Long-Term Evolution of Uranium Mobility within Sulfated Mill Tailings in Arid Regions: A Reactive Transport Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Geographic Zone of Interest
2.2. Materials
2.2.1. Mineralogy
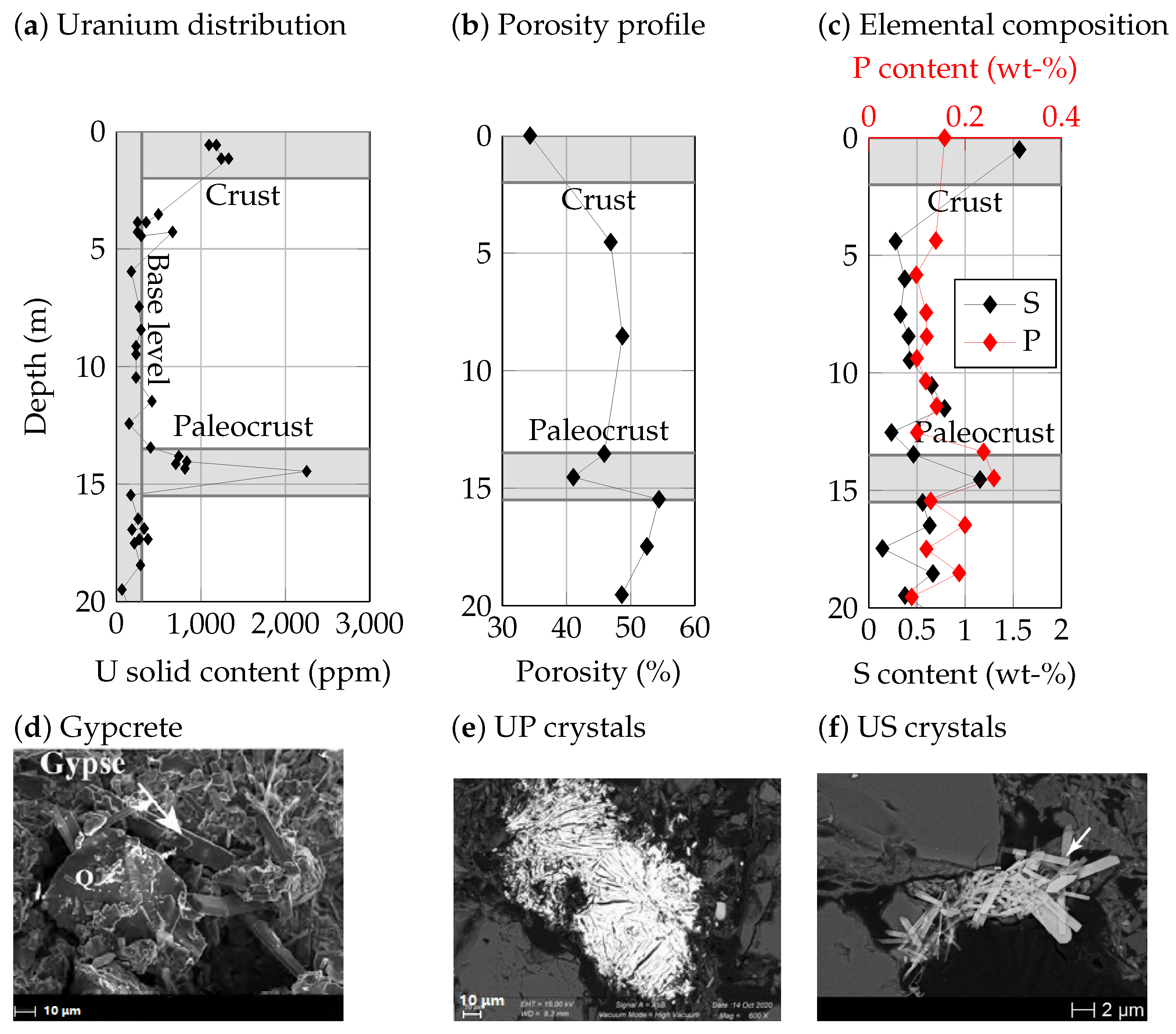
2.2.2. Tailings Evolution
2.3. Methods
2.3.1. Mathematical Description
2.3.2. Evaporation and Flow
2.3.3. Geochemical System and Thermodynamic Database
2.3.4. Simulation Descriptions
3. Results
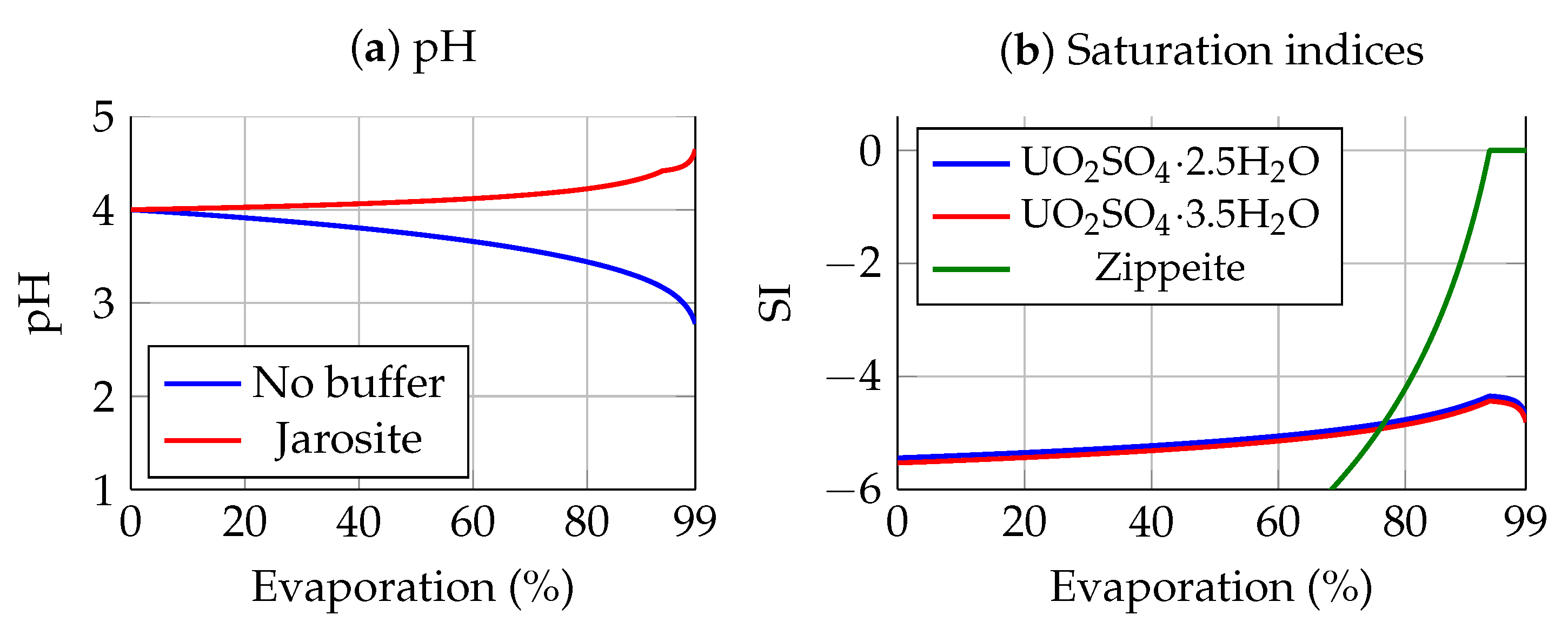
3.1. Geochemical Evolution during Evaporation
3.2. Gypcrete Formation
3.3. Apatite Dissolution and Passivation
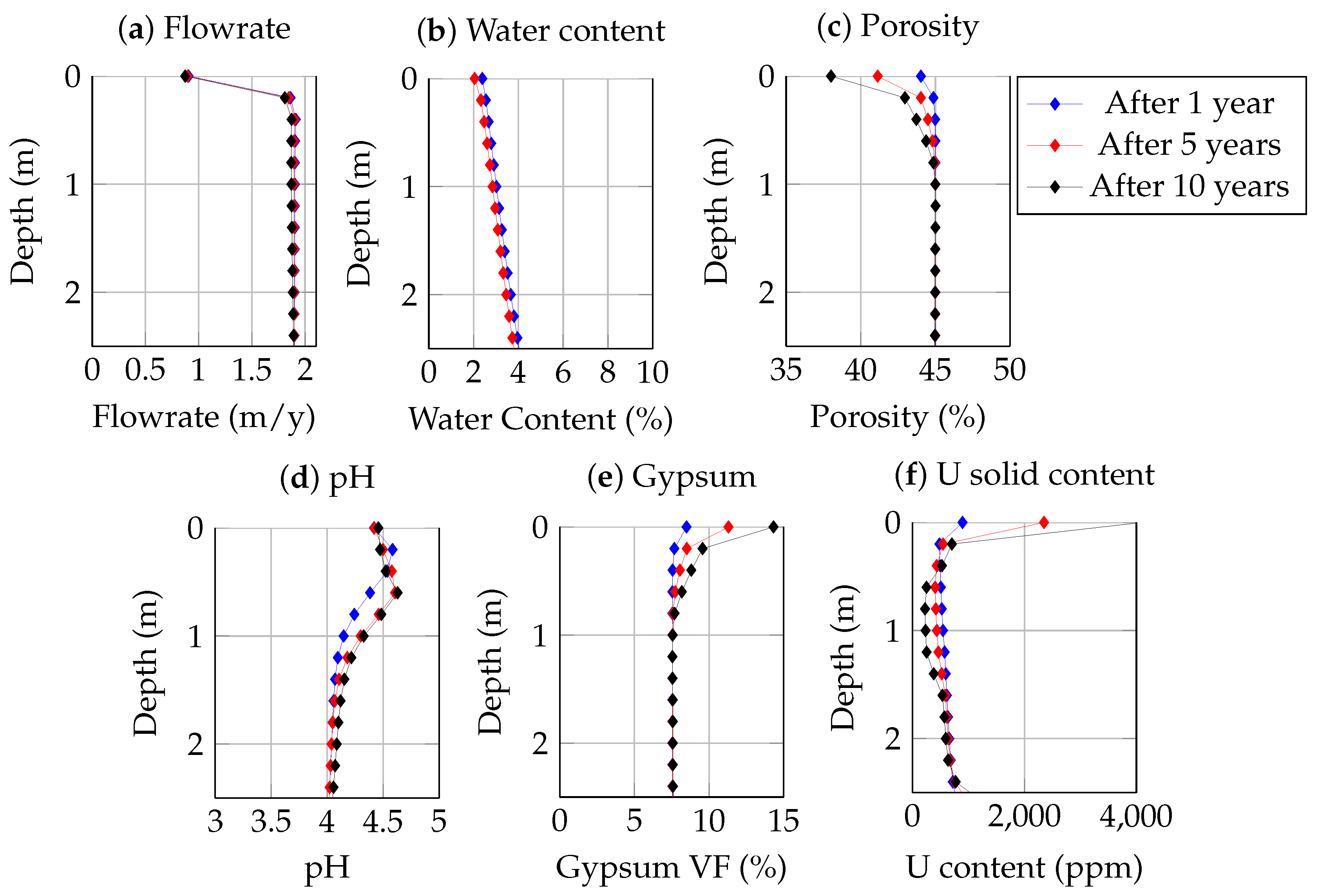
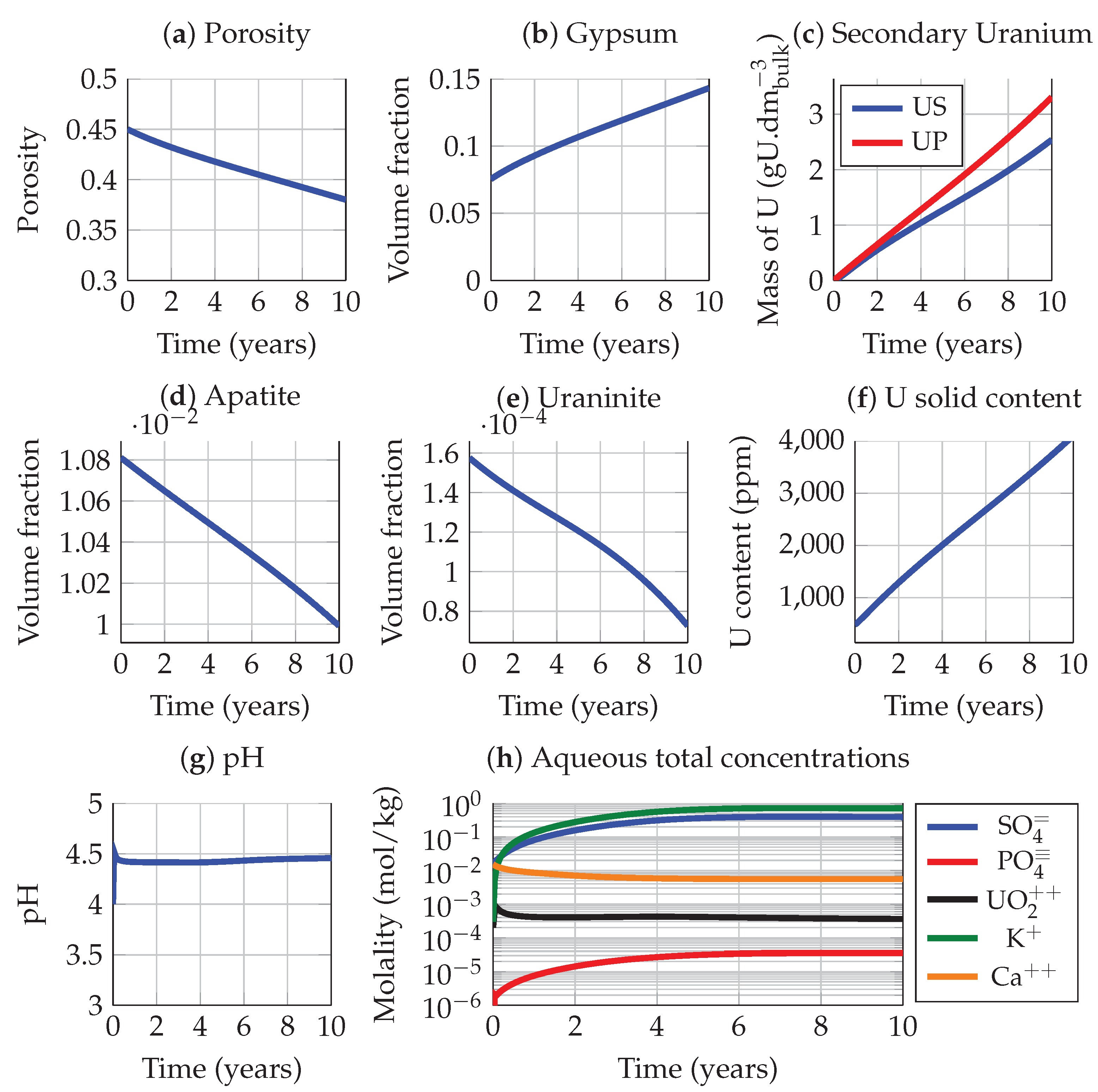
3.4. System Evolution after Burying the Tailings
4. Discussion
5. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CK | Cominak |
| US | Uranyl sulfate (mineral) |
| UP | Uranyl phosphate (mineral) |
| VF | Volume fraction |
References
- Blowes, D.W. The environmental effects of mine wastes. Proc. Explor. 1997, 97, 887–892. [Google Scholar]
- Jamieson, H.E. Geochemistry and mineralogy of solid mine waste: Essential knowledge for predicting environmental impact. Elements 2011, 7, 381–386. [Google Scholar] [CrossRef]
- Vriens, B.; Plante, B.; Seigneur, N.; Jamieson, H. Mine waste rock: Insights for sustainable hydrogeochemical management. Minerals 2020, 10, 728. [Google Scholar] [CrossRef]
- Wolkersdorfer, C.; Nordstrom, D.K.; Beckie, R.D.; Cicerone, D.S.; Elliot, T.; Edraki, M.; Valente, T.; França, S.C.A.; Kumar, P.; Lucero, R.A.O.; et al. Guidance for the integrated use of hydrological, geochemical, and isotopic tools in mining operations. Mine Water Environ. 2020, 39, 204–228. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, B.; Mayya, Y.; Sapra, B.; Gaware, J.; Banerjee, K.; Kushwaha, H. Radon exhalation studies in an Indian uranium tailings pile. Radiat. Meas. 2010, 45, 237–241. [Google Scholar] [CrossRef]
- Abdelouas, A. Uranium mill tailings: Geochemistry, mineralogy, and environmental impact. Elements 2006, 2, 335–341. [Google Scholar] [CrossRef]
- Morin, K.A.; Cherry, J.A.; Dave, N.K.; Lim, T.P.; Vivyurka, A.J. Migration of acidic groundwater seepage from uranium-tailings impoundments, 1. Field study and conceptual hydrogeochemical model. J. Contam. Hydrol. 1988, 2, 271–303. [Google Scholar] [CrossRef]
- Dubrovsky, N.; Morin, K.; Cherry, J.; Smyth, D. Uranium tailings acidification and subsurface contaminant migration in a sand aquifer. Water Qual. Res. J. 1984, 19, 55–89. [Google Scholar] [CrossRef]
- Liu, B.; Peng, T.; Sun, H.; Yue, H. Release behavior of uranium in uranium mill tailings under environmental conditions. J. Environ. Radioact. 2017, 171, 160–168. [Google Scholar] [CrossRef]
- Bernhard, G.; Geipel, G.; Brendler, V.; Nitsche, H. Speciation of uranium in seepage waters of a mine tailing pile studied by time-resolved laser-induced fluorescence spectroscopy (TRLFS). Radiochim. Acta 1996, 74, 87–92. [Google Scholar] [CrossRef]
- Geipel, G.; Brachmann, A.; Brendler, V.; Bernhard, G.; Nitsche, H. Uranium (VI) sulfate complexation studied by time-resolved laser-induced fluorescence spectroscopy (TRLFS). Radiochim. Acta 1996, 75, 199–204. [Google Scholar] [CrossRef]
- Bernhard, G.; Geipel, G.; Brendler, V.; Nitsche, H. Uranium speciation in waters of different uranium mining areas. J. Alloys Compd. 1998, 271, 201–205. [Google Scholar] [CrossRef]
- Reiller, P.E.; Descostes, M. Development and application of the thermodynamic database PRODATA dedicated to the monitoring of mining activities from exploration to remediation. Chemosphere 2020, 251, 126301. [Google Scholar] [CrossRef] [PubMed]
- Déjeant, A.; Galoisy, L.; Roy, R.; Calas, G.; Boekhout, F.; Phrommavanh, V.; Descostes, M. Evolution of uranium distribution and speciation in mill tailings, COMINAK Mine, Niger. Sci. Total Environ. 2016, 545, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Déjeant, A.; Bourva, L.; Sia, R.; Galoisy, L.; Calas, G.; Phrommavanh, V.; Descostes, M. Field analyses of 238U and 226Ra in two uranium mill tailings piles from Niger using portable HPGe detector. J. Environ. Radioact. 2014, 137, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.; Hendry, M.J.; Kotzer, T.; Hughes, K.A. Geochemistry of uranium mill tailings in the Athabasca basin, Saskatchewan, Canada: A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1237–1293. [Google Scholar] [CrossRef]
- Ballini, M.; Chautard, C.; Nos, J.; Phrommavanh, V.; Beaucaire, C.; Besancon, C.; Boizard, A.; Cathelineau, M.; Peiffert, C.; Vercouter, T.; et al. A multi-scalar study of the long-term reactivity of uranium mill tailings from Bellezane site (France). J. Environ. Radioact. 2020, 218, 106223. [Google Scholar] [CrossRef]
- Chautard, C.; Beaucaire, C.; Gerard, M.; Roy, R.; Savoye, S.; Descostes, M. Geochemical characterization of uranium mill tailings (Bois Noirs Limouzat, France) highlighting the U and 226Ra retention. J. Environ. Radioact. 2020, 218, 106251. [Google Scholar] [CrossRef] [PubMed]
- Bigham, J.; Nordstrom, D.K. Iron and aluminum hydroxysulfates from acid sulfate waters. Rev. Mineral. Geochem. 2000, 40, 351–403. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; Blowes, D.W.; Ptacek, C.J. Hydrogeochemistry and microbiology of mine drainage: An update. Appl. Geochem. 2015, 57, 3–16. [Google Scholar] [CrossRef]
- Husson, A.; Leermakers, M.; Descostes, M.; Lagneau, V. Environmental geochemistry and bioaccumulation/bioavailability of uranium in a post-mining context–The Bois-Noirs Limouzat mine (France). Chemosphere 2019, 236, 124341. [Google Scholar] [CrossRef]
- Badía, D.; Martí, C.; Casanova, J.; Gillot, T.; Cuchí, J.A.; Palacio, J.; Andrés, R. A Quaternary soil chronosequence study on the terraces of the Alcanadre River (semiarid Ebro Basin, NE Spain). Geoderma 2015, 241, 158–167. [Google Scholar] [CrossRef]
- Jacobson, G.; Arakel, A.; Yijian, C. The central Australian groundwater discharge zone: Evolution of associated calcrete and gypcrete deposits. Aust. J. Earth Sci. 1988, 35, 549–565. [Google Scholar] [CrossRef]
- Chen, X.Y. Pedogenic gypcrete formation in arid central Australia. Geoderma 1997, 77, 39–61. [Google Scholar] [CrossRef]
- Aref, M.A. Classification and depositional environments of Quaternary pedogenic gypsum crusts (gypcrete) from east of the Fayum Depression, Egypt. Sediment. Geol. 2003, 155, 87–108. [Google Scholar] [CrossRef]
- Carlisle, D. Concentration of uranium and vanadium in calcretes and gypcretes. Geol. Soc. Lond. Spec. Publ. 1983, 11, 185–195. [Google Scholar] [CrossRef]
- Molson, J.; Aubertin, M.; Bussière, B. Reactive transport modelling of acid mine drainage within discretely fractured porous media: Plume evolution from a surface source zone. Environ. Model. Softw. 2012, 38, 259–270. [Google Scholar] [CrossRef]
- Wilson, D.; Amos, R.T.; Blowes, D.W.; Langman, J.B.; Smith, L.; Sego, D.C. Diavik Waste Rock Project: Scale-up of a reactive transport model for temperature and sulfide-content dependent geochemical evolution of waste rock. Appl. Geochem. 2018, 96, 177–190. [Google Scholar] [CrossRef]
- Vriens, B.; Seigneur, N.; Mayer, K.U.; Beckie, R.D. Scale dependence of effective geochemical rates in weathering mine waste rock. J. Contam. Hydrol. 2020, 234, 103699. [Google Scholar] [CrossRef] [PubMed]
- Seigneur, N.; Vriens, B.; Beckie, R.; Mayer, K. Reactive transport modelling to investigate multi-scale waste rock weathering processes. J. Contam. Hydrol. 2021, 236, 103752. [Google Scholar] [CrossRef] [PubMed]
- Muniruzzaman, M.; Karlsson, T.; Ahmadi, N.; Kauppila, P.M.; Kauppila, T.; Rolle, M. Weathering of unsaturated waste rocks from Kevitsa and Hitura mines: Pilot-scale lysimeter experiments and reactive transport modeling. Appl. Geochem. 2021, 130, 104984. [Google Scholar] [CrossRef]
- Yi, X.; Su, D.; Seigneur, N.; Mayer, K.U. Modeling of Thermal-Hydrological-Chemical (THC) processes during waste rock weathering under permafrost conditions. Front. Water 2021, 3, 645675. [Google Scholar] [CrossRef]
- Garcia-Rios, M.; De Windt, L.; Luquot, L.; Casiot, C. Modeling of microbial kinetics and mass transfer in bioreactors simulating the natural attenuation of arsenic and iron in acid mine drainage. J. Hazard. Mater. 2021, 405, 124133. [Google Scholar] [CrossRef] [PubMed]
- Bain, J.; Mayer, K.; Blowes, D.; Frind, E.; Molson, J.; Kahnt, R.; Jenk, U. Modelling the closure-related geochemical evolution of groundwater at a former uranium mine. J. Contam. Hydrol. 2001, 52, 109–135. [Google Scholar] [CrossRef]
- Zhu, C.; Hu, F.Q.; Burden, D.S. Multi-component reactive transport modeling of natural attenuation of an acid groundwater plume at a uranium mill tailings site. J. Contam. Hydrol. 2001, 52, 85–108. [Google Scholar] [CrossRef]
- De Windt, L.; Burnol, A.; Montarnal, P.; van der Lee, J. Intercomparison of reactive transport models applied to UO2 oxidative dissolution and uranium migration. J. Contam. Hydrol. 2003, 61, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Montarnal, P.; Mügler, C.; Colin, J.; Descostes, M.; Dimier, A.; Jacquot, E. Presentation and use of a reactive transport code in porous media. Phys. Chem. Earth Parts A/B/C 2007, 32, 507–517. [Google Scholar] [CrossRef]
- Fang, Y.; Yabusaki, S.B.; Morrison, S.J.; Amonette, J.P.; Long, P.E. Multicomponent reactive transport modeling of uranium bioremediation field experiments. Geochim. Cosmochim. Acta 2009, 73, 6029–6051. [Google Scholar] [CrossRef]
- Avasarala, S.; Lichtner, P.C.; Ali, A.M.S.; González-Pinzón, R.; Blake, J.M.; Cerrato, J.M. Reactive transport of U and V from abandoned uranium mine wastes. Environ. Sci. Technol. 2017, 51, 12385–12393. [Google Scholar] [CrossRef] [Green Version]
- de Boissezon, H.; Levy, L.; Jakymiw, C.; Distinguin, M.; Guerin, F.; Descostes, M. Modeling uranium and 226Ra mobility during and after an acidic in situ recovery test (Dulaan Uul, Mongolia). J. Contam. Hydrol. 2020, 235, 103711. [Google Scholar] [CrossRef] [PubMed]
- Besançon, C.; Chautard, C.; Beaucaire, C.; Savoye, S.; Sardini, P.; Gérard, M.; Descostes, M. The role of barite in the post-mining stabilization of radium-226: A modeling contribution for sequential extractions. Minerals 2020, 10, 497. [Google Scholar] [CrossRef]
- Lagneau, V.; Regnault, O.; Descostes, M. Industrial deployment of reactive transport simulation: An application to uranium in situ recovery. Rev. Mineral. Geochem. 2019, 85, 499–528. [Google Scholar] [CrossRef] [Green Version]
- Collet, A.; Regnault, O.; Ozhogin, A.; Imantayeva, A.; L., G. 3D reactive transport simulation of Uranium In situ Recovery. Large scale well-field application in Shu Saryssu Bassin, Tortkuduk deposit (Kazakhstan). Hydrometallurgy 2021. submitted. [Google Scholar]
- Steefel, C.I.; Beckingham, L.E.; Landrot, G. Micro-continuum approaches for modeling pore-scale geochemical processes. Rev. Mineral. Geochem. 2015, 80, 217–246. [Google Scholar] [CrossRef]
- Gran, M.; Carrera, J.; Olivella, S.; Saaltink, M.W. Modeling evaporation processes in a saline soil from saturation to oven dry conditions. Hydrol. Earth Syst. Sci. 2011, 15, 2077–2089. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, K.; Hosokawa, T.; Wada, S.I.; Momii, K.; Jinno, K.; Berndtsson, R. Modelling reactive solute transport from groundwater to soil surface under evaporation. Hydrol. Process. Int. J. 2010, 24, 608–617. [Google Scholar] [CrossRef]
- Seigneur, N.; Lagneau, V.; Corvisier, J.; Dauzères, A. Recoupling flow and chemistry in variably saturated reactive transport modelling-An algorithm to accurately couple the feedback of chemistry on water consumption, variable porosity and flow. Adv. Water Resour. 2018, 122, 355–366. [Google Scholar] [CrossRef]
- Van Der Lee, J.; De Windt, L.; Lagneau, V.; Goblet, P. Module-oriented modeling of reactive transport with HYTEC. Comput. Geosci. 2003, 29, 265–275. [Google Scholar] [CrossRef]
- van der Lee, J.; De Windt, L.; Lagneau, V.; Goblet, P. Presentation and application of the reactive transport code HYTEC. In Developments in Water Science; Elsevier: Amsterdam, The Netherlands, 2002; Volume 47, pp. 599–606. [Google Scholar]
- Lagneau, V.; Van Der Lee, J. HYTEC results of the MoMas reactive transport benchmark. Comput. Geosci. 2010, 14, 435–449. [Google Scholar] [CrossRef] [Green Version]
- Déjeant, A. Réactivité de Résidus Miniers Après leur Entreposage: Conséquence sur la Spéciation de l’Uranium (Mine Uranifère de COMINAK, Niger. Ph.D. Thesis, Paris Diderot University, Paris, France, 2014. [Google Scholar]
- Dodo, A.; Zuppi, G.M. Variabilité climatique durant le Quaternaire dans la nappe du Tarat (Arlit, Niger). Comptes Rendus L’Académie Sci.-Ser. IIA-Earth Planet. Sci. 1999, 328, 371–379. [Google Scholar] [CrossRef]
- Brunel, J.; Bouron, B. Evaporation from free water tables in Sahelian and tropical Africa. In Evaporation from Free Water Tables in Sahelian and Tropical Africa; Centre for Agriculture and Biosciences International: Wallingford, UK, 1992. [Google Scholar]
- Alazard, M.; Leduc, C.; Travi, Y.; Boulet, G.; Salem, A.B. Estimating evaporation in semi-arid areas facing data scarcity: Example of the El Haouareb dam (Merguellil catchment, Central Tunisia). J. Hydrol. Reg. Stud. 2015, 3, 265–284. [Google Scholar] [CrossRef] [Green Version]
- Lahrouch, F.; Baptiste, B.; Dardenne, K.; Rothe, J.; Elkaim, E.; Descostes, M.; Gerard, M. Uranium speciation control by uranyl sulfate and phosphate in tailings subject to a Sahelian climate, Cominak, Niger. Chemosphere 2022, 287, 132–139. [Google Scholar] [CrossRef]
- Finch, R.; Murakami, T. Systematics and paragenesis of Uranium minerals. Rev. Mineral. 1999, 38, 91–179. [Google Scholar]
- Cretaz, F.; Szenknect, S.; Clavier, N.; Vitorge, P.; Mesbah, A.; Descostes, M.; Poinssot, C.; Dacheux, N. Solubility properties of synthetic and natural meta-torbernite. J. Nucl. Mater. 2013, 442, 195–207. [Google Scholar] [CrossRef]
- Boekhout, F.; Gérard, M.; Kanzari, A.; Michel, A.; Déjeant, A.; Galoisy, L.; Calas, G.; Descostes, M. Uranium migration and retention during weathering of a granitic waste rock pile. Appl. Geochem. 2015, 58, 123–135. [Google Scholar] [CrossRef]
- Kanzari, A.; Gérard, M.; Boekhout, F.; Galoisy, L.; Calas, G.; Descostes, M. Impact of incipient weathering on uranium migration in granitic waste rock piles from former U mines (Limousin, France). J. Geochem. Explor. 2017, 183, 114–126. [Google Scholar] [CrossRef] [Green Version]
- Reinoso-Maset, E.; Perdrial, N.; Steefel, C.I.; Um, W.; Chorover, J.; O’Day, P.A. Dissolved Carbonate and pH Control the Dissolution of Uranyl Phosphate Minerals in Flow-Through Porous Media. Environ. Sci. Technol. 2020, 54, 6031–6042. [Google Scholar] [CrossRef]
- Morrow, N.R. Physics and thermodynamics of capillary action in porous media. Ind. Eng. Chem. 1970, 62, 32–56. [Google Scholar] [CrossRef]
- Van Genuchten, M.T. A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef] [Green Version]
- Sharifironizi, M.; Szymanowski, J.E.; Sigmon, G.E.; Navrotsky, A.; Fein, J.B.; Burns, P.C. Thermodynamic studies of zippeite, a uranyl sulfate common in mine wastes. Chem. Geol. 2016, 447, 54–58. [Google Scholar] [CrossRef] [Green Version]
- Palandri, J.; Kharaka, Y. A Compilation of Rate Parameters of Water–Mineral Interaction Kinetics for Application to Geochemical Modeling; Open File Report 2004-1068; US Geological Survey: Reston, VA, USA, 2004.

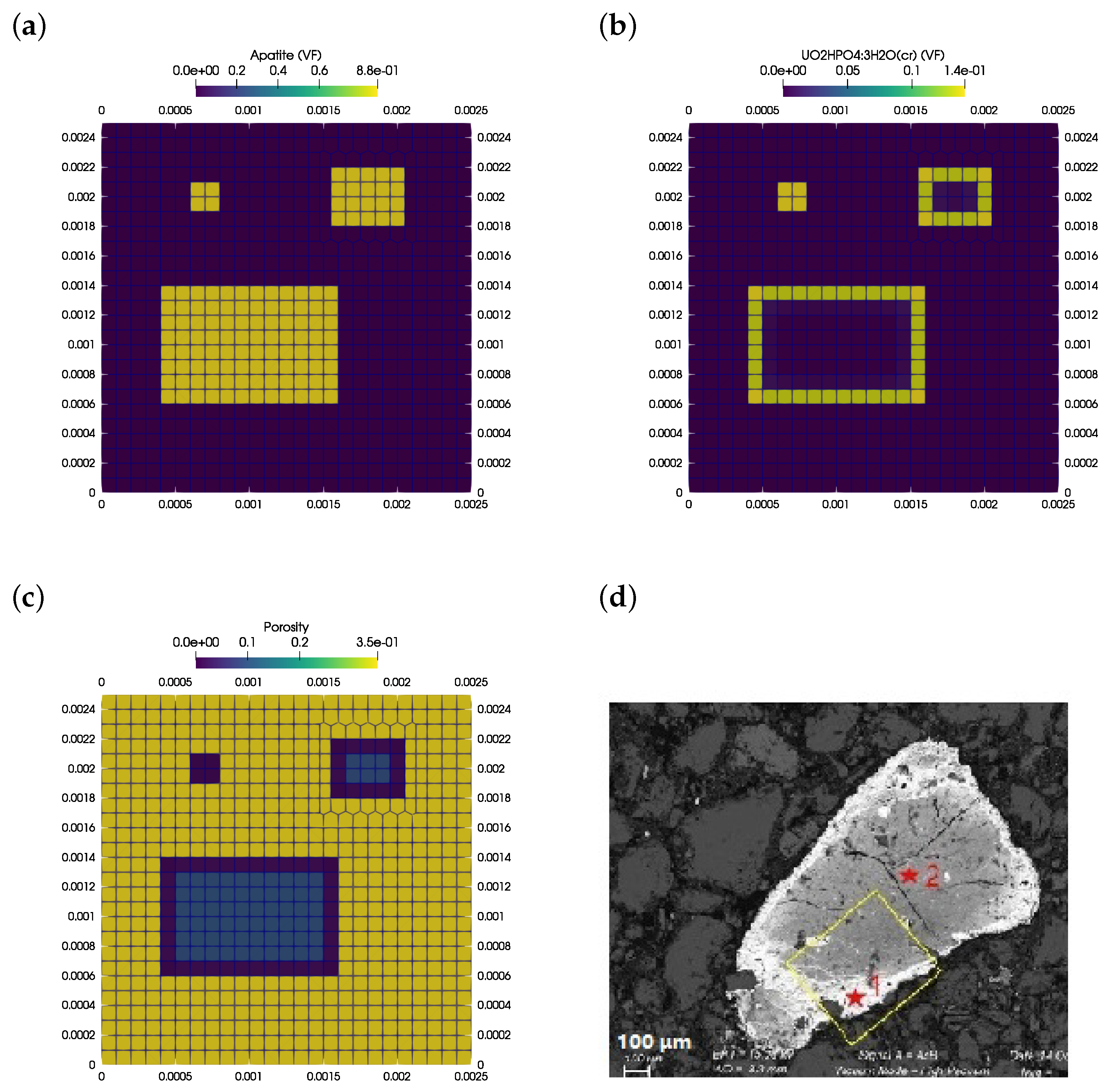
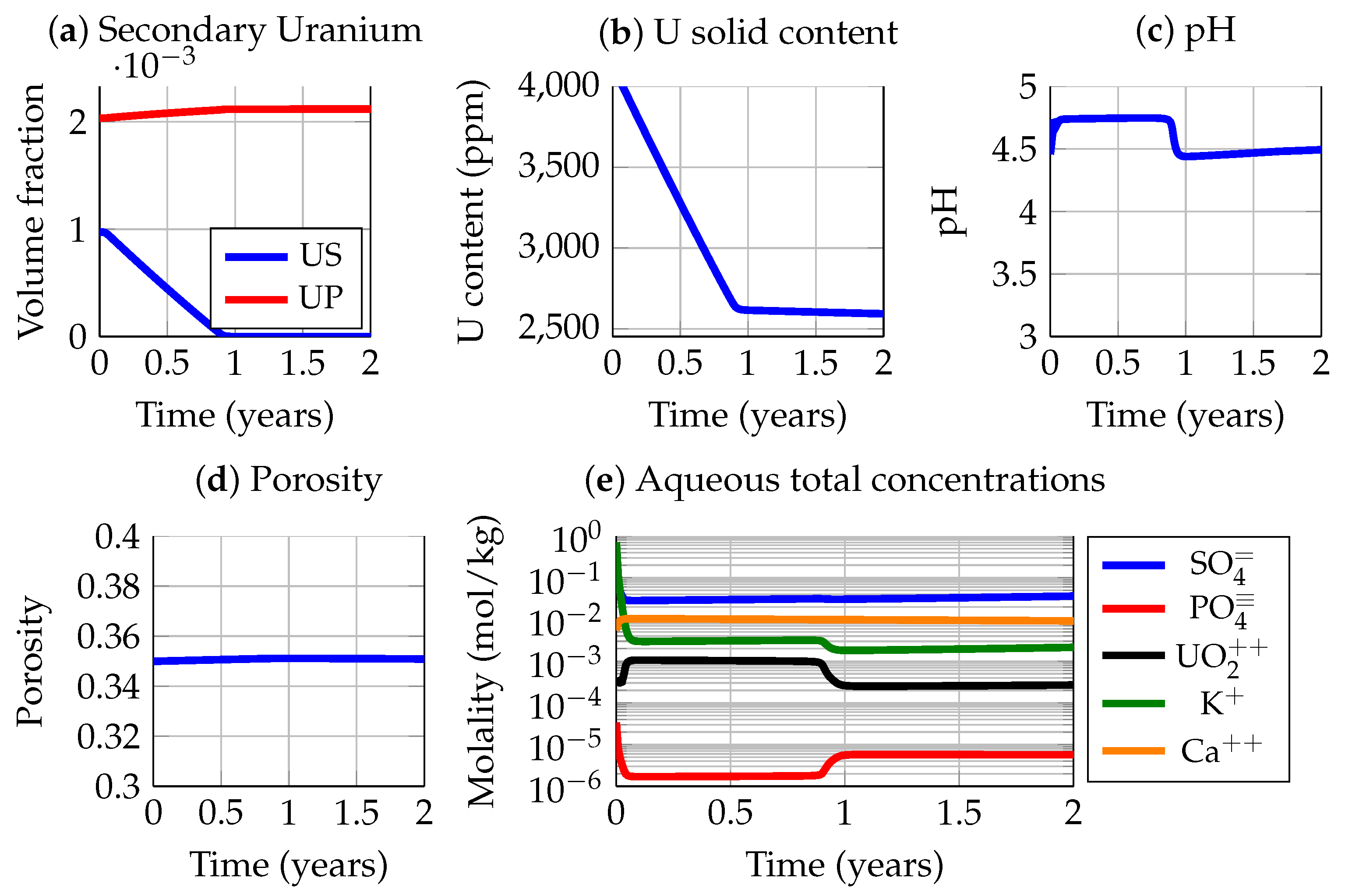
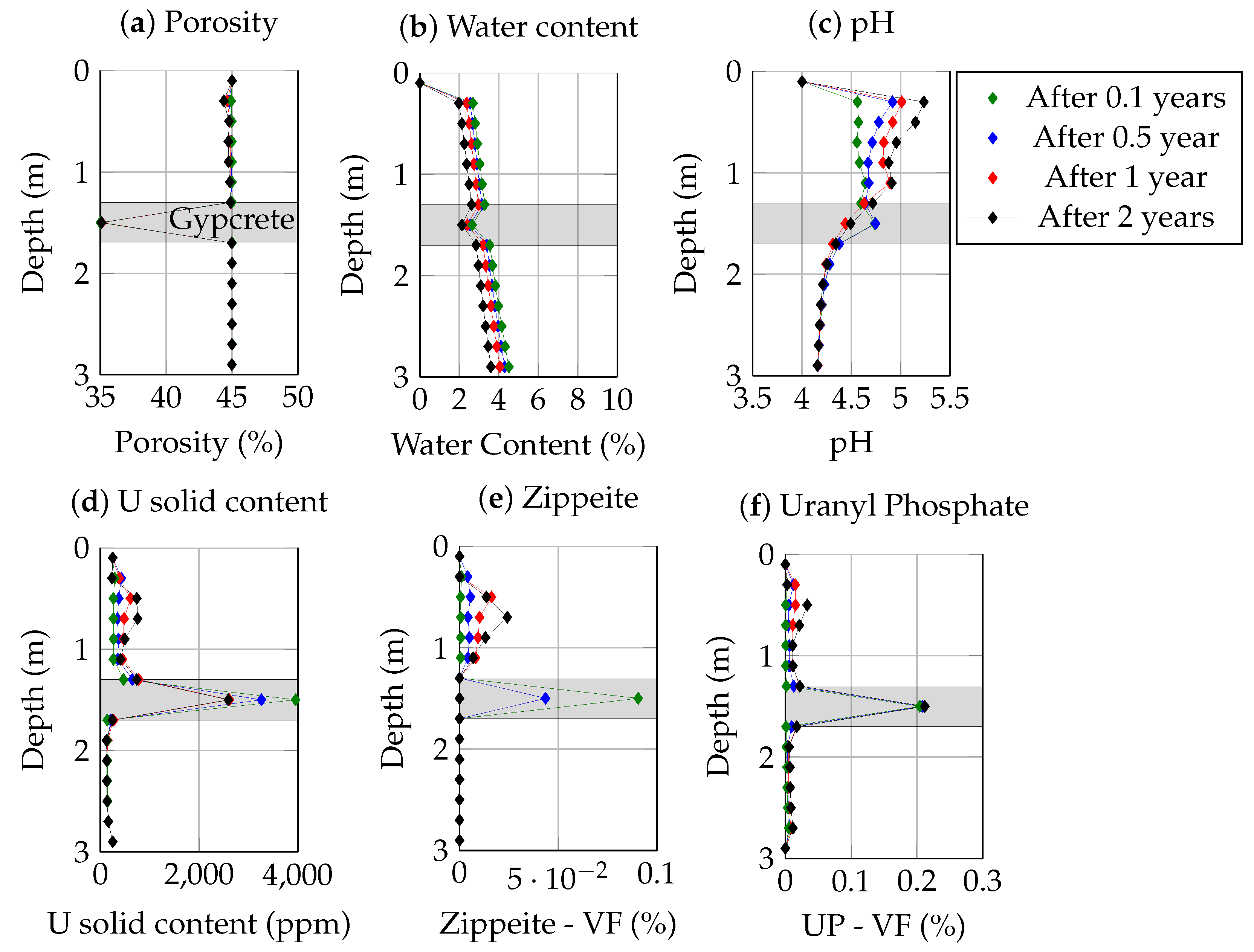
| Parameter | Value | Unit |
|---|---|---|
| Hydraulic conductivity K | 0.1 | m·s |
| Porosity | 0.45 | - |
| 10 | m·s | |
| 4 × 10 | m·s | |
| 0.1 | m | |
| 0.5 | m | |
| 2 | - |
| Mineral | Formation Reaction | logK | MM | Density | Reactivity | Content (wt %) |
|---|---|---|---|---|---|---|
| Apatite | 5 Ca + 3 PO + HO − H | 57.32 | 502.3 | 3180 | Kinetics (diss) | 2.5 |
| Gypsum | Ca + 2 SO + 2 HO | 4.54 | 136 | 2305 | Equilibrium | 10 |
| Quartz | Si(OH) − 2 HO | 4 | 60 | 2650 | Kinetics (diss) | 85.365 |
| Ferrihydrite | Fe + 3 HO − 3 H | −3.03 | 107 | 3800 | Equilibrium | 0.1 |
| Uraninite | U − 4 H + 2 HO | 4.85 | 322 | 5000 | Kinetics (diss) | 0.035 |
| Jarosite-K | K − 6 H + 3 Fe + 6 HO + 2 SO | 11 | 500 | 3269 | Kinetics (diss) | 2 |
| Zippeite | 3 K + 4 UO − 7 H + 7.3 HO + 2 SO | −4.1 [63] | 1514 | 3600 | Equilibrium | 0 |
| Uranyl Phosphate | UO + PO + H + 3 HO | 25.52 | 420 | 3000 | Equilibrium | 0 |
| Uranyl Sulfate | UO + SO + 2.5 HO | 1.59 | - | - | Equilibrium | 0 |
| Mineral | Rate Constant k | Specific Surface |
|---|---|---|
| (mol·m·s) | (m·g) | |
| Apatite | 10 | 0.1 |
| Quartz | 10 | 0.001 |
| Uraninite | 2 × 10 | 0.01 |
| Jarosite-K | 10 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seigneur, N.; De Windt, L.; Déjeant, A.; Lagneau, V.; Descostes, M. Long-Term Evolution of Uranium Mobility within Sulfated Mill Tailings in Arid Regions: A Reactive Transport Study. Minerals 2021, 11, 1201. https://doi.org/10.3390/min11111201
Seigneur N, De Windt L, Déjeant A, Lagneau V, Descostes M. Long-Term Evolution of Uranium Mobility within Sulfated Mill Tailings in Arid Regions: A Reactive Transport Study. Minerals. 2021; 11(11):1201. https://doi.org/10.3390/min11111201
Chicago/Turabian StyleSeigneur, Nicolas, Laurent De Windt, Adrien Déjeant, Vincent Lagneau, and Michaël Descostes. 2021. "Long-Term Evolution of Uranium Mobility within Sulfated Mill Tailings in Arid Regions: A Reactive Transport Study" Minerals 11, no. 11: 1201. https://doi.org/10.3390/min11111201
APA StyleSeigneur, N., De Windt, L., Déjeant, A., Lagneau, V., & Descostes, M. (2021). Long-Term Evolution of Uranium Mobility within Sulfated Mill Tailings in Arid Regions: A Reactive Transport Study. Minerals, 11(11), 1201. https://doi.org/10.3390/min11111201







