Effective Removal of Barrier Layer on the Surface of Low-Nickel Matte in an FeCl3-HCl-H2O Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemical Measurements
2.2. Characterization
3. Results and Discussion
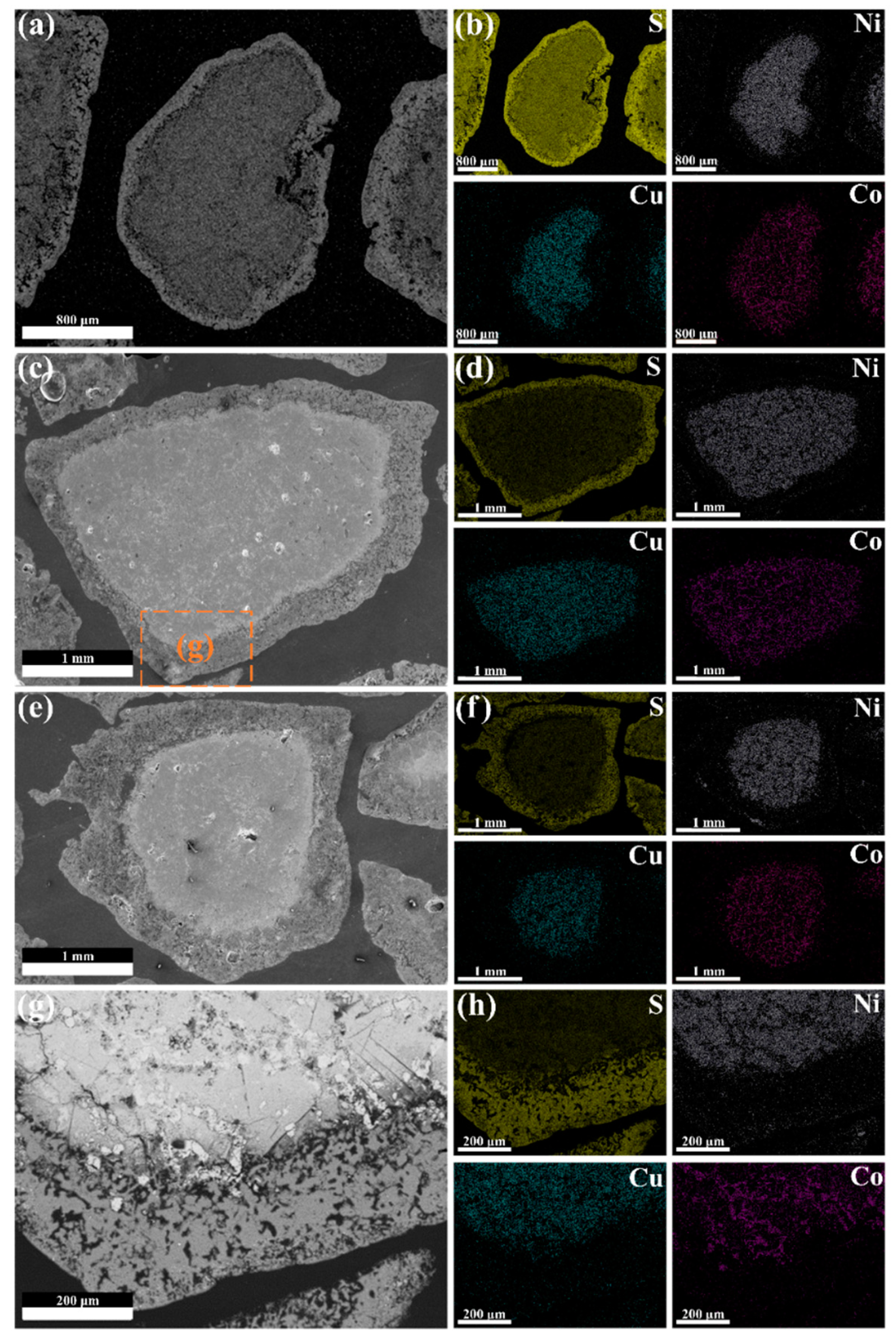
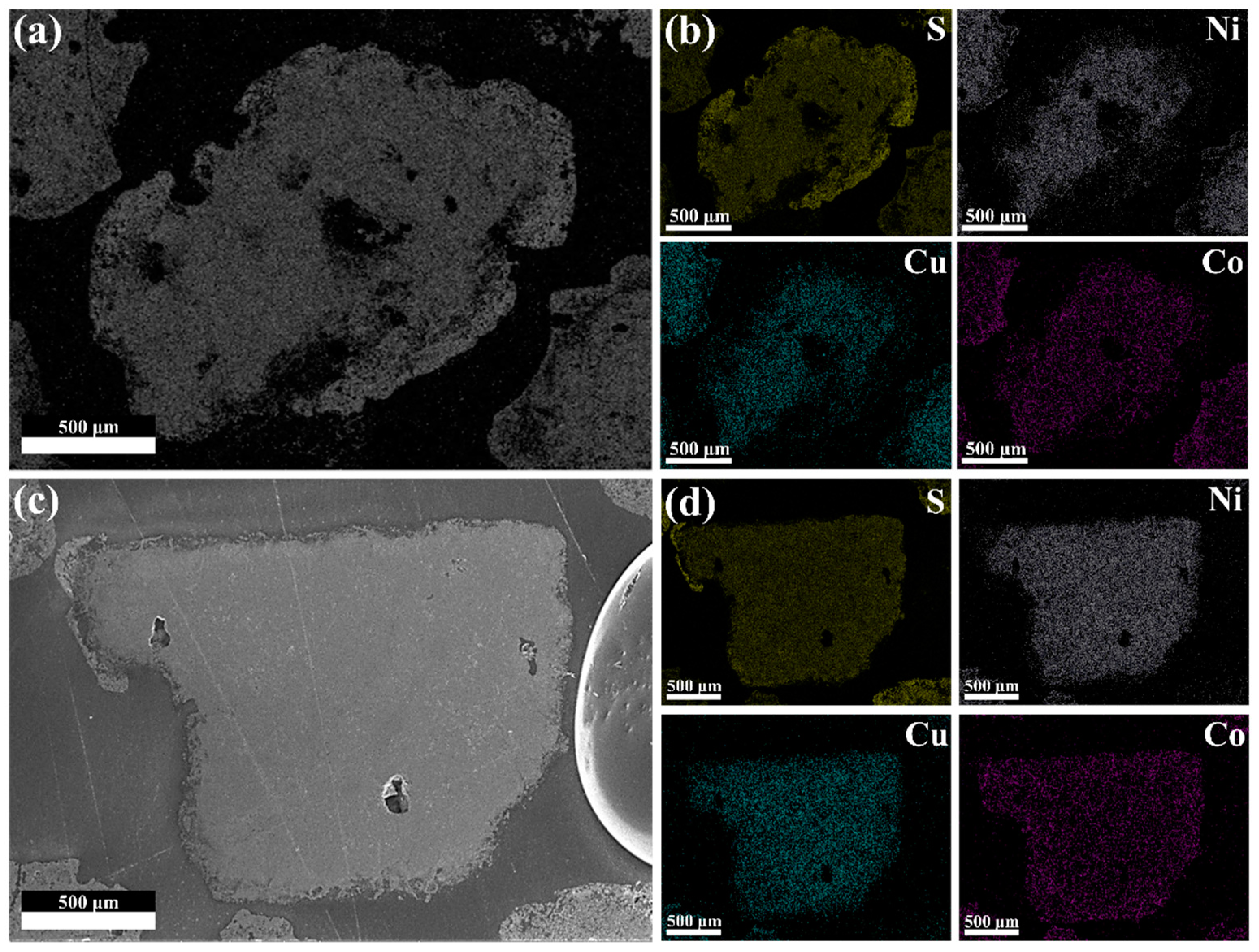
3.1. Characterization of the Low-Nickel Matte
3.2. Chemical Feasibility of the Leaching Process
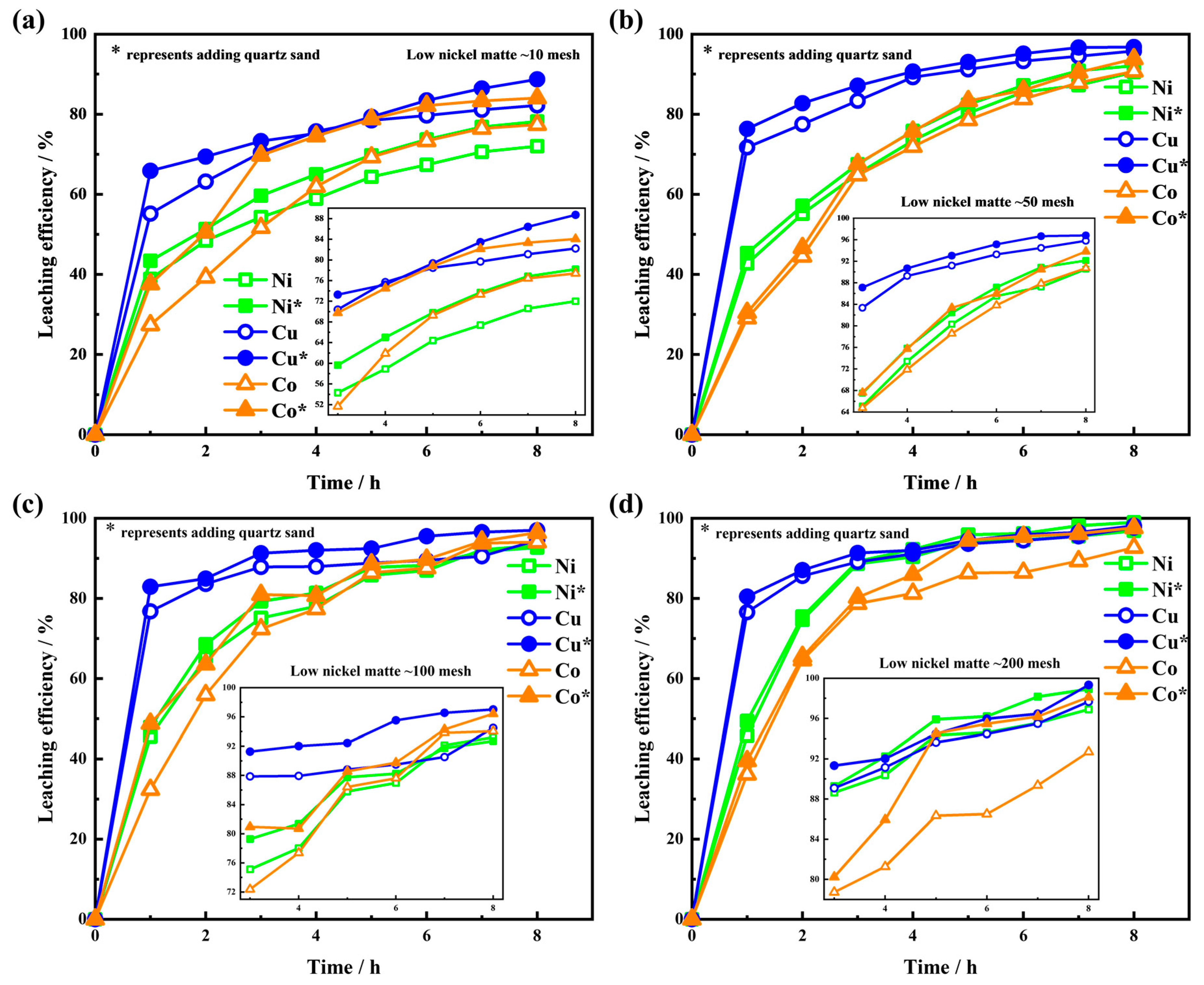
3.3. Leaching Results of the Low-Nickel Matte Particles
3.4. Leaching Results of the Low-Nickel Matte Interface with Quartz Sand
3.5. Leaching Efficiency of Low-Nickel Matte with Different Particle Sizes
3.6. Characterization of Quartz Sand
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mu, W.; Cui, F.; Xin, H.; Zhai, Y.; Xu, Q. A novel process for simultaneously extracting Ni and Cu from mixed oxide-sulfide copper-nickel ore with highly alkaline gangue via FeCl3∙6H2O chlorination and water leaching. Hydrometallurgy 2020, 191, 105187. [Google Scholar] [CrossRef]
- Finkelstein, N.P. The activation of sulphide minerals for flotation: A review. Int. J. Miner. Process. 1997, 52, 81–120. [Google Scholar] [CrossRef]
- Cui, F.; Mu, W.; Wang, S.; Xin, H.; Xu, Q.; Zhai, Y. A sustainable and selective roasting and water-leaching process to simultaneously extract valuable metals from low-grade Ni-Cu matte. JOM 2018, 70, 1977–1984. [Google Scholar] [CrossRef]
- Moskalyk, R.R.; Alfantazi, A.M. Nickel laterite processing and electrowinning practice. Miner. Eng. 2002, 15, 593–605. [Google Scholar] [CrossRef]
- Plasket, R.P.; Romanchuk, S. Recovery of nickel and copper from high-grade matte at Impala Platinum by the Sherritt process. Hydrometallurgy 1978, 3, 135–151. [Google Scholar] [CrossRef]
- Lamya, R.M.; Lorenzen, L. A study of factors influencing the kinetics of copper cementation during atmospheric leaching of converter matte. J. South Afr. Inst. Min. Metall. 2005, 3, 21–28. [Google Scholar]
- Rademan, J.A.M.; Lorenzen, L.; van Deventer, J.S.J. The leaching characteristics of Ni-Cu matte in the acid-oxygen pressure leach process at Impala Platinum. Hydrometallurgy 1999, 52, 231–252. [Google Scholar] [CrossRef]
- Sui, C.C.; Brienne, S.H.R.; Rao, S.R.; Xu, Z.; Finch, J.A. Metal ion production and transfer between sulphide minerals. Miner. Eng. 1995, 8, 1523–1539. [Google Scholar] [CrossRef]
- Niu, Y.; Sun, F.; Xu, Y.; Cong, Z.; Wang, E. Applications of electrochemical techniques in mineral analysis. Talanta 2014, 127, 211–218. [Google Scholar] [CrossRef]
- Chen, G.; Gao, J.; Guo, M.Z.M. Efficient and selective recovery of Ni, Cu, and Co from low-nickel matte via a hydrometallurgical process. Int. J. Min. Met. Mater. 2017, 24, 249–256. [Google Scholar] [CrossRef]
- Xiao, W.; Chen, X.; Liu, X.; Zhao, Z.; Li, Y. A method for extracting valuable metals from low nickel matte by non-oxidative leaching with H2SO4. Sep. Purif. Technol. 2021, 270, 118789. [Google Scholar] [CrossRef]
- Xiao, W.; Liu, X.; Zhao, Z. Kinetics of nickel leaching from low-nickel matte in sulfuric acid solution under atmospheric pressure. Hydrometallurgy 2020, 194, 105353. [Google Scholar] [CrossRef]
- Fu, Y.; Li, B.; Fan, C.; Zhai, X.; Zhang, X.; Li, D. Selective leaching of nickel from low-sulfur Ni-Cu matte at atmospheric pressure. Trans. Nonferr. Met. Soc. 2010, 20, 71–76. [Google Scholar] [CrossRef]
- Huang, K.; Li, Q.; Chen, J. Recovery of copper, nickel and cobalt from acidic pressure leaching solutions of low-grade sulfide flotation concentrates. Miner. Eng. 2007, 20, 722–728. [Google Scholar] [CrossRef]
- Park, K.H.; Mohapatra, D.; Nam, C.W.; Kim, H.I. A comparative study of different leaching processes for the extraction of Cu, Ni and Co from a complex matte. Korean. J. Chem. Eng. 2007, 24, 835–842. [Google Scholar] [CrossRef]
- Park, K.H.; Mohapatra, D.; Reddy, B.R.; Nam, C.W. A study on the oxidative ammonia/ammonium sulphate leaching of a complex (Cu-Ni-Co-Fe) matte. Hydrometallurgy 2007, 86, 164–171. [Google Scholar] [CrossRef]
- Park, K.H.; Mohapatra, D.; Reddy, B.R. A study on the acidified ferric chloride leaching of a complex (Cu–Ni–Co–Fe) matte. Sep. Purif. Technol. 2006, 51, 332–337. [Google Scholar] [CrossRef]
- Muzenda, E.; Ramatsa, I.M.; Ntuli, F.; Abdulkareem, A.S.; Afolabi, A.S. Parametric effects on leaching behavior of nickel-copper matte in ammonia. Particul. Sci. Technol. 2013, 31, 319–325. [Google Scholar] [CrossRef]
- Chen, G.; Wang, H.; Zhang, M.; Guo, M. High efficient leaching of Ni, Cu and Co from low nickel matte. Chin. J. Nonferrous Met. 2017, 27, 1936–1942. [Google Scholar]
- Anand, S.; Pao, P.K.; Jena, P.K. Recovery of metal values from copper converter and smelter slags by ferric chloride leaching. Hydrometallurgy 1980, 5, 355–365. [Google Scholar] [CrossRef]
- Hashemzadeh, M.; Dixon, D.G.; Liu, W. Modelling the kinetics of chalcocite leaching in acidified ferric chloride media under fully controlled pH and potential. Hydrometallurgy 2019, 186, 275–283. [Google Scholar] [CrossRef]
- Harahsheh, M.A.; Kingman, S.; Harahsheh, A.A. Ferric chloride leaching of chalcopyrite: Synergetic effect of CuCl2. Hydrometallurgy 2008, 91, 89–97. [Google Scholar] [CrossRef]
- Almeida, V.O.; Schneider, I.A.H. Production of a ferric chloride coagulant by leaching an iron ore tailing. Miner. Eng. 2020, 156, 106511. [Google Scholar] [CrossRef]
- Carneiro, M.F.C.; Leão, V.A. The role of sodium chloride on surface properties of chalcopyrite leached with ferric sulphate. Hydrometallurgy 2007, 87, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Maurice, D.; Hawk, J.A. Ferric chloride leaching of a mechanically activated pentlandite-chalcopyrite concentrate. Hydrometallurgy 1999, 52, 289–312. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Jeffrey, M.I.; Zhu, Y.; Lawson, F. Studies of pentlandite leaching in mixed oxygenated acidic chloride-sulfate solutions. Hydrometallurgy 2000, 56, 63–74. [Google Scholar] [CrossRef]
- Adamou, A.; Nicolaides, A.; Varotsis, C. Bio-hydrometallurgy dynamics of copper sulfide-minerals probed by microFTIR mapping and Raman microspectroscopy. Miner. Eng. 2019, 132, 39–47. [Google Scholar] [CrossRef]
- Sasaki, K.; Nakamuta, Y.; Hirajima, T.; Tuovinen, O.H. Raman characterization of secondary minerals formed during chalcopyrite leaching with Acidithiobacillus ferrooxidans. Hydrometallurgy 2009, 95, 153–158. [Google Scholar] [CrossRef]
- Parker, G.K.; Hope, G.A.; Woods, R. Gold-enhanced Raman observation of chalcopyrite leaching, Colloid. Surf. A 2008, 325, 132–140. [Google Scholar] [CrossRef]
- Zhu, J.; Tao, W.; Chen, J.; Xu, Q.; Chen, H.; Zou, X.; Lu, X. Experimental and first principle analysis accounting for oxidative reactions of low nickel matte in FeCl3-HCl-H2O solution. Chin. J. Nonferrous Met. 2020, 30, 2658–2671. [Google Scholar]
- Hope, G.A.; Woods, R.; Munce, C.G. Raman microprobe mineral identification. Miner. Eng. 2001, 14, 1565–1577. [Google Scholar] [CrossRef]
- Barkov, A.Y.; Shvedov, G.I.; Nikiforov, A.A.; Martin, R.F. Platinum-group minerals from Seyba, Eastern Sayans, Russia, and substitutions in the PGE-rich pentlandite and ferhodsite series. Miner. Mag. 2019, 83, 531–538. [Google Scholar] [CrossRef]
- Giebel, R.J.; Gauert, C.D.K.; Marks, M.A.W.; Costin, G.; Markl, G. Multi-stage formation of REE minerals in the Palabora Carbonatite Complex, South Africa. Am. Mineral. 2017, 102, 1218–1233. [Google Scholar] [CrossRef]
- Pashkov, G.L.; Mikhlina, E.V.; Kholmogorov, A.G.; Mikhlin, Y.L. Effect of potential and ferric ions on lead sulfide dissolution in nitric acid. Hydrometallurgy 2002, 63, 171–179. [Google Scholar] [CrossRef]
- Mohammadrezsa, F.; Mohammad, N.; Ziaeddin, S.S. Nickel extraction from low grade laterite by agitation leaching at atmospheric pressure. Int. J. Min. Sci. Technol. 2014, 24, 543–548. [Google Scholar] [CrossRef]
- Muir, D.M.; Ho, E. Process review and electrochemistry of nickel sulphides and nickel mattes in acidic sulphate and chloride media. Min. Proc. Ext. Met. Rev. 2006, 115, 57–65. [Google Scholar] [CrossRef]
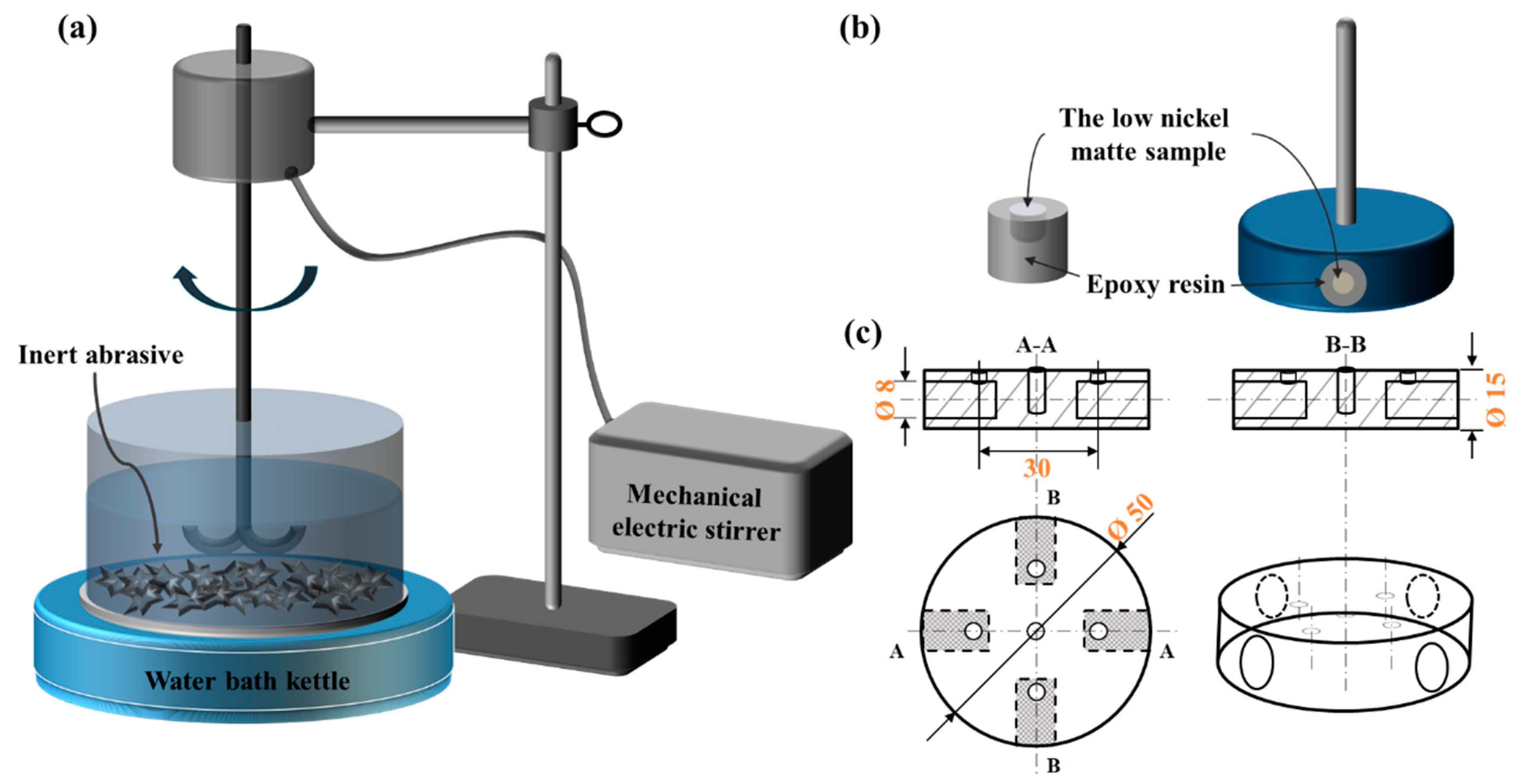






| Revolutions per Minute (RPM)/n (r/min) | Relative Velocity/v (m/s) |
|---|---|
| 200 | 0.52 |
| 350 | 0.84 |
| 500 | 1.31 |
| Component | Mass Fraction/% |
|---|---|
| Ni | 25.51 |
| Cu | 14.02 |
| Fe | 29.67 |
| Co | 1.39 |
| S | 27.84 |
| Others | 1.57 |
| Mineral | % by Weight |
|---|---|
| Pentlandite (Fe5Ni4S8) | 63.96 |
| Bornite (Cu5FeS4) | 23.85 |
| Magnetite (Fe3O4) | 3.29 |
| Ferronickel (Ni3Fe) | 6.83 |
| Others | 2.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Lei, Y.; Hu, X.; Xu, Q.; Zou, X.; Cheng, H.; Lu, X. Effective Removal of Barrier Layer on the Surface of Low-Nickel Matte in an FeCl3-HCl-H2O Solution. Minerals 2021, 11, 1219. https://doi.org/10.3390/min11111219
Zhu C, Lei Y, Hu X, Xu Q, Zou X, Cheng H, Lu X. Effective Removal of Barrier Layer on the Surface of Low-Nickel Matte in an FeCl3-HCl-H2O Solution. Minerals. 2021; 11(11):1219. https://doi.org/10.3390/min11111219
Chicago/Turabian StyleZhu, Chuncheng, Yu Lei, Xinbo Hu, Qian Xu, Xingli Zou, Hongwei Cheng, and Xionggang Lu. 2021. "Effective Removal of Barrier Layer on the Surface of Low-Nickel Matte in an FeCl3-HCl-H2O Solution" Minerals 11, no. 11: 1219. https://doi.org/10.3390/min11111219
APA StyleZhu, C., Lei, Y., Hu, X., Xu, Q., Zou, X., Cheng, H., & Lu, X. (2021). Effective Removal of Barrier Layer on the Surface of Low-Nickel Matte in an FeCl3-HCl-H2O Solution. Minerals, 11(11), 1219. https://doi.org/10.3390/min11111219






