Preferential Elimination of Ba2+ through Irreversible Biogenic Manganese Oxide Sequestration
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Exogenous Mn2+ Oxidation by Newly Formed BMOs
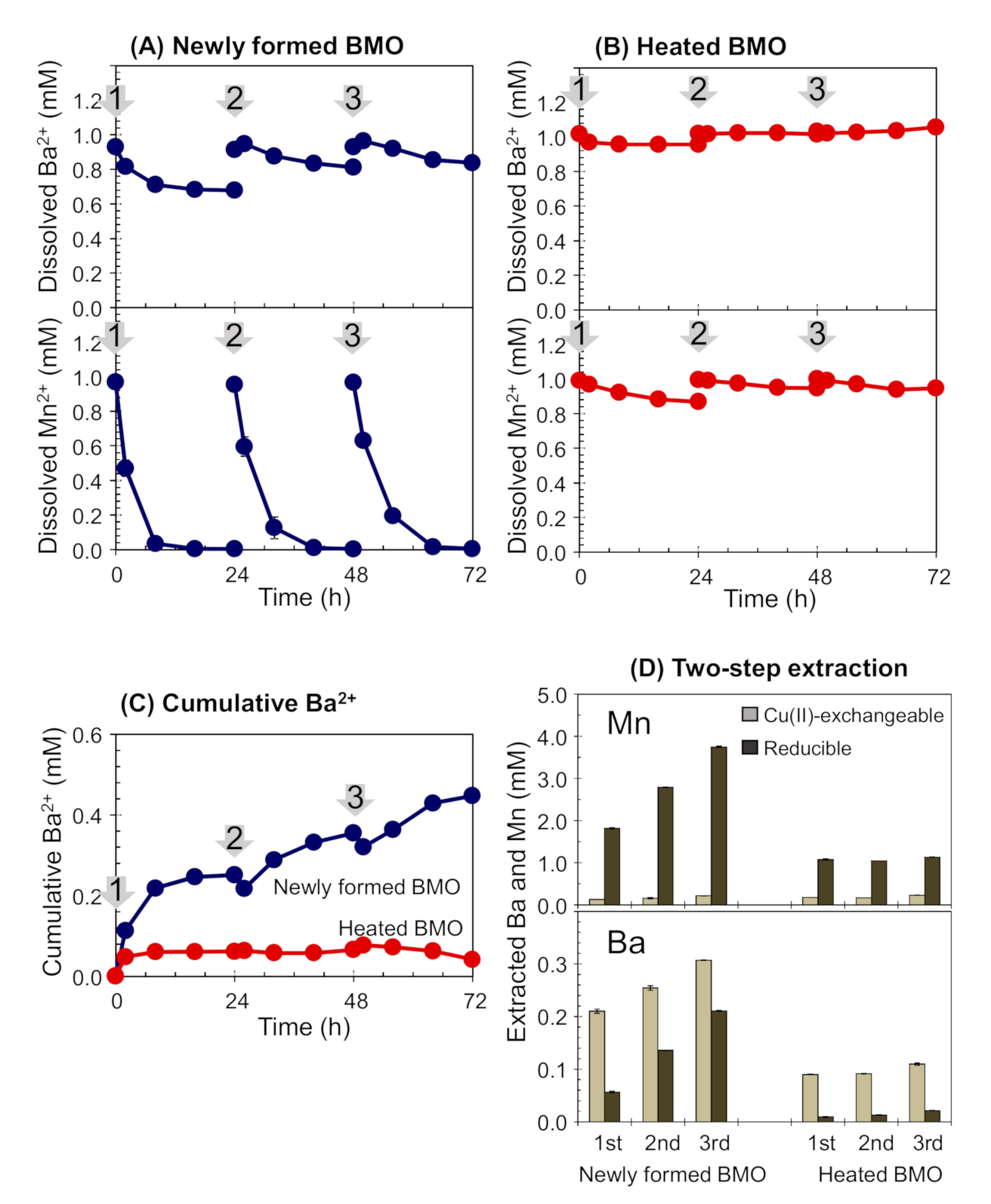
3.2. Ba2+ Sequestration by Newly Formed or Heated BMOs with Exogenous Mn2+
3.3. BMO Alteration from Turbostratic to Tightly Stacked Birnessite
3.4. Sr2+, Ca2+, and Mg2+ Sequestration by Newly Formed or Heated BMOs Involving Exogenous Mn2+
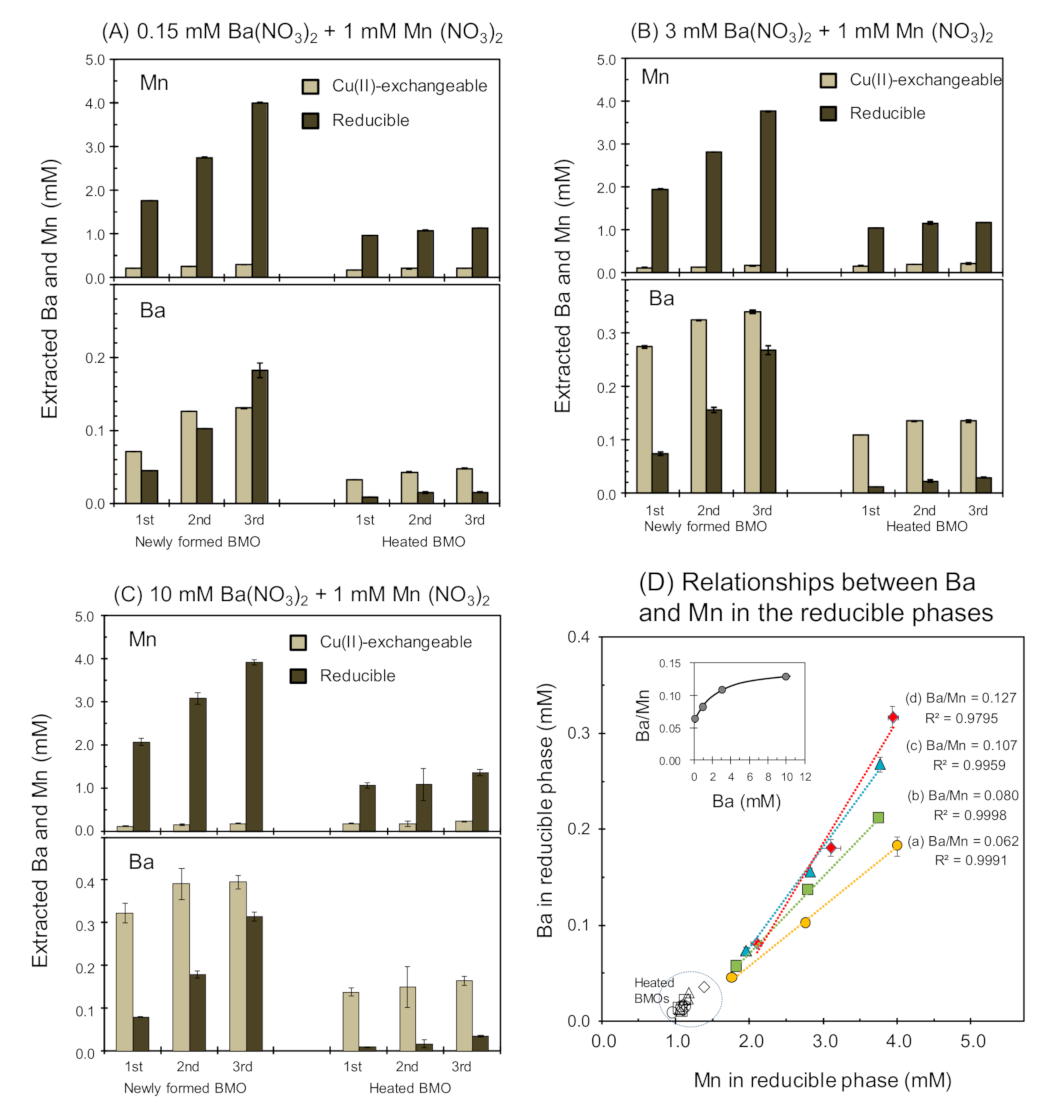
3.5. Active Mn2+ Oxidation Sequestration Selectivity Enhancement for Ba2+
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suwa, R.; Jayachandran, K.; Nguyen, N.T.; Boulenouar, A.; Fujita, K.; Saneoka, H. Barium toxicity effects in soybean plants. Arch. Environ. Contam. Toxicol. 2008, 55, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Golding, L.A.; McKnight, K.; Binet, M.; Adams, M.; Apte, S.C. Toxicity of dissolved and precipitated forms of barium to a freshwater alga (Chlorella sp. 12) and water flea (Ceriodaphnia dubia). Environ. Toxicol. Chem. 2018, 37, 1632–1642. [Google Scholar] [CrossRef]
- Lu, Q.; Xu, X.; Xu, Z.; Liang, L.; Shang, L.; Xiao, D.; Zhang, S.; Jiang, Y.; Qiu, G. Barium concentrations and speciation in surface waters collected from an active barium mining area in Guizhou Province, southwestern China. Environ. Sci. Pollut. Res. Int. 2018, 25, 7608–7617. [Google Scholar] [CrossRef] [PubMed]
- Rebello, C.A.; Couperthwaite, S.J.; Millar, G.J.; Dawes, L.A. Coal seam water quality and the impact upon management strategies. J. Petrol. Sci. Eng. 2017, 150, 323–333. [Google Scholar] [CrossRef] [Green Version]
- Warner, N.R.; Christie, C.A.; Jackson, R.B.; Vengosh, A. Impacts of shale gas wastewater disposal on water quality in western Pennsylvania. Environ. Sci. Technol. 2013, 47, 11849–11857. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, D.; Tsang, D.C.W.; Wang, L.; Ok, Y.S.; Feng, Y. A critical review of risks, characteristics, and treatment strategies for potentially toxic elements in wastewater from shale gas extraction. Environ. Int. 2019, 125, 452–469. [Google Scholar] [CrossRef] [PubMed]
- Post, J.E. Manganese oxide minerals: Crystal structures and economic and environmental significance. Proc. Natl. Acad. Sci. USA 1999, 96, 3447–3454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manceau, A.; Kersten, M.; Marcus, M.A.; Geoffroy, N.; Granina, L. Ba and Ni speciation in a nodule of binary Mn oxide phase composition from Lake Baikal. Geochim. Cosmochim. Acta 2007, 71, 1967–1981. [Google Scholar] [CrossRef]
- Bohu, T.; Akob, D.M.; Abratis, M.; Lazar, C.S.; Küsel, K. Biological low-pH Mn(II) oxidation in a manganese deposit influenced by metal-rich groundwater. Appl. Environ. Microbiol. 2016, 82, 3009–3021. [Google Scholar] [CrossRef] [Green Version]
- Tani, Y.; Miyata, N.; Iwahori, K.; Soma, M.; Tokuda, S.; Seyama, H.; Theng, B.K.G. Biogeochemistry of manganese oxide coatings on pebble surfaces in the Kikukawa River System, Shizuoka, Japan. Appl. Geochem. 2003, 18, 1541–1554. [Google Scholar] [CrossRef]
- Manceau, A.; Lanson, M.; Geoffroy, N. Natural speciation of Ni, Zn, Ba, and as in ferromanganese coatings on quartz using X-ray fluorescence, absorption, and diffraction. Geochim. Cosmochim. Acta 2007, 71, 95–128. [Google Scholar] [CrossRef]
- Adams, J.P.; Kirst, R.; Kearns, L.E.; Krekeler, M.P.S. Mn-oxides and sequestration of heavy metals in a suburban catchment basin of the Chesapeake Bay watershed. Environ. Geol. 2009, 58, 1269–1280. [Google Scholar] [CrossRef]
- Sasaki, K.; Kaseyama, T.; Hirajima, T. Selective sorption of Co(II) over Ni(II) using biogenic manganese oxides. Mater. Trans. 2009, 50, 2643–2648. [Google Scholar] [CrossRef] [Green Version]
- Mayanna, S.; Peacock, C.L.; Schäffner, F.; Grawunder, A.; Merten, D.; Kothe, E.; Büchel, G. Biogenic precipitation of manganese oxides and enrichment of heavy metals at acidic soil pH. Chem. Geol. 2015, 402, 6–17. [Google Scholar] [CrossRef]
- Yuan, L.; Sun, L.; Fortin, D.; Wang, Y.; Yin, X. Microscale characterization and trace element distribution in bacteriogenic ferromanganese coatings on sand grains from an intertidal zone of the East China Sea. PLoS ONE 2015, 10, e0119080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tebo, B.M.; Bargar, J.R.; Clement, B.G.; Dick, G.J.; Murray, K.J.; Parker, D.; Verity, R.; Webb, S.M. Biogenic manganese oxides: Properties and mechanisms of formation. Annu. Rev. Earth Planet. Sci. 2004, 32, 287–328. [Google Scholar] [CrossRef] [Green Version]
- Villalobos, M.; Bargar, J.; Sposito, G. Trace metal retention on biogenic manganese oxide nanoparticles. Elements 2005, 1, 223–226. [Google Scholar] [CrossRef]
- Furuta, S.; Ikegaya, H.; Hashimoto, H.; Ichise, S.; Kohno, T.; Miyata, N.; Takada, J. Formation of filamentous Mn oxide particles by the alphaproteobacterium Bosea sp. strain BIWAKO-01. Geomicrobiol. J. 2015, 32, 666–676. [Google Scholar] [CrossRef]
- Nelson, Y.M.; Lion, L.W.; Ghiorse, W.C.; Shuler, M.L. Production of biogenic Mn oxides by Leptothrix discophora SS-1 in a chemically defined growth medium and evaluation of their Pb adsorption characteristics. Appl. Environ. Microbiol. 1999, 65, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Nelson, Y.M.; Lion, L.W.; Shuler, M.L.; Ghiorse, W.C. Effect of oxide formation mechanisms on lead adsorption by biogenic manganese (hydr)oxides, iron (hydr)oxides, and their mixtures. Environ. Sci. Technol. 2002, 36, 421–425. [Google Scholar] [CrossRef]
- Villalobos, M.; Bargar, J.; Sposito, G. Mechanisms of Pb(II) sorption on a biogenic manganese oxide. Environ. Sci. Technol. 2005, 39, 569–576. [Google Scholar] [CrossRef]
- Toner, B.; Manceau, A.; Webb, S.M.; Sposito, G. Zinc sorption to biogenic hexagonal- birnessite particles within a hydrated bacterial biofilm. Geochim. Cosmochim. Acta 2006, 70, 27–43. [Google Scholar] [CrossRef]
- Miyata, N.; Tani, Y.; Sakata, M.; Iwahori, K. Microbial manganese oxide formation and interaction with toxic metal ions. J. Biosci. Bioeng. 2007, 104, 1–8. [Google Scholar] [CrossRef]
- Meng, Y.T.; Zheng, Y.M.; Zhang, L.M.; He, J.Z. Biogenic Mn oxides for effective adsorption of Cd from aquatic environment. Environ. Pollut. 2009, 157, 2577–2583. [Google Scholar] [CrossRef]
- Sasaki, K.; Uejima, Y.; Sakamoto, A.; Yu, Q.; Ishibashi, J.; Okibe, N.; Hirajima, T. Geochemical and microbiological analysis of Sambe Hot Springs, Shimane Prefecture, Japan. Res. Geol. 2013, 63, 155–165. [Google Scholar] [CrossRef]
- Wang, W.M.; Shao, Z.Z.; Liu, Y.J.; Wang, G.J. Removal of multi-heavy metals using biogenic manganese oxides generated by a deep-sea sedimentary bacterium Brachybacterium sp. strain Mn32. Microbiology 2009, 155, 1989–1996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Q.; Sasaki, K.; Tanaka, K.; Ohnuki, T.; Hirajima, T. Zinc sorption during bio-oxidation and precipitation of manganese modifies the layer stacking of biogenic birnessite. Geomicrobiol. J. 2013, 30, 829–839. [Google Scholar] [CrossRef]
- Grangeon, S.; Bataillard, P.; Coussy, S. The nature of manganese oxides in soils and their role as scavengers of trace elements: Implication for soil remediation. In Environmental Soil Remediation and Rehabilitation; Van Hullebusch, E.D., Huguenot, D., Pechaud, Y., Simonnot, M.-O., Colombano, S., Eds.; Springer Nature: Cham, Switzerland, 2020; Chapter 7; pp. 399–429. [Google Scholar] [CrossRef]
- Chang, J.; Tani, Y.; Naitou, H.; Miyata, N.; Seyama, H. Fungal Mn oxides supporting Mn(II) oxidase activity as effective Mn(II) sequestering materials. Environ. Technol. 2013, 34, 2781–2787. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.; Tani, Y.; Chang, J.; Miyata, N.; Naitou, H.; Seyama, H. As(III) oxidation kinetics of biogenic manganese oxides formed by Acremonium strictum strain KR21-2. Chem. Geol. 2013, 347, 227–232. [Google Scholar] [CrossRef]
- Tojo, F.; Kitayama, A.; Miyata, N.; Okano, K.; Fukushima, J.; Suzuki, R.; Tani, Y. Molecular cloning and heterologous expression of manganese(II)-oxidizing enzyme from Acremonium strictum strain KR21-2. Catalysts 2020, 10, 686. [Google Scholar] [CrossRef]
- Chang, J.; Tani, Y.; Naitou, H.; Miyata, N.; Tojo, F.; Seyama, H. Zn(II) sequestration by fungal biogenic manganese oxide through enzymatic and abiotic processes. Chem. Geol. 2014, 383, 155–163. [Google Scholar] [CrossRef]
- Chang, J.; Tani, Y.; Naitou, H.; Miyata, N.; Seyama, H.; Tanaka, K. Cobalt(II) sequestration on fungal biogenic manganese oxide enhanced by manganese(II) oxidase activity. Appl. Geochem. 2013, 37, 170–178. [Google Scholar] [CrossRef]
- Chang, J.; Tani, Y.; Naitou, H.; Miyata, N.; Seyama, H.; Tanaka, K. Sequestration of Cd(II) and Ni(II) ions on fungal manganese oxides associated with Mn(II) oxidase activity. Appl. Geochem. 2014, 47, 198–208. [Google Scholar] [CrossRef]
- Inthorn, D.; Tani, Y.; Chang, J.; Naitou, H.; Miyata, N. Magnetically modified fungal Mn oxides with high sequestration efficiency for simultaneously removing multiple heavy metal ions from wastewater. J. Environ. Chem. Eng. 2014, 2, 1635–1641. [Google Scholar] [CrossRef]
- Zheng, H.; Tani, Y.; Naitou, H.; Miyata, N.; Tojo, F.; Seyama, H. Sequestration of La3+ by fungal manganese oxides and the effect of Mn(II) oxidase activity. J. Environ. Chem. Eng. 2017, 5, 735–743. [Google Scholar] [CrossRef]
- Zheng, H.; Tani, Y.; Naitou, H.; Miyata, N.; Tojo, F. Oxidative Ce3+ sequestration by fungal manganese oxides with an associated Mn(II) oxidase activity. Appl. Geochem. 2016, 71, 110–122. [Google Scholar] [CrossRef]
- Suzuki, R.; Tani, Y.; Naitou, H.; Miyata, N.; Tanaka, K. Sequestration and oxidation of Cr(III) by fungal Mn oxides with Mn(II) oxidizing activity. Catalysts 2020, 10, 44. [Google Scholar] [CrossRef] [Green Version]
- Miyata, N.; Tani, Y.; Iwahori, K.; Soma, M. Enzymatic formation of manganese oxides by an Acremonium-like hyphomycete fungus, strain KR21-2. FEMS Microbiol. Ecol. 2004, 47, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Miyata, N.; Tani, Y.; Maruo, K.; Tsuno, H.; Sakata, M.; Iwahori, K. Manganese(IV) oxide production by Acremonium sp. strain KR21-2 and extracellular Mn(II) oxidase activity. Appl. Environ. Microbiol. 2006, 72, 6467–6473. [Google Scholar] [CrossRef] [Green Version]
- Tani, Y.; Miyata, N.; Ohashi, M.; Ohnuki, T.; Seyama, H.; Iwahori, K.; Soma, M. Interaction of inorganic arsenic with biogenic manganese oxide produced by a Mn-oxidizing fungus, strain KR21-2. Environ. Sci. Technol. 2004, 38, 6618–6624. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry. Chemical Equilibria and Rates in Natural Waters, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1995; pp. 976–983. [Google Scholar]
- Rosson, R.A.; Nealson, K.H. Manganese binding and oxidation by spores of a marine bacillus. J. Bacteriol. 1982, 151, 1027–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vrind, J.P.; De Vrind-de Jong, E.W.; De Voogt, J.W.; Westbroek, P.; Boogerd, F.C.; Rosson, R.A. Manganese oxidation by spores and spore coats of a marine Bacillus species. Appl. Environ. Microbiol. 1986, 52, 1096–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, J.; Tani, Y.; Miyata, N.; Seyama, H.; Mitsunobu, S.; Naitou, H. Concurrent sorption of As(V) and Mn(II) during biogenic manganese oxide formation. Chem. Geol. 2012, 306–307, 123–128. [Google Scholar] [CrossRef]
- Reimann, C.; De Caritat, P. Chemical Elements in the Environment: Factsheets for the Geochemist and Environmental Scientist; Springer: Berlin/Heidenburg, Germany, 1998; p. 397. [Google Scholar]
- Grangeon, S.; Lanson, B.; Miyata, N.; Tani, Y.; Manceau, A. Structure of nanocrystalline phyllomanganates produced by freshwater fungi. Am. Mineral. 2010, 95, 1608–1616. [Google Scholar] [CrossRef] [Green Version]
- Webb, S.M.; Fuller, C.C.; Tebo, B.M.; Bargar, J.R. Determination of uranyl incorporation into biogenic manganese oxides using X-ray absorption spectroscopy and scattering. Environ. Sci. Technol. 2006, 40, 771–777. [Google Scholar] [CrossRef]
- Saratovsky, I.; Gurr, S.J.; Hayward, M.A. The structure of manganese oxide formed by the fungus Acremonium sp. strain KR21-2. Geochim. Cosmochim. Acta 2009, 73, 3291–3300. [Google Scholar] [CrossRef]
- Xhaxhiu, K. Synthetic birnessites and buserites as heavy metal cation traps and environmental remedies. J. Metal. Nanotechnol. 2015, 3, 23–32. [Google Scholar]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tani, Y.; Kakinuma, S.; Chang, J.; Tanaka, K.; Miyata, N. Preferential Elimination of Ba2+ through Irreversible Biogenic Manganese Oxide Sequestration. Minerals 2021, 11, 53. https://doi.org/10.3390/min11010053
Tani Y, Kakinuma S, Chang J, Tanaka K, Miyata N. Preferential Elimination of Ba2+ through Irreversible Biogenic Manganese Oxide Sequestration. Minerals. 2021; 11(1):53. https://doi.org/10.3390/min11010053
Chicago/Turabian StyleTani, Yukinori, Satomi Kakinuma, Jianing Chang, Kazuya Tanaka, and Naoyuki Miyata. 2021. "Preferential Elimination of Ba2+ through Irreversible Biogenic Manganese Oxide Sequestration" Minerals 11, no. 1: 53. https://doi.org/10.3390/min11010053
APA StyleTani, Y., Kakinuma, S., Chang, J., Tanaka, K., & Miyata, N. (2021). Preferential Elimination of Ba2+ through Irreversible Biogenic Manganese Oxide Sequestration. Minerals, 11(1), 53. https://doi.org/10.3390/min11010053




