Fly Ash Utilisation in Mullite Fabrication: Development of Novel Percolated Mullite
Abstract
1. Introduction
- a-axis: α20–1000 = 5.39 × 10−6 °C−1
- b-axis: α20–1000 = 7.73 × 10−6 °C−1
- c-axis: α20–1000 = 7.10 × 10−6 °C−1
- Bulk: α20–1000 = 6.74 × 10−6 °C−1
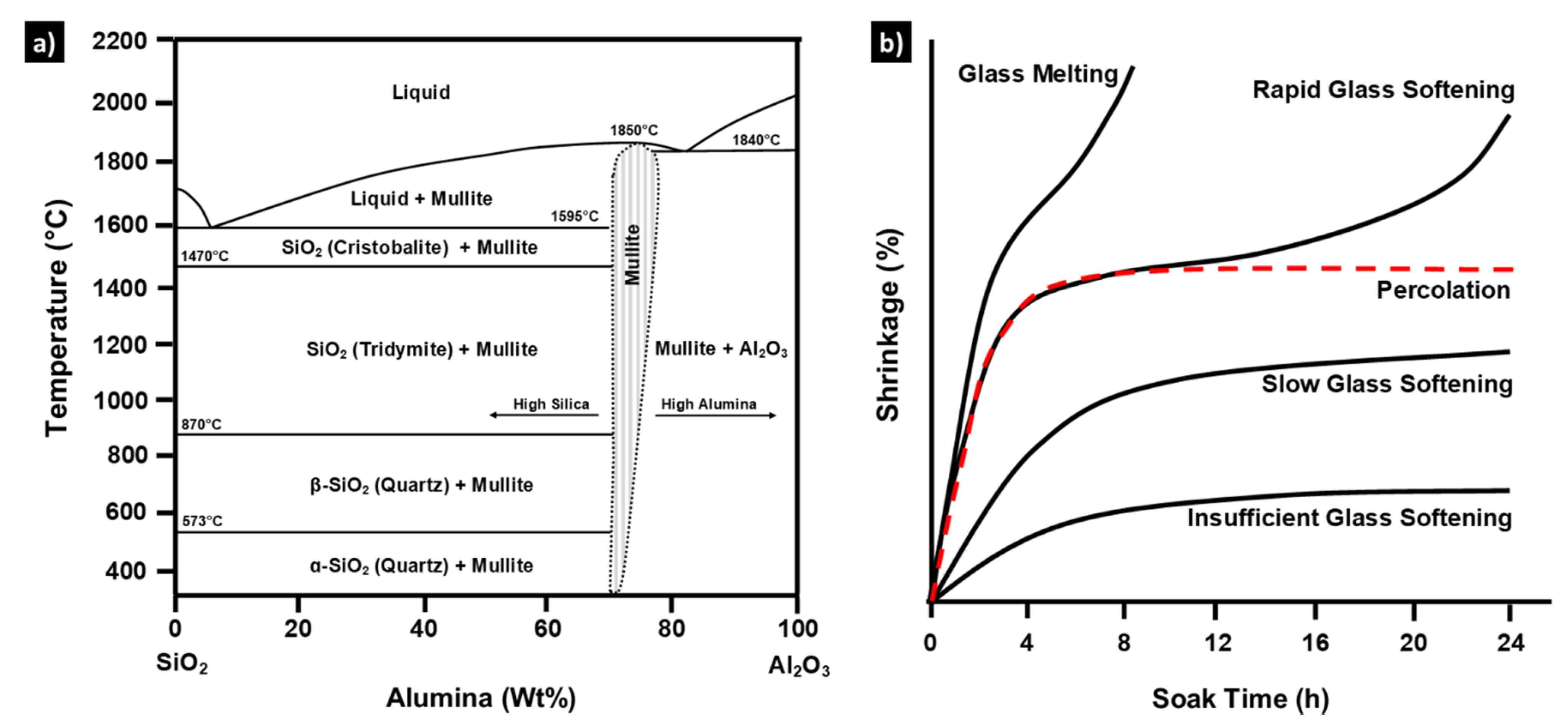
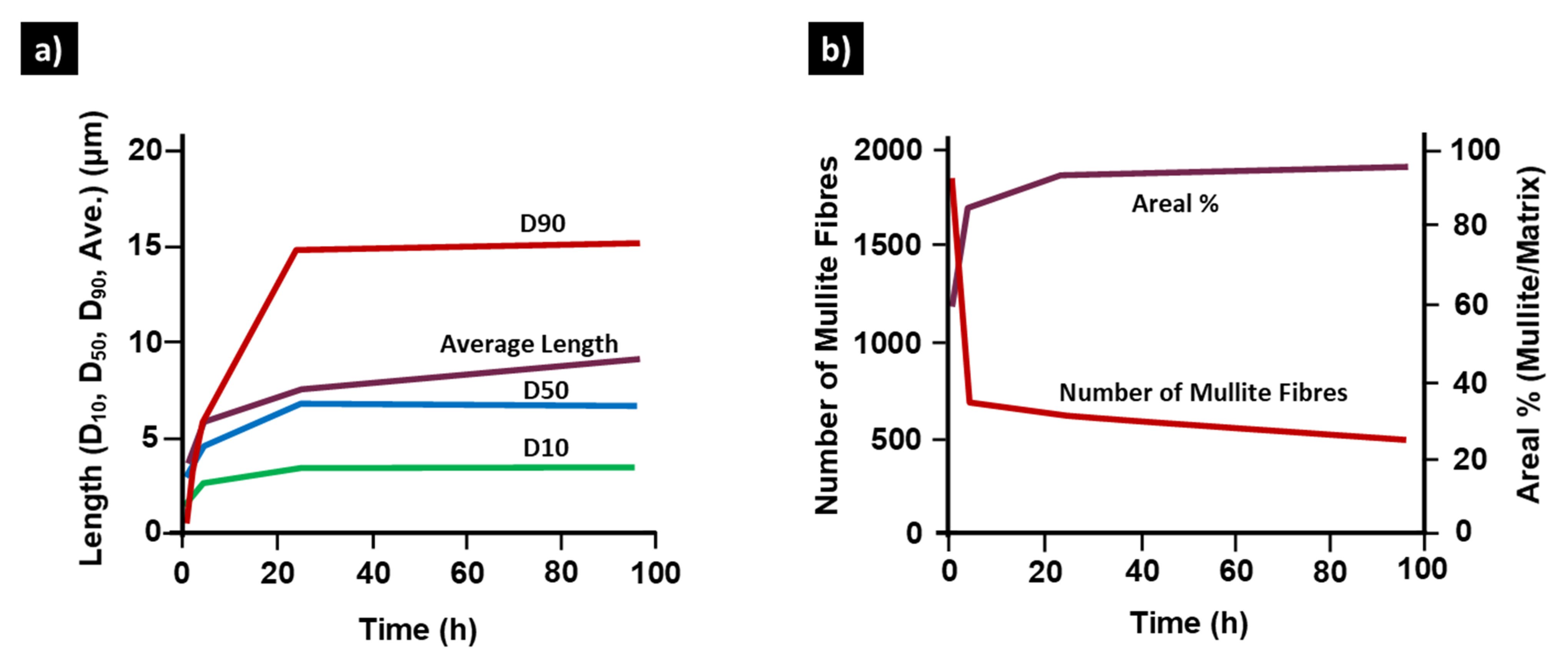
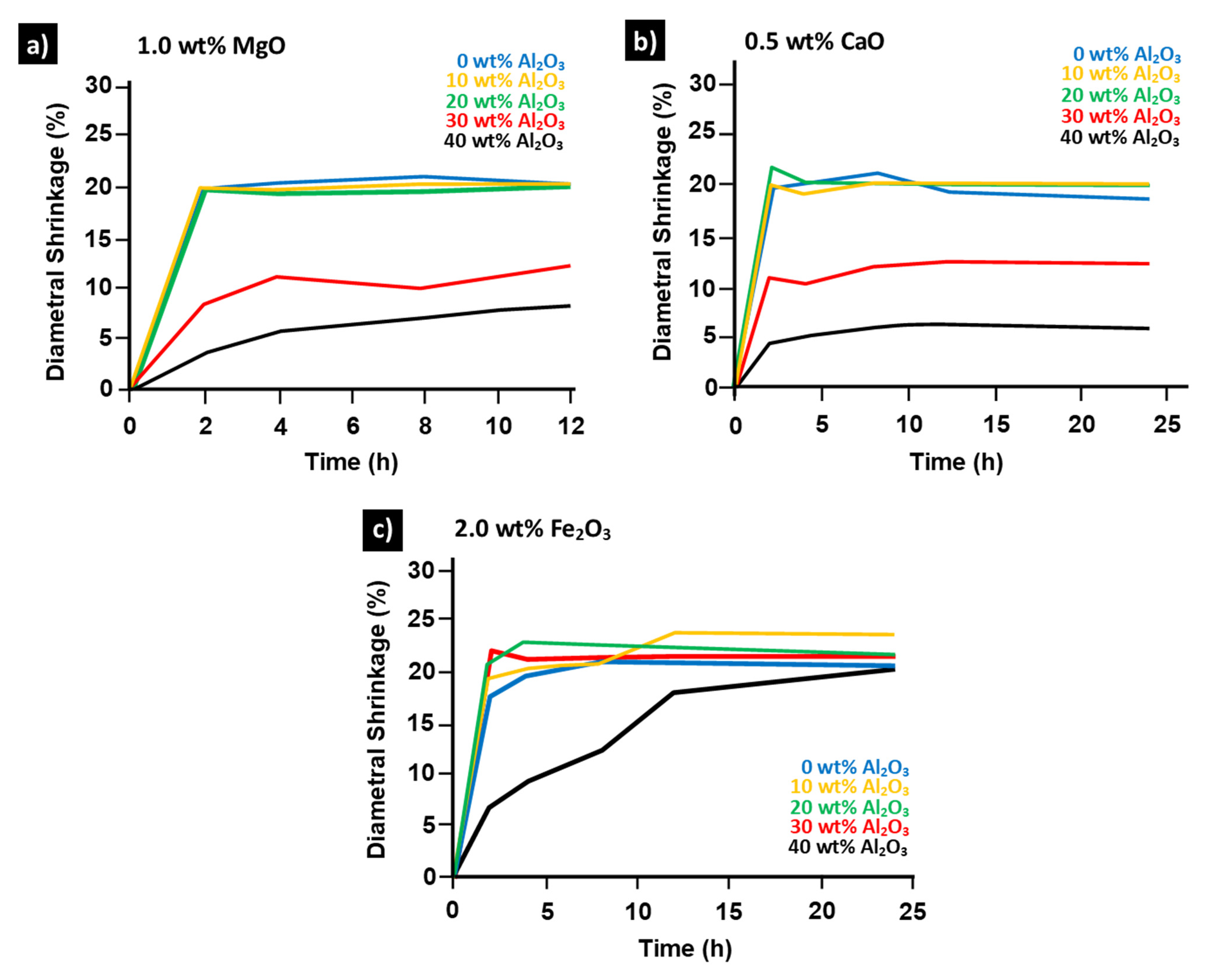
2. Percolated Mullite
| FeO-Al2O3-SiO2 | 1083 °C | (1.8 wt% addition) | [60] |
| Fe2O3-Al2O3-SiO2 | 1382 °C | (2.0 wt% addition) | [61] |
| MgO-Al2O3-SiO2 | 1355 °C | (1.0 wt% addition) | [60] |
| CaO-Al2O3-SiO2 | 1170 °C | (0.5 wt% addition) | [60] |
3. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Zhuang, X.; Querol, X.; Font, O.; Moreno, N. A Review on the Applications of Coal Combustion Products in China. Int. Geol. Rev. 2018, 60, 671–716. [Google Scholar] [CrossRef]
- Fisher, G.L.; Chang, D.P.Y.; Brummer, M. Fly Ash Collected from Electrostatic Precipitators: Microcrystalline Structures and the Mystery of the Spheres. Science 1976, 192, 553–555. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.; Priyadarshini, S.; Mohankrishnan, A.A.; Abri, A.; Sattler, M.; Techapaphawit, S. Physical, Chemical, and Geotechnical Properties of Coal Fly Ash: A Global Review. Case Stud. Constr. Mater. 2019, 11, e00263. [Google Scholar] [CrossRef]
- Ash Development Association of Australia. Coal Combustion Products Handbook, 2nd ed.; Ward, C., Heidrich, C., Yeatman, O., Eds.; HBM Group: Port Kembla, NSW, Australia, 2014. [Google Scholar]
- Qadir, S.U.; Raja, V.; Siddiqui, W.A. Morphological and Biochemical Changes in Azadirachta Indica from Coal Combustion Fly Ash Dumping Site from a Thermal Power Plant in Delhi, India. Ecotoxicol. Environ. Saf. 2016, 129, 320–328. [Google Scholar] [CrossRef]
- Papadakis, V.G. Effect of Fly Ash on Portland Cement Systems: Part O: Low-Calcium Fly Ash. Cem. Concr. Res. 1999, 29, 1727–1736. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. A Review on the Utilization of Fly Ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- ASTM C618-05. Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete; ASTM International: West Conshohocken, PA, USA, 2005. [Google Scholar]
- Schneider, H.; Schreuer, J.; Hildmann, B. Structure and Properties of Mullite—A Review. J. Eur. Ceram. Soc. 2008, 28, 329–344. [Google Scholar] [CrossRef]
- Aramaki, S.; Roy, R. Revised Phase Diagram for the System Al2O3-SiO2. J. Am. Ceram. Soc. 1962, 45, 229–242. [Google Scholar] [CrossRef]
- Parvizi-Majidi, A. 1/06—Whiskers and Particulates. In Comprehensive Composite Materials; Kelly, A., Zweben, C.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 1, pp. 175–198. [Google Scholar]
- Schneider, H.; Komarneni, S. Mullite; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2005. [Google Scholar]
- Morrell, R. Handbook of Properties of Technical & Engineering Ceramics. Part 1: An Introduction for the Engineer and Designer; HMSO: London, UK, 1989. [Google Scholar]
- Ward, C.R.; French, D. Determination of Glass Content and Estimation of Glass Composition in Fly Ash using Quantitative X-Ray Diffractometry. Fuel 2006, 85, 2268–2277. [Google Scholar] [CrossRef]
- Roeder, P.L.; Glasser, F.P.; Osborn, E.F. The System Al2O3-Cr2O3-SiO2. J. Am. Ceram. Soc. 1968, 51, 585–593. [Google Scholar] [CrossRef]
- Katsuki, H.; Furuta, S.; Ichinose, H.; Nakao, H. Preparation and Some Properties of Porous Ceramics Sheet Composed of Needle-Like Mullite. J. Ceram. Soc. Jpn. 1988, 96, 1081–1086. [Google Scholar] [CrossRef]
- Kim, B.M.; Cho, Y.K.; Yoon, S.Y.; Stevens, R.; Park, H.C. Mullite Whiskers Derived from Kaolin. Ceram. Int. 2009, 35, 579–583. [Google Scholar] [CrossRef]
- Abdullayev, A.; Zemke, F.; Gurlo, A.; Bekheet, M.F. Low-Temperature Fluoride-Assisted Synthesis of Mullite Whiskers. RSC Adv. 2020, 10, 31180–31186. [Google Scholar] [CrossRef]
- Kriven, W.M.; Palko, J.W.; Sinogeikin, S.; Bass, J.D.; Sayir, A.; Brunaue, G.; Boysen, H.; Frey, F.; Schneider, J. High Temperature Single Crystal Properties of Mullite. J. Eur. Ceram. Soc. 1999, 19, 2529–2541. [Google Scholar] [CrossRef]
- Nishikawa, A. Technology of Monolithic Refractories; Plibrico Japan Company Limited: Tokyo, Japan, 1984. [Google Scholar]
- Chesters, J.H. Refractories: Production and Properties; Iron and Steel Institute: London, UK, 1973. [Google Scholar]
- Orton Ceramic. Cone Equivalent Temperatures. Available online: https://www.ortonceramic.com/files/2676/File/cone-equivalent-temperatures-C.pdf (accessed on 8 January 2021).
- Carniglia, S.C.; Barna, G.L. Handbook of Industrial Refractories Technology: Principles, Types, Properties and Applications; Noyes Publications: Park Ridge, NJ, USA, 1992. [Google Scholar]
- Koshy, P.; Gupta, S.; Sahajwalla, V.; Edwards, P. Effect of Silica on High-Temperature Interfacial Phenomena of Monolithic Refractories with Al-Alloy. Metall. Mater. Trans. B 2008, 39, 331–339. [Google Scholar] [CrossRef]
- Adabifiroozjaei, E.; Koshy, P.; Sorrell, C.C. Effects of AlPO4 Addition on the Corrosion Resistance of Andalusite-Based Low-Cement Castables with Molten Al-Alloy. J. Eur. Ceram. Soc. 2013, 33, 1067–1075. [Google Scholar] [CrossRef]
- Adabifiroozjaei, E.; Saidi, A.; Monshi, A.; Koshy, P. Effect of Different Calcium Compounds on the Corrosion Resistance of Andalusite-Based Low-Cement Castables in Contact with Molten Al-Alloy. Metall. Mater. Trans. B 2011, 42, 400–411. [Google Scholar] [CrossRef]
- Koshy, P.; Gupta, S.; Sahajwalla, V.; Edwards, P. Effect of CaF2 on Interfacial Phenomena of High Alumina Refractories with Al-Alloy. Metall. Mater. Trans. B 2008, 39, 603–611. [Google Scholar] [CrossRef]
- Adabifiroozjaei, E.; Koshy, P.; Sorrell, C.C. Effects of V2O5 Addition on the Corrosion Resistance of Andalusite Based Low-Cement Castables with Molten Al-Alloy. J. Eur. Ceram. Soc. 2012, 32, 1463–1471. [Google Scholar] [CrossRef]
- Adabifiroozjaei, E.; Koshy, P.; Sorrell, C.C. Effects of Different Boron Compounds on the Corrosion Resistance of Andalusite-Based Low Cement Castables in Contact with Molten Al-Alloy. Metall. Mater. Trans. B 2012, 43, 5–13. [Google Scholar] [CrossRef]
- Sorrell, C.C.; Koshy, P.; Koszo, S. Percolated Mullite and a Method of Forming Same. U.S. Patent 9,527,775, 27 December 2016. [Google Scholar]
- Dong, Y.; Feng, X.; Feng, X.; Ding, Y.; Liu, X.; Meng, G. Preparation of Low-Cost Mullite Ceramics from Natural Bauxite and Industrial Waste Fly Ash. J. Alloys Compd. 2008, 460, 599–606. [Google Scholar] [CrossRef]
- Park, Y.M.; Yang, T.Y.; Yoon, S.Y.; Stevens, R.; Park, H.C. Mullite Whiskers Derived from Coal Fly Ash. Mater. Sci. Eng. A 2007, 454–455, 518–522. [Google Scholar] [CrossRef]
- Cao, J.; Dong, X.; Li, L.; Dong, Y.; Hampshire, S. Recycling of Waste Fly Ash for Production of Porous Mullite Ceramic Membrane Supports with Increased Porosity. J. Eur. Ceram. Soc. 2014, 34, 3181–3194. [Google Scholar] [CrossRef]
- Dong, Y. Recycling of Fly Ash for Preparing Porous Mullite Membrane Supports with Titania Addition. J. Hazard. Mater. 2010, 180, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Du, H.; Guo, A.; Xu, H.; Yang, D. Preparation of Self-Reinforcement of Porous Mullite Ceramics Through In Situ Synthesis of Mullite Whisker in Flyash Body. Ceram. Int. 2012, 38, 1027–1032. [Google Scholar] [CrossRef]
- Li, J.-H.; Ma, H.-W.; Huang, W.-H. Effect of V2O5 on the Properties of Mullite Ceramics Synthesized from High-Aluminum Fly Ash and Bauxite. J. Hazard. Mater. 2009, 166, 1535–1539. [Google Scholar] [CrossRef]
- Chen, A.-N.; Li, M.; Xu, J.; Lou, C.-H.; Wu, J.; Cheng, L.; Shi, Y.; Li, C. High-Porosity Mullite Ceramic Foams Prepared by Selective Laser Sintering using Fly Ash Hollow Spheres as Raw Materials. J. Eur. Ceram. Soc. 2018, 38, 4553–4559. [Google Scholar] [CrossRef]
- Dong, Y.; Hampshire, S.; Zhou, J.; Li, Z.; Wang, J.; Meng, G. Sintering and Characterization of Flyash-Based Mullite with MgO Addition. J. Eur. Ceram. Soc. 2011, 31, 687–695. [Google Scholar] [CrossRef]
- Guo, A.; Liu, J.; Xu, R.; Xu, H.; Wang, C. Preparation of Mullite from Desilication-Flyash. Fuel 2010, 89, 3630–3636. [Google Scholar] [CrossRef]
- Lin, B.; Li, S.; Hou, X.; Li, H. Preparation of High Performance Mullite Ceramics from High-Aluminum Fly Ash by an Effective Method. J. Alloys Compd. 2015, 623, 359–361. [Google Scholar] [CrossRef]
- Ma, B.; Su, C.; Ren, X.; Qian, F.; Yang, W.; Liu, G.; Li, H.; Yu, J.; Zhu, Q. Preparation and Properties of Porous Mullite Ceramics with High-Closed Porosity and High Strength from Fly Ash via Reaction Synthesis Process. J. Alloys Compd 2019, 803, 981–991. [Google Scholar] [CrossRef]
- Das, D.; Nijhuma, K.; Gabriel, A.M.; Daniel, G.P.F.; de Murilo, D.M.I. Recycling of Coal Fly Ash for Fabrication of Elongated Mullite Rod Bonded Porous SiC Ceramic Membrane and its Application in Filtration. J. Eur. Ceram. Soc. 2020, 40, 2163–2172. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J.; Zeng, Y.; Liu, Z.; Wang, C. Preparation and Characterization of Mullite Powders from Coal Fly Ash by the Mullitization and Hydrothermal Processes. Mater. Chem. Phys. 2018, 213, 518–524. [Google Scholar] [CrossRef]
- Fu, M.; Liu, J.; Dong, X.; Zhu, L.; Dong, Y.; Hampshire, S. Waste Recycling of Coal Fly Ash for Design of Highly Porous Whisker-Structured Mullite Ceramic Membranes. J. Eur. Ceram. Soc. 2019, 39, 5320–5331. [Google Scholar] [CrossRef]
- Luo, Y.; Ma, S.; Zheng, S.; Liu, C.; Han, D.; Wang, X. Mullite-Based Ceramic Tiles Produced Solely from High-Alumina Fly Ash: Preparation and Sintering Mechanism. J. Alloys Compd. 2018, 732, 828–837. [Google Scholar] [CrossRef]
- Li, C.; Zhou, Y.; Tian, Y.; Zhao, Y.; Wang, K.; Li, G.; Chai, Y. Preparation and Characterization of Mullite Whisker Reinforced Ceramics Made from Coal Fly Ash. Ceram. Int. 2019, 45, 5613–5616. [Google Scholar] [CrossRef]
- Tan, H. Preparation of Mullite Whiskers from Coal Fly Ash using Sodium Sulfate Flux. Int. J. Miner. Process. 2011, 100, 188–189. [Google Scholar] [CrossRef]
- Chen, X.; Li, T.; Ren, Q.; Wu, X.; Li, H.; Dang, A.; Zhao, T.; Shang, Y.; Zhang, Y. Mullite Whisker Network Reinforced Ceramic with High Strength and Lightweight. J. Alloys Compd. 2017, 700, 37–42. [Google Scholar] [CrossRef]
- Wang, T.; Ma, S.; Wang, X.; Hong, T.; Luo, Y. A 100% High-Aluminum Fly Ash-Based High-Density Mullite Ceramic with a Triple Microstructure: Preparation and Mechanical Characterization. Constr. Build. Mater. 2020, 239, 117761. [Google Scholar] [CrossRef]
- Ren, S.; Tao, X.; Xu, X.; Guo, A.; Liu, J.; Fan, J.; Ge, J.; Fang, D.; Liang, J. Preparation and Characteristic of the Fly Ash Cenospheres/Mullite Composite for High-Temperature Application. Fuel 2018, 233, 335–345. [Google Scholar] [CrossRef]
- Chen, J.; Shao, L.; Lu, J. Synthesis of Mullite from High-Alumina Fly Ash: A Case from the Jungar Power Plant in Inner Mongolia, Northern China. Acta Geol. Sin. Engl. Ed. 2008, 82, 99–104. [Google Scholar]
- Han, G.; Yang, S.; Peng, W.; Huang, Y.; Wu, H.; Chai, W.; Liu, J. Enhanced Recycling and Utilization of Mullite from Coal Fly Ash with a Flotation and Metallurgy Process. J. Clean. Prod. 2018, 178, 804–813. [Google Scholar] [CrossRef]
- Foo, C.T.; Salleh, M.A.M.; Ying, K.K.; Matori, K.A. Mineralogy and Thermal Expansion Study of Mullite-Based Ceramics Synthesized From Coal Fly Ash and Aluminum Dross Industrial Wastes. Ceram. Int. 2019, 45, 7488–7494. [Google Scholar] [CrossRef]
- Yadav, A.K.; Patel, S.; Bhattacharyya, S. Preparation of Low-Cost Porous Mullite Ceramics by Recycling Fly Ash. AIP Conf. Proc. 2019, 2142, 30004. [Google Scholar]
- Zhu, J.; Yan, H. Microstructure and Properties of Mullite-Based Porous Ceramics Produced from Coal Fly Ash with Added Al2O3. Int. J. Miner. Metall. Mater. 2017, 24, 309–315. [Google Scholar] [CrossRef]
- Koshy, P.; Koszo, S.A.; Severin, E.; Sorrell, C.C. High-Performance Refractory Ceramics of Percolated Mullite from Waste Materials. Am. Ceram. Soc. Bull. 2018, 97, 29–33. [Google Scholar]
- Adabifiroozjaei, E.; Hart, J.N.; Koshy, P.; Mitchell, D.R.G.; Sorrell, C.C. Mullite-Glass and Mullite-Mullite Interfaces: Analysis by Molecular Dynamics (MD) Simulation and High-Resolution TEM. J. Am. Ceram. Soc. 2017, 101, 428–439. [Google Scholar] [CrossRef]
- Uchino, K. Piezoelectric Composite Materials. In Reference Module in Materials Science and Materials Engineering; Hashmi, S., Ed.; Elsevier: Oxford, UK, 2016; pp. 1–12. [Google Scholar]
- Newnham, R.E.; Skinner, D.P.; Cross, L.E. Connectivity and Piezoelectric-Pyroelectric Composites. Mater. Res. Bull. 1978, 13, 525–536. [Google Scholar] [CrossRef]
- Levin, E.M.; Robbins, C.R.; McMurdie, H.F. (Eds.) Figures 630, 696, and 712. In Phase Diagrams for Ceramists; American Ceramic Society: Columbus, OH, USA, 1964. [Google Scholar]
- Jak, E.; Hayes, P.; Pelton, A.; Dectorov, S. Thermodynamic Modelling of the Al2O3-CaO-FeO-Fe2O3-PbO-SiO2-ZnO System with Addition of K and Na with Metallurgical Applications. In Proceedings of the VIII International Conference on Molten Slags, Fluxes and Salts—MOLTEN 2009, Santiago, Chile, 18–21 January 2009; Sánchez, M., Parra, R., Riveros, G., Diaz, C., Eds.; Gecamin Ltd.: Santiago, Chile, 2009; pp. 473–490. [Google Scholar]
- Brownell, W.E. Structural Clay Products; Springer-Verlag: Vienna, Austria, 1976. [Google Scholar]
- Kingery, W.D.; Bowen, H.K.; Uhlmann, D.R. Introduction to Ceramics, 2nd ed.; John Wiley: New York, NY, USA, 1976. [Google Scholar]



| No. | Raw Materials | Sintering Temperatures (°C)/Time | Microstructural Features and Mullite Morphologies | Observations | Ref. |
|---|---|---|---|---|---|
| 1 | Fly Ash/Bauxite | 1000°–1600 °C/4 h |
|
| [31] |
| 2 | Fly Ash/Ammonium Alum/Sodium Dihydrogen Phosphate | 1300 °C/10 h |
|
| [32] |
| 3 | Fly Ash/ Bauxite/V2O5/AlF3 | 1200°–1500 °C/2.5 h |
|
| [33] |
| 4 | Fly Ash/Bauxite/TiO2 | 1300°–1500 °C/2 h |
|
| [34] |
| 5 | Fly Ash/ Al(OH)3/Al2O3/AlF3 | 1400°–1600 °C/4 h |
|
| [35] |
| 6 | Fly Ash/ Bauxite/V2O5 | 1100°–1500 °C/4 h |
|
| [36] |
| 7 | Fly Ash Hollow Spheres (FAHSs) | 1250°–1400 °C/3 h |
|
| [37] |
| 8 | Fly Ash/Bauxite/MgO | 1300°–1500 °C/ 2 h |
|
| [38] |
| 9 | Desilicified Fly Ash | 1300°–1600 °C/ 4 h |
|
| [39] |
| 10 | High-Aluminium Fly Ash | 1200°–1600 °C/2 h |
|
| [40] |
| 11 | Fly Ash/ Bauxite/Potash Feldspar/SiC/V2O5 | 1450°–1550 °C/2 h |
|
| [41] |
| 12 | Fly Ash/SiC/MoO3 | 850 °C/2 h 1000 °C/1 h |
|
| [42] |
| 13 | Fly Ash/Boehmite Sol | 900°–1300 °C/2 h |
|
| [43] |
| 14 | Fly Ash/Al(OH)3/MoO3 | 1100°–1500 °C/2 h |
|
| [44] |
| 15 | High-Alumina Fly Ash/HF/NaOH | 1100°–1400 °C/2 h |
|
| [45] |
| 16 | Fly Ash/Alumina/AlF3 | 1000°–1400 °C/2 h |
|
| [46] |
| 17 | Fly Ash/Na2SO4/ Aluminium Sulfate/HF | 1000 °C/3 h |
|
| [47] |
| 18 | Fly Ash/Bauxite/ Kaolin/Potash Feldspar/ Talc/BaCO3/Pyrolusite | 1390 °C/2 h |
|
| [48] |
| 19 | High-Aluminium Fly Ash/NaOH | 1400 °C/100 min |
|
| [49] |
| 20 | Fly Ash Cenospheres (FACs)/Kaolin | 900°–1300 °C/3 h |
|
| [50] |
| 21 | High-Aluminium Fly Ash/HCl | 1300°–1500 °C/2–4 h |
|
| [51] |
| 22 | Fly Ash + Alumina + Calcite | 1400°–1800 °C/ Time not specified |
|
| [52] |
| 23 | Fly Ash/Aluminium Dross/ HCl | 1200°, 1500 °C/4 h |
|
| [53] |
| 24 | Fly Ash/Bauxite | 1300°–1500 °C/Time not specified |
|
| [54] |
| 25 | Fly Ash/Al2O3/Starch Filler | 1600 °C/4 h |
|
| [55] |
| Sample | SiO2 | Al2O3 | Fe2O3 | Na2O | K2O | CaO | MgO | TiO2 | LOI | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| Fly Ash 1 | 72.2 | 22.6 | 1.1 | 0.1 | 0.5 | 0.0 | 0.2 | 1.3 | 2.1 | 0.0 |
| Fly Ash 2 | 33.8 | 31.2 | 2.5 | 0.1 | 0.4 | 1.6 | 0.4 | 1.3 | 25.0 | 3.7 |
| Fly Ash 3 | 68.1 | 23.6 | 1.3 | 0.2 | 2.2 | 0.7 | 0.3 | 0.9 | 2.4 | 0.3 |
| General Range | 33–75 | 18–36 | 0.8–6.0 | 0.0–0.3 | 0.4–2.6 | 0.1–4.0 | 0.2–1.0 | 0.4–1.8 | 0.5–25 | 0.5–4.0 |
| Mullite | 28.2 | 71.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Time | Shrinkage | Glass Viscosity | Dominant Effect |
|---|---|---|---|
| <2 h | Significant contraction | High | Volumetric shrinkage from softening of solid-like glass with little effect of dispersed mullite needles in deformable matrix |
| 2–4 h | Minor expansion | High | Volumetric expansion from thermal expansion of solid-like glass and semi-percolated mullite needle skeleton |
| >4 h | Continued contraction | Decreasing | Gradual volumetric shrinkage from resultant progressively decreasing glass viscosity, and increasingly liquid-like behaviour |
| Refractory | Bulk Density (kg·m−3) | Apparent Porosity (%) |
|---|---|---|
| Dense and Porous Shapes | ~1600–2000 | ~5–30 |
| Sintered Aggregates | ~900–1100 | ~40–50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koshy, P.; Ho, N.; Zhong, V.; Schreck, L.; Koszo, S.A.; Severin, E.J.; Sorrell, C.C. Fly Ash Utilisation in Mullite Fabrication: Development of Novel Percolated Mullite. Minerals 2021, 11, 84. https://doi.org/10.3390/min11010084
Koshy P, Ho N, Zhong V, Schreck L, Koszo SA, Severin EJ, Sorrell CC. Fly Ash Utilisation in Mullite Fabrication: Development of Novel Percolated Mullite. Minerals. 2021; 11(1):84. https://doi.org/10.3390/min11010084
Chicago/Turabian StyleKoshy, Pramod, Naomi Ho, Vicki Zhong, Luisa Schreck, Sandor Alex Koszo, Erik J. Severin, and Charles Christopher Sorrell. 2021. "Fly Ash Utilisation in Mullite Fabrication: Development of Novel Percolated Mullite" Minerals 11, no. 1: 84. https://doi.org/10.3390/min11010084
APA StyleKoshy, P., Ho, N., Zhong, V., Schreck, L., Koszo, S. A., Severin, E. J., & Sorrell, C. C. (2021). Fly Ash Utilisation in Mullite Fabrication: Development of Novel Percolated Mullite. Minerals, 11(1), 84. https://doi.org/10.3390/min11010084






