Characterization of Natural Consolidated Halloysite Nanotube Structures
Abstract
:1. Introduction
1.1. Halloysite Structure, a Geometric Variation of Kaolinite
1.2. Surface Chemistry
1.3. Objectives of the Study: Multiscale Characterization
2. Materials and Methods
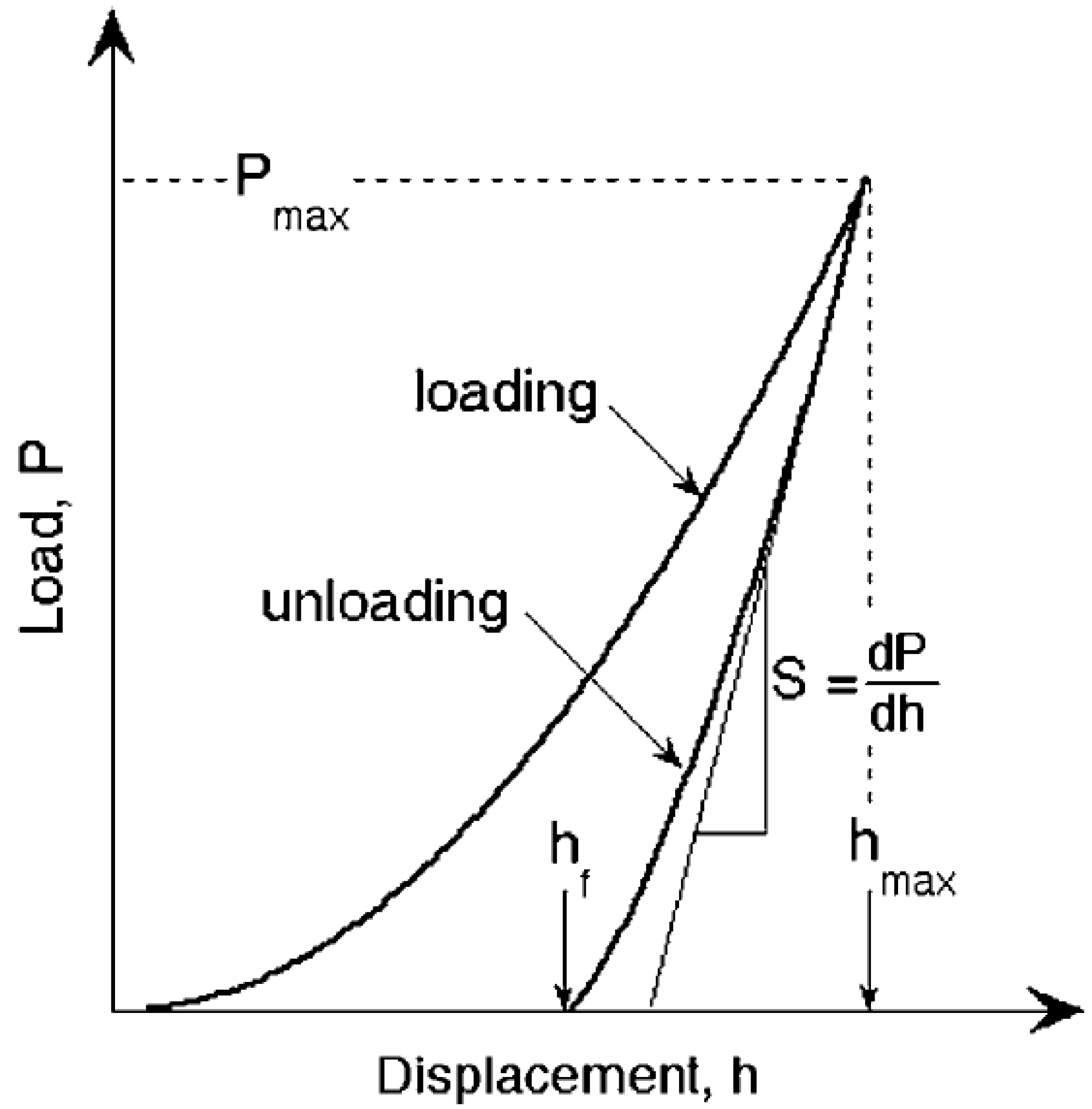
2.1. Mechanical Properties Measurements by Nanoindentation
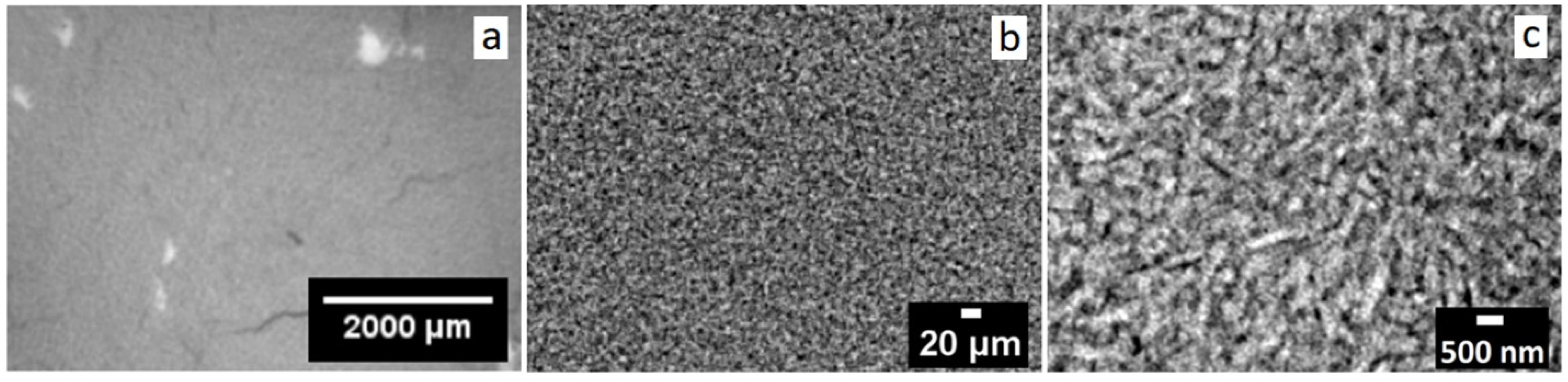
2.2. Porosity by X-ray Attenuation in Micro-X-ray Computed Tomography (Micro-XCT)
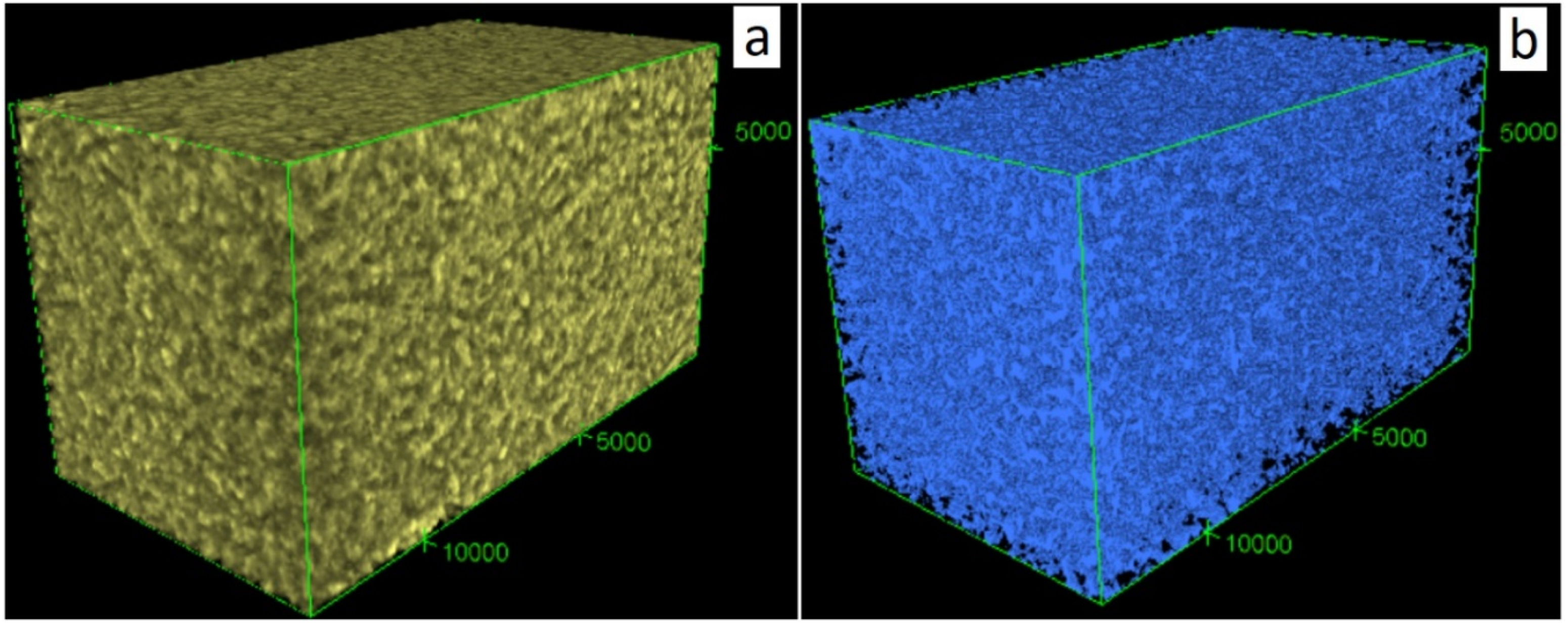
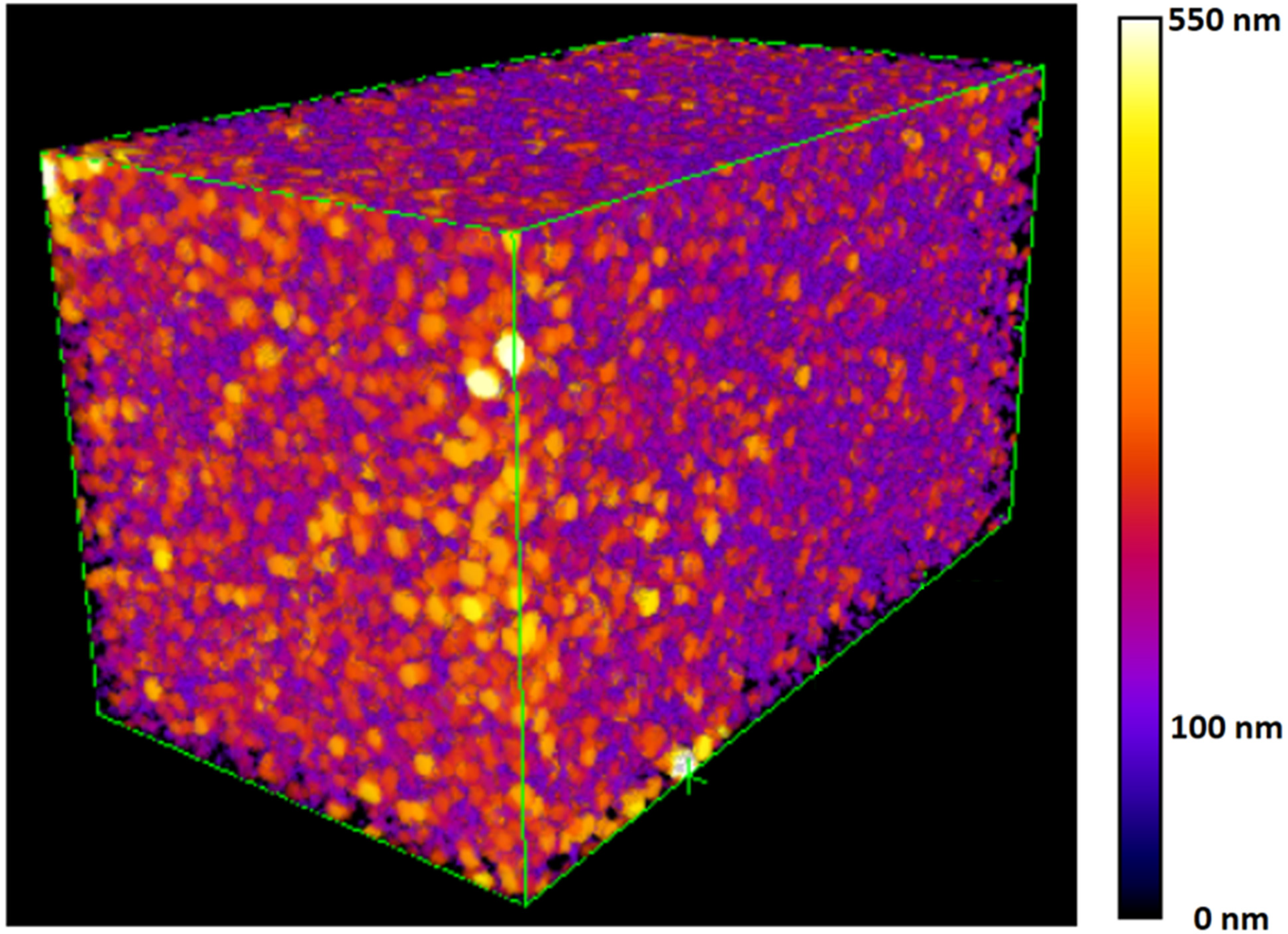
2.3. Pore Network Characterization by Nano-XCT
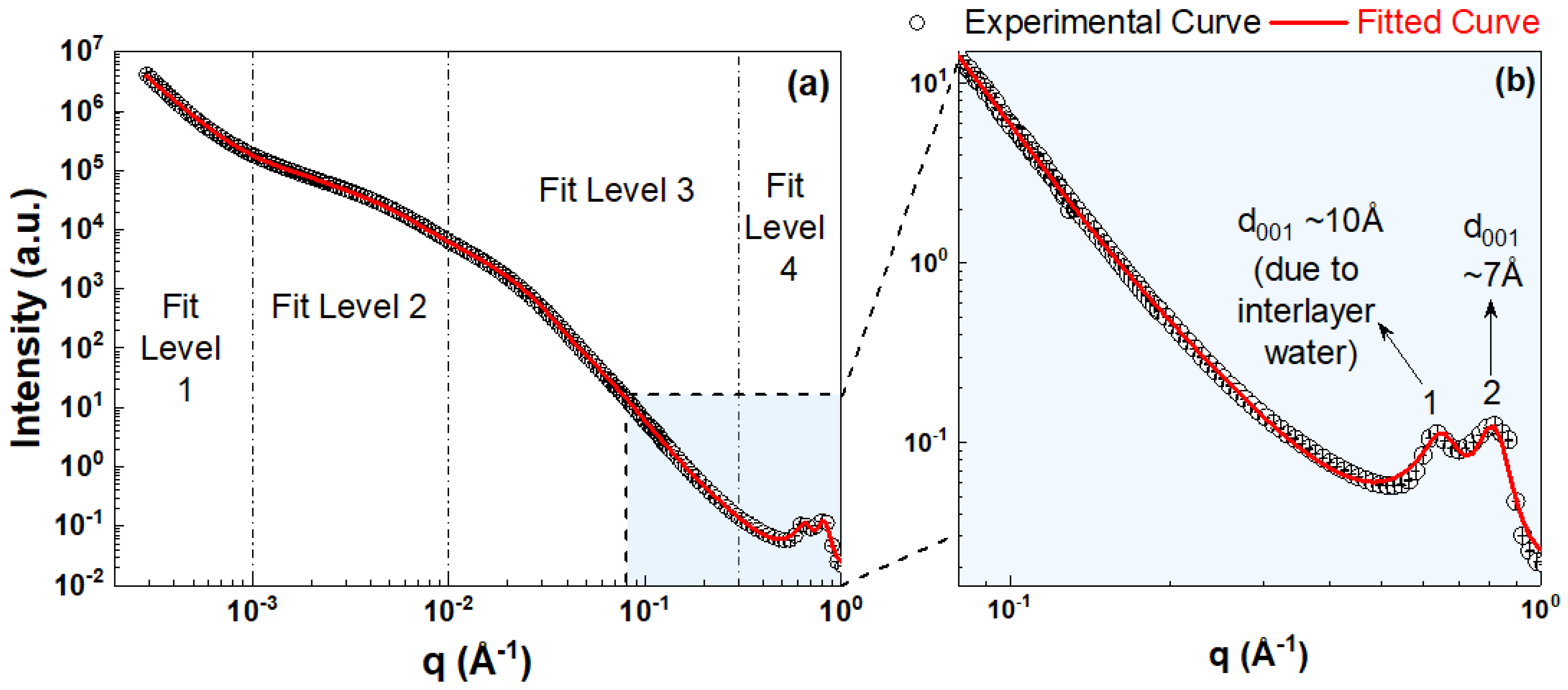
2.4. Small-Angle X-ray Scattering (SAXS)
2.5. Sedimentation Field-Flow Fractionation (SdFFF)
3. Results and Discussion
3.1. Mechanical Properties of Consolidated Halloysite
3.2. Porosity of Natural Consolidated Halloysite
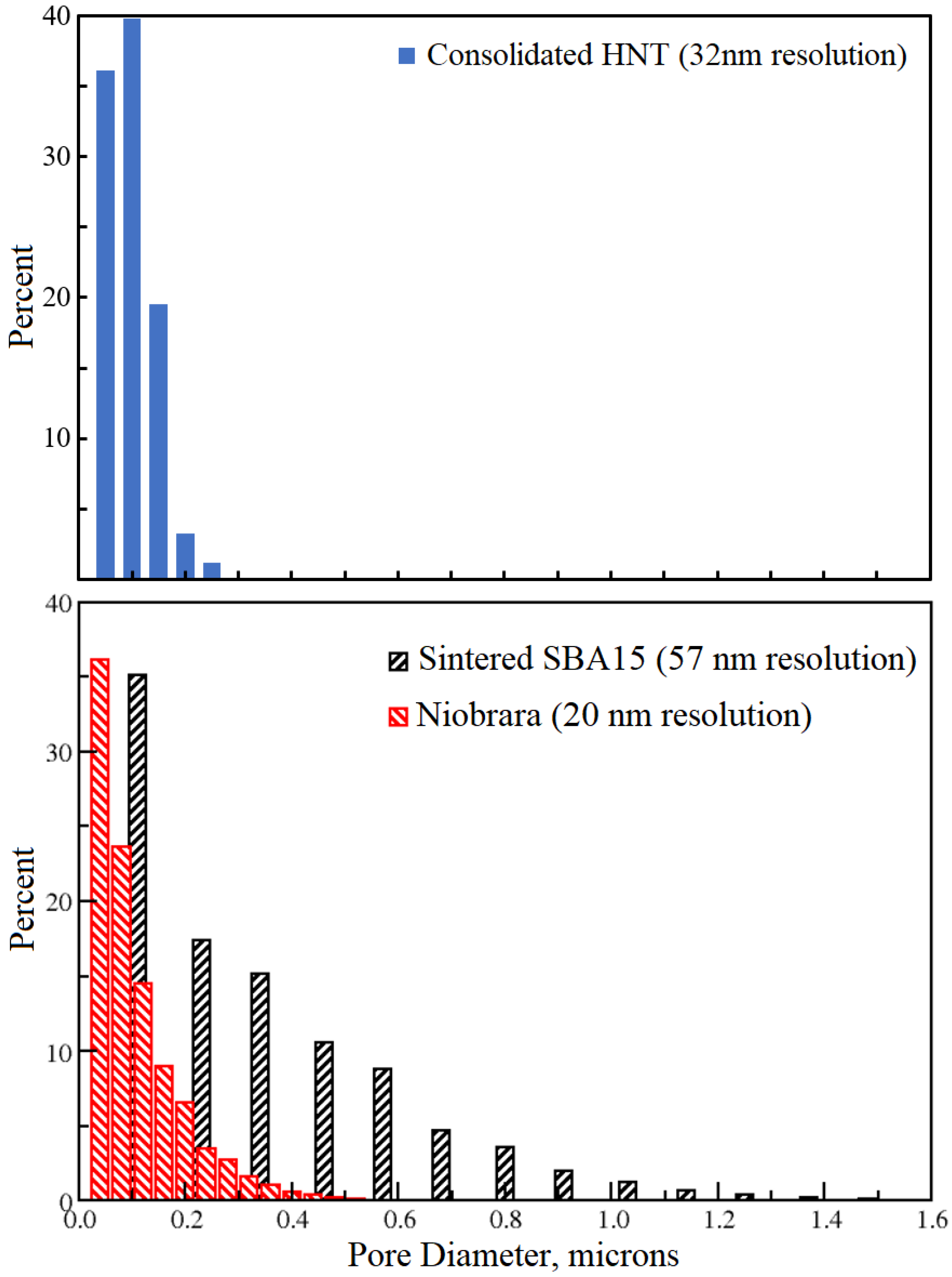
3.3. Pore Size Distribution
3.3.1. Inter-HNT Pore Size Measured by Nano-XCT
3.3.2. Intra-HNT Pore Size Measured by Combined USAXS/SAXS
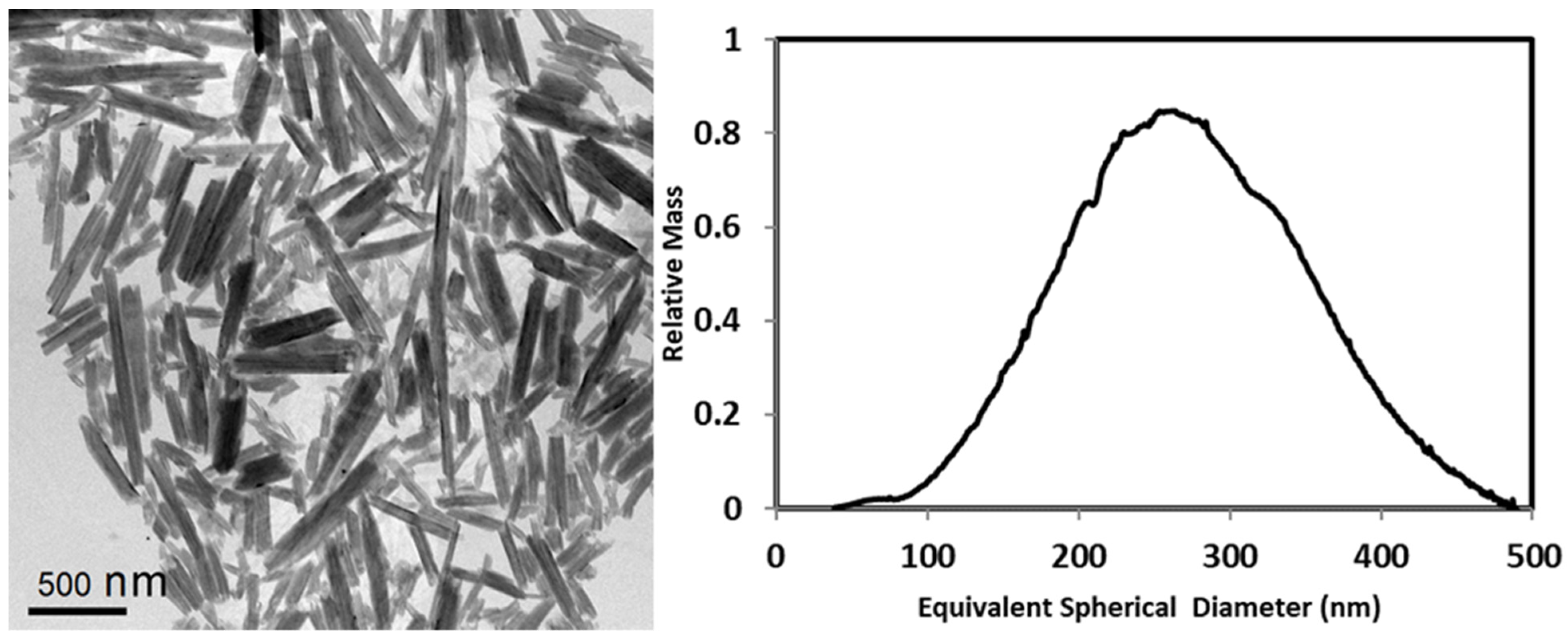
3.4. Individual HNTs of the Natural Consolidated Structures
3.4.1. Size of Individual HNTs
3.4.2. Halloysite Interlayer Spacing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Secchi, E.; Marbach, S.; Niguès, A.; Stein, D.; Siria, A.; Bocquet, L. Massive radius-dependent flow slippage in carbon nanotubes. Nature 2016, 537, 210–213. [Google Scholar] [CrossRef]
- Rogers, B.J.; Wirth, M.J. Slip flow through colloidal crystals of varying particle diameter. ACS Nano 2013, 7, 725–731. [Google Scholar] [CrossRef] [Green Version]
- Joussein, E.; Petit, S.; Churchman, J.; Theng, B.; Right, D.; Delvaux, B. Halloysite clay minerals—A review. Clay Miner. 2005, 40, 383–426. [Google Scholar] [CrossRef]
- Vinokurov, V.A.; Stavitskaya, A.V.; Chudakov, Y.A.; Ivanov, E.V.; Shrestha, L.K.; Ariga, K.; Darrat, Y.A.; Lvov, Y.M. Formation of metal clusters in halloysite clay nanotubes. Sci. Technol. Adv. Mater. 2017, 18, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Brindley, G.W. Structural mineralogy of clays. Clays Clay Miner. 1952, 1, 33–43. [Google Scholar] [CrossRef]
- Yuan, P.; Thill, A.; Bergaya, F. Nanosized Tubular Clay Minerals: Halloysite and Imogolite. In Developments in Clay Science 7; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–754. [Google Scholar]
- Churchman, G.J.; Pasbakhsh, P.; Hillier, S. The rise and rise of halloysite. Clay Miner. 2016, 51, 303–308. [Google Scholar] [CrossRef] [Green Version]
- Lampropoulou, P.; Papoulis, D. Halloysite in different ceramic products: A review. Materials 2021, 14, 5501. [Google Scholar] [CrossRef] [PubMed]
- Yahaya, S.; Jikan, S.S.; Badarulzaman, N.A.; Adamu, A.D. Chemical composition and particle size analysis of kaolinite. Path Sci. 2017, 3, 1001–1004. [Google Scholar] [CrossRef]
- Gupta, V.; Miller, J.D. Surface force measurements at the basal planes of ordered kaolinite particles. J. Colloid Interface Sci. 2010, 344, 362–371. [Google Scholar] [CrossRef]
- Yin, X.; Miller, J.D. Wettability of kaolinite basal planes based on surface force measurements using atomic force microscopy. Miner. Metall. Process. 2012, 29, 13–19. [Google Scholar] [CrossRef]
- Yin, X. Anisotropic Surface Features of Selected Phyllosilicates. Ph.D. Thesis, The University of Utah, Salt Lake City, UT, USA, 2012. [Google Scholar]
- Dutta, A.K. Mechanical Properties of Granular Materials using Nanoindentation and Modeling with Distinct Element Method. Ph.D. Thesis, University of Tennessee, Knoxville, TN, USA, 2006. [Google Scholar]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sending indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 2004, 19, 3–20. [Google Scholar] [CrossRef]
- McCullough, E.C. Photon attenuation in computed tomography. Med. Phys. 1975, 2, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Videla, A.R.; Yu, Q.; Miller, J.D. Characterization and analysis of porous, brittle solid structures by X-ray micro computed tomography. JOM 2010, 62, 86–89. [Google Scholar] [CrossRef]
- Hildebrand, T.; Rüegsegger, P. A new method for the model-independent assessment of thickness in three-dimensional images. J. Microsc. 1997, 185, 67–75. [Google Scholar] [CrossRef]
- Bonse, U.; Hart, M. Tailless x-ray single-crystal reflection curves obtained by multiple reflection. Appl. Phys. Lett. 1965, 7, 238–240. [Google Scholar] [CrossRef]
- Ilavsky, J.; Zhang, F.; Allen, A.J.; Levine, L.E.; Jemian, P.R.; Long, G.G. Ultra-Small-Angle X-ray scattering instrument at the advanced photon source: History, recent development, and current status. Metall. Mater. Trans. A 2013, 44, 68–76. [Google Scholar] [CrossRef]
- Nyam-Osor, M.; Soloviov, D.V.; Kovalev, Y.S.; Zhigunov, A.; Rogachev, A.V.; Ivankov, O.I.; Erhan, R.V.; Kuklin, A.I. Silver behenate and silver stearate powders for calibration of SAS instruments. J. Phys. Conf. Ser. 2012, 351, 012024. [Google Scholar] [CrossRef]
- Ilavsky, J.; Jemian, P.R. Irena: Tool suite for modeling and analysis of small-angle scattering. J. Appl. Crystallogr. 2009, 42, 347–353. [Google Scholar] [CrossRef]
- Ilavsky, J. Nika: Software for two-dimensional data reduction. J. Appl. Crystallogr. 2012, 45, 324–328. [Google Scholar] [CrossRef]
- Gadikota, G.; Zhang, F.; Allen, A. In situ angstrom-to-micrometer characterization of the structural and microstructural changes in kaolinite on heating using ultra small-angle, small-angle, and wide-angle x-ray scattering (USAXS/SAXS/WAXS). Ind. Eng. Chem. Res. 2017, 56, 11791–11801. [Google Scholar] [CrossRef] [PubMed]
- Gadikota, G.; Zhang, F.; Allen, A.J. Towards understanding the microstructural and structural changes in natural hierarchical materials for energy recovery: In-operando multi-scale X-ray scattering characterization of Na-and Ca-montmorillonite on heating to 1150 °C. Fuel 2017, 196, 195–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadikota, G.; Allen, A. Microstructural and structural characterization of materials for CO2 storage using multi-scale X-ray scattering methods. In Materials and Processes for CO2 Capture, Conversion, and Sequestration; Wiley Online: Hoboken, NJ, USA, 2018; pp. 296–318. [Google Scholar]
- Glatter, O.; Kratky, O.; Kratky, H.C. Small Angle X-ray Scattering; Academic Press: Cambridge, MA, USA, 1982. [Google Scholar]
- Beaucage, G. Approximations leading to a unified exponential/power-law approach to small-angle scattering. J. Appl. Crystallogr. 1995, 28, 717–728. [Google Scholar] [CrossRef]
- Beaucage, G. Small-angle scattering from polymeric mass fractals of arbitrary mass-fractal dimension. J. Appl. Crystallogr. 1996, 29, 134–146. [Google Scholar] [CrossRef] [Green Version]
- Asgar, H.; Jin, J.; Miller, J.; Kuzmenko, I.; Gadikota, G. Contrasting thermally-induced structural and microstructural evolution of alumino-silicates with tubular and planar arrangements: Case study of halloysite and kaolinite. Colloid Surf. A Physicochem. Eng. Asp. 2021, 613, 126106. [Google Scholar] [CrossRef]
- García, F.J.; Sergio, G.R.; Andreas, K.; Armin, R. Study of natural halloysite from the Dragon Mine, Utah (USA). Z. Für Anorg. Und Allg. Chem. 2009, 635, 790–795. [Google Scholar] [CrossRef]
- Pasbakhsh, P.; Churchman, G.J.; Keeling, J.L. Characterisation of properties of various halloysites relevant to their use as nanotubes and microfibre fillers. Appl. Clay Sci. 2013, 74, 47–57. [Google Scholar] [CrossRef]
- Samsonov, G.V. Mechanical properties of the elements. In Handbook of the Physicochemical Properties of the Elements; IFI_Plenum: New York, NY, USA, 1968; p. 432. [Google Scholar]
- Lin, C.L.; Miller, J.D. Spatial characterization of heterogeneous nanopore surfaces from XCT scans of Niobrara shale. Colloid Surf. A 2019, 572, 120–137. [Google Scholar] [CrossRef] [Green Version]
- Maheshwari, H.; Roehling, J.D.; Turner, B.A.; Abdinor, J.; Tran-Roehling, T.B.; Deo, M.D.; Bartl, M.H.; Risbud, S.H.; Van Benthem, K. Robust mesoporous silica compacts: Multi-scale characterization of microstructural changes related to physical–mechanical properties. J. Mater. Sci. 2016, 51, 4470–4480. [Google Scholar] [CrossRef]
- Hillier, S.; Brydson, R.; Delbos, E.; Fraser, T.; Gray, N.; Pendlowski, H.; Phillips, I.; Robertson, J.; Wilson, I. Correlations among the mineralogical and physical properties of halloysite nanotubes (HNTs). Clay Miner. 2016, 51, 325–350. [Google Scholar] [CrossRef]
- Bish, D.L.; Von Dreele, R.B. Rietveld refinement of non-hydrogen atomic positions in kaolinite. Clays Clay Miner. 1989, 37, 289–296. [Google Scholar] [CrossRef]
- Wada, N.; Raythatha, R.; Minomura, S. Pressure effects on water-intercalated kaolinite. Solid State Commun. 1987, 63, 783–786. [Google Scholar] [CrossRef]
- Frost, R.L.; Kristof, J.; Paroz, G.N.; Kloprogge, J.T. Role of water in the intercalation of kaolinite with hydrazine. J. Colloid Interface Sci. 1998, 208, 216–225. [Google Scholar] [CrossRef] [PubMed]



| Nanoindentation Results | Alunite Content (ppm) | Mohs Scale | ||||
|---|---|---|---|---|---|---|
| Mineral Sample | Average Ea (GPa) | SD Ea (GPa) | Average H (GPa) | SD. H (GPa) | ||
| Consolidated HNT 01 | 1.53 | 0.50 | 0.03 | 0.01 | <0.06 | – |
| Consolidated HNT 02 | 4.60 | 1.13 | 0.28 | 0.14 | 0.33 | 2–2.5 |
| Consolidated HNT 03 | 14.28 | 3.27 | 0.41 | 0.23 | 0.94 | – |
| Kaolinite 01 | 0.03 | 0.03 | 0.00 | 0.00 | – | 2–2.5 |
| Kaolinite 02 | 3.86 | 1.27 | 0.07 | 0.05 | – | |
| Calcite | 47.20 | 2.40 | 2.28 | 0.18 | – | 3 |
| Quartz | 77.82 | – | 10.8 | – | – | 7 |
| Method | X-ray Attenuation Micro-XCT (19.32 μm) | He Porosimetry | 3D Pore Network Nano-XCT (32 nm) |
|---|---|---|---|
| Total Porosity | 40.6% | 40.4% | – |
| Inter-HNT Porosity > 32 nm | – | – | 13.2% |
| Method/Source | Intra-HNT Pore Size (nm) |
|---|---|
| SAXS Rg | 12.8 |
| BET N2 Adsorption | 10.4 |
| BET N2 Desorption | 10.7 |
| BJH N2 Adsorption | 12.3 |
| Literature [4,5] | 12~15 |
| Sample | ESD Range (nm) | Peak ESD (nm) | Mean Diameter (nm) |
|---|---|---|---|
| Dragon A | 90~500 | 246 | – |
| Dragon B | 100~400 | 211 | – |
| Dragon C | 100~500 | 262 | – |
| SAXS | – | – | 70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, J.; Assemi, S.; Asgar, H.; Gadikota, G.; Tran, T.; Nguyen, W.; McLennan, J.D.; Miller, J.D. Characterization of Natural Consolidated Halloysite Nanotube Structures. Minerals 2021, 11, 1308. https://doi.org/10.3390/min11121308
Jin J, Assemi S, Asgar H, Gadikota G, Tran T, Nguyen W, McLennan JD, Miller JD. Characterization of Natural Consolidated Halloysite Nanotube Structures. Minerals. 2021; 11(12):1308. https://doi.org/10.3390/min11121308
Chicago/Turabian StyleJin, Jiaqi, Shoeleh Assemi, Hassnain Asgar, Greeshma Gadikota, Thang Tran, William Nguyen, John D. McLennan, and Jan D. Miller. 2021. "Characterization of Natural Consolidated Halloysite Nanotube Structures" Minerals 11, no. 12: 1308. https://doi.org/10.3390/min11121308
APA StyleJin, J., Assemi, S., Asgar, H., Gadikota, G., Tran, T., Nguyen, W., McLennan, J. D., & Miller, J. D. (2021). Characterization of Natural Consolidated Halloysite Nanotube Structures. Minerals, 11(12), 1308. https://doi.org/10.3390/min11121308








