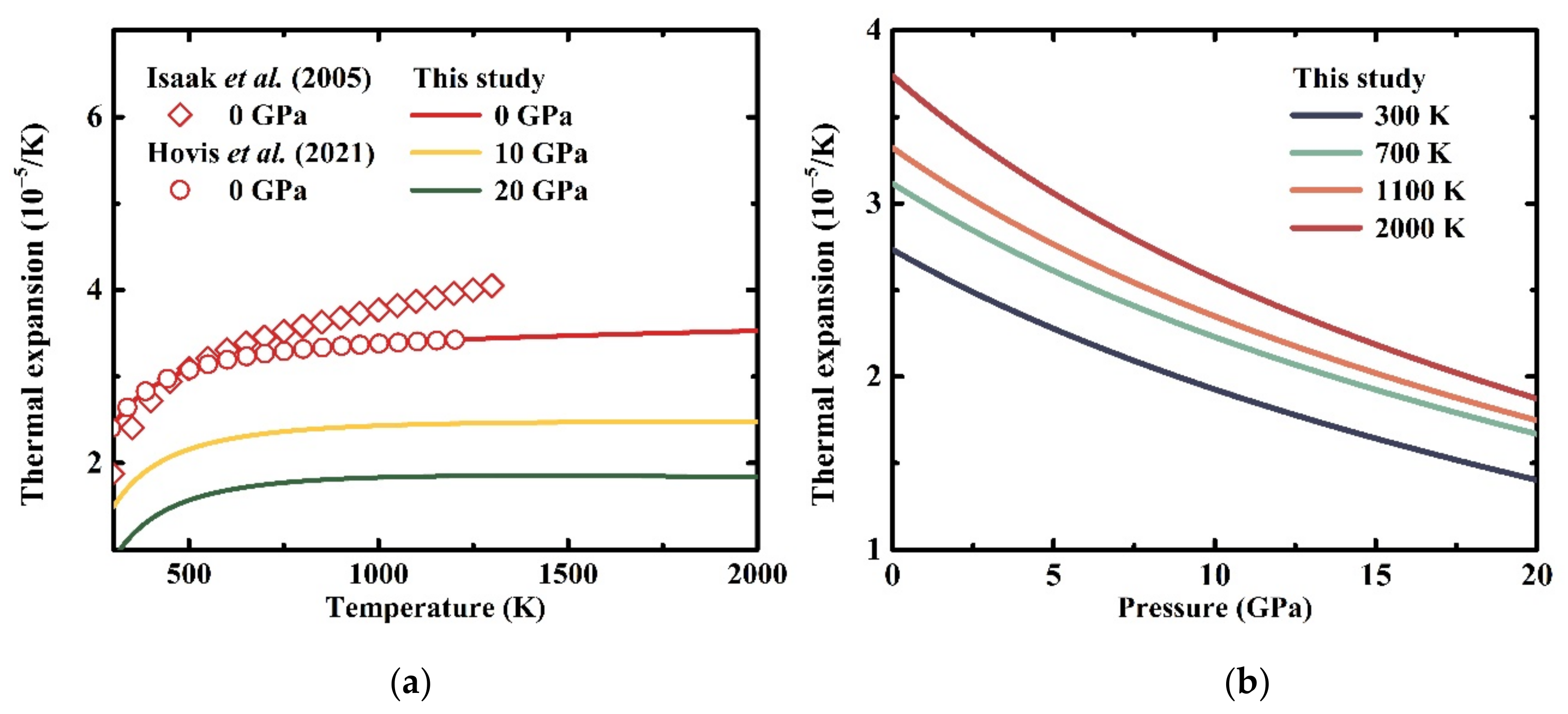
3.3. Thermodynamic Properties at High Temperatures and High Pressures
4. Discussion
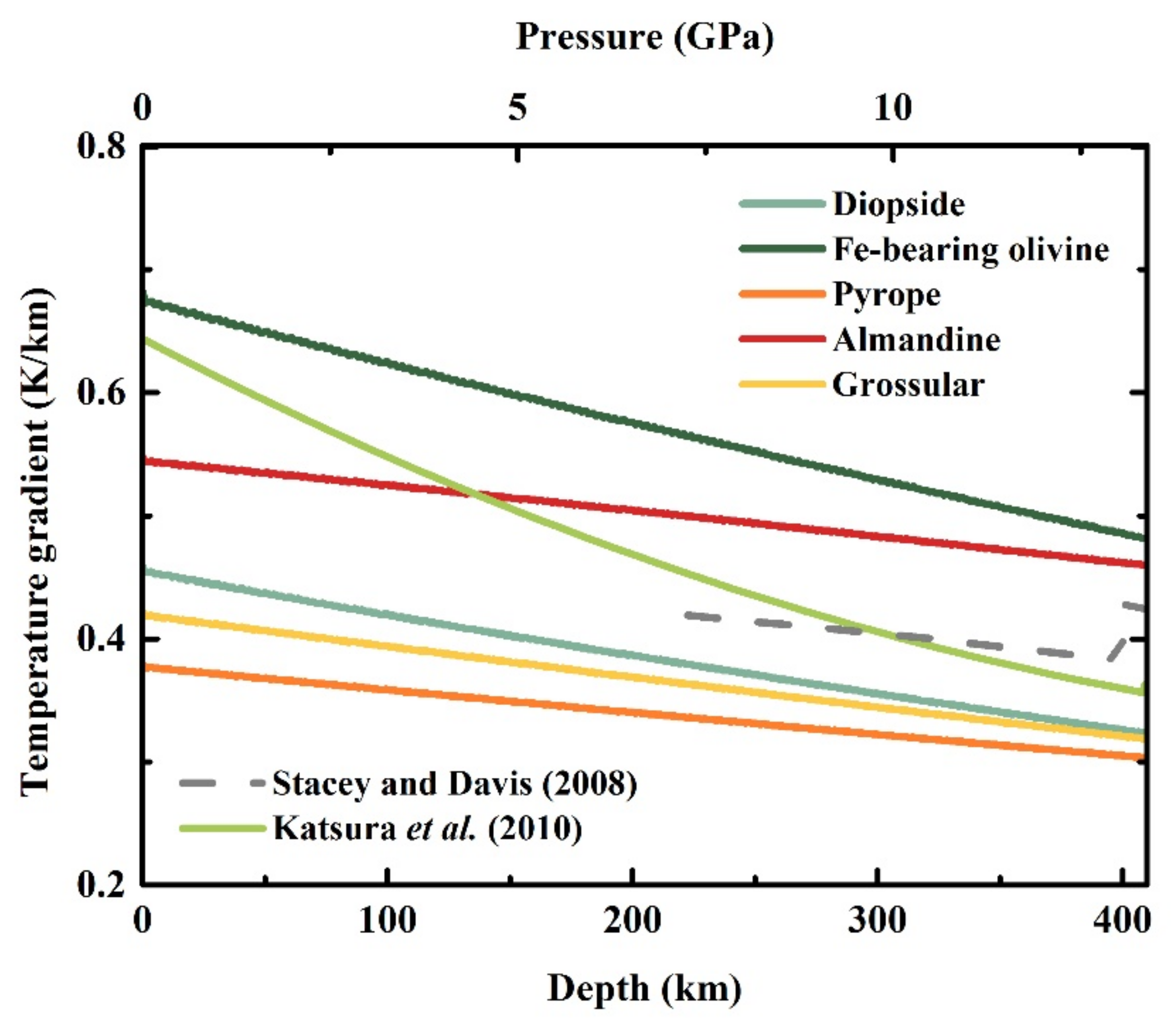
4.1. Effect of Mineral Composition on the Adiabatic Temperature Gradient
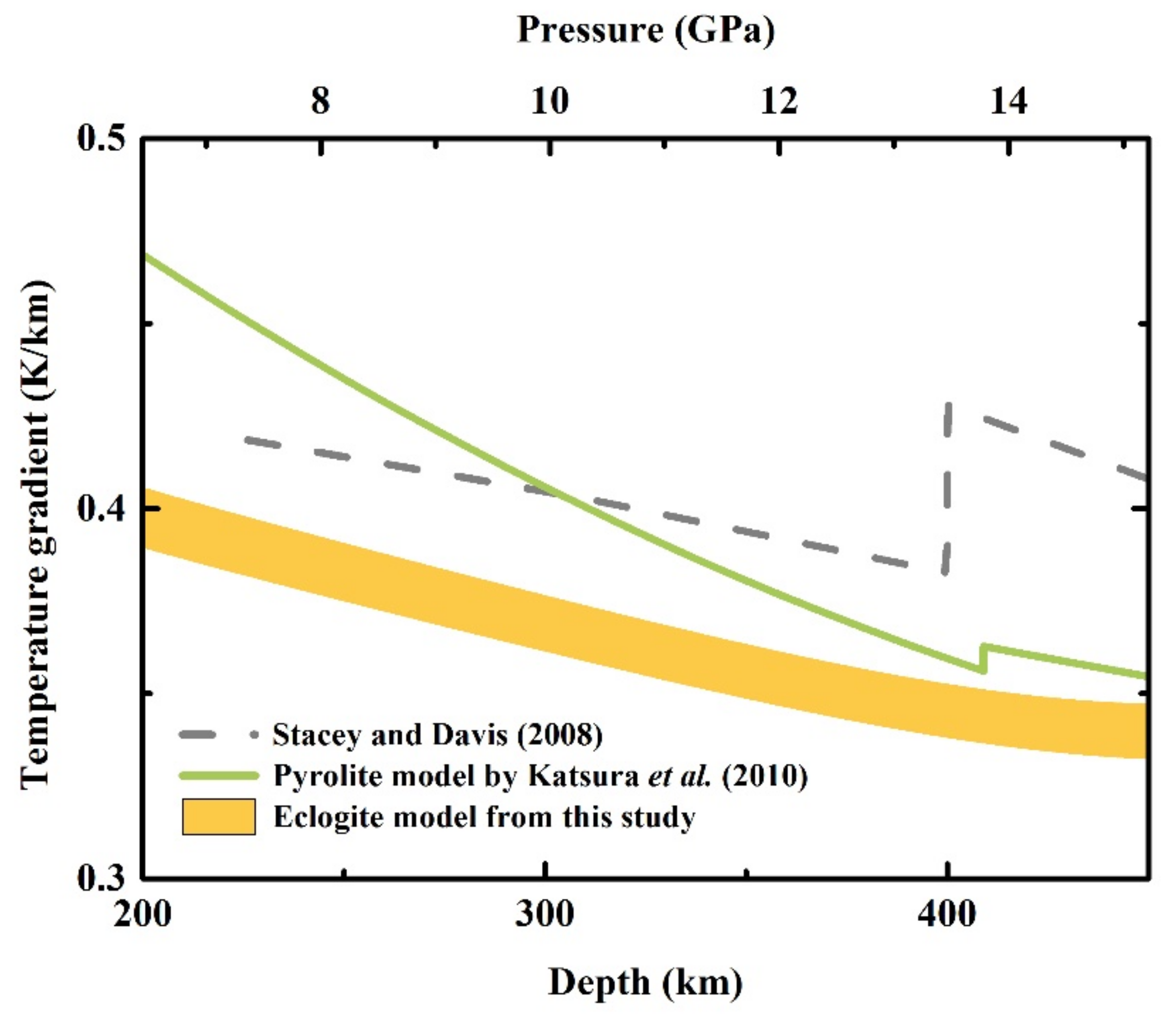
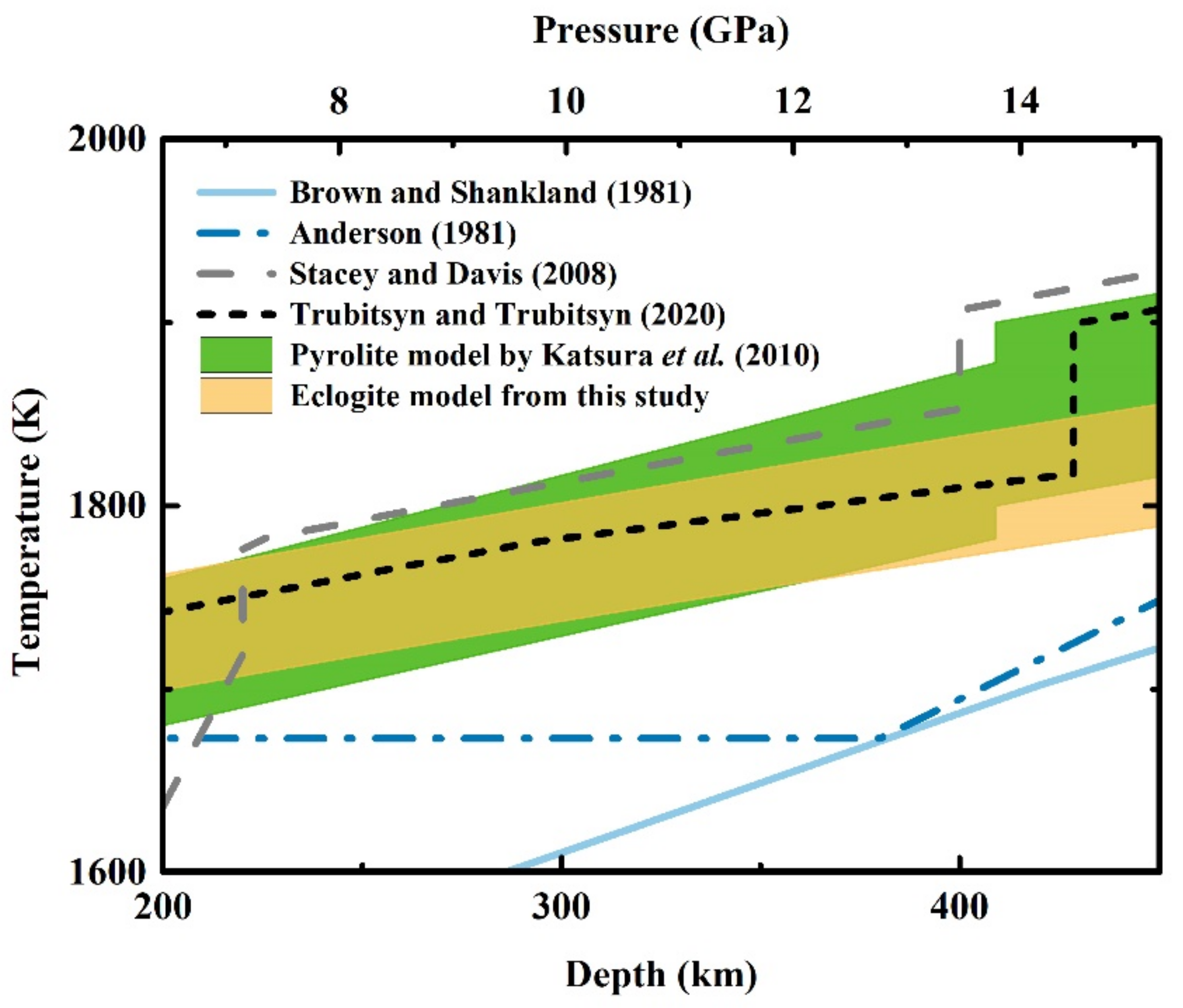
4.2. Adiabatic Geotherm of the Eclogite Model
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weidner, D.J.; Wang, Y. Phase transformations: Implications for mantle structure. In Earth’s Deep Interior: Mineral. Physics and Tomography From the Atomic to the Global Scale; American Geophysical Union: Washington, DC, USA, 2000; pp. 215–235. [Google Scholar]
- Akaogi, M.; Ito, E.; Navrotsky, A. Olivine-modified spinel-spinel transitions in the system Mg2SiO4-Fe2SiO4: Calorimetric measurements, thermochemical calculation, and geophysical application. J. Geophys. Res. Solid Earth 1989, 94, 15671–15685. [Google Scholar] [CrossRef]
- Ono, S. Experimental constraints on the temperature profile in the lower mantle. Phys. Earth Planet. Inter. 2008, 170, 267–273. [Google Scholar] [CrossRef]
- Stacey, F.; Davis, P. Physics of the Earth; Cambridge Univ. Press: Cambridge, UK, 2008. [Google Scholar]
- Turcotte, D.L.; Schubert, G. Geodynamics, 2nd ed.; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Dziewonski, A.M.; Hales, A.L.; Lapwood, E.R. Parametrically simple earth models consistent with geophysical data. Phys. Earth Planet. Inter. 1975, 10, 12–48. [Google Scholar] [CrossRef]
- Stacey, F.D. A thermal model of the earth. Phys. Earth Planet. Inter. 1977, 15, 341–348. [Google Scholar] [CrossRef]
- Anderson, O.L. Temperature profiles in the Earth. Evol. Earth 1981, 5, 19–27. [Google Scholar]
- Brown, J.M.; Shankland, T.J. Thermodynamic parameters in the Earth as determined from seismic profiles. Geophys. J. Int. 1981, 66, 579–596. [Google Scholar] [CrossRef]
- Jeanloz, R.; Richter, F.M. Convection, composition, and the thermal state of the lower mantle. J. Geophys. Res. 1979, 84, 5497–5504. [Google Scholar] [CrossRef]
- Ringwood, A.E. A model for the upper mantle. J. Geophys. Res. 1962, 67, 4473–4478. [Google Scholar] [CrossRef]
- Bass, J.D.; Anderson, D.L. Composition of the upper mantle: Geophysical tests of two petrological models. Geophys. Res. Lett. 1984, 11, 229–232. [Google Scholar] [CrossRef]
- Katsura, T. Thermal expansion of Mg2SiO4 ringwoodite at high pressures. J. Geophys. Res. 2004, 109, 209–218. [Google Scholar] [CrossRef]
- Katsura, T.; Shatskiy, A.; Manthilake, M.A.G.M.; Zhai, S.; Fukui, H.; Yamazaki, D.; Matsuzaki, T.; Yoneda, A.; Ito, E.; Kuwata, A.; et al. Thermal expansion of forsterite at high pressures determined by in situ X-ray diffraction: The adiabatic geotherm in the upper mantle. Phys. Earth Planet. Inter. 2009, 174, 86–92. [Google Scholar] [CrossRef]
- Katsura, T.; Shatskiy, A.; Manthilake, M.A.G.M.; Zhai, S.; Yamazaki, D.; Matsuzaki, T.; Yoshino, T.; Yoneda, A.; Ito, E.; Sugita, M.; et al. P-V-T relations of wadsleyite determined by in situ X-ray diffraction in a large-volume high-pressure apparatus. Geophys. Res. Lett. 2009, 36, 1–5. [Google Scholar]
- Katsura, T.; Yamada, H.; Nishikawa, O.; Song, M.; Kubo, A.; Shinmei, T.; Yokoshi, S.; Aizawa, Y.; Yoshino, T.; Walter, M.J.; et al. Olivine-wadsleyite transition in the system (Mg,Fe)2SiO4. J. Geophys. Res. Solid Earth 2004, 109, 1–12. [Google Scholar] [CrossRef]
- Katsura, T.; Yokoshi, S.; Kawabe, K.; Shatskiy, A.; Manthilake, M.A.G.M.; Zhai, S.; Fukui, H.; Hegoda, H.A.C.I.; Yoshino, T.; Yamazaki, D.; et al. P-V-T relations of MgSiO3 perovskite determined by in situ X-ray diffraction using a large-volume high-pressure apparatus. Geophys. Res. Lett. 2009, 36, 1–6. [Google Scholar]
- Katsura, T.; Yoneda, A.; Yamazaki, D.; Yoshino, T.; Ito, E. Adiabatic temperature profile in the mantle. Phys. Earth Planet. Inter. 2010, 183, 212–218. [Google Scholar] [CrossRef]
- Su, C.; Liu, Y. Thermal expansion, heat capacity and Grüneisen parameter of grossular at high temperature and high pressure. High. Temp.-High. Press. 2021, 50, 105–119. [Google Scholar]
- Su, C.; Liu, Y.; Fan, D.; Song, W.; Yang, G. Self-consistent thermodynamic parameters of pyrope and almandine at high-temperature and high-pressure conditions: Implication on the adiabatic temperature gradient. Phys. Earth Planet. Inter. 2021, 106789. [Google Scholar] [CrossRef]
- Su, C.; Liu, Y.; Song, W.; Fan, D.; Wang, Z.; Tang, H. Thermodynamic properties of San Carlos olivine at high temperature and high pressure. Acta Geochim. 2018, 37, 171–179. [Google Scholar] [CrossRef]
- Ringwood, A.E. Phase transformations and the constitution of the mantle. Phys. Earth Planet. Inter. 1970, 3, 109–155. [Google Scholar] [CrossRef]
- Li, B.; Neuville, D.R. Elasticity of diopside to 8 GPa and 1073 K and implications for the upper mantle. Phys. Earth Planet. Inter. 2010, 183, 398–403. [Google Scholar] [CrossRef]
- Zou, F.; Wu, Z.; Wang, W.; Wentzcovitch, R.M. An extended semianalytical approach for thermoelasticity of monoclinic crystals: Application to diopside. J. Geophys. Res. Solid Earth 2018, 123, 7629–7643. [Google Scholar] [CrossRef]
- Zhao, Y.; Dreele, R.B.V.; Zhang, J.Z.; Weidner, D.J. Thermoelastic Equation of State of Monoclinic Pyroxene: CaMgSi2O6 Diopside. Rev. High. Press. Sci. Technol. 1998, 7, 25–27. [Google Scholar] [CrossRef]
- Birch, F. Finite Elastic Strain of Cubic Crystals. Phys. Rev. 1947, 71, 809–824. [Google Scholar] [CrossRef]
- Jackson, I.; Rigden, S.M. Analysis of P-V-T data: Constraints on the thermoelastic properties of high-pressure minerals. Phys. Earth Planet. Inter. 1996, 96, 85–112. [Google Scholar] [CrossRef]
- Pandolfo, F.; Cámara, F.; Domeneghetti, M.C.; Alvaro, M.; Nestola, F.; Karato, S.-I.; Amulele, G. Volume thermal expansion along the jadeite–diopside join. Phys. Chem. Miner. 2015, 42, 1–14. [Google Scholar] [CrossRef]
- Hovis, G.L.; Tribaudino, M.; Leaman, A.; Almer, C.; Altomare, C.; Morris, M.; Maksymiw, N.; Morris, D.; Jackson, K.; Scott, B.; et al. Thermal expansion of minerals in the pyroxene system and examination of various thermal expansion models. Am. Mineral. 2021, 106, 883–899. [Google Scholar] [CrossRef]
- Krupka, K.M.; Hemingway, B.S.; Robie, R.A.; Kerrick, D.M. High-temperature heat capacities and derived thermodynamic properties of anthophyllite, diopside, dolomite, enstatite, bronzite, talc, tremolite and wollastonite. Am. Mineral. 1985, 70, 261–271. [Google Scholar]
- Richet, P.; Fiquet, G. High-temperature heat capacity and premelting of minerals in the system MgO-CaO-Al2O3-SiO2. J. Geophys. Res. 1991, 96, 445–456. [Google Scholar] [CrossRef]
- Stebbins, J.F.; Carmichael, I.S.E.; Weill, D.E. The high temperature liquid and glass heat contents and the heats of fusion of diopside, albite, sanidine and nepheline. Am. Mineral. 1983, 68, 717–730. [Google Scholar]
- Isaak, D.G.; Ohno, I.; Lee, P.C. The elastic constants of monoclinic single-crystal chrome-diopside to 1300 K. Phys. Chem. Miner. 2006, 32, 691–699. [Google Scholar] [CrossRef]
- De Koker, N.; Stixrude, L. Self-consistent thermodynamic description of silicate liquids, with application to shock melting of MgO periclase and MgSiO3 perovskite. Geophys. J. Int. 2009, 178, 162–179. [Google Scholar] [CrossRef]
- Sokolova, T.S.; Dorogokupets, P.I. Equations of State of Ca-Silicates and Phase Diagram of the CaSiO3 System under Upper Mantle Conditions. Minerals 2021, 11, 322. [Google Scholar] [CrossRef]
- Angel, R.J. Equations of State. Rev. Mineral. Geochem. 2000, 41, 35–59. [Google Scholar] [CrossRef]
- Sang, L.; Bass, J.D. Single-crystal elasticity of diopside to 14 GPa by Brillouin scattering. Phys. Earth Planet. Inter. 2014, 228, 75–79. [Google Scholar] [CrossRef]
- Cameron, M.; Sueno, S.; Prewitt, C.T.; Papike, J.J. High-Temperature Crystal Chemistry of Acmite, Diopside, Hedenbergite Jadeite, Spodumene and Ureyite. Am. Mineral. 1973, 58, 594–618. [Google Scholar]
- Finger, L.W.; Ohashi, Y. The thermal expansion of diopside to 800 °C and a refinement of the crystal structure at 700 °C. Am. Mineral. 1976, 61, 303–310. [Google Scholar]
- Gonzalez-Platas, J.; Alvaro, M.; Nestola, F.; Angel, R. EosFit7-GUI: A new graphical user interface for equation of state calculations, analyses and teaching. J. Appl. Crystallogr. 2016, 49, 1377–1382. [Google Scholar] [CrossRef]
- Fei, Y. Thermal Expansion. In Mineral. Physics & Crystallography; Ahrens, T.J., Ed.; American Geophysical Union: Washington, DC, USA, 1995; pp. 29–44. [Google Scholar]
- Thompson, R.M.; Downs, R.T. The crystal structure of diopside at pressure to 10 GPa. Am. Mineral. 2008, 93, 177–186. [Google Scholar] [CrossRef]
- Tribaudino, M.; Prencipe, M.; Bruno, M.; Levy, D. High-pressure behaviour of Ca-rich C2/c clinopyroxenes along the join diopside-enstatite (CaMgSi2O6-Mg2Si2O6). Phys. Chem. Miner. 2000, 27, 656–664. [Google Scholar] [CrossRef]
- Levien, L.; Prewitt, C.T. High-pressure structural study of diopside. Am. Mineral. 1981, 66, 315–323. [Google Scholar]
- Sang, L.; Vanpeteghem, C.B.; Sinogeikin, S.V.; Bass, J.D. The elastic properties of diopside, CaMgSi2O6. Am. Mineral. 2011, 96, 224–227. [Google Scholar] [CrossRef]
- Walker, A.M.; Tyer, R.P.; Bruin, R.P.; Dove, M.T. The compressibility and high pressure structure of diopside from first principles simulation. Phys. Chem. Miner. 2008, 35, 359–366. [Google Scholar] [CrossRef][Green Version]
- Saxena, S.K.; Chatterjee, N.; Fei, Y.; Shen, G. Thermodynamic Data on Oxides and Silicates. An Assessed Data Set Based on Thermochemistry and High Pressure Phase Equilibrium, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- Vočadlo, N.L.; Price, G.D. The Grüneisen parameter-computer calculations via lattice dynamics. Phys. Earth Planet. Inter. 1994, 82, 261–270. [Google Scholar] [CrossRef]
- Wu, Z.; Wentzcovitch, R.M. Vibrational and thermodynamic properties of wadsleyite: A density functional study. J. Geophys. Res. 2007, 112. [Google Scholar] [CrossRef]
- McKenzie, D.; Bickle, M.J. The volume and composition of melt generated by extension of the lithosphere. J. Petrol. 1988, 29, 625–679. [Google Scholar] [CrossRef]
- Ringwood, A.E. Phase transformations and their bearing on the constitution and dynamics of the mantle. Geochim. Et Cosmochim. Acta 1991, 55, 2083–2110. [Google Scholar] [CrossRef]
- Ringwood, A.E. Phase transformations in the mantle. Earth Planet. Sci. Lett. 1968, 5, 401–412. [Google Scholar] [CrossRef]
- Muir, J.M.R.; Zhang, F.; Brodholt, J.P. The effect of water on the post-spinel transition and evidence for extreme water contents at the bottom of the transition zone. Earth Planet. Sci. Lett. 2021, 565, 116909. [Google Scholar] [CrossRef]
- Ringwood, A.E. Phase Transformations and Differentiation in Subducted Lithosphere: Implications for Mantle Dynamics, Basalt Petrogenesis, and Crustal Evolution. J. Geol. 1982, 90, 611–643. [Google Scholar] [CrossRef]
- Ringwood, A.E.; Irifune, T. Nature of the 650–km seismic discontinuity: Implications for mantle dynamics and differentiation. Nature 1988, 331, 131–136. [Google Scholar] [CrossRef]
- Ishii, T.; Kojitani, H.; Akaogi, M. Phase Relations of Harzburgite and MORB up to the Uppermost Lower Mantle Conditions: Precise Comparison with Pyrolite by Multisample Cell High-Pressure Experiments with Implication to Dynamics of Subducted Slabs. J. Geophys. Res. Solid Earth 2019, 124, 3491–3507. [Google Scholar] [CrossRef]
- Green, D.H.; Ringwood, A.E. An experimental investigation of the gabbro to eclogite transformation and its petrological applications. Geochim. Et Cosmochim. Acta 1967, 31, 767–833. [Google Scholar] [CrossRef]
- Poli, S. The amphibolite-eclogite transformation; an experimental study on basalt. Am. J. Sci. 1993, 293, 1061–1107. [Google Scholar] [CrossRef]
- Akaogi, M.; Akimoto, S. Pyroxene-garnet solid-solution equilibria in the systems Mg4Si4O12-Mg3Al2Si3O12 and Fe4Si4O12-Fe3Al2Si3O12 at high pressures and temperatures. Phys. Earth Planet. Inter. 1977, 15, 90–106. [Google Scholar] [CrossRef]
- Irifune, T. Phase transformations in the earth’s mantle and subducting slabs: Implications for their compositions, seismic velocity and density structures and dynamics. Isl. Arc. 1993, 2, 55–71. [Google Scholar] [CrossRef]
- Kiseeva, E.S.; Yaxley, G.M.; Stepanov, A.S.; Tkalčić, H.; Litasov, K.D.; Kamenetsky, V.S. Metapyroxenite in the mantle transition zone revealed from majorite inclusions in diamonds. Geology 2013, 41, 883–886. [Google Scholar] [CrossRef]
- Presnall, D.C.; Gudfinnsson, G.H.; Walter, M.J. Generation of mid-ocean ridge basalts at pressures from 1 to 7 GPa. Geochim. Et Cosmochim. Acta 2002, 66, 2073–2090. [Google Scholar] [CrossRef]
- Putirka, K.D. Mantle potential temperatures at Hawaii, Iceland, and the mid-ocean ridge system, as inferred from olivine phenocrysts: Evidence for thermally driven mantle plumes. Geochem. Geophys. Geosystems 2005, 6, 1–14. [Google Scholar] [CrossRef]
- Dalton, C.A.; Langmuir, C.H.; Gale, A. Geophysical and geochemical evidence for deep temperature variations beneath mid-ocean ridges. Science 2014, 344, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Sarafian, E.; Gaetani, G.A.; Hauri, E.H.; Sarafian, A.R. Experimental constraints on the damp peridotite solidus and oceanic mantle potential temperature. Science 2017, 355, 942–945. [Google Scholar] [CrossRef] [PubMed]
- Irifune, T.; Sekine, T.; Ringwood, A.E.; Hibberson, W.O. The eclogite-garnetite transformation at high pressure and some geophysical implications. Earth Planet. Sci. Lett. 1986, 77, 245–256. [Google Scholar] [CrossRef]
- Duan, L.; Wang, W.; Wu, Z.; Qian, W. Thermodynamic and Elastic Properties of Grossular at High Pressures and High Temperatures: A First-Principles Study. J. Geophys. Res. Solid Earth 2019, 124, 7792–7805. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, D.; Fan, D.; Dera, P.K.; Shi, F.; Zhou, W. Thermoelastic Properties of Eclogitic Garnets and Omphacites: Implications for Deep Subduction of Oceanic Crust and Density Anomalies in the Upper Mantle. Geophys. Res. Lett. 2019, 46, 179–188. [Google Scholar] [CrossRef]
- Trubitsyn, A.P.; Trubitsyn, V.P. Temperature Distribution in the Earth’s Mantle. Dokl. Earth Sci. 2020, 495, 905–909. [Google Scholar] [CrossRef]
- Green, D.H.; Ringwood, A.E. Mineralogy of peridotitic compositions under upper mantle conditions. Phys. Earth Planet. Inter. 1970, 3, 359–371. [Google Scholar] [CrossRef]
- Liu, L.-G. The mineralogy of an eclogitic earth mantle. Phys. Earth Planet. Inter. 1980, 23, 262–267. [Google Scholar] [CrossRef]
- Ringwood, A.E.; Major, A. The system Mg2SiO4-Fe2SiO4 at high pressures and temperatures. Phys. Earth Planet. Inter. 1970, 3, 89–108. [Google Scholar] [CrossRef]
- Canil, D.; O’Neill, H.S.C. Distribution of Ferric Iron in some Upper-Mantle Assemblages. J. Petrol. 1996, 37, 609–635. [Google Scholar] [CrossRef]


| m0 | m1 | m2 | m3 | |
|---|---|---|---|---|
| vP | 8061.78 (±13.41) | −0.402 (±0.019) | 101.21 (±4.42) | −1.771 (±0.317) |
| vS | 4712.23 (±13.78) | −0.329 (±0.019) | 33.60 (±4.55) | −0.388 (±0.325) |
| T K | α0 10–5/K | ∂α/∂P 10–6/K GPa | ∂2α/∂P2 10−8/K GPa2 |
| 300 | 2.71 | −0.92 | 1.4 |
| 700 | 3.09 | −1.02 | 1.6 |
| 1100 | 3.29 | −1.13 | 1.8 |
| 2000 | 3.70 | −1.37 | 2.4 |
| T K | CP0 J/mol K | ∂CP/∂P J/mol K GPa | ∂2CP/∂P2 10−3 J/mol K GPa2 |
| 300 | 167.15 | −0.417 | 2.3 |
| 700 | 231.57 | −0.275 | 4.5 |
| 1100 | 250.91 | −0.372 | 7.2 |
| 2000 | 274.09 | −0.688 | 14.2 |
| T K | γ0 | ∂γ/∂P 10−3/GPa | ∂2γ/∂P2 10−4/GPa2 |
| 300 | 1.254 | −3.4 | −5.2 |
| 700 | 1.004 | −2.7 | −3.8 |
| 1100 | 0.961 | −3.4 | −3.5 |
| 2000 | 0.928 | −4.8 | −3.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, C.; Fan, D.; Jiang, J.; Sun, Z.; Liu, Y.; Song, W.; Wan, Y.; Yang, G.; Qiu, W. Self-Consistent Thermodynamic Parameters of Diopside at High Temperatures and High Pressures: Implications for the Adiabatic Geotherm of an Eclogitic Upper Mantle. Minerals 2021, 11, 1322. https://doi.org/10.3390/min11121322
Su C, Fan D, Jiang J, Sun Z, Liu Y, Song W, Wan Y, Yang G, Qiu W. Self-Consistent Thermodynamic Parameters of Diopside at High Temperatures and High Pressures: Implications for the Adiabatic Geotherm of an Eclogitic Upper Mantle. Minerals. 2021; 11(12):1322. https://doi.org/10.3390/min11121322
Chicago/Turabian StyleSu, Chang, Dawei Fan, Jiyi Jiang, Zhenjun Sun, Yonggang Liu, Wei Song, Yongge Wan, Guang Yang, and Wuxueying Qiu. 2021. "Self-Consistent Thermodynamic Parameters of Diopside at High Temperatures and High Pressures: Implications for the Adiabatic Geotherm of an Eclogitic Upper Mantle" Minerals 11, no. 12: 1322. https://doi.org/10.3390/min11121322
APA StyleSu, C., Fan, D., Jiang, J., Sun, Z., Liu, Y., Song, W., Wan, Y., Yang, G., & Qiu, W. (2021). Self-Consistent Thermodynamic Parameters of Diopside at High Temperatures and High Pressures: Implications for the Adiabatic Geotherm of an Eclogitic Upper Mantle. Minerals, 11(12), 1322. https://doi.org/10.3390/min11121322







