X-ray Fluorescence and Laser-Induced Breakdown Spectroscopy Analysis of Li-Rich Minerals in Veins from Argemela Tin Mine, Central Portugal
Abstract
:1. Introduction
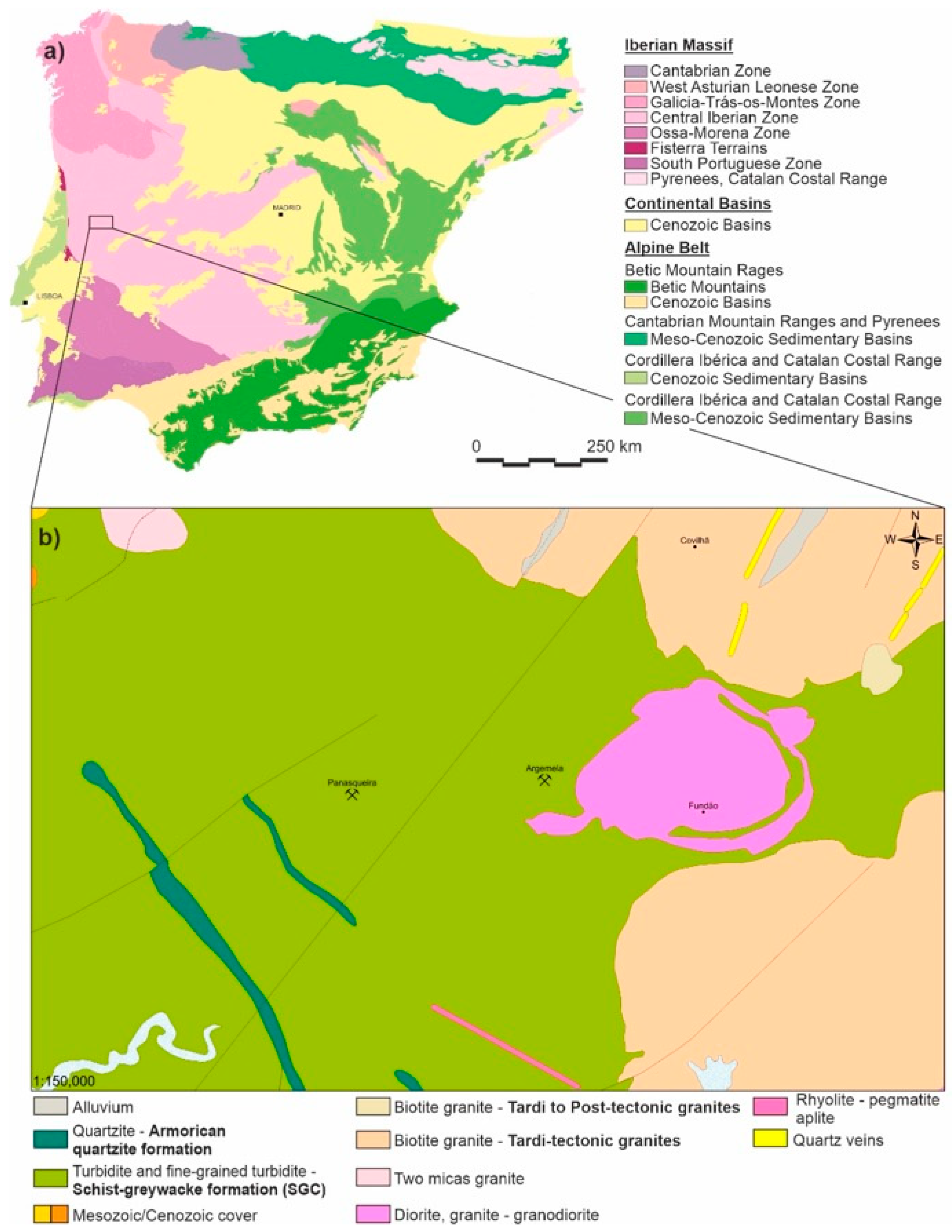
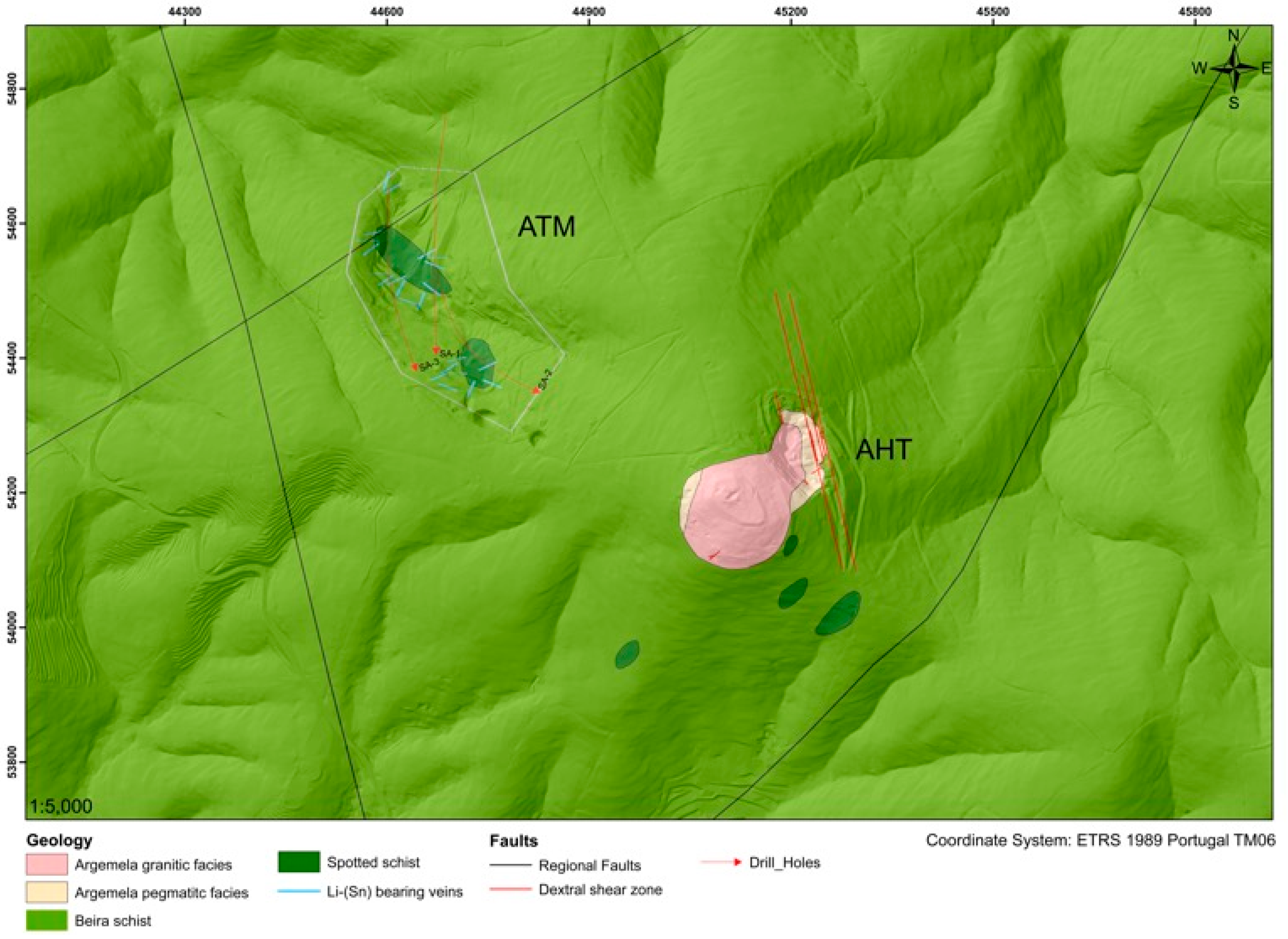
2. Geological Setting
3. Methodology
3.1. Samples
3.2. Petrography
3.3. XRF Instrumentation
3.4. LIBS Instrumentation
4. Results
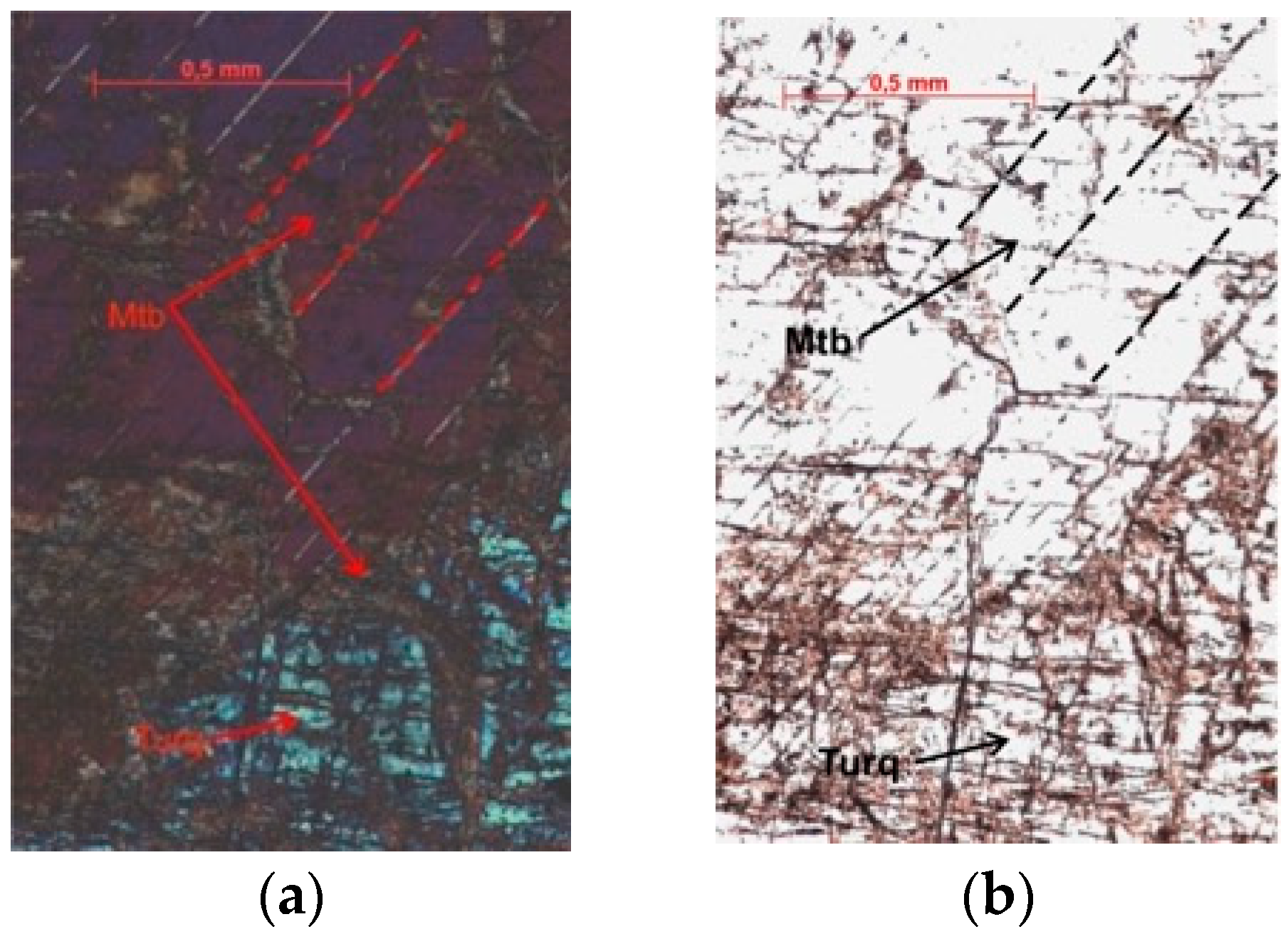
4.1. Petrography and Mineralogy
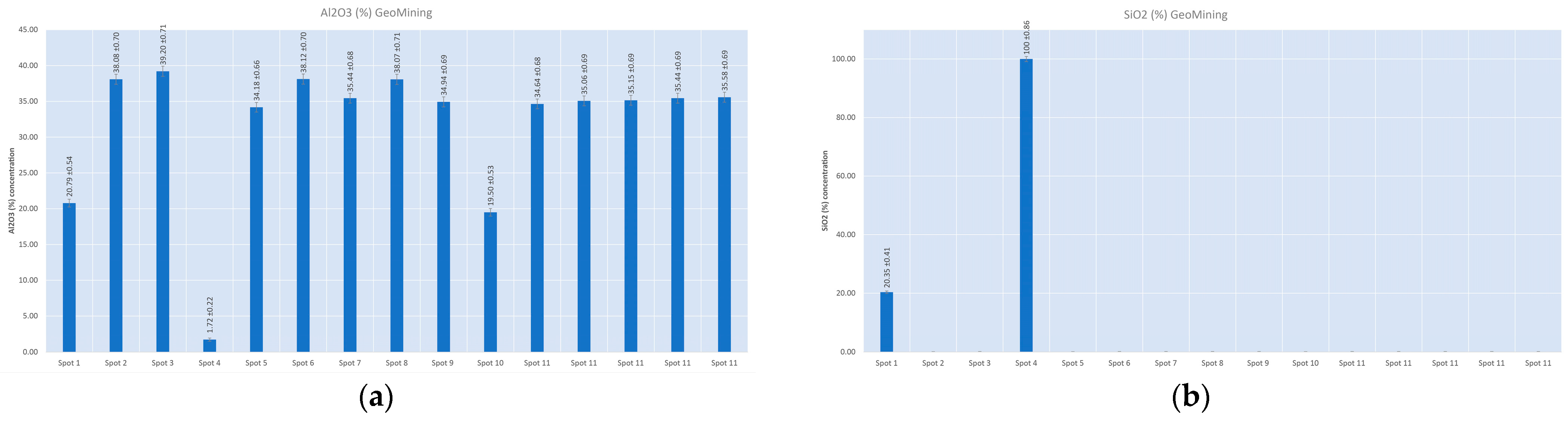
4.2. X-ray Fluorescence Quantitative Analysis
4.3. LIBS Qualitative Analysis
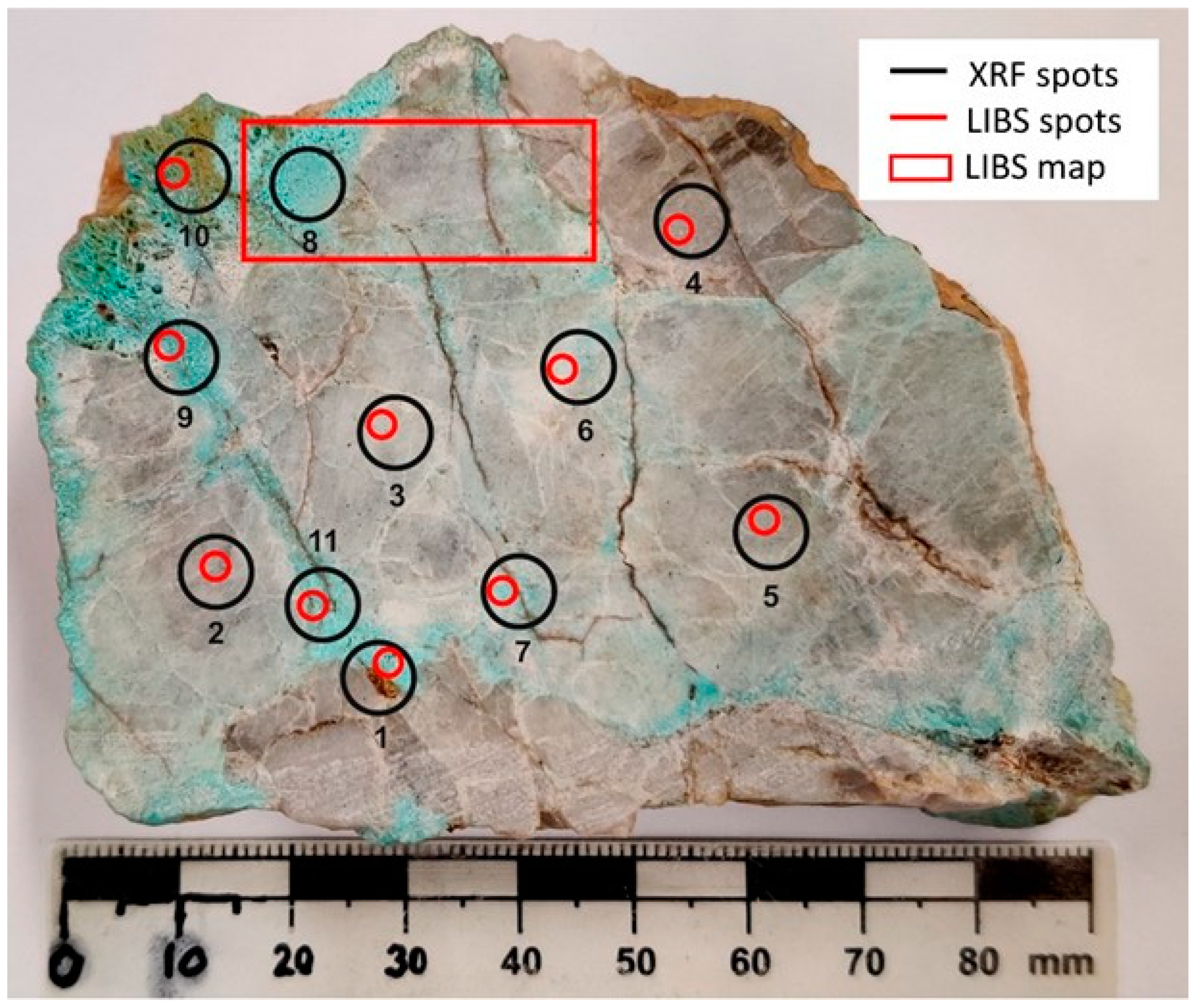
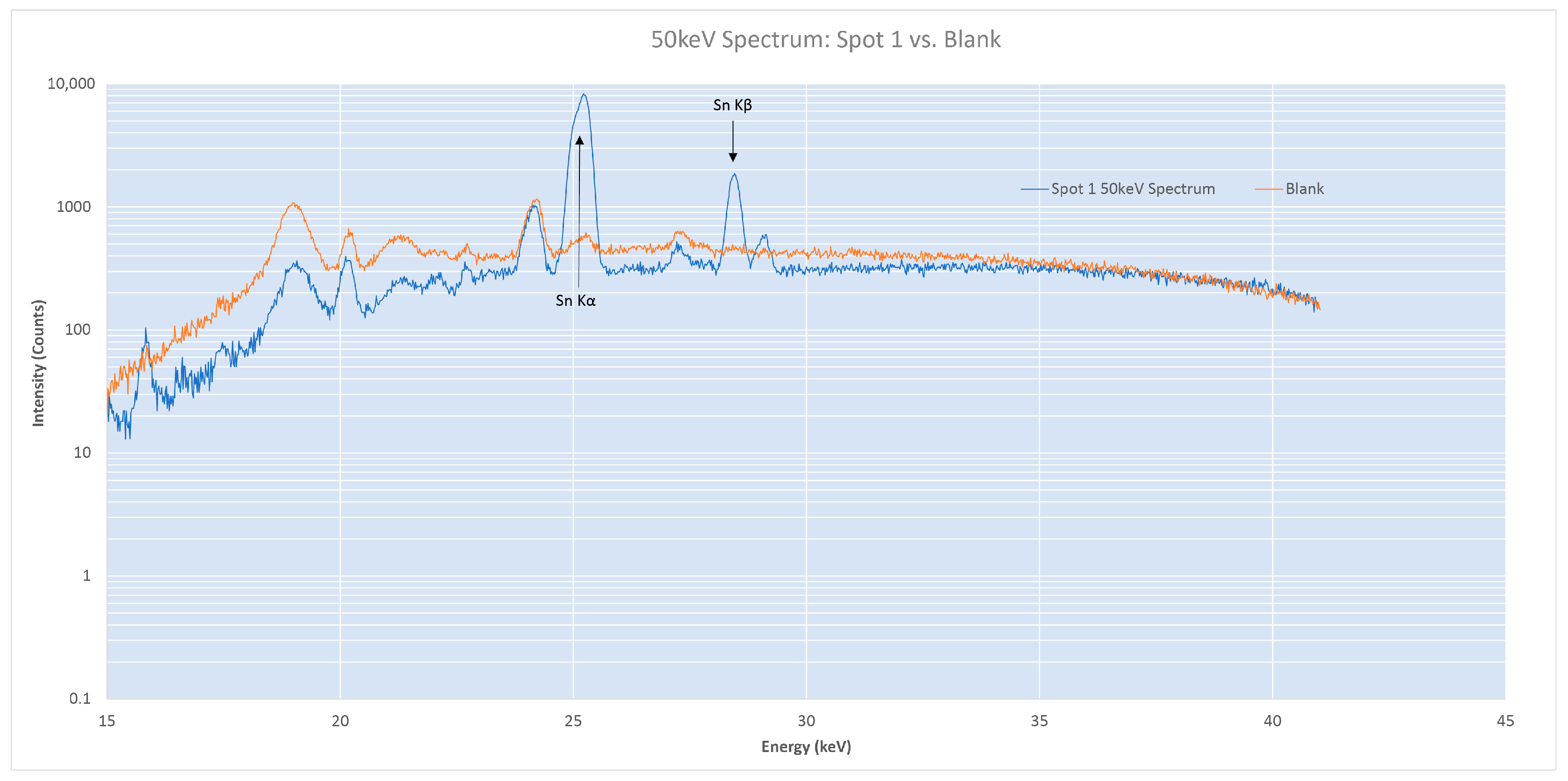
4.3.1. Hand Sample: Individual Spots
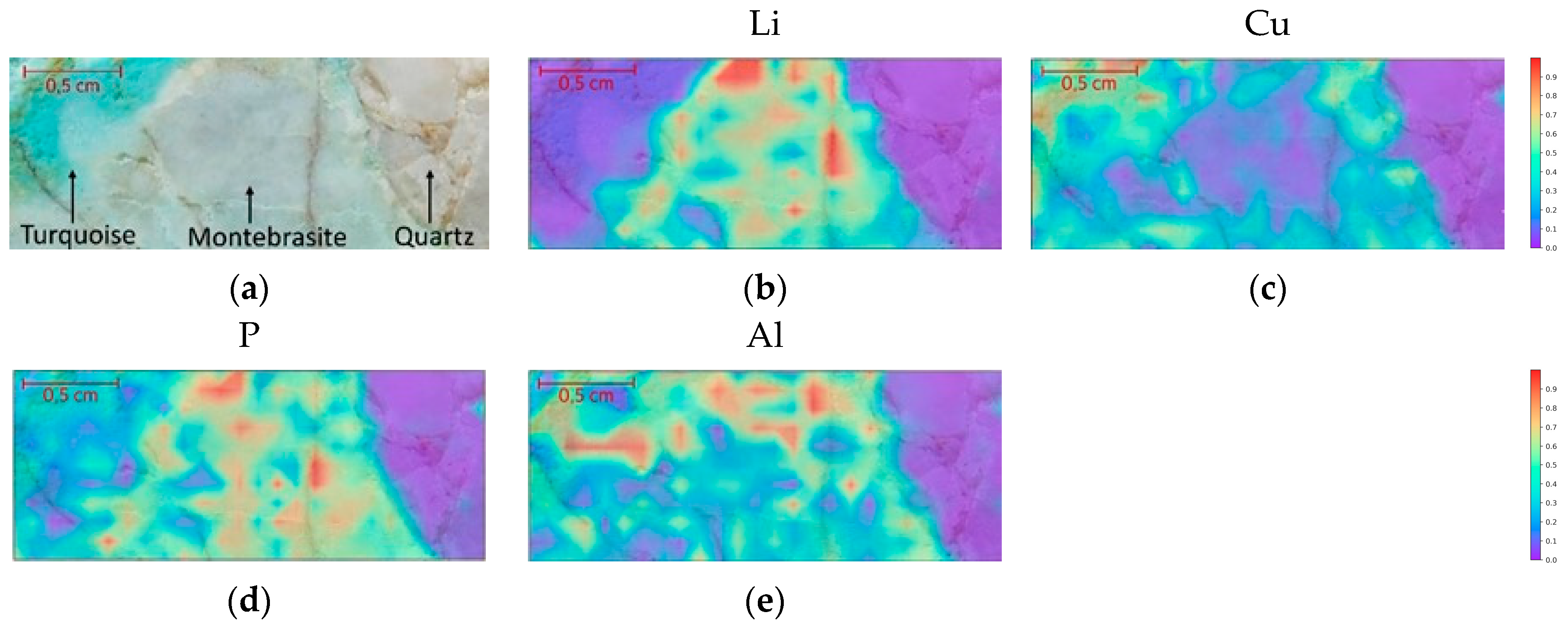
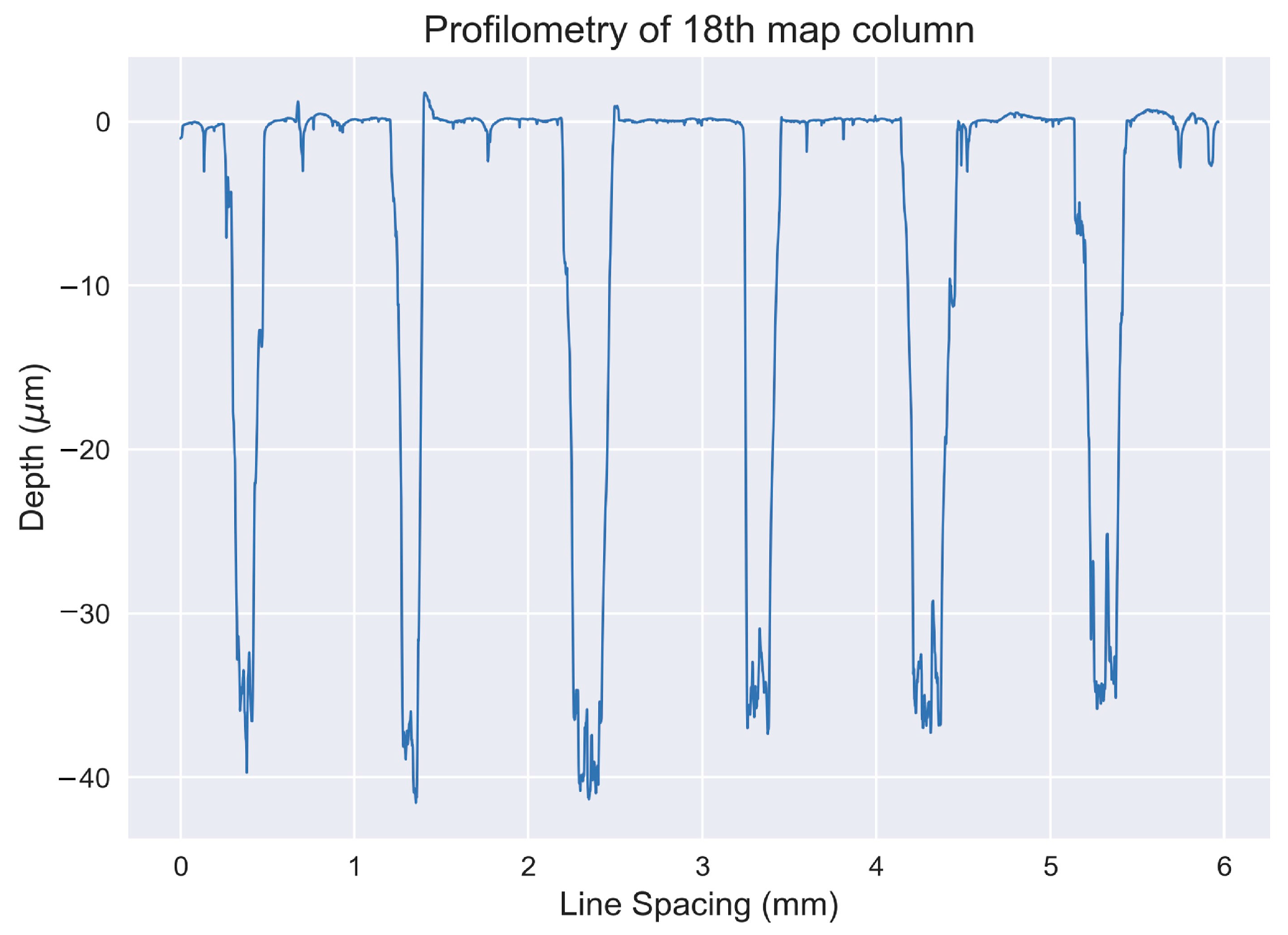
4.3.2. Hand Sample: Mapping
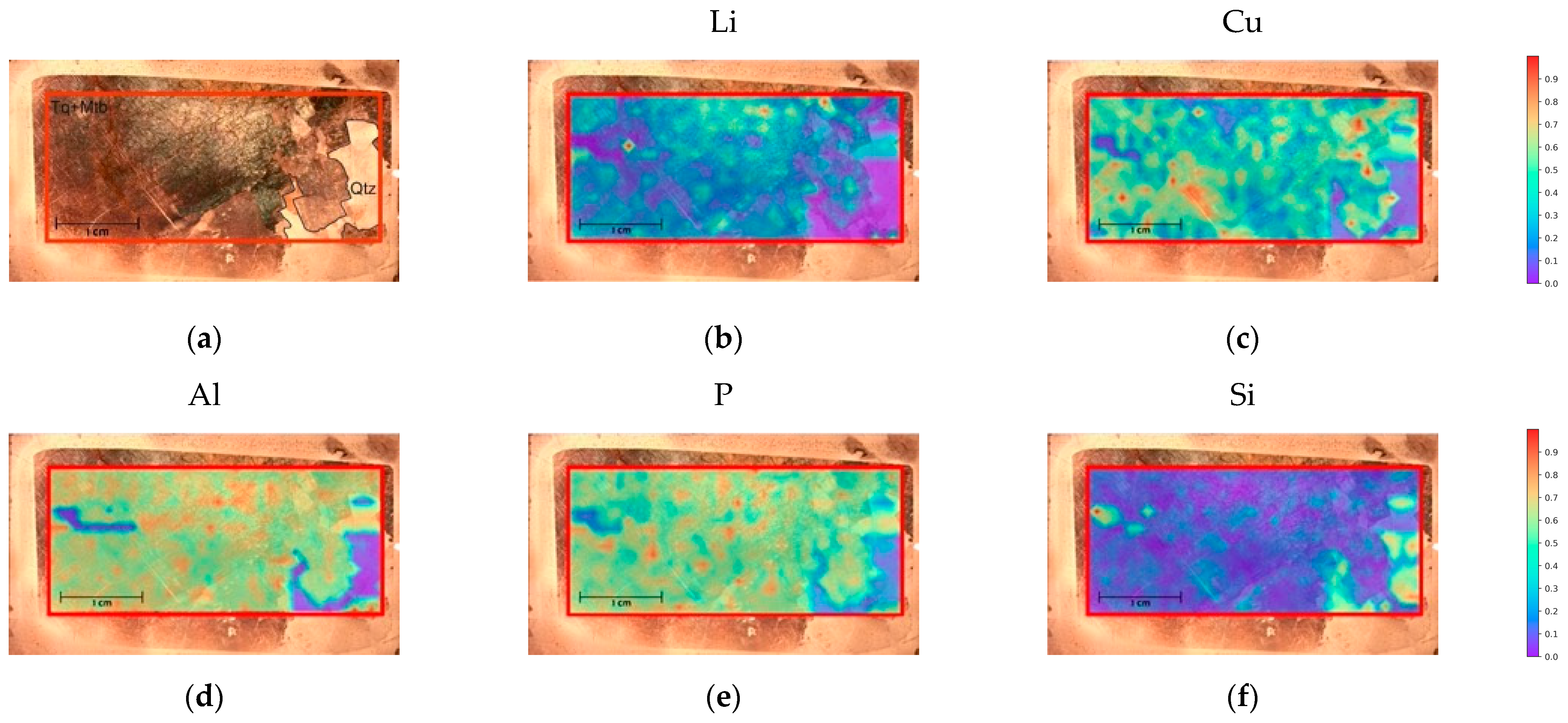
4.3.3. Thin Section Mapping
5. Interpretation and Discussion
5.1. Hand Sample Analysis
5.2. Thin Section Mapping
6. Main Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions, Critical Raw Materials Resilience: Charting a Path towards Greater Security and Sustainability; European Commission: Brussels, Belgium, 2020; p. 23. [Google Scholar]
- Kesler, S.E.; Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Everson, M.P.; Wallington, T.J. Global lithium resources: Relative importance of pegmatite, brine and other deposits. Ore Geol. Rev. 2012, 48, 55–69. [Google Scholar] [CrossRef]
- Gourcerol, B.; Gloaguen, E.; Melleton, J.; Tuduri, J.; Galiegue, X. Re-assessing the European lithium resource potential—A review of hard-rock resources and metallogeny. Ore Geol. Rev. 2019, 109, 494–519. [Google Scholar] [CrossRef] [Green Version]
- Roda-Robles, E.; Pesquera, A.; Gil-Crespo, P.P.; Vieira, R.; Lima, A.; Garate-Olave, I.; Martins, T.; Torres-Ruiz, J. Geology and mineralogy of Li mineralization in the Central Iberian Zone (Spain and Portugal). Mineral. Mag. 2016, 80, 103–126. [Google Scholar] [CrossRef] [Green Version]
- Charoy, B.; Noronha, F. Multistage Growth of a Rare-Element, Volatile-Rich Microgranite at Argemela (Portugal). J. Petrol. 1996, 37, 73–94. [Google Scholar] [CrossRef]
- Martín-Izard, A.; Reguilón, R.; Palero, F. Las mineralizaciones litiniferas del oeste de Salamanca y Zamora. Estud. Geológicos 1992, 48, 19–30. [Google Scholar] [CrossRef]
- Pesquera, A.; Torres-Ruiz, J.; Velilla, N.; Gil-Crespo, P.P. Chemistry and genetic implications of tourmaline and Li-F-Cs micas from the Valdeflores area (Caceres, Spain). Am. Mineral. 1999, 84, 55–69. [Google Scholar] [CrossRef]
- Gallego Garrido, M. Las Mineralizaciones de Litio Asociadas a Magmatismo Acido en Extremadura y su Encuadra en la Zona Centro-Iberica. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 1992. [Google Scholar]
- Carvalho, J.M.F.; Farinha, J.A.L.B. Lithium Potentialities in Northern Portugal. In Proceedings of the 17th Industrial Minerals International Congress, Barcelona, Spain, 28–31 March 2004; pp. 1–10. [Google Scholar]
- Pirard, C.; Hatert, F.; Fransolet, A.-M. Alteration sequences of aluminium phosphates from Montebras pegmatite, Massif Central, France. In Proceedings of the Granitic Pegmatites: The State of the Art—International Symposium, Porto, Portugal, 6–12 May 2007. [Google Scholar]
- De Carvalho, C.N.; Hamilton, T.G. Notas Sobre a Ocorrência Excepcional de Turquesas (Fosfato Hidratado de Cobre e Alumínio) no Cabeço da Argemela (Fundão/Covilhã). AÇAFA On Line. 2018. Available online: https://www.researchgate.net/publication/325011942_NOTAS_SOBRE_A_OCORRENCIA_EXCEPCIONAL_DE_TURQUESAS_FOSFATO_HIDRATADO_DE_COBRE_E_ALUMINIO_NO_CABECO_DA_ARGEMELA_FUNDAOCOVILHA_Notes_on_the_Exceptional_Finding_of_Turquoises_Hydrated_Phosphate_of_Copper_link (accessed on 16 March 2020).
- Margui, E.; Van Grieken, R. X-ray Fluorescence Spectrometry and Related Techniques; Momentum Press: New York, NY, USA, 2013. [Google Scholar]
- Potts, P.J. X-ray Fluorescence Analysis: Principles and Practice of Wavelength Dispersive Spectrometry. In A Handbook of Silicate Rock Analysis; Blackie & Son Ltd.: London, UK, 1987; pp. 226–285. [Google Scholar]
- Cremers, D.A.; Radziemski, L.J. Handbook of Laser-Induced Breakdown Spectroscopy, 2nd ed.; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2013. [Google Scholar]
- Rai, V.N.; Thakur, S.N. Physics of plasma in laser-induced breakdown spectroscopy. In Laser-Induced Breakdown Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2007; pp. 83–111. [Google Scholar]
- Anabitarte, F.; Cobo, A.; López-Higuera, J. Laser-Induced Breakdown Spectroscopy: Fundamentals, Applications, and Challenges. ISRN Spectrosc. 2012, 2012, 285240. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.K.H.; Iqbal, J.; Ahmad, P.; Khandaker, M.U.; Haq, S.; Naeem, M. Laser induced breakdown spectroscopy methods and applications: A comprehensive review. Radiat. Phys. Chem. 2020, 170, 108666. [Google Scholar] [CrossRef]
- Gazley, M.F.; Tutt, C.M.; Fisher, L.A.; Latham, A.R.; Duclaux, G.; Taylor, M.D.; de Beer, S.J. Objective geological logging using portable XRF geochemical multi-element data at Plutonic Gold Mine, Marymia Inlier, Western Australia. J. Geochem. Explor. 2014, 143, 74–83. [Google Scholar] [CrossRef]
- Jones, M.C.; Williams-Thorpe, O.; Potts, P.J.; Webb, P.C. Using Field-Portable XRF to Assess Geochemical Variations Within and Between Dolerite Outcrops of Preseli, South Wales. Geostand. Geoanal. Res. 2005, 29, 251–269. [Google Scholar] [CrossRef]
- Sarala, P.; Taivalkoski, A.; Valkama, J. Portable XRF: An Advanced On-Site Analysis Method in Till Geochemical Exploration. Geol. Surv. Finl. 2015, 57, 63–86. [Google Scholar]
- Guimarães, D.; Ferreira, M.F.S.; Ribeiro, R.; Dias, C.; Lima, A.; Martins, R.C.; Jorge, P.A.S. Application of a novel LIBS prototype as an analytical grade tool for Li quantification in pegmatite samples. In Proceedings of the Fourth International Conference on Applications of Optics and Photonics, Lisbon, Portugal, 31 May–4 June 2019; Volume 11207, p. 1120725. [Google Scholar]
- Harmon, R.; Lawley, C.; Watts, J.; Harraden, C.; Somers, A.; Hark, R. Laser-Induced Breakdown Spectroscopy—An Emerging Analytical Tool for Mineral Exploration. Minerals 2019, 9, 718. [Google Scholar] [CrossRef] [Green Version]
- McMillan, N.J.; Rees, S.; Kochelek, K.; McManus, C. Geological Applications of Laser-Induced Breakdown Spectroscopy. Geostand. Geoanal. Res. 2014, 38, 329–343. [Google Scholar] [CrossRef]
- Streubel, L.; Jacobsen, L.; Merk, S.; Thees, M.; Rammlmair, D.; Meima, J.; Mory, D. Rapid Analysis of Geological Drill-Cores with LIBS. Opt. Photon. 2016, 11, 23–27. [Google Scholar] [CrossRef]
- Julivert, M.; Fontbote, J.M.; Ribeiro, A.; Conde, L. Mapa Tectónico de la Península Ibérica y Baleares E. 1:1.000.000; Servicio de Publicaciones del Ministerio de Industria: Madrid, Spain, 1972. [Google Scholar]
- Farias, P.; Gallastegui, G.; González Lodeiro, F.; Marquínez, J.; Martín Parra, L.M.; Martínez Catalán, J.R.; De Pablo Maciá, J.G.; Rodríguez Fernández, L.R. Aportaciones al conocimiento de la litoestratigrafia y estructura de Galicia Central. In IX Reunião Sobre a Geologia do Oeste Peninsular; Universidade do Porto, Faculdade de Ciências, Museu e Laboratório Mineralógico e Geológico: Porto, Portugal, 1987; Volume I, pp. 410–432. [Google Scholar]
- Thadeu, D. Hercynian paragenetic units of the portuguese part of the hesperic massif. Bol. Soc. Geológica Port. 1977, 20, 247–276. [Google Scholar]
- Pereira, E.; Ribeiro, A.; Meireles, C. Variscan shear zones and control of Sn-W, Au, U mineralizations in the central-Iberian Zone in Portugal. Cuad. Lab. Xeol. Laxe 1993, 18, 89–119. [Google Scholar]
- Rodríguez Fernández, L.R.; López Olmedo, F.; Oliveira, J.T.; Medialdea, T.; Terrinha, P.; Matas, J.; Martín-Serrano, A.; Martín Parra, L.M.; Rubio, F.; Marín, C.; et al. Mapa Geológico de la Península Ibérica, Baleares y Canarias a Escala 1/1.000.000. 2015. © Instituto Geológico y Minero de España (IGME); © Laboratório Nacional de Energia e Geologia (LNEG). Available online: https://info.igme.es/cartografiadigital/geologica/ (accessed on 29 January 2021).
- Michaud, J. Rare Metal Granites—Origin, Emplacement and Mechanisms of the Magmatic-Hydrothermal Transition. Ph.D. Thesis, University of Orleans, Orleans, France, 2019. [Google Scholar]
- Inverno, C.; Ribeiro, M.L. Fracturação e cortejo filoneano nas Minas da Argemela (Fundão). Comun. Serv. Geolo. Port. 1980, 66, 185–193. [Google Scholar]
- Braga, P.F.A.; França, S.C.A.; Gonçalves, C.C.; Ferraz, P.F.V.; Neumann, R. Extraction of lithium from a montebrasite concentrate: Applied mineralogy, pyro- and hydrometallurgy. Hydrometallurgy 2020, 191, 105249. [Google Scholar] [CrossRef]
- Michaud, J.; Gumiaux, C.; Pichavant, M.; Gloaguen, E.; Marcoux, E. From magmatic to hydrothermal Sn-Li-(Nb-Ta-W) mineralization: The Argemela area (Central Portugal). Ore Geol. Rev. 2019, 116, 103215. [Google Scholar] [CrossRef]
- Sweetapple, M.T.; Tassios, S. Laser-induced breakdown spectroscopy (LIBS) as a tool for in situ mapping and textural interpretation of lithium in pegmatite minerals. Am. Mineral. 2015, 100, 2141–2151. [Google Scholar] [CrossRef]
- Guimarães, D.; Ferreira, M.F.S.; Dias, C.; Ribeiro, R.; Lima, A.; Martins, R.C.; Jorge, P.A.S. Lithium bearing minerals differentiation using Laser-Induced Breakdown Spectroscopy. In Proceedings of the 10th Euro-Mediterranean Symposium on Laser-Induced Breakdown Spectroscopy (EMSLIBS 2019), Brno, Czech Republic, 8–13 September 2019; pp. 212–213. [Google Scholar]
- Wiens, R.; Maurice, S.; Lasue, J.; Forni, O.; Anderson, R.; Clegg, S.; Bender, S.; Blaney, D.; Barraclough, B.; Cousin, A.; et al. Pre-flight calibration and initial data processing for the ChemCam laser-induced breakdown spectroscopy instrument on the Mars Science Laboratory rover. Spectrochim. Acta Part B At. Spectrosc. 2013, 82, 1–27. [Google Scholar] [CrossRef]



| GeoMining (3 Phase Results) | MgO | Al2O3 | SiO2 | P | S | Cl | K2O | Ca | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | As | Se | Rb | Sr | Y | Zr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LOD (ppm) on pure SiO2 matrix | 4900 | 1250 | N/A | 39 | 60 | 135 | 44 | 35 | 40 | 22 | 16 | 13 | 20 | 3 | 6 | 3 | 2 | 1 | 3 | 2 | 1 | 2 | 1 | 7 |
| Upper range (wt%) | 70 | 69 | 100 | 13.2 | 41 | 4 | 15.3 | 31 | 60 | 56 | 27 | 45.4 | 70 | 72.4 | 79 | 80 | 80 | 0.02 | 76 | 0.05 | 0.07 | 1.02 | 0.22 | 39 |
| GeoMining (3 Phase Results) | Nb | Mo | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | Ba(K) | La(K) | Ce (K) | Hf | Ta | W | Pt | Au | Hg | Tl | Pb | Bi | Th | U |
| LOD (ppm) on pure SiO2 matrix Upper range (wt%) | 1 70 | 10 67 | 14 0.03 | 13 0.08 | 10 0.66 | 10 0.78 | 13 0 | 69 88 | 26 19 | 7 0.02 | 62 10.7 | 161 0.7 | 128 4.8 | 9 1.05 | 43 82 | 33 80 | 11 0.16 | 1 0.1 | 1 86 | 15 0.02 | 6 93 | 4 90 | 4 0.68 | 9 0.17 |
| Number of Measurements | Al2O3 (wt%) | Err (wt%) | SiO2 (wt%) | Err (wt%) | K2O (wt%) | Err (wt%) | Mn (wt%) | Err (wt%) | Fe (wt%) | Err (wt%) | Cu (wt%) | Err (wt%) | Rb (wt%) | Err (wt%) | Pb (wt%) | Err (wt%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measurements during the sample analysis | 1 | 11.8 | 0.4 | 70.1 | 0.7 | 4.90 | 0.04 | 0.116 | 0.007 | 1.77 | 0.02 | 0.023 | 0.002 | 0.0149 | 0.0009 | 0.087 | 0.004 |
| 2 | 11.6 | 0.4 | 69.0 | 0.7 | 4.83 | 0.04 | 0.119 | 0.007 | 1.80 | 0.02 | 0.022 | 0.002 | 0.0142 | 0.0009 | 0.093 | 0.004 | |
| 3 | 11.0 | 0.4 | 66.5 | 0.7 | 4.77 | 0.04 | 0.116 | 0.007 | 1.81 | 0.02 | 0.023 | 0.002 | 0.0147 | 0.0009 | 0.100 | 0.005 | |
| 4 | 11.4 | 0.4 | 66.8 | 0.7 | 4.71 | 0.04 | 0.118 | 0.007 | 1.79 | 0.02 | 0.023 | 0.002 | 0.0143 | 0.0009 | 0.095 | 0.005 | |
| 5 | 11.8 | 0.4 | 68.9 | 0.7 | 4.83 | 0.04 | 0.118 | 0.007 | 1.80 | 0.02 | 0.023 | 0.002 | 0.0142 | 0.0009 | 0.090 | 0.004 | |
| 6 | 11.4 | 0.4 | 67.0 | 0.7 | 4.75 | 0.04 | 0.113 | 0.007 | 1.76 | 0.02 | 0.022 | 0.002 | 0.0145 | 0.0009 | 0.098 | 0.005 | |
| 7 | 11.6 | 0.4 | 68.2 | 0.7 | 4.80 | 0.04 | 0.108 | 0.006 | 1.75 | 0.02 | 0.022 | 0.002 | 0.0144 | 0.0009 | 0.089 | 0.004 | |
| QC reference | Target Mean | 11.82 | 68.79 | 4.78 | 0.110 | 1.75 | 0.0218 | 0.0142 | 0.0886 | ||||||||
| S. Deviation | 0.19 | 1.00 | 0.08 | 0.003 | 0.01 | 0.0006 | 0.0004 | 0.0026 | |||||||||
| Limit MIN | 10.89 | 63.82 | 4.36 | 0.095 | 1.70 | 0.0189 | 0.0122 | 0.0758 | |||||||||
| Limit MAX | 12.74 | 73.77 | 5.19 | 0.126 | 1.80 | 0.0247 | 0.0162 | 0.1013 |
| Quartz | Chemical Formula | Si | O | ||||
| SiO2 | 46.74% | 53.26% | |||||
| Turquoise | Chemical Formula | Al | Cu | P | H | O | |
| CuAl6(PO4)4(OH)8 4(H2O) | 19.90% | 7.81% | 15.23% | 1.98% | 55.07% | ||
| Ambligonite–Montebrasite | Chemical Formula | Li | Al | P | H | O | F |
| LiAl(PO4)(OH,F) | 4.74% | 18.43% | 21.16% | 0.52% | 51.91% | 3.24% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, R.; Capela, D.; Ferreira, M.; Martins, R.; Jorge, P.; Guimarães, D.; Lima, A. X-ray Fluorescence and Laser-Induced Breakdown Spectroscopy Analysis of Li-Rich Minerals in Veins from Argemela Tin Mine, Central Portugal. Minerals 2021, 11, 1169. https://doi.org/10.3390/min11111169
Ribeiro R, Capela D, Ferreira M, Martins R, Jorge P, Guimarães D, Lima A. X-ray Fluorescence and Laser-Induced Breakdown Spectroscopy Analysis of Li-Rich Minerals in Veins from Argemela Tin Mine, Central Portugal. Minerals. 2021; 11(11):1169. https://doi.org/10.3390/min11111169
Chicago/Turabian StyleRibeiro, Ricardo, Diana Capela, Miguel Ferreira, Rui Martins, Pedro Jorge, Diana Guimarães, and Alexandre Lima. 2021. "X-ray Fluorescence and Laser-Induced Breakdown Spectroscopy Analysis of Li-Rich Minerals in Veins from Argemela Tin Mine, Central Portugal" Minerals 11, no. 11: 1169. https://doi.org/10.3390/min11111169
APA StyleRibeiro, R., Capela, D., Ferreira, M., Martins, R., Jorge, P., Guimarães, D., & Lima, A. (2021). X-ray Fluorescence and Laser-Induced Breakdown Spectroscopy Analysis of Li-Rich Minerals in Veins from Argemela Tin Mine, Central Portugal. Minerals, 11(11), 1169. https://doi.org/10.3390/min11111169










