Human Health Risk Assessment of Trace Elements in Tap Water and the Factors Influencing Its Value
Abstract
:1. Introduction
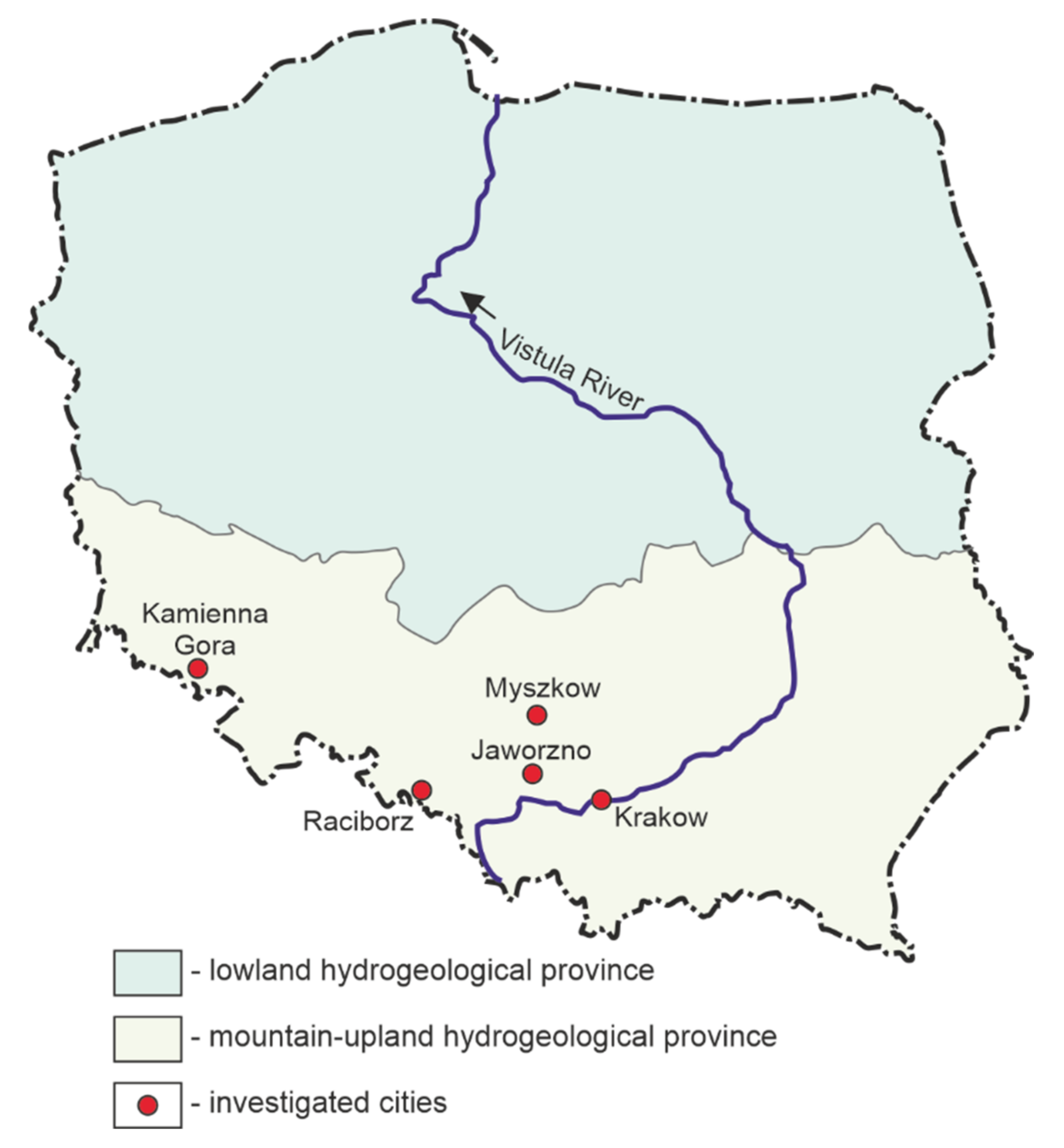
2. Study Area
3. Materials and Methods
3.1. Data Analysis
3.2. Risk Assessment
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Khan, Z.I.; Ahmad, K.; Rehman, S.; Siddique, S.; Bashir, H.; Zafar, A.; Sohail, M.; Ali, S.A.; Cazzato, E.; De Mastro, G. Health risk assessment of heavy metals in wheat using different water qualities: Implication for human health. Environ. Sci. Pollut. Res. 2017, 24, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Krewski, D.; Yokel, R.A.; Nieboer, E.; Borchelt, D.; Cohen, J.; Harry, J.; Kacew, S.; Lindsay, J.; Mahfouz, A.M.; Rondeau, V. Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J. Toxicol. Environ. Health B 2007, 10, 1–269. [Google Scholar] [CrossRef]
- Saha, N.; Rahman, M.S.; Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Industrial metal pollution in water and probabilistic assessment of human health risk. J. Environ. Manag. 2017, 185, 70–78. [Google Scholar] [CrossRef]
- Amirah, M.N.; Afiza, A.S.; Faizal, W.I.W.; Nurliyana, M.H.; Laili, S. Human health risk assessment of metal contamination through consumption of fish. J. Environ. Pollut. Hum. Health 2013, 1, 1–5. [Google Scholar] [CrossRef]
- Giri, S.; Singh, A.K. Human health risk assessment via drinking water pathway due to metal contamination in the groundwater of Subarnarekha River Basin, India. Environ. Monit. Assess. 2015, 187, 63. [Google Scholar] [CrossRef]
- Duda, R.; Zdechlik, R.; Kania, J. Semiquantitative Risk Assessment Method for Groundwater Source Protection Using a Process-based Interdisciplinary Approach. Water Resour. Manag. 2021, 35, 3373–3394. [Google Scholar] [CrossRef]
- Wongsasuluk, P.; Chotpantarat, S.; Siriwong, W.; Robson, M. Heavy metal contamination and human health risk assessment in drinking water from shallow groundwater wells in an agricultural area in Ubon Ratchathani province, Thailand. Environ. Geochem. Health 2014, 36, 169–182. [Google Scholar] [CrossRef]
- Yi, Y.; Yang, Z.; Zhang, S. Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environ. Pollut. 2011, 159, 2575–2585. [Google Scholar] [CrossRef]
- Strzebońska, M.; Gruszecka-Kosowska, A.; Kostka, A. Chemistry and Microbiology of Urban Roof Runoff in Kraków, Poland with Ecological and Health Risk Implications. Appl. Sci. 2020, 10, 8554. [Google Scholar] [CrossRef]
- Gruszecka-Kosowska, A. Significance of Environmental Input Data in Risk Assessment Analyses. J. Xenobiot. 2020, 10, 36–38. [Google Scholar] [CrossRef]
- Fallahzadeh, R.A.; Ghaneian, M.T.; Miri, M.; Dashti, M.M. Spatial analysis and health risk assessment of heavy metals concentration in drinking water resources. Environ. Sci. Pollut. Res. 2017, 24, 24790–24802. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.A.; Zarei, A.; Majidi, S.; Ghaderpoury, A.; Hashempour, Y.; Saghi, M.H.; Alinejad, A.; Yousefi, M.; Hosseingholizadeh, N.; Ghaderpoori, M. Carcinogenic and non-carcinogenic health risk assessment of heavy metals in drinking water of Khorramabad, Iran. MethodsX 2019, 6, 1642–1651. [Google Scholar] [CrossRef] [PubMed]
- Mirzabeygi, M.; Abbasnia, A.; Yunesian, M.; Nodehi, R.N.; Yousefi, N.; Hadi, M.; Mahvi, A.H. Heavy metal contamination and health risk assessment in drinking water of Sistan and Baluchistan, Southeastern Iran. Hum. Ecol. Risk Assess 2017, 23, 1893–1905. [Google Scholar] [CrossRef]
- Directive. Council Directive 98/83/EC of 3 November 1998 on the Quality of Water Intended for Human Consumption. 1998. Available online: http://data.europa.eu/eli/dir/1998/83/oj (accessed on 8 November 2021).
- Regulation. Regulation (EC) No 1882/2003 of the European Parliament and of the Council of 29 September 2003 Adapting to Council Decision 1999/468/EC the Provisions Relating to Committees which Assist the Commission in the Exercise of its Implementing Powers Laid Down in Instruments Subject to the Procedure Referred to in Article 251 of the EC Treaty. 2003. Available online: http://data.europa.eu/eli/reg/2003/1882/oj (accessed on 8 November 2021).
- Regulation. Regulation (EC) No 596/2009 of the European Parliament and of the Council of 18 June 2009 Adapting a Number of Instruments Subject to the Procedure Referred to in Article 251 of the Treaty to Council Decision 1999/468/EC with Regard to the Regulatory Procedure with Scrutiny. Adaptation to the Regulatory Procedure with Scrutiny—Part Four. 2009. Available online: http://data.europa.eu/eli/reg/2009/596/oj (accessed on 8 November 2021).
- Directive. Commission Directive (EU) 2015/1787 of 6 October 2015 Amending Annexes II and III to Council Directive 98/83/EC on the Quality of Water Intended for Human Consumption. 2005. Available online: http://data.europa.eu/eli/dir/2015/1787/oj (accessed on 8 November 2021).
- RMH. Regulation of the Minister of the Health of 7th December 2017 on the Quality of Water Intended for Human Consumption. J. Law 2017, 2294. Available online: https://oeil.secure.europarl.europa.eu/oeil/popups/ficheprocedure.do?lang=enreference=2017/0332(COD) (accessed on 8 November 2021).
- Directive. Directive (EU) 2020/2184 of the European Parliament and of the Council on the Quality of Water Intended for Human Consumption (Recast). 2020. Available online: https://eur-lex.europa.eu/eli/dir/2020/2184/oj (accessed on 21 March 2021).
- Rusiniak, P.; Wątor, K.; Kmiecik, E.; Postawa, A. The influence of selected factors on the concentration of heavy metals in drinking water. In Proceedings of the International Multidisciplinary Scientific GeoConference: Science and Technologies in Geology, Exploration and Mining, Albena, Bulgaria, 29 June–5 July 2017; Volume 17, pp. 921–928. [Google Scholar]
- Wątor, K.; Kmiecik, E.; Postawa, A. Analysis of factors influencing changes of drinking water chemical composition. Przegląd Geol. 2017, 65, 1388–1392. (In Polish) [Google Scholar]
- ISO. ISO 5667-5:2006—Water Quality—Sampling—Part 5: Guidance on Sampling of Drinking Water from Treatment Works and Piped Distribution Systems; International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- Postawa, A. Best Practice Guide on Sampling and Monitoring of Metals in Drinking Water; IWA Publishing: London, UK, 2012; 156p. [Google Scholar]
- Boyd, G.R.; Pierson, G.L.; Kirmeyer, G.J.; Britton, M.D.; English, R.J. Lead release from new end-use plumbing components in Seattle Public Schools. J. Am. Water Work. Ass. 2008, 100, 105–114. [Google Scholar] [CrossRef]
- Cartier, C.; Arnold, R.B., Jr.; Triantafyllidou, S.; Prévost, M.; Edwards, M. Effect of flow rate and lead/copper pipe sequence on lead release from service lines. Water Res. 2012, 46, 4142–4152. [Google Scholar] [CrossRef] [PubMed]
- Hayes, C. Best Practice Guide on the Control of Lead in Drinking Water; IWA Publishing: London, UK, 2010; 85p. [Google Scholar]
- Bhattacharya, P.; Polya, D.; Jovanovic, D. Best Practice Guide on the Control of Arsenic in Drinking Water; IWA Publishing: London, UK, 2017; 300p. [Google Scholar]
- Postawa, A.; Hayes, C. Best Practice Guide on the Control of Iron and Manganese in Water Supply; IWA Publishing: London, UK, 2017; 96p. [Google Scholar]
- Toczyłowska, B. Wpływ Instalacji Wodociągowych Z Miedzi Na Jakość Wody; Europejski Instytut Miedzi: Wrocław, Poland, 2016; pp. 1–32. (In Polish) [Google Scholar]
- Mika, A.; Sekuła, K.; Dendys, M.; Ptaszek, W.; Postawa, A. Drinking of tap water is smart, but how do it better?—A tap water quality research. In Proceedings of the E3S Web of Conferences, Krakow, Poland, 12–13 September 2017; Volume 30, p. 01007. [Google Scholar] [CrossRef] [Green Version]
- Postawa, A. The Influence of Sampling Methodology on the Results of Metals Determination in Groundwaters and Drinking Waters. In Water Supply and Water Quality; Dymaczewski, Z., Jeż-Walkowiak, J., Nowak, M., Eds.; Polskie Zrzeszenie Inżynierów i Techników Sanitarnych: Poznań, Poland, 2014; pp. 349–366. [Google Scholar]
- Wątor, K.; Mika, A.; Postawa, A. Influence of Installation Materials and Water. In Water Supply and Water Quality; Dymaczewski, Z., Jeż-Walkowiak, J., Nowak, M., Eds.; Polskie Zrzeszenie Inżynierów i Techników Sanitarnych: Poznań, Poland, 2016; pp. 877–889. (In Polish) [Google Scholar]
- Dong, W.; Zhang, Y.; Quan, X. Health risk assessment of heavy metals and pesticides: A case study in the main drinking water source in Dalian, China. Chemosphere 2019, 242, 125113. [Google Scholar] [CrossRef]
- Hu, G.; Rana, A.; Mian, H.R.; Saleem, S.; Mohseni, M.; Jasim, S.; Hewage, K.; Sadiq, R. Human health risk-based life cycle assessment of drinking water treatment for heavy metal (loids) removal. J. Clean Prod. 2020, 267, 121980. [Google Scholar] [CrossRef]
- Hashmi, M.Z.; Yu, C.; Shen, H.; Duan, D.; Shen, C.; Lou, L.; Chen, Y. Concentrations and Human Health Risk Assessment of Selected Heavy Metals in Surface Water of the Siling Reservoir Watershed in Zhejiang Province, China. Pol. J. Environ. Stud. 2014, 23, 801–811. [Google Scholar]
- Ukah, B.U.; Egbueri, J.C.; Unigwe, C.O.; Ubido, O.E. Extent of heavy metals pollution and health risk assessment of groundwater in a densely populated industrial area, Lagos, Nigeria. Int. J. Energy Water Res. 2019, 3, 291–303. [Google Scholar] [CrossRef]
- Jehan, S.; Khattak, S.A.; Muhammad, S.; Ali, L.; Rashid, A.; Hussain, M.L. Human health risks by potentially toxic metals in drinking water along the Hattar Industrial Estate, Pakistan. Environ. Sci. Pollut. Res. 2020, 27, 2677–2690. [Google Scholar] [CrossRef] [PubMed]
- Aithani, D.; Jyethi, D.S.; Siddiqui, Z.; Yadav, A.K.; Khillare, P.S. Source apportionment, pollution assessment, and ecological and human health risk assessment due to trace metals contaminated groundwater along urban river floodplain. Groundw. Sustain. Dev. 2020, 11, 100445. [Google Scholar] [CrossRef]
- Bodrud-Doza, M.; Islam, S.D.U.; Rume, T.; Quraishi, S.B.; Rahman, M.S.; Bhuiyan, M.A.H. Groundwater quality and human health risk assessment for safe and sustainable water supply of Dhaka City dwellers in Bangladesh. Groundw. Sustain. Dev. 2020, 10, 100374. [Google Scholar] [CrossRef]
- Ji, Y.; Wu, J.; Wang, Y.; Elumalai, V.; Subramani, T. Seasonal variation of drinking water quality and human health risk assessment in Hancheng City of Guanzhong Plain, China. Expos. Health 2020, 12, 469–485. [Google Scholar] [CrossRef]
- Janoska, O.; Gruszecka-Kosowska, A. Water quality and human health risk assessment: A case study of the Czarna Przemsza River source in Zawiercie, Poland. Hum. Ecol. Risk Assess. Int. J. 2020, 26, 757–781. [Google Scholar] [CrossRef]
- Al-Husseini, A.H.E. Risk assessment of heavy metals in tap drinking water in different age group; in Baghdad city, Iraq. Mesop. Environ. J. 2018, 4, 89–102. [Google Scholar]
- Tian, Y.; Li, J.; Jia, S.; Zhao, W. Co-release potential and human health risk of heavy metals from galvanized steel pipe scales under stagnation conditions of drinking water. Chemosphere 2021, 267, 129270. [Google Scholar] [CrossRef]
- Singh, K.P.; Malik, A.; Singh, V.K.; Mohan, D.; Sinha, S. Chemometric analysis of groundwater quality data of alluvial aquifer of Gangetic plain, North India. Anal. Chim. Acta 2005, 550, 82–91. [Google Scholar] [CrossRef]
- Raju, N.J.; Patel, P.; Gurung, D.; Ram, P.; Gossel, W.; Wycisk, P. Geochemical assessment of groundwater quality in the Dun valley of central Nepal using chemometric method and geochemical modeling. Groundw. Sustain. Dev. 2015, 1, 135–145. [Google Scholar] [CrossRef]
- Li, L.; He, Z.; Shields, M.R.; Bianchi, T.S.; Pain, A.; Stoffella, P.J. Partial least squares analysis to describe the interactions between sediment properties and water quality in an agricultural watershed. J. Hydrol. 2018, 566, 386–395. [Google Scholar] [CrossRef]
- Singh, K.P.; Malik, A.; Basant, N.; Saxena, P. Multi-way partial least squares modeling of water quality data. Anal. Chim. Acta 2007, 584, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Apetrei, C.; Iticescu, C.; Georgescu, L.P. Multisensory System Used for the Analysis of the Water in the Lower Area of River Danube. Nanomaterials 2019, 9, 891. [Google Scholar] [CrossRef] [Green Version]
- Siepak, M.; Sojka, M. Application of multivariate statistical approach to identify trace elements sources in surface waters: A case study of Kowalskie and Stare Miasto reservoirs, Poland. Environ. Monit. Assess. 2017, 189, 364. [Google Scholar] [CrossRef] [Green Version]
- Singh, K.P.; Malik, A.; Mohan, D.; Sinha, S.; Singh, V.K. Chemometric data analysis of pollutants in wastewater—A case study. Anal. Chim. Acta 2005, 532, 15–25. [Google Scholar] [CrossRef]
- Sridharan, M.; Nathan, D.S. Chemometric tool to study the mechanism of arsenic contamination in groundwater of Puducherry region, South East coast of India. Chemosphere 2018, 208, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Bucci, A.; Franchino, E.; De Luca, D.A.; Lasagna, M.; Malandrino, M.; Prevot, A.B.; Bianco Prevot, A.; Hernandez Sac, H.O.; Coyoy, I.M.; Sac Escobar, E.O.; et al. Groundwater chemistry characterization using multi-criteria approach: The upper Samalá River basin (SW Guatemala). J. South Am. Earth. Sci. 2017, 78, 150–163. [Google Scholar] [CrossRef]
- Yu, Y.; Song, X.; Zhang, Y.; Zheng, F.; Liang, J.; Han, D.; Ma, Y.; Bu, H. Identification of key factors governing chemistry in groundwater near the water course recharged by reclaimed water at Miyun County, Northern China. J. Environ. Sci. 2013, 25, 1754–1763. [Google Scholar] [CrossRef]
- Elumalai, V.; Nwabisa, D.P.; Rajmohan, N. Evaluation of high fluoride contaminated fractured rock aquifer in South Africa–Geochemical and chemometric approaches. Chemosphere 2019, 235, 1–11. [Google Scholar] [CrossRef]
- Wątor, K.; Kmiecik, E.; Lipiec, I. The use of principal component analysis for the assessment of the spatial variability of curative waters from the Busko-Zdrój and Solec-Zdrój region (Poland)–preliminary results. Water Suppl. 2018, 19, 1137–1143. [Google Scholar] [CrossRef]
- Rwoo, M.A.; Juahir, H.; Roslan, N.M.; Endut, A.; Kamarudin, M.K.A.; Amran, M.A. Assessment of drinking water quality using principal component analysis and partial least square discriminant analysis: A case study at water treatment plants, Selangor. J. Fundam. Appl. Sci. 2017, 9, 157–173. [Google Scholar] [CrossRef] [Green Version]
- Garboś, S.; Święcicka, D. Application of bimodal distribution to the detection of changes in uranium concentration in drinking water collected by random daytime sampling method from a large water supply zone. Chemosphere 2015, 138, 377–382. [Google Scholar] [CrossRef]
- Hyk, W.; Święcicka, D.; Garboś, S. Application of mixed (bimodal) distribution to human health risk assessment of Cu and Ni in drinking water collected by RDT sampling method from a large water supply zone. Microchem. J. 2013, 110, 465–472. [Google Scholar] [CrossRef]
- Górski, J.; Siepak, M. Metals and related substances in drinking water at consumers in Poznań. Biul. Państwowego Inst. Geol. 2011, 445, 139–148. (In Polish) [Google Scholar]
- Górski, J.; Siepak, M. Metals in Drinking Water at Consumers in the Light of Investigations Conducted in the Area of Poznań, Szczecin and Choszczno. In Water Supply and Water Quality; Dymaczewski, Z., Jeż-Walkowiak, J., Eds.; Zrzeszenie Inżynierów i Techników Sanitarnych: Poznań, Poland, 2012; pp. 317–335. (In Polish) [Google Scholar]
- Górski, J.; Siepak, M. Assessment of metal concentrations in tap-water–from source to the tap: A case study from Szczecin, Poland. Geologos 2014, 20, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Górski, J.; Siepak, M. Metals in Drinking Water at the Consumers Based on the Study Conducted in Selected Cities in Poland. In Oldest Hydrogeologists of the World; Belaruskaya Navuka: Minsk, Belarus, 2016; pp. 155–168. [Google Scholar]
- Górski, J.; Siepak, M.; Garboś, S.; Święcicka, D. Preliminary Assessment of Metal Concentrations in Drinking Water in the City of Szczecin (Poland): Human Health Aspects. In Metals and Related Substances in Drinking Water; Hayes, C., Ersoz, M., Barott, L., Postawa, A., Bhattacharya, P., Rosborg, I., Sandhi, A., Joao Benoliel, M., Eds.; IWA Publishing: London, UK, 2012; pp. 91–99. [Google Scholar]
- Blazejczyk, K. Natural and Human Environment of Poland. A Geographical Overview. In Climate and Bioclimate of Poland; Institute of Geography and Spatial Organization, Polish Academy of Sciences: Warszawa, Poland, 2006; pp. 31–48. [Google Scholar]
- Postawa, A.; Witczak, S. (Eds.) Metals and Related Substances in Drinking Water in Poland; IWA Publishing: London, UK, 2011. [Google Scholar]
- Witczak, S.; Szczepański, A.; Mikołajków, J.; Skrzypczyk, L. Protection of groundwater quality and quantity of strategic groundwater resources of the Major Groundwater Basins. Przegląd Geol. 2010, 58, 754–761. [Google Scholar]
- ISO. ISO 5667-3:2018 Water Quality—Sampling—Part 3: Preservation and Handling of Water Samples; International Organization for Standardization: Geneva, Switzerland, 2018. [Google Scholar]
- ISO. ISO 10523:2008 Water Quality—Determination of pH; International Organization for Standardization: Geneva, Switzerland, 2008. [Google Scholar]
- ISO. EN 27888:1993. Water Quality—Determination of Electrical Conductivity; International Organization for Standardization: Geneva, Switzerland, 1993. [Google Scholar]
- ISO. ISO 17294-2:2016 Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)—Part 2: Determination of Selected Elements Including Uranium Isotopes; International Organization for Standardization: Geneva, Switzerland, 2016. [Google Scholar]
- ISO. ISO 11885:2007 Water Quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES); International Organization for Standardization: Geneva, Switzerland, 2007. [Google Scholar]
- Tabachnick, B.; Fidell, L.S. Using Multivarite Statistics, 6th ed.; Pearson: Boston, MA, USA, 2012. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 8 November 2021).
- Egbueri, J.C.; Mgbenu, C.N. Chemometric analysis for pollution source identification and human health risk assessment of water resources in Ojoto Province, southeast Nigeria. Appl. Water Sci. 2020, 10, 98. [Google Scholar] [CrossRef] [Green Version]
- USEPA. Risk Assessment Guidance for Superfund. In Human Health Evaluation Manual (Part A); Office of Emergency and Remedial Respons: Washington, DC, USA, 1989; Volume 1. [Google Scholar]
- Egbueri, J.C. Heavy metals pollution source identification and probabilistic health risk assessment of shallow groundwater in Onitsha. Anal. Lett. 2020, 53, 1620–1638. [Google Scholar] [CrossRef]
- USEPA. Exposure Factors Handbook; Office of Research and Development, National Center for Environmental Assessment: Washington, DC, USA, 1997. [Google Scholar]
- USEPA. Exposure Factors Handbook; (EPA/600/R-09/052F); National Center for Environmental Assessment: Washington, DC, USA, 2011. [Google Scholar]
- Bortey-Sam, N.; Nakayama, S.M.; Ikenaka, Y.; Akoto, O.; Baidoo, E.; Mizukawa, H.; Ishizuka, M. Health risk assessment of heavy metals and metalloid in drinking water from communities near gold mines in Tarkwa, Ghana. Environ. Monit. Assess. 2015, 187, 397. [Google Scholar] [CrossRef]
- USEPA. Supplemental Guidance for Developing Soil Screening Levels for Superfund Sites; OSWER 9355; Office of Emergency and Remedial Response: Washington, DC, USA, 2002. [Google Scholar]
- Hayes, C. Internal Corrosion Control of Water Supply Systems; IWA Publishing: London, UK, 2012; 92p. [Google Scholar]
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Driscoll, C.T.; Letterman, R.D. Chemistry and fate of Al (III) in treated drinking water. J. Environ. Eng. 1988, 114, 21–37. [Google Scholar] [CrossRef]
- Sollars, C.J.; Bragg, S.; Simpson, A.M.; Perry, R. Aluminium in European drinking water. Environ. Technol. 1989, 10, 131–150. [Google Scholar] [CrossRef]
- Cui, F.Y. Investigation on aluminum concentration in drinking water in part of China’s cities. China Water Wastewater 2002, 18, 5–8. [Google Scholar]
- Alam, I.A.; Sadiq, M. Metal contamination of drinking water from corrosion of distribution pipes. Environ. Pollut. 1989, 57, 167–178. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Q.; Song, L.; Shi, B. Risk assessment of heavy metals in pipe scales and loose deposits formed in drinking water distribution systems. Sci. Total Environ. 2019, 652, 1387–1395. [Google Scholar] [CrossRef]
- Sarin, P.; Snoeyink, V.L.; Bebee, J.; Jim, K.K.; Beckett, M.A.; Kriven, W.M.; Clement, J.A. Iron release from corroded iron pipes in drinking water distribution systems: Effect of dissolved oxygen. Water Res. 2004, 38, 1259–1269. [Google Scholar] [CrossRef]
- Lytle, D.A.; Schock, M.R. Stagnation Time, Composition, pH, and Orthophosphate Effects on Metal Leaching from Brass; National Risk Management Research Laboratory, Office of Research and Development, US Environmental Protection Agency: Cincinnati, OH, USA, 1996; 184p.
- Sarver, E.; Zhang, Y.; Edwards, M. Review of brass dezincification corrosion in potable water systems. Corros. Rev. 2010, 28, 155–196. [Google Scholar] [CrossRef]
- Kimbrough, D.E. Brass corrosion as a source of lead and copper in traditional and all-plastic distribution systems. J. Am. Water Work. Ass. 2007, 99, 70–76. [Google Scholar] [CrossRef]
- Ng, D.Q.; Chen, C.Y.; Lin, Y.P. A new scenario of lead contamination in potable water distribution systems: Galvanic corrosion between lead and stainless steel. Sci. Total Environ. 2018, 637, 1423–1431. [Google Scholar] [CrossRef]
- Cartier, C.; Nour, S.; Richer, B.; Deshommes, E.; Prévost, M. Impact of water treatment on the contribution of faucets to dissolved and particulate lead release at the tap. Water Res. 2012, 46, 5205–5216. [Google Scholar] [CrossRef] [PubMed]
- Doré, E.; Deshommes, E.; Laroche, L.; Nour, S.; Prévost, M. Lead and copper release from full and partially replaced harvested lead service lines: Impact of stagnation time prior to sampling and water quality. Water Res. 2019, 150, 380–391. [Google Scholar] [CrossRef]
- Elfland, C.; Scardina, P.; Edwards, M. Lead-contaminated water from brass plumbing devices in new buildings. J. Am. Water Work. Ass. 2010, 102, 66–76. [Google Scholar] [CrossRef]
- Lytle, D.A.; Schock, M.R. Impact of stagnation time on metal dissolution from plumbing materials in drinking water. J. Water Supply Res. 2000, 49, 243–257. [Google Scholar] [CrossRef]
- Zlatanović, L.; Van Der Hoek, J.P.; Vreeburg, J.H.G. An experimental study on the influence of water stagnation and temperature change on water quality in a full-scale domestic drinking water system. Water Res. 2017, 123, 761–772. [Google Scholar] [CrossRef]
- Sakamoto, A.; Yamasaki, T.; Matsumura, M. Erosion-corrosion tests on copper alloys for water tap use. Wear 1995, 186, 548–554. [Google Scholar] [CrossRef]
- Andersen, K.E.; Nielsen, G.D.; Flyvholm, M.A.; Fregert, S.; Gruvberge, B. Nickel in tap water. Contact Dermat. 1983, 9, 140–143. [Google Scholar] [CrossRef]
- Gonzalez, S.; Lopez-Roldan, R.; Cortina, J.L. Presence of metals in drinking water distribution networks due to pipe material leaching: A review. Toxicol. Environ. Chem. 2013, 95, 870–889. [Google Scholar] [CrossRef]
- McNeill, L.S.; Edwards, M. Iron pipe corrosion in distribution systems. J. Am. Water Work. Ass. 2001, 93, 88–100. [Google Scholar] [CrossRef]
- Zietz, B.P.; Richter, K.; Laß, J.; Suchenwirth, R.; Huppmann, R. Release of metals from different sections of domestic drinking water installations. Water Qual. Expo. Health 2015, 7, 193–204. [Google Scholar] [CrossRef]
- Zhang, Y.; Griffin, A.; Edwards, M. Effect of Nitrification on Corrosion of Galvanized Iron, Copper, and Concrete. J. Am. Water Work. Ass. 2010, 102, 83–93. [Google Scholar] [CrossRef]
- Clark, B.N.; Masters, S.V.; Edwards, M.A. Lead release to drinking water from galvanized steel pipe coatings. Environ. Eng. Sci. 2015, 32, 713–721. [Google Scholar] [CrossRef]





| City | Geological Setting |
|---|---|
| Myszkow | Carbonate series of Middle Triassic (Muschelkalk), formed as cracked karstic fissured dolomites and limestones |
| Jaworzno | Formations of carbonate series of Lower and Middle Triassic and sandstone series of Upper Carboniferous |
| Raciborz | Pleistocene groundwater reservoir with sandy and gravely formations |
| Kamienna Gora | Pleistocene groundwater reservoir with gravely formations, covered with loams, silts, and contemporary river alluvia |
| City | Water Source | Number of Samples | TDS [mg/L] | pH | EC [µS/cm] | Ca2+ [mg/L] | Mg2+ [mg/L] | SO42− [mg/L] | HCO3− [mg/L] | Water Treatment Process |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | ||||||||||
| Krakow | Surface water | 101 | 178 ± 19 | 7.1 ± 0.4 | 294 ± 24 | 41.7 ± 5.0 | 5.8 ± 0.7 | 45.8 ± 7.7 | 111 ± 12 | ozonation coagulation sedimentation filtration disinfection |
| Jaworzno | groundwater | 100 | 450 ± 37 | 7.5 ± 0.1 | 760 ± 17 | 74.9 ± 8.0 | 40.4 ± 3.8 | 123 ± 16 | 266 ± 18 | disinfection |
| Kamienna Gora | 100 | 175 ± 7 | 6.8 ± 0.1 | 270 ± 6 | 32.6 ± 2.1 | 9.1 ± 0.9 | 62.2 ± 7.2 | 81.7 ± 6.5 | untreated | |
| Raciborz | 100 | 315 ± 46 | 7.4 ± 0.2 | 560 ± 64 | 82.6 ± 13.2 | 16.1 ± 3.1 | 47.8 ± 8.0 | 274 ± 43 | aeration filtration disinfection | |
| Myszkow | 100 | 346 ± 21 | 7.3 ± 0.1 | 568 ± 26 | 71.0 ± 9.0 | 35.5 ± 4.3 | 58.1 ± 14.4 | 322 ± 15 | aeration disinfection (if necessary) | |
| Factor | Categories |
|---|---|
| City | Krakow Jaworzno Kamienna Gora Raciborz Myszkow |
| Kind of household | public building block of flats house |
| Age of connection | 10 years 10–30 years 30 years |
| Age of pipe-work | 10 years 10–30 years 30 years |
| Age of tap | 10 years 10–30 years 30 years |
| Stagnation time | 1 h 1 h |
| Material of connection | stainless steel iron plastic galvanised steel other |
| Material of pipe-work | stainless steel copper iron plastic galvanised steel other |
| Material of appliance | stainless steel other |
| Water rating by end-user | good unacceptable |
| Parameters | Unit | Value | |
|---|---|---|---|
| Children | Adult | ||
| IRW | L | 1 | 2 |
| EF | day/year | 365 | 365 |
| ED | Year | 6 | 30 |
| BW | Kg | 15 | 70 |
| AT (non-carcinogenic) | Days | 2190 | 25,550 |
| AT (carcinogenic) | Days | 25,550 | |
| RfD (As) | mg/kg/day | 0.0003 | |
| RfD (Al) | 1 | ||
| RfD (Cd) | 0.001 | ||
| RfD (Cr) | 0.003 | ||
| RfD (Cu) | 0.04 | ||
| provisional RfD (Fe) | 0.7 | ||
| RfD (Mn) | 0.14 | ||
| RfD (Ni) | 0.02 | ||
| RfD (Pb) | 0.014 | ||
| RfD (Zn) | 0.3 | ||
| SF (As) | µg/g/day | 1.5 | |
| SF (Al) | not assessed | ||
| SF (Cd) | 6.3 | ||
| SF (Cr) | 0.5 | ||
| SF (Cu) | not assessed | ||
| SF (Fe) | not assessed | ||
| SF (Mn) | not assessed | ||
| SF (Ni) | 1.7 | ||
| SF (Pb) | 0.0085 | ||
| SF (Zn) | not assessed | ||
| Variable | Parametric Value [7] [µg/L] | Guideline Value [47] [µg/L] | Mean [µg/L] | 5% Trimmed Mean [µg/L] | Median [µg/L] | Standard Deviation [µg/L] | Minimum [µg/L] | Maximum [µg/L] |
|---|---|---|---|---|---|---|---|---|
| As | 10 | 10 | 0.72 | 0.70 | 0.65 | 0.38 | 0.18 | 3.39 |
| Cr | 50 | 50 | 4.85 | 4.64 | 4.45 | 2.97 | 0.03 | 44.26 |
| Zn | — 1 | — | 650 | 525 | 365 | 930 | 5 | 11,377 |
| Al | 200 | — | 11.64 | 10.52 | 3.19 | 17.42 | 0.15 | 149.4 |
| Cd | 5 | 3 | 0.53 | 0.42 | 0.18 | 0.74 | 0.002 | 4.73 |
| Mn | 50 | — | 16.26 | 12.00 | 6.08 | 28.34 | 0.04 | 336.9 |
| Cu | 2000 | 2000 | 38.21 | 18.92 | 11.01 | 118.1 | 0.09 | 1610.0 |
| Ni | 20 | 70 | 3.73 | 2.28 | 1.93 | 19.91 | 0.02 | 433.8 |
| Pb | 10 | 10 | 5.43 | 4.21 | 1.77 | 8.68 | 0.10 | 75.69 |
| Fe | 200 | — | 202 | 159 | 104 | 282 | 13 | 2331 |
| Element | Statistic | CDI (Non-CarcinoGenic) | HQ | HI | CR | ||||
|---|---|---|---|---|---|---|---|---|---|
| Children | Adults | Children | Adults | Children | Adults | Children | Adults | ||
| As | range | 1.2 × 10–5–2.3 × 10−4 | 2.2 × 10–6–4.2 × 10−5 | 4.0 × 10−2–7.5 × 10−1 | 7.3 × 10−3–1.4 × 10−1 | range: 9.3 × 10−2–4.4 mean: 5.8 × 10−1 | range: 1.7 × 10−2–8.1 × 10−1 mean: 1.1 × 10−1 | 1.5 × 10−6–2.9 × 10−5 | 3.3 × 10−6–6.2 × 10−5 |
| mean | 4.8 × 10−5 | 8.8 × 10−6 | 1.6 × 10−1 | 2.9 × 10−2 | 6.2 × 10−6 | 1.3 × 10−5 | |||
| Cr | range | n.c. –3 × 10–3 | n.c. –5.4 × 10−4 | n.c.–9.8 × 10−1 | n.c. –1.8 × 10−1 | n.c. –1.3 × 10−4 | n.c. –2.7 × 10−4 | ||
| mean | 3.2 × 10−4 | 5.9 × 10−5 | 1.1 × 10−1 | 2.0 × 10−2 | 1.4 × 10−5 | 3.0 × 10−5 | |||
| Zn | range | 3.1 × 10−4-7.6 × 10−1 | 5.7E × 10−5–1.4 × 10−1 | 1.0 × 10−3–2.53 | 1.9 × 10−4–4.6 × 10−1 | not assessed | |||
| mean | 4.3 × 10−2 | 8.0 × 10−3 | 1.4 × 10−1 | 2.7 × 10−2 | |||||
| Al | range | 1.0 × 10−5–1.0 × 10−2 | 1.9 × 10−6–1.8 × 10−3 | 1.0 × 10−5–1.0 × 10−2 | 1.9 × 10−6–1.8 × 10−3 | not assessed | |||
| mean | 8.4 × 10−4 | 1.5 × 10−4 | 8.4 × 10−4 | 1.5 × 10−4 | |||||
| Cd | range | 1.3 × 10−7–3.2 × 10−4 | 2.4 × 10−8–5.8 × 10−5 | 1.3 × 10−4–3.2 × 10−1 | 2.4 × 10−5–6.02 × 10−2 | 7.2 × 10−8–1.7 × 10−4 | 1.5 × 10−7–3.7 × 10−4 | ||
| mean | 3.5 × 10−5 | 6.5 × 10−6 | 3.5 × 10−2 | 6.5 × 10−3 | 1.9 × 10−5 | 4.1 × 10−5 | |||
| Mn | range | 2.9 × 10−6–2.3 × 10−2 | 5.3 × 10−7–4.1 × 10−3 | 2.0 × 10−5–1.6 × 10−1 | 3.8 × 10−6–3.0 × 10−2 | not assessed | |||
| mean | 1.1 × 10−3 | 2.0 × 10−4 | 7.7 × 10−3 | 1.4 × 10−3 | |||||
| Cu | range | 5.9 × 10−6–1.1 × 10−1 | 1.1 × 10−6–2.0 × 10−2 | 1.5 × 10−4–2.7 | 2.7 × 10−5–4.9 × 10−1 | not assessed | |||
| mean | 2.6 × 10−3 | 4.7 × 10−4 | 6.4 × 10−2 | 1.2 × 10−2 | |||||
| Ni | range | n.c. –2.9 × 10−2 | n.c. –5.3 × 10−3 | n.c. –1.5 | n.c. –2.7 × 10−1 | n.c. –4.2 × 10−3 | n.c. –9.0 × 10−3 | ||
| mean | 2.5 × 10−4 | 4.6 × 10−5 | 1.2 × 10−2 | 2.3 × 10−3 | 4.0 × 10−5 | 8.0 × 10−5 | |||
| Pb | range | n.c. –5.1 × 10−3 | n.c. –9.3 × 10−5 | n.c. –3.6 × 10−1 | n.c. –6.6 × 10−2 | n.c. –3.7 × 10−6 | n.c. –7.9 × 10−6 | ||
| mean | 3.6 × 10−4 | 6.6 × 10−5 | 2.6 × 10−2 | 4.7 × 10−3 | 2.6 × 10−7 | 5.6 × 10−7 | |||
| Fe | range | n.c. –1.6 × 10−1 | n.c. –2.9 × 10−2 | n.c. –2.2 × 10−1 | n.c. –4.0 × 10−2 | not assessed | |||
| mean | 1.3 × 10−2 | 2.5 × 10−3 | 1.9 × 10−2 | 3.5 × 10−3 | |||||
| Factor | Variables | MANOVA p-Value |
|---|---|---|
| City | Zn, Mn | p 0.05 |
| Age of connection | As, Cr, Cu, Ni | p 0.05 |
| Age of pipe-work | Cr, Cu, Ni | p 0.05 |
| Age of tap | Cr, Zn, Fe | p 0.05 |
| Material of connection | As, Zn, Cu, Ni, Pb, Fe | p 0.05 |
| Material of pipe-work | Zn, Ni, Fe | p 0.05 |
| Material of appliance | Zn, Cu, Ni, Fe | p 0.05 |
| Water rating by end-user | Cr, Zn, Ni, Fe | p 0.05 |
| City | Element | HQ | HI | CR | |||
|---|---|---|---|---|---|---|---|
| Children | Adults | Children | Adults | Children | Adults | ||
| Jaworzno | As | 1.6 × 10−1 | 3.0 × 10−2 | 7.8 × 10−1 | 1.4 × 10−1 | 6.2 × 10−6 | 1.3 × 10−5 |
| Cr | 1.6 × 10−1 | 2.9 × 10−2 | 2.0 × 10−5 | 4.3 × 10−5 | |||
| Zn | 2.4 × 10 | 4.4 × 10−2 | n.a. | n.a. | |||
| Al | 1.7 × 10−3 | 3.2 × 10−4 | n.a. | n.a. | |||
| Cd | 8.2 × 10−2 | 1.5 × 10−2 | 4.4 × 10−5 | 9.4 × 10−5 | |||
| Mn | 5.0 × 10−3 | 9.2 × 10−4 | n.a. | n.a. | |||
| Cu | 3.7 × 10−2 | 6.8 × 10−3 | n.a. | n.a. | |||
| Ni | 9.1 × 10−2 | 1.7 × 10−3 | 2.6 × 10−5 | 5.7 × 10−5 | |||
| Pb | 6.9 × 10−2 | 1.3 × 10−2 | 7.0 × 10−7 | 1.5 × 10−6 | |||
| Fe | 2.2 × 10−2 | 4.1 × 10−3 | n.a. | n.a. | |||
| Kamienna Gora | As | 6.2 × 10−2 | 1.1 × 10−2 | 6.2 × 10−1 | 1.1 × 10−1 | 2.4 × 10−6 | 5.2 × 10−6 |
| Cr | 9.4 × 10−2 | 1.7 × 10−2 | 1.2 × 10−5 | 2.6 × 10−5 | |||
| Zn | 1.8 × 10−1 | 3.4 × 10−2 | n.a. | n.a. | |||
| Al | 2.0 × 10−4 | 3.6 × 10−5 | n.a. | n.a. | |||
| Cd | 2.5 × 10−2 | 4.5 × 10−3 | 1.3 × 10−5 | 2.9 × 10−5 | |||
| Mn | 1.2 × 10−2 | 2.2 × 10−3 | n.a. | n.a. | |||
| Cu | 1.8 × 10−1 | 3.2 × 10−2 | n.a. | n.a. | |||
| Ni | 2.3 × 10−2 | 4.2 × 10−3 | 6.6 × 10−5 | 1.4 × 10−4 | |||
| Pb | 2.7 × 10−2 | 4.9 × 10−3 | 2.7 × 10−7 | 5.8 × 10−7 | |||
| Fe | 2.2 × 10−2 | 4.0 × 10−3 | n.a. | n.a. | |||
| Krakow | As | 1.7 × 10−1 | 3.1 × 10−2 | 5.1 × 10−1 | 9.4 × 10−2 | 6.6 × 10−6 | 1.4 × 10−5 |
| Cr | 1.1 × 10−1 | 2.0 × 10−2 | 1.4 × 10−5 | 3.0 × 10−5 | |||
| Zn | 1.1 × 10−1 | 2.1 × 10−2 | n.a. | n.a. | |||
| Al | 2.0 × 10−3 | 3.6 × 10−4 | n.a. | n.a. | |||
| Cd | 1.9 × 10−2 | 3.6 × 10−3 | 1.1 × 10−5 | 2.3 × 10−5 | |||
| Mn | 2.0 × 10−3 | 3.6 × 10−4 | n.a. | n.a. | |||
| Cu | 6.7 × 10−2 | 1.2 × 10−2 | n.a. | n.a. | |||
| Ni | 7.5 × 10−3 | 1.4 × 10−3 | 2.2 × 10−5 | 1.4 × 10−3 | |||
| Pb | 8.8 × 10−3 | 1.6 × 10−3 | 9.0 × 10−8 | 1.9 × 10−7 | |||
| Fe | 1.3 × 10−2 | 2.5 × 10−3 | n.a. | n.a. | |||
| Myszkow | As | 1.7 × 10−1 | 3.2 × 10−2 | 5.5 × 10−1 | 1.0 × 10−1 | 6.7 × 10−6 | 1.4 × 10−5 |
| Cr | 9.6 × 10−2 | 1.8 × 10−2 | 1.2 × 10−5 | 2.7 × 10−5 | |||
| Zn | 1.4 × 10−1 | 2.6 × 10−2 | n.a. | n.a. | |||
| Al | 2.4 × 10−4 | 4.4 × 10−5 | n.a. | n.a. | |||
| Cd | 4.1 × 10−2 | 7.6 × 10−3 | 2.2 × 10−5 | 4.8 × 10−5 | |||
| Mn | 1.9 × 10−2 | 3.4 × 10−3 | n.a. | n.a. | |||
| Cu | 1.2 × 10−2 | 2.2 × 10−3 | n.a. | n.a. | |||
| Ni | 7.4 × 10−3 | 1.4 × 10−3 | 2.2 × 10−5 | 4.6 × 10−5 | |||
| Pb | 2.1 × 10−2 | 3.8 × 10−3 | 2.1 × 10−7 | 4.5 × 10−7 | |||
| Fe | 3.3 × 10−2 | 6.0 × 10−3 | n.a. | n.a. | |||
| Raciborz | As | 2.3 × 10−1 | 4.3 × 10−2 | 4.3 × 10−1 | 7.9 × 10−2 | 9.0 × 10−6 | 1.9 × 10−5 |
| Cr | 8.9 × 10−2 | 1.6 × 10−2 | 1.1 × 10−5 | 2.5 × 10−5 | |||
| Zn | 4.6 × 10−2 | 8.4 × 10−3 | n.a. | n.a. | |||
| Al | 8.0 × 10−5 | 1.5 × 10−5 | n.a. | n.a. | |||
| Cd | 9.3 × 10−3 | 1.7 × 10–3 | 5.0 × 10−6 | 1.1 × 10−5 | |||
| Mn | 8.7 × 10−4 | 1.6 × 10−4 | n.a. | n.a. | |||
| Cu | 2.7 × 10−2 | 4.9 × 10−3 | n.a. | n.a. | |||
| Ni | 1.6 × 10−2 | 2.9 × 10−3 | 4.6 × 10−5 | 9.8 × 10−5 | |||
| Pb | 5.4 × 10−3 | 1.0 × 10−3 | 5.5 × 10−8 | 1.2 × 10−7 | |||
| Fe | 5.9 × 10−3 | 1.1 × 10−3 | n.a. | n.a. | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wątor, K.; Rusiniak, P.; Martyna, A.; Kmiecik, E.; Postawa, A. Human Health Risk Assessment of Trace Elements in Tap Water and the Factors Influencing Its Value. Minerals 2021, 11, 1291. https://doi.org/10.3390/min11111291
Wątor K, Rusiniak P, Martyna A, Kmiecik E, Postawa A. Human Health Risk Assessment of Trace Elements in Tap Water and the Factors Influencing Its Value. Minerals. 2021; 11(11):1291. https://doi.org/10.3390/min11111291
Chicago/Turabian StyleWątor, Katarzyna, Piotr Rusiniak, Agnieszka Martyna, Ewa Kmiecik, and Adam Postawa. 2021. "Human Health Risk Assessment of Trace Elements in Tap Water and the Factors Influencing Its Value" Minerals 11, no. 11: 1291. https://doi.org/10.3390/min11111291
APA StyleWątor, K., Rusiniak, P., Martyna, A., Kmiecik, E., & Postawa, A. (2021). Human Health Risk Assessment of Trace Elements in Tap Water and the Factors Influencing Its Value. Minerals, 11(11), 1291. https://doi.org/10.3390/min11111291







