Substitution of Cement with Granulated Blast Furnace Slag in Cemented Paste Backfill: Evaluation of Technical and Chemical Properties
Abstract
:1. Introduction
- a.
- Study the leaching behavior and changes in phase composition of the five different recipes;
- b.
- Test if the compressive strength of the alternative CPB recipes meets the structural requirements and how the different binder mixtures affect attainment of CPB strength;
- c.
- Study how the changes in the recipes affect the internal microstructure of the specimens.
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Process Water and Mine Tailings Porewater
2.2.2. Mine Tailings, Slag, Cement and Five Cemented Paste Backfill (CPB) Recipes
2.2.3. Preparation of CPB Test Pieces
2.2.4. Compressive Strength Tests of the CBP Blocks
2.2.5. X-ray Tomography
3. Results and Discussion
3.1. Chemical Composition of Parent Materials and the CPB Recipes
3.1.1. Process Water and Mine Tailings Porewater
3.1.2. Solid Materials
3.2. Mineral and Secondary Phase Composition of Parent Materials and the CPB Recipes
3.3. Compressive Strength
3.4. X-ray Tomography
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- EUROSTAT. Waste Generation, 2018. Online publication. Data Extracted in October 2020. 2018. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/Waste_statistics#Total_waste_generation (accessed on 20 September 2021).
- Hund, K.; La Porta, D.; Fabregas, T.; Laing, T.; Drexhage, J.; World Bank Group. Minerals for Climate Action: The Mineral Intensity of the Clean Energy Transition. 2020. Available online: https://pubdocs.worldbank.org/en/961711588875536384/Minerals-for-Climate-Action-The-Mineral-Intensity-of-the-Clean-Energy-Transition.pdf (accessed on 20 September 2021).
- Blowes, D.W.; Ptacek, C.J.; Jurjovec, J. Mill tailings: Hydrogeology and geochemistry. Environ. Asp. Mine Wastes 2003, 31, 95–116. [Google Scholar]
- Dold, B. Evolution of Acid Mine Drainage Formation in Sulphidic Mine Tailings. Minerals 2014, 4, 621–641. [Google Scholar] [CrossRef] [Green Version]
- Benzaazoua, M.; Marion, P.; Picquet, I.; Bussière, B. The use of pastefill as a solidification and stabilization process for the control of acid mine drainage. Miner. Eng. 2004, 17, 233–243. [Google Scholar] [CrossRef]
- Belem, T.; Benzaazoua, M. Design and Application of Underground Mine Paste Backfill Technology. Geotech. Geol. Eng. 2007, 26, 147–174. [Google Scholar] [CrossRef]
- Mitchell, R.J. Stability of Cemented Tailings Backfill; Balasubramaniam, A.S., Rantucci, G., Chandra, S., Bergado, D.T., Phienweja, N., Saran, S., Nutalaya, P., Eds.; CRC Press: Bangkok, Thailand, 1989; pp. 501–507. ISBN 9789061918646. [Google Scholar]
- Coates, D.F. Caving, Subsidence, and Ground Control. In Rock Mechanics Principles, CANMET; Chapters 5, 5.1–5.42; Department of Energy, Mines and Resources: Ottawa, QC, Canada, 1981. [Google Scholar]
- Taylor, H.F. Cement Chemistry; Thomas Telford: London, UK, 1997; p. 459. ISBN 0-7277-2592-0. [Google Scholar]
- Deboucha, W.; Leklou, N.; Khelidj, A.; Oudjit, M.N. Hydration development of mineral additives blended cement using thermogravimetric analysis (TGA): Methodology of calculating the degree of hydration. Constr. Build. Mater. 2017, 146, 687–701. [Google Scholar] [CrossRef]
- Deboucha, W.; Leklou, N.; Khelidj, A. Blast Furnace Slag Addition Effects on Delayed Ettringite Formation in Heat-cured Mortars. KSCE J. Civ. Eng. 2018, 22, 3484–3490. [Google Scholar] [CrossRef]
- Li, W.; Fall, M. Strength and self-desiccation of slag-cemented paste backfill at early ages: Link to initial sulphate concentration. Cem. Concr. Compos. 2018, 89, 160–168. [Google Scholar] [CrossRef]
- Cihangir, F.; Ercikdi, B.; Kesimal, A.; Turan, A.; Deveci, H. Utilisation of alkali-activated blast furnace slag in paste backfill of high-sulphide mill tailings: Effect of binder type and dosage. Miner. Eng. 2012, 30, 33–43. [Google Scholar] [CrossRef]
- MEND Report 10.2–Paste Backfill Geochemistry—Environmental Effects of Leaching and Weathering; Mine Environment Neutral Drainage (MEND) Program: Vancouver, BC, Canada, 2006; 62p.
- Al-Abed, S.R.; Jegadeesan, G.; Purandare, J.; Allen, D. Arsenic release from iron rich mineral processing waste: Influence of pH and redox potential. Chemosphere 2007, 66, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Hamberg, R.; Maurice, C.; Alakangas, L. The use of low binder proportions in cemented paste backfill—Effects on As-leaching. Miner. Eng. 2015, 78, 74–82. [Google Scholar] [CrossRef]
- Wyche, N.; Eilu, P.; Koppström, K.; Kortelainen, V.; Niiranen, T.; Välimaa, J. Chapter 5.2—The Suurikuusikko Gold Deposit (Kittilä Mine), Northern Finland; Maier, W.D., Lahtinen, R., O’Brien, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 411–433. ISBN 9780124104389. [Google Scholar] [CrossRef]
- Agnico Eagle Finland Oy. Rikastamon Syötemäärän Sekä NP Rikastushiekan Varastointikapasiteetin Kasvattaminen Kittilän Kultakaivoksella; Environmental Impact Assessment Report 16X260196.720E14; Pöyry Finland Oy: Oulu, Finland, 2016; 381p. [Google Scholar]
- The SFS-EN ISO Standard: 11885; Finnish Environmental Instutute: Helsinki, Finland, 2009.
- The SFS-EN ISO Standard: 10304-1; Finnish Environmental Instutute: Helsinki, Finland, 2009.
- The SFS-EN ISO/IEC Standard: 17025:2017; Finnish Standards Association: Helsinki, Finland, 2017.
- Karlsson, T.; Alakangas, L.; Kauppila, P.; Räisänen, M.L. A Test of Two Methods for Waste Rock Drainage Quality Prediction: Aqua Regia Extraction and Single-addition Net-acid Generation Test Leachate Analysis. Mine Water Environ. 2021, 1–16. [Google Scholar] [CrossRef]
- Doležal, J.; Povondra, P.; Šulcek, Z. Decomposition Techniques in Inorganic Analysis; London Iliffe Books Ltd.: London, UK, 1968; p. 224. [Google Scholar]
- Räisänen, M.; Tenhola, M.; Mäkinen, J. Relationship between mineralogy and the physico-chemical properties of till in central Finland. Bull. Geol. Soc. Finl. 1992, 64, 35–58. [Google Scholar] [CrossRef]
- The SFS-EN Standard: 12457-3; General Industry Federation: Helsinki, Finland, 2002.
- Sylvester, P.J. Use of the mineral liberation analyzer (MLA) for mineralogical studies of sediments and sedimentary rocks. Mineral. Assoc. Can. 2012, 1, 1–16. [Google Scholar]
- The SFS-EN Standard: 12390-3:2019; Finnish Association of Construction Product Industries RTT: Helsinki, Finland, 2019.
- The SFS-EN Standard: 12504-1:2019; Finnish Association of Construction Product Industries RTT: Helsinki, Finland, 2019.
- The FINAS T301, SFS-EN ISO/IEC Standard: 17025; Finnish Standards Association: Helsinki, Finland, 2005.
- VNa 214/2007. Available online: https://www.finlex.fi/fi/laki/alkup/2007/20070214 (accessed on 20 September 2021).
- Shukla, J.; Mohandas, V.P.; Kumar, A. Effect of pH on the Solubility of CaSO4·2H2O in Aqueous NaCl Solutions and Physicochemical Solution Properties at 35 °C. J. Chem. Eng. Data 2008, 53, 2797–2800. [Google Scholar] [CrossRef]
- VNa 331/2013. Available online: https://www.finlex.fi/fi/laki/ajantasa/2013/20130331 (accessed on 20 September 2021).
- Vriens, B.; Skierszkan, E.K.; St-Arnault, M.; Salzsauler, K.; Aranda, C.; Mayer, K.U.; Beckie, R.D. Mobilization of Metal(oid) Oxyanions through Circumneutral Mine Waste-Rock Drainage. ACS Omega 2019, 4, 10205–10215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, A.E. Review of metal sulphide precipitation. Hydrometallurgy 2010, 104, 222–234. [Google Scholar] [CrossRef]
- Bellmann, F.; Stark, J. Activation of blast furnace slag by a new method. Cem. Concr. Res. 2009, 39, 644–650. [Google Scholar] [CrossRef]
- Ercikdi, B.; Cihangir, F.; Kesimal, A.; Deveci, H.; Alp, I. Utilization of industrial waste products as pozzolanic material in cemented paste backfill of high sulphide mill tailings. J. Hazard. Mater. 2009, 168, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Bowell, R.J. A review of sulphate removal options for mine waters. In Mine Water 2004–Proceedings International Mine Water Association Symposium; Jarvis, A.P., Dudgeon, B.A., Younger, P.L., Eds.; International Mine Water Association: Newcastle upon Tyne, UK, 2004; pp. 75–91. Available online: http://www.mwen.info/docs/imwa_2004/IMWA2004_43_Bowell.pdf (accessed on 20 September 2021).
- Lukas, W. Substitution of Si in the lattice of ettringite. Cem. Concr. Res. 1976, 6, 225–233. [Google Scholar] [CrossRef]
- Matschei, T.; Bellmann, F.; Stark, J. Hydration behaviour of sulphate-activated slag cements. Adv. Cem. Res. 2005, 17, 167–178. [Google Scholar] [CrossRef]
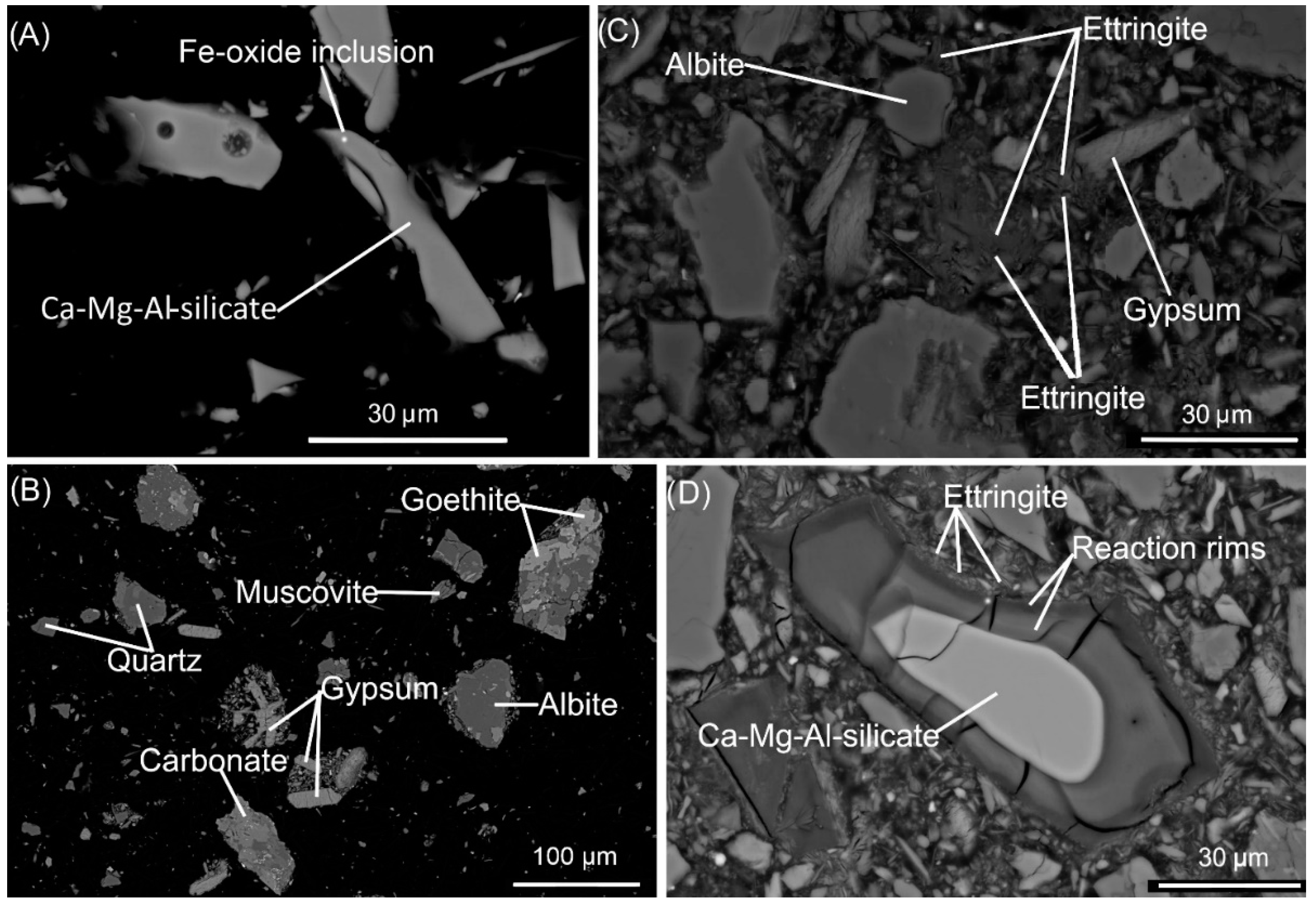
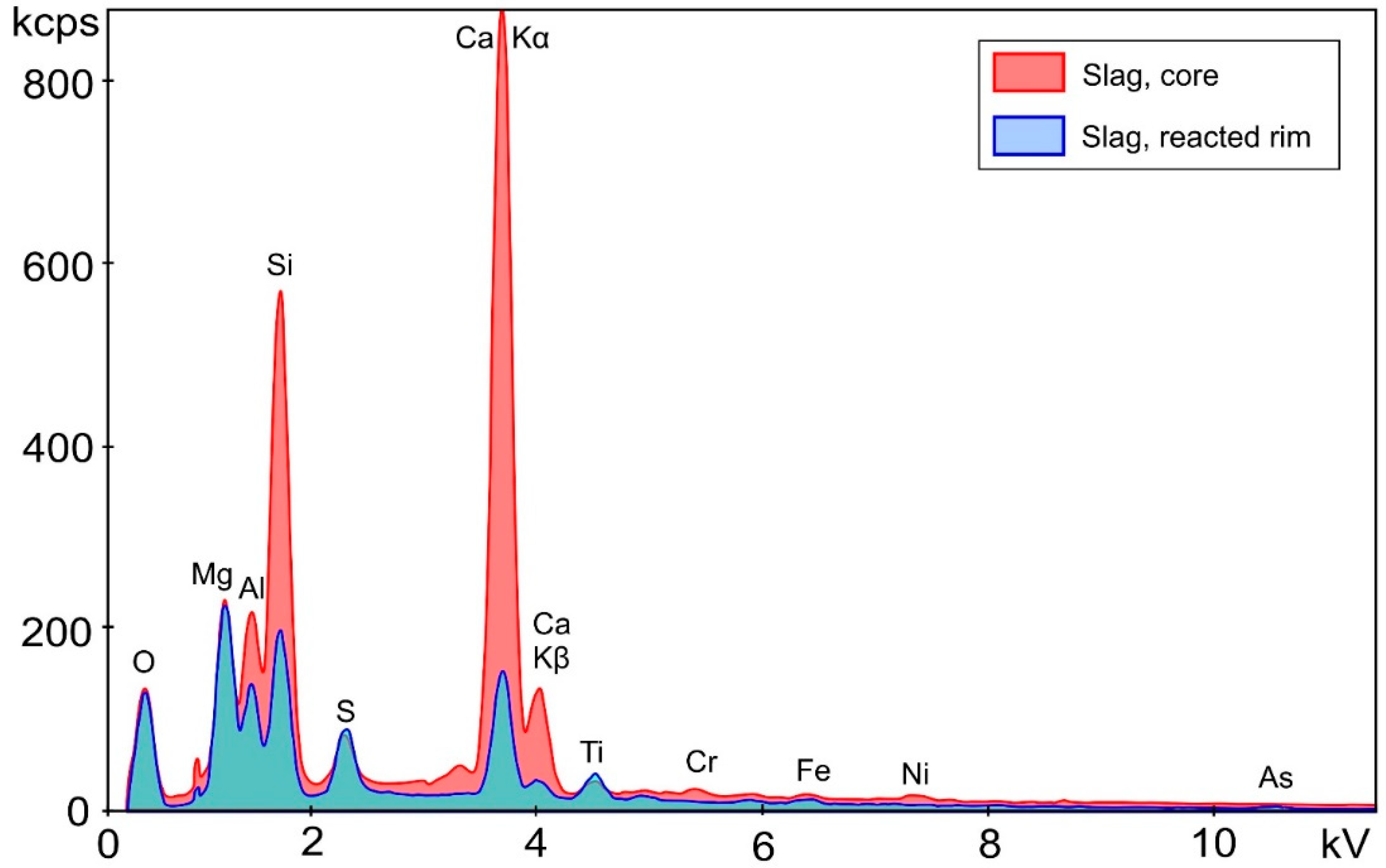
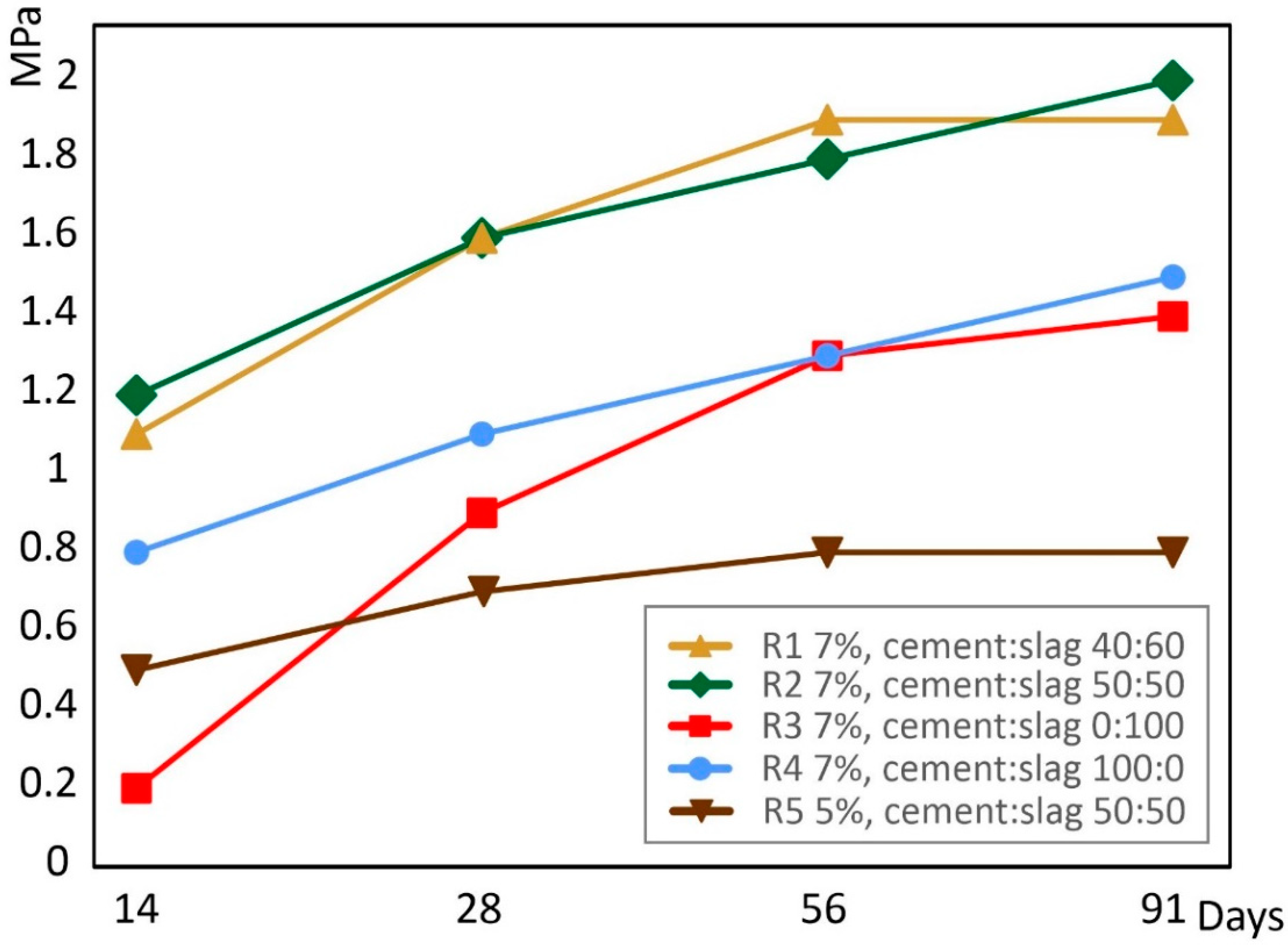


| Starting Materials | Cemented Paste Backfill Specimens (Cement: Slag Ratio) | ||||
|---|---|---|---|---|---|
| R1 (40:60) | R2 (50:50) | R3 (0:100) | R4 (100:0) | R5 (50:50) | |
| Mine tailings (%) | 63 | 63 | 63 | 63 | 65 |
| Water tot. (%) | 30 | 30 | 30 | 30 | 30 |
| Share of pore water (%) | 96 | 96 | 96 | 96 | 100 |
| Share of process water (%) | 4 | 4 | 4 | 4 | 0 |
| Binder (%) | 7 | 7 | 7 | 7 | 5 |
| Share of cement from binder (%) | 40 | 50 | 0 | 100 | 50 |
| Share of slag from binder (%) | 60 | 50 | 100 | 0 | 50 |
| Consistency slump mm | 200 | 220 | 210 | 190 | 200 |
| Measured Variable | Detection Limit | Unit | Pore Water | Process Water |
|---|---|---|---|---|
| Alkalinity | 0.1 | mmol/L | 1.4 | 4.7 |
| pH | - | pH | 8.0 | 7.8 |
| SO42− | 50 | mg/L | 7900.0 | 2300.0 |
| S | 1 | mg/L | 2260.0 | 676.0 |
| Mg | 0.05 | mg/L | 1410.0 | 103.0 |
| Ca | 0.1 | mg/L | 357.0 | 578.0 |
| K | 0.01 | mg/L | 114.0 | 79.0 |
| Na | 0.2 | mg/L | 101.0 | 117.0 |
| Cl | 10 | mg/L | 25.0 | 28.0 |
| F | 1 | µg/L | 1800.0 | <1.0 |
| Mn | 0.02 | µg/L | 1350.0 | 1440.0 |
| Sr | 0.1 | µg/L | 1180.0 | 1950.0 |
| Li | 0.1 | µg/L | 149.0 | 233.0 |
| Rb | 0.01 | µg/L | 134.0 | 138.0 |
| P | 0.05 | µg/L | 100.0 | 500.0 |
| As | 0.05 | µg/L | 58.9 | 17.5 |
| Mo | 0.02 | µg/L | 35.2 | 6.3 |
| Ba | 0.05 | µg/L | 20.7 | 55.8 |
| Al | 1 | µg/L | 17.7 | 13.2 |
| Ni | 0.05 | µg/L | 11.2 | 63.5 |
| Cu | 0.1 | µg/L | 10.6 | 3.8 |
| Co | 0.02 | µg/L | 2.7 | 6.1 |
| Zn | 0.2 | µg/L | 0.4 | 9.5 |
| V | 0.05 | µg/L | 0.1 | 0.4 |
| Cr | 0.2 | µg/L | <0.2 | 6.0 |
| Cd | 0.02 | µg/L | 0.0 | 0.1 |
| Element | Starting Materials | Cemented Paste Backfill Specimens (Cement:Slag Ratio) | ||||||
|---|---|---|---|---|---|---|---|---|
| Cement | Slag | Tailings | R1 (40:60) | R2 (50:50) | R3 (0:100) | R4 (100:0) | R5 (50:50) | |
| Al % | 3.1 | 6 | 5.1 | 4.4 | 4.4 | 4.4 | 4.2 | 4.4 |
| Fe % | 2.2 | 9.7 | 9 | 5.9 | 5.9 | 5.8 | 5.9 | 6.1 |
| Mg % | 2.3 | 7.2 | 2 | 3.2 | 3.2 | 3.3 | 3 | 3.2 |
| Ca % | 38.5 | 25.2 | 6.2 | 7.8 | 7.9 | 7.4 | 8.1 | 7.5 |
| Na % | 0.5 | 0.4 | 1.9 | 1 | 1 | 1 | 1 | 1 |
| K % | 0.8 | 0.7 | 1.1 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 |
| S % | 1.4 | 0.8 | 2 | 1.7 | 1.8 | 1.7 | 1.7 | 1.8 |
| As (ppm) | 22 | 1 | 1560 | 1570 | 1580 | 1550 | 1565 | 1640 |
| Ba (ppm) | 260 | 723 | 202 | 194 | 193 | 205 | 175 | 188 |
| Cd (ppm) | 0 | <0.1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Co (ppm) | 38 | <2.0 | 29 | 34 | 35 | 33 | 36 | 35 |
| Cr (ppm) | 52 | 31 | 46 | 257 | 263 | 258 | 265 | 265 |
| Cu (ppm) | 136 | 3 | 265 | 259 | 264 | 255 | 263 | 267 |
| Mo (ppm) | 18 | <2.0 | <2.0 | <2.0 | <2.0 | <2.0 | 3 | <2.0 |
| Ni (ppm) | 58 | 3 | 50 | 158 | 161 | 156 | 159 | 167 |
| Pb (ppm) | 20 | <10.0 | <10 | 13 | <11.0 | 13 | 16 | 12 |
| Sb (ppm) | 4 | <0.2 | 14 | 21 | 21 | 21 | 21 | 20 |
| V (ppm) | 142 | 355 | 88 | 198 | 200 | 204 | 189 | 199 |
| Zn (ppm) | 325 | <2.0 | 97 | 96 | 101 | 95 | 107 | 99 |
| Sb mg/kg | As mg/kg | Cd mg/kg | Co mg/kg | Cr mg/kg | Cu mg/kg | Pb mg/kg | Ni mg/kg | Zn mg/kg | V mg/kg | S % | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treshold value | 2 | 5 | 1 | 20 | 100 | 100 | 60 | 50 | 200 | 100 | |
| Lower guideline value | 10 | 50 | 10 | 100 | 200 | 150 | 200 | 100 | 250 | 150 | |
| Upper guideline value | 50 | 100 | 20 | 250 | 300 | 200 | 750 | 150 | 400 | 250 | |
| Blast-furnace slag | <0.02 | 1.15 | <0.01 | <1 | 29.6 | 2 | <0.1 | 2.2 | <1 | 321 | 0.63 |
| Cement | 3.65 | 18.3 | 0.22 | 27.4 | 60.8 | 117 | 20 | 48.3 | 287 | 102 | 0.12 |
| Mine tailings | 20.4 | 1730 | 0.26 | 35.1 | 78.4 | 288 | 3.85 | 164 | 81 | 42.8 | 1.81 |
| R1 (40:60) | 16.8 | 1500 | 0.24 | 33.9 | 85.9 | 260 | 4.57 | 157 | 89 | 63.5 | 1.73 |
| R1 (40:60), D | 17.2 | 1490 | 0.23 | 32.7 | 86.2 | 260 | 4.25 | 155 | 89 | 63.9 | 1.73 |
| R2 (50:50) | 19.4 | 1490 | 0.24 | 31.4 | 82.9 | 256 | 4.24 | 151 | 86 | 57.8 | 1.63 |
| R2 (50:50), D | 17 | 1510 | 0.23 | 33.5 | 82.9 | 259 | 4.29 | 155 | 89 | 60.3 | 1.73 |
| R3 (0:100) | 19.4 | 1510 | 0.23 | 32.6 | 83.7 | 261 | 3.61 | 157 | 81 | 70.2 | 1.75 |
| R3 (0:100), D | 17.9 | 1500 | 0.24 | 31.5 | 81.2 | 252 | 3.63 | 151 | 78 | 67 | 1.69 |
| R4 (100:0) | 20.4 | 1540 | 0.26 | 35.5 | 87.6 | 271 | 2.44 | 163 | 103 | 55.9 | 1.77 |
| R4 (100:0), D | 19.1 | 1510 | 0.24 | 34.2 | 86.8 | 271 | 2.37 | 161 | 102 | 54.6 | 1.76 |
| R5 (50:50 5%) | 19.3 | 1520 | 0.25 | 34.5 | 89.3 | 272 | 4.09 | 162 | 91 | 61.1 | 1.79 |
| R5 (50:50 5%), D | 18.6 | 1520 | 0.23 | 33.8 | 87 | 267 | 4.04 | 161 | 90 | 59.1 | 1.79 |
| As mg/kg | Ba mg/kg | Cr mg/kg | Cu mg/kg | Mo mg/kg | F− mg/kg | Cl− mg/kg | SO42− mg/kg | DOC mg/kg | |
|---|---|---|---|---|---|---|---|---|---|
| Inert waste | 0.5 | 20 | 0.5 | 2 | 0.5 | 10 | 800 | 1000 | 500 |
| Non-hazardous waste | 2 | 100 | 10 | 50 | 10 | 150 | 15,000 | 20,000 | 800 |
| Hazardous waste | 25 | 300 | 70 | 100 | 30 | 500 | 25,000 | 50,000 | 1000 |
| Blast-furnace slag | <0.09 | 1.2 | <0.1 | <0.05 | <0.05 | 1.5 | <1 | 129 | 8.2 |
| Cement | <0.05 | 16.6 | 0.4 | <0.05 | 6.9 | 18.4 | 176.6 | 2697 | 81.5 |
| Mine tailings | 0.6 | 0.1 | <0.1 | <0.05 | <0.05 | 8.4 | 15.8 | 19,470 | 5.9 |
| R1 (40:60) | 1.9 | 0.6 | <0.07 | 0.1 | 0.3 | 1.1 | 26.5 | 13,219 | 11.5 |
| R1 (40:60), D | 1.9 | 0.6 | <0.07 | 0.1 | 0.4 | 4.3 | 30.2 | 12,896 | 10.8 |
| R2 (50:50) | 1.6 | 0.6 | <0.06 | 0.1 | 0.5 | 4.8 | 31 | 13,175 | 12.4 |
| R2 (50:50), D | 1.7 | 0.6 | <0.06 | 0.1 | 0.4 | 4.6 | 31.9 | 13,613 | 11.9 |
| R3 (0:100) | 1.4 | 0.7 | <0.06 | <0.06 | 0.1 | 4.1 | 23.2 | 12,409 | 6.9 |
| R3 (0:100), D | 1.5 | 0.7 | <0.06 | <0.05 | 0.1 | 4.2 | 23.4 | 12,583 | 7.6 |
| R4 (100:0) | 1.7 | 0.5 | 0.1 | <0.06 | 0.7 | 6.6 | 40.6 | 12,321 | 12.9 |
| R4 (100:0), D | 1.6 | 0.5 | <0.05 | <0.05 | 0.8 | 6.5 | 40 | 12,825 | 12.6 |
| R5 (50:50) | 2.2 | 0.5 | 0.1 | <0.05 | 0.3 | 4.4 | 30.6 | 12,127 | 8.8 |
| R5 (50:50), D | 2.2 | 0.5 | 0.1 | <0.05 | 0.3 | 4.4 | 30.1 | 12,882 | 9.5 |
| Minerals and Phases (wt.%) | Starting Materials | CPB Samples (Cement: Slag) | ||||||
|---|---|---|---|---|---|---|---|---|
| Tailings | Slag | Cement | R1 | R2 | R3 | R4 | R5 | |
| (40:60) | (50:50) | (0:100) | (100:0) | (50:50) | ||||
| Quartz | 20 | - | 0.3 | 21.3 | 24.4 | 21.9 | 21.3 | 22.8 |
| Feldspars | 21.1 | - | - | 19.9 | 19.4 | 19.3 | 16.1 | 19.4 |
| Mica and chlorite | 11.4 | - | - | 9.3 | 7 | 9.4 | 6.6 | 9.1 |
| Clay minerals | 4.3 | - | - | 1.6 | 1.4 | 1 | 0.9 | 1.8 |
| Minor silicates | 0.5 | 0.1 | - | 2.9 | 2.4 | 2.3 | 1.6 | 2.3 |
| Carbonates (Ca, Mg, Fe) | 21.1 | 0.1 | 5.0 (1) | 19.6 | 15.2 | 19 | 17.1 | 18.5 |
| Gypsum | 9.7 | - | 2.9 | 5.2 | 5.7 | 5.1 | 3.5 | 6.4 |
| Ca-phosphate | 0.5 | - | - | 0 | 0 | 0 | 0 | 0 |
| Ettringite | - | - | 0.8 | 12.2 | 17.5 | 10.1 | 29.5 | 12.1 |
| Ca-Al-Mg-silicate (slag) | - | 99.1 | 20.7 | 6.3 | 5.3 | 10.3 | 1.9 | 5.9 |
| Metal oxides and hydroxides | 10.3 | 0.6 | 3.1 | 0.9 | 0.9 | 0.8 | 0.9 | 0.6 |
| Metal sulphides | 0.4 | - | - | 0.1 | 0.2 | 0.2 | 0.2 | 0.5 |
| Ca-silicates (C2S, C3S) (2) | - | - | 53.2 | - | - | - | - | - |
| Ca-aluminate (C3A)(2) | - | - | 9.3 | - | - | - | - | - |
| Ca-Al-Fe-oxide (C4AF) (2) | - | - | 4.7 | - | - | - | - | - |
| Unclassified | 0.7 | 0.1 | 0.1 | 0.6 | 0.5 | 0.7 | 0.4 | 0.6 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Matrix (<10 µm), not included in results | - | - | - | 25.7 | 28.8 | 21.3 | 25.2 | 26.2 |
| CPB Specimens (Cement:Slag Ratio) | |||||
|---|---|---|---|---|---|
| R1 (40:60) | R2 (50:50) | R3 (0:100) | R4 (100:0) | R5 (50:50) | |
| Sample 1 | 0.22% | 0.31% | 0.06% | 0.56% | 0.21% |
| Sample 2 | 0.21% | 0.32% | 0.04% | 0.48% | 0.22% |
| Average | 0.22% | 0.32% | 0.05% | 0.52% | 0.22% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solismaa, S.; Torppa, A.; Kuva, J.; Heikkilä, P.; Hyvönen, S.; Juntunen, P.; Benzaazoua, M.; Kauppila, T. Substitution of Cement with Granulated Blast Furnace Slag in Cemented Paste Backfill: Evaluation of Technical and Chemical Properties. Minerals 2021, 11, 1068. https://doi.org/10.3390/min11101068
Solismaa S, Torppa A, Kuva J, Heikkilä P, Hyvönen S, Juntunen P, Benzaazoua M, Kauppila T. Substitution of Cement with Granulated Blast Furnace Slag in Cemented Paste Backfill: Evaluation of Technical and Chemical Properties. Minerals. 2021; 11(10):1068. https://doi.org/10.3390/min11101068
Chicago/Turabian StyleSolismaa, Soili, Akseli Torppa, Jukka Kuva, Pasi Heikkilä, Simo Hyvönen, Petri Juntunen, Mostafa Benzaazoua, and Tommi Kauppila. 2021. "Substitution of Cement with Granulated Blast Furnace Slag in Cemented Paste Backfill: Evaluation of Technical and Chemical Properties" Minerals 11, no. 10: 1068. https://doi.org/10.3390/min11101068
APA StyleSolismaa, S., Torppa, A., Kuva, J., Heikkilä, P., Hyvönen, S., Juntunen, P., Benzaazoua, M., & Kauppila, T. (2021). Substitution of Cement with Granulated Blast Furnace Slag in Cemented Paste Backfill: Evaluation of Technical and Chemical Properties. Minerals, 11(10), 1068. https://doi.org/10.3390/min11101068








