Applications of Chemically Modified Clay Minerals and Clays to Water Purification and Slow Release Formulations of Herbicides
Abstract
1. Introduction
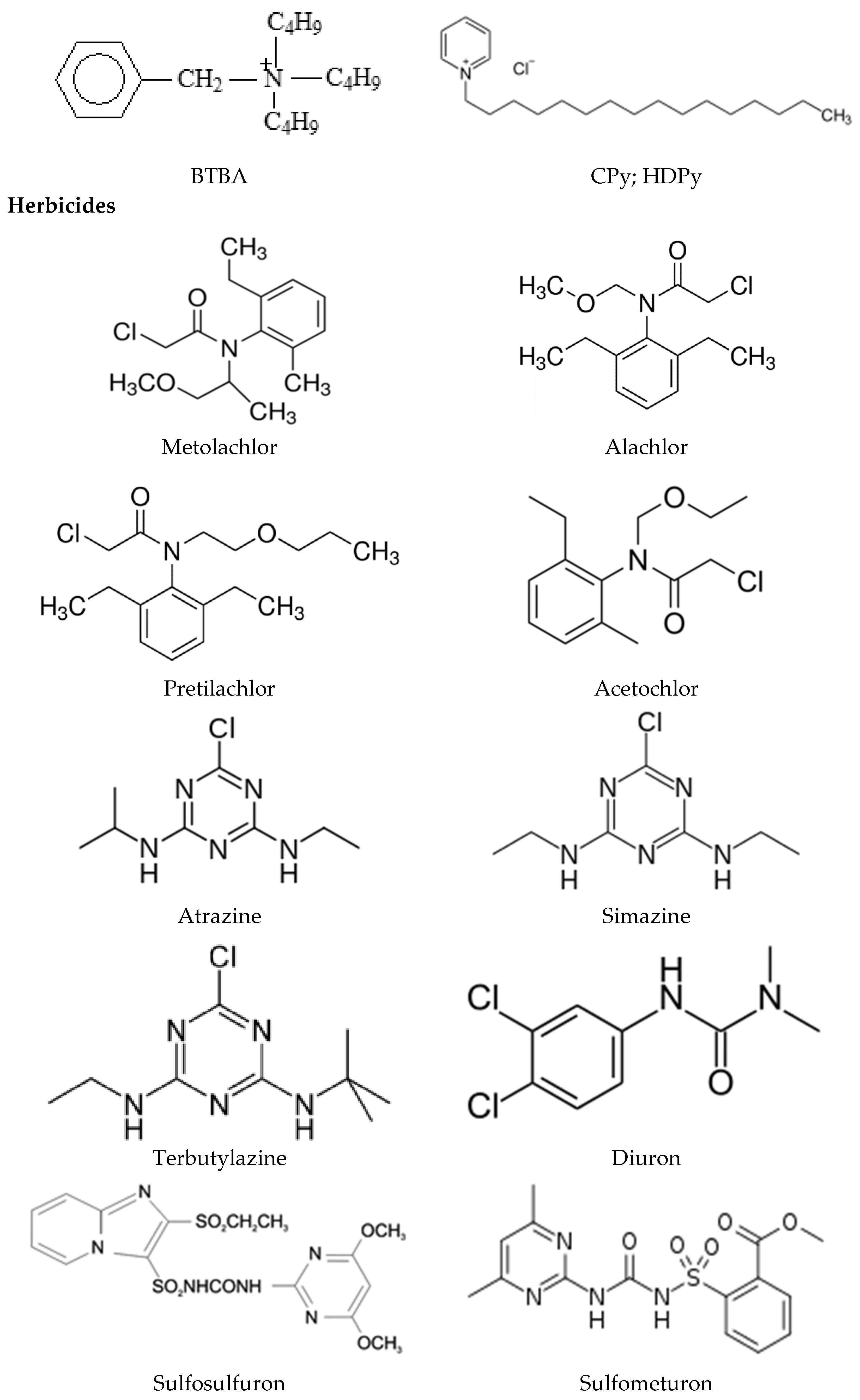
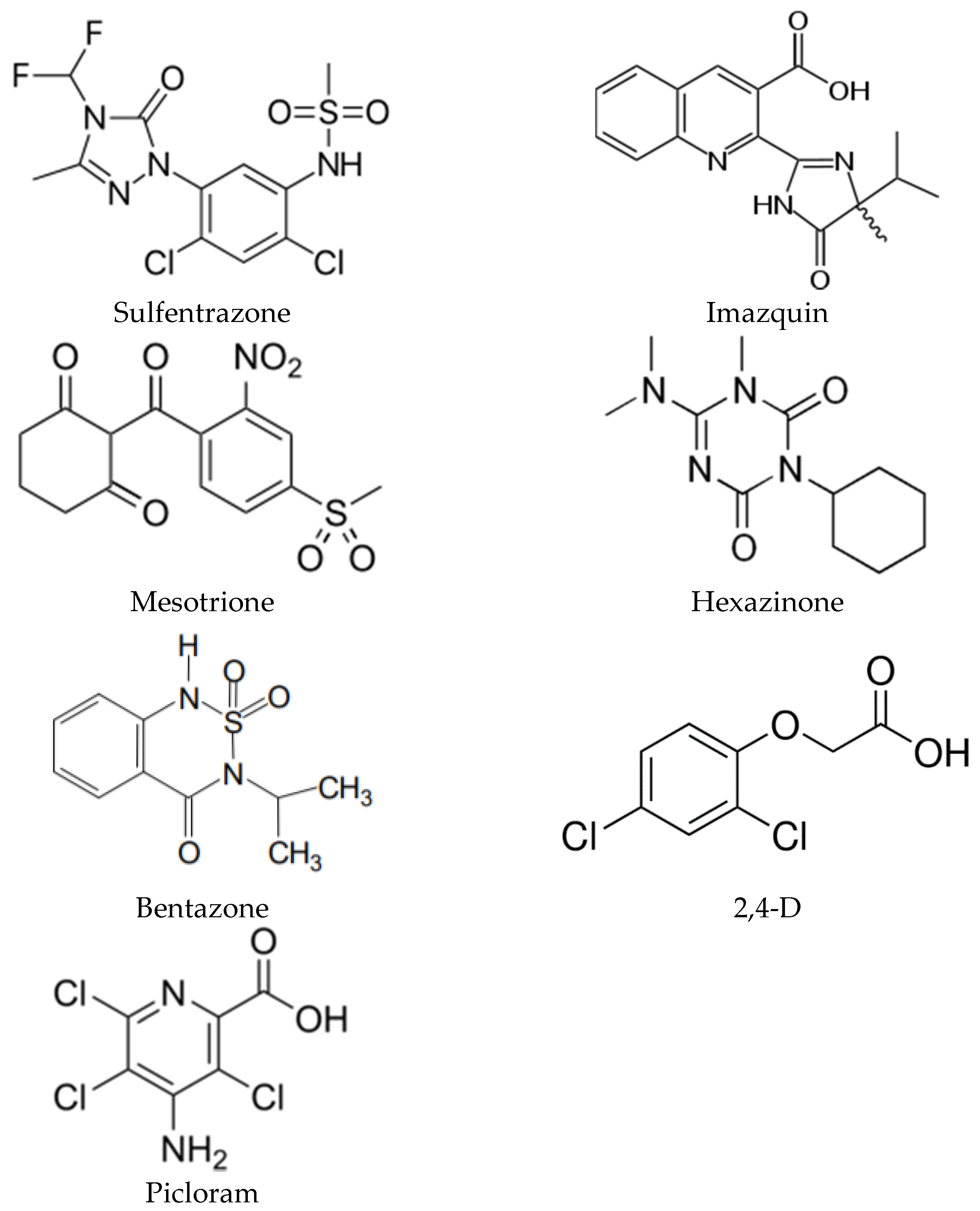
2. Slow Release Formulations of Herbicides
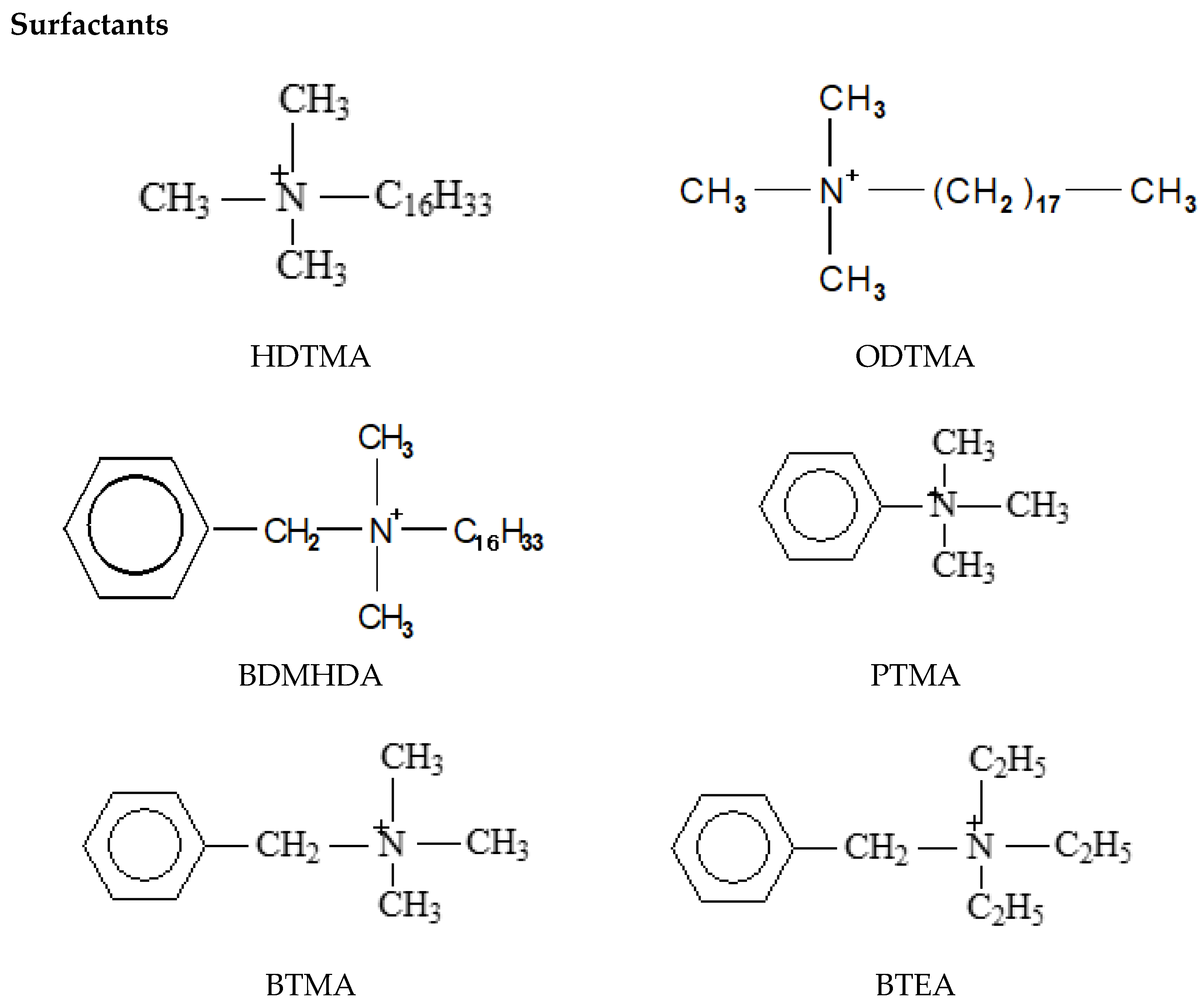
2.1. Modified Surfaces of Clay-Minerals and Clays by Organic Cations: Nomenclature
- (1)
- Organo-clays.
- (2)
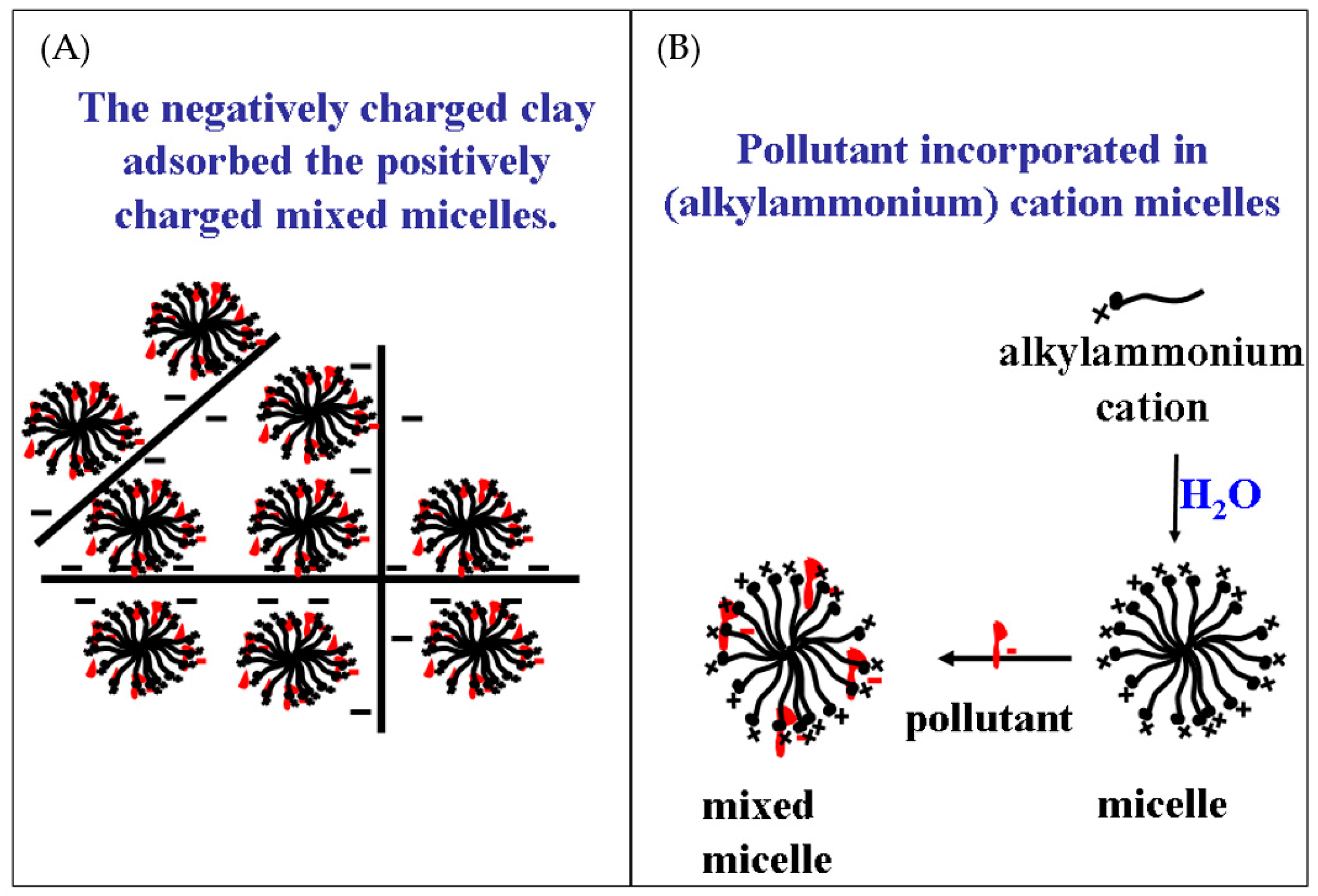
- Micelle- and liposome-clays. In (i) the adsorbing organic cations reside initially as monomers in a water solution, whereas in (ii) most of the organic cations are initially in micelles, or vesicles (Figure 1).
2.2. Preparation and Tests of Herbicide Formulations of Slow Release
- Preparation of the herbicide formulation.
- Determination of release of the herbicide from the formulation in a water medium and in a soil environment.
- Determination of leaching.
- Determination of weed control in pot or column experiments.
- Field experiment which includes determination of weed control, leaching, and resulting crop yield.
2.3. Slow Release Formulations Hydrophobic and Neutral Herbicides
- Formation of organo-clays by adsorbing the chosen cations on the clay mineral montmorillonite.
- Addition of an herbicide solution to the organo-clay particles. This stage can optionally occur in the same solution used in (a), and it involves a sorption of a fraction of the herbicide molecules, followed by possible stages of centrifugation, collection and drying of the pellet, followed by its grinding.
- (1)
- The total amount of the cation adsorbed consists of: (A) cations residing in the double layer region, and (B) cations bound to surface sites.
- (2)
- The electrostatic Gouy–Chapman equations are solved for a solution or dispersion containing ions of several valences and particles whose surfaces are charged and partially neutralized by cation binding.
- (3)
- The concentration of surface sites is explicitly considered, since it affects the depletion of cations from solution as a result of adsorption.
- (4)
- The extension to interactions of organic cations with clays, or clay-minerals [29,30,31,32], considered that monovalent organic cations can form neutral complex, in which one cation is complexed with one surface site, and positively charged complexes, where two cations form a complex with one surface sites of a clay-mineral, such as montmorillonite.
2.4. The Micelle– and Liposome–Clay Complexes
2.4.1. Release Experiments
2.4.2. Field Experiments
3. Removal of Pollutants from Water
3.1. Granulation of Clay-Composites
- (1)
- Incubation of ODTMA-Br in tap water at temperatures between 38 °C and 50 °C for 1–3 h, which was the time needed for complete dissolution of the cation ODTMA. At the end of the dissolution most of the cation ODTMA was in micellar form and the solution became clear. At lower temperatures, e.g., 22–24 °C the liquid looked opaque.
- (2)
- A gradual addition of the clay (sodium-bentonite) during 1 h while stirring the solution for 3 h. This procedure produced a micelle-clay complex which was left to sediment for 1 h.
- (3)
- Filtration of the sediment (or cake) in a membrane filter press to a final water content of 45 to 50%; heating was applied when the water content in the cake was larger by placing the cake in an oven at temperatures between 60 to 100 °C. When needed, the cake was broken by a knife crusher or by a mixer.
- (4)
- Granulation of the crushed cake was accomplished by a two-stage machine. First a shredder was used to obtain the desired granule size; the second stage involved production of spherical granules.
- (5)
- Drying of the granular micelle-clay was in an oven at 50 to 60 °C for about 24 h or more, until no further weight loss was observed, corresponding to 3% humidity.
- (6)
- The granules were sieved by a vibrational equipment for selection of suitable particle size fractions, whose dimensions were 400 × 400 mm. The sizes of the sieve holes chosen were based on the required particle sizes and the diameters were between 0.3, or 0.4 and 2 mm. More than 85% of the material was granules in the range of the chosen sizes. The choice of particle size requires a compromise. The smaller particles exhibited a larger efficiency of adsorption of pollutants, but the presence of smaller particles in a filter results in a larger chance of clogging. The granulated particles which deviate from above stated standards can be reprocessed.
3.2. Modeling of Filtration Kinetics
3.2.1. Introduction
3.2.2. Adsorption and Convection in a Column Filter
3.2.3. Effect of Volume Filtered, Flow Rate, Flow Velocity, Filter Length, Concentration of Surface Sites, and Pollutant Concentration on Solute Removal
- (i)
- In this pattern, the calculations [20] showed that the volume of pollutant solution that can be purified by the filter when reducing the pollutant concentration 100-fold (from 10 ppm to 0.1 ppm) is increased 4.76-fold, or vice versa, a 100-fold increase of pollutant concentration increases the price of the filtration 4.76-fold. More examples are provided by our experimental results.
- (ii)
- The second pattern occurs for a very large K-value. In this pattern the volume of solution that can be purified by such a filter can decrease linearly or even steeper with an increase in pollutant concentration.
3.2.4. Peculiar Effects in Kinetics of Filtration of a Solution Including Several Pollutants
3.3. Removal of Pollutants from Water
3.3.1. Removal of Herbicides from Water
3.3.2. Removal of DOM from Water by Filtration
3.4. Removal of Pharmaceuticals
3.4.1. Removal of Antibiotics
3.4.2. Removal of Several Non-Steroidal Anti-Inflammatory (NSAID) Drugs
3.5. Removal of Inorganic Anions—Perchlorate
3.5.1. Perchlorate Removal: Laboratory and Pilot Experiments
3.5.2. Removal of Ferricyanide
3.6. Removal of Bacteria from Water
3.6.1. Removal of Bacteria from Water by Filtration with Granulated Complex and Effect of Free Cations
3.6.2. Removal of Cyanobacteria and Their Toxins from Water
3.7. Collaboration of Technologies: Purification of Greywater
Author Contributions
Funding
Conflicts of Interest
References
- Hedlund, J.; Longo, S.B.; York, R. Agriculture, Pesticide Use, and Economic Development: A Global Examination (1990–2014). Rural. Sociol. 2019, 85, 519–544. [Google Scholar] [CrossRef]
- Leng, M.L.; Leovey, E.M.; Zubkoff, P.L. Agrochemical Environmental Fate: State of the Art; Lewis Publishers: Boca Raton, FL, USA, 1995; p. 410. [Google Scholar]
- Tominack, R.L. Herbicide Formulations. J. Toxicol. Clin. Toxicol. 2000, 38, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Seaman, D. Trends in the formulation of pesticides-an overview. Pestic. Sci. 1990, 29, 437–449. [Google Scholar] [CrossRef]
- Huang, B.; Chen, F.; Shen, Y.; Qian, K.; Wang, Y.; Sun, C.; Zhao, X.; Cui, B.; Gao, F.; Zeng, Z.; et al. Advances in Targeted Pesticides with Environmentally Responsive Controlled Release by Nanotechnology. Nanomaterials 2018, 8, 102. [Google Scholar] [CrossRef]
- Mishael, Y.G.; Undabeytia, T.; Rabinovitz, O.; Rubin, B.; Nir, S. Slow-Release Formulations of Sulfometuron Incorporated in Micelles Adsorbed on Montmorillonite. J. Agric. Food Chem. 2002, 50, 2864–2869. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mishael, Y.G.; Undabeytia, T.; Rytwo, G.; Papahadjpoulos-Steinberg, B.; Rubin, B.; Nir, S. Sulfometuron adsorption via alkylammonium cations adsorption as monomers and micelles on montmorillonite. J. Agric. Food Chem. 2002, 50, 2856–2863. [Google Scholar] [CrossRef][Green Version]
- Margulies, L.; Stern, T.; Rubin, B.; Ruzo, L.O. Photostabilization of trifluralin adsorbed on a clay matrix. J. Agric. Food Chem. 1992, 40, 152–155. [Google Scholar] [CrossRef]
- Undabeytia, T.; Nir, S.; Tel-Or, E.; Rubin, B. Photo-stabilization of the herbicide norflurazon by using organo-clays. J. Agric. Food Chem. 2000, 48, 4774–4779. [Google Scholar] [CrossRef]
- Margulies, L.; Stern, T.; Rubin, B. Slow Release of S-Ethyl Dipropylcarbamothioate from Clay Surfaces. J. Agric. Food Chem. 1994, 42, 1223–1227. [Google Scholar] [CrossRef]
- El-Nahhal, Y.; Nir, S.; Serban, C.; Rabinovitch, O.; Rubin, B. Montmorillonite-phenyltrimethylammonium yields environmentally improved formulations of hydrophobic herbicides. J. Agric. Food Chem. 2000, 48, 4791–4801. [Google Scholar] [CrossRef]
- El-Nahhal, Y.; Nir, S.; Serban, C.; Rabinovitz, O.; Rubin, B. Organo-clay formulation of acetochlor for reduced movement in soil. J. Agric. Food Chem. 2001, 49, 5364–5371. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.D. Herbicide movement in soils: Principles, pathways and processes. Weed Res. 2000, 40, 113–122. [Google Scholar] [CrossRef]
- Ternes, T. Occurrence of drugs in German sewage treatment plants and rivers1Dedicated to Professor Dr. Klaus Haberer on the occasion of his 70th birthday.1. Water Res. 1998, 32, 3245–3260. [Google Scholar] [CrossRef]
- Ternes, T.A.; Meisenheimer, M.; McDowell, D.; Sacher, F.; Brauch, H.-J.; Haist-Gulde, B.; Preuss, G.; Wilme, U.; Zulei-Seibert, N. Removal of Pharmaceuticals during Drinking Water Treatment. Environ. Sci. Technol. 2002, 36, 3855–3863. [Google Scholar] [CrossRef] [PubMed]
- Stackelberg, P.E.; Furlong, E.T.; Meyer, M.T.; Zaugg, S.D.; Henderson, A.K.; Reissman, D.B. Persistence of pharmaceutical compounds and other organic wastewater contaminants in a conventional drinking-water-treatment plant. Sci. Total. Environ. 2004, 329, 99–113. [Google Scholar] [CrossRef]
- Stackelberg, P.E.; Gibs, J.; Furlong, E.T.; Meyer, M.T.; Zaugg, S.D.; Lippincott, R.L. Efficiency of conventional drinking-water-treatment processes in removal of pharmaceuticals and other organic compounds. Sci. Total. Environ. 2007, 377, 255–272. [Google Scholar] [CrossRef]
- Cirja, M.; Ivashechkin, P.; Schäffer, A.; Corvini, P.F.-X. Factors affecting the removal of organic micropollutants from wastewater in conventional treatment plants (CTP) and membrane bioreactors (MBR). Rev. Environ. Sci. Bio Technol. 2007, 7, 61–78. [Google Scholar] [CrossRef]
- Shtarker-Sasi, A.; Castro Sowinski, A.; Matan, O.; Kagan, T.; Nir, S.; Okon, Y.; Nasser, A.M. Removal of bacteria and cryptosporidium from water by micelle—Montmorillonite complexes. Desalin. Water Treat. 2013, 51, 7672–7680. [Google Scholar] [CrossRef]
- Nir, S.; Shuali, U. Water Purification by Micelle-Clay Nano-Particles; Nova Science Publishers: New York, NY, USA, 2019. [Google Scholar]
- Hwang, B.-F.; Magnus, P.; Jaakkola, M.S. Risk of specific birth defects in relation to chlorination and the amount of natural organic matter in the water supply. Am. J. Epidemiol. 2002, 156, 374–382. [Google Scholar] [CrossRef]
- Charrois, J.W.; Boyd, J.M.; Froese, K.L.E.; Hrudey, S. Occurrence of N-nitrosamines in Alberta public drinking-water distribution systems. J. Environ. Eng. Sci. 2007, 6, 103–114. [Google Scholar] [CrossRef]
- Asami, M.; Oya, M.; Kosaka, K. A nationwide survey of NDMA in raw and drinking water in Japan. Sci. Total. Environ. 2009, 407, 3540–3545. [Google Scholar] [CrossRef] [PubMed]
- Bond, T.; Huang, J.; Templeton, M.R.; Graham, N. Occurrence and control of nitrogenous disinfection by-products in drinking water—A review. Water Res. 2011, 45, 4341–4354. [Google Scholar] [CrossRef] [PubMed]
- Lukasik, J.; Cheng, Y.-F.; Lu, F.; Tamplin, M.; Farrah, S.R. Removal of microorganisms from water by columns containing sand coated with ferric and aluminum hydroxides. Water Res. 1999, 33, 769–777. [Google Scholar] [CrossRef]
- Torkelson, A.; Da Silva, A.; Love, D.; Kim, J.; Alper, J.; Coox, B.; Dahm, J.; Kozodoy, P.; Maboudian, R.; Nelson, K.L. Investigation of quaternary ammonium silane-coated sand filter for the removal of bacteria and viruses from drinking water. J. Appl. Microbiol. 2012, 113, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Undabeytia, T.; Posada, R.; Nir, S.; Galindo, I.; Laiz, L.; Saiz-Jimenez, C.; Morillo, E. Removal of waterborne microorganisms by filtration using clay–polymer complexes. J. Hazard. Mater. 2014, 279, 190–196. [Google Scholar] [CrossRef]
- Nir, S. Cation adsorption to clay particles: Specific and nonspecific adsorption and associated changes in solution concentrations and surface potentials. Soil Sci. Soc. Am. J. 1986, 50, 52–57. [Google Scholar] [CrossRef]
- Margulies, L.; Rozen, H.; Nir, S. A model for competitive adsorption of organic cations and clays. Clays Clay Miner. 1988, 36, 270–276. [Google Scholar] [CrossRef]
- Rytwo, G.; Nir, S.; Margulies, L. Interactions of Monovalent Organic Cations with Montmorillonite: Adsorption Studies and Model Calculations. Soil Sci. Soc. Am. J. 1995, 59, 554–564. [Google Scholar] [CrossRef]
- Rytwo, G.; Nir, S.; Margulies, L. Adsorption and interaction studies of the divalent cationic herbicides diquat and paraquat with montmorillonite. Soil Sci. Soc. Am. J. 1996, 60, 601–610. [Google Scholar] [CrossRef]
- Rytwo, G.; Nir, S.; Margulies, L. A Model for Adsorption of Divalent Organic Cations to Montmorillonite. J. Colloid Interface Sci. 1996, 181, 551–560. [Google Scholar] [CrossRef]
- Cruz-Guzmán, M.; Celis, R.; Hermosín, M.C.; Cornejo, J. Adsorption of the Herbicide Simazine by Montmorillonite Modified with Natural Organic Cations. Environ. Sci. Technol. 2004, 38, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Singh, N. Surfactant-modified bentonite clays: Preparation, characterization, and atrazine removal. Environ. Sci. Pollut. Res. 2015, 22, 3876–3885. [Google Scholar] [CrossRef] [PubMed]
- Polubesova, T. Adsorption of Benzyltrimethylammonium and Benzyltriethylammonium on Montmorillonite: Experimental Studies and Model Calculations. Clays Clay Miner. 1997, 45, 834–841. [Google Scholar] [CrossRef]
- Nir, S.; Undabeytia, T.; Yaron-Marcovich, D.; El-Nahhal, Y.; Polubesova, T.; Serban, C.; Rytwo, G.; Lagaly, G.; Rubin, B. Optimization of Adsorption of Hydrophobic Herbicides on Montmorillonite Preadsorbed by Monovalent Organic Cations: Interaction between Phenyl Rings. Environ. Sci. Technol. 2000, 34, 1269–1274. [Google Scholar] [CrossRef]
- Huang, A.; Huang, Z.; Dong, Y.; Chen, L.; Fu, L.; Li, L.; Ma, L. Controlled release of phoxim from organobentonite based formulation. Appl. Clay Sci. 2013, 63–68. [Google Scholar] [CrossRef]
- Zhu, L.; Zhu, R.; Xu, L.; Ruan, X. Influence of clay charge densities and surfactant loading amount on the microstructure of CTMA–montmorillonite hybrids. Colloids Surfaces A Physicochem. Eng. Asp. 2007, 304, 41–48. [Google Scholar] [CrossRef]
- Cornejo, J.; Celis, R.; Pavlovic, I.; Ulibarri, M.A. Interactions of pesticides with clays and layered double hydroxides: A review. Clay Miner. 2008, 43, 155–175. [Google Scholar] [CrossRef]
- Carrizosa, M.; Calderón, M.; Hermosín, M.C.; Cornejo, J. Organosmectites as sorbent and carrier of the herbicide bentazone. Sci. Total. Environ. 2000, 247, 285–293. [Google Scholar] [CrossRef]
- Celis, R.; Hermosín, C.; Cornejo, L.; Carrizosa, J.; Cornejo, J.; Hermosín, M.C. Clay-Herbicide Complexes to Retard Picloram Leaching in Soil. Int. J. Environ. Anal. Chem. 2002, 82, 503–517. [Google Scholar] [CrossRef]
- Celis, R.; Facenda, G.; Hermosín, M.C.; Cornejo, J. Assessing factors influencing the release of hexazinone from clay-based formulations. Int. J. Environ. Anal. Chem. 2007, 85, 1153–1164. [Google Scholar] [CrossRef]
- Celis, R.; Trigo, C.; Facenda, G.; Hermosín, M.D.C.; Cornejo, J. Selective Modification of Clay Minerals for the Adsorption of Herbicides Widely Used in Olive Groves. J. Agric. Food Chem. 2007, 55, 6650–6658. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, C.D.; Celis, R.; Domínguez, C.; Hermosín, L.C. Use of modified montmorillonites to reduce herbicide leaching in sports turf surfaces: Laboratory and field experiments. Appl. Clay Sci. 2008, 42, 284–291. [Google Scholar] [CrossRef]
- Trigo, C.; Koskinen, W.C.; Celis, R.; Sadowsky, M.J.; Hermosín, M.C.; Cornejo, J. Bioavailability of Organoclay Formulations of Atrazine in Soil. J. Agric. Food Chem. 2010, 58, 11857–11863. [Google Scholar] [CrossRef]
- Chevillard, A.; Angellier-Cpoussy, H.; Peyron, S.; Gontard, N.; Gastaldi, E. Investigating ethofumeste-clay interactions for pesticide-controlled release. Soil Sci. Soc. Am. J. 2012, 76, 420–430. [Google Scholar] [CrossRef]
- Pool, C.F.; Du-Toit, D. Leaching depth of imazamethabenz methyl and chlorsulfuron and metsulfuron methyl in different soils. Appl. Plant. Sci. 1995, 9, 43–47. [Google Scholar]
- Sarmah, A.K.; Kookana, R.S.; Alston, A.M. Fate and behavior of trisulfusulfuron, metsulfuron-methyl, and chlorsulfuron in Australian soil environment: A review. Aust. J. Agric. Res. 1998, 49, 775–790. [Google Scholar] [CrossRef]
- Undabeytia, T.; Mishael, Y.G.; Nir, S.; Papahadjopoulos-Sternberg, B.; Rubin, B.; Morillo, E.; Maqueda, C. A Novel System for Reducing Leaching from Formulations of Anionic Herbicides: Clay-Liposomes. Environ. Sci. Technol. 2003, 37, 4475–4480. [Google Scholar] [CrossRef]
- Mishael, Y.G.; Undabeytia, T.; Rabinovitz, O.; Rubin, A.B.; Nir, S. Sulfosulfuron Incorporated in Micelles Adsorbed on Montmorillonite for Slow Release Formulations. J. Agric. Food Chem. 2003, 51, 2253–2259. [Google Scholar] [CrossRef][Green Version]
- Polubesova, T.; Nir, S.; Rabinovitz, O.; Borisover, A.M.; Rubin, B. Sulfentrazone Adsorbed on Micelle−Montmorillonite Complexes for Slow Release in Soil. J. Agric. Food Chem. 2003, 51, 3410–3414. [Google Scholar] [CrossRef]
- Ziv, D.; Mishael, Y.G. Herbicide solubilization in micelle-clay composites as a basis for controlled release formulations. J. Agric. Food Chem. 2008, 56, 9159–9165. [Google Scholar] [CrossRef]
- Galán-Jiménez, M.D.C.; Mishael, Y.-G.; Nir, S.; Morillo, E.; Undabeytia, T. Factors Affecting the Design of Slow Release Formulations of Herbicides Based on Clay-Surfactant Systems. A Methodological Approach. PLoS ONE 2013, 8, e59060. [Google Scholar] [CrossRef]
- Galán-Jiménez, M.D.C.; Morillo, E.; Bonnemoy, F.; Mallet, C.; Undabeytia, T. A sepiolite-based formulation for slow release of the herbicide mesotrione. Appl. Clay Sci. 2020, 189, 105503. [Google Scholar] [CrossRef]
- Galán-Jiménez, M.; Gómez-Pantoja, E.; Morillo, E.; Undabeytia, T. Solubilization of herbicides by single and mixed commercial surfactants. Sci. Total. Environ. 2015, 538, 262–269. [Google Scholar] [CrossRef]
- Undabeytia, T.; Nir, S.; Gómara, M.J. Clay−Vesicle Interactions: Fluorescence Measurements and Structural Implications for Slow Release Formulations of Herbicides. Langmuir 2004, 20, 6605–6610. [Google Scholar] [CrossRef] [PubMed]
- Undabeytia, T.; Nir, S.; Sánchez-Verdejo, T.; Villaverde, J.; Maqueda, C.; Morillo, E. A clay–vesicle system for water purification from organic pollutants. Water Res. 2008, 42, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Verdejo, T.; Undabeytia, T.; Nir, S.; Maqueda, C.; Morillo, E. Environmentally Friendly Slow Release Formulations of Alachlor Based on Clay-Phosphatidylcholine. Environ. Sci. Technol. 2008, 42, 5779–5784. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sánchez-Verdejo, T.; Undabeytia, T.; Nir, S.; Villaverde, J.; Maqueda, C.; Morillo, E. Environmentally Friendly Formulations of Alachlor and Atrazine: Preparation, Characterization, and Reduced Leaching. J. Agric. Food Chem. 2008, 56, 10192–10199. [Google Scholar] [CrossRef] [PubMed]
- Undabeytia, T.; Sopeña, F.; Sánchez-Verdejo, T.; Villaverde, J.; Nir, S.; Morillo, E.; Maqueda, C. Performance of Slow-Release Formulations of Alachlor. Soil Sci. Soc. Am. J. 2010, 74, 898–905. [Google Scholar] [CrossRef]
- Undabeytia, T.; Recio, E.; Maqueda, C.; Morillo, E.; Gómez-Pantoja, E.; Sánchez-Verdejo, T. Reduced metribuzin pollution with phosphatidylcholine-clay formulations. Pest. Manag. Sci. 2010, 67, 271–278. [Google Scholar] [CrossRef]
- Undabeytia, T.; Recio, E.; Maqueda, C.; Sánchez-Verdejo, T.; Balek, V. Slow diuron release formulations based on clay–phosphatidylcholine complexes. Appl. Clay Sci. 2012, 55, 53–61. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority. Document 4982). Guidance document for predicting environmental concentrations of active substances of plant protection products and transformation products of these active substances in soil. EFSA J. 2017, 15, 1–115. [Google Scholar]
- Celis, R.; Hermosín, M.C.; Carrizosa, M.J.; Cornejo, J. Inorganic and Organic Clays as Carriers for Controlled Release of the Herbicide Hexazinone. J. Agric. Food Chem. 2002, 50, 2324–2330. [Google Scholar] [CrossRef] [PubMed]
- Kaluđerović, L.M.; Tomić, Z.P.; Đurović-Pejčev, R.D.; Vulić, P.J.; Asanin, D. Influence of the organic complex concentration on adsorption of herbicide in organic modified montmorillonite. J. Environ. Sci. Health Part. B 2017, 52, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Lou, X.; Xu, X.; Huang, A.; Zhang, M.; Ma, L. Thermodynamics and Kinetics of Pretilachlor Adsorption on Organobentonites for Controlled Release. ACS Omega 2020, 5, 4191–4199. [Google Scholar] [CrossRef] [PubMed]
- Shirvani, M.; Farajollahi, E.; Bakhtiari, S.; Ogunseitan, O.A. Mobility and efficacy of 2,4-D herbicide from slow-release delivery systems based on organo-zeolite and organo-bentonite complexes. J. Environ. Sci. Health Part. B 2014, 49, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Hermosín, M.; Celis, R.; Facenda, G.; Carrizosa, M.; Ortega-Calvo, J.; Cornejo, J. Bioavailability of the herbicide 2,4-D formulated with organoclays. Soil Biol. Biochem. 2006, 38, 2117–2124. [Google Scholar] [CrossRef]
- Tejada, M.; Morillo, E.; Gómez, I.; Madrid, F.; Undabeytia, T. Effect of controlled release formulations of diuron and alachlor herbicides on the biochemical activity of agricultural soils. J. Hazard. Mater. 2017, 322, 334–347. [Google Scholar] [CrossRef]
- Polubesova, T.; Nir, S.; Zadaka, D.; Rabinovitz, O.; Serban, C.; Groisman, A.L.; Rubin, B. Water Purification from Organic Pollutants by Optimized Micelle−Clay Systems. Environ. Sci. Technol. 2005, 39, 2343–2348. [Google Scholar] [CrossRef] [PubMed]
- Nir, S.; Brook, I.; Anavi, Y.; Ben-Ari, J.; Sheveky-Huterer, R.; Etkin, H.; Zadaka-Amir, D.; Shuali, U. Water purification from perchlorate micelle-clay complex. Laboratory and pilot experiments. Appl. Clay Sci. 2015, 114, 151–156. [Google Scholar] [CrossRef]
- Nir, S.; Ryskin, M. Method of Production of Granulated Micelle-Clay Complexes: Application for Removal of Organic, Inorganic Anionic Pollutants and Microorganisms from Contaminated Water. U.S. Patent 10,384,959, 20 August 2019. [Google Scholar]
- Nir, S.; Zadaka-Amir, D.; Kartaginer, A.; Gonen, Y. Simulation of adsorption and flow of pollutants in a column filter: Application to Micelle-clay mixtures with sand. Appl. Clay Sci. 2012, 67–68, 134–140. [Google Scholar] [CrossRef]
- Simunek, J.; Van Genuchten, M.T.; Sejna, M.; Toride, N.; Leij, F.J. The STANMOD Computer Software for Evaluating Solute Transport. In Porous Media Using Analytical Solutions of Convection-Dispersion Equation; U.S. Salinity Laboratory Agricultural Research Service: Riverside, CA, USA, 1999. [Google Scholar]
- Rakovitsky, N.; Brook, I.; Van Rijn, J.; Ryskin, M.; Mkhweli, Z.; Etkin, H.; Nir, S. Purification of greywater by a moving bed reactor followed by a filter including a granulated micelle-clay composite. Appl. Clay Sci. 2016, 132, 267–272. [Google Scholar] [CrossRef]
- US EPA (United States Environmental Protection Agency). Exposure Factors Handbook: 2011 Edition; Office of Research and Development, United States Environmental Protection Agency: Washington, DC, USA, 2011.
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Lelario, F.; Gardi, I.; Mishael, Y.; Dolev, N.; Undabeytia, T.; Nir, S.; Scrano, L.; Bufo, S.A. Pairing micropollutants and clay-composite sorbents for efficient water treatment: Filtration and modeling at a pilot scale. Appl. Clay Sci. 2017, 137, 225–232. [Google Scholar] [CrossRef]
- Zadaka, D.; Polubesova, T.; Mishael, Y.; Spitzy, A.; Koehler, H.; Wakshal, E.; Rabinovitz, O.; Nir, S. Determination of release of organic cations from micelle–clay complexes and their re-adsorption in sand/clay columns. Appl. Clay Sci. 2005, 29, 282–286. [Google Scholar] [CrossRef]
- Kalfa, A.; Rakovitsky, N.; Tavassi, M.; Ryskin, M.; Ben-Ari, J.; Etkin, H.; Shuali, U.; Nir, S. Removal of Escherichia coli and total bacteria from water by granulated micelle-clay complexes: Filter regeneration and modeling of filtration kinetics. Appl. Clay Sci. 2017, 147, 63–68. [Google Scholar] [CrossRef]
- Radian, A.; Carmeli, M.; Zadaka-Amir, D.; Nir, S.; Wakshal, E.; Mishael, Y.G. Enhanced removal of humic acid from water by micelle-montmorillonite composites: Comparison to granulated activated carbon. Appl. Clay Sci. 2011, 54, 258–263. [Google Scholar] [CrossRef]
- Schaumann, G.E. Soil organic matter beyond molecular structure Part I: Macromolecular and supramolecular characteristics. J. Plant. Nutr. Soil Sci. 2006, 169, 145–156. [Google Scholar] [CrossRef]
- Polubesova, T.; Zadaka, D.; Groisman, L.; Nir, S. Water remediation by micelle–clay system: Case study for tetracycline and sulfonamide antibiotics. Water Res. 2006, 40, 2369–2374. [Google Scholar] [CrossRef] [PubMed]
- Joss, A.; Keller, E.; Alder, A.C.; Göbel, A.; McArdell, C.S.; Ternes, T.; Siegrist, H. Removal of pharmaceuticals and fragrances in biological wastewater treatment. Water Res. 2005, 39, 3139–3152. [Google Scholar] [CrossRef] [PubMed]
- Pérez, S.; Eichhorn, P.; Aga, D.S. Evaluating the biodegradability of sulfamethazine, sulfamethoxazole, sulfathiazole, and trimethoprim at different stages of sewage treatment. Environ. Toxicol. Chem. 2005, 24, 1361–1367. [Google Scholar] [CrossRef]
- Awwad, M.; Al-Rimawi, F.; Dajani, K.J.K.; Khamis, M.; Nir, S.; Karaman, R. Removal of amoxicillin and cefuroxime axetil by advanced membranes technology, activated carbon and micelle–clay complex. Environ. Technol. 2015, 36, 2069–2078. [Google Scholar] [CrossRef]
- Karaman, R.; Khamis, M.; Quried, M.; Halabieh, R.; Makharzeh, I.; Manassra, A.; Abbadi, J.; Qtait, A.; Bufo, S.A.; Nasser, A.; et al. Removal of diclofenac potassium from wastewater using clay-micelle complex. Environ. Technol. 2012, 33, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Morales, V.; Gardi, I.; Nir, S.; Undabeytia, T. Removal of pharmaceuticals from water by clay-cationic starch sorbents. J. Clean. Prod. 2018, 190, 703–711. [Google Scholar] [CrossRef]
- Khalaf, S.; Al-Rimawi, F.; Khamis, M.; Zimmerman, D.; Shuali, U.; Nir, S.; Scrano, L.; Bufo, S.A.; Karaman, R. Efficiency of advanced wastewater treatment plant system and laboratory-scale micelle-clay filtration for the removal of ibuprofen residues. J. Environ. Sci. Health Part. B 2013, 48, 814–821. [Google Scholar] [CrossRef]
- Zimmerman, D. Removal of Micropollutants from Water Using Clay and Micelle-Clay Complexes Packed in Adsorption Columns. Master’s Thesis, The Robert, H. Smith Faculty of Agriculture, Food and Environment of Rehovot, The Hebrew University of Jerusalem, Jerusalem, Israel, 2020. [Google Scholar]
- Khalaf, S.; Al-Rimawi, F.; Khamis, M.; Nir, S.; Bufo, S.A.; Scrano, L.; Mecca, G.; Karaman, R. Efficiency of membrane technology, activated charcoal, and a micelle-clay complex for removal of the acidic pharmaceutical mefenamic acid. J. Environ. Sci. Health Part A 2013, 48, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Qurie, M.; Khamis, M.; Malek, F.; Nir, S.; Bufo, S.A.; Abbadi, J.; Scrano, L.; Karaman, R. Stability and Removal of Naproxen and Its Metabolite by Advanced Membrane Wastewater Treatment Plant and Micelle-Clay Complex. CLEAN Soil Air Water 2013, 42, 594–600. [Google Scholar] [CrossRef]
- Sulaiman, S.; Khamis, M.; Nir, S.; Lelario, F.; Scrano, L.; Bufo, S.A.; Mecca, G.; Karaman, R. Stability and removal of atorvastatin, rosuvastatin and simvastatin from wastewater. Environ. Technol. 2015, 36, 3232–3242. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, S.; Khamis, M.; Nir, S.; Lelario, F.; Scrano, L.; Bufo, S.A.; Karaman, R. Stability and removal of spironolactone from wastewater. J. Environ. Sci. Health Part A 2015, 50, 1127–1135. [Google Scholar] [CrossRef]
- Karaman, R.; Khamis, M.; Abbadi, J.; Amro, A.; Qurie, M.; Ayyad, I.; Ayyash, F.; Hamarsheh, O.; Yaqmour, R.; Nir, S.; et al. Paracetamol biodegradation by activated sludge and photocatalysis and its removal by a micelle–clay complex, activated charcoal, and reverse osmosis membranes. Environ. Technol. 2016, 37, 2414–2427. [Google Scholar] [CrossRef]
- Khamis, M.; Karaman, R.; Ayyash, F.; Qtait, A.; Deeb, O.; Manssra, A. Efficiency of advanced membrane wastewater treatment plant towards removal of aspirin, salicylic acid, paracetamol and p-aminophenol. J. Environ. Sci. Eng. 2011, 5, 121–137. [Google Scholar]
- Coates, J.D.; Michaelidou, U.; O’Connor, S.M.; Bruce, R.A.; Achenbach, L.A. The Diverse Microbiology of (Per)Chlorate Reduction. In Perchlorate in the Environment; Springer Science and Business Media LLC: Berlin, Germany, 2000; pp. 257–270. [Google Scholar]
- Tan, K.; Anderson, T.A.; Jones, M.W.; Smith, P.N.; Jackson, W.A. Accumulation of Perchlorate in Aquatic and Terrestrial Plants at a Field Scale. J. Environ. Qual. 2004, 33, 1638–1646. [Google Scholar] [CrossRef]
- USEPA. Drinking Water. Regulatory Determination on Perchlorate; Federal Register: Washington, DC, USA, 2011.
- Urbansky, E.T. Perchlorate as an environmental contaminant. Environ. Sci. Pollut. Res. 2002, 9, 187–192. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, C.L.; Blake, S.; Hall, T.; Harman, M.; Kanda, R.; Hunt, J.; Rumsby, P.C. Perchlorate in raw and drinking water in England and Wales. Water Environ. J. 2011, 25, 456–465. [Google Scholar] [CrossRef]
- Brown, G.M.; Gu, B.; Moyer, B.A.; Bonnensen, P.V. Regeneration of Strong-Base Anion Exchange Resin by Sequential Chemical Displacement. U.S. Patent No. 6,448,229 B1, 10 September 2002. [Google Scholar]
- Gu, B.; Coats, D.J. (Eds.) Perchlorate: Environmental Occurrence, Interactions and Treatments; Springer: New York, NY, USA, 2006. [Google Scholar]
- Xiong, Z.; Zhao, A.D.; Harper, J.W.F. Sorption and Desorption of Perchlorate with Various Classes of Ion Exchangers: A Comparative Study. Ind. Eng. Chem. Res. 2007, 46, 9213–9222. [Google Scholar] [CrossRef]
- Lutes, C.C.; Patterson, J.; Goltz, M.; Graham, J. Tailored Granular Activated Carbon Treatment of Perchlorate in Drinking Water; Environmental security Technology Program (ESTCP) project ER-200546; Department of Defense (DoD): Washington, DC, USA, 2010. [Google Scholar]
- Velizarov, S.; Matos, C.T.; Reis, M.A.; Crespo, J.G. Removal of inorganic charged micropollutants in an ion-exchange membrane bioreactor. Desalination 2005, 178, 203–210. [Google Scholar] [CrossRef]
- Fox, S.; Oren, Y.; Ronen, Z.; Gilron, J. Ion exchange membrane bioreactor for treating groundwater contaminated with high perchlorate concentrations. J. Hazard. Mater. 2014, 264, 552–559. [Google Scholar] [CrossRef]
- Gu, B.; Brown, G.M.; Alexandratos, S.D.; Ober, R.; Patel, V. Selective Anion Exchange Resins for the Removal of Perchlorate ClO4−; Publication No. 4863; Oak Ridge National Library, Environmental Sciences Division: Oak Ridge, TN, USA, 1999. [Google Scholar]
- Gu, B.; Brown, G.M.; Maya, L.; Lance, M.J.; Moyer, B.A. Regeneration of perchlorate (ClO4-)-loaded anion exchange resins by a novel tetrachloroferrate (FeCl4-) displacement technique. Environ. Sci. Technol. 2001, 35, 3363–3368. [Google Scholar] [CrossRef]
- Maillard, J.-Y. Bacterial target sites for biocide action. J. Appl. Microbiol. 2002, 92, 16–27. [Google Scholar] [CrossRef]
- Ferreira, C.; Pereira, A.M.; Pereira, M.C.; Melo, L.F.; Simões, M. Physiological changes induced by the quaternary ammonium compound benzyldimethyldodecylammonium chloride on Pseudomonas fluorescens. J. Antimicrob. Chemother. 2011, 66, 1036–1043. [Google Scholar] [CrossRef]
- Inácio, Â.S.; Domingues, N.S.; Nunes, A.; Martins, P.T.; Moreno, M.J.; Estronca, L.M.; Fernandes, R.; Moreno, A.J.M.; Borrego, M.J.; Gomes, J.P.; et al. Quaternary ammonium surfactant structure determines selective toxicity towards bacteria: Mechanisms of action and clinical implications in antibacterial prophylaxis. J. Antimicrob. Chemother. 2015, 71, 641–654. [Google Scholar] [CrossRef]
- Sukenik, A.; Viner-Mozzini, Y.; Tavassi, M.; Nir, S. Removal of cyanobacteria and cyanotoxins from lake water by composites of bentonite with micelles of the cation octadecyltrimethylammonium (ODTMA). Water Res. 2017, 120, 165–173. [Google Scholar] [CrossRef]
- Shuali, U.; Nir, S. Prepublication: Role of micelle-clay complexes and quaternary amine cations in removal of bacteria from water: Adsorption, biostatic and biocidal effects. Clays Clay Miner. 2018, 66, 485–492. [Google Scholar] [CrossRef]
- Nir, S. Water Purification by a Micelle-Clay Composite Alone and its Collaboration with other Technologies. Open Access J. Waste Manag. Xenobiotics 2019, 2. [Google Scholar] [CrossRef]
- Kaya, A.U.; Güner, S.; Ryskin, M.; Lameck, A.S.; Benitez, A.R.; Shuali, U.; Nir, S. Effect of Microwave Radiation on Regeneration of a Granulated Micelle–Clay Complex after Adsorption of Bacteria. Appl. Sci. 2020, 10, 2530. [Google Scholar] [CrossRef]
- Benitez, A.R. Application of Granulated Micelle-Clay Complexes for Removal from Water of (i) Bacteria (ii) Non-Ionic Herbicide: Regeneration and Effects of Organic Cation Loading on Complex. Capacity. M.Sc. Thesis. The Robert, H. Smith Faculty of Agriculture, Food and Environment; The Hebrew University: Jerusalem, Israel, 2020. [Google Scholar]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Waste Water, 21st ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2005. [Google Scholar]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Chen, W.; Peng, L.; Wan, N.; Gan, N.; Zhang, X. Distribution and bioaccumulation of microcystins in water columns: A systematic investigation into the environmental fate and the risks associated with microcystins in Meiliang Bay, Lake Taihu. Water Res. 2007, 41, 2853–2864. [Google Scholar] [CrossRef]
- Szlag, D.; Sinclair, J.L.; Southwell, B.; Westrick, J.A. Cyanobacteria and Cyanotoxins Occurrence and Removal from Five High-Risk Conventional Treatment Drinking Water Plants. Toxins 2015, 7, 2198–2220. [Google Scholar] [CrossRef]
- Wang, H.; Ho, L.; Lewis, D.M.; Brookes, J.D.; Newcombe, G. Discriminating and assessing adsorption and biodegradation removal mechanisms during granular activated carbon filtration of microcystin toxins. Water Res. 2007, 41, 4262–4270. [Google Scholar] [CrossRef]
- Cheriker, H.; Bauer, T.S.; Oren, Y.; Nir, S.; Hayouka, Z. Immobilized random peptide mixtures exhibit broad antimicrobial activity with high selectivity. Chem. Commun. 2020, 56, 11022. [Google Scholar] [CrossRef]
- Gerchman, Y.; Vasker, B.; Tavasi, M.; Mishael, Y.; Kinel-Tahan, Y.; Yehoshua, Y. Effective harvesting of microalgae: Comparison of different polymeric flocculants. Bioresour. Technol. 2017, 228, 141–146. [Google Scholar] [CrossRef]
- Rytwo, G.; Lavi, R.; Rytwo, Y.; Monchase, H.; Dultz, S.; König, T.N. Clarification of olive mill and winery wastewater by means of clay–polymer nanocomposites. Sci. Total Environ. 2013, 442, 134–142. [Google Scholar] [CrossRef]
- Rytwo, G. Hybrid Clay-Polymer Nanocomposites for the Clarification of Water and Effluents. Recent Patents Nanotechnol. 2017, 11, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Lyck, S. Simultaneous changes in cell quotas of microcystin, chlorophyll a, protein and carbohydrate during different growth phases of a batch culture experiment with Microcystis aeruginosa. J. Plankton Res. 2004, 26, 727–736. [Google Scholar] [CrossRef]
- Alfiya, Y.; Damti, O.; Stoler-Katz, A.; Zoubi, A.; Shaviv, A.; Friedler, E. Potential impacts of on-site greywater reuse in landscape irrigation. Water Sci. Technol. 2012, 65, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.; Maimon, A.; Alfiya, Y.; Friedler, E. Greywater Reuse; CRC Press. Taylor & Francis Group, Inc.: London, UK, 2015; p. 306. [Google Scholar]
- Winward, G.P.; Avery, L.M.; Stephenson, T.; Jefferson, B. Chlorine disinfection of grey water for reuse: Effect of organics and particles. Water Res. 2008, 42, 483–491. [Google Scholar] [CrossRef]
- Winward, G.P.; Avery, L.M.; Frazer-Williams, R.; Pidou, M.; Jeffrey, P.; Stephenson, T.; Jefferson, B. A study of the microbial quality of grey water and an evaluation of treatment technologies for reuse. Ecol. Eng. 2008, 32, 187–197. [Google Scholar] [CrossRef]
- Maimon, A.; Friedler, E.; Gross, A. Parameters affecting greywater quality and its safety for use. Sci. Total Environ. 2014, 487, 20–25. [Google Scholar] [CrossRef]
- Friedler, E.F.; Kovalio, R.; Ben-Zvi, A. Comparative Study of the Microbial Quality of Greywater treated by Three On-Site Treatment Systems. Environ. Technol. 2006, 27, 653–663. [Google Scholar] [CrossRef]
- Gilboa, Y.; Friedler, E.F. UV disinfection of RBC-treated light greywater effluent: Kinetics, survival and regrowth of selected microorganisms. Water Res. 2008, 42, 1043–1050. [Google Scholar] [CrossRef]
- Benami, M.; Gross, A.; Zhang, W.; Orlofsky, E.; Vonshak, A.; Gillor, O. Assessment of pathogenic bacteria in treated graywater and irrigated soils. Sci. Total Environ. 2013, 458, 298–302. [Google Scholar] [CrossRef]
- Travis, M.J.; Wiel-Shafran, A.; Weisbrod, N.; Adar, E.; Gross, A. Greywater reuse for irrigation: Effect on soil properties. Sci. Total Environ. 2010, 408, 2501–2508. [Google Scholar] [CrossRef]
- Brook, I.; Malchi, T.; Nir, S. Removal of anionic detergents from water and treatment of gray water by micelle–clay composites. Desalination Water Treat. 2013, 53, 2184–2192. [Google Scholar] [CrossRef]
- WHO. Overview of Greywater Management, Health Considerations. Regional Office for the Eastern Mediterranean Centre for Environmental Health Activities; Eco Design Ideal: Amman, Jordan, 2006. [Google Scholar]
- Biswas, K.; Turner, S.J. Microbial Community Composition and Dynamics of Moving Bed Biofilm Reactor Systems Treating Municipal Sewage. Appl. Environ. Microbiol. 2011, 78, 855–864. [Google Scholar] [CrossRef]
- Brienza, M.; Nir, S.; Plantard, G.; Goetz, V.; Chiron, S. Combining micelle-clay sorption to solar photo-Fenton processes for domestic wastewater treatment. Environ. Sci. Pollut. Res. 2018, 26, 18971–18978. [Google Scholar] [CrossRef]
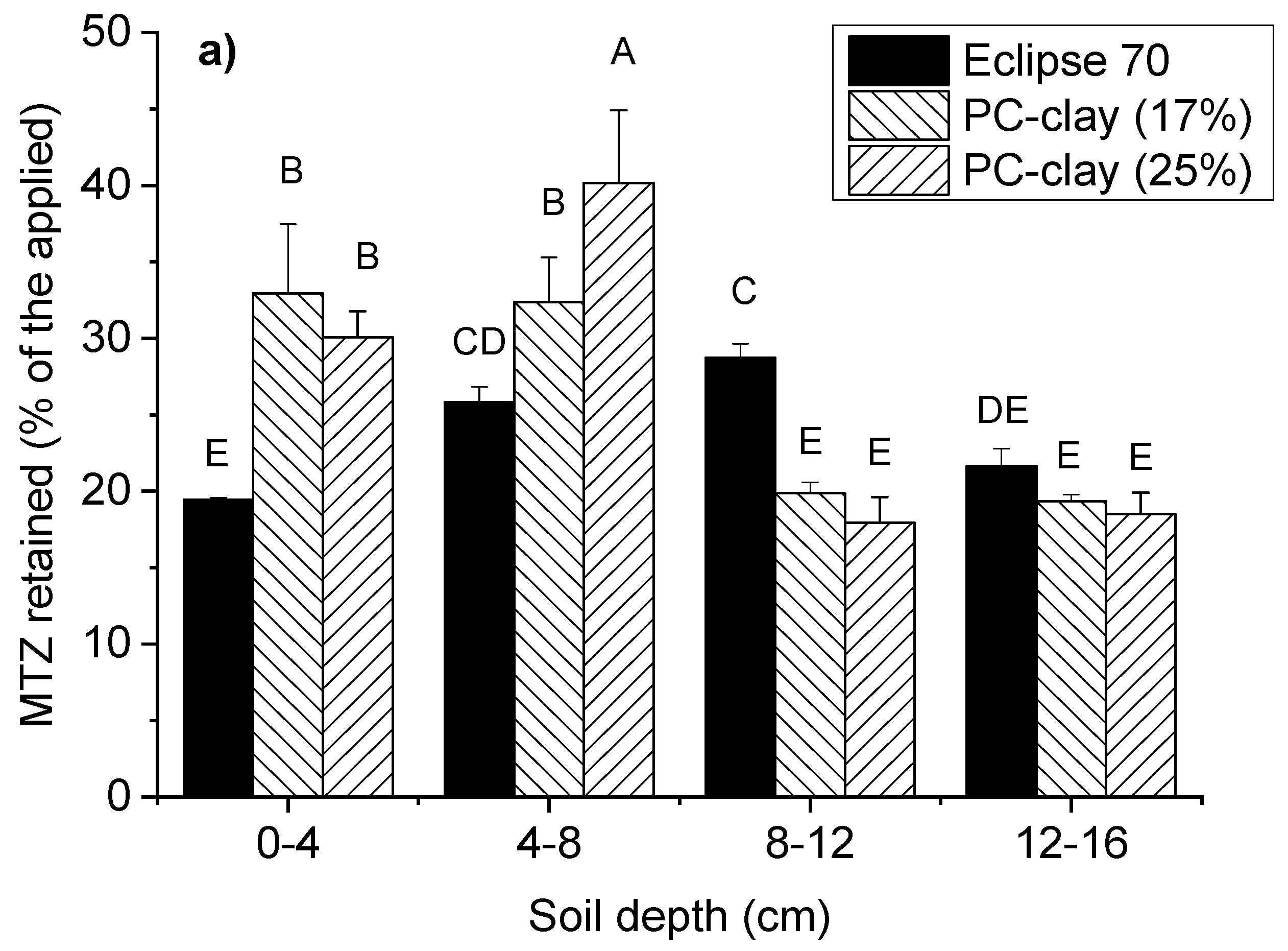

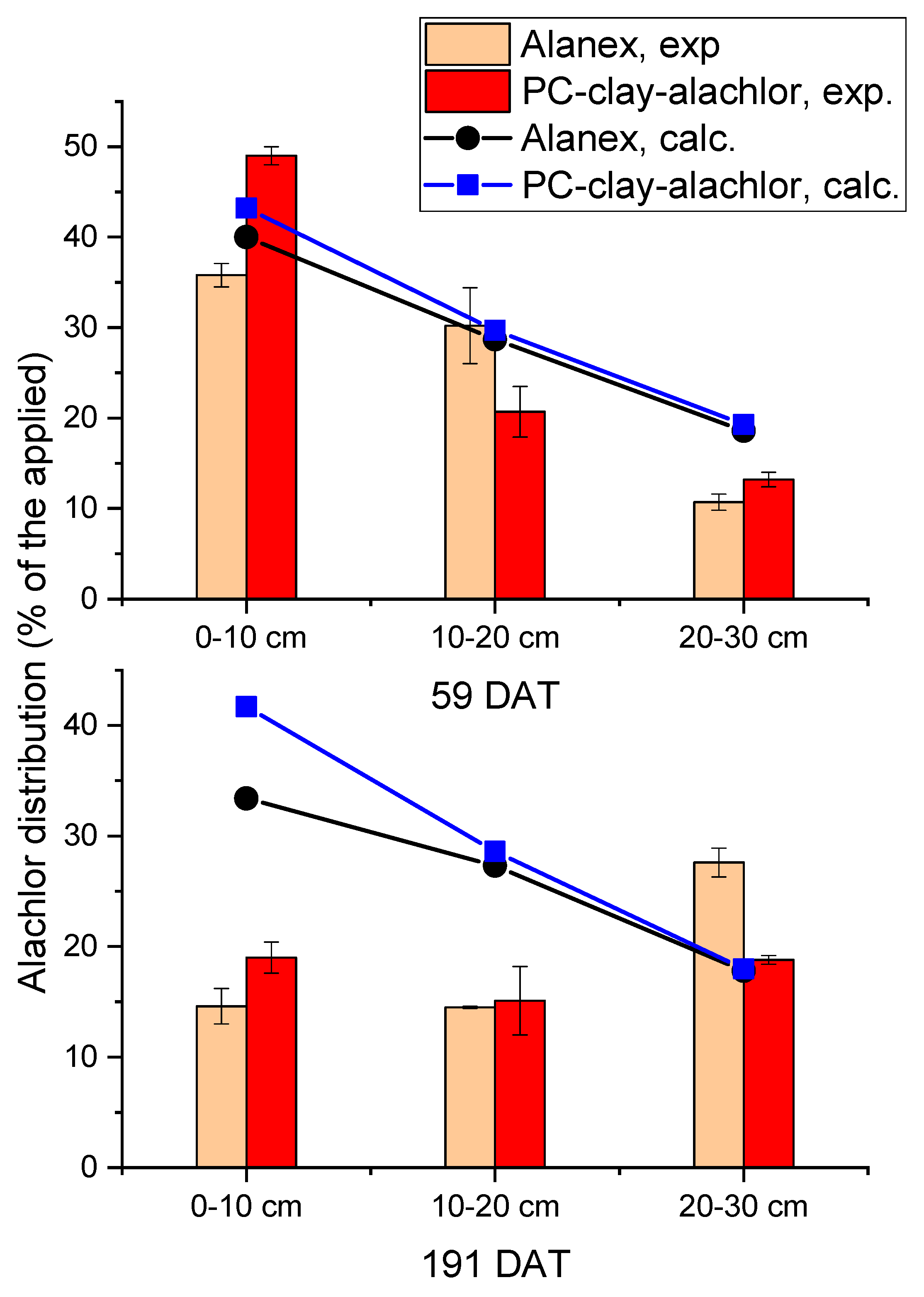
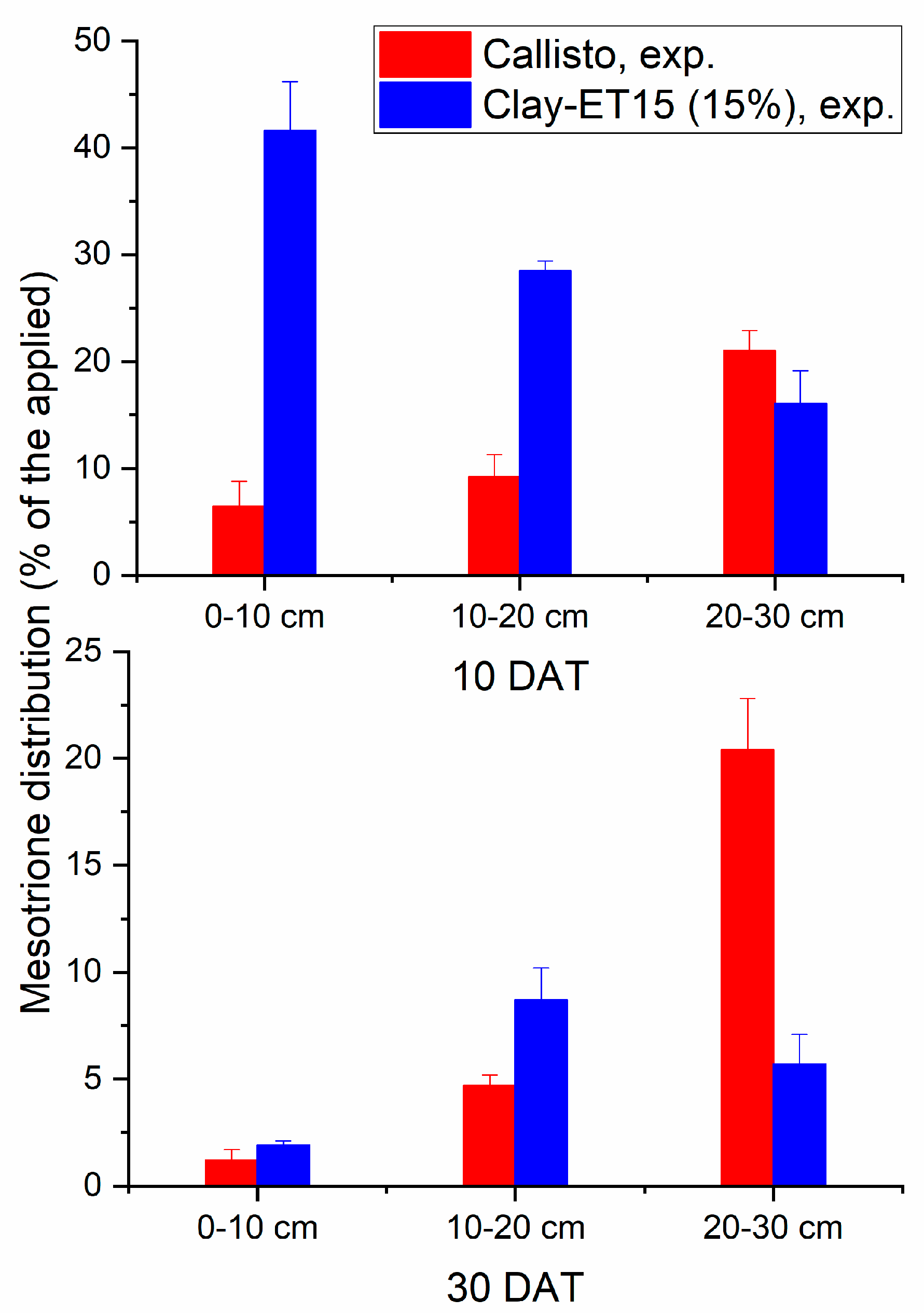

| Free Alachlor | Band of C Aromatic- H 793 |
|---|---|
| Clay | 809 |
| Clay-BTMA 0.5 | 815 |
| Clay-BTMA 0.8 | 809 |
| Clay-BTEA 0.5 | 810 |
| Clay-BTEA 0.8 | 810 |
| Wash # | Commercial | Micelle-Clay |
|---|---|---|
| 1 | 77.60 | 0.45 |
| 2 | 14.97 | 0.42 |
| 3 | 1.45 | 0.40 |
| 4 | 0.93 | 0.39 |
| Total | 99.91 | 3.67 |
| Time (Weeks) | % Growth Inhibition | |
|---|---|---|
| Clay-PTMA 0.5 | Commercial Formulation (EC) | |
| 2 | 100 | 38 ± 7 |
| 4 | 93 ± 3 | 6 ± 6 |
| 6 | 74 ± 6 | 2 ± 5 |
| Surfactant Modifier | Herbicide | Clay/Mineral Clay | Reference; [Remarks] |
|---|---|---|---|
| Hexadecyltrimethyl-ammonium (HDTMA) | Bentazone | MMT | Carrisoza et al., 2000 [40] |
| Hexazinone | MMT | Celis et al., 2002 [64] | |
| Celis et al., 2005 [42] | |||
| Acetochlor | MMT | Kaluderovich et al. [65] | |
| Picloram | MMT | Celis et al., 2002 [41] | |
| Pretilachlor | Bentonite | Wu et al., 2020 [66] | |
| Sulfometuron | MMT | Mishael et al., 2002 [6] [Micelle-clay] | |
| Sulfosulfuron | MMT | Mishael et al., 2003 [50] [Micelle-clay] | |
| Sulfentrazone | MMT | Polubesova et al., 2003 [51] [Micelle-clay] | |
| MMT | Ziv and Mishael, 2008 [52] [Micelle-clay] | ||
| Diuron | MMT | Celis et al., 2007 [43] | |
| Terbutylazine | MMT | Celis et al., 2007 | |
| MCPA | MMT | Celis et al., 2007 | |
| 2,4-D | Bentonite (also zeolite, composed mainly by clinopetilolite) MMT MMT | Shirvani et al., 2014 [67] | |
| Atrazine | Bentonite | Hermosin et al., 2006 [68]. Trigo et al., 2010 [45]. Dutta et al., 2015 [34] | |
| Benzyldimethyl-hexadecylammonium (BDMHDA) | Sulfentrazone | MMT | Polubesova et al., 2003 [51] [Micelle-clay] |
| Octadecyltrimethyl-ammonium (ODTMA) | Sulfometuron | MMT | Mishael et al., 2002 [6,7] [Micelle-clay] |
| Sulfosulfuron | MMT | Mishael et al., 2003 [50] [Micelle-clay] | |
| Sulfentrazone | MMT | Polubesova et al., 2003 [51] [Micelle-clay] | |
| Metolachlor | MMT | Ziv and Mishael, 2007 [52] [Micelle-clay] | |
| Dioctadecyldimethyl-ammonium (DDOB) | Bentazone | MMT | Carrizosa et al., 2000 [40] |
| Hexazinoe | MMT | Celis et al., 2002 [64] | |
| Didodecydimethyl-ammonium (DDAB) | Sulfometuron | MMT | Undabeytia et al., 2003 [49] [Vesicle-clay] |
| Sulfosulfuron | MMT | Undabeytia et al., 2003 [Vesicle-clay] | |
| N-cetylpyridine | 2,4-D | Bentonite (also zeolite: clinopetiolite) | Shirvani et al., 2014 [67] |
| Phosphatidylcholine (PC): 74% distearyl PC and 26% 1-palmitoyl-2stearyl PC | Alachlor | MMT | Sanchez-Verdejo et al., 2008 [58,59] [Vesicle-clay] |
| MMT | Undabeytia et al., 2008 [57] [Vesicle-clay] | ||
| Atrazine | MMT | Tejada et al., 2017 [69] [Vesicle-clay] | |
| MMT | Sanchez-Verdejo et al., 2008 [69] [Vesicle-clay] | ||
| Diuron | MMT | Undabeytia et al., 2008 [57] [Vesicle-clay] | |
| Meribuzin | MMT | Tejada et al., 2017 [69] [Vesicle-clay] | |
| MMT | Undabeytia et al., 2012 [62] [Vesicle-clay] | ||
| MMT | Undabeytia et al., 2010 [60] [Vesicle-clay] | ||
| Phenytrimethylammonium (PTMA) | Metolachlor | MMT | El-Nahhal et al., 2000 [11] |
| Alachlor | MMT | El-Nahhal et al., 2000 | |
| Acetochlor | MMT | El-Nahhal et al., 2001 [12] | |
| Bentazone | MMT | Carrisoza et al., 2000 [40] | |
| Hexazinone | MMT | Celis et al., 2002 [64] | |
| MMT | Celis et al. 2005 [42] | ||
| Diuron | MMT | Celis et al., 2007 [43] | |
| Terbutylazine | MMT | Celis et al., 2007 | |
| MCPA | MMT | Celis et al., 2007 | |
| Atrazine | MMT | Trigo et al., 2010 [45] | |
| Bentonite | Dutta et al., 2015 [34] | ||
| Octadecylammonium (ODA) | Bentazone | MMT | Carrisoza et al., 2000 [40] |
| Hexazinone | MMT | Celis et al., 2002 [64] | |
| Celis et al., 2005 [42] | |||
| Picloram | MMT | Celis et al., 2002 [41] | |
| Trioctylmethylammonium (TOMA) | Atrazine | Bentonite | Dutta et al., 2015 [34] |
| Tallow alkyl ethoxylated amine (ET15) | Mesotrione | Sepiolite | Galan-Jimenez et al., 2020 [54] |
| V(L) | Emerging Concentration ppb | % Removed Exp. | % Removed Calc.a |
|---|---|---|---|
| Column 1 | |||
| 9.0 | 2.3 | 97.9 | 92.3 |
| 15.0 | 5.3 | 95.2 | 86.5 |
| 30.0 | 41.6 | 62.1 | 70.0 |
| Column 2 | |||
| 9.0 | 0.32 | 99.7 | 99.6 |
| 15.0 | 0.22 | 99.8 | 99.1 |
| 30.0 | 0.5 | 99.5 | 96.0 |
| Cumulative Volume Passed (m3) | Perchlorate Conc. at Filter Inlet (ppb) | Perchlorate Conc. in Water Emerging from Filter (ppb) | Calculated Emerging Perchlorate Conc. (ppb) |
|---|---|---|---|
| 164 | 858 | 0–4 | 0.3 |
| 182.2 | 0–4 | 1 | |
| 189.6 | 6 | 2 (1.6) | |
| 211 | 12 | 10 (6) | |
| 211.7 | 12 | 10 (7) | |
| 220 | 787 | 16 | 14 (10) |
| 231 | 806 | 22 | 23 (19) |
| 235 | 832 | 25 | 30 (25) |
| 239.5 | 911 | 32 | 32 (27) |
| 244.5 | 898 | 44 | 44 (42) |
| 252 | 763 | 46 | 58 (48) |
| Number of Bacteria mL−1 in Initial Solution | Number of m3 Water Purified to Less Than 1 Emerging Bacterium 100 mL−1 1kg−1 of Granulated Complex, and after First Regeneration (in Parenthesis). |
|---|---|
| 10,000 | 74 (137) |
| 100,000 | 55 (104) |
| 1,000,000 | 22 (41) |
| 5,000,000 | 5 (9) |
| Bacteria | Volume (µm3) | Molecular R0 (M) | C1 (M−1 min−1) | D1 (min−1) |
|---|---|---|---|---|
| E. coli S-17 | 1 | 5.8 × 10−12 | 9.5 × 1011 | 1.0 × 10−4 |
| Microcystis | 14 | 7.94 × 10−13 | 2 × 1012 | 1.0 × 10−4 |
| Aphanizomenon | 4500 | >2.5 × 10−14 | 6 × 1013 | 1.5 × 10−5 |
| Initial Number of Cells mL−1 | Number of Cell mL−1 after Coagulation/Sedimentation | Capacity of the Filter:Volume (m3) of Water Which Can Be Purified from Microcystis Cells Removed kg−1 of Complex |
|---|---|---|
| 107 | 106 | 3 |
| 106 | 105 | 22 |
| 2 × 105 | 2 × 104 | 45 |
| 105 | 104 | 67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Undabeytia, T.; Shuali, U.; Nir, S.; Rubin, B. Applications of Chemically Modified Clay Minerals and Clays to Water Purification and Slow Release Formulations of Herbicides. Minerals 2021, 11, 9. https://doi.org/10.3390/min11010009
Undabeytia T, Shuali U, Nir S, Rubin B. Applications of Chemically Modified Clay Minerals and Clays to Water Purification and Slow Release Formulations of Herbicides. Minerals. 2021; 11(1):9. https://doi.org/10.3390/min11010009
Chicago/Turabian StyleUndabeytia, Tomas, Uri Shuali, Shlomo Nir, and Baruch Rubin. 2021. "Applications of Chemically Modified Clay Minerals and Clays to Water Purification and Slow Release Formulations of Herbicides" Minerals 11, no. 1: 9. https://doi.org/10.3390/min11010009
APA StyleUndabeytia, T., Shuali, U., Nir, S., & Rubin, B. (2021). Applications of Chemically Modified Clay Minerals and Clays to Water Purification and Slow Release Formulations of Herbicides. Minerals, 11(1), 9. https://doi.org/10.3390/min11010009







