A Combined EPMA and LA-ICP-MS Investigation on Bi-Cu-Au Mineralization from the Kizhnica Ore Field (Vardar Zone, Kosovo)
Abstract
:1. Introduction
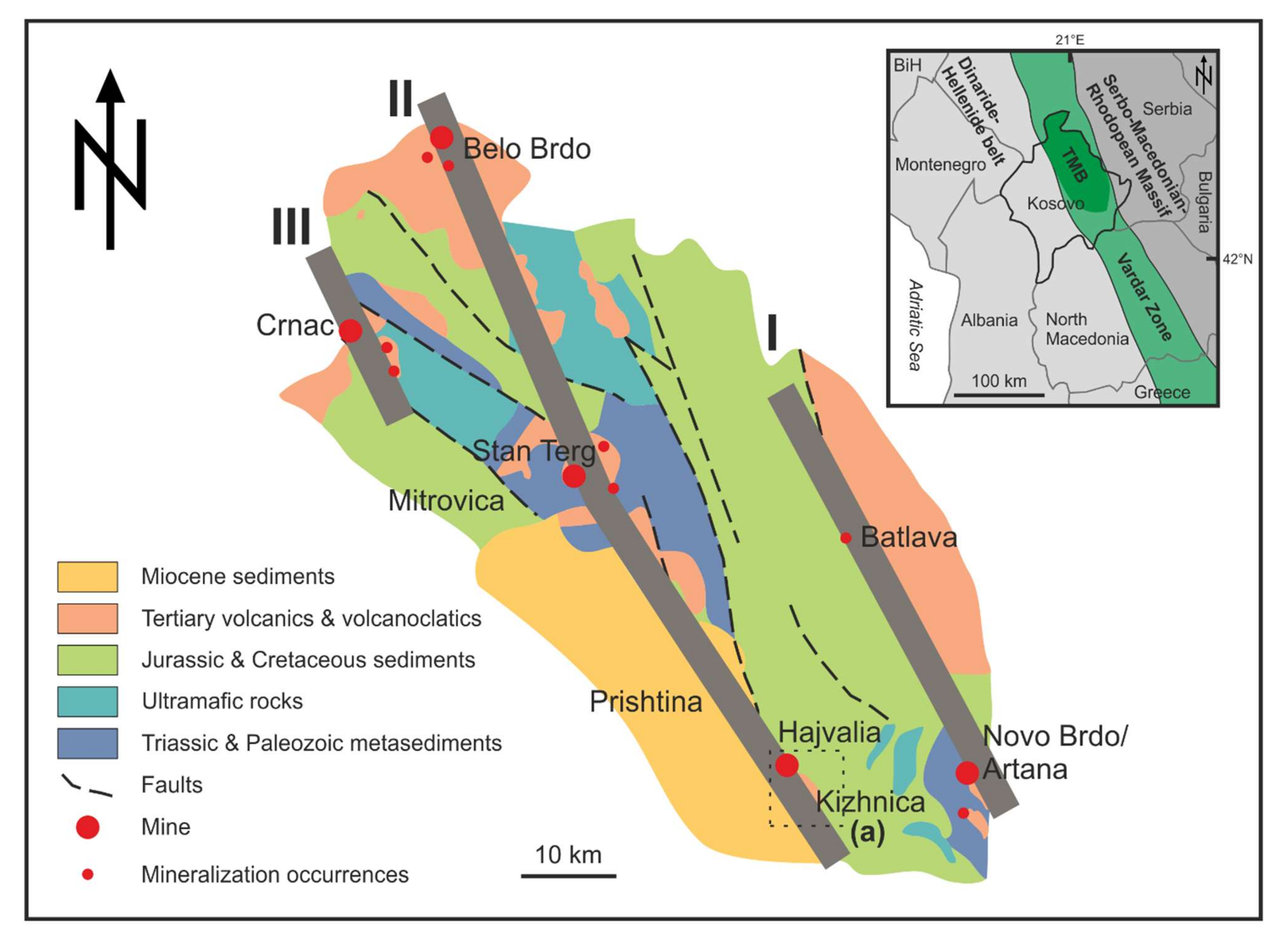
2. Geological Setting
2.1. Trepça Mineral Belt
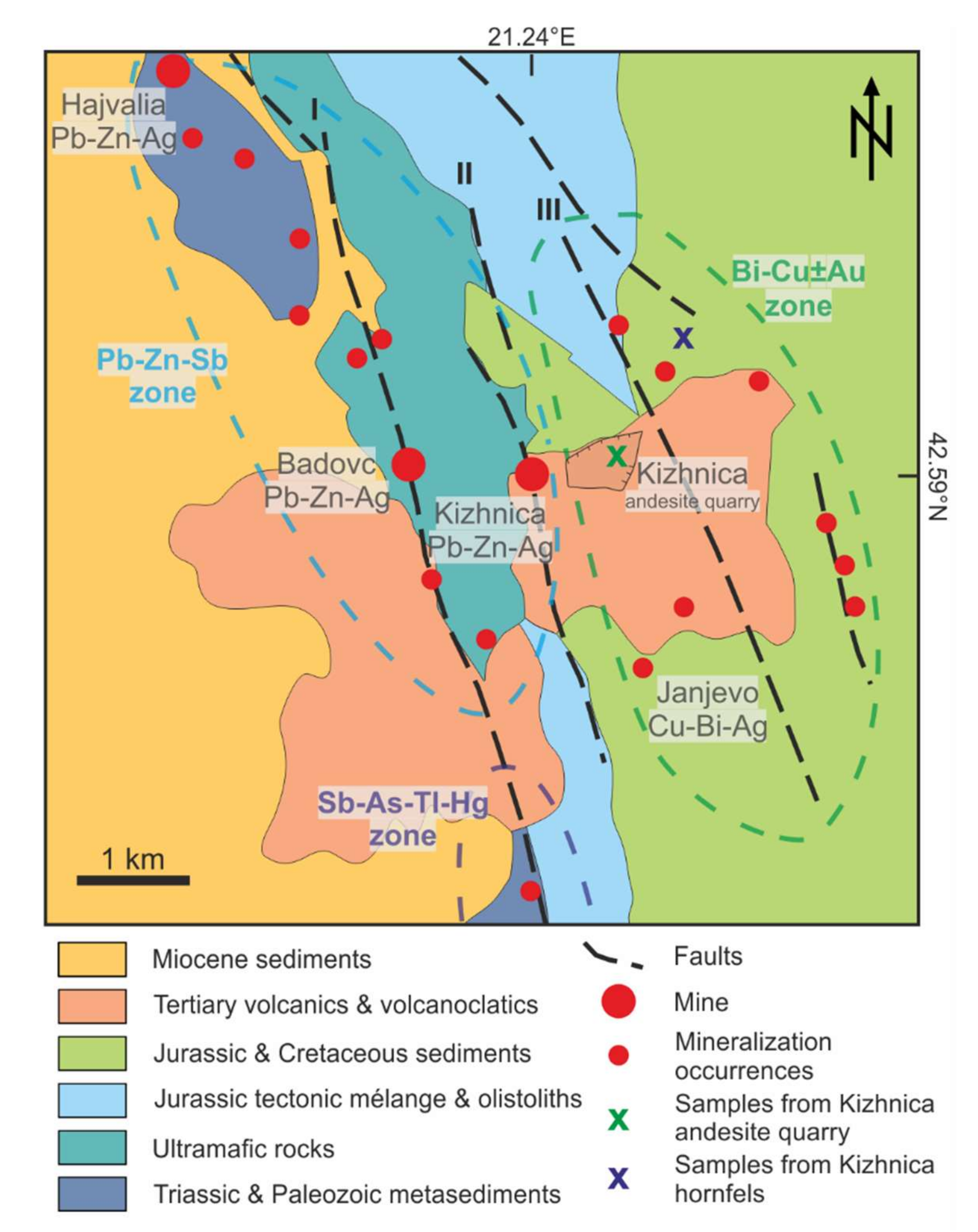
2.2. Kizhnica-Hajvalia-Badovc Ore Field
3. Samples and Methods
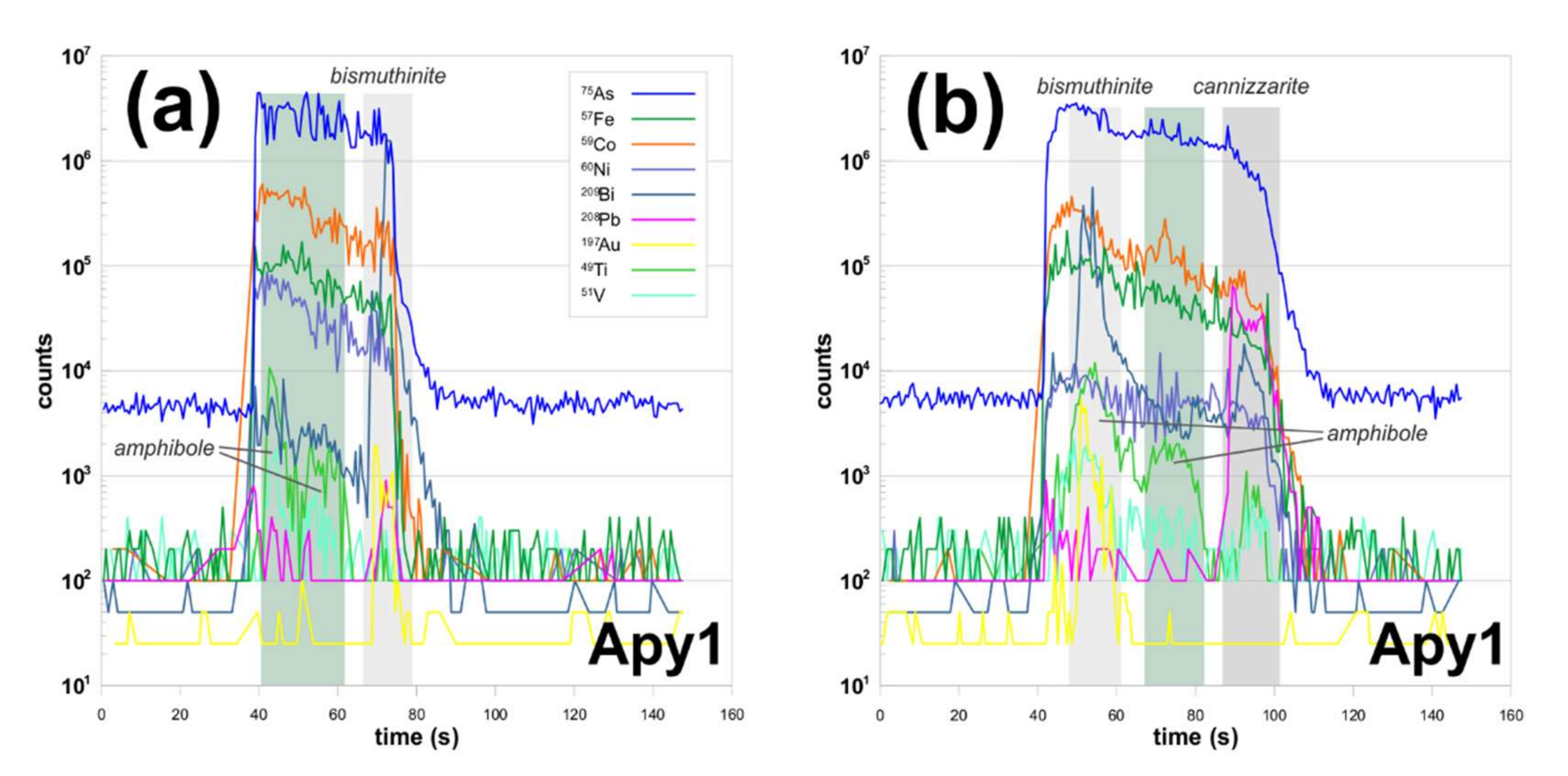
4. Results
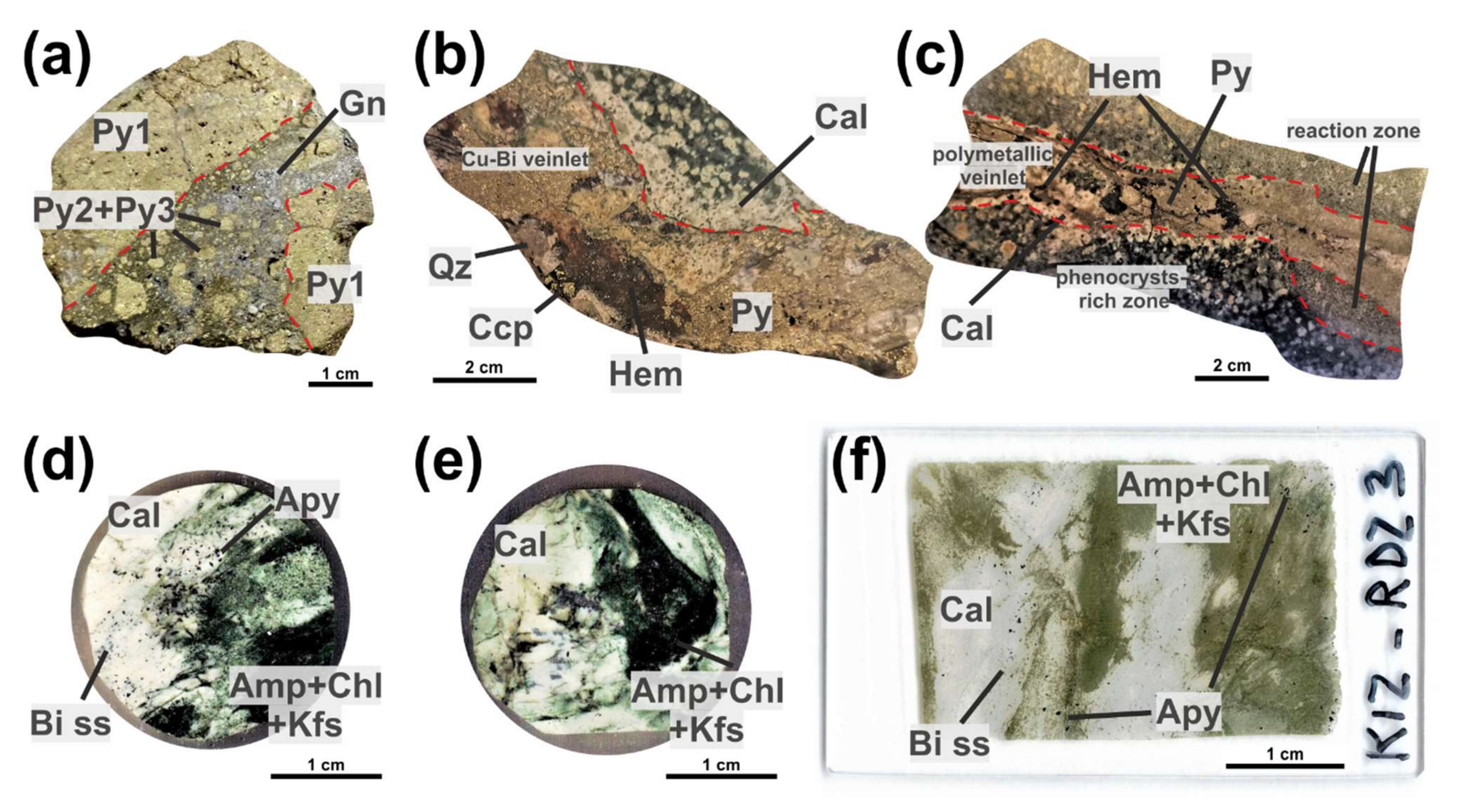
4.1. Kizhnica Quarry
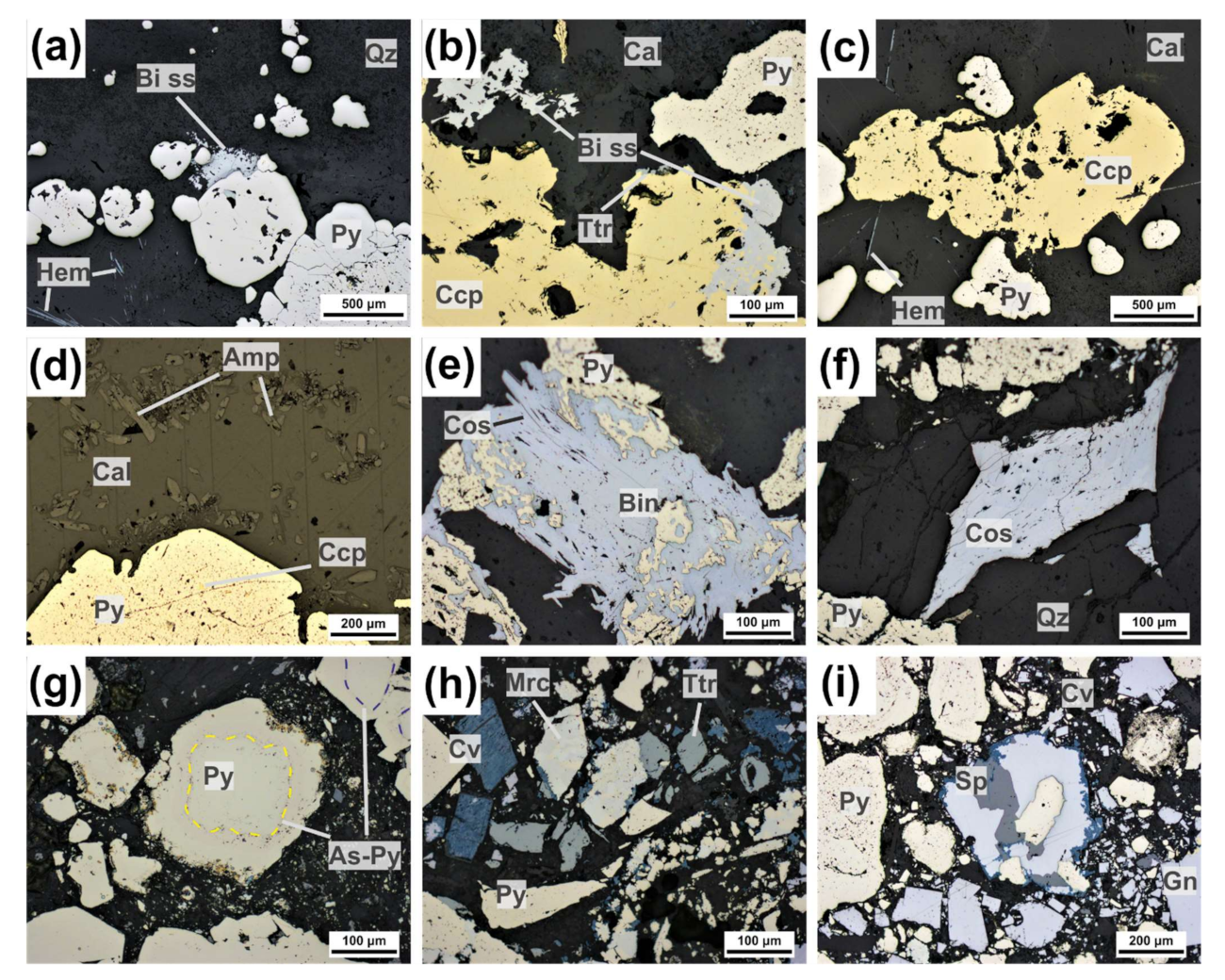
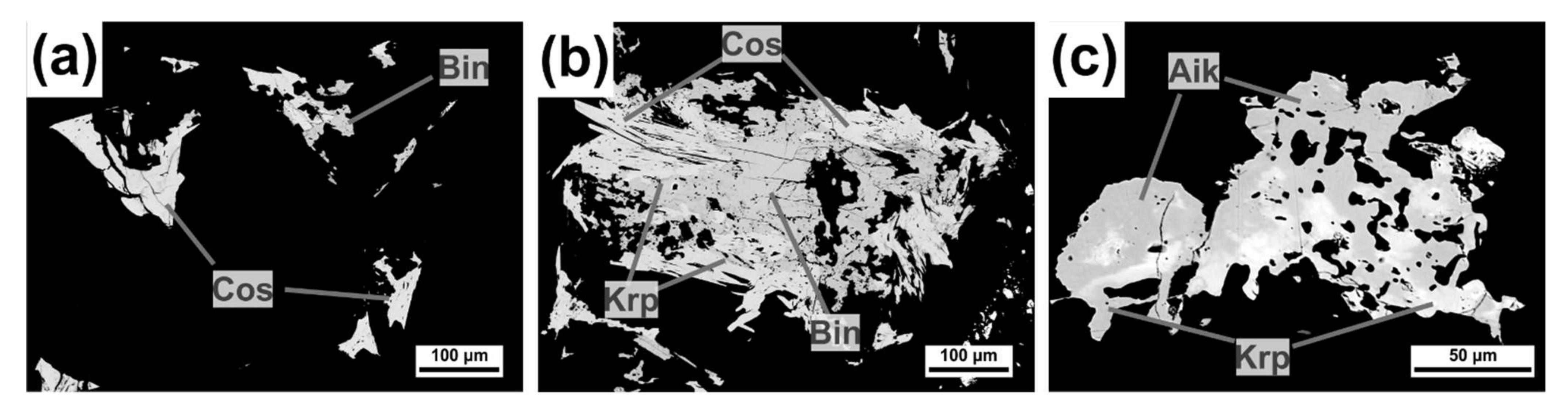
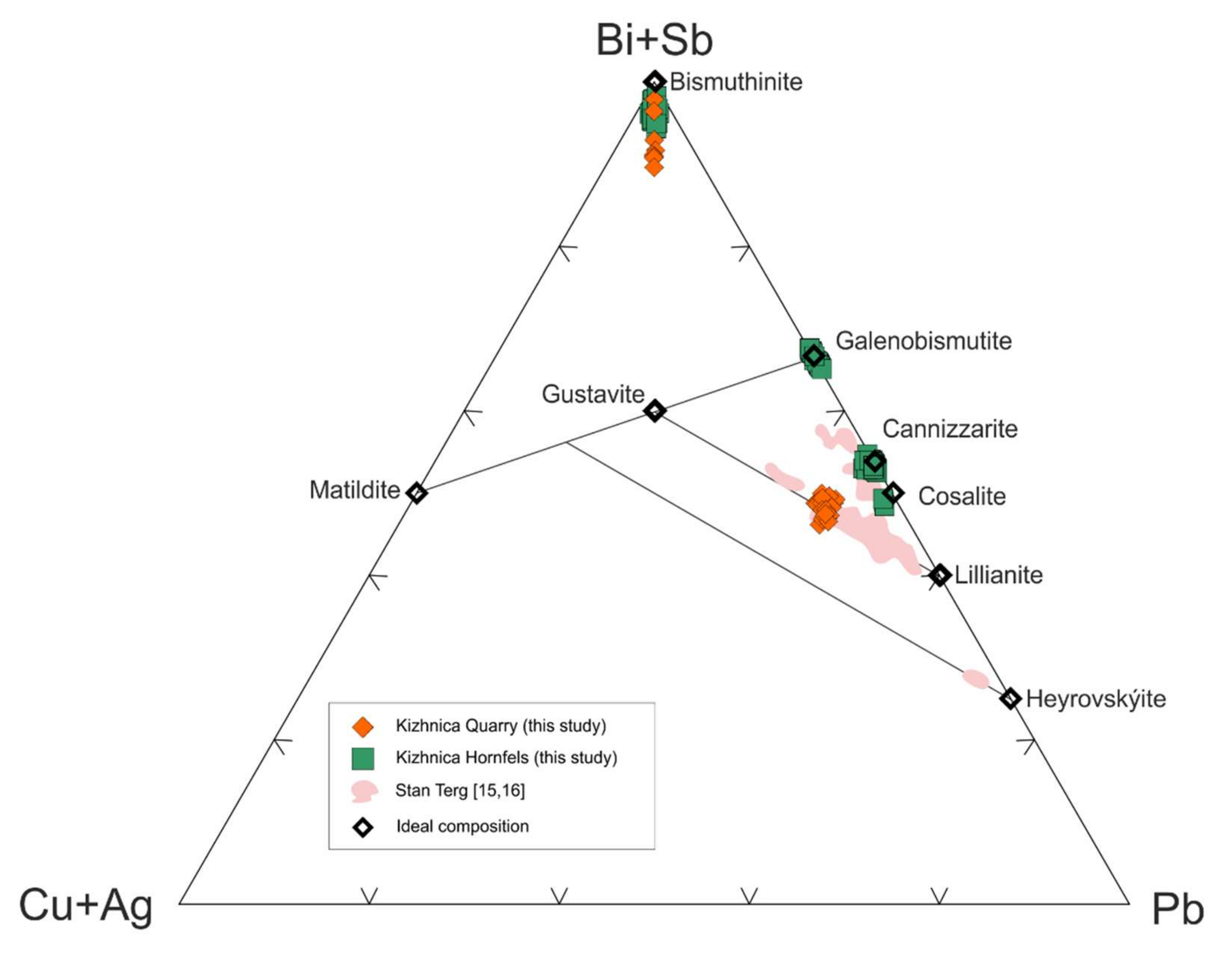
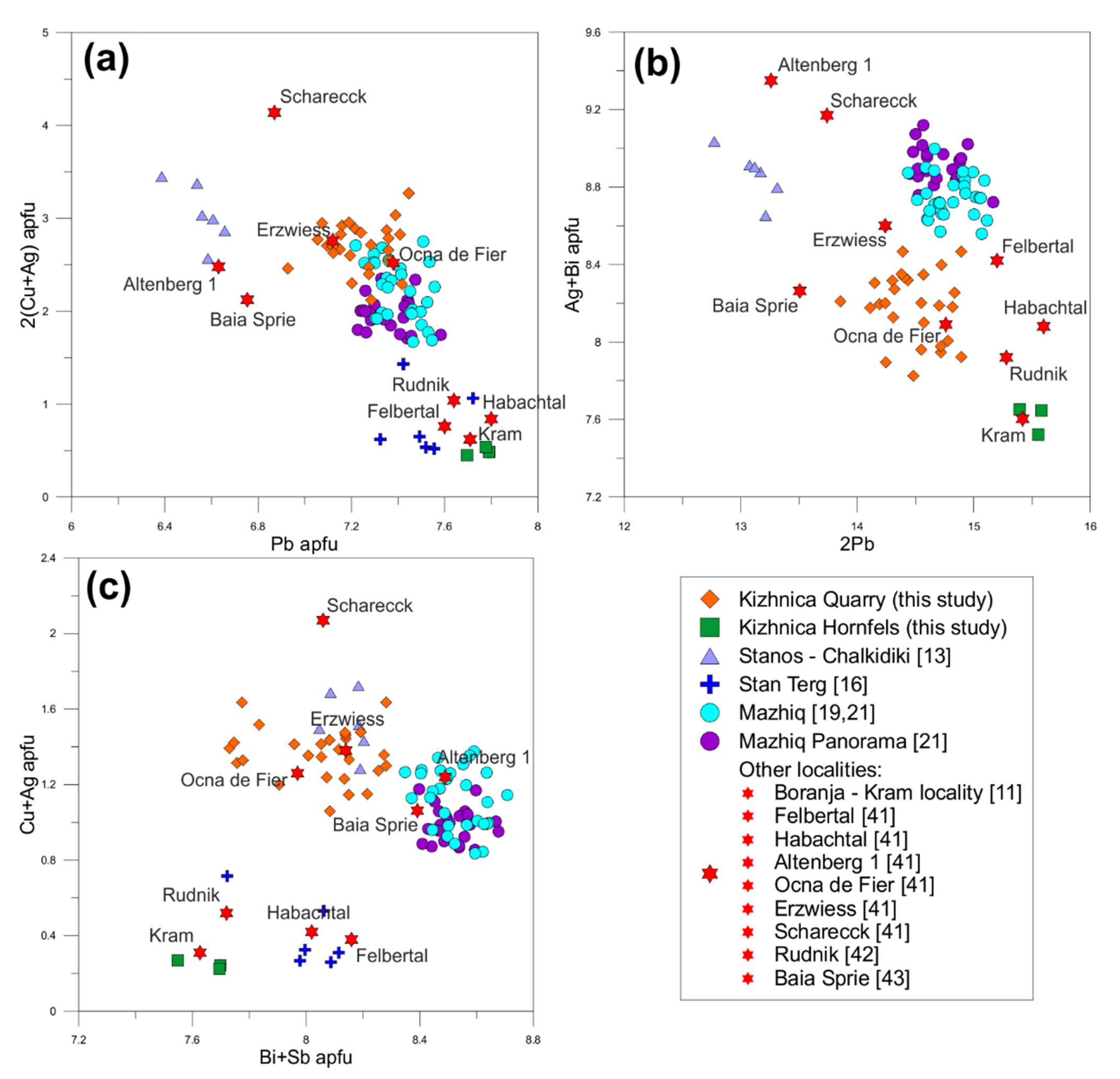
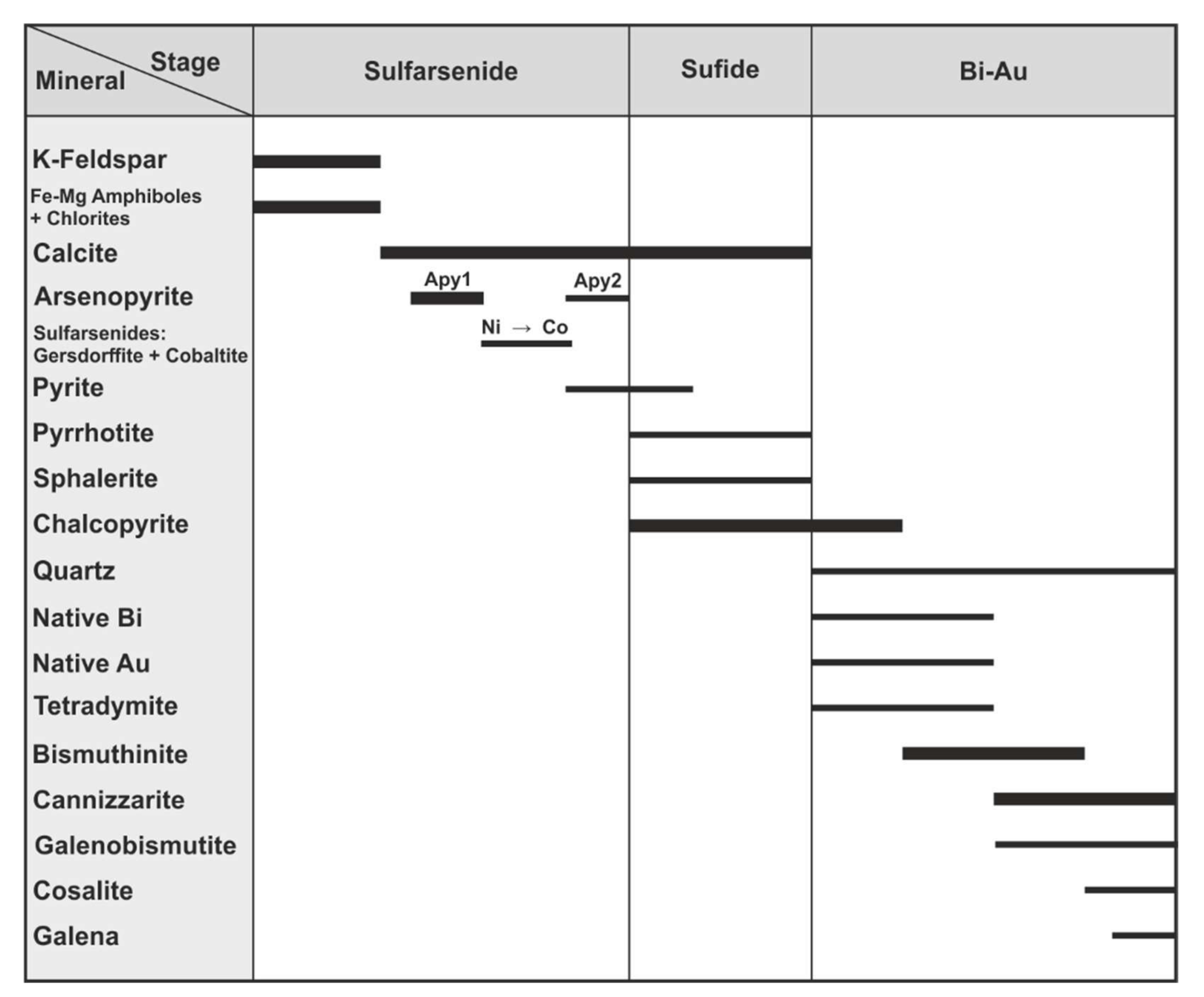
4.1.1. Bismuth Sulfosalts
Bismuthinite-Aikinite Series
Cosalite
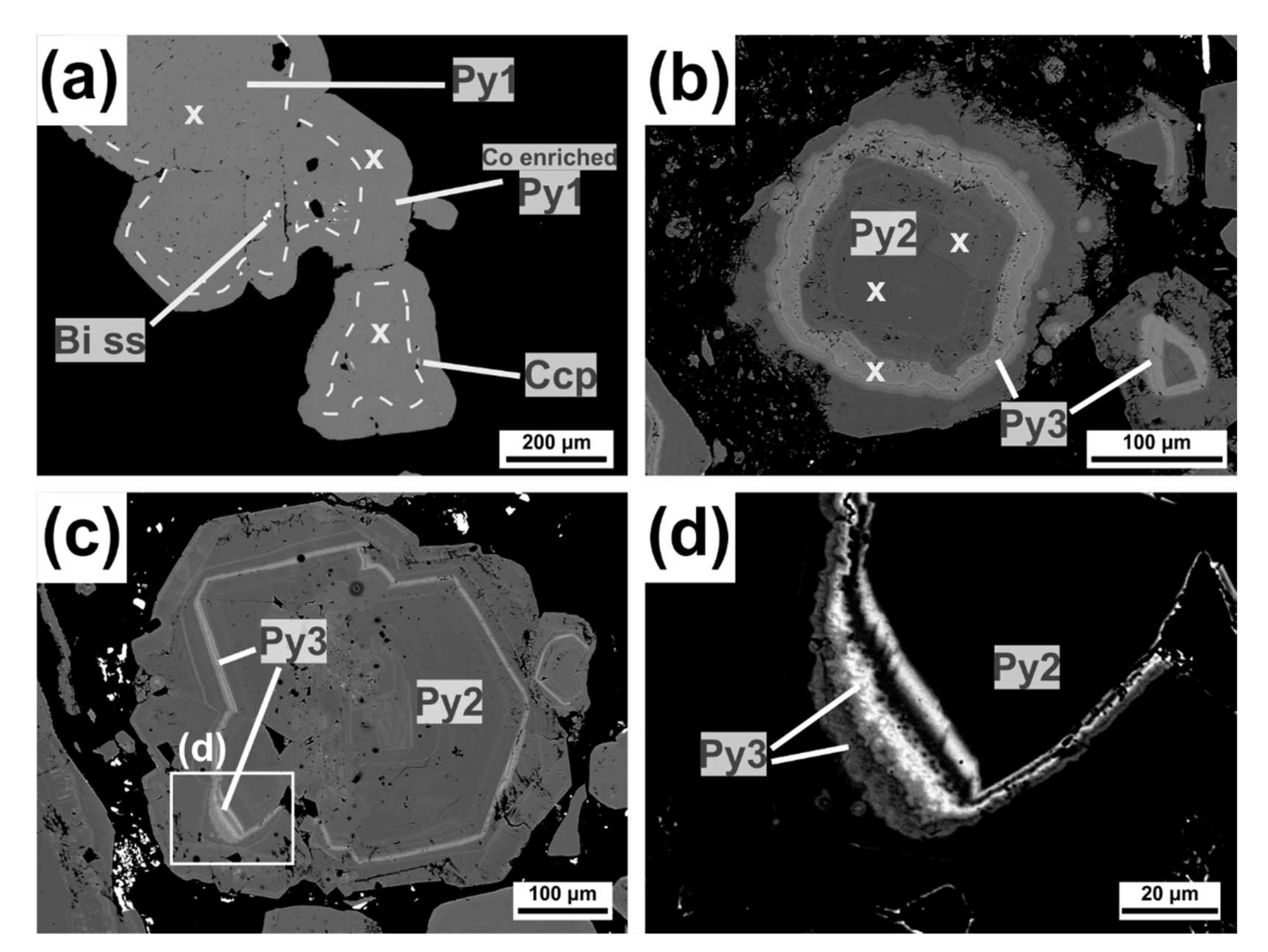
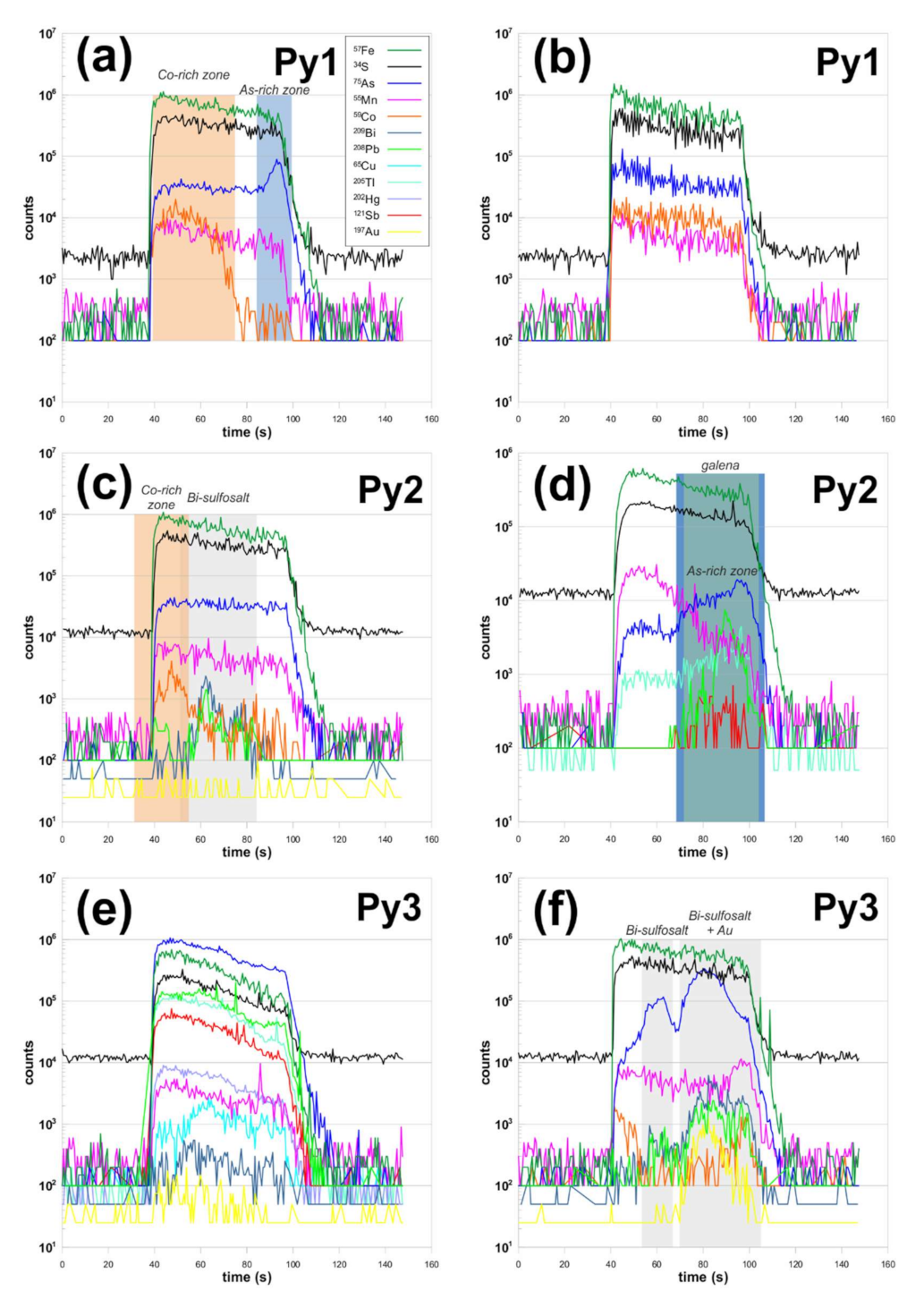
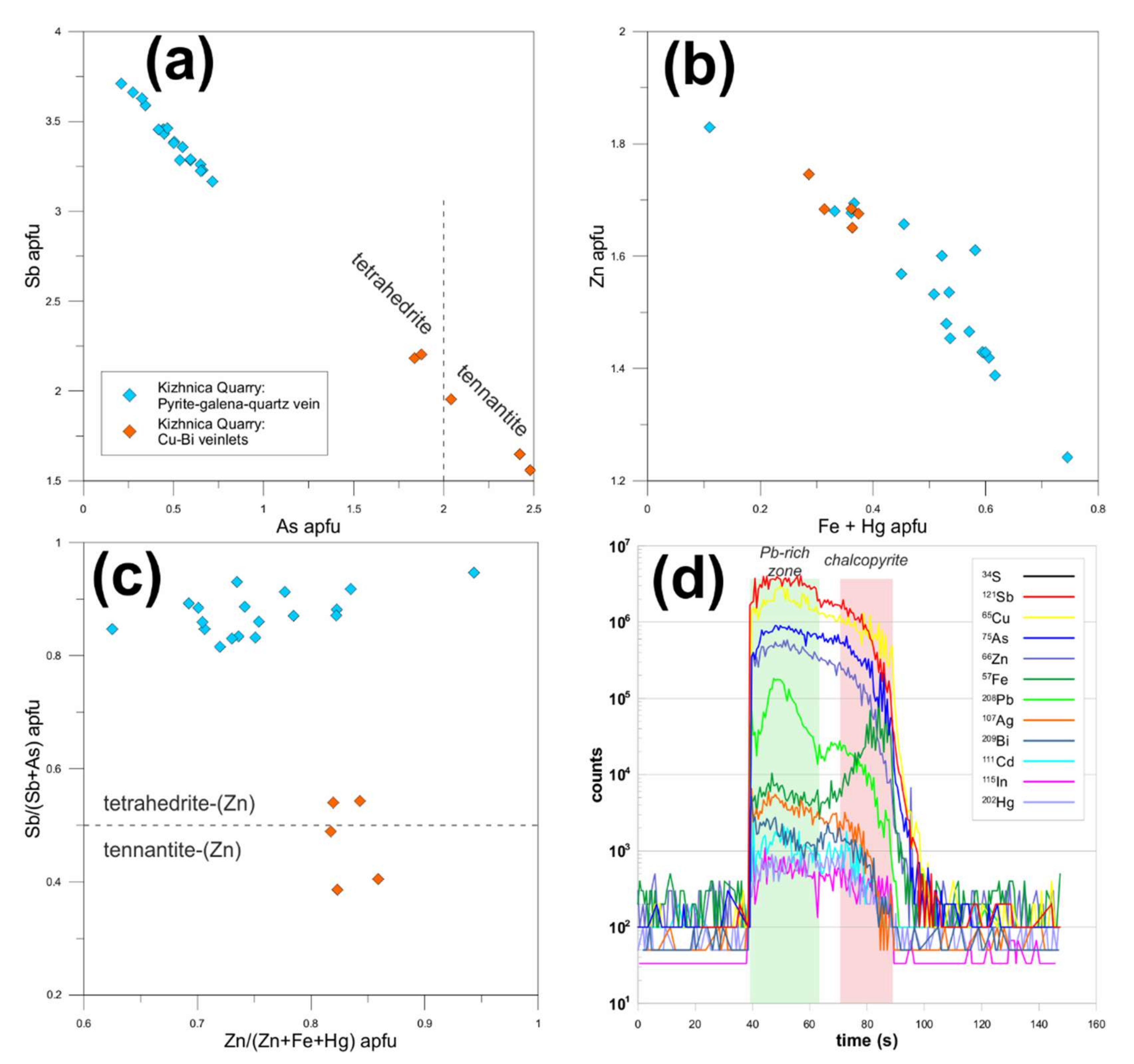
4.1.2. Pyrite
4.1.3. Chalcopyrite
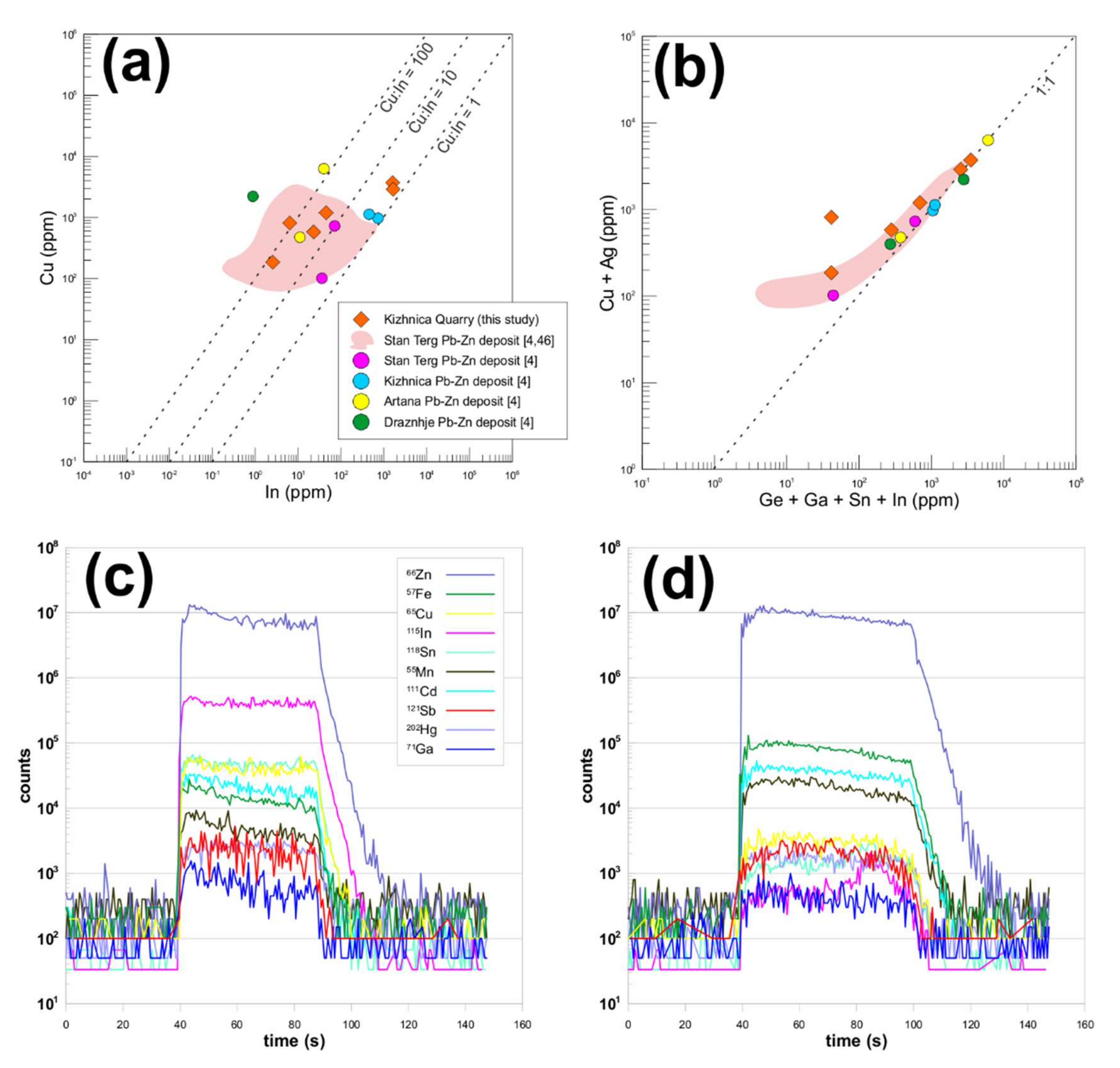
4.1.4. Sphalerite
4.1.5. Tetrahedrite Group Minerals
4.1.6. Associated minerals
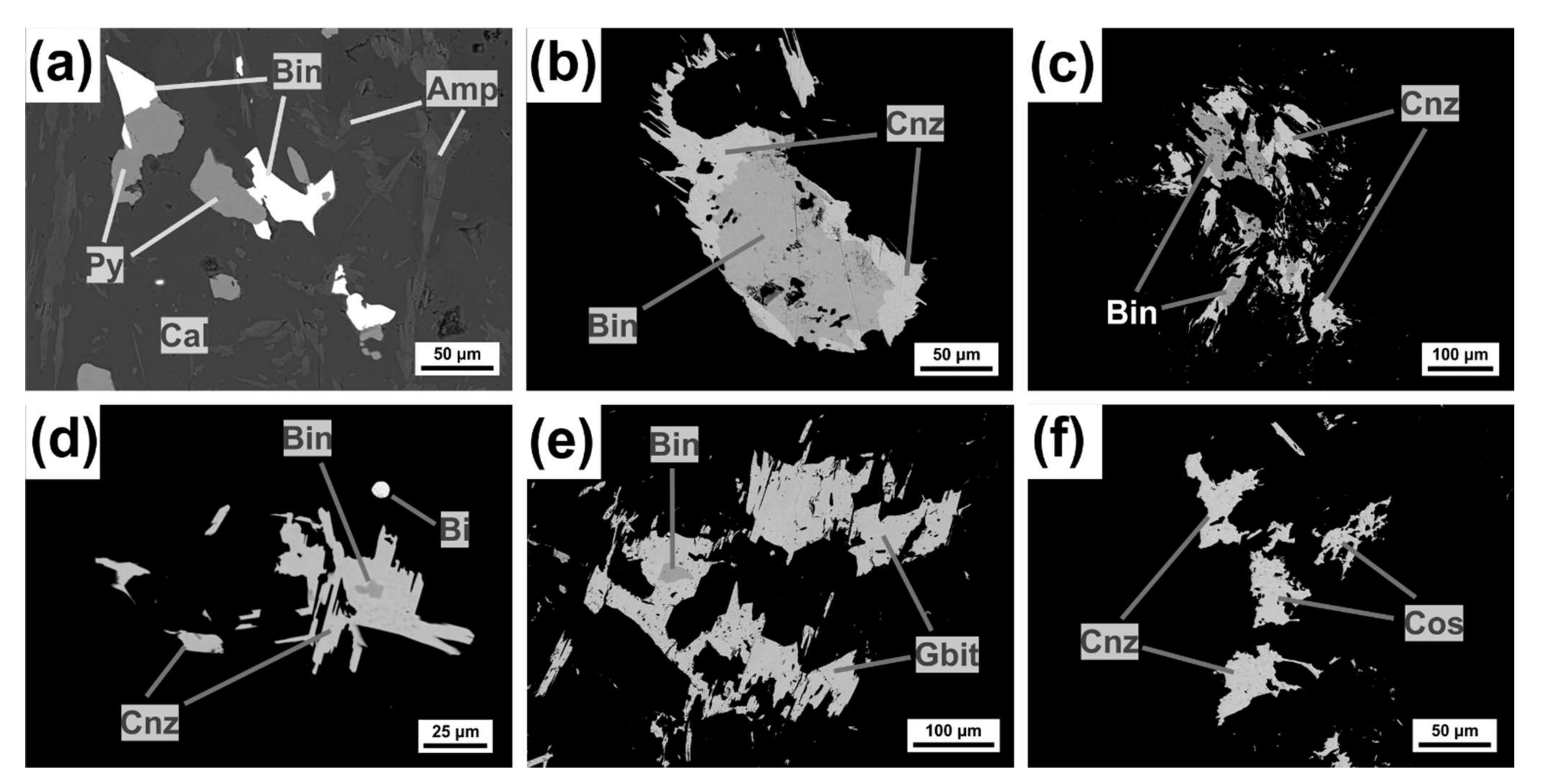
4.2. Kizhnica Hornfels
4.2.1. Bismuth Sulfosalts
Bismuthinite-Aikinite Series
Cannizzarite
Galenobismutite
Cosalite
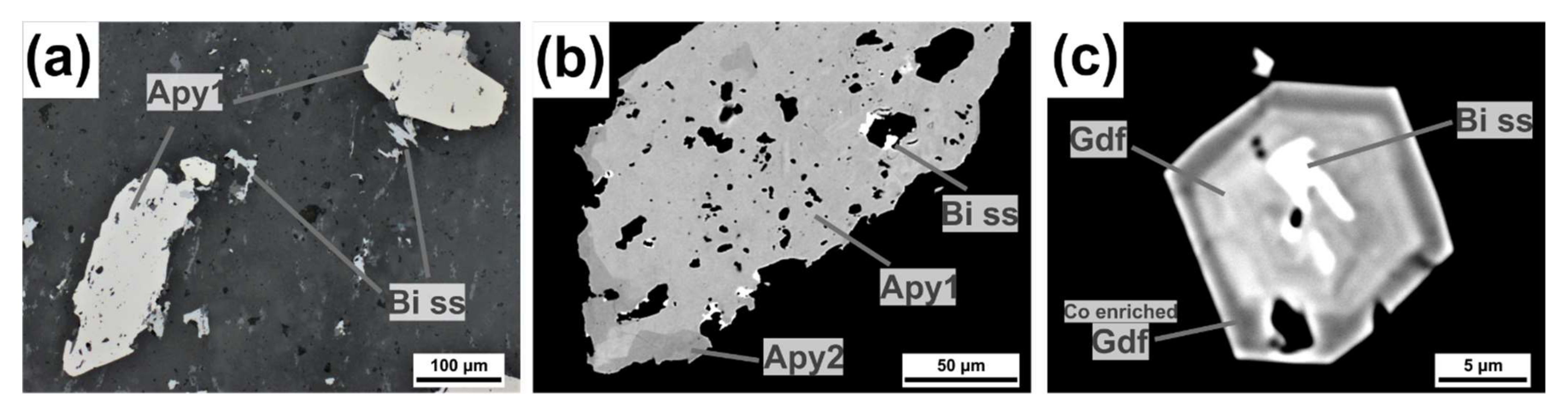
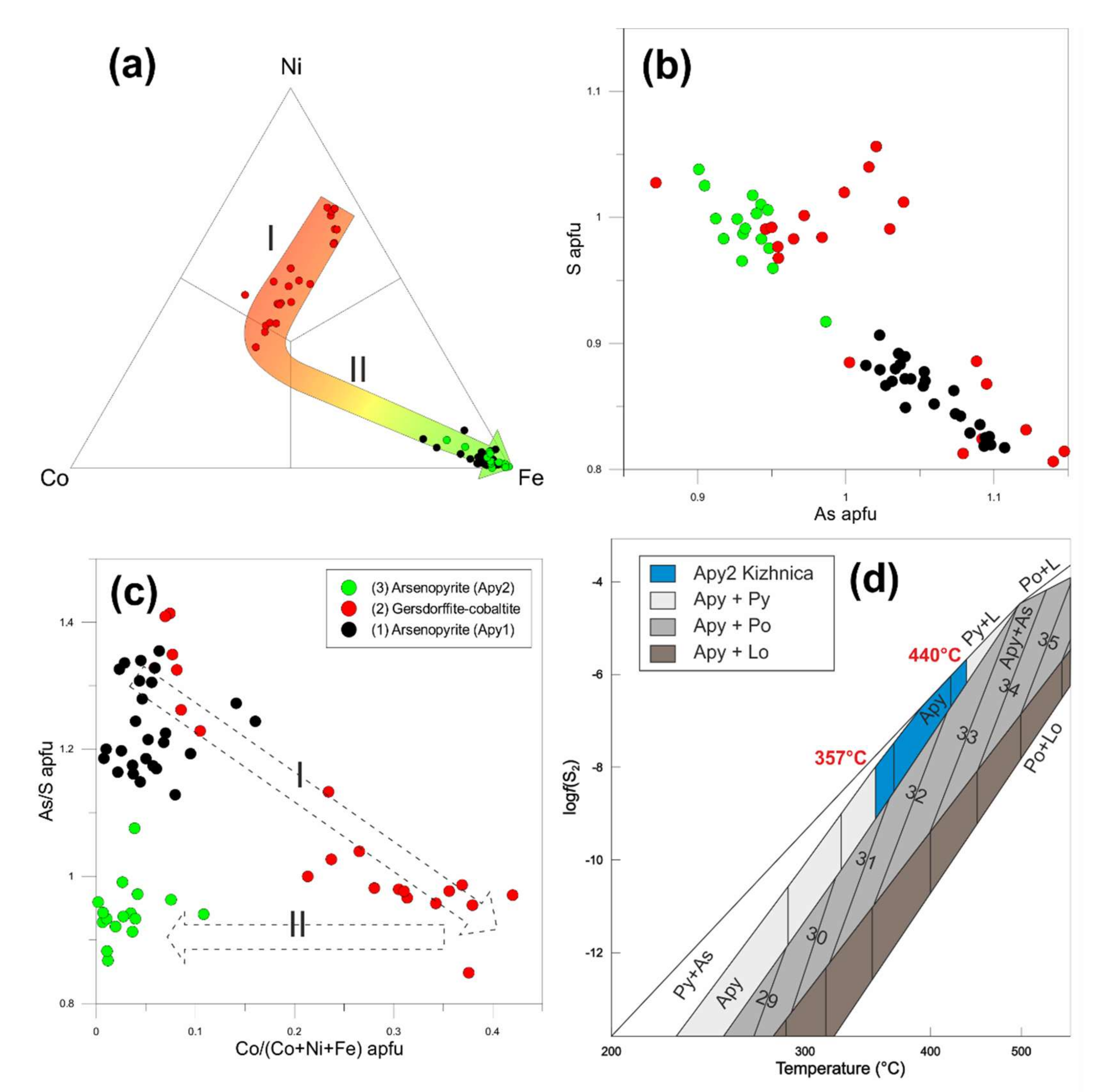
4.2.2. Sulfarsenides: Arsenopyrite-Gersdorffite-Cobaltite
4.2.3. Associated Minerals
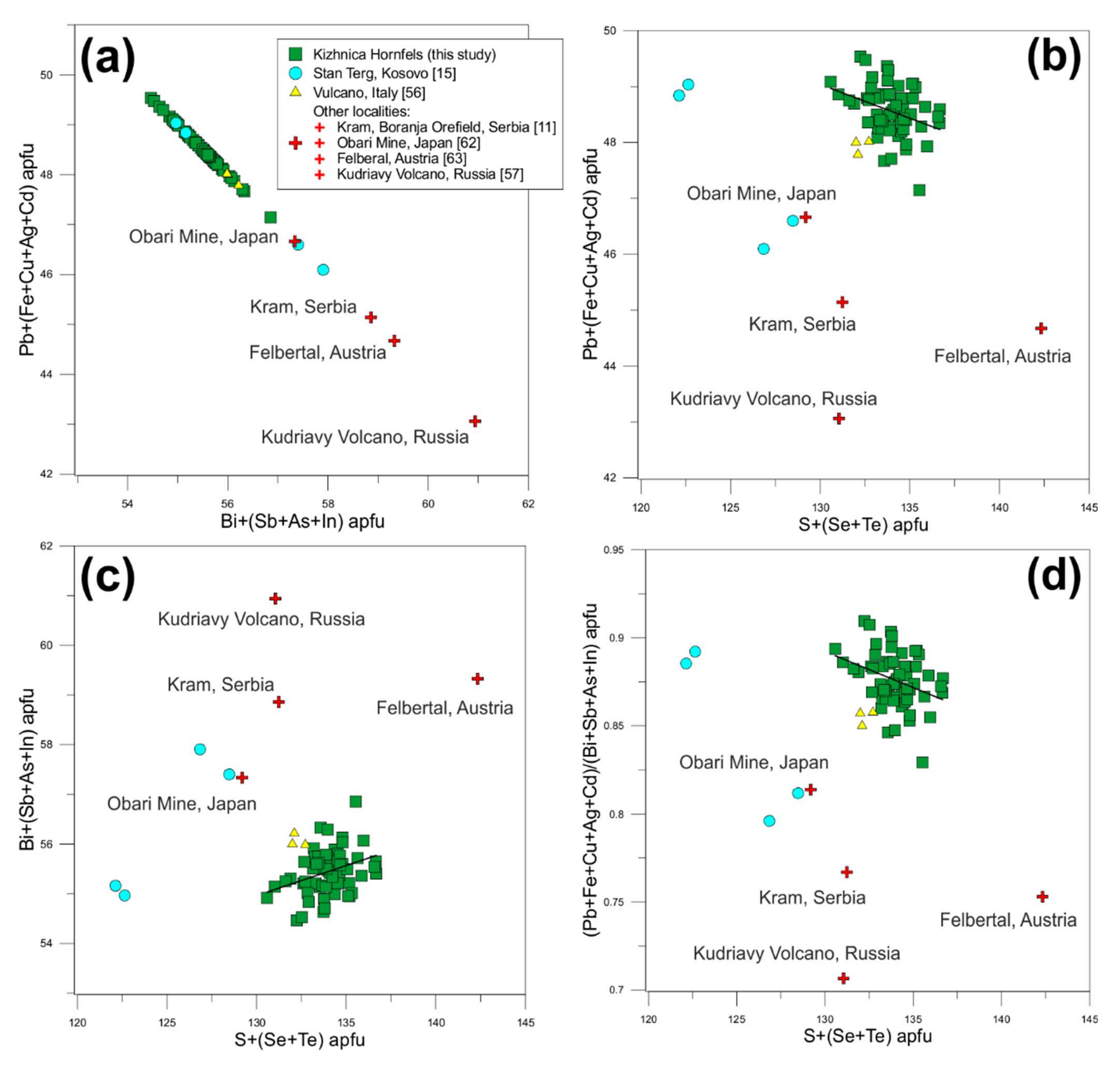
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baker, T. Gold ± copper endowment and deposit diversity in the Western Tethyan magmatic belt, southeast Europe: Implications for exploration. Econ. Geol. 2019, 114, 1237–1250. [Google Scholar] [CrossRef]
- Janković, S. The Carpatho-Balkanides and adjacent area: A sector of the Tethyan Eurasian metallogenic belt. Miner. Depos. 1997, 32, 426–433. [Google Scholar] [CrossRef]
- Borojević Šoštarić, S.B.; Cvetković, V.; Neubauer, F.; Palinkaš, L.A.; Bernroider, M.; Genser, J. Oligocene shoshonitic rocks of the Rogozna Mts. (Central Balkan Peninsula): Evidence of petrogenetic links to the formation of Pb–Zn–Ag ore deposits. Lithos 2012, 148, 176–195. [Google Scholar] [CrossRef]
- Kołodziejczyk, J.; Pršek, J.; Voudouris, P.; Melfos, V.; Asllani, B. Sn-bearing minerals and associated sphalerite from lead-zinc deposits, Kosovo: An electron microprobe and LA-ICP-MS study. Minerals 2016, 6, 42. [Google Scholar] [CrossRef] [Green Version]
- Frenzel, M.; Voudouris, P.; Cook, N.J.; Ciobanu, C.L.; Gilbert, S.; Wade, B.P. Evolution of a hydrothermal ore-forming system recorded by sulfide mineral chemistry: A case study from the Plaka Pb–Zn–Ag Deposit, Lavrion, Greece. Miner. Depos. 2021, 1–22. [Google Scholar] [CrossRef]
- Kroll, T.; Müller, D.; Seifert, T.; Herzig, P.M.; Schneider, A. Petrology and geochemistry of the shoshonite-hosted Skouries porphyry Cu–Au deposit, Chalkidiki, Greece. Miner. Depos. 2002, 37, 137–144. [Google Scholar] [CrossRef]
- Serafimovski, T.; Stefanova, V.; Volkov, A.V. Dwarf copper-gold porphyry deposits of the Buchim-Damjan-Borov Dol ore district, Republic of Macedonia (FYROM). Geol. Ore Depos. 2010, 52, 179–195. [Google Scholar] [CrossRef]
- Stefanova, V.; Volkov, A.V.; Serafimovski, T.; Sidorov, A.A. Native gold from the Plavica epithermal deposit, Republic of Macedonia. Dokl. Earth Sci. 2013, 451, 818–823. [Google Scholar] [CrossRef]
- Siron, C.R.; Thompson, J.F.; Baker, T.; Darling, R.; Dipple, G. Origin of Au-rich carbonate-hosted replacement deposits of the Kassandra mining district, northern Greece: Evidence for Late Oligocene, structurally controlled, and zoned hydrothermal systems. Econ. Geol. 2019, 114, 1389–1414. [Google Scholar] [CrossRef]
- Voudouris, P.; Mavrogonatos, C.; Spry, P.G.; Baker, T.; Melfos, V.; Klemd, R.; Haase, K.; Repstock, A.; Djiba, A.; Bismayer, U.; et al. Porphyry and epithermal deposits in Greece: An overview, new discoveries, and mineralogical constraints on their genesis. Ore Geol. Rev. 2019, 107, 654–691. [Google Scholar] [CrossRef]
- Radosavljević-Mihajlović, A.S.; Stojanovic, J.N.; Dimitrijevic, R.Z.; Radosavljević, S.A. Rare Pb-Bi sulfosalt mineralization from the Boranja orefield (Podrinje district, Serbia). Neues Jahrbuch für Mineralogie-Abhandlungen 2007, 184, 217–224. [Google Scholar] [CrossRef]
- Serafimovski, T.; Tasev, G.; Stefanova, V. Rare mineral phases related with major sulphide minerals in the Bučim porphyry copper deposit, R. Macedonia. Geol. Maced. 2013, 27, 43–54. [Google Scholar]
- Voudouris, P.C.; Spry, P.G.; Mavrogonatos, C.; Sakellaris, G.A.; Bristol, S.K.; Melfos, V.; Fornadel, A.P. Bismuthinite derivatives, lillianite homologues, and bismuth sulfotellurides as indicators of gold mineralization in the Stanos shear-zone related deposit, Chalkidiki, Northern Greece. Can. Miner. 2013, 51, 119–142. [Google Scholar] [CrossRef]
- Voudouris, P.; Mavrogonatos, C.; Rieck, B.; Kolitsch, U.; Spry, P.G.; Scheffer, C.; Tarantola, A.; Vanderhaeghe, O.; Galanos, E.; Melfos, V.; et al. The gersdorffite-bismuthinite-native gold association and the skarn-porphyry mineralization in the Kamariza mining district, Lavrion, Greece. Minerals 2018, 8, 531. [Google Scholar] [CrossRef] [Green Version]
- Kołodziejczyk, J.; Pršek, J.; Melfos, V.; Voudouris, P.C.; Maliqi, F.; Kozub-Budzyń, G. Bismuth minerals from the Stan Terg deposit (Trepça, Kosovo). Neues Jahrbuch für Mineralogie-Abhandlungen 2015, 192, 317–333. [Google Scholar] [CrossRef]
- Kołodziejczyk, J.; Pršek, J.; Voudouris, P.C.; Melfos, V. Bi-sulphotellurides associated with Pb-Bi-(Sb±Ag, Cu, Fe) sulphosalts: An example from the Stan Terg deposit in Kosovo. Geol. Carpath. 2017, 68, 366–381. [Google Scholar] [CrossRef] [Green Version]
- Radosavljević, S.A.; Stojanović, J.N.; Radosavljević-Mihajlović, A.S.; Kašić, V.D. Polymetallic mineralization of the Boranja orefield, Podrinje Metallogenic District, Serbia: Zonality, mineral associations and genetic features. Perdiod Miner. 2013, 82, 61–87. [Google Scholar] [CrossRef]
- Buerger, R.; Giroux, G. NI 43-101 Technical Report on the Slivovo Gold–Silver Project; Avrupa-Minerals: Pristina, Kosovo, 2016; pp. 1–117. [Google Scholar]
- Mederski, S.; Pršek, J.; Asllani, B.; Kozub-Budzyń, G. Bi-sulphosalts from the Mazhiq, Stan Terg area, Kosovo. In Proceedings of the 5th Central-European Mineralogical Conference and 7th Mineral Sciences in the Carpathians Conference, Banska Štiavnica, Slovakia, 26–30 June 2018. [Google Scholar]
- Mederski, S.; Wojsław, M.; Pršek, S.; Majzlan, J.; Kiefer, S.; Asllani, B. A geochemical study of gersdorffite from the Trepça Mineral Belt, Vardar Zone, Kosovo. J. Geosci. 2021, 66, 97–115. [Google Scholar] [CrossRef]
- Węgrzynowicz, J.; Pršek, J.; Mederski, S.; Asllani, B.; Kwiecień, K.; Kanigowski, J. Pb−Bi(−Cu) and Pb−Sb sulfosalts from Stan Terg area, Kosovo. In Proceedings of the Life with Ore Deposits on Earth 15th Biennial SGA Meeting, Advances in Understanding Hydrothermal Processes, Glasgow, Scotland, 27–30 August 2019; University of Glasgow Publicity Services: Glasgow, Scotland, 2019; Volume 1, pp. 380–383. [Google Scholar]
- Janković, S. The principal metallogenic features of the Kopaonik District. In Proceedings of the Geology and Metallogeny of the Kopaonik Mt Symposium, Belgrade, Serbia, 19–22 June 1995; pp. 79–101. [Google Scholar]
- Elezaj, Z. Geodynamic evolution of Kosovo during the Triassic and Jurassic. Yerbilimleri 2009, 30, 113–126. [Google Scholar]
- Strmić Palinkaš, S.; Palinkaš, L.A.; Renac, C.; Spangenberg, J.E.; Lüders, V.; Molnar, F.; Maliqi, G. Metallogenic model of the Trepca Pb-Zn-Ag Skarn deposit, Kosovo: Evidence from fluid inclusions, rare earth elements, and stable isotope data. Econ. Geol. 2013, 108, 135–162. [Google Scholar] [CrossRef] [Green Version]
- Hyseni, M.; Durmishaj, B.; Fetahaj, B.; Shala, F.; Berisha, A.; Large, D. Trepça Ore Belt and Stan Terg mine—Geological overview and interpretation, Kosovo (SE Europe). Geologija 2010, 51, 87–92. [Google Scholar] [CrossRef]
- Strmić Palinkaš, S.A.; Palinkaš, L.; Mandić, M.; Roller-Lutz, Z.; Pécskay, Z.; Maliqi, G.; Bermanec, V. Origin and K-Ar age of the phreatomagmatic breccia at the Trepča Pb-Zn-Ag skarn deposit, Kosovo: Implications for ore-forming processes. Geol. Croat. 2016, 69, 121–142. [Google Scholar] [CrossRef] [Green Version]
- Féraud, J.; Deschamps, Y. French Scientific Cooperation 2007–2008 on the Trepça Lead-Zinc-Silver Mine and the Gold Potential of Novo Brdo/Artana Tailings (Kosovo); BRGM Report No RP-57204-FR; BRGM: Orléans, France, 2009; pp. 1–99. [Google Scholar]
- Radosavljević, S.A.; Stojanović, J.N.; Vuković, N.S.; Radosavljević-Mihajlović, A.S.; Kašić, V.D. Low-temperature Ni-As-Sb-S mineralization of the Pb (Ag)-Zn deposits within the Rogozna Orefield, Serbo-Macedonian Metallogenic Province: Ore mineralogy, crystal chemistry and paragenetic relationships. Ore Geol. Rev. 2015, 65, 213–227. [Google Scholar] [CrossRef]
- Dangić, A. Minor element distribution between galena and sphalerite as a geothermometer—Application to two lead-zinc areas in Yugoslavia. Econ. Geol. 1985, 80, 180–183. [Google Scholar] [CrossRef]
- Borojević Šoštarić, S.B.; Palinkaš, L.A.; Topa, D.; Spangenberg, J.E.; Prochaska, W. Silver–base metal epithermal vein and listwaenite types of deposit Crnac, Rogozna Mts., Kosovo. Part I: Ore mineral geochemistry and sulfur isotope study. Ore Geol. Rev. 2011, 40, 65–80. [Google Scholar] [CrossRef]
- Borojević Šoštarić, S.B.; Palinkaš, L.A.; Neubauer, F.; Hurai, V.; Cvetković, V.; Roller-Lutz, Z.; Mandić, J.; Genser, J. Silver-base metal epithermal vein and listwanite hosted deposit Crnac, Rogozna Mts., Kosovo, part II: A link between magmatic rocks and epithermal mineralization. Ore Geol. Rev. 2013, 50, 98–117. [Google Scholar] [CrossRef]
- Kołodziejczyk, J.; Prsek, J.; Asllani, B.; Maliqi, F. The paragenesis of silver minerals in the Pb-Zn Stan Terg deposit, Kosovo: An example of precious metal epithermal mineralization. Geol. Geophys. Environ. 2016, 42, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Durmishaj, B.; Hyseni, S.; Kelmendi, M. Geochemical association of the sulfides of lead-zinc mineralization in Trepça mineral belt-Kizhnica mine, Kosovo. Int. J. Geol. Agric. 2015, 3, 1–4. [Google Scholar]
- Durmishaj, B.; Hyseni, S.; Tashko, A. The main geochemical association of the sulfides of lead-zinc mineralization in Trepça Mineral Belt- Hajvalia mine, Kosovo. ARPN J. Eng. Appl. Sci. 2014, 9, 1376–1380. [Google Scholar]
- Mederski, S.; Pršek, J.; Hincyngier, K. Pb−Zn−Sb−Ni−Au mineralization from the Kizhnica area, central Kosovo: New data on the listwaenite type mineralization. In Proceedings of the Life with Ore Deposits on Earth 15th Biennial SGA Meeting, Magmatic Sulfide and Oxide Systems; Gold—From Orogenesis to Alluvial; Supergenes, Gems and Non-Metallic Ores, Glasgow, Scotland, 27–30 August 2019; University of Glasgow Publicity Services: Glasgow, Scotland, 2019; Volume 2, pp. 834–837. [Google Scholar]
- Westner, K. Roman Mining and Metal Production Near the Antique City of ULPIANA (Kosovo). Ph.D. Thesis, Goethe-Universität, Frankfurt, Germany, 2017. [Google Scholar]
- Mederski, S.; Pršek, J.; Dimitrova, D. Geochemistry of tetrahedrite group minerals from the Janjevo Cu-Bi-Ag(Pb,W) locality: Results of EPMA and LA-ICP-MS investigations. Acta Miner. Petrogr. 2021, 11, 29. [Google Scholar]
- Guillong, M.; Meier, D.L.; Allan, M.M.; Heinrich, C.A.; Yardley, B.W.D. Appendix A6: SILLS: A MATLAB-based program for the reduction of laser ablation ICP-MS data of homogeneous materials and inclusions. In Laser Ablation ICP–MS in the Earth Sciences: Current Practices and Outstanding Issues; Sylvester, P., Ed.; Mineralogical Association of Canada Short Course 40: Vancouver, BC, Canada, 2008; pp. 328–333. [Google Scholar]
- Topa, D.; Makovicky, E.; Paar, W.H. Composition ranges and exsolution pairs for the members of the bismuthinite–aikinite series from Felbertal, Austria. Can. Miner. 2002, 40, 849–869. [Google Scholar] [CrossRef]
- Pršek, J.; Ozdín, D.; Sejkora, J. Eclarite and associated Bi sulfosalts from the Brezno-Hviezda occurrence (Nízke Tatry Mts, Slovak Republic). Neues Jahrbuch für Mineralogie-Abhandlungen 2008, 185, 117–130. [Google Scholar] [CrossRef]
- Topa, D.; Makovicky, E. The crystal chemistry of cosalite based on new electron-microprobe data and single-crystal determinations of the structure. Can. Miner. 2010, 48, 1081–1107. [Google Scholar] [CrossRef] [Green Version]
- Stojanovic, J.N.; Radosavljević, S.A.; Karanović, L.; Cvetković, L. Mineralogy of WPbBi ores from Rudnik Mt., Serbia. Neues Jb. Miner. Abh. 2006, 182, 299–306. [Google Scholar] [CrossRef]
- Buzatu, A.; Damian, G.; Dill, H.G.; Buzgar, N.; Apopei, A.I. Mineralogy and geochemistry of sulfosalts from Baia Sprie ore deposit (Romania)—New bismuth minerals occurrence. Ore Geol. Rev. 2015, 65, 132–147. [Google Scholar] [CrossRef]
- George, L.L.; Biagioni, C.; Lepore, G.O.; Lacalamita, M.; Agrosì, G.; Capitani, G.C.; Bonaccorsia, E.; d’Acapito, F. The speciation of thallium in (Tl, Sb, As)-rich pyrite. Ore Geol. Rev. 2019, 107, 364–380. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Cook, N.J.; Pring, A.; Brugger, J.; Danyushevsky, L.V.; Shimizu, M. ‘Invisible gold’ in bismuth chalcogenides. Geochim. Cosmochim. Acta 2009, 73, 1970–1999. [Google Scholar] [CrossRef]
- Kołodziejczyk, J. Mineralogical and Geochemical Diversity Within the Stan Terg Deposit, Kosovo. Ph.D. Thesis, AGH University of Science and Technology, Kraków, Poland, 2016. [Google Scholar]
- Cook, N.J.; Ciobanu, C.L.; Pring, A.; Skinner, W.; Shimizu, M.; Danyushevsky, L.; Melcher, F. Trace and minor elements in sphalerite: A LAICPMS study. Geochim. Cosmochim. Acta 2009, 73, 4761–4791. [Google Scholar] [CrossRef]
- Cook, N.J.; Sundblad, K.; Valkama, M.; Nygård, R.; Ciobanu, C.L.; Danyushevsky, L. Indium mineralisation in A-type granites in southeastern Finland: Insights into mineralogy and partitioning between coexisting minerals. Chem. Geol. 2011, 284, 62–73. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Brugger, J.; Etschmann, B.; Howard, D.L.; de Jonge, M.D.; Paterson, D. Determination of the oxidation state of Cu in substituted Cu-In-Fe-bearing sphalerite via μ-XANES spectroscopy. Am. Miner. 2012, 97, 476–479. [Google Scholar] [CrossRef]
- Murakami, H.; Ishihara, S. Trace elements of Indium-bearing sphalerite from tin-polymetallic deposits in Bolivia, China and Japan: A femto-second LA-ICPMS study. Ore Geol. Rev. 2013, 53, 223–243. [Google Scholar] [CrossRef]
- Belissont, R.; Boiron, M.C.; Luais, B.; Cathelineau, M. LA-ICP-MS analyses of minor and trace elements and bulk Ge isotopes in zoned Ge-rich sphalerites from the Noailhac–Saint-Salvy deposit (France): Insights into incorporation mechanisms and ore deposition processes. Geochim. Cosmochim. Acta 2014, 126, 518–540. [Google Scholar] [CrossRef]
- Frenzel, M.; Hirsch, T.; Gutzmer, J. Gallium, germanium, indium, and other trace and minor elements in sphalerite as a function of deposit type—a meta-analysis. Ore Geol. Rev. 2016, 76, 52–78. [Google Scholar] [CrossRef]
- Xu, J.; Cook, N.J.; Ciobanu, C.L.; Li, X.; Kontonikas-Charos, A.; Gilbert, S.; Lv, Y. Indium distribution in sphalerite from sulfide–oxide–silicate skarn assemblages: A case study of the Dulong Zn–Sn–In deposit, Southwest China. Miner. Depos. 2021, 56, 307–324. [Google Scholar] [CrossRef]
- Biagioni, C.; George, L.L.; Cook, N.J.; Makovicky, E.; Moëlo, Y.; Pasero, M.; Sejkora, J.; Stanley, C.J.; Welch, M.D.; Bosi, F. The tetrahedrite group: Nomenclature and classification. Am. Miner. 2020, 105, 109–122. [Google Scholar] [CrossRef]
- George, L.L.; Cook, N.J.; Ciobanu, C.L. Minor and trace elements in natural tetrahedrite-tennantite: Effects on element partitioning among base metal sulphides. Minerals 2017, 7, 17. [Google Scholar] [CrossRef] [Green Version]
- Topa, D.; Makovicky, E.; Dittrich, H. The crystal structure of 7H: 12Q cannizzarite from Vulcano, Italy. Can. Miner. 2010, 48, 483–495. [Google Scholar] [CrossRef]
- Borisov, S.V.; Pervukhina, N.V.; Magarill, S.A.; Kuratieva, N.V.; Bryzgalov, I.A.; Mozgova, N.N.; Chaplygin, I.V. The crystal structure of (Cd, In)-rich cannizzarite from Kudriavy volcano, Iturup island, Kuriles, Russia. Can. Miner. 2012, 50, 387–395. [Google Scholar] [CrossRef]
- Matzat, E. Cannizzarite. Acta Cryst. 1979, 35, 133–136. [Google Scholar] [CrossRef]
- Makovicky, E.; Hyde, B.G. Non-commensurate (misfit) layer structures. Struct. Bond. 1981, 46, 101–170. [Google Scholar]
- Mozgova, N.N.; Kuzmina, O.V.; Organova, N.I.; Laputina, I.P. New data on sulphosalt assemblages at Vulcano (Italy). Rend. Soc. Ital. Miner. Petrol. 1985, 40, 277–283. [Google Scholar]
- Borodaev, Y.S.; Garavelli, A.; Garbarino, C.; Grillo, S.M.; Mozgova, N.N.; Organova, N.I.; Trubkin, N.V.; Vurro, F. Rare sulfosalts from Vulcano, Aeolian islands, Italy. III. Wittite and cannizzarite. Can. Miner. 2000, 38, 23–34. [Google Scholar] [CrossRef]
- Izumino, Y.; Nakashima, K.; Nagashima, M. Cuprobismutite group minerals (cuprobismutite, hodrušhite, kupčíkite and padĕraite), other Bi–sulfosalts and Bi–tellurides from the Obari mine, Yamagata Prefecture, Japan. J. Miner. Petrol. Sci. 2014, 109, 177–190. [Google Scholar] [CrossRef] [Green Version]
- Topa, D.; Makovicky, E.; Criddle, A.J.; Paar, W.H.; Balić-Žunić, T. Felbertalite, Cu2Pb6Bi8S19, an new mineral species from Felbertal, Salzburg Province, Austria. Eur. J. Miner. 2001, 13, 961–972. [Google Scholar] [CrossRef]
- Garavelli, A.; Laviano, R.; Vurro, F. Sublimate deposition from hydrothermal fluids at the Fossa crater-Vulcano, Italy. Eur. J. Miner. 1997, 9, 423–432. [Google Scholar] [CrossRef] [Green Version]
- Kretschmar, U.; Scott, S.D. Phase relations involving arsenopyrite in the system Fe-As-S and their application. Can. Miner. 1976, 14, 364–386. [Google Scholar]
- Sharp, Z.D.; Essene, E.J.; Kelly, W.C. A re-examination of the arsenopyrite geobarometry: Pressure considerations and applications to natural assemblages. Can. Miner. 1985, 23, 517–534. [Google Scholar]
- Scharrer, M.; Kreissl, S.; Markl, G. The mineralogical variability of hydrothermal native element-arsenide (five-element) associations and the role of physicochemical and kinetic factors concerning sulfur and arsenic. Ore Geol. Rev. 2019, 113, 103025. [Google Scholar] [CrossRef]
- Yund, R.A. The system Ni-As-S; phase relations and mineralogical significance. Am. J. Sci. 1962, 260, 761–782. [Google Scholar] [CrossRef]
- Klemm, D.D. Synthesen und Analysen in den Dreiecksdiagrammen FeAsS-CoAsS-NiAsS und FeS2-CoS2-NiS2. Neues Jb. Miner. Abh. 1965, 205–255. [Google Scholar] [CrossRef]
- Hem, S.R.; Makovicky, E. The system Fe–Co–Ni–As–SI Phase relations in the (Fe,Co,Ni)As0.5S1.5 section at 650 and 500 °C. Can. Miner. 2004, 42, 43–62. [Google Scholar] [CrossRef]
- Hem, S.R.; Makovicky, E. The system Fe–Co–Ni–As–S. II. Phase relations in the (Fe,Co,Ni)As1.5S0.5 section at 650 and 500 °C. Can. Miner. 2004, 42, 63–86. [Google Scholar] [CrossRef]
- Stojanović, J.N.; Radosavljević, S.A.; Tošović, R.D.; Pačevski, A.M.; Radosavljević-Mihajlović, A.S.; Kašić, V.D.; Vuković, N.S. A review of the Pb-Zn-Cu-Ag-Bi-W polymetallic ore from the Rudnik orefield, Central Serbia. Geol. An. Balk. Poluos. 2018, 79, 47–69. [Google Scholar] [CrossRef]
- Budinov, Z.D.; Yonezu, K.; Tindell, T.; Gabo-Ratio, J.A.; Milutinovic, S.; Boyce, A.J.; Watanabe, K. Copper–gold skarn mineralization at the Karavansalija ore zone, Rogozna Mountain, Southwestern Serbia. Resour. Geol. 2015, 65, 328–344. [Google Scholar] [CrossRef]
- Voudouris, P.; Melfos, V.; Spry, P.G.; Bonsall, T.A.; Tarkian, M.; Solomos, C. Carbonate-replacement Pb–Zn–Ag±Au mineralization in the Kamariza area, Lavrion, Greece: Mineralogy and thermochemical conditions of formation. Miner. Petrol. 2008, 94, 85–106. [Google Scholar] [CrossRef]



| Analyses | wt.% | apfu | Cations | naik | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | Fe | Cu | Ag | Sb | Pb | As | Bi | Total | S | Fe | Cu | Ag | Sb | Pb | As | Bi | |||
| 1 | 19.09 | b.d.l. | 0.30 | b.d.l. | 0.08 | 0.81 | b.d.l. | 81.85 | 102.14 | 12.01 | - | 0.09 | - | 0.01 | 0.08 | - | 7.90 | 8.09 | 2.22 |
| 2 | 18.94 | 0.45 | 1.10 | b.d.l. | 0.06 | 3.58 | b.d.l. | 78.85 | 102.99 | 11.72 | 0.16 | 0.34 | - | 0.01 | 0.34 | - | 7.49 | 8.34 | 8.78 |
| 3 | 17.97 | b.d.l. | 5.54 | b.d.l. | 0.23 | 17.93 | b.d.l. | 59.89 | 101.58 | 11.94 | - | 1.86 | - | 0.04 | 1.84 | - | 6.10 | 9.85 | 46.51 |
| 4 | 17.74 | b.d.l. | 5.69 | b.d.l. | 0.08 | 18.65 | b.d.l. | 58.60 | 100.76 | 11.94 | - | 1.93 | - | 0.01 | 1.94 | - | 6.05 | 9.94 | 48.65 |
| 5 | 16.92 | 0.14 | 10.57 | b.d.l. | b.d.l. | 32.57 | b.d.l. | 42.35 | 102.58 | 11.50 | 0.05 | 3.62 | - | - | 3.42 | - | 4.42 | 11.52 | 91.95 |
| 6 | 16.81 | 0.06 | 10.60 | b.d.l. | b.d.l. | 34.47 | b.d.l. | 40.51 | 102.48 | 11.59 | 0.03 | 3.69 | - | - | 3.68 | - | 4.29 | 11.68 | 95.30 |
| 7 | 16.91 | 0.11 | 10.94 | b.d.l. | b.d.l. | 34.44 | b.d.l. | 39.57 | 102.01 | 11.70 | 0.04 | 3.82 | - | - | 3.69 | - | 4.20 | 11.75 | 96.19 |
| 8 | 16.74 | b.d.l. | 10.78 | b.d.l. | 0.12 | 34.57 | b.d.l. | 39.78 | 101.99 | 11.61 | - | 3.77 | - | 0.02 | 3.71 | - | 4.23 | 11.74 | 96.66 |
| Analyses | wt.% | apfu | Cations | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | Fe | Cu | Ag | Sb | Pb | As | Bi | Total | S | Fe | Cu | Ag | Sb | Pb | As | Bi | ||
| 1 | 16.76 | b.d.l. | 1.73 | 0.77 | 0.43 | 38.58 | b.d.l. | 41.65 | 99.93 | 20.00 | - | 1.04 | 0.27 | 0.13 | 7.12 | - | 7.62 | 16.21 |
| 2 | 16.72 | 0.07 | 1.79 | 0.77 | 0.27 | 38.34 | b.d.l. | 43.17 | 101.13 | 20.00 | 0.05 | 1.08 | 0.27 | 0.09 | 7.10 | - | 7.92 | 16.50 |
| 3 | 16.66 | b.d.l. | 1.43 | 0.55 | 0.57 | 39.23 | b.d.l. | 42.93 | 101.39 | 20.00 | - | 0.87 | 0.19 | 0.18 | 7.29 | - | 7.90 | 16.44 |
| 4 | 16.63 | b.d.l. | 1.81 | 0.69 | 0.32 | 38.27 | b.d.l. | 43.11 | 100.84 | 20.00 | - | 1.10 | 0.25 | 0.10 | 7.12 | - | 7.95 | 16.52 |
| 5 | 16.56 | b.d.l. | 1.53 | 0.74 | 0.67 | 38.93 | b.d.l. | 41.52 | 99.99 | 20.00 | - | 0.93 | 0.27 | 0.21 | 7.28 | - | 7.69 | 16.39 |
| 6 | 16.53 | 0.05 | 1.45 | 0.75 | 0.47 | 38.48 | b.d.l. | 43.46 | 101.19 | 20.00 | 0.04 | 0.88 | 0.27 | 0.15 | 7.20 | - | 8.07 | 16.60 |
| 7 | 16.52 | b.d.l. | 1.91 | 0.72 | 0.56 | 38.65 | b.d.l. | 40.74 | 99.11 | 20.00 | - | 1.16 | 0.26 | 0.18 | 7.24 | - | 7.57 | 16.43 |
| 8 | 16.49 | 0.05 | 1.79 | 0.83 | 0.26 | 39.20 | b.d.l. | 41.08 | 99.70 | 20.00 | 0.03 | 1.09 | 0.30 | 0.08 | 7.36 | - | 7.65 | 16.52 |
| 9 | 16.45 | b.d.l. | 1.71 | 0.78 | 0.25 | 39.12 | b.d.l. | 41.28 | 99.61 | 20.00 | - | 1.05 | 0.28 | 0.08 | 7.36 | - | 7.70 | 16.47 |
| 10 | 16.31 | b.d.l. | 2.16 | 0.83 | 0.48 | 39.26 | b.d.l. | 40.52 | 99.56 | 20.00 | - | 1.33 | 0.30 | 0.15 | 7.45 | - | 7.62 | 16.87 |
| 11 | 16.31 | b.d.l. | 1.95 | 0.86 | 0.44 | 38.95 | b.d.l. | 40.89 | 99.45 | 20.00 | - | 1.20 | 0.31 | 0.14 | 7.39 | - | 7.69 | 16.77 |
| 12 | 16.26 | 0.18 | 1.65 | 0.75 | 0.28 | 37.80 | b.d.l. | 43.41 | 100.32 | 20.00 | 0.13 | 1.03 | 0.27 | 0.09 | 7.19 | - | 8.19 | 16.90 |
| Mineralization Type | Hydrothermal Veinlets | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Veinlets (n = 5); Py1 | Disseminated (n = 8); Py2 | Disseminated (n = 6); Py3 | |||||||||||||
| Element | MIN | MAX | ST DEV | AVG | na.d.l. | MIN | MAX | ST DEV | AVG | na.d.l. | MIN | MAX | ST DEV | AVG | na.d.l. |
| Ti | 15 | 24 | 3.70 | 18 | 5 | 10 | 29 | 6.36 | 19 | 8 | 18 | 38 | 9.24 | 25 | 4 |
| V | 0.66 | 0.66 | n.c. | n.c. | 1 | 2.57 | 3.01 | 0.31 | 2.79 | 2 | 5.90 | 5.90 | n.c. | n.c. | 1 |
| Cr | 34 | 51 | 6.04 | 41 | 5 | 30 | 44 | 5.50 | 36 | 8 | 23 | 41 | 6.06 | 33 | 6 |
| Mn | 52 | 56 | 2.13 | 54 | 5 | 45 | 295 | 87 | 87 | 8 | 44 | 57 | 4.52 | 48 | 6 |
| Fe | 46.25 | 46.80 | 0.23 | 46.65 | 5 | 46.12 | 46.76 | 0.23 | 46.42 | 8 | 44.29 | 46.91 | 0.97 | 46.22 | 6 |
| Co | 5.26 | 121 | 52 | 49 | 5 | 3.73 | 24 | 6.88 | 12 | 7 | 0.96 | 7.91 | 2.89 | 3.57 | 6 |
| Ni | 8.62 | 8.62 | n.c. | n.c. | 1 | 3.39 | 17 | 8.25 | 8.29 | 3 | 6.83 | 6.83 | n.c. | n.c. | 1 |
| Cu | 2.57 | 3.77 | 0.85 | 3.17 | 2 | 2.48 | 7.36 | 2.67 | 5.54 | 3 | 5.53 | 130 | 71 | 48 | 3 |
| Zn | 5.84 | 7.08 | 0.87 | 6.46 | 2 | 8.70 | 11 | 1.95 | 10 | 3 | 7.25 | 11 | 2.74 | 9.18 | 2 |
| Ga | b.d.l. | b.d.l. | n.c. | n.c. | 0 | 20 | 20 | n.c. | n.c. | 1 | 1.46 | 1.46 | n.c. | n.c. | 1 |
| Ge | b.d.l. | b.d.l. | n.c. | n.c. | 0 | 1.43 | 2.57 | 0.63 | 1.84 | 3 | 4.80 | 4.80 | n.c. | n.c. | 1 |
| As | 743 | 10131 | 3831 | 3501 | 5 | 235 | 1298 | 366 | 672 | 8 | 1215 | 49609 | 19406 | 10018 | 6 |
| Se | 4.79 | 6.19 | 0.99 | 5.49 | 2 | 9.54 | 9.54 | n.c. | n.c. | 1 | b.d.l. | b.d.l. | n.c. | n.c. | 0 |
| Mo | b.d.l. | b.d.l. | n.c. | n.c. | 0 | 1.39 | 1.39 | n.c. | n.c. | 1 | 6.47 | 6.47 | n.c. | n.c. | 1 |
| Ag | b.d.l. | b.d.l. | n.c. | n.c. | 0 | 0.20 | 0.25 | 0.03 | 0.23 | 2 | 3.11 | 3.11 | n.c. | n.c. | 1 |
| In | 0.24 | 0.24 | n.c. | n.c. | 1 | 0.06 | 0.06 | n.c. | n.c. | 1 | 0.34 | 0.34 | n.c. | n.c. | 1 |
| Sn | b.d.l. | b.d.l. | n.c. | n.c. | 0 | b.d.l. | b.d.l. | n.c. | n.c. | 0 | 8.66 | 8.66 | n.c. | n.c. | 1 |
| Sb | b.d.l. | b.d.l. | n.c. | n.c. | 0 | b.d.l. | b.d.l. | n.c. | n.c. | 0 | 0.35 | 1298 | 645 | 330 | 4 |
| Te | b.d.l. | b.d.l. | n.c. | n.c. | 0 | 2.10 | 2.10 | n.c. | n.c. | 1 | 3.34 | 3.34 | n.c. | n.c. | 1 |
| W | b.d.l. | b.d.l. | n.c. | n.c. | 0 | 0.54 | 74 | 52 | 37 | 2 | 0.97 | 0.97 | n.c. | n.c. | 1 |
| Au | 0.19 | 0.79 | 0.42 | 0.49 | 2 | 0.55 | 0.55 | n.c. | n.c. | 1 | 0.70 | 2.06 | 0.73 | 1.53 | 3 |
| Hg | b.d.l. | b.d.l. | n.c. | n.c. | 0 | 0.44 | 0.44 | n.c. | n.c. | 1 | 178 | 178 | n.c. | n.c. | 1 |
| Tl | b.d.l. | b.d.l. | n.c. | n.c. | 0 | 8.60 | 8.60 | n.c. | n.c. | 1 | 1022 | 1022 | n.c. | n.c. | 1 |
| Pb | 0.76 | 0.76 | n.c. | n.c. | 1 | 0.30 | 2.89 | 1.03 | 1.29 | 5 | 0.70 | 1794 | 724 | 317 | 6 |
| Bi | 0.11 | 0.70 | 0.30 | 0.37 | 3 | 0.20 | 19.52 | 6.95 | 4.27 | 7 | 0.51 | 8.67 | 2.92 | 3.46 | 6 |
| Mineralization Type | Hydrothermal Veinlets | Hornfels | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Veinlets (n = 14); Chalcopyrite (Ccp) | Disseminated (n = 6); Sphalerite (Sph) | Veinlets (n = 1); Tetrahedrite | Disseminated (n = 10); Arsenopyrite 1 (Apy1) | |||||||||||||
| Element | MIN | MAX | ST DEV | AVG | na.d.l. | MIN | MAX | ST DEV | AVG | na.d.l. | MIN | MAX | ST DEV | AVG | na.d.l. | |
| Ti | 9.55 | 24 | 4.93 | 14 | 11 | 8.50 | 9.78 | 0.57 | 8.96 | 4 | b.d.l. | 42 | 3509 | 1201 | 1037 | 8 |
| V | b.d.l. | b.d.l. | n.c. | n.c. | 0 | b.d.l. | b.d.l. | n.c. | n.c. | 0 | b.d.l. | 2.90 | 24 | 9.06 | 13 | 9 |
| Cr | 23 | 37 | 4.97 | 28 | 10 | 5.53 | 5.53 | n.c. | n.c. | 1 | b.d.l. | 48 | 120 | 39 | 76 | 3 |
| Mn | 26 | 31 | 1.33 | 28 | 14 | 18 | 329 | 125 | 174 | 6 | 5.60 | 25 | 60 | 10 | 37 | 10 |
| Fe | 30.14 | 30.61 | 0.12 | 30.46 | 14 | 4309 | 43,188 | 18,299 | 30,493 | 6 | 10,421 | 29.43 | 33.75 | 1.23 | 31.93 | 10 |
| Co | 0.48 | 0.58 | 0.07 | 0.53 | 2 | b.d.l. | b.d.l. | n.c. | n.c. | 0 | 18 | 11,159 | 20,282 | 2960 | 16,011 | 10 |
| Ni | b.d.l. | b.d.l. | n.c. | n.c. | 0 | b.d.l. | b.d.l. | n.c. | n.c. | 0 | b.d.l. | 3205 | 15,365 | 4476 | 6865 | 10 |
| Cu | 372,279 | 426,230 | 14,482 | 405,036 | 14 | 185 | 3717 | 1413 | 1565 | 6 | 40.15 | 25 | 25 | n.c. | n.c. | 1 |
| Zn | 117 | 359 | 79 | 242 | 14 | 57.75 | 64.69 | 2.94 | 61.00 | 6 | 97,254 | b.d.l. | b.d.l. | n.c. | n.c. | 0 |
| Ga | b.d.l. | b.d.l. | n.c. | n.c. | 0 | 3.62 | 12 | 3.23 | 8.34 | 6 | 2.40 | 11 | 11 | n.c. | n.c. | 1 |
| Ge | b.d.l. | b.d.l. | n.c. | n.c. | 0 | 2.50 | 7.27 | 2.32 | 4.70 | 5 | 8.83 | 31 | 51 | 7.16 | 41 | 10 |
| As | b.d.l. | b.d.l. | n.c. | n.c. | 0 | 1.38 | 10 | 4.13 | 4.46 | 4 | 84,912 | 327,229 | 574,790 | 66,586 | 433,605 | 10 |
| Se | b.d.l. | b.d.l. | n.c. | n.c. | 0 | b.d.l. | b.d.l. | n.c. | n.c. | 0 | b.d.l. | 41 | 41 | n.c. | n.c. | 1 |
| Mo | 1.85 | 1.85 | n.c. | n.c. | 1 | b.d.l. | b.d.l. | n.c. | n.c. | 0 | b.d.l. | 7.93 | 7.93 | n.c. | n.c. | 1 |
| Ag | b.d.l. | b.d.l. | n.c. | n.c. | 0 | 0.65 | 2.36 | 0.65 | 1.13 | 6 | 150 | b.d.l. | b.d.l. | n.c. | n.c. | 0 |
| Cd | 4.21 | 4.21 | n.c. | n.c. | 1 | 1364 | 2530 | 428 | 1857 | 6 | 300 | b.d.l. | b.d.l. | n.c. | n.c. | 0 |
| In | 10 | 25 | 4.09 | 17 | 14 | 2.55 | 1655 | 832 | 556 | 6 | 7.65 | 0.37 | 0.72 | 0.12 | 0.53 | 10 |
| Sn | b.d.l. | b.d.l. | n.c. | n.c. | 0 | 21 | 1895 | 714 | 611 | 6 | b.d.l. | 2.98 | 2.98 | n.c. | n.c. | 1 |
| Sb | b.d.l. | b.d.l. | n.c. | n.c. | 0 | 13 | 94 | 28 | 41 | 6 | 174,331 | 8.05 | 22 | 4.60 | 13 | 10 |
| Te | b.d.l. | b.d.l. | n.c. | n.c. | 0 | b.d.l. | b.d.l. | n.c. | n.c. | 0 | b.d.l. | 28 | 28 | n.c. | n.c. | 1 |
| W | b.d.l. | b.d.l. | n.c. | n.c. | 0 | 0.79 | 0.79 | n.c. | n.c. | 1 | b.d.l. | 3.65 | 6.69 | 2.15 | 5.17 | 2 |
| Au | 0.28 | 0.28 | n.c. | n.c. | 1 | b.d.l. | b.d.l. | n.c. | n.c. | 0 | b.d.l. | b.d.l. | b.d.l. | n.c. | n.c. | 0 |
| Hg | 0.73 | 0.96 | 0.13 | 0.81 | 2 | 19 | 40 | 8.31 | 25 | 6 | 30 | b.d.l. | b.d.l. | n.c. | n.c. | 0 |
| Pb | 0.53 | 1.81 | 0.51 | 1.17 | 9 | 4.29 | 19 | 5.72 | 7.67 | 6 | 2609 | 1.27 | 4.68 | 1.25 | 2.84 | 7 |
| Bi | 0.35 | 7.09 | 2.19 | 1.26 | 9 | 0.16 | 0.16 | n.c. | n.c. | 1 | 31 | 13 | 313 | 91 | 61 | 10 |
| Analyses | wt.% | apfu Ʃ16 Cation | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | Cu | Sb | Bi | Fe | Zn | Hg | Ag | As | Total | S | Cu | Sb | Bi | Fe | Zn | Hg | Ag | As | |
| 1 | 25.58 | 38.55 | 26.73 | 0.10 | 1.03 | 6.65 | 0.32 | 0.02 | 1.48 | 100.44 | 13.18 | 10.02 | 3.63 | 0.01 | 0.31 | 1.68 | 0.03 | 0.00 | 0.33 |
| 2 | 25.85 | 39.12 | 25.52 | 0.11 | 1.92 | 5.67 | 0.52 | 0.04 | 2.05 | 100.78 | 13.20 | 10.08 | 3.43 | 0.01 | 0.56 | 1.42 | 0.04 | 0.01 | 0.45 |
| 3 | 25.80 | 38.94 | 25.10 | 0.10 | 1.18 | 6.76 | 0.26 | 0.04 | 2.29 | 100.45 | 13.19 | 10.04 | 3.38 | 0.01 | 0.34 | 1.69 | 0.02 | 0.01 | 0.50 |
| 4 | 25.72 | 38.86 | 24.28 | 0.08 | 1.80 | 5.67 | 0.78 | b.d.l. | 2.70 | 99.89 | 13.23 | 10.08 | 3.29 | 0.01 | 0.53 | 1.43 | 0.06 | - | 0.59 |
| 5 | 26.35 | 38.74 | 24.08 | 0.11 | 1.77 | 4.89 | 2.64 | 0.02 | 2.68 | 101.29 | 13.65 | 10.12 | 3.28 | 0.01 | 0.53 | 1.24 | 0.22 | 0.00 | 0.59 |
| 6 | 25.79 | 38.75 | 23.33 | 0.08 | 1.70 | 5.80 | 0.84 | b.d.l. | 3.25 | 99.55 | 13.29 | 10.07 | 3.17 | 0.01 | 0.50 | 1.47 | 0.07 | - | 0.72 |
| 7 | 26.64 | 40.15 | 16.86 | 0.13 | 1.11 | 6.99 | b.d.l. | 0.09 | 8.74 | 100.70 | 13.10 | 9.96 | 2.18 | 0.01 | 0.31 | 1.68 | - | 0.01 | 1.84 |
| 8 | 26.75 | 40.31 | 15.17 | 0.12 | 1.32 | 6.99 | 0.06 | b.d.l. | 9.75 | 100.46 | 13.09 | 9.95 | 1.95 | 0.01 | 0.37 | 1.68 | 0.00 | - | 2.04 |
| 9 | 26.75 | 40.44 | 12.92 | 0.14 | 1.03 | 7.35 | b.d.l. | b.d.l. | 11.68 | 100.32 | 12.96 | 9.89 | 1.65 | 0.01 | 0.29 | 1.75 | - | - | 2.42 |
| 10 | 26.85 | 41.01 | 12.37 | 0.08 | 1.32 | 7.18 | b.d.l. | 0.03 | 12.10 | 100.94 | 12.85 | 9.90 | 1.56 | 0.01 | 0.36 | 1.68 | - | 0.00 | 2.48 |
| Analyses | wt.% | apfu | Cations | naik | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | Fe | Cu | Ag | Sb | Pb | As | Bi | Total | S | Fe | Cu | Ag | Sb | Pb | As | Bi | |||
| 1 | 19.20 | b.d.l. | 0.36 | b.d.l. | 0.24 | 0.66 | b.d.l. | 79.24 | 99.77 | 12.41 | - | 0.12 | - | 0.04 | 0.07 | - | 7.86 | 8.09 | 2.21 |
| 2 | 19.26 | b.d.l. | 0.37 | b.d.l. | 0.26 | 0.66 | b.d.l. | 79.23 | 99.86 | 12.43 | - | 0.12 | - | 0.04 | 0.07 | - | 7.84 | 8.09 | 2.42 |
| 3 | 19.05 | b.d.l. | 0.35 | b.d.l. | 0.22 | 1.56 | b.d.l. | 78.76 | 99.96 | 12.33 | - | 0.11 | - | 0.04 | 0.16 | - | 7.82 | 8.13 | 3.33 |
| 4 | 19.03 | b.d.l. | 0.57 | b.d.l. | 0.22 | 1.48 | b.d.l. | 77.78 | 99.09 | 12.42 | - | 0.19 | - | 0.04 | 0.15 | - | 7.79 | 8.17 | 4.08 |
| 5 | 18.89 | b.d.l. | 0.58 | b.d.l. | 0.18 | 1.30 | b.d.l. | 78.52 | 99.54 | 12.23 | - | 0.19 | - | 0.03 | 0.13 | - | 7.80 | 8.16 | 4.21 |
| 6 | 19.12 | b.d.l. | 0.58 | b.d.l. | 0.20 | 2.00 | b.d.l. | 77.90 | 99.81 | 12.43 | - | 0.19 | - | 0.03 | 0.20 | - | 7.77 | 8.20 | 4.74 |
| 7 | 18.93 | b.d.l. | 0.68 | b.d.l. | 0.22 | 1.57 | 0.20 | 77.75 | 99.37 | 12.24 | - | 0.22 | - | 0.04 | 0.16 | 0.03 | 7.71 | 8.17 | 4.80 |
| 8 | 18.89 | b.d.l. | 0.70 | b.d.l. | 0.21 | 2.37 | 0.23 | 77.43 | 99.84 | 12.19 | - | 0.23 | - | 0.04 | 0.24 | 0.04 | 7.67 | 8.21 | 5.73 |
| Analyses | wt.% | apfu | Anions | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | Fe | Cu | Ag | Sb | Pb | As | Bi | Total | S | Fe | Cu | Ag | Sb | Pb | As | Bi | ||
| 1 | 16.72 | 0.11 | 0.06 | b.d.l. | 0.06 | 38.42 | b.d.l. | 44.61 | 100.26 | 134.53 | 0.52 | 0.23 | - | 0.14 | 47.85 | - | 55.08 | 134.53 |
| 2 | 16.69 | 0.10 | b.d.l. | 0.11 | b.d.l. | 37.92 | b.d.l. | 45.03 | 99.99 | 134.40 | 0.44 | - | 0.26 | - | 47.25 | - | 55.63 | 134.40 |
| 3 | 16.93 | 0.07 | 0.10 | 0.10 | 0.16 | 37.94 | b.d.l. | 44.48 | 99.94 | 136.65 | 0.32 | 0.41 | 0.23 | 0.33 | 47.39 | - | 55.09 | 136.65 |
| 4 | 16.57 | b.d.l. | 0.07 | b.d.l. | 0.06 | 38.40 | b.d.l. | 44.55 | 99.73 | 134.08 | - | 0.30 | - | 0.13 | 48.07 | - | 55.29 | 134.08 |
| 5 | 16.63 | b.d.l. | 0.10 | 0.10 | 0.06 | 38.03 | b.d.l. | 44.63 | 99.60 | 134.65 | - | 0.40 | 0.25 | 0.12 | 47.66 | - | 55.46 | 134.65 |
| 6 | 16.41 | b.d.l. | 0.07 | 0.12 | 0.07 | 38.41 | b.d.l. | 44.39 | 99.51 | 132.71 | - | 0.30 | 0.28 | 0.16 | 48.07 | - | 55.09 | 132.71 |
| 7 | 16.69 | b.d.l. | 0.08 | b.d.l. | 0.07 | 37.59 | b.d.l. | 44.74 | 99.32 | 135.97 | - | 0.33 | - | 0.16 | 47.37 | - | 55.91 | 135.97 |
| 8 | 16.65 | b.d.l. | 0.08 | b.d.l. | 0.13 | 38.02 | b.d.l. | 44.36 | 99.30 | 135.64 | - | 0.31 | - | 0.27 | 47.93 | - | 55.45 | 135.64 |
| 9 | 16.61 | 0.18 | 0.05 | 0.15 | b.d.l. | 37.36 | b.d.l. | 44.86 | 99.28 | 134.53 | 0.82 | 0.20 | 0.36 | - | 46.82 | - | 55.73 | 134.53 |
| 10 | 16.53 | b.d.l. | 0.06 | b.d.l. | 0.09 | 37.84 | b.d.l. | 44.47 | 99.11 | 134.65 | - | 0.25 | - | 0.20 | 47.69 | - | 55.57 | 134.65 |
| 11 | 16.38 | b.d.l. | 0.10 | b.d.l. | 0.07 | 38.00 | b.d.l. | 44.40 | 99.10 | 133.16 | - | 0.42 | - | 0.14 | 47.79 | - | 55.36 | 133.16 |
| 12 | 16.45 | b.d.l. | 0.09 | 0.10 | 0.06 | 38.62 | b.d.l. | 43.75 | 99.09 | 133.80 | - | 0.37 | 0.23 | 0.13 | 48.60 | - | 54.58 | 133.80 |
| Analyses | wt.% | apfu | Cations | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | Fe | Cu | Ag | Sb | Pb | As | Bi | Total | S | Fe | Cu | Ag | Sb | Pb | As | Bi | ||
| 1 | 16.97 | b.d.l. | b.d.l. | b.d.l. | 0.10 | 28.54 | b.d.l. | 54.31 | 99.97 | 3.98 | - | - | - | 0.01 | 1.04 | - | 1.95 | 3.00 |
| 2 | 17.25 | b.d.l. | b.d.l. | b.d.l. | 0.05 | 27.74 | 0.14 | 54.54 | 99.76 | 4.06 | - | - | - | 0.00 | 1.01 | 0.01 | 1.97 | 3.00 |
| 3 | 17.27 | b.d.l. | 0.07 | b.d.l. | 0.09 | 27.63 | b.d.l. | 54.57 | 99.62 | 4.08 | - | 0.01 | - | 0.01 | 1.01 | - | 1.98 | 3.00 |
| 4 | 17.16 | b.d.l. | b.d.l. | b.d.l. | - | 28.23 | b.d.l. | 54.17 | 99.57 | 4.06 | - | - | - | - | 1.03 | - | 1.97 | 3.00 |
| 5 | 17.44 | b.d.l. | b.d.l. | b.d.l. | 0.10 | 28.03 | b.d.l. | 53.87 | 99.56 | 4.13 | - | - | - | 0.01 | 1.03 | - | 1.95 | 3.00 |
| 6 | 17.14 | b.d.l. | b.d.l. | b.d.l. | 0.15 | 28.01 | b.d.l. | 54.12 | 99.41 | 4.05 | - | - | - | 0.01 | 1.03 | - | 1.96 | 3.00 |
| 7 | 17.33 | 0.10 | b.d.l. | b.d.l. | 0.07 | 27.99 | b.d.l. | 53.90 | 99.40 | 4.10 | 0.01 | - | - | 0.00 | 1.02 | - | 1.96 | 3.00 |
| 8 | 17.32 | b.d.l. | b.d.l. | b.d.l. | 0.12 | 28.40 | b.d.l. | 53.51 | 99.36 | 4.11 | - | - | - | 0.01 | 1.04 | - | 1.95 | 3.00 |
| 9 | 17.37 | b.d.l. | b.d.l. | b.d.l. | 0.13 | 28.09 | b.d.l. | 53.71 | 99.33 | 4.12 | - | - | - | 0.01 | 1.03 | - | 1.96 | 3.00 |
| 10 | 17.28 | b.d.l. | b.d.l. | b.d.l. | 0.07 | 28.20 | b.d.l. | 53.71 | 99.31 | 4.10 | - | - | - | 0.00 | 1.04 | - | 1.96 | 3.00 |
| Analyses | wt.% | apfu | Cations | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | Fe | Cu | Ag | Sb | Pb | As | Bi | Total | S | Fe | Cu | Ag | Sb | Pb | As | Bi | ||
| 1 | 16.61 | 0.07 | 0.40 | b.d.l. | 0.17 | 41.82 | b.d.l. | 41.39 | 100.46 | 20.00 | 0.05 | 0.24 | - | 0.05 | 7.79 | - | 7.65 | 15.78 |
| 2 | 16.64 | 0.06 | 0.41 | b.d.l. | 0.15 | 41.82 | b.d.l. | 40.68 | 99.87 | 20.00 | 0.04 | 0.25 | - | 0.05 | 7.78 | - | 7.50 | 15.66 |
| 3 | 16.45 | 0.09 | 0.34 | b.d.l. | 0.18 | 40.91 | b.d.l. | 40.94 | 98.94 | 20.00 | 0.06 | 0.21 | - | 0.06 | 7.70 | - | 7.64 | 15.68 |
| Analyses | wt.% | apfu Ʃ1 Cation | Cations | Anions | As/S | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Ni | Co | As | Sb | Bi | S | Total | Fe | Ni | Co | As | Sb | Bi | S | ||||
| 1 | 32.18 | 1.77 | 0.35 | 48.32 | 0.05 | 0.02 | 17.23 | 99.94 | 0.94 | 0.05 | 0.01 | 1.05 | 0.00 | 0.00 | 0.88 | 1.00 | 1.93 | 1.20 |
| 2 | 30.81 | 1.50 | 1.60 | 49.72 | b.d.l. | b.d.l. | 15.88 | 99.55 | 0.91 | 0.04 | 0.04 | 1.10 | - | - | 0.82 | 1.00 | 1.92 | 1.34 |
| 3 | 32.54 | 0.53 | 1.61 | 47.02 | b.d.l. | b.d.l. | 17.52 | 99.29 | 0.94 | 0.01 | 0.04 | 1.01 | - | - | 0.88 | 1.00 | 1.90 | 1.15 |
| 4 | 32.71 | 0.29 | 1.83 | 47.88 | b.d.l. | b.d.l. | 17.29 | 100.05 | 0.94 | 0.01 | 0.05 | 1.03 | - | - | 0.87 | 1.00 | 1.90 | 1.19 |
| 5 | 30.66 | 1.03 | 2.10 | 49.47 | b.d.l. | b.d.l. | 15.94 | 99.20 | 0.91 | 0.03 | 0.06 | 1.10 | - | - | 0.83 | 1.00 | 1.92 | 1.33 |
| 6 | 30.81 | 0.66 | 2.24 | 49.86 | b.d.l. | b.d.l. | 15.75 | 99.36 | 0.92 | 0.02 | 0.06 | 1.11 | - | - | 0.82 | 1.00 | 1.93 | 1.35 |
| 7 | 30.90 | 0.88 | 2.89 | 47.30 | b.d.l. | b.d.l. | 17.94 | 99.91 | 0.90 | 0.02 | 0.08 | 1.02 | - | - | 0.91 | 1.00 | 1.93 | 1.13 |
| 8 | 29.66 | 1.34 | 3.43 | 47.68 | b.d.l. | b.d.l. | 17.11 | 99.22 | 0.87 | 0.04 | 0.10 | 1.04 | - | - | 0.87 | 1.00 | 1.91 | 1.19 |
| 9 | 26.10 | 2.75 | 5.79 | 48.63 | b.d.l. | b.d.l. | 16.73 | 100.03 | 0.76 | 0.08 | 0.16 | 1.06 | - | - | 0.85 | 1.00 | 1.91 | 1.24 |
| 10 | 9.10 | 13.04 | 13.88 | 44.00 | b.d.l. | b.d.l. | 19.72 | 99.80 | 0.26 | 0.36 | 0.38 | 0.95 | - | - | 0.99 | 1.00 | 1.94 | 0.95 |
| 11 | 8.80 | 11.22 | 14.87 | 44.86 | b.d.l. | 0.02 | 19.78 | 100.50 | 0.26 | 0.32 | 0.42 | 1.00 | - | 0.00 | 1.03 | 1.00 | 2.02 | 0.97 |
| 12 | 8.35 | 23.15 | 2.39 | 50.26 | b.d.l. | b.d.l. | 15.26 | 99.43 | 0.26 | 0.67 | 0.07 | 1.15 | - | - | 0.81 | 1.00 | 1.96 | 1.41 |
| 13 | 9.78 | 20.08 | 3.56 | 48.40 | b.d.l. | b.d.l. | 16.86 | 99.70 | 0.30 | 0.59 | 0.10 | 1.12 | - | - | 0.91 | 1.00 | 2.03 | 1.23 |
| 14 | 9.57 | 13.81 | 12.48 | 44.01 | b.d.l. | b.d.l. | 19.67 | 99.55 | 0.28 | 0.38 | 0.34 | 0.95 | - | - | 0.99 | 1.00 | 1.94 | 0.96 |
| 15 | 8.86 | 13.61 | 13.46 | 44.28 | b.d.l. | b.d.l. | 19.21 | 99.47 | 0.26 | 0.37 | 0.37 | 0.95 | - | - | 0.97 | 1.00 | 1.92 | 0.99 |
| 16 | 35.08 | 0.15 | 0.07 | 44.65 | b.d.l. | 0.03 | 19.91 | 99.89 | 0.99 | 0.00 | 0.00 | 0.94 | - | 0.00 | 0.98 | 1.00 | 1.93 | 0.96 |
| 17 | 35.02 | 0.08 | 0.40 | 43.10 | b.d.l. | 0.05 | 20.90 | 99.59 | 0.99 | 0.00 | 0.01 | 0.91 | - | 0.00 | 1.03 | 1.00 | 1.93 | 0.88 |
| 18 | 33.68 | 0.56 | 0.71 | 43.89 | b.d.l. | 0.04 | 20.39 | 99.28 | 0.97 | 0.02 | 0.02 | 0.94 | - | 0.00 | 1.02 | 1.00 | 1.96 | 0.92 |
| 19 | 33.75 | 0.69 | 1.37 | 43.68 | b.d.l. | b.d.l. | 20.47 | 99.96 | 0.95 | 0.02 | 0.04 | 0.91 | - | - | 1.00 | 1.00 | 1.91 | 0.91 |
| 20 | 33.07 | 1.04 | 1.47 | 43.64 | b.d.l. | 0.02 | 20.01 | 99.26 | 0.93 | 0.03 | 0.04 | 0.92 | - | 0.00 | 0.98 | 1.00 | 1.90 | 0.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mederski, S.; Pršek, J.; Dimitrova, D.; Hyseni, B. A Combined EPMA and LA-ICP-MS Investigation on Bi-Cu-Au Mineralization from the Kizhnica Ore Field (Vardar Zone, Kosovo). Minerals 2021, 11, 1223. https://doi.org/10.3390/min11111223
Mederski S, Pršek J, Dimitrova D, Hyseni B. A Combined EPMA and LA-ICP-MS Investigation on Bi-Cu-Au Mineralization from the Kizhnica Ore Field (Vardar Zone, Kosovo). Minerals. 2021; 11(11):1223. https://doi.org/10.3390/min11111223
Chicago/Turabian StyleMederski, Sławomir, Jaroslav Pršek, Dimitrina Dimitrova, and Bahri Hyseni. 2021. "A Combined EPMA and LA-ICP-MS Investigation on Bi-Cu-Au Mineralization from the Kizhnica Ore Field (Vardar Zone, Kosovo)" Minerals 11, no. 11: 1223. https://doi.org/10.3390/min11111223
APA StyleMederski, S., Pršek, J., Dimitrova, D., & Hyseni, B. (2021). A Combined EPMA and LA-ICP-MS Investigation on Bi-Cu-Au Mineralization from the Kizhnica Ore Field (Vardar Zone, Kosovo). Minerals, 11(11), 1223. https://doi.org/10.3390/min11111223






