Genomic Analysis of a Newly Isolated Acidithiobacillus ferridurans JAGS Strain Reveals Its Adaptation to Acid Mine Drainage
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture Media, Phenotypic and Growth Observations
2.2. Comparative Genomics
2.3. Pan-Genome Analysis
2.4. Prediction of Mobile Genetic Elements
3. Results and Discussion
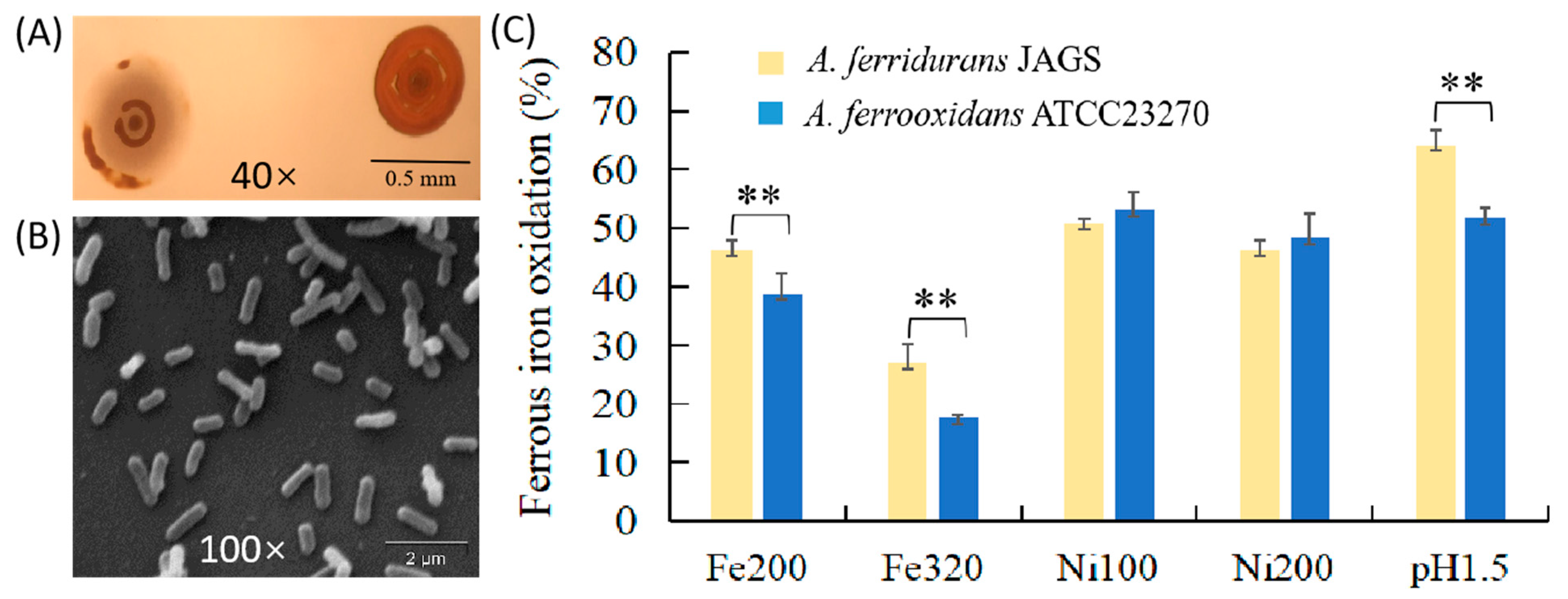
3.1. Phenotypic and Growth Features
3.2. Genomic Features
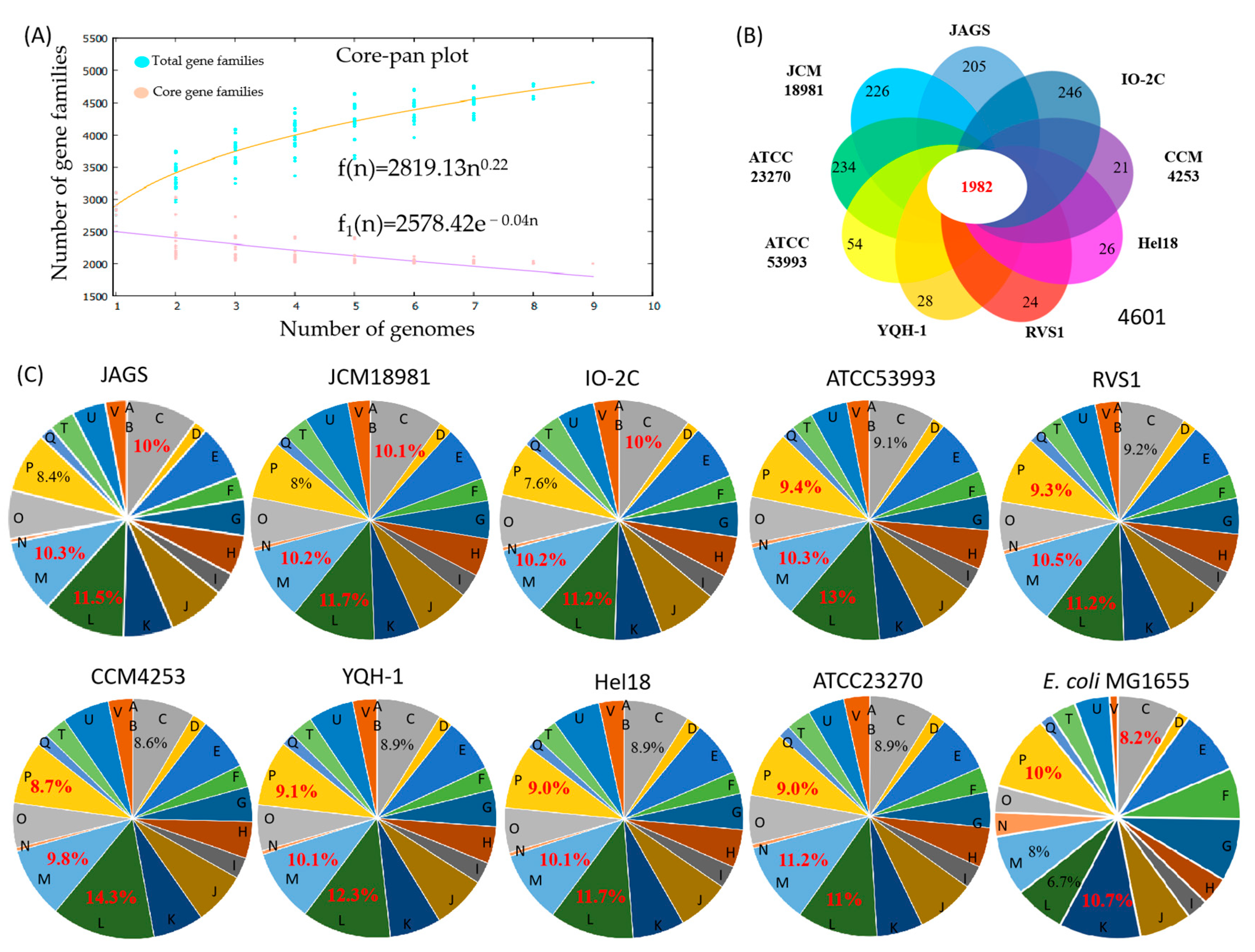
3.3. Pan-Genome and Functional Gene Analysis
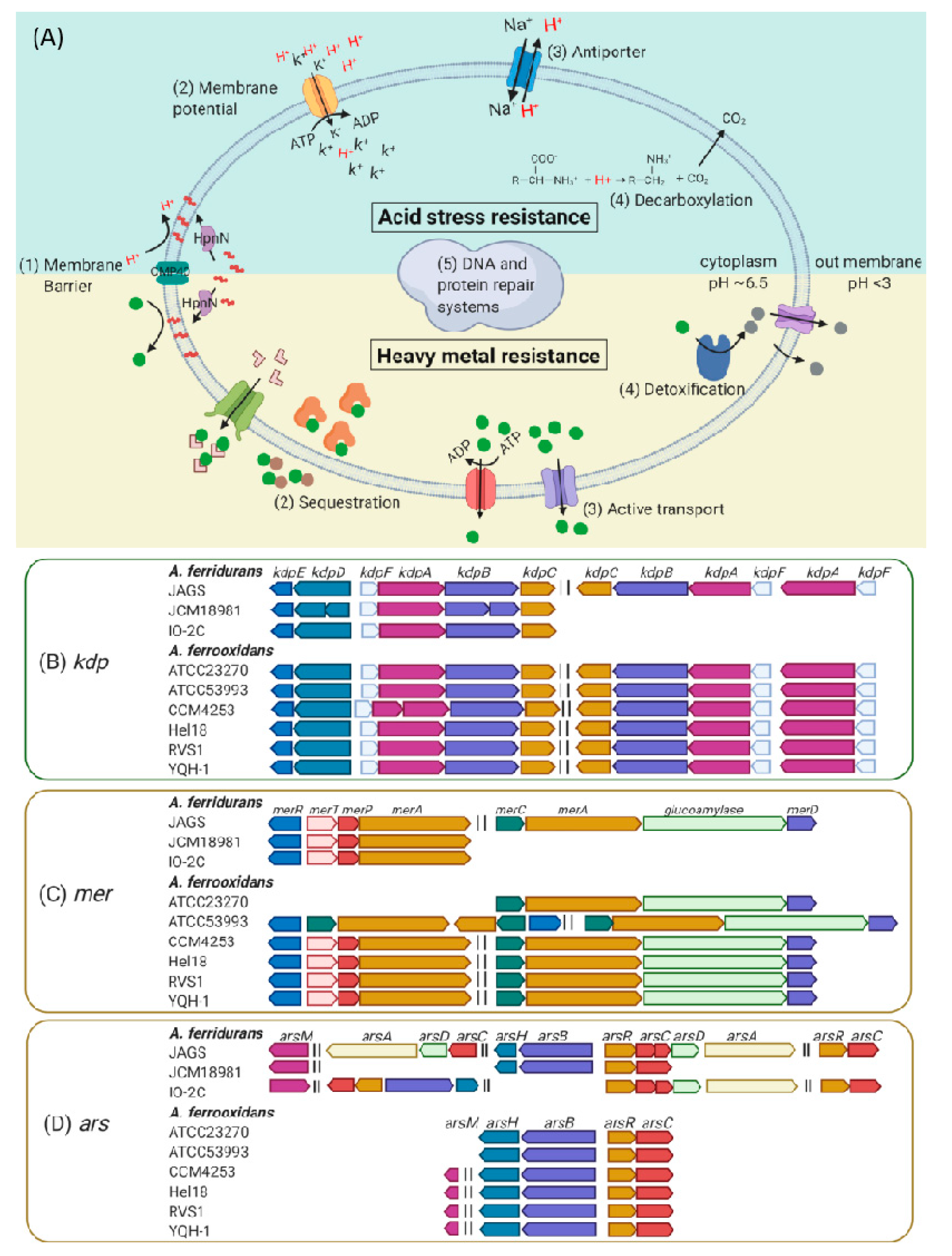
3.4. Genetic Mechanisms of Acid Stress and Metal Resistance.
3.5. Mobile Genetic Elements Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Igarashi, T.; Herrera, P.S.; Uchiyama, H.; Miyamae, H.; Iyatomi, N.; Hashimoto, K.; Tabelin, C.B. The two-step neutralization ferrite-formation process for sustainable acid mine drainage treatment: Removal of copper, zinc and arsenic, and the influence of coexisting ions on ferritization. Sci. Total Environ. 2020, 715, 136877. [Google Scholar] [CrossRef]
- Thao, N.T.; Tsuji, S.; Jeon, S.; Park, I.; Tabelin, C.B.; Ito, M.; Hiroyoshi, N. Redox potential-dependent chalcopyrite leaching in acidic ferric chloride solutions: Leaching experiments. Hydrometallurgy 2020, 194, 105299. [Google Scholar] [CrossRef]
- Baniasadi, M.; Vakilchap, F.; Bahaloo-Horeh, N.; Mousavi, S.M.; Farnaud, S. Advances in bioleaching as a sustainable method for metal recovery from e-waste: A review. J. Ind. Eng. Chem. 2019, 76, 75–90. [Google Scholar] [CrossRef]
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.; Seno, K.; Ito, M.; Hiroyoshi, N. A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 2019, 219, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Vera, M.; Schippers, A.; Sand, W. Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation—part A. Appl. Microbiol. Biotechnol. 2013, 97, 7529–7541. [Google Scholar] [CrossRef] [PubMed]
- Bosecker, K. Bioleaching: Metal solubilization by microorganisms. FEMS Microbiol. Rev. 1997, 20, 591–604. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Corpuz, R.D.; Igarashi, T.; Villacorte-Tabelin, M.; Alorro, R.D.; Yoo, K.; Raval, S.; Ito, M.; Hiroyoshi, N. Acid mine drainage formation and arsenic mobility under strongly acidic conditions: Importance of soluble phases, iron oxyhydroxides/oxides and nature of oxidation layer on pyrite. J. Hazard. Mater. 2020, 399, 122844. [Google Scholar] [CrossRef]
- Rasoulnia, P.; Barthen, R.; Lakaniemi, A.M. A critical review of bioleaching of rare earth elements: The mechanisms and effect of process parameters. Crit. Rev. Environ. Sci. Technol. 2020, 1–50. [Google Scholar] [CrossRef]
- Park, I.; Tabelin, C.B.; Magaribuchi, K.; Seno, K.; Ito, M.; Hiroyoshi, N. Suppression of the release of arsenic from arsenopyrite by carrier-microencapsulation using Ti-catechol complex. J. Hazard. Mater. 2018, 344, 322–332. [Google Scholar] [CrossRef]
- Wu, C.; Jiang, M.; Hsieh, L.; Cai, Y.; Shen, Y.; Wang, H.; Lin, Q.; Shen, C.; Hu, B.; Lou, L. Feasibility of bioleaching of heavy metals from sediment with indigenous bacteria using agricultural sulfur soil conditioners. Sci. Total Environ. 2020, 703, 134812. [Google Scholar] [CrossRef]
- Camargo, F.P.; do Prado, P.F.; Tonello, P.S.; Dos Santos, A.C.A.; Duarte, I.C.S. Bioleaching of toxic metals from sewage sludge by co-inoculation of Acidithiobacillus and the biosurfactant-producing yeast Meyerozyma guilliermondii. J. Environ. Manag. 2018, 211, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Giese, E.C.; Carpen, H.L.; Bertolino, L.C.; Schneider, C.L. Characterization and bioleaching of nickel laterite ore using Bacillus subtilis strain. Biotechnol. Prog. 2019, 35, e2860. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yan, L.; Xing, W.; Chen, P.; Zhang, Y.; Wang, W. Acidithiobacillus ferrooxidans and its potential application. Extremophiles 2018, 22, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, Z.; Meng, D.; Liu, X.; Li, X.; Zhang, M.; Tao, J.; Gu, Y.; Zhong, S.; Yin, H. Comparative genomic analysis reveals the distribution, organization, and evolution of metal resistance genes in the Genus Acidithiobacillus. Appl. Environ. Microbiol. 2019, 85, e02153-18. [Google Scholar] [CrossRef] [PubMed]
- Valdés, J.; Pedroso, I.; Quatrini, R.; Dodson, R.J.; Tettelin, H.; Blake, R.; Eisen, J.A.; Holmes, D.S. Acidithiobacillus ferrooxidans metabolism: From genome sequence to industrial applications. BMC Genom. 2008, 9, 597. [Google Scholar] [CrossRef] [PubMed]
- Campodonico, M.A.; Vaisman, D.; Castro, J.F.; Razmilic, V.; Mercado, F.; Andrews, B.A.; Feist, A.M.; Asenjo, J.A. Acidithiobacillus ferrooxidans’s comprehensive model driven analysis of the electron transfer metabolism and synthetic strain design for biomining applications. Metab. Eng. Commun. 2016, 3, 84–96. [Google Scholar] [CrossRef]
- Hedrich, S.; Johnson, D.B. Acidithiobacillus ferridurans sp. nov., an acidophilic iron-, sulfur-and hydrogen-metabolizing chemolithotrophic gammaproteobacterium. Int. J. Syst. Evol. Microbiol. 2013, 63, 4018–4025. [Google Scholar]
- Falagán, C.; Moya-Beltrán, A.; Castro, M.; Quatrini, R.; Johnson, D.B. Acidithiobacillus sulfuriphilus sp. nov.: An extremely acidophilic sulfur-oxidizing chemolithotroph isolated from a neutral pH environment. Int. J. Syst. Evol. Microbiol. 2019, 69, 2907–2913. [Google Scholar]
- Jalali, F.; Fakhari, J.; Zolfaghari, A. Response surface modeling for lab-scale column bioleaching of low-grade uranium ore using a new isolated strain of Acidithiobacillus Ferridurans. Hydrometallurgy 2019, 185, 194–203. [Google Scholar] [CrossRef]
- Jalali, F.; Fakhar, J.; Zolfaghari, A. On using a new strain of Acidithiobacillus ferridurans for bioleaching of low-grade uranium. Sep. Sci. Technol. 2020, 55, 994–1004. [Google Scholar] [CrossRef]
- Miyauchi, T.; Kouzuma, A.; Abe, T.; Watanabe, K. Complete genome sequence of Acidithiobacillus ferridurans JCM 18981. Microbiol. Resour Announc 2018, 7, e01028-18. [Google Scholar] [CrossRef]
- Garg, S. Abiotic and Biotic Leaching Characteristics of Pyrrhotite Tailings from the Sudbury, Ontario Area. Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 2017. [Google Scholar]
- Chen, J.; Liu, Y.; Diep, P.; Jo, A.; Nesbø, C.; Edwards, E.; Papangelakis, V.; Mahadevan, R. Complete genome sequence of Acidithiobacillus ferridurans JAGS, isolated from acidic mine drainage. Microbiol. Resour. Announc. 2020, 9, 9. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Liu, S.; Yu, Y.; Lin, J.; Lin, J.; Pang, X.; Zhao, J. Development of a markerless gene replacement system for Acidithiobacillus ferrooxidans and construction of a pfkB mutant. Appl. Environ. Microbiol. 2012, 78, 1826–1835. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stookey, L.L. Ferrozine—A new spectrophotometric reagent for iron. Anal. Chem. 1970, 42, 779–781. [Google Scholar] [CrossRef]
- Daoud, J.; Karamanev, D. Formation of jarosite during Fe2+ oxidation by Acidithiobacillus ferrooxidans. Miner. Eng. 2006, 19, 960–967. [Google Scholar] [CrossRef]
- Kolmert, Å.; Wikström, P.; Hallberg, K.B. A fast and simple turbidimetric method for the determination of sulfate in sulfate-reducing bacterial cultures. J. Microbiol. Methods 2000, 41, 179–184. [Google Scholar] [CrossRef]
- Wattam, A.R.; Davis, J.J.; Assaf, R.; Boisvert, S.; Brettin, T.; Bun, C.; Conrad, N.; Dietrich, E.M.; Disz, T.; Gabbard, J.L. Improvements to PATRIC, the all-bacterial bioinformatics database and analysis resource center. Nucleic Acids Res. 2017, 45, D535–D542. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Rosselló-Móra, R.; Oliver Glöckner, F.; Peplies, J. JSpeciesWS: A web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics 2016, 32, 929–931. [Google Scholar] [CrossRef]
- Darling, A.E.; Mau, B.; Perna, N.T. progressiveMauve: Multiple genome alignment with gene gain, loss and rearrangement. PloS ONE 2010, 5, e11147. [Google Scholar] [CrossRef]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef]
- Chaudhari, N.M.; Gupta, V.K.; Dutta, C. BPGA-an ultra-fast pan-genome analysis pipeline. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Huerta-Cepas, J.; Szklarczyk, D.; Heller, D.; Hernández-Plaza, A.; Forslund, S.K.; Cook, H.; Mende, D.R.; Letunic, I.; Rattei, T.; Jensen, L.J. eggNOG 5.0: A hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 2019, 47, D309–D314. [Google Scholar] [CrossRef]
- Siguier, P.; Pérochon, J.; Lestrade, L.; Mahillon, J.; Chandler, M. ISfinder: The reference centre for bacterial insertion sequences. Nucleic Acids Res. 2006, 34, D32–D36. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, C.; Laird, M.R.; Williams, K.P.; Group, S.F.U.R.C.; Lau, B.Y.; Hoad, G.; Winsor, G.L.; Brinkman, F.S. IslandViewer 4: Expanded prediction of genomic islands for larger-scale datasets. Nucleic Acids Res. 2017, 45, W30–W35. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.; Liu, F.; Marcuson, S.; Muinonen, M.; Lakshmanan, V.; Sridhar, R.; Barati, M. Canadian pyrrhotite treatment: The history, inventory and potential for tailings processing. Can. Metall. Q. 2017, 56, 410–417. [Google Scholar] [CrossRef]
- Kernan, T.; Majumdar, S.; Li, X.; Guan, J.; West, A.C.; Banta, S. Engineering the iron-oxidizing chemolithoautotroph Acidithiobacillus ferrooxidans for biochemical production. Biotechnol. Bioeng. 2016, 113, 189–197. [Google Scholar] [CrossRef]
- Beard, S.D.; Paradela, A.D.; Albar, J.P.D.; Jerez, C.A.D. Growth of Acidithiobacillus ferrooxidans ATCC 23270 in thiosulfate under oxygen-limiting conditions generates extracellular sulfur globules by means of a secreted tetrathionate hydrolase. Front. Microbiol. 2011, 2, 79. [Google Scholar] [CrossRef]
- Shen, X.; Hu, H.; Peng, H.; Wang, W.; Zhang, X. Comparative genomic analysis of four representative plant growth-promoting rhizobacteria in Pseudomonas. BMC Genom. 2013, 14, 271. [Google Scholar] [CrossRef]
- Vernikos, G.; Medini, D.; Riley, D.R.; Tettelin, H. Ten years of pan-genome analyses. Curr. Opin. Microbiol. 2015, 23, 148–154. [Google Scholar] [CrossRef]
- Hinger, I.; Ansorge, R.; Mussmann, M.; Romano, S. Phylogenomic analyses of members of the widespread marine heterotrophic genus Pseudovibrio suggest distinct evolutionary trajectories and a novel genus, Polycladidibacter gen. nov. Appl. Environ. Microbiol. 2020, 86, 86. [Google Scholar] [CrossRef]
- Mi, S.; Song, J.; Lin, J.; Che, Y.; Zheng, H.; Lin, J. Complete genome of Leptospirillum ferriphilum ML-04 provides insight into its physiology and environmental adaptation. J. Microbiol. 2011, 49, 890–901. [Google Scholar] [CrossRef]
- Mirete, S.; Morgante, V.; González-Pastor, J.E. Acidophiles: Diversity and mechanisms of adaptation to acidic environments. In Adaption of Microbial Life to Environmental Extremes; Springer: Cham, Switzerland, 2017; pp. 227–251. [Google Scholar]
- Vergara, E.; Neira, G.; González, C.; Cortez, D.; Dopson, M.; Holmes, D.S. Evolution of predicted acid resistance mechanisms in the extremely acidophilic Leptospirillum genus. Genes 2020, 11, 389. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Liang, Y.; Fan, F.; Zhang, X.; Yin, H. Metabolic diversity and adaptive mechanisms of iron-and/or sulfur-oxidizing autotrophic acidophiles in extremely acidic environments. Environ. Microbiol. Rep. 2016, 8, 738–751. [Google Scholar] [CrossRef] [PubMed]
- Guiliani, N.; Jerez, C.A. Molecular Cloning, Sequencing, and Expression ofomp-40, the gene coding for the major outer membrane protein from the acidophilic bacterium Thiobacillus ferrooxidans. Appl. Environ. Microbiol. 2000, 66, 2318–2324. [Google Scholar] [CrossRef] [PubMed]
- Belin, B.J.; Busset, N.; Giraud, E.; Molinaro, A.; Silipo, A.; Newman, D.K. Hopanoid lipids: From membranes to plant–bacteria interactions. Nat. Rev. Microbiol. 2018, 16, 304. [Google Scholar] [CrossRef] [PubMed]
- Nanatani, K.; Shijuku, T.; Takano, Y.; Zulkifli, L.; Yamazaki, T.; Tominaga, A.; Souma, S.; Onai, K.; Morishita, M.; Ishiura, M. Comparative analysis of kdp and ktr mutants reveals distinct roles of the potassium transporters in the model cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 2015, 197, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Nies, D.H. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol. Rev. 2003, 27, 313–339. [Google Scholar] [CrossRef]
- Tang, Y.-C.; Amon, A. Gene copy-number alterations: A cost-benefit analysis. Cell 2013, 152, 394–405. [Google Scholar] [CrossRef]
- Shapiro, J. Mobile Genetic Elements; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Couvin, D.; Bernheim, A.; Toffano-Nioche, C.; Touchon, M.; Michalik, J.; Néron, B.; Rocha, E.P.; Vergnaud, G.; Gautheret, D.; Pourcel, C. CRISPRCasFinder, an update of CRISRFinder, includes a portable version, enhanced performance and integrates search for Cas proteins. Nucleic Acids Res. 2018, 46, W246–W251. [Google Scholar] [CrossRef]
- Makarova, K.S.; Haft, D.H.; Barrangou, R.; Brouns, S.J.; Charpentier, E.; Horvath, P.; Moineau, S.; Mojica, F.J.; Wolf, Y.I.; Yakunin, A.F. Evolution and classification of the CRISPR–Cas systems. Nat. Rev. Microbiol. 2011, 9, 467. [Google Scholar] [CrossRef]

| Genome Name | Geographic Origin | Contigs | Genome Size | GC% | No. of CDS | BioProject Accession | Mash Distance | ANIb | ANIm |
|---|---|---|---|---|---|---|---|---|---|
| A. ferridurans JAGS | Acid mine drainage, Canada | 1 | 2,933,811 | 58.6 | 3001 | PRJNA573091 | - | - | - |
| A. ferridurans JCM18981 | Uranium mine drainage water, Japan | 1 | 2,921,399 | 58.4 | 3026 | PRJDB7175 | 0.0090 | 99.13 | 99.66 |
| A. ferrooxidans IO-2C | Acid seep soil, USA | 23 | 2,716,894 | 58.7 | 2634 | PRJNA432283 | 0.0136 | 98.69 | 99.23 |
| A. ferrooxidans ATCC53993 | - | 1 | 2,885,038 | 58.9 | 2826 | PRJNA16689 | 0.0446 | 94.98 | 95.51 |
| A. ferrooxidans RVS1 | Andacollo gold mining area, Argentina | 49 | 2,826,311 | 58.8 | 2705 | PRJNA499028 | 0.0463 | 94.68 | 95.33 |
| A. ferrooxidans CCM4253 | Mine waters, Czech Republic | 15 | 3,196,562 | 58.6 | 3059 | PRJNA475418 | 0.0480 | 94.70 | 95.32 |
| A. ferrooxidans YQH-1 | Wudalianchi volcano water, China | 96 | 3,111,222 | 58.6 | 3089 | PRJNA294114 | 0.0482 | 94.71 | 95.34 |
| A. ferrooxidans Hel18 | Flue dust | 123 | 3,109,160 | 58.6 | 3179 | PRJNA308169 | 0.0484 | 94.69 | 95.34 |
| A. ferrooxidans ATCC23270 | bituminous coal mine effluent | 1 | 2,982,397 | 58.8 | 3147 | PRJNA53 | 0.0493 | 94.76 | 95.37 |
| A. The Putative Insertion Sequences | |||||||
| IS Family | JAGS | JCM18981 | IO-2C | ATCC53993 | CCM4253 | ATCC23270 | RVS1 |
| IS110 | 3 | 3 | 1 | 4 | 4 | 3 | 1 |
| IS1182 | 0 | 1 | 0 | 1 | 0 | 0 | - |
| IS1595 | 15 | 1 | 3 | 6 | 0 | 1 | - |
| IS1634 | 2 | 6 | 4 | 2 | 1 | 6 | - |
| IS200/IS605 | 3 | 2 | 0 | 1 | 0 | 2 | - |
| IS21 | 7 | 17 | 3 | 9 | 6 | 17 | 1 |
| IS256 | 7 | 7 | 0 | 2 | 2 | 7 | - |
| IS3 | 21 | 23 | 14 | 21 | 22 | 23 | - |
| IS481 | 1 | 0 | 0 | 1 | 1 | 0 | - |
| IS5 | 1 | 1 | 2 | 12 | 2 | 1 | - |
| IS66 | 2 | 2 | 2 | 2 | 2 | 2 | - |
| IS7 | 1 | 1 | 1 | 1 | 1 | 1 | - |
| ISL3 | 8 | 8 | 7 | 7 | 8 | 8 | - |
| Tn | 7 | 6 | 1 | 5 | 3 | 6 | - |
| In total | 78 | 78 | 38 | 74 | 52 | 77 | - |
| B. The Predicted Genomic Islands | |||||||
| GI No. | 22 | 22 | 20 | 21 | 26 | 21 | 19 |
| Size range (Kb) | 4–42 | 4–46 | 4–28 | 4–158 | 4–63 | 4–25 | 4–35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Liu, Y.; Diep, P.; Mahadevan, R. Genomic Analysis of a Newly Isolated Acidithiobacillus ferridurans JAGS Strain Reveals Its Adaptation to Acid Mine Drainage. Minerals 2021, 11, 74. https://doi.org/10.3390/min11010074
Chen J, Liu Y, Diep P, Mahadevan R. Genomic Analysis of a Newly Isolated Acidithiobacillus ferridurans JAGS Strain Reveals Its Adaptation to Acid Mine Drainage. Minerals. 2021; 11(1):74. https://doi.org/10.3390/min11010074
Chicago/Turabian StyleChen, Jinjin, Yilan Liu, Patrick Diep, and Radhakrishnan Mahadevan. 2021. "Genomic Analysis of a Newly Isolated Acidithiobacillus ferridurans JAGS Strain Reveals Its Adaptation to Acid Mine Drainage" Minerals 11, no. 1: 74. https://doi.org/10.3390/min11010074
APA StyleChen, J., Liu, Y., Diep, P., & Mahadevan, R. (2021). Genomic Analysis of a Newly Isolated Acidithiobacillus ferridurans JAGS Strain Reveals Its Adaptation to Acid Mine Drainage. Minerals, 11(1), 74. https://doi.org/10.3390/min11010074






