Phosphate Rocks: A Review of Sedimentary and Igneous Occurrences in Morocco
Abstract
:1. Introduction
2. Key Features of Sedimentary and Igneous Phosphate Rocks
2.1. Marine Sedimentary Phosphate Deposits
- (i)
- Pristine phosphate, corresponding to the authigenic facies as deposited originally without any subsequent reworking or transport. This lithofacies usually takes the form of finely laminated sediment with disseminated authigenic francolite. It contains high content of organic matter and low phosphate concentrations, ranging from 2 to 10 wt.% P2O5;
- (ii)
- Reworked phosphate or Granular phosphate results from reworking and re-sedimentation of the primary phosphate under high-energy conditions induced by storm waves and currents. These reworking events can occur in situ or at different parts of the depositional system, allowing the formation of a densely packed and cleaned phosphate with high P2O5 content (up to 35 wt.%).
2.2. Igneous Phosphates
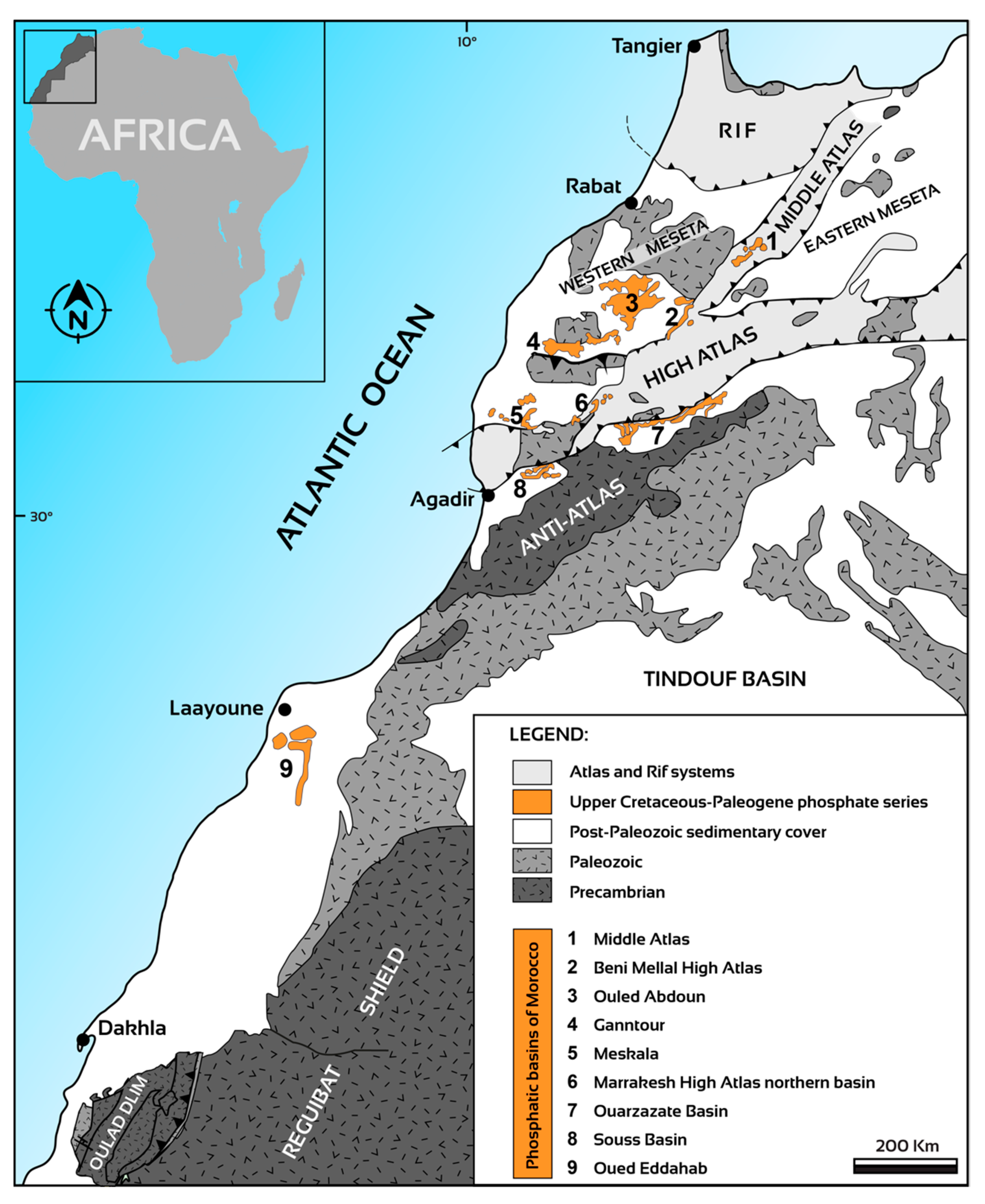
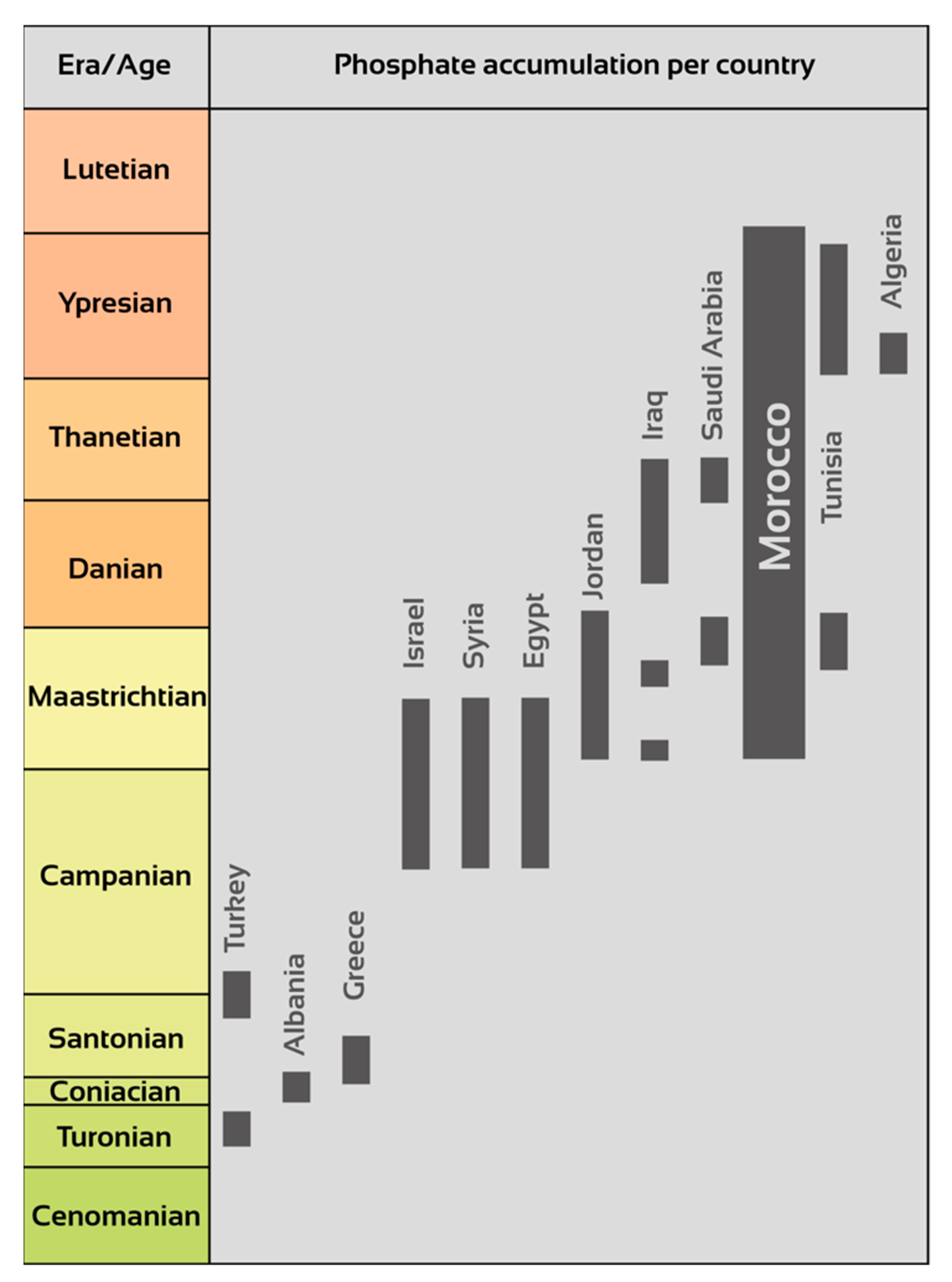
3. Moroccan Sedimentary Phosphates: A Unique Geological Heritage
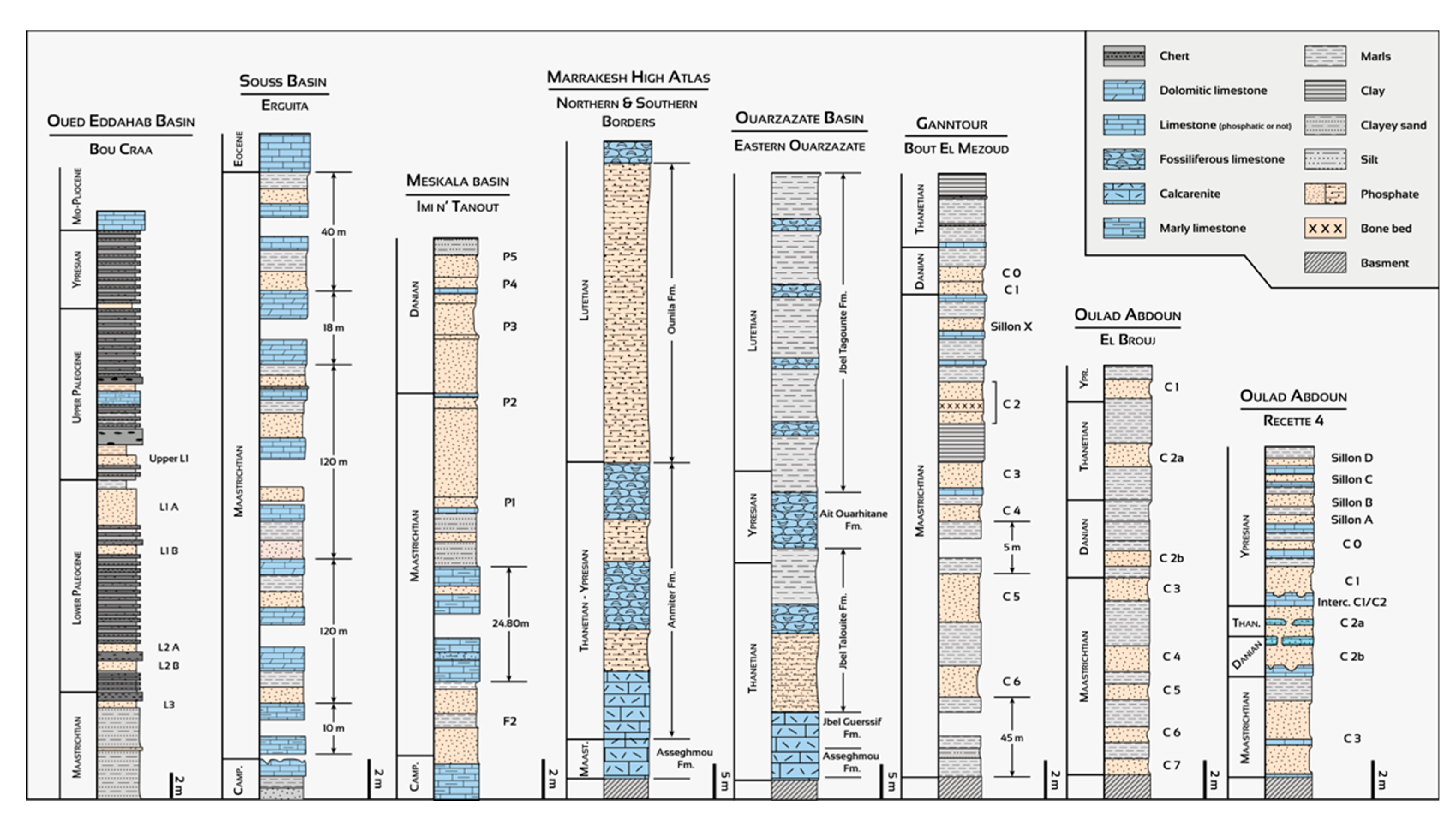
3.1. Geological and Depositional Setting of Moroccan Sedimentary Phosphate
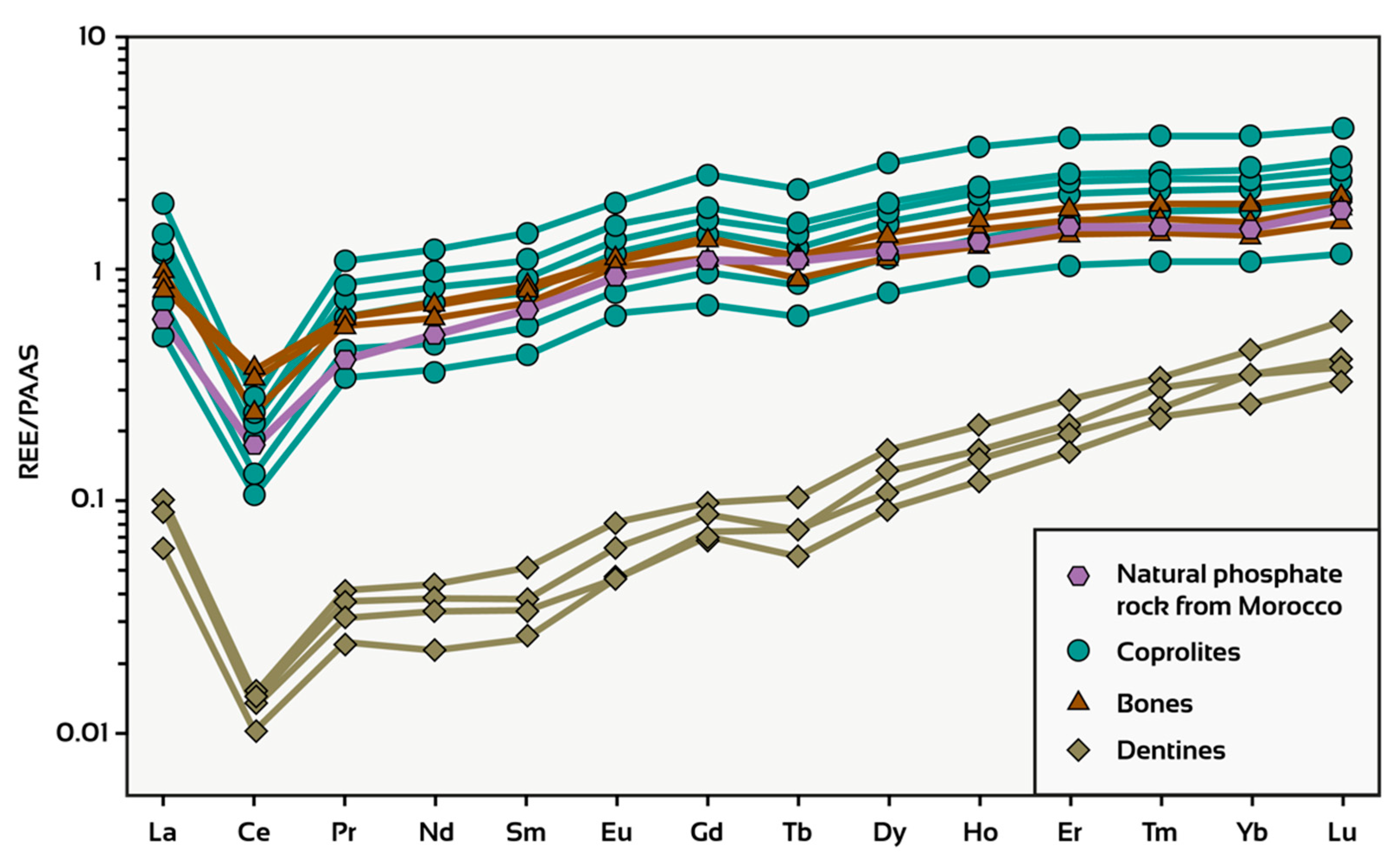
3.2. Mineralogical and Geochemical Signatures of Moroccan Sedimentary Phosphates
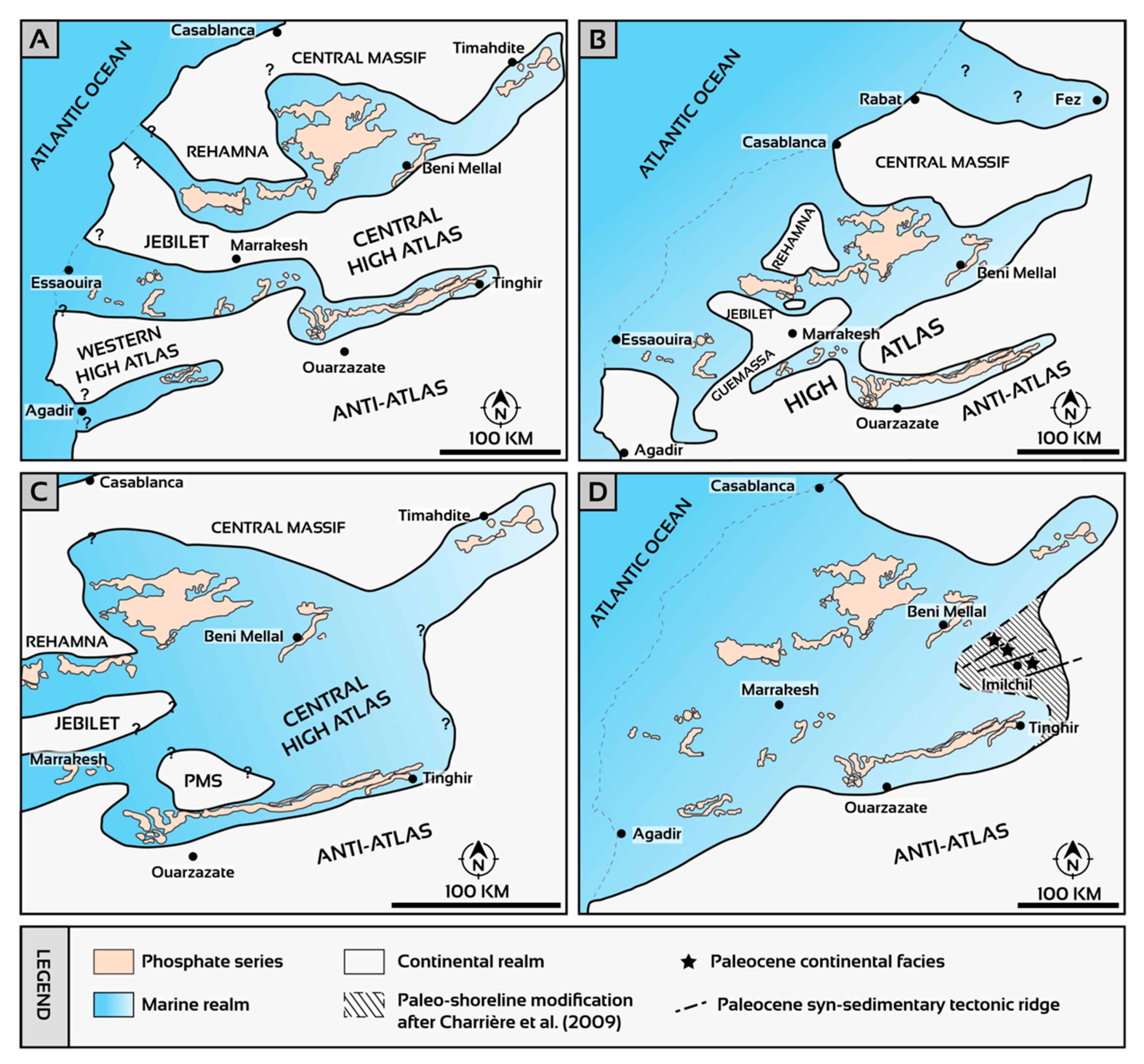
3.3. Phosphogenesis and Paleogeography of Phosphate Deposits in Morocco
- (i)
- A system of narrow gulfs separated by emerged lands (Figure 8A,B; Rehamna and Jebilet Hercynian massifs) [89]. In this paleogeographic configuration, the opening to the Atlantic would correspond to several narrow corridors [89]. For some authors, the opening to the Atlantic was through at least three distinct gulfs (Ouled Abdoun–Ganntour, Ouarzazate–Essaouira, and Souss) [88,89].
- (ii)
- (iii)
- (iv)
- A vast epicontinental sea (Phosphate Sea) without the presence of islands, in direct connection with the Atlantic Ocean to the west (Figure 8D) [91]. In this paleogeographic configuration, landforms did not occur during phosphogenic periods. Charrière et al. (2009) [107] suggested that the position of the paleo-coastline should be shifted to the east at the Imilchil area following the discovery of charophytes and ostracods in the Lutetian (Figure 8D). The presence of terrestrial vertebrates (dinosaurs, pterosaurs, mammals) in the northeastern parts of the Ouled Abdoun Basin suggests that this area corresponded to a proximal high-energy environment in the vicinity of the Paleozoic central massif [108]. The phosphate series at the Ganntour Basin, where the most complete sequence was recorded, was deposited in a more subsident and quieter setting [25,89].
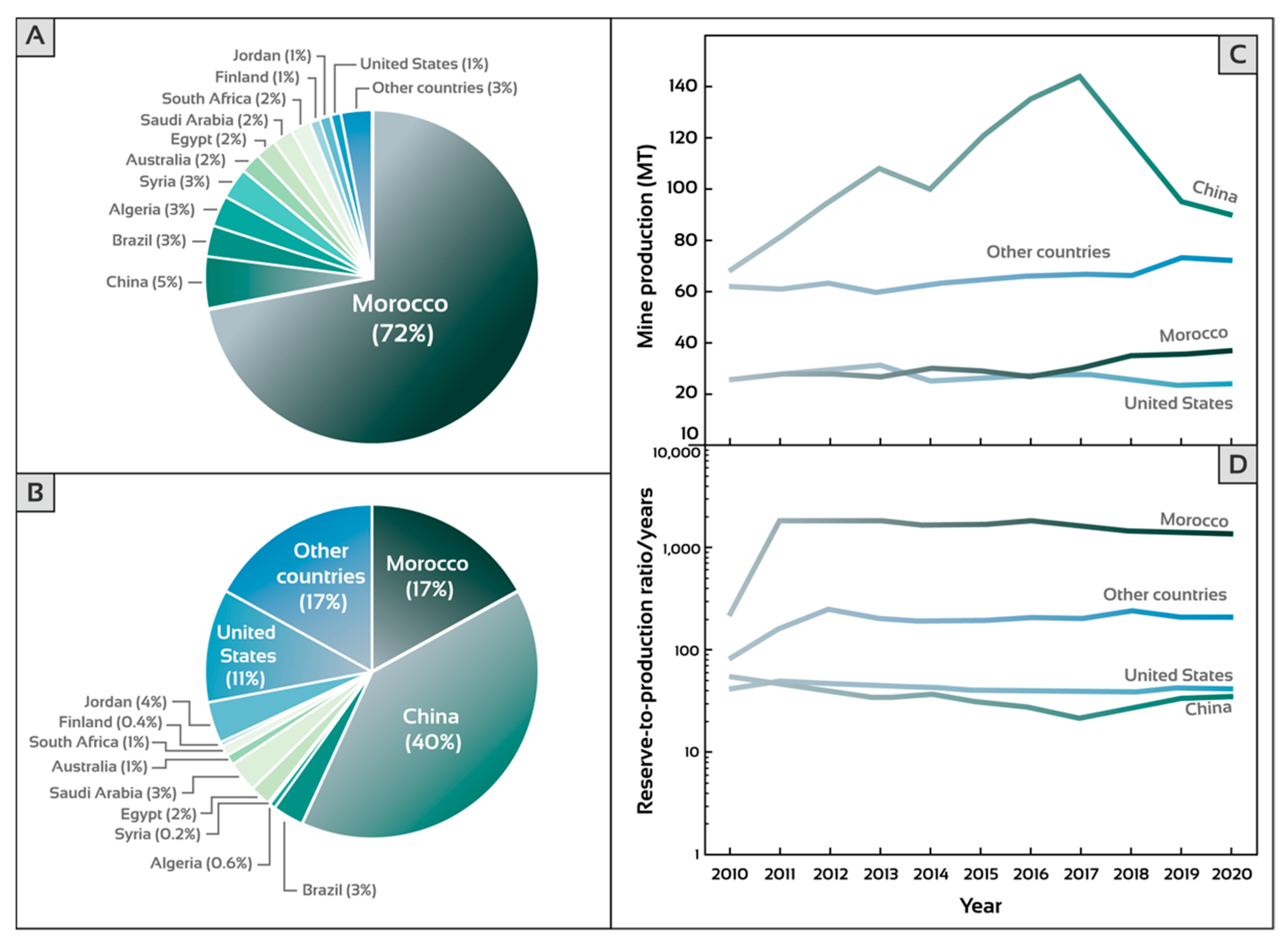
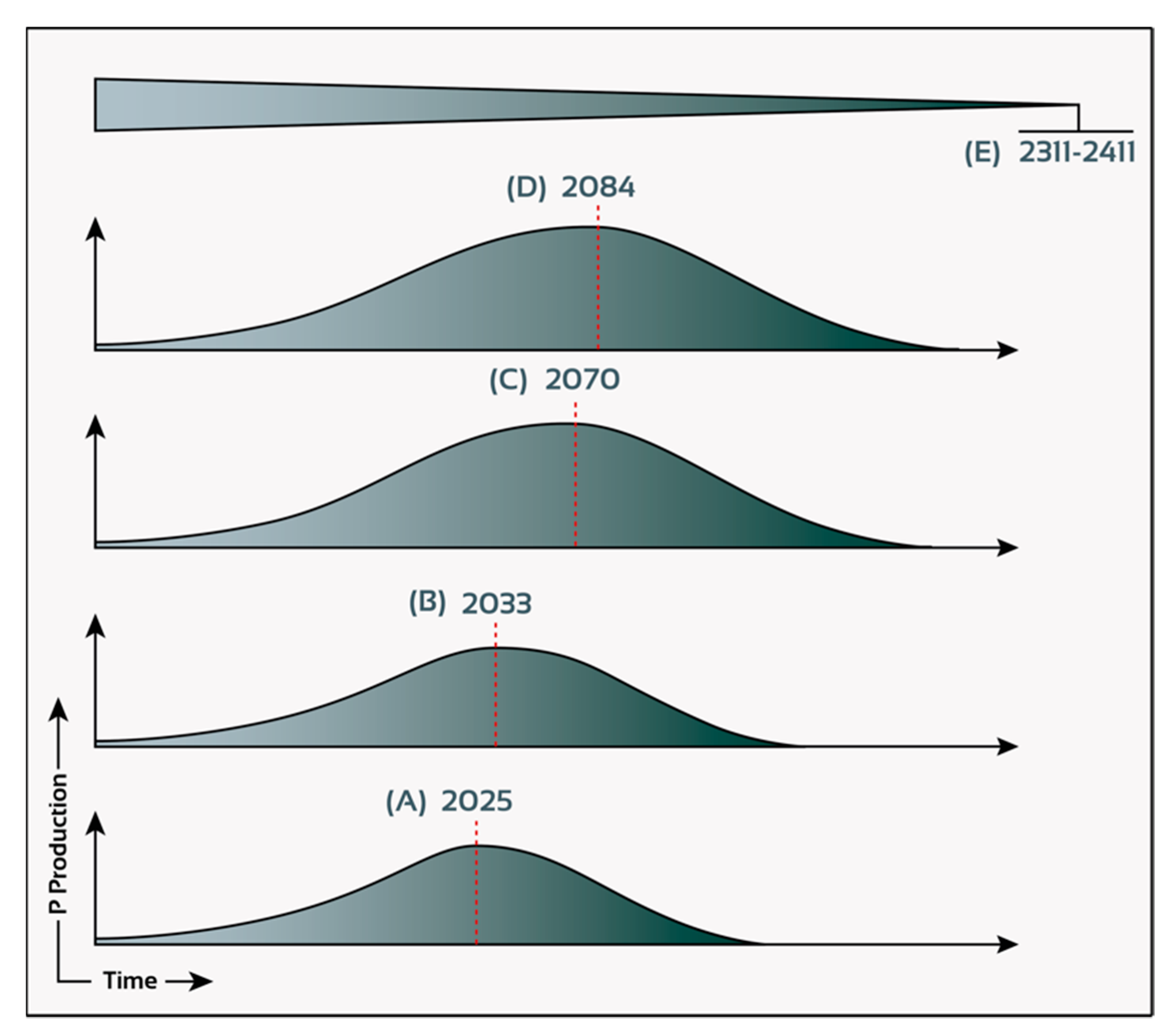
3.4. Morocco Hosting the Largest Phosphate Reserves
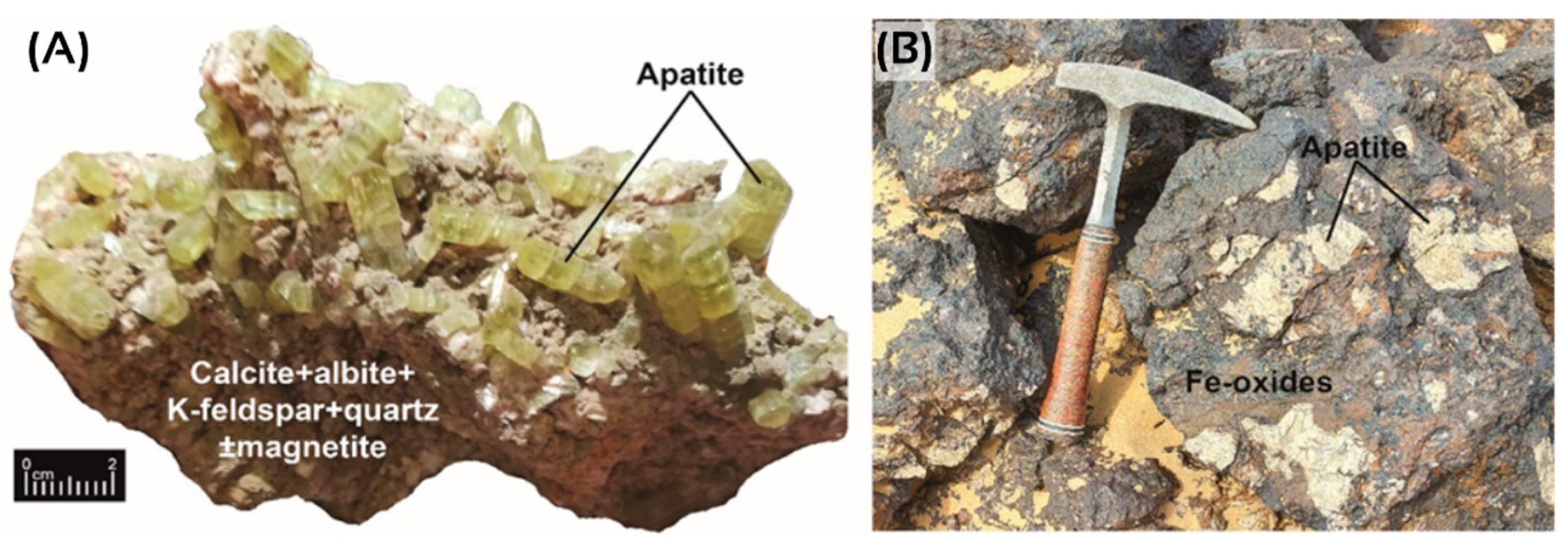
4. Moroccan Igneous Phosphates: An Underexplored Resource

5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Filippelli, G.M. Phosphate Rock Formation and Marine Phosphorus Geochemistry: The Deep Time Perspective. Chemosphere 2011, 84, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Pufahl, P.K.; Groat, L.A. Sedimentary and Igneous Phosphate Deposits: Formation and Exploration: An Invited Paper. Econ. Geol. 2017, 112, 483–516. [Google Scholar] [CrossRef]
- Emsbo, P.; McLaughlin, P.I.; Breit, G.N.; du Bray, E.A.; Koenig, A.E. Rare Earth Elements in Sedimentary Phosphate Deposits: Solution to the Global REE Crisis? Gondwana Res. 2015, 27, 776–785. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Wang, L.; Zhao, L.; Zhang, P.; El-Shall, H.; Moudgil, B.; Huang, X.; Zhang, L. Recovery of Rare Earth Elements from Phosphate Rock by Hydrometallurgical Processes—A Critical Review. Chem. Eng. J. 2018, 335, 774–800. [Google Scholar] [CrossRef]
- Gabriel, S.; Baschwitz, A.; Mathonnière, G.; Eleouet, T.; Fizaine, F. A Critical Assessment of Global Uranium Resources, Including Uranium in Phosphate Rocks, and the Possible Impact of Uranium Shortages on Nuclear Power Fleets. Ann. Nucl. Energy 2013, 58, 213–220. [Google Scholar] [CrossRef]
- Mar, S.S.; Okazaki, M. Investigation of Cd Contents in Several Phosphate Rocks Used for the Production of Fertilizer. Microchem. J. 2012, 104, 17–21. [Google Scholar] [CrossRef]
- Menzel, R.G. Uranium, Radium, and Thorium Content in Phosphate Rocks and Their Possible Radiation Hazard. Available online: https://pubs.acs.org/doi/pdf/10.1021/jf60156a002 (accessed on 25 September 2021).
- Kent, J.A. Phosphorus and Phosphates. In Riegel’s Handbook of Industrial Chemistry; Kent, J.A., Ed.; Springer: Boston, MA, USA, 2003; pp. 362–385. ISBN 978-0-387-23816-6. [Google Scholar]
- Daneshgar, S.; Callegari, A.; Capodaglio, A.G.; Vaccari, D. The Potential Phosphorus Crisis: Resource Conservation and Possible Escape Technologies: A Review. Resources 2018, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- Desmidt, E.; Ghyselbrecht, K.; Zhang, Y.; Pinoy, L.; Van der Bruggen, B.; Verstraete, W.; Rabaey, K.; Meesschaert, B. Global Phosphorus Scarcity and Full-Scale P-Recovery Techniques: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 336–384. [Google Scholar] [CrossRef]
- Cordell, D.; White, S. Peak Phosphorus: Clarifying the Key Issues of a Vigorous Debate about Long-Term Phosphorus Security. Sustainability 2011, 3, 2027–2049. [Google Scholar] [CrossRef] [Green Version]
- Broom-Fendley, S.; Siegfried, P.R.; Wall, F.; O’Neill, M.; Brooker, R.A.; Fallon, E.K.; Pickles, J.R.; Banks, D.A. The Origin and Composition of Carbonatite-Derived Carbonate-Bearing Fluorapatite Deposits. Min. Depos. 2021, 56, 863–884. [Google Scholar] [CrossRef]
- Föllmi, K.B. The Phosphorus Cycle, Phosphogenesis and Marine Phosphate-Rich Deposits. Earth-Sci. Rev. 1996, 40, 55–124. [Google Scholar] [CrossRef]
- Piper, D.Z.; Codispoti, L.A. Marine Phosphorite Deposits and the Nitrogen Cycle. Science 1975, 188, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Schuffert, J.D.; Kastner, M.; Jahnke, R.A. Carbon and Phosphorus Burial Associated with Modern Phosphorite Formation. Mar. Geol. 1998, 146, 21–31. [Google Scholar] [CrossRef]
- Chew, D.M.; Spikings, R.A. Geochronology and Thermochronology Using Apatite: Time and Temperature, Lower Crust to Surface. Elements 2015, 11, 189–194. [Google Scholar] [CrossRef]
- Jasinski, S.M. Mineral Commodity Summaries: Phosphate Rock. US Geol. Surv. 2021. [Google Scholar]
- El Bamiki, R.; Séranne, M.; Chellaï, E.H.; Merzeraud, G.; Marzoqi, M.; Melinte-Dobrinescu, M.C. The Moroccan High Atlas Phosphate-Rich Sediments: Unraveling the Accumulation and Differentiation Processes. Sediment. Geol. 2020, 403, 105655. [Google Scholar] [CrossRef]
- Ouabid, M.; Raji, O.; Dautria, J.-M.; Bodinier, J.-L.; Parat, F.; El Messbahi, H.; Garrido, C.J.; Ahechach, Y. Petrological and Geochemical Constraints on the Origin of Apatite Ores from Mesozoic Alkaline Intrusive Complexes, Central High-Atlas, Morocco. Ore Geol. Rev. 2021, 136, 104250. [Google Scholar] [CrossRef]
- Malainine, C.-E.; Raji, O.; Ouabid, M.; Khouakhi, A.; Bodinier, J.-L.; Laamrani, A.; Messbahi, H.E.; Youbi, N.; Boumehdi, M.A. An Integrated ASTER-Based Approach for Mapping Carbonatite and Iron Oxide-Apatite Deposits. Geocarto Int. 2021, 1–19. [Google Scholar] [CrossRef]
- Benaouda, R.; Kraemer, D.; Sitnikova, M.; Goldmann, S.; Freitag, R.; Bouali, A.; Mouttaqi, A.; El Haloui, R.; Essaadaoui, M.; Bau, M. Thorium-Poor Monazite and Columbite-(Fe) Mineralization in the Gleibat Lafhouda Carbonatite and Its Associated Iron-Oxide-Apatite Deposit of the Ouled Dlim Massif, South Morocco. Gondwana Res. 2020, 77, 19–39. [Google Scholar] [CrossRef]
- ONHYM Glibat Lafhouda Carbonatites (Southern Provinces, Morocco). 2016. Available online: http://www.onhym.com/pdf/en/PromotionEn/1_Glibat%20Lafhouda_2018_Ang.pdf (accessed on 25 September 2021).
- ONHYM Annular Structure of Twihinate (REE, Nb, Fe, U; Southern Provinces, Morocco) 2016. Available online: http://www.onhym.com/pdf/en/MiningPromotion_2021ENG/02_Twihinate_Lamlaga_2021Eng.pdf (accessed on 25 September 2021).
- Trappe, J. A Nomenclature System for Granular Phosphate Rocks According to Depositional Texture. Sediment. Geol. 2001, 145, 135–150. [Google Scholar] [CrossRef]
- Lucas, J.; Prevot-Lucas, L. On the Genesis of Sedimentary Apatite and Phosphate-Rich Sediments. In Soils and Sediments: Mineralogy and Geochemistry; Paquet, H., Clauer, N., Eds.; Springer: Berlin/Heidelberg, Germany, 1997; pp. 249–268. ISBN 978-3-642-60525-3. [Google Scholar]
- Nathan, Y. The Mineralogy and Geochemistry of Phosphorites. In Phosphate Minerals; Nriagu, J.O., Moore, P.B., Eds.; Springer: Berlin/Heidelberg, Germany, 1984; pp. 275–291. ISBN 978-3-642-61736-2. [Google Scholar]
- McClellan, G.H.; Kauwenbergh, S.J.V. Mineralogy of Sedimentary Apatites. Geol. Soc. Lond. Spec. Publ. 1990, 52, 23–31. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Nanosized and Nanocrystalline Calcium Orthophosphates. Acta Biomater. 2010, 6, 715–734. [Google Scholar] [CrossRef]
- Burnett, W.C.; Roe, K.K.; Piper, D.Z. Upwelling and Phosphorite Formation in the Ocean. In Coastal Upwelling Its Sediment Record: Part A: Responses of the Sedimentary Regime to Present Coastal Upwelling; Suess, E., Thiede, J., Eds.; NATO Conference Series; Springer: Boston, MA, USA, 1983; pp. 377–397. ISBN 978-1-4615-6651-9. [Google Scholar]
- Schöllhorn, I.; Houben, A.; Gertsch, B.; Adatte, T.; Alexey, U.; de Kaenel, E.; Spangenberg, J.E.; Janssen, N.; Schwennicke, T.; Föllmi, K.B. Enhanced Upwelling and Phosphorite Formation in the Northeastern Pacific during the Late Oligocene: Depositional Mechanisms, Environmental Conditions, and the Impact of Glacio-Eustacy. GSA Bull. 2020, 132, 687–709. [Google Scholar] [CrossRef]
- Ruttenberg, K.C. 8.13—The Global Phosphorus Cycle. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Pergamon: Oxford, UK, 2003; pp. 585–643. ISBN 978-0-08-043751-4. [Google Scholar]
- Lumiste, K.; Mänd, K.; Bailey, J.; Stüeken, E.E.; Paiste, K.; Lang, L.; Sepp, H.; Lepland, A.; Kirsimäe, K. Constraining the Conditions of Phosphogenesis: Stable Isotope and Trace Element Systematics of Recent Namibian Phosphatic Sediments. Geochim. Et Cosmochim. Acta 2021, 302, 141–159. [Google Scholar] [CrossRef]
- Diaz, J.; Ingall, E.; Benitez-Nelson, C.; Paterson, D.; de Jonge, M.D.; McNulty, I.; Brandes, J.A. Marine Polyphosphate: A Key Player in Geologic Phosphorus Sequestration. Science 2008, 320, 652–655. [Google Scholar] [CrossRef]
- Crosby, C.H.; Bailey, J.V.; Sharma, M. Fossil Evidence of Iron-Oxidizing Chemolithotrophy Linked to Phosphogenesis in the Wake of the Great Oxidation Event. Geology 2014, 42, 1015–1018. [Google Scholar] [CrossRef]
- März, C.; Riedinger, N.; Sena, C.; Kasten, S. Phosphorus Dynamics around the Sulphate-Methane Transition in Continental Margin Sediments: Authigenic Apatite and Fe(II) Phosphates. Mar. Geol. 2018, 404, 84–96. [Google Scholar] [CrossRef] [Green Version]
- Wan, B.; Yang, P.; Jung, H.; Zhu, M.; Diaz, J.M.; Tang, Y. Iron Oxides Catalyze the Hydrolysis of Polyphosphate and Precipitation of Calcium Phosphate Minerals. Geochim. Et Cosmochim. Acta 2021, 305, 49–65. [Google Scholar] [CrossRef]
- Schwid, M.F.; Xiao, S.; Hiatt, E.E.; Fang, Y.; Nolan, M.R. Iron Phosphate in the Ediacaran Doushantuo Formation of South China: A Previously Undocumented Marine Phosphate Sink. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2020, 560, 109993. [Google Scholar] [CrossRef]
- Fang, L.; Zeng, W.; Xu, L.; Huang, L.-Z. Green Rusts as a New Solution to Sequester and Stabilize Phosphate in Sediments under Anoxic Conditions and Their Implication for Eutrophication Control. Chem. Eng. J. 2020, 388, 124198. [Google Scholar] [CrossRef]
- Refait, P.; Reffass, M.; Landoulsi, J.; Sabot, R.; Jeannin, M. Role of Phosphate Species during the Formation and Transformation of the Fe(II–III) Hydroxycarbonate Green Rust. Colloids Surf. A Physicochem. Eng. Asp. 2007, 299, 29–37. [Google Scholar] [CrossRef]
- Jiang, X.-D.; Sun, X.-M.; Chou, Y.-M.; Hein, J.R.; He, G.-W.; Fu, Y.; Li, D.; Liao, J.-L.; Ren, J.-B. Geochemistry and Origins of Carbonate Fluorapatite in Seamount FeMn Crusts from the Pacific Ocean. Mar. Geol. 2020, 423, 106135. [Google Scholar] [CrossRef]
- Lusty, P.A.; Hein, J.R.; Josso, P. Formation and Occurrence of Ferromanganese Crusts: Earth’s Storehouse for Critical Metals. Elements 2019, 14, 313318. [Google Scholar] [CrossRef]
- Hein, J.R.; Conrad, T.; Mizell, K.; Banakar, V.K.; Frey, F.A.; Sager, W.W. Controls on Ferromanganese Crust Composition and Reconnaissance Resource Potential, Ninetyeast Ridge, Indian Ocean. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2016, 110, 1–19. [Google Scholar] [CrossRef]
- Rudmin, M.; Banerjee, S.; Abdullayev, E.; Ruban, A.; Filimonenko, E.; Lyapina, E.; Kashapov, R.; Mazurov, A. Ooidal Ironstones in the Meso-Cenozoic Sequences in Western Siberia: Assessment of Formation Processes and Relationship with Regional and Global Earth Processes. J. Palaeogeogr. 2020, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Todd, S.E.; Pufahl, P.K.; Murphy, J.B.; Taylor, K.G. Sedimentology and Oceanography of Early Ordovician Ironstone, Bell Island, Newfoundland: Ferruginous Seawater and Upwelling in the Rheic Ocean. Sediment. Geol. 2019, 379, 1–15. [Google Scholar] [CrossRef]
- Reinhard, C.T.; Planavsky, N.J.; Gill, B.C.; Ozaki, K.; Robbins, L.J.; Lyons, T.W.; Fischer, W.W.; Wang, C.; Cole, D.B.; Konhauser, K.O. Evolution of the Global Phosphorus Cycle. Nature 2017, 541, 386–389. [Google Scholar] [CrossRef]
- Jones, C.; Nomosatryo, S.; Crowe, S.A.; Bjerrum, C.J.; Canfield, D.E. Iron Oxides, Divalent Cations, Silica, and the Early Earth Phosphorus Crisis. Geology 2015, 43, 135–138. [Google Scholar] [CrossRef]
- Pufahl, P.K.; Grimm, K.A. Coated Phosphate Grains: Proxy for Physical, Chemical, and Ecological Changes in Seawater. Geology 2003, 31, 801–804. [Google Scholar] [CrossRef]
- Schuffert, J.D.; Jahnke, R.A.; Kastner, M.; Leather, J.; Sturz, A.; Wing, M.R. Rates of Formation of Modern Phosphorite off Western Mexico. Geochim. Et Cosmochim. Acta 1994, 58, 5001–5010. [Google Scholar] [CrossRef]
- Trappe, J. Phanerozoic Phosphorite Depositional Systems: A Dynamic Model for a Sedimentary Resource System; Lecture Notes in Earth Sciences; Springer: Berlin/Heidelberg, Germany, 1998; ISBN 978-3-540-63581-9. [Google Scholar]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock-Forming Minerals; GeoscienceWorld: McLean, VA, USA, 2013. [Google Scholar] [CrossRef]
- Maria Barros de Oliveira, S.; Aparecida Liguori Imbernon, R. Weathering Alteration and Related REE Concentration in the Catalão I Carbonatite Complex, Central Brazil. J. S. Am. Earth Sci. 1998, 11, 379–388. [Google Scholar] [CrossRef]
- Dymek, R.F.; Owens, B.E. Petrogenesis of Apatite-Rich Rocks (Nelsonites and Oxide-Apatite Gabbronorites) Associated with Massif Anorthosites. Econ. Geol. 2001, 96, 797–815. [Google Scholar] [CrossRef]
- Simandl, G.J.; Paradis, S. Carbonatites: Related Ore Deposits, Resources, Footprint, and Exploration Methods. Appl. Earth Sci. 2018, 127, 123–152. [Google Scholar] [CrossRef] [Green Version]
- Krasnova, N.I.; Petrov, T.G.; Balaganskaya, E.G.; Garcia, D.; Moutte, J.; Zaitsev, A.N.; Wall, F. Introduction to Phoscorites: Occurrence, Composition, Nomenclature and Petrogenesis; GeoscienceWorld: McLean, VA, USA, 2004. [Google Scholar] [CrossRef]
- Arzamastsev, A.A.; Arzamastseva, L.V.; Zhirova, A.M.; Glaznev, V.N. Model of Formation of the Khibiny-Lovozero Ore-Bearing Volcanic-Plutonic Complex. Geol. Ore Depos. 2013, 55, 341–356. [Google Scholar] [CrossRef]
- Zaitsev, A.N.; Terry Williams, C.; Jeffries, T.E.; Strekopytov, S.; Moutte, J.; Ivashchenkova, O.V.; Spratt, J.; Petrov, S.V.; Wall, F.; Seltmann, R.; et al. Rare Earth Elements in Phoscorites and Carbonatites of the Devonian Kola Alkaline Province, Russia: Examples from Kovdor, Khibina, Vuoriyarvi and Turiy Mys Complexes. Ore Geol. Rev. 2014, 61, 204–225. [Google Scholar] [CrossRef]
- Kogarko, L. Chemical Composition and Petrogenetic Implications of Apatite in the Khibiny Apatite-Nepheline Deposits (Kola Peninsula). Minerals 2018, 8, 532. [Google Scholar] [CrossRef] [Green Version]
- Cerva-Alves, T.; Remus, M.V.D.; Dani, N.; Basei, M.A.S. Integrated Field, Mineralogical and Geochemical Characteristics of Caçapava Do Sul Alvikite and Beforsite Intrusions: A New Ediacaran Carbonatite Complex in Southernmost Brazil. Ore Geol. Rev. 2017, 88, 352–369. [Google Scholar] [CrossRef]
- Laznicka, P. Giant Metallic Deposits: Future Sources of Industrial Metals, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 978-3-642-12404-4. [Google Scholar]
- Xu, C.; Wang, L.; Song, W.; Wu, M. Carbonatites in China: A Review for Genesis and Mineralization. Geosci. Front. 2010, 1, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Lai, X.; Yang, X.; Liu, Y.; Yan, Z. Genesis of the Bayan Obo Fe–REE–Nb Deposit: Evidences from Pb–Pb Age and Microanalysis of the H8 Formation in Inner Mongolia, North China Craton. J. Asian Earth Sci. 2016, 120, 87–99. [Google Scholar] [CrossRef]
- Castor, S.B. The mountain pass rare-earth carbonatite and associated ultrapotassic rocks, California. Can. Mineral. 2008, 46, 779–806. [Google Scholar] [CrossRef]
- Pitawala, A.; Lottermoser, B.G. Petrogenesis of the Eppawala Carbonatites, Sri Lanka: A Cathodoluminescence and Electron Microprobe Study. Mineral. Petrol. 2012, 105, 57–70. [Google Scholar] [CrossRef]
- Bühn, B.; Dörr, W.; Brauns, C.M. Petrology and Age of the Otjisazu Carbonatite Complex, Namibia: Implications for the Pre- and Synorogenic Damaran Evolution. J. Afr. Earth Sci. 2001, 32, 1–17. [Google Scholar] [CrossRef]
- Bühn, B. The Role of the Volatile Phase for REE and Y Fractionation in Low-Silica Carbonate Magmas: Implications from Natural Carbonatites, Namibia. Mineral. Petrol. 2008, 92, 453–470. [Google Scholar] [CrossRef]
- Antonini, P.; Comin-chiaramonti, P.; Gomes, C.B.; Censi, P.; Riffel, B.F.; Yamamoto, E. The Early Proterozoic Carbonatite Complex of Angico Dos Dias, Bahia State, Brazil: Geochemical and Sr-Nd Isotopic Evidence for an Enriched Mantle Origin. Mineral. Mag. 2003, 67, 1039–1057. [Google Scholar] [CrossRef]
- O’Brien, H.; Heilimo, E.; Heino, P. Chapter 4.3—The Archean Siilinjärvi Carbonatite Complex. In Mineral Deposits of Finland; Maier, W.D., Lahtinen, R., O’Brien, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 327–343. ISBN 978-0-12-410438-9. [Google Scholar]
- Lee, W.-J.; Wyllie, P.J. Processes of Crustal Carbonatite Formation by Liquid Immiscibility and Differentiation, Elucidated by Model Systems. J. Petrol. 1998, 39, 2005–2013. [Google Scholar] [CrossRef]
- Bell, K.; Kjarsgaard, B.A.; Simonetti, A. Carbonatites—Into the Twenty-First Century. J. Petrol. 1998, 39, 1839–1845. [Google Scholar] [CrossRef]
- Veksler, I.; Lentz, D. Parental Magmas of Plutonic Carbonatites, Carbonate-Silicate Immiscibility and Decarbonation Reactions: Evidence from Melt and Fluid Inclusions. Melt Incl. Plutonic Rocks 2006, 123–150. [Google Scholar]
- Bell, K.; Rukhlov, A.S. Carbonatites from the Kola Alkaline Province: Origin, Evolution and Source Characteristics; GeoscienceWorld: McLean, VA, USA, 2004. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Reguir, E.P.; Zaitsev, A.N.; Couëslan, C.; Xu, C.; Kynický, J.; Mumin, A.H.; Yang, P. Apatite in Carbonatitic Rocks: Compositional Variation, Zoning, Element Partitioning and Petrogenetic Significance. Lithos 2017, 274–275, 188–213. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.P.; Campbell, L.S.; Kynicky, J. A Review of the Genesis of the World Class Bayan Obo Fe–REE–Nb Deposits, Inner Mongolia, China: Multistage Processes and Outstanding Questions. Ore Geol. Rev. 2015, 64, 459–476. [Google Scholar] [CrossRef]
- Migdisov, A.; Williams-Jones, A.E.; Brugger, J.; Caporuscio, F.A. Hydrothermal Transport, Deposition, and Fractionation of the REE: Experimental Data and Thermodynamic Calculations. Chem. Geol. 2016, 439, 13–42. [Google Scholar] [CrossRef] [Green Version]
- Prokopyev, I.R.; Doroshkevich, A.G.; Ponomarchuk, A.V.; Sergeev, S.A. Mineralogy, Age and Genesis of Apatite-Dolomite Ores at the Seligdar Apatite Deposit (Central Aldan, Russia). Ore Geol. Rev. 2017, 81, 296–308. [Google Scholar] [CrossRef]
- Toledo, M.C.M.D.; Lenharo, S.L.R.; Ferrari, V.C.; Fontan, F.; Parseval, P.D.; Leroy, G. The compositional evolution of apatite in the weathering profile of the Catalão i alkaline-carbonatitic complex, Goias, Brazil. Can. Mineral. 2004, 42, 1139–1158. [Google Scholar] [CrossRef] [Green Version]
- Lottermoser, B.G. Rare-Earth Element Mineralisation within the Mt. Weld Carbonatite Laterite, Western Australia. Lithos 1990, 24, 151–167. [Google Scholar] [CrossRef]
- Walter, A.-V.; Nahon, D.; Flicoteaux, R.; Girard, J.P.; Melfi, A. Behaviour of Major and Trace Elements and Fractionation of REE under Tropical Weathering of a Typical Apatite-Rich Carbonatite from Brazil. Earth Planet. Sci. Lett. 1995, 136, 591–602. [Google Scholar] [CrossRef]
- Flicoteaux, R.; Lucas, J. Weathering of Phosphate Minerals. In Phosphate Minerals; Nriagu, J.O., Moore, P.B., Eds.; Springer: Berlin/Heidelberg, Germany, 1984; pp. 292–317. ISBN 978-3-642-61736-2. [Google Scholar]
- Notholt, A.J.G. Phosphorite Resources in the Mediterranean (Tethyan) Phosphogenic Province: A Progress Report. Sci. Géologiques Bull. Et Mémoires 1985, 77, 9–17. [Google Scholar]
- Piqué, A. Géologie Du Maroc: Les Domaines Régionaux et Leur Évolution Structurale; Imprimerie el Maarif al Jadida: Rabat, Morocco, 1994. [Google Scholar]
- Cappetta, H. Un nouveau genre de Sélacien (Batomorphii, Myliobatiformes) de l’Yprésien des Ouled Abdoun, Maroc. Geobios 1986, 19, 635–640. [Google Scholar] [CrossRef]
- Rauscher, R.; Doubinger, J. Les dinokystes du Maestrichtien phosphaté du Maroc. Sci. Géologiques Bull. Et Mémoires 1982, 35, 97–116. [Google Scholar] [CrossRef]
- Ollivier-Pierre, M.-F. La microflore du Paléocène et de l’Eocène des séries phosphatées des Ganntour (Maroc). Sci. Géologiques Bull. Et Mémoires 1982, 35, 117–127. [Google Scholar] [CrossRef]
- Soncini, M.-J. Three New Dinoflagellate Cysts from the Moroccan Paleocene-Eocene Phosphates. Rev. Palaeobot. Palynol. 1992, 70, 325–338. [Google Scholar] [CrossRef]
- Prévôt, L. Géochimie et pétrographie de la formation à phosphate des Ganntour (Maroc): Utilisation pour une explication de la genèse des phosphorites Cretacé-Eocènes. Ph.D. Thesis, Université Louis Pasteur, Strasbourg, France, 1988. [Google Scholar]
- Zouhri, S.; Kchikach, A.; Saddiqi, O.; Haïmer, F.Z.E.; Baidder, L.; Michard, A. The Cretaceous-Tertiary Plateaus. In Continental Evolution: The Geology of Morocco; Michard, A., Saddiqi, O., Chalouan, A., de Lamotte, D.F., Eds.; Lecture Notes in Earth Sciences; Springer: Berlin/Heidelberg, Germany, 2008; Volume 116, pp. 331–358. ISBN 978-3-540-77075-6. [Google Scholar]
- Belfkira, O. Evolutions Sédimentologiques et Géochimiques de la Série Phosphatée du Maestrichtien des Ouled Abdoun (Maroc). Ph.D Thesis, Université Scientifique et Médicale de Grenoble, Saint-Martin-d’Hères, France, 1980. [Google Scholar]
- Boujo, A. Contribution à l’étude géologique du gisement de phosphate crétacé-éocène des Ganntour (Maroc occidental). Sci. Géologiques Bull. Et Mémoires 1976, 43, 1. [Google Scholar]
- Jourani, E. Anatomie Séquentielle et Géochimie Des Phosphates de Bouabout (Gisement de Meskala, Maroc): Eléments Pour Un Modèle Génétique. Ph.D. Thesis, Université De Pau et Des Pays De L’Adour, Pau, France, 1988. [Google Scholar]
- Trappe, J. Stratigraphy, facies distribution and Paleogeography of the marine Paleogene from the Western High Atlas, Morocco. Neues Jahrb. Geol. Paläontologie. Abh. 1991, 180, 279–321. [Google Scholar]
- Chellai, E.H.; Marzoqi, M.; Pascal, A.; Mouflih, M. Stratigraphy and Evolution of Upper Cretaceous-Palaeogene Sedimentary Systems in the Marrakesh High Atlas (Morocco). Comptes Rendus-Acad. Des Sci. Paris Ser. 2 Sci. De La Terre Et Des Planetes Fasc. A 1995, 321, 745. [Google Scholar]
- Marzoqi, M.; Pascal, A.; Chellai, E.H.; Lang, J. Les séquences de depôts sur la rampe carbonatée maastrichtienne-paléogène en bordure nord orientale du Golfe Atlantique dans la région d’Aït-Ourir (Atlas de Marrakech, Maroc). Bull. des Centres de Rech. Explor. -Prod. Elf-Aquitaine Mémoire 1996, 511–520. [Google Scholar]
- Nguidi, M.A.; Mouflih, M.; Benbouziane, A.; Kocsis, L.; El Ouariti, S.; El Boukhari, H.; Aquit, M.; Yazami, O.K. Lithofacies Analysis, Sedimentary Dynamics and Genesis of Maastrichtian-Eocene Phosphorites of BouCraa Deposit (Southern Morocco). J. Afr. Earth Sci. 2021, 177, 104161. [Google Scholar] [CrossRef]
- Herbig, H.-G. Das Paläogen Am Südrand Zantralen Hohen Atlas Und Mittleren Atlas Marokkos. Stratigraphie, Fazies, Paläogeographie Und Paläotektonik. Berl. Geowiss. Abh. 1991, 135, 1–289. [Google Scholar]
- Noubhani, A.; Cappetta, H. Révision Des Rhombodontidae (Neoselachii, Batomorphii) Des Bassins à Phosphate Du Maroc. Palaeovertebrata 1994, 23, 1–49. [Google Scholar]
- Marzoqi, M. Les Systèmes Sédimentaires Marins Du Crétacé Terminal-Paleogene Dans l’Atlas de Marrakech et Le Bassin de Ouarzazate. Thèse d’état, Université Cadi Ayyad, Marrakech, Morocco, 2001. [Google Scholar]
- Kocsis, L.; Gheerbrant, E.; Mouflih, M.; Cappetta, H.; Ulianov, A.; Chiaradia, M.; Bardet, N. Gradual Changes in Upwelled Seawater Conditions (Redox, PH) from the Late Cretaceous through Early Paleogene at the Northwest Coast of Africa: Negative Ce Anomaly Trend Recorded in Fossil Bio-Apatite. Chem. Geol. 2016, 421, 44–54. [Google Scholar] [CrossRef]
- Kocsis, L.; Ulianov, A.; Mouflih, M.; Khaldoune, F.; Gheerbrant, E. Geochemical Investigation of the Taphonomy, Stratigraphy, and Palaeoecology of the Mammals from the Ouled Abdoun Basin (Paleocene-Eocene of Morocco). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2021, 577, 110523. [Google Scholar] [CrossRef]
- Nathan, Y.; Benalioulhaj, N.; Prévôt, L.; Lucas, J. The Geochemistry of Cadmium in the Phosphate-Rich and Organic-Rich Sediments of the Oulad-Abdoun and Timahdit Basins (Morocco). J. Afr. Earth Sci. 1996, 22, 17–27. [Google Scholar] [CrossRef]
- McArthur, J.M.; Walsh, J.N. Rare-Earth Geochemistry of Phosphorites. Chem. Geol. 1984, 47, 191–220. [Google Scholar] [CrossRef]
- Amine, M.; Asafar, F.; Bilali, L.; Nadifiyine, M. Hydrochloric Acid Leaching Study of Rare Earth Elements from Moroccan Phosphate. J. Chem. 2019, 2019, e4675276. [Google Scholar] [CrossRef] [Green Version]
- Moutaouakil, D.; Giresse, P. Petrologie et Environnements Sedimentaires Des Phosphates Mesocenozoiques Du Bassin Des Ouled Abdoun (Maroc). Bull. De La Société Géologique De Fr. 1993, 164, 473–491. [Google Scholar]
- Salvan, H. Géologie Des Gîtes Mineraux Marocains, Vol. 3. Phosphates. Notes Et Mémoires Du Serv. Géologique Du Maroc 1986, 276, 392. [Google Scholar]
- Herbig, H.-G.; Trappe, J. Stratigraphy of the Subatlas Group (Maastrichtian—Middle Eocene, Morocco). Newsl. Stratigr. 1994, 30, 125–165. [Google Scholar] [CrossRef]
- Herbig, H.-G. Lithostratigraphisch-Fazielle Untersuchungen Im Marinen Alttertiär Südlich Des Zentralen Hohen Atlas (Marokko). Berl. Geowiss. abh. Reihe. Geol. Palaeontol 1986, 66, 343–380. [Google Scholar]
- Charrière, A.; Haddoumi, H.; Mojon, P.-O.; Ferrière, J.; Cuche, D.; Zili, L. Mise en évidence par charophytes et ostracodes de l’âge Paléocène des dépôts discordants sur les rides anticlinales de la région d’Imilchil (Haut Atlas, Maroc): Conséquences paléogéographiques et structurales. Comptes Rendus Palevol. 2009, 8, 9–19. [Google Scholar] [CrossRef]
- Bardet, N.; Gheerbrant, E.; Noubhani, A.; Cappetta, H.; Jouve, S.; Bourdon, E.; Suberbiola, X.P.; Jalil, N.-E.; Vincent, P.; Houssaye, A.; et al. Les Vertébrés Des Phosphates Crétacés-Paléogènes (72, 1–47, 8 Ma) Du Maroc. Mémoire De La Société Géologique De Fr. 2017, 180, 351–452. [Google Scholar]
- Scholz, R.W.; Ulrich, A.E.; Eilittä, M.; Roy, A. Sustainable Use of Phosphorus: A Finite Resource. Sci. Total. Environ. 2013, 461–462, 799–803. [Google Scholar] [CrossRef]
- Geissler, B.; Steiner, G.; Mew, M.C. Clearing the Fog on Phosphate Rock Data—Uncertainties, Fuzziness, and Misunderstandings. Sci. Total. Environ. 2018, 642, 250–263. [Google Scholar] [CrossRef]
- Van Kauwenbergh, S.J. World Phosphate Rock Reserves and Resources; IFDC: Muscle Shoals, AL, USA, 2010. [Google Scholar]
- Cooper, J.; Lombardi, R.; Boardman, D.; Carliell-Marquet, C. The Future Distribution and Production of Global Phosphate Rock Reserves. Resour. Conserv. Recycl. 2011, 57, 78–86. [Google Scholar] [CrossRef]
- Mohr, S.; Evans, G. Projections of Future Phosphorus Production. Philica 2013, 380. [Google Scholar]
- Cordell, D.; Drangert, J.-O.; White, S. The Story of Phosphorus: Global Food Security and Food for Thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Walan, P. Modeling of Peak Phosphorus: A Study of Bottlenecks and Implications for Future Production. Master’s Thesis, Uppsala University, Uppsala, Sweden, 2013. [Google Scholar]
- Bouabdellah, M.; Hoernle, K.; Kchit, A.; Duggen, S.; Hauff, F.; Klügel, A.; Lowry, D.; Beaudoin, G. Petrogenesis of the Eocene Tamazert Continental Carbonatites (Central High Atlas, Morocco): Implications for a Common Source for the Tamazert and Canary and Cape Verde Island Carbonatites. J. Petrol. 2010, 51, 1655–1686. [Google Scholar] [CrossRef]
- Montero, P.; Haissen, F.; Mouttaqi, A.; Molina, J.F.; Errami, A.; Sadki, O.; Cambeses, A.; Bea, F. Contrasting SHRIMP U–Pb Zircon Ages of Two Carbonatite Complexes from the Peri-Cratonic Terranes of the Reguibat Shield: Implications for the Lateral Extension of the West African Craton. Gondwana Res. 2016, 38, 238–250. [Google Scholar] [CrossRef]
- Bouabdli, A.; Dupuy, C.; Dostal, J. Geochemistry of Mesozoic Alkaline Lamprophyres and Related Rocks from the Tamazert Massif, High Atlas (Morocco). Lithos 1988, 22, 43–58. [Google Scholar] [CrossRef]
- Marks, M.A.W.; Schilling, J.; Coulson, I.M.; Wenzel, T.; Markl, G. The Alkaline–Peralkaline Tamazeght Complex, High Atlas Mountains, Morocco: Mineral Chemistry and Petrological Constraints for Derivation from a Compositionally Heterogeneous Mantle Source. J. Petrol. 2008, 49, 1097–1131. [Google Scholar] [CrossRef] [Green Version]
- Kchit, A. Le Complexe Plutonique Alcalin Du Tamazert, Haut-Atlas de Midelt (Maroc): Pétrologie et Structurologie. Ph.D. Thesis, Université Toulouse III, Toulouse, France, 1990. [Google Scholar]
- Armando, G. Intracontinental Alkaline Magmatism: Geology, Petrography, Mineralogy and Geochemistry of the Jebel Hayim Massif (Central High Atlas-Morocco); Université de Lausanne: Lausanne, Sweden, 1999. [Google Scholar]
- Lhachmi, A.; Lorand, J.-P.; Fabries, J. Pétrologie de l’intrusion alcaline mésozoïque de la région d’Anemzi, Haut Atlas Central, Maroc. J. Afr. Earth Sci. 2001, 32, 741–764. [Google Scholar] [CrossRef]
- Essaifi, A.; Zayane, R. Petrogenesis and Origin of the Upper Jurassic-Lower Cretaceous Magmatism in Central High Atlas (Morocco): Major, Trace Element and Isotopic (Sr-Nd) Constraints. J. Afr. Earth Sci. 2018, 137, 229–245. [Google Scholar] [CrossRef]
- Dumańska-Słowik, M.; Wesełucha-Birczyńska, A.; Heflik, W.; Maksymiuk, W.; Sikorska-Jaworowska, M. Organic Inclusions Evidence, Composition, and Cathodoluminescence Behaviour for the Formation Conditions of Fluorapatite from Anemzi (Morocco). J. Raman Spectrosc. 2018, 49, 2008–2020. [Google Scholar] [CrossRef]
- Rakovan, J. Connoisseur’s Choice: Fluorapatite, Acushnet Quarry, Bristol County, Massachusetts. Rocks Miner. 2015, 90, 244–259. [Google Scholar] [CrossRef]
- Michard, A.; Ibouh, H.; Charrière, A. Syncline-Topped Anticlinal Ridges from the High Atlas: A Moroccan Conundrum, and Inspiring Structures from the Syrian Arc, Israel. Terra Nova 2011, 23, 314–323. [Google Scholar] [CrossRef]
- Bea, F.; Montero, P.; Haissen, F.; El Archi, A. 2.46 Ga Kalsilite and Nepheline Syenites from the Awsard Pluton, Reguibat Rise of the West African Craton, Morocco. Generation of Extremely K-Rich Magmas at the Archean–Proterozoic Transition. Precambrian Res. 2013, 224, 242–254. [Google Scholar] [CrossRef]
- Bea, F.; Montero, P.; Haissen, F.; Rjimati, E.; Molina, J.F.; Scarrow, J.H. Kalsilite-Bearing Plutonic Rocks: The Deep-Seated Archean Awsard Massif of the Reguibat Rise, South Morocco, West African Craton. Earth-Sci. Rev. 2014, 138, 1–24. [Google Scholar] [CrossRef]
- Bea, F.; Montero, P.; Haissen, F.; Molina, J.F.; Michard, A.; Lazaro, C.; Mouttaqi, A.; Errami, A.; Sadki, O. First Evidence for Cambrian Rift-Related Magmatism in the West African Craton Margin: The Derraman Peralkaline Felsic Complex. Gondwana Res. 2016, 36, 423–438. [Google Scholar] [CrossRef]
- Benaouda, R.; Kraemer, D.; Sitnikova, M.; Goldmann, S.; Schwarz-Schampera, U.; Errami, A.; Mouttaqi, A.; Bau, M. Discovery of High-Grade REE-Nb-Fe Mineralization Associated with Calciocarbonatite in South Morocco. Ore Geol. Rev. 2020, 124, 103631. [Google Scholar] [CrossRef]
- Raji, O.; Ouabid, M.; Bodinier, J.-L.; El Messbahi, H.; Malainine, C.E.; Tabbakh, Z. An Integrated Approach for Rapid Delineation of K-Rich Syenites Suitable as Unconventional Potash Resources. Nat. Resour. Res. 2021, 30, 3219–3239. [Google Scholar] [CrossRef]



| Country | Ore | Age | P2O5 Content (%) | Major Associated Commodity | Rock in Deposit |
|---|---|---|---|---|---|
| Russia | Khibina (Kola Peninsula) | Devonian (385–360 Ma) | 15 | Nepheline (Al) | Carbonatite, eruptive breccia, foyaite, ijolite, melteigite, nepheline syenite, phoscorite, urtite |
| Kovdor (Kola Peninsula) | Devonian (385–360 Ma) | 6–7 | Magnetite (Fe), vermiculite, baddeleyite (Zr) | Carbonatite, dunite, ijolite, melteigite, phoskorite, pyroxenite | |
| South Africa | Palabora | Paleoproterozoic (~2 Ga) | 7–9 | Vermiculite, chalcopyrite (Cu), magnetite (Fe), thorite (U), baddeleyite (Zr) | Carbonatite, phoscorite, micaceous pyroxenite, pyroxene-phlogopite-apatite pegmatoid |
| Glenover | Upper Proterozoic (~1 Ga) | 25–29 | Apatite-hematite breccia, carbonatite, pyroxenite | ||
| Brazil | Jacupiranga | Jurassic-Cretaceous (161–125 Ma) | ~5 | Lime (calcite) | Carbonatite, ijolite, peridotite, jacupirangite, nepheline syenite |
| Araxá | Cretaceous (~87 Ma) | 15 | Pyrochlore (Nb) | Carbonatite, glimmerite, lamprophyre, phoscorite | |
| Catalão I | Cretaceous (~83 Ma) | 5–17 | Pyrochlore (Nb), Ti | Carbonatite, dunite, glimmerite, pyroxenite | |
| Tapira | Cretaceous (~70 Ma) | ~8 | Anatase (Ti) | Carbonatite, dunite, bebedourite, jacupirangite, peridotite, syenite, silexite, trachyte, tuff | |
| Angico dos Dias | Paleoproterozoic (2 Ga) | ~15 | Carbonatite, syenite, pyroxenite | ||
| Anitápolis | Cretaceous (131–104 Ma) | 6–35 | Ijolite, biotite pyroxenite, nepheline syenite, carbonatite | ||
| Ipanema | Cretaceous (138–121 Ma) | ~7 | Glimmerite, carbonatite, aegirinite, syenite | ||
| Miacuru | Ediacaran (~589 Ma) | 15 | Pyroxenite, syenite, glimmerite, carbonatite | ||
| Finland | Siilinjärvi | Archean (~2.6 Ga) | >3.5 | Lime (calcite), phlogopite | Glimmerite, carbonatite, fenite |
| Sokli | Devonian (410–362 Ma) | ~16 | Carbonatite, phoscorite, fenite | ||
| Uganda | Bukusu | Cenozoic (~40 Ma) | ~15 | Carbonatite, melteigite, ijolite, pyroxenite, syenite | |
| Sukulu | Cenozoic (~40 Ma) | 11–13 | Magnetite (Fe), pyrochlore (Nb) | Carbonatite, syenite | |
| Zimbabwe | Dorowa | Mesozoic | 5–7 | Magnetite (Fe) | Carbonatite, ijolite, syenite, fenite, nephelinite |
| Sri Lanka | Eppawala | Ediacaran (~550 Ma) | 38 | Carbonatite | |
| Canada | Lackner Lake (Ontario) | Neoproterozoic (~1.1 Ga) | ~9 | Pyrochlore (Nb), magnetite (Fe), REE | Carbonatite, ijolite, syenite, lamprophyre |
| Cargill (Ontario) | Neoproterozoic (~1.7 Ga) | ~20 | Carbonatite, pyroxenite | ||
| Martinson (Ontario) | 20–23 | Pyrochlore (Nb) | Carbonatite, ultramafic breccia | ||
| Namibia | Ondurakorume | Cretaceous | 7 | REE, Sr, Nb | Carbonatite, syenite, volcanic breccia |
| Otjisazu | Neoproterozoic (~837 Ma) | 3–5 | Carbonatite, pyroxenite, syenite, fenite | ||
| Zambia | Nkombwa Hill | Neoproterozoic (~679 Ma) | 7–8 | Pyrochlore (Nb), REE | Carbonatite, fenite |
| Kaluwe | Cretaceous (100–103 Ma) | 3–5 | Carbonatite | ||
| Burundi | Matongo | Neoproterozoic (739–780 Ma) | ~11 | Carbonatite, syenite, gabbro, diorite |
| Age | Mean CaO Wt.% | S.D. | Mean P2O5 Wt.% | S.D. |
|---|---|---|---|---|
| Ypresian (n = 7) | 36.41 | 11.65 | 22.17 | 8.05 |
| Thanetian (n = 4) | 36.50 | 6.33 | 15.83 | 2.46 |
| Danian (n = 5) | 46.04 | 6.15 | 24.26 | 6.53 |
| Maastrichtian (n = 14) | 39.90 | 7.26 | 20.89 | 5.80 |
| SiO2 | Al2O3 | MgO | CaO | Fe2O3 | TiO2 | Na2O | K2O | P2O5 | L.O.I | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (n = 146) | 14.73 | 2.49 | 4.08 | 37.07 | 0.99 | 0.15 | 0.33 | 0.46 | 11.68 | 24.72 | ||||
| S.D. (n = 146) | 8.59 | 1.97 | 3.84 | 9.82 | 0.82 | 0.09 | 0.18 | 0.43 | 9.12 | 8.66 | ||||
| Sr | Ba | V | Ni | Co | Cr | B | Mn | Zn | Ga | Cu | Pb | Sn | Cd | |
| Mean (n = 146) | 762 | 74 | 80 | 95 | 0.27 | 181 | 8 | 36 | 158 | 0.33 | 43 | 14 | 0.32 | 9 |
| S.D. (n = 146) | 392 | 46 | 142 | 53 | 2 | 91 | 17 | 47 | 97 | 2 | 21 | 44 | 1 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Bamiki, R.; Raji, O.; Ouabid, M.; Elghali, A.; Khadiri Yazami, O.; Bodinier, J.-L. Phosphate Rocks: A Review of Sedimentary and Igneous Occurrences in Morocco. Minerals 2021, 11, 1137. https://doi.org/10.3390/min11101137
El Bamiki R, Raji O, Ouabid M, Elghali A, Khadiri Yazami O, Bodinier J-L. Phosphate Rocks: A Review of Sedimentary and Igneous Occurrences in Morocco. Minerals. 2021; 11(10):1137. https://doi.org/10.3390/min11101137
Chicago/Turabian StyleEl Bamiki, Radouan, Otmane Raji, Muhammad Ouabid, Abdellatif Elghali, Oussama Khadiri Yazami, and Jean-Louis Bodinier. 2021. "Phosphate Rocks: A Review of Sedimentary and Igneous Occurrences in Morocco" Minerals 11, no. 10: 1137. https://doi.org/10.3390/min11101137
APA StyleEl Bamiki, R., Raji, O., Ouabid, M., Elghali, A., Khadiri Yazami, O., & Bodinier, J.-L. (2021). Phosphate Rocks: A Review of Sedimentary and Igneous Occurrences in Morocco. Minerals, 11(10), 1137. https://doi.org/10.3390/min11101137