Industrial Heap Bioleaching of Copper Sulfide Ore Started with Only Water Irrigation
Abstract
:1. Introduction
2. Mineralogy and Methods
2.1. Mineralogy of Letpadaung Ore
2.2. Heap Sample Collection and Test
3. Laboratory Testing and Industrial Steps of Water Start Heap Bioleaching
3.1. Sulfuric Acid Generating Potential Based on Minerology
3.2. Labrotory Column Tests Summary
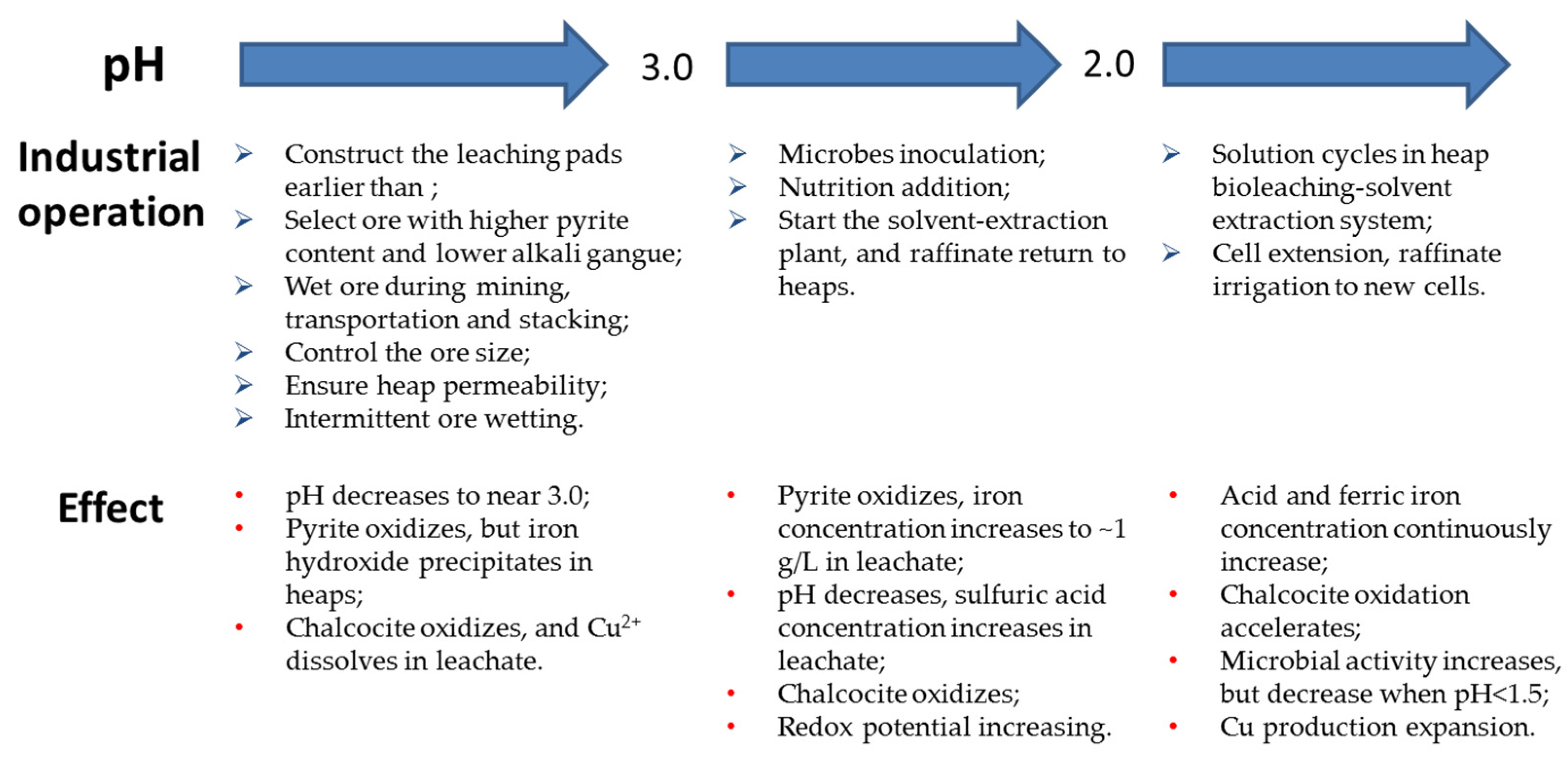
3.3. Steps for Water Start Heap Bioleaching
4. Heap Bioleaching Performance
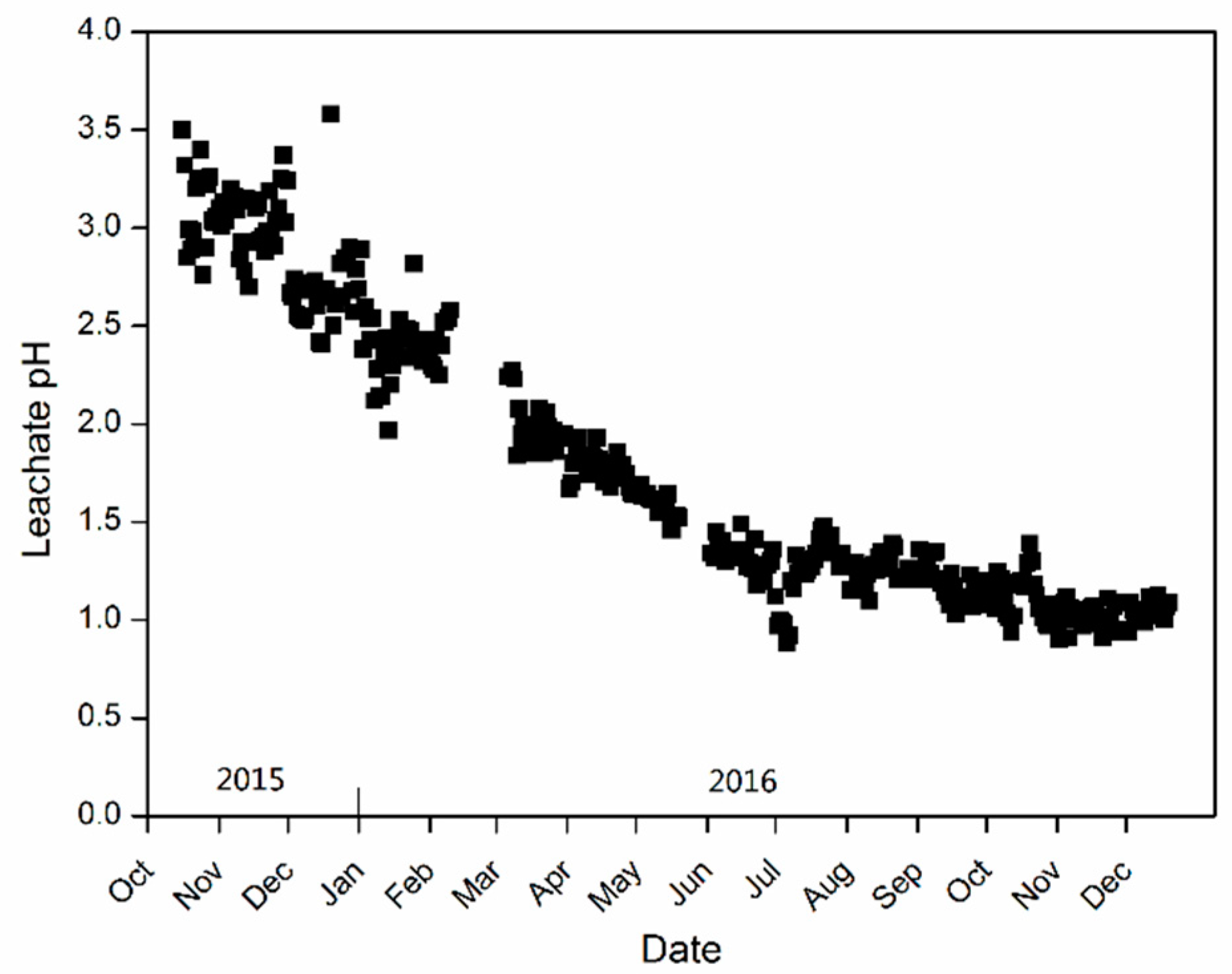
4.1. Leachate pH Dropping during the Heap Leaching
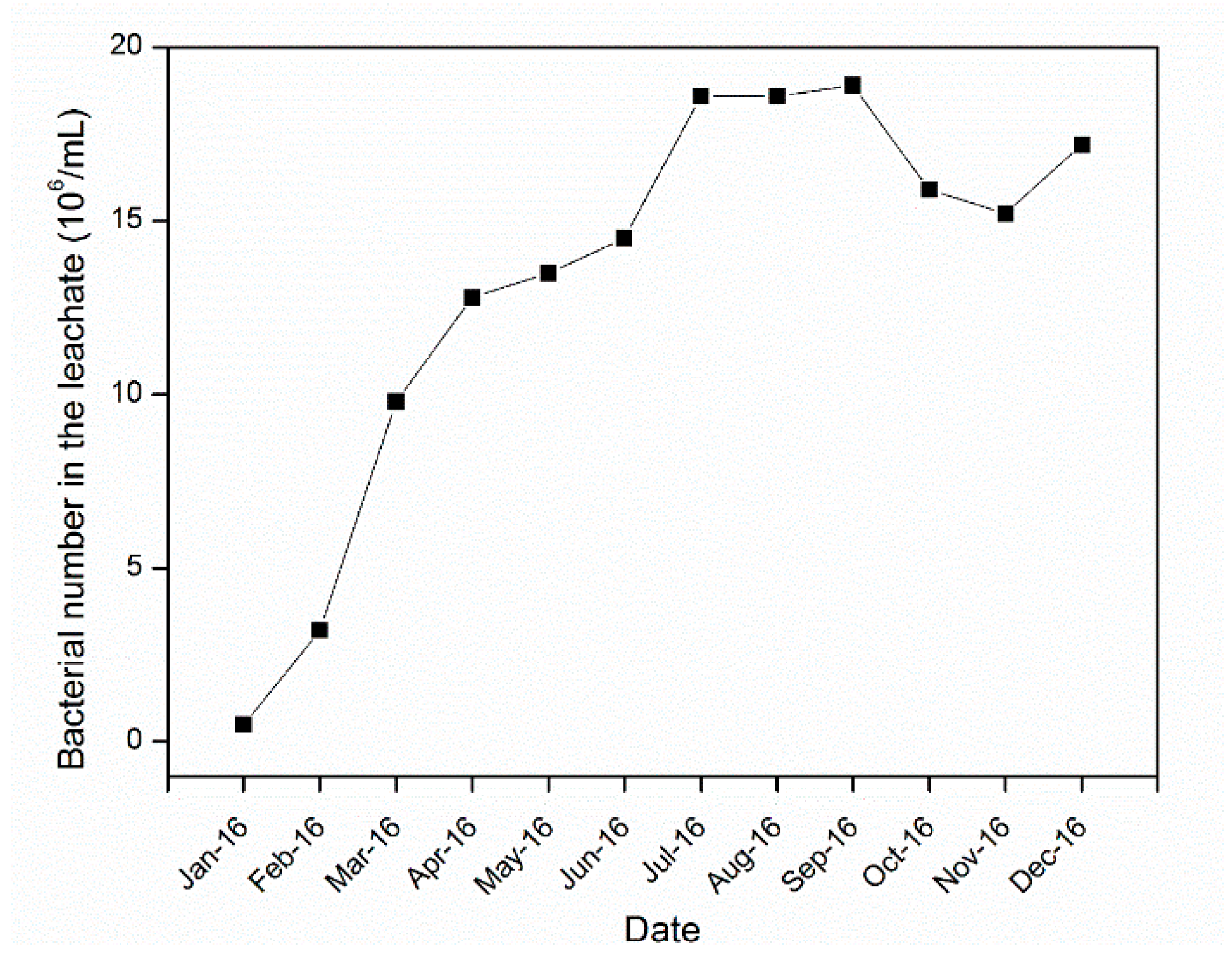
4.2. Microbial Community and Activity
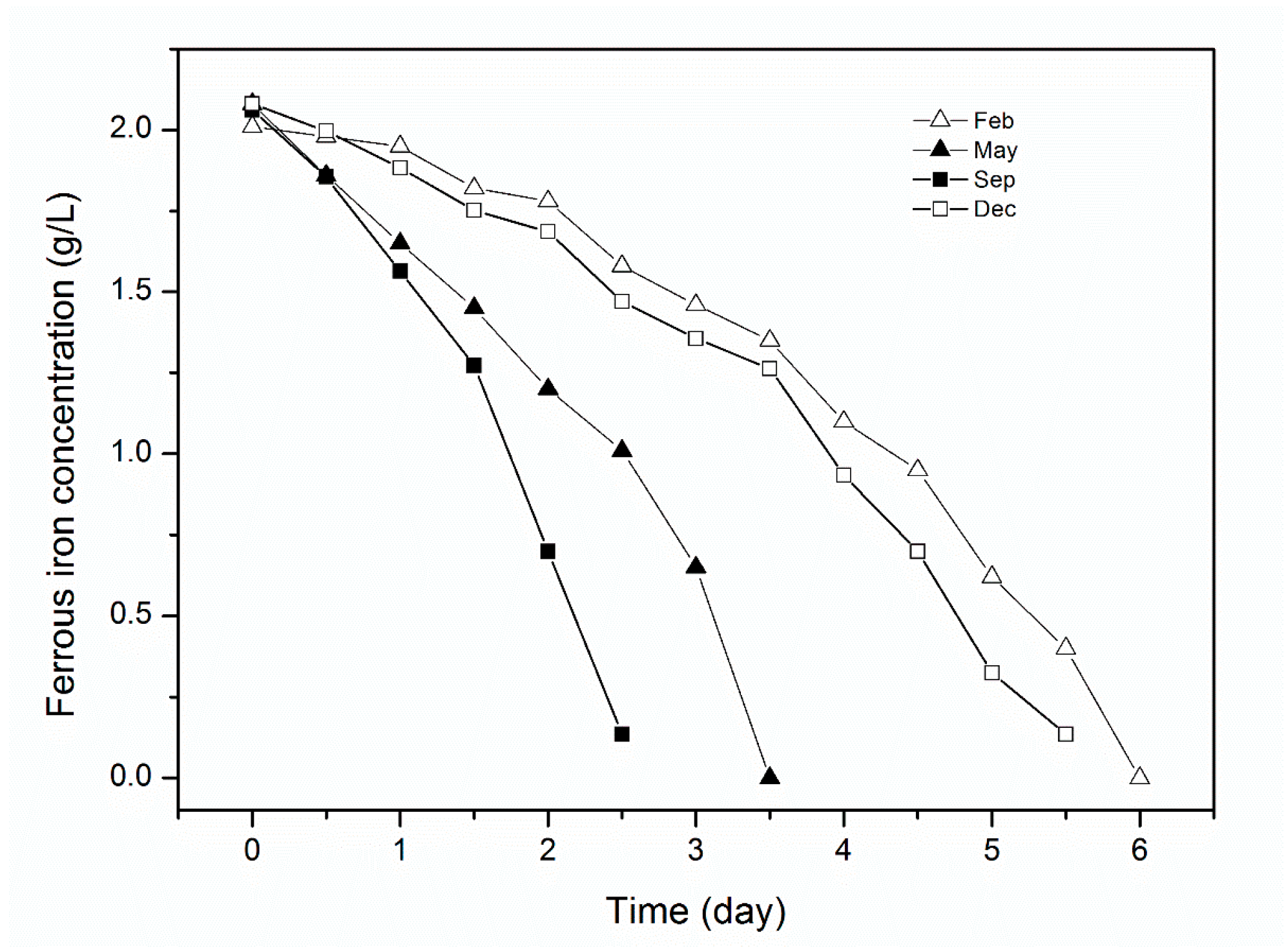
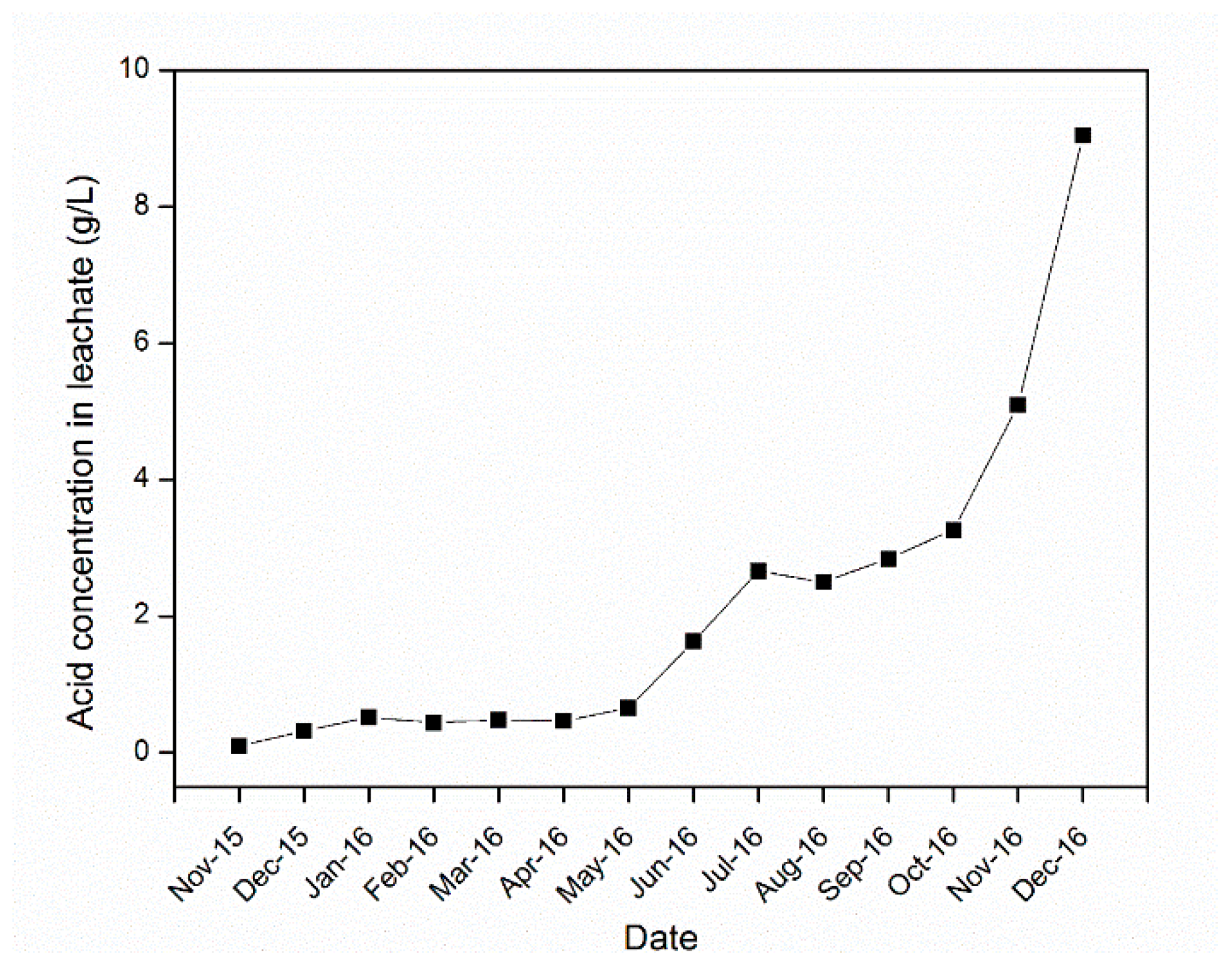
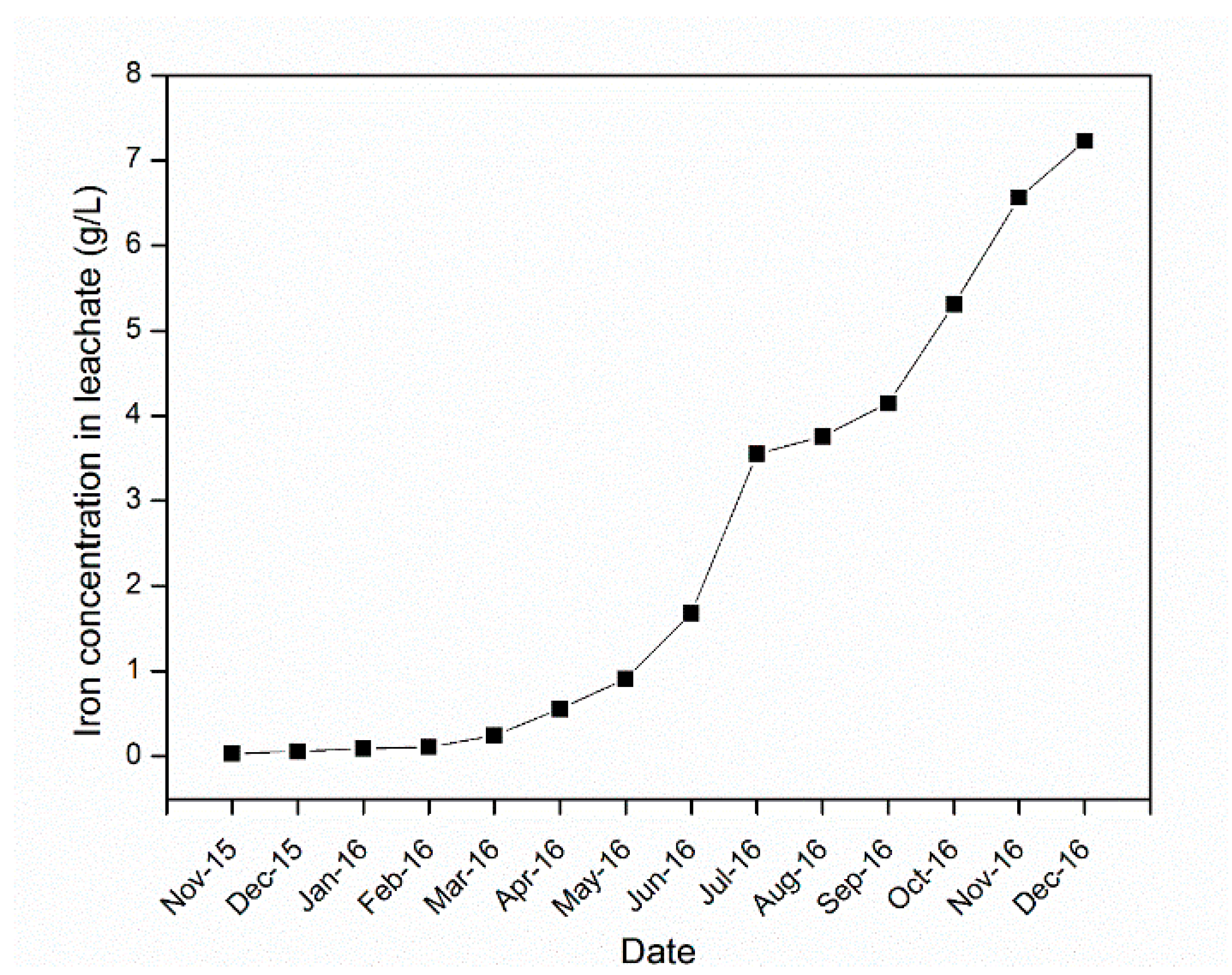
4.3. Sulfuric Acid and Iron Concentration in the Leachate
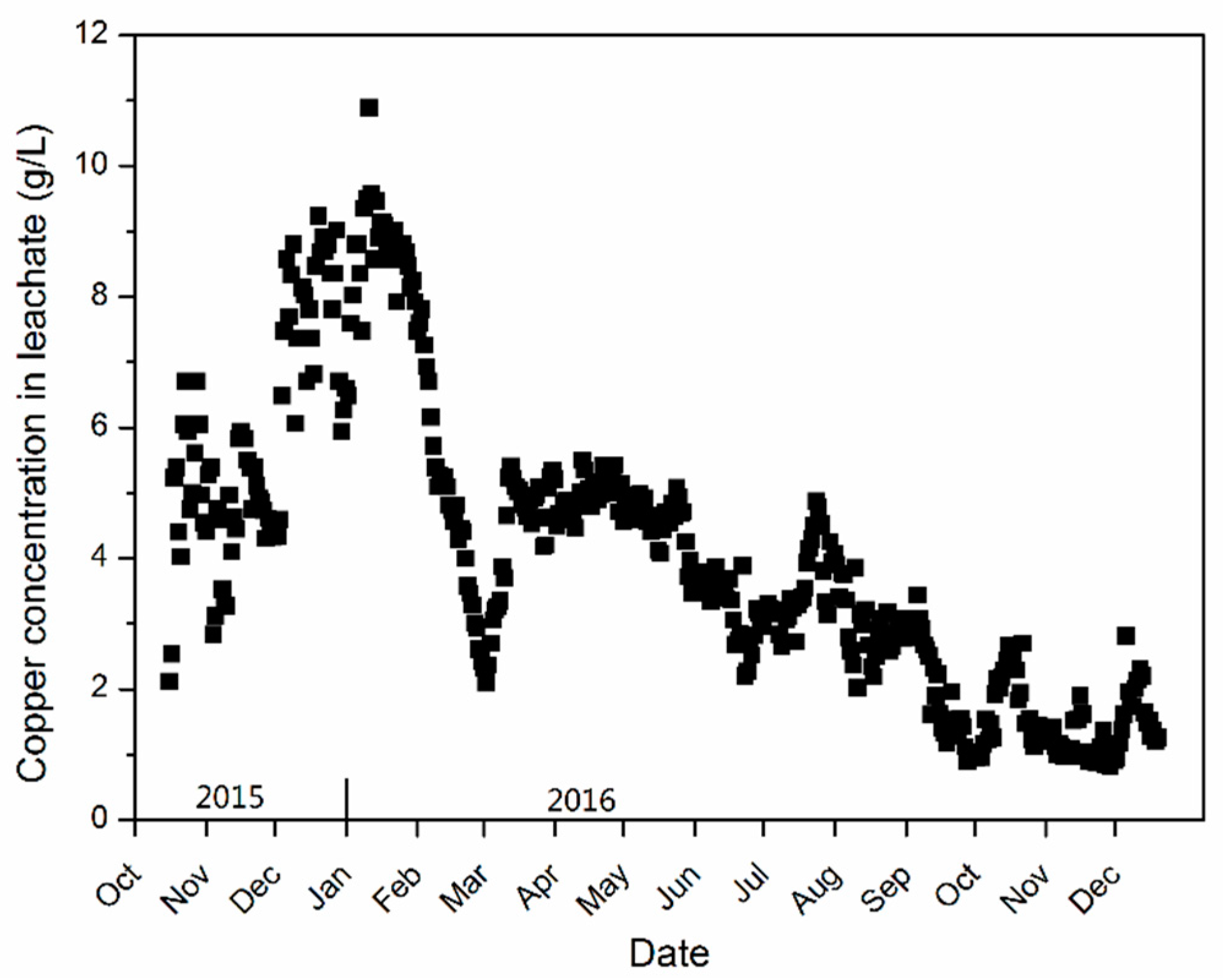
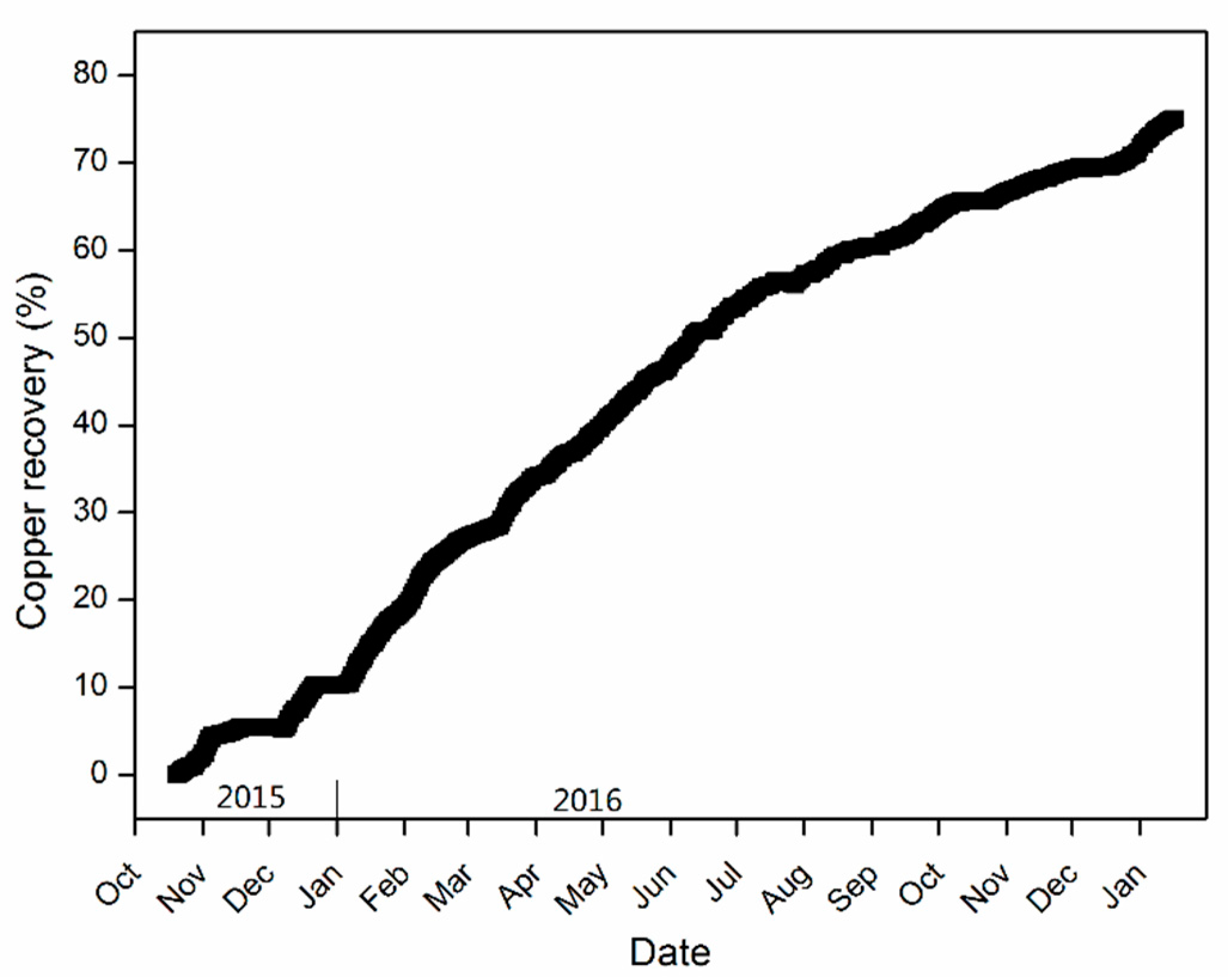
4.4. Copper Leaching
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, H.; Zhang, Y.; Zhang, X.; Qian, L.; Sun, M.; Yang, Y.; Qiu, G. The dissolution and passivation mechanism of chalcopyrite in bioleaching: An overview. Miner. Eng. 2019, 136, 140–154. [Google Scholar] [CrossRef]
- Watling, H.R. The bioleaching of sulphide minerals with emphasis on copper sulphides: A review. Hydrometallurgy 2006, 84, 81–108. [Google Scholar] [CrossRef]
- Domic, E.M. A Review of the Development and Current Status of Copper Bioleaching Operations in Chile: 25 Years of Successful Commercial Implementation, in Biomining; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2007; pp. 81–96. [Google Scholar]
- Schippers, J.A. (Bio)chemistry of bacterial leaching—Direct vs. indirect bioleaching. Hydrometallurgy 2001, 59, 159–175. [Google Scholar]
- Jia, Y.; Tan, Q.; Sun, H.; Zhang, Y.P.; Gao, H.S.; Ruan, R.M. Sulfide mineral dissolution microbes: Community structure and function in industrial bioleaching heaps. Green Energy Environ. 2018, 4, 29–37. [Google Scholar] [CrossRef]
- Ngoma, I.E.; Ojumu, T.V.; Harrison, S. Investigating the effect of acid stress on selected mesophilic micro-organisms implicated in bioleaching. Miner. Eng. 2015, 75, 6–13. [Google Scholar] [CrossRef]
- Gericke, M.; Neale, J.W.; Staden, P. A mintek perspective of the past 25 years in minerals bioleaching. J. S. Afr. Inst. Min. Metall. 2009, 109, 567–585. [Google Scholar]
- Templeton, J.H.; Schlitt, W.J. Method for determining the sulfuric acid balance in copper-leach systems. Min. Met. Explor. 1997, 14, 1–7. [Google Scholar] [CrossRef]
- Chen, J.H.; Guo, X.J.; Li, H.X. Implementation and practice of an integrated process to recover copper from low grade ore at Zijinshan mine. Hydrometallurgy 2020, 195, 105394. [Google Scholar] [CrossRef]
- Jia, Y.; Ruan, R.M.; Gen, Y.; Sun, H.Y.; Liu, C. Acceleration and inhibition of pyrite oxidation during heap bioleaching of copper sulfides. In Heap Leach Mining Solutions; InfoMine: Vancouver, BC, Canada, 2016; pp. 83–93. [Google Scholar]
- Jia, Y.; Sun, H.Y.; Tan, Q.Y.; Gao, H.S.; Feng, X.L.; Ruan, R.M. Linking leach chemistry and microbiology of low-grade copper ore bioleaching at different temperatures. Int. J. Min. Met. Mater. 2018, 25, 271–279. [Google Scholar] [CrossRef]
- Ruan, R.; Liu, X.; Zou, G.; Chen, J.; Wen, J.; Wang, D. Industrial practice of a distinct bioleaching system operated at low pH, high ferric concentration, elevated temperature and low redox potential for secondary copper sulfide. Hydrometallurgy 2011, 108, 130–135. [Google Scholar] [CrossRef]
- Jia, Y.; Sun, H.Y.; Chen, D.F.; Gao, H.S.; Ruan, R.M. Characterization of microbial community in industrial bioleaching heap of copper sulfide ore at Monywa mine, Myanmar. Hydrometallurgy 2016, 164, 355–361. [Google Scholar] [CrossRef]
- Mitchell, A.; Myint, W.; Lynn, K.; Htay, M.T.; Zaw, T. Geology of the high sulfidation copper deposits, Monywa mine, Myanmar. Resour. Geol. 2010, 61, 1–29. [Google Scholar] [CrossRef]
- Bates, S.; Berglyons, D.; Caporaso, J.; Walters, W.; Knight, R.; Fierer, N. Examining the global distribution of dominant archaeal populations in soil. ISME J. 2011, 5, 908–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.Q.; Chen, J.H.; Chen, Y.; Zhao, C.; Zhang, Y.; Ke, B. Interactions of oxygen and water molecules with pyrite surface: A new insight. Langmuir 2018, 34, 1941–1952. [Google Scholar] [CrossRef] [PubMed]
- Moses, C.O.; Nordstrom, D.K.; Herman, J.S.; Mills, A.L. Aqueous pyrite oxidation by dissolved oxygen and by ferric iron. Geochim. Cosmochim. Acta 1987, 51, 1561–1571. [Google Scholar] [CrossRef]
- Singer, P.C.; Stumm, W. Acidic mine drainage: The rate-determining step. Science 1970, 167, 1121–1123. [Google Scholar] [CrossRef]
- Boon, M.; Ras, C.; Heijnen, J.J. The ferrous iron oxidation kinetics of thiobacillus ferrooxidans in batch cultures. Appl. Microbiol. Biotechnol. 1999, 51, 813–819. [Google Scholar] [CrossRef]
- van Scherpenzeel, D.A.; Boon, M.; Ras, C.; Hansford, G.S.; Heijnen, J.J. Kinetics of ferrous iron oxidation by leptospirillum bacteria in continuous cultures. Biotechnol. Prog. 1998, 14, 425–433. [Google Scholar] [CrossRef]
- Ramprakash, Y.; Koch, D.; Woods, R. The interaction of iron species with pyrite surfaces. J. Appl. Electrochem. 1991, 21, 531–536. [Google Scholar] [CrossRef]
- Laajalehto, K.; Kartio, I.; Suoninen, E. XPS and SR-XPS techniques applied to sulphide mineral surfaces. Int. J. Miner. Process. 1997, 51, 163–170. [Google Scholar] [CrossRef]
- Usher, C.R.; Cleveland, C.A.; Strongin, D.R.; Schoonen, M.A. Origin of oxygen in sulfate during pyrite oxidation with water and dissolved oxygen: An in situ horizontal attenuated total reflectance infrared spectroscopy isotope study. Environ. Sci. Technol. 2004, 38, 5604–5606. [Google Scholar] [CrossRef]
- Heidel, C.; Tichomirowa, M. The isotopic composition of sulfate from anaerobic and low oxygen pyrite oxidation experiments with ferric iron—New insights into oxidation mechanisms. Chem. Geol. 2011, 281, 305–316. [Google Scholar] [CrossRef]
- Bond, P.L.; Smriga, S.P.; Banfield, J.F. Phylogeny of microorganisms populating a thick, subaerial, predominantly lithotrophic biofilm at an extreme acid mine drainage site. Appl. Environ. Microbiol. 2000, 66, 3842–3849. [Google Scholar] [CrossRef] [Green Version]
- Edwards, K.J.; Goebel, B.M.; Rodgers, T.M.; Schrenk, M.O.; Gihring, T.M.; Cardona, M.M.; Mcguire, M.M.; Hamers, R.J.; Pace, N.R.; Banfield, J.F. Geomicrobiology of pyrite (FeS2) dissolution: Case study at Iron Mountain, California. Geomicrobiol. J. 1999, 16, 155–179. [Google Scholar]
- Okibe, N.; Gericke, M.; Hallberg, K.B.; Johnson, D.B. Enumeration and characterization of acidophilic microorganisms isolated from a pilot plant stirred-tank bioleaching operation. Appl. Environ. Microbiol. 2003, 69, 1936–1943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boon, M.; Heijnen, J.J.; Hansford, G.S. The mechanism and kinetics of bioleaching sulphide minerals. Miner. Process. Extr. Metall. Rev. 1998, 19, 107–115. [Google Scholar]
- Nicol, M.J.; Miki, H.; Zhang, S.; Basson, P. The effects of sulfate ions and temperaturemon the leaching of pyrite. 1. Electrochemistry. Hydrometallurgy 2013, 133, 188–196. [Google Scholar] [CrossRef] [Green Version]
- Nicol, M.; Miki, H.; Basson, P. The effects of sulfate ions and temperature on the leaching of pyrite. 2. Dissolution rates. Hydrometallurgy 2013, 133, 182–187. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Jia, Y.; Sun, H.; Tan, Q.; Niu, X.; Leng, X.; Ruan, R. Limited role of sessile acidophiles in pyrite oxidation below redox potential of 650 mV. Sci. Rep. 2017, 7, 5032. [Google Scholar] [CrossRef]
- Dong, B.; Jia, Y.; Tan, Q.; Sun, H.; Ruan, R. Contributions of microbial “contact leaching” to pyrite oxidation under different controlled redox potentials. Minerals 2020, 10, 856. [Google Scholar] [CrossRef]
- Sun, H.Y.; Chen, M.; Zou, L.C.; Shu, R.B.; Ruan, R.M. Study of the kinetics of pyrite oxidation under controlled redox potential. Hydrometallurgy 2015, 155, 13–19. [Google Scholar] [CrossRef]
- Nicholson, R.V.; Gillham, R.W.; Reardon, E.J. Pyrite oxidation in carbonate-buffered solution: 1. Experimental kinetics. Geochim. Cosmochim. Acta 1988, 52, 1077–1085. [Google Scholar] [CrossRef]
- Holmes, P.R.; Crundwell, F.K. The kinetics of the oxidation of pyrite by ferric ions and dissolved oxygen: An electrochemical study. Geochim. Cosmochim. Acta 2000, 64, 263–274. [Google Scholar] [CrossRef]
- Niu, X.P.; Ruan, R.M.; Tan, Q.Y.; Jia, Y.; Sun, H.Y. Study on the second stage of chalcocite leaching in column with redox potential control and its implications. Hydrometallurgy 2015, 155, 141–152. [Google Scholar] [CrossRef]
- Zepeda, V.J.; Cautivo, D.; Galleguillos, P.A.; Salazar, C.N.; Velásquez, A.; Pinilla, C.; Demergasso, C.S. Effect of increased acid concentration on the microbial population inhabiting an industrial heap bioleaching plant. Adv. Mater. Res. 2013, 825, 348–351. [Google Scholar] [CrossRef]
- Todd, E.C.; Sherman, D.M. Surface oxidation of chalcocite (Cu2S) under aqueous (pH = 2–11) and ambient atmospheric conditions: Mineralogy from Cu L- and O K-edge X-ray absorption spectroscopy. Am. Mineral. 2003, 88, 1652–1656. [Google Scholar] [CrossRef]


| Minerals | Formula | Percentage (%) |
|---|---|---|
| Chalcocite | CuS2 | 0.52 |
| Chalcopyrite | CuFeS2 | 0.10 |
| Pyrite | FeS2 | 12.13 |
| Silica | SiO2 | 56.46 |
| Alunite | KAl3(SO4)2(OH)6 | 12.93 |
| Sericite-Illite-Montmorillonite | K2(AlFeMg)4(SiAl)8O20(OH)4∙nH2O, Al4Si8O20(OH)4∙nH2O, Al4SiO4O10(OH)8 | 8.32 |
| Alkali gangue | CaMg(CO3)2, CaCO3 | <1.0 |
| Chemical assay: Cu 0.45%, TFe 6.59% (Fe(III) 0.82%, Fe(II) 5.77%), TS 8.63% | ||
| Processes | Value | |
|---|---|---|
| Acid generation | Pyrite oxidation-Jarosite formation: 4FeS2 + 15O2 + 2H2O→2Fe2(SO4)3 + 2H2SO4 3Fe3+ + 2SO42- + 7H2O→(H3O)Fe3(SO4)2(OH)6↓ + 5H+ | 1.09 kg/kg FeS2 |
| Cu solvent extraction: CuSO4 + 2HR→CuR2 + H2SO4 | 1.54 kg/kg cathode copper | |
| Acid consumption | Gangue mineral acid consumption | 7.5 kg/t ore |
| Chalcocite oxidation: Cu2S + 2Fe3+→Cu2+ + 2Fe2+ + CuS CuS + 2Fe3+→Cu2+ + 2Fe2+ + S0 Cu2S + 5O2 + H2SO4→2CuSO4 + H2O | 0.77 kg/kg cathode copper |
| Genus | Species | Percentage (%) |
|---|---|---|
| Acidithiobacillus | At.ferrooxidans | 57.1 |
| At. thiooxidans | 4.2 | |
| At. caldus | 8.2 | |
| Leptospirillum | L. ferriphilum | 10.5 |
| L. ferrooxidans | 3.6 | |
| Sulfobacillus | S. acidophilus | 1.2 |
| Ferroplasma | F.acidiphilium | 5.2 |
| Acidiphilium | Acidiphiliumsp. | 8.3 |
| Other | 1.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Y.; Sun, H.; Tan, Q.; Xu, J.; Feng, X.; Ruan, R. Industrial Heap Bioleaching of Copper Sulfide Ore Started with Only Water Irrigation. Minerals 2021, 11, 1299. https://doi.org/10.3390/min11111299
Jia Y, Sun H, Tan Q, Xu J, Feng X, Ruan R. Industrial Heap Bioleaching of Copper Sulfide Ore Started with Only Water Irrigation. Minerals. 2021; 11(11):1299. https://doi.org/10.3390/min11111299
Chicago/Turabian StyleJia, Yan, Heyun Sun, Qiaoyi Tan, Jingyuan Xu, Xinliang Feng, and Renman Ruan. 2021. "Industrial Heap Bioleaching of Copper Sulfide Ore Started with Only Water Irrigation" Minerals 11, no. 11: 1299. https://doi.org/10.3390/min11111299
APA StyleJia, Y., Sun, H., Tan, Q., Xu, J., Feng, X., & Ruan, R. (2021). Industrial Heap Bioleaching of Copper Sulfide Ore Started with Only Water Irrigation. Minerals, 11(11), 1299. https://doi.org/10.3390/min11111299





