Abstract
Rhizofiltration experiments were conducted using uranium-contaminated groundwater and lettuce (Lactuca sativa), Chinese cabbage (Brassica campestris L.), radish (Raphanus sativus L.), and buttercup (Oenanthe javanica), which are commonly grown and consumed in South Korea. The results of the rhizofiltration experiments with artificial solutions with different initial uranium concentrations (18, 32, 84, 116, 173, and 263 μg/L) show that the uranium accumulation and bioconcentration factor (BCF) of plant roots increase with increasing uranium concentration in the groundwater. Among the four plants, the uranium concentration in the roots of Raphanus sativus L. is 1215.8 μg/g dry weight, with a maximum BCF value of 2692.7. The BCF value of the artificial solutions with various pH values (pH 3, 5, 7, and 9) is the highest under acidic conditions (pH 3) for all four plants. The uranium BCF values based on different hydroponic conditions range from 170.5 to 11580.3 and the results are comparable with those of other studies using similar methods; the highest BCF value was determined for Brassica campestris L. at pH 3. The BCF values of Raphanus sativus L. after the rhizofiltration experiments with genuine groundwater contaminated with uranium are the highest among the four species; that is, 1684.7 and 1700.1 in Oesam-dong and Bugokdong groundwater samples with uranium concentrations of 83 and 173 μg/L, respectively. The results of the scanning electron microscope/electron dispersive X-ray spectroscope analyses show that uranium in contaminated groundwater is adsorbed as a solid phase on the root surface. These results demonstrate that Raphanus sativus L. has a high tolerance to high concentrations of uranium and low pH conditions and a remarkable potential for uranium accumulation.
1. Introduction
Phytoremediation is an economical and eco-friendly remediation method that can simultaneously restore and protect ecosystems [1,2,3,4]. Among the phytoremediation techniques, rhizofiltration is a method based on which heavy metals or radionuclides are adsorbed from the groundwater by the roots of plants [5,6]. The mechanisms by contaminant accumulation in plant cell are as follows: extracellular precipitation, cell wall precipitation and adsorption, and adsorption within cells by cytoplasmic compartments or vacuoles. Additionally, most cell walls or epidermal layers of plants are negatively charged [7] and can adsorb cations from groundwater for effective remediation [8].
The uranium speciation strongly depends on the pH of groundwater and is known to be sensitive to the carbon dioxide partial pressure, uranium concentrations, and ligands that form complex compounds [9,10]. Generally, the uranyl cation, which is absorbed effectively to the plant cell, is acting actively in under pH 5. In addition, hydroxide complexes were presented in pH 6 and carbonate complexes above pH 7.5 [11]. Among plants used for phytoremediation, sunflowers, legumes, and mustards have been applied to the removal of radionuclides such as cesium, strontium, and uranium [12,13,14,15]. The roots of these plants strongly adsorb uranium, which is mainly adsorbed to the surface of the root in combination with the carboxyl group (COO−) of the cell wall [16,17].
Most studies related to phytoremediation have been conducted on uranium in soil [11,18,19,20,21,22], whereas only a few phytoremediation studies focused on uranium in ground- or surface water. Eapen et al. (2003) [23] performed hydroponic experiments with different initial concentrations (10–5000 μM) using hairy root cultures of Brassica juncea and Chenopodium amaranticolor to assess the feasibility for rhizofiltration in uranium-contaminated water. Soudek et al. (2011) [20] also conducted hydroponic experiments with artificially contaminated uranium solutions (0.1 and 0.5 μM) using 20 species of crop plants. To study the accumulation of uranium in artificially contaminated water under various pH conditions, Helianthus annuus L. [14,24,25], Pisum sativum [11], Hydrilla verticillate [26], Phaseolus vulgaris L. var. vulgaris [27] have been used in the past. These studies suggest that the accumulation of uranium in the roots of plants is the highest when the contaminated water is acidic. With respect to the accumulation of uranium in plants in genuine groundwater and surface water, Lee and Yang (2010) [24] and Yang et al. (2015) [27] demonstrated the uranium removal by rhizofiltration using Helianthus annuus L. and Phaseolus vulgaris L. var. vulgaris in genuine groundwater with high uranium concentrations (270 and 240 μg/L). As mentioned above, various types of plants have been used to investigate the accumulation of uranium in plants, but there is little information on uranium accumulation in commonly consumed crops and vegetables, which are generally grown using groundwater irrigation.
In this study, we investigated the uranium accumulation capacity in lettuce (Lactuca sativa), Chinese cabbage (Brassica campestris L.), radish (Raphanus sativus L.), and buttercup (Oenanthe javanica), which are species that have not been used for uranium accumulation studies in the past and are commonly grown and consumed in South Korea. Rhizofiltration experiments were performed using artificial uranium solutions with different concentrations and pH values to determine the amount of uranium accumulated in each plant. We also conducted rhizofiltration experiments using genuine groundwater contaminated with uranium. The amount of uranium accumulated in the rhizofiltration experiments was quantified by using the uranium bioconcentration factor (BCF) to verify the relative accumulation capacity of these plants by comparing it with previous research results.
2. Materials and Methods
2.1. Plant Cultivation
Four species, lettuce (Lactuca sativa L.), Chinese cabbage (Brassica campestris L.), radish sprouts (Raphanus sativus L.), and buttercup (Oenanthe javanica), were used in this study to investigate their uranium accumulation capability. Seeds of plants, except for buttercups, were germinated and grown for three weeks in hydroponic tanks (glass; 12 cm × 12 cm × 8 cm) with a support net for plant roots in a phytotron. The stems of buttercups were cut into 10 cm pieces, immersed in hydroponic glass tanks, and roots were grown for three weeks. The following plant growth conditions, which are optimal for plant germination and growth, were maintained: temperature of 25 °C, 70% humidity, 4000 Lux (16 h/day), and 600 mg/L of CO2.
2.2. Uranium Rhizofiltration Experiments
In this study, all rhizofiltration experiments were conducted for a total of 72 h after sufficiently soaking the roots of the plants in a hydroponic tank (12 cm × 12 cm × 8 cm) filled with 350 mL contaminated water (Figure S1a). To ensure the reliability of the experimental results, the experiments were repeated three times under the same conditions for each plant. Control experiments without uranium were also performed for comparison with uranium accumulation in each plant root and shoot. After the experiments, the plants were separated into roots and leaves (Figure S1b) and pretreated by wet decomposition to analyze the concentration of uranium adsorbed on each part of the plant. Detailed descriptions of the wet decomposition are provided below (Section 2.3). Finally, the BCFs of uranium were calculated as the ratios of the mean uranium concentrations in the plant to the mean concentration in the groundwater. The mean differences of uranium concentrations among plant species were compared by one-way analysis of variance (ANOVA) test using SAS version 9.4 (SAS Inc., Cary, NC, USA).
2.2.1. Rhizofiltration Experiments with Different Initial Uranium Concentrations and pH Values
Batch rhizofiltration experiments with artificially contaminated solutions with different uranium concentrations (15, 30, 80, 120, 180, and 450 μg/L) were conducted to investigate the uranium accumulation capacities of the four species. For the batch experiments, ultrapure water purified with a Mili-Q water purification system (Millipore, Molsheim, France) was titrated with a uranium standard solution (1000 g/mL in 1% (v/v) HNO3; Sigma–Aldrich (Saint Louis, MO, USA)).
In addition, batch rhizofiltration experiments were conducted under different pH conditions to investigate the changes in uranium accumulation of the plant depending on the pH of the water. The pH of the contaminated water was adjusted by adding HCl and NaOH. The uranium concentrations adsorbed on the plants were compared after hydroponic cultivation under four pH conditions (pH 3, 5, 7, and 9). The initial uranium concentration of the contaminated water was set to 116 μg/L.
2.2.2. Rhizofiltration Experiment with Genuine Groundwater
Genuine groundwater with uranium concentrations of 86 and 173 μg/L was sampled from two different sites in South Korea: Oesam-dong, Deajeon, and Bugok-dong, Busan. To determine the chemical properties of the groundwater used in this study, the pH, oxidation-reduction potential (ORP), dissolved oxygen (DO), electric conductivity (EC), and heavy metal concentrations were measured. Table S1 shows the chemical characteristics of the two types of genuine groundwater used for the rhizofiltration experiments.
Rhizofiltration experiments using the four plant species were carried out with two groundwater samples. The phytotron conditions and procedures of the rhizofiltration experiments were the same as those used in the previous experiments.
2.3. Analysis of Uranium Accumulation in Plants
To analyze the uranium accumulation in the plants after 72 h of rhizofiltration experiments, the plants were separated into leaves (including stems) and roots, soaked in 0.1% HClO4 solution for 3 min and washed with distilled water. The uranium concentration of the plant was determined by wet decomposition. In the wet decomposition process after the experiment, 3 g plant samples were separated into roots (1 g) and shoots (2 g) and placed in a 300 mL beaker to which 10 mL nitric acid (HNO3) was added for 10 h [28]. First, acid decomposition was carried out at 180 °C by adding 1 mL of hydrogen peroxide (H2O2) solution to the samples until all the brown smoke was disappeared (organic decomposition). Subsequently, 20 mL of the ternary solution (HNO3:H2SO4:HClO4 = 10:1:4), the final decomposition solution, was added and slowly heated at 160–200 °C until the solution turned pale yellow or colorless. After adjusting the volume of the final digestion solution to 10 mL using 0.5% nitric acid, the uranium concentration of the filtrate (filter paper: Whatman No. 40) was analyzed using inductively coupled plasma mass spectrometry (Elan 6100, FIMS 400, Perkin Elmer, Waltham, MA, USA). For the control experiment, a blank reagent without plant roots and shoots was used for the decomposition and the results were compared with those of the rhizofiltration experiments.
In this study, the bioconcentration factor (BCF) is defined as the ratio of the uranium concentration in the plant to that in the solution. The BCF indicates an index of the capability of the plant to accumulate uranium with respect to its concentration in the solution [29]. The capability of the plant to accumulate uranium may be evaluated using the BCF value when compared between different accumulation periods and plant species [30]. The uranium BCF for each plant species can be expressed in dimensionless form as
2.4. Scanning Electron Microscopy and Energy-Dispersive X-ray Spectrometry
Scanning electron microscope (SEM) and energy dispersive X-ray spectrometer (EDS) were performed to determine the uranium adsorption pattern on the plant root surface. After hydroponically cultivating the plants in 350 mL of artificially contaminated water (pH 5) with an initial uranium concentration of 100 mg/L for 24 h, the root surfaces of the plants were coated with gold and SEM (HITACHI S-2700, Tokyo, Japan) and EDS (KEVEX Ltd. SIGMA, Newark, DF, USA) analysis were carried out using a voltage of 20 kV and 5000-fold magnification. In the SEM image of the root surface, three spots with solid phase crystals were randomly selected and subjected to EDS analysis to determine the presence of uranium on the plant root surface.
3. Results and Discussion
3.1. Rhizofiltration Experiments with Different Initial Uranium Concentrations and pH Values
The results for the uranium accumulation in the roots and shoots of four plant species for six different uranium solutions (15, 30, 80, 120, 180, and 450 μg/L) are shown in Figure 1. The uranium contents of the roots and shoots of all four plant species increase with increasing uranium concentration in the solution. At all solution concentrations, most of the uranium is accumulated in the roots, with an average of 98%, despite the small volume and weight of roots compared with those of the shoots. At an initial uranium concentration of 15 μg/L, the highest uranium concentration of 32.3 μg/g dry weight (DW) was observed in the roots of Raphanus sativus L. (Figure 1a). The uranium concentrations in the roots of Brassica campestris L. and Lactuca sativa L. are 26.8 and 18.4 μg/g DW and Oenanthe javanica contains the lowest amount of uranium (17.9 μg/g DW). At the maximum initial uranium concentration (450 μg/L), the uranium accumulation in the roots of Brassica campestris L. and Raphanus sativus L. is high (1321.9 ± 125.2 and 1215.8 ± 80 μg/g DW, respectively). The roots of Lactuca sativa L. and Oenanthe javanica contain lower amounts of uranium at 699.1 ± 35.4 and 548.1 ± 66.8 μg/g DW, respectively. The amount of uranium accumulated in the leaves of all the species is very low, less than 10 μg/g DW, with an initial uranium concentration in the solution from 15 μg/L to 180 μg/L (Figure 1b). Even at a high concentration of uranium (450 μg/L) in the solution, the uranium accumulation in the leaves of all species is on average 14.4 μg/g DW. Lactuca sativa L. leaves show the highest uranium concentration of 23.7 ± 14.5 μg/g DW, which is ~3.8 times higher than that of Oenanthe javanica leaves (6.2 ± 0.8 μg/g DW). For different initial concentrations in the solution, there was a significant difference in the uranium accumulation in both shoots and roots of all species, based on the result of the statistical test by ANOVA (p < 0.05) (Table S2), indicating that a higher uranium concentration in the solution leads to grater uranium accumulation in the roots of all species.
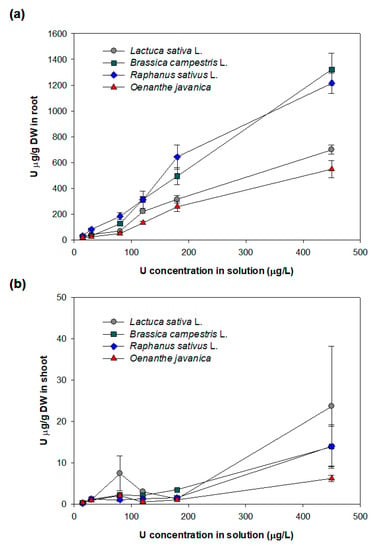
Figure 1.
Uranium accumulation in the roots (a) and shoots (b) of four different plant species after 72 h of treatment with solutions with different concentrations (15, 30, 80, 120, 180, and 450 μg/L).
The uranium BCFs for each plant calculated based on the rhizofiltration experiments with various initial uranium concentrations in the solution are illustrated in Figure 2. The uranium BCF values of the four species depend on the initial uranium concentration in the solution. The uranium BCFs of Brassica campestris L. and Raphanus sativus L. are high (1147.5–2968.4 and 2146.7–3578.3, respectively), whereas those of Lactuca sativa L. and Oenanthe javanica are low (863.7–1860.7 and 606–1444.4, respectively). The average BCF value of Raphanus sativus L. is 2683.5, which is the highest among the four species, followed by the BCF of Brassica campestris L. (2141.5), Lactuca sativa (1447.2), and Oenanthe javanica (1076).
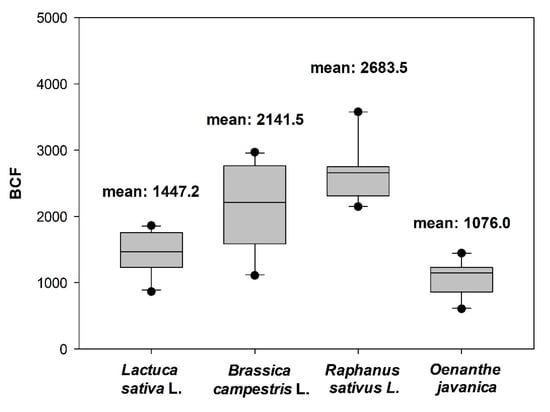
Figure 2.
Box-and-whisker plot of bioaccumulation factors of four species in artificial solutions with different initial uranium concentrations (15, 30, 80, 120, 180, and 450 μg/L) after 72 h of treatment.
The uranium BCF values of the four species depending on the pH conditions are shown in Figure 3. The average uranium BCF values of all species are 6820.9 at pH 3, 2121.5 at pH 5, 365.9 at pH 7, and 422.1 at pH 9, indicating that a lower pH leads to greater uranium accumulation in the plants (Table S3). In ANOVA analysis, statistically significant differences were found in the uranium accumulation in both shoots and roots of all species with different pH conditions (p < 0.05) (Table S2). The uranium BCF values of Raphanus sativus L. and Brassica campestris L. under acidic condition (pH 3) are 11061.3 and 11580.3, and their uranium concentrations in the roots are 1320.2 and 1259.0 μg/g, respectively. They are significantly higher than the values under other pH conditions. Similar results were previously reported for sunflower and bean plants at pH 3 and pH 5 [14,24,27]. Under acidic conditions below pH 5, the dissolved species of uranium in the solution do not form bonds with other anions (e.g., OH and CO32−) but mainly exist in the form of the cationic UO22+ (uranyl cation). It has been speculated that the uranium BCF of the plants significantly increases due to the adsorption of the uranyl cation to the carboxyl group (COO−) of the negatively charged plant cell wall [16,17].
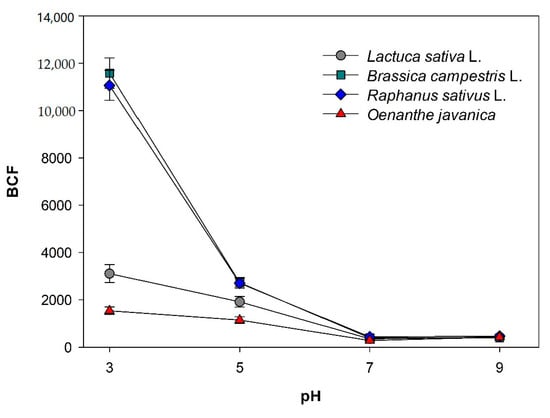
Figure 3.
Uranium bioconcentration factors of four different plant species in artificially contaminated solutions with four different pH values after 72 h treatment.
3.2. Rhizofiltration Experiment with Genuine Groundwater
The uranium accumulation and removal efficiency for the four species based on the groundwater are shown in Table S4. For the Oesam-dong groundwater with the uranium concentration of 83 μg/L, the ratios (accumulation efficiency) of the uranium mass in solution to the uranium accumulated mass in each plant were as follows: Raphanus sativus L. (88.8%), Brassica campestris L. (82.9%), Lactuca sativa (66.1%), and Oenanthe javanica (32.6%). For the Bugok-dong groundwater with the uranium concentration of 173 μg/L, the accumulation efficiency of Raphanus sativus L. is the highest (82.8%), followed by Brassica campestris L. (77%), Lactuca sativa (46.9%), and Oenanthe javanica (29.5%). The average uranium BCF of Raphanus sativus L. (1692.4) from the two rhizofiltration experiments is the highest among the four plants, followed by Brassica campestris L. (1160.2), Lactuca sativa (422.8) and Oenanthe javanica (170.5) (Figure 4). This order is the same as that of the results of the rhizofiltration experiments with artificially contaminated groundwater. For the Oesam-dong groundwater, the uranium BCF can be ranked as follows: Raphanus sativus L. (1684.7) > Brassica campestris L. (781.1) > Lactuca sativa (487.6) > Oenanthe javanica (213.5). In the groundwater of Bugok-dong, the uranium BCF of Raphanus sativus L. is the highest (1700.1), followed by Brassica campestris L. (1539.3), Lactuca sativa (358), and Oenanthe javanica (127.5). Because chemical characteristics of genuine groundwater were quite different from those of artificial groundwater, the uranium BCFs in genuine groundwater were not as high as those of the artificially contaminated groundwater. However, these results confirm that Raphanus sativus L. has a remarkable potential to accumulate uranium from contaminated groundwater despite the different water characteristics and ionic strength caused by competitive ions in the genuine groundwater.
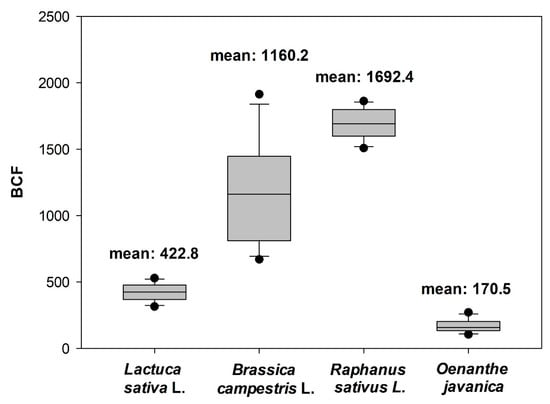
Figure 4.
Box-and-whisker plot of bioaccumulation factors of four plant species in genuine groundwater samples with different initial uranium concentrations (83 and 173 μg/L) after 72 h of treatment.
3.3. SEM/EDS Analysis of Uranium Adsorbed on Plant Roots
The results of SEM and EDS analyses, which confirm the adsorption of uranium onto the roots of Raphanus sativus L. after the uranium rhizofiltration experiment, are shown in Figure 5. The SEM micrographs provide direct optical evidence for the uranium adsorption onto the surface of the plant roots. Many clusters of small white particles (Figure 5a) and a relatively big white crystal (Figure 5b) can be observed. The EDS spectra of the white crystals in the white circle shown in Figure 5a,b were obtained. The uranium peaks in the EDS spectra indicate significantly high uranium concentrations, accounting for 79.6% and 68.2% of the total component weight, respectively (Figure 5c,d). These findings suggest that adsorption or precipitation may be the dominant mechanism of uranium removal from groundwater. Considering that the phosphate contents account for 17.4% and 18% of the total component weight based on the EDS analysis (Figure 5c,d), the white crystals are presumed to be phosphate complexes such as UO2HPO40 and UO2(HPO4)22− [11,31]. These findings are consistent with the results of previous studies in which optical evidence of white particles was provided using SEM and EDS analyses; the authors suggested that adsorption on the root surface of sunflower and bean is the dominant mechanism for uranium removal from the solution [24,27]. However, the size of the white particles in the SEM micrographs was much smaller (<1 μm) than those of the particles (>10 μm) observed in this study and the uranium concentration of the particles was also much smaller (<26% of the total composition weight) than that determined in this study (>68% of the total composition weight) [24,27].
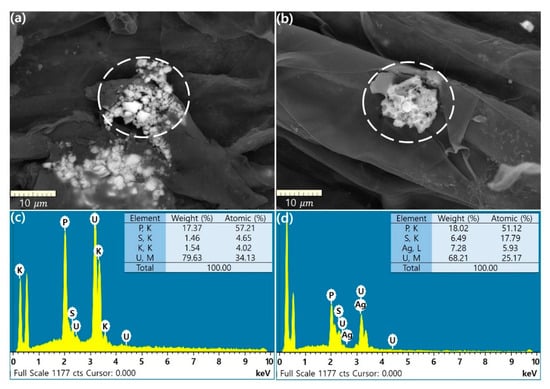
Figure 5.
SEM micrographs of Raphanus sativus L. roots after rhizofiltration and EDS spectra for a cluster of white crystalline particles (a,c) and a big white crystal particle (b,d) in the dashed white circle.
3.4. Uranium BCF Trends in Plants
The uranium BCFs reflect the capacities of plants to accumulate uranium from a solution. Because the BCF values depend on the uranium concentrations of the individual plants and solution, they allow a comparison of the uranium accumulation in different plant species [20,22,23,25,27,32,33,34,35,36,37,38,39]. Note that the uranium BCF values of previous studies are compared with the results of this study in Figure 6, Figure 7 and Figure 8. The rhizofiltration time required for bioaccumulation to remove uranium in artificially contaminated solutions significantly varies among the plants in previous studies, as shown in Figure 6. The BCF values are the highest in studies using longer reaction times (more than nine days; [32,33,34,35]). Although the reaction time used to determine the BCF values in this study is three days, the four species used in this study show intermediate BCFs ranging from >1000 to <3000. The BCF of Raphanus sativus L. (2683.5) is more than three times higher than that reported for Phaseolus vulgaris L. var. vulgaris (889.3) by Yang et al. (2015) [27], who used the same reaction time for the rhizofiltration experiments (three days).
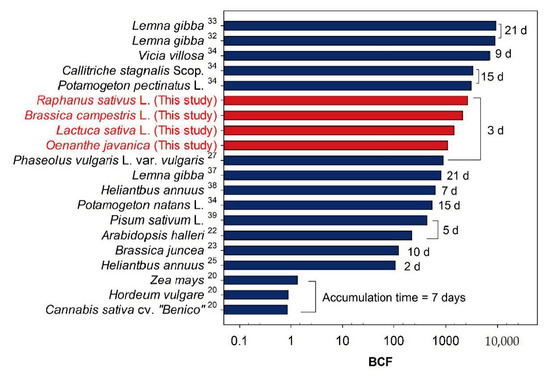
Figure 6.
Average uranium BCFs of various plants after rhizofiltration experiments using artificially contaminated solution. The BCF values and accumulation times were obtained from previous studies.
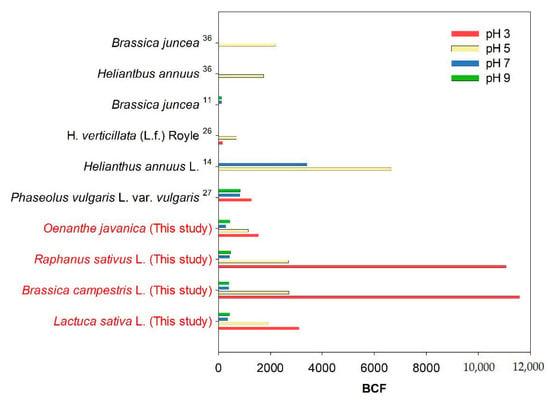
Figure 7.
Average uranium BCFs of various plants after accumulation experiments using solutions with different pH values. The BCF values were obtained from previous studies.
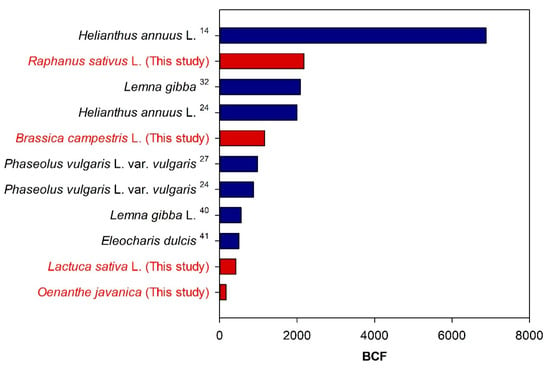
Figure 8.
Average uranium BCFs of various plants after accumulation experiments using genuine groundwater and surface water. The BCF values were obtained from previous studies.
The dependency of the BCF on pH conditions for previous studies and this study are summarized in Figure 7 [11,14,26,27,36]. At a pH of 5 or higher, the BCF values of the four plants in this study are lower than those reported for Phaseolus vulgaris L. var. vulgaris (pH 9 = 831.6, pH 7 = 808.5) by Yang et al. (2015) [27] and Helianthus annuus L. (pH 7 = 3406.8, pH 5 = 6654.5) by Dushenkov et al. (1997) [14]. However, the BCF values determined for Raphanus sativus L. and Brassica campestris L. at a pH of 3 in this study are 11061.3 and 11580.3, respectively, which are remarkably higher than those of plants reported in previous studies. These results suggest that the uranium accumulation in plants is enhanced under acidic conditions (pH 3–5). In addition, the results show that Raphanus sativus L. and Brassica campestris L. can be employed to remove uranium using rhizofiltration, even in acidic environments such as acid mine drainage.
The uranium BCF values determined for various plants during rhizofiltration experiments using genuine ground- and surface water are summarized in Figure 8; they range from >102 to <104 [14,24,27,32,40,41]. Even discounting the BCF of approximately 7000 reported for Helianthus annuus L. by Dushenkov et al. (1997) [14], who conducted rhizofiltration experiments under continuous injection of uranium-contaminated surface water at a flow rate of 1.05 L/min for 14 days, Raphanus sativus L. in this study, Lemna gibba (Mkandawire et al., 2004b) [33], and Helianthus annuus L. (Lee and Yang, 2010) [24] exhibit average BCFs of 2179, 2085, and 2000, respectively, indicating their great rhizofiltration ability. The BCF of Raphanus sativus L. in this study is more than two times higher than the values reported for Phaseolus vulgaris L. var. vulgaris by Lee and Yang (2010; 875) [24] and Yang et al. (2015; 976) [27], who used the same experiment time of three days.
However, this work used a simplified approach to compare between the obtained and measured BCF values. Although the value of BCF provides robust estimates of uranium accumulation capacity for plants from a solution, the associated parameters used in previous studies such as different initial concentrations, exposure times, and competitive ions in a solution varied. There is a possibility that longer exposure periods and high uranium concentration in a solution may lead to physiological damage that results in a reduced ability to adsorb contaminants and allows the adsorbed contaminants to be excreted again. In addition, this work posits that chemical characteristics of the genuine groundwater used in this study are similar to those used in previous studies. Various assessments are needed to evaluate the relationship between BCFs and chemical characteristics, such as competitive adsorption of cations onto the root surface.
4. Conclusions and Implications
Based on the results of the rhizofiltration experiments with artificially contaminated solutions with different initial uranium concentrations, the highest uranium accumulation is observed for Raphanus sativus L., with an average BCF of 2683.5, followed by Brassica campestris L., Lactuca sativa, and Oenanthe javanica. At all uranium concentrations, the uranium accumulations in the roots of all species are two orders of magnitude higher than that in the shoots. Under acidic conditions (pH 3), the BCF values of both Raphanus sativus L. and Brassica campestris L. are more than 4 times higher than those at pH 5 and more than 25 times higher than those at pH 7 and 9. The results of the SEM and EDS analyses using the roots of Raphanus sativus L. demonstrate that the primary rhizofiltration mechanisms are the adsorption and precipitation of uranium phosphate onto the root surface. When compared with uranium BCFs of various plant species from previous studies, Raphanus sativus L. demonstrates a remarkable potential for uranium accumulation with a high tolerance to low pH and high uranium concentrations in the solution.
The results of various rhizofiltration experiments confirm the outstanding uranium accumulation capacities of Lactuca sativa, Brassica campestris L., Raphanus sativus L., and Oenanthe javanica, which have not been used in previous uranium accumulation studies. Among the four species, Raphanus sativus L. is a plant species capable of accumulating a large amount of uranium in a relatively short time. Although this work conducted laboratory-scale experiments, the rhizofiltration approach can be applied to remove uranium at larger scales, such as continuous clean-up rhizofiltration systems; each rhizofiltration reservoir is connected to another as a series circuit [14,42]. For example, in the rhizofiltration experiment with uranium solution (116 μg/L) under the acidic condition for 72 h, 0.1 g DW of Raphanus sativus L. roots accumulated approximately 40 μg of uranium mass from the solution. Assuming 1000 L of groundwater having a uranium concentration of 116 μg/L is treated using the continuous clean-up system, approximately 300 g DW of Raphanus sativus L. roots would be sufficient to remove uranium in the groundwater. Therefore, it can be applied for uranium removal studies in the future. In the future, potential harmful effects of uranium accumulation in plants on human health should be studied. In contrast to the plants utilized in previous studies, the four plant species used in this study are all edible crops. If local uranium-contaminated groundwater is used as water source for these plants, humans will be exposed to high levels of uranium. Hence, more research is needed regarding the uranium contamination of crops and vegetables and the expected uranium intake.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-163X/11/1/41/s1, Figure S1: photographs of Lactuca sative L., Brassica campestirs L., Raphanus sativus L., and Oenanthe javanica grown for 4 weeks in the phytotron; Table S1: chemical properties of genuine groundwater samples from two sites; Table S2: the results of one-way ANOVA analysis for uranium accumulation in four plant species with the different initial concentrations and pH values in the solution; Table S3: Uranium concentration in roots and shoots of Lactuca sativa L., Brassica campestris L., Raphanus sativus L., and Oenanathe javanica at four different pH values. Each point represents the mean ± standard deviation of triplicates, Table S4: uranium concentration in roots and shoots and removal efficiency of four different plants exposed to two genuine groundwaters (Oesam-dong = 80 μg/L, Bugok-dong = 173 μg/L).
Author Contributions
Writing—original draft preparation, Y.H. and M.Y.; formal analysis, Y.H. and J.L.; investigation, Y.H.; conceptualization, M.L., J.L. and M.Y.; software, J.P., C.K., and J.L.; visualization, J.P., C.K., and J.L.; writing—review and editing, J.L. and M.Y.; supervision, M.L. and M.Y; funding acquisi-tion, M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the grant (2019002470002) from Korea Ministry of Environment as “The SEM (Subsurface Environmental Management) projects” and by the Basic Research Program through the National Research Foundation of Korea (NRF) funded by the MSIT (2020R1F1A1072122).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or supplementary material.
Acknowledgments
The authors are grateful to researchers at Environmental Hydrogeology Laboratory (EHL) in Pukyong National University, South Korea for their valuable feedback, suggestions, and discussions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Antonkiewicz, J.; Jasiewicz, C. The use of plants accumulating heavy metals for detoxication of chemically polluted soils. Electron. J. Polish Agric. Univ. 2002, 5, 121–143. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, H.; Wang, J.; Fang, W.; Yuan, J.; Yang, Z. Heavy Metal Accumulations of 24 Asparagus Bean Cultivars Grown in Soil Contaminated with Cd Alone and with Multiple Metals (Cd, Pb, and Zn). J. Agric. Food Chem. 2007, 55, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhou, Q.; Zhang, Z.; Hua, T.; Cai, Z. Evaluation of Cadmium Phytoremediation Potential in Chinese Cabbage Cultivars. J. Agric. Food Chem. 2011, 59, 8324–8330. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Zhou, L.; Chen, L.; Li, Z.; Wu, L.; Christie, P.; Luo, Y. Oxytetracycline Toxicity and Its Effect on Phytoremediation by Sedum plumbizincicola and Medicago sativain Metal-Contaminated Soil. J. Agric. Food Chem. 2016, 64, 8045–8053. [Google Scholar] [CrossRef] [PubMed]
- Gramss, G.; Voigt, K.-D. Regulation of the mineral concentrations in pea seeds from uranium mine and reference soils diverging extremely in their heavy metal load. Sci. Hortic. 2015, 194, 255–266. [Google Scholar] [CrossRef]
- Wang, N.; Wei, Q.; Yan, T.; Pan, Z.; Liu, Y.; Peng, S. Improving the boron uptake of boron-deficient navel orange plants under low boron conditions by inarching boron-efficient rootstock. Sci. Hortic. 2016, 199, 49–55. [Google Scholar] [CrossRef]
- Beveridge, T.J.; Murray, R.G. Sites of metal deposition in the cell wall of Bacillus subtilis. J. Bacteriol. 1980, 141, 876–887. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kelly, S.D.; Kemner, K.M.; Banfield, J.F. Nanometre-size products of uranium bioreduction. Nat. Cell Biol. 2002, 419, 134. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Althoff, H.W.; Bartel, H.; Jekel, M. The effect of groundwater composition on uranium(VI) sorption onto bacteriogenic iron oxides. Water Res. 2006, 40, 3646–3652. [Google Scholar] [CrossRef]
- Pabalan, R.T.; Turner, D.R. Uranium(6+) sorption on montmorillonite: Experimental and surface complexation modeling study. Aquat. Geochem. 1997, 2, 203–226. [Google Scholar] [CrossRef]
- Ebbs, S.D.; Brady, D.; Kochian, L.V. Role of uranium speciation in the uptake and translocation of uranium by plants. J. Exp. Bot. 1998, 49, 1183–1190. [Google Scholar] [CrossRef]
- Cornish, J.E. Evaluation of in situ phytoremediation of uranium-contaminated soils in Ohio and Montana. In Proceedings of the Hazardous Management’95 Conference Proceeding, Tuscon, AZ, USA, 26 February–3 March 1995. [Google Scholar]
- Dushenkov, V.; Kumar, P.B.A.N.; Motto, H.; Raskin, I. Rhizofiltration: The Use of Plants to Remove Heavy Metals from Aqueous Streams. Environ. Sci. Technol. 1995, 29, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Dushenkov, S.; Vasudev, D.; Kapulnik, Y.; Gleba, D.; Fleisher, D.; Ting, K.C.; Ensley, B. Removal of Uranium from Water Using Terrestrial Plants. Environ. Sci. Technol. 1997, 31, 3468–3474. [Google Scholar] [CrossRef]
- Willey, N.; Collins, C. Phytoremediation of soil contaminated with low concentration of radionuclides. Water Air Soil Pollut. 1996, 88, 167–176. [Google Scholar]
- Langmuir, D. Aqueous Environmental Geochemistry; Prentice Hall: Upper Saddle River, NJ, USA, 1997. [Google Scholar]
- Waite, T.; Davis, J.; Payne, T.; Waychunas, G.; Xu, N. Uranium(VI) adsorption to ferrihydrite: Application of a surface complexation model. Geochim. Cosmochim. Acta 1994, 58, 5465–5478. [Google Scholar] [CrossRef]
- Ebbs, S.D.; Brady, D.; Norvell, W.; Kochian, L.V. Uranium Speciation, Plant Uptake, and Phytoremediation. In Environmental and Pipeline Engineering 2000; American Society of Civil Engineers (ASCE): Reston, VA, USA, 2000; pp. 466–475. [Google Scholar]
- Serre, N.B.; Alban, C.; Bourguignon, J.; Ravanel, S. Uncovering the physiological and cellular effects of uranium on the root system of Arabidopsis thaliana. Environ. Exp. Bot. 2019, 157, 121–130. [Google Scholar] [CrossRef]
- Soudek, P.; Petrova, S.; Benešová, D.; Dvorakova, M.; Vanek, T. Uranium uptake by hydroponically cultivated crop plants. J. Environ. Radioact. 2011, 102, 598–604. [Google Scholar] [CrossRef]
- Stojanović, M.D.; Mihajlović, M.L.; Milojković, J.; Lopičić, Z.R.; Adamović, M.; Stanković, S. Efficient phytoremediation of uranium mine tailings by tobacco. Environ. Chem. Lett. 2012, 10, 377–381. [Google Scholar] [CrossRef]
- Viehweger, K.; Geipel, G. Uranium accumulation and tolerance in Arabidopsis halleri under native versus hydroponic conditions. Environ. Exp. Bot. 2010, 69, 39–46. [Google Scholar] [CrossRef]
- Eapen, S.; Suseelan, K.; Tivarekar, S.; Kotwal, S.; Mitra, R. Potential for rhizofiltration of uranium using hairy root cultures of Brassica juncea and Chenopodium amaranticolor. Environ. Res. 2003, 91, 127–133. [Google Scholar] [CrossRef]
- Lee, M.; Yang, M. Rhizofiltration using sunflower (Helianthus annuus L.) and bean (Phaseolus vulgaris L. var. vulgaris) to remediate uranium contaminated groundwater. J. Hazard. Mater. 2010, 173, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Tomé, F.V.; Rodríguez, P.B.; Lozano, J. Elimination of natural uranium and 226 Ra from contaminated waters by rhizofiltration using Helianthus annuus L. Sci. Total. Environ. 2008, 393, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Bhainsa, K.; D’Souza, S.F. Investigation of uranium accumulation potential and biochemical responses of an aquatic weed Hydrilla verticillata (L.f.) Royle. Bioresour. Technol. 2010, 101, 2573–2579. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Jawitz, J.W.; Lee, M. Uranium and cesium accumulation in bean (Phaseolus vulgaris L. var. vulgaris) and its potential for uranium rhizofiltration. J. Environ. Radioact. 2015, 140, 42–49. [Google Scholar] [CrossRef]
- NIAST. Methods of Soil and Plant Analysis; National Institute of Agricultural Science and Technology: Suwon, Korea, 2000. [Google Scholar]
- Zayed, A.; Gowthaman, S.; Terry, N. Phytoaccumulation of trace elements by wetland plants: I. Duckweed. J. Environ. Qual. 1998, 27, 715–721. [Google Scholar] [CrossRef]
- Ghosh, M.; Singh, S. A comparative study of cadmium phytoextraction by accumulator and weed species. Environ. Pollut. 2005, 133, 365–371. [Google Scholar] [CrossRef]
- Langmuir, D. Uranium solution-mineral equilibria at low temperatures with applications to sedimentary ore deposits. Geochim. Cosmochim. Acta 1978, 42, 547–569. [Google Scholar] [CrossRef]
- Mkandawire, M.; Dudel, E.G.; Taubert, B. Accumulation of uranium in Lemna gibba L. in relation to milieu conditions of tailing waters in abandoned uranium mines in Germany. Mine Water Process Policy Prog. 2004, 2, 9–18. [Google Scholar]
- Mkandawire, M.; Taubert, B.; Dudel, E.G. Capacity of Lemna gibba L. (Duckweed) for Uranium and Arsenic Phytoremediation in Mine Tailing Waters. Int. J. Phytoremediat. 2004, 6, 347–362. [Google Scholar] [CrossRef]
- Pratas, J.; Paulo, C.; Favas, P.J.; Venkatachalam, P. Potential of aquatic plants for phytofiltration of uranium-contaminated waters in laboratory conditions. Ecol. Eng. 2014, 69, 170–176. [Google Scholar] [CrossRef]
- Ramaswami, A.P.A.; Carr, P.; Burkhardt, M. Plant-Uptake of Uranium: Hydroponic and Soil System Studies. Int. J. Phytoremediat. 2001, 3, 189–201. [Google Scholar] [CrossRef]
- Tomé, F.V.; Rodríguez, P.B.; Lozano, J. The ability of Helianthus annuus L. and Brassica juncea to uptake and translocate natural uranium and 226Ra under different milieu conditions. Chemosphere 2009, 74, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Mkandawire, M.; Taubert, B.; Dudel, E.G. Resource manipulation in uranium and arsenic attenuation by Lemna gibba L.(duckweed) in tailing water of a former uranium mine. Water Air Soil Pollut. 2005, 166, 83–101. [Google Scholar] [CrossRef]
- Rodríguez, P.B.; Tomé, F.V.; Fernández, M.P.; Lozano, J. Linearity assumption in soil-to-plant transfer factors of natural uranium and radium in Helianthus annuus L. Sci. Total Environ. 2006, 361, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.K.; Vuković, A.; Semenishchev, V.S.; Inouhe, M.; Walther, C. Uranium accumulation and its phytotoxicity symptoms in Pisum sativum L. Environ. Sci. Pollut. Res. 2020, 27, 3513–3522. [Google Scholar] [CrossRef] [PubMed]
- Sasmaz, A.; Obek, E. The accumulation of arsenic, uranium, and boron in Lemna gibba L. exposed to secondary effluents. Ecol. Eng. 2009, 35, 1564–1567. [Google Scholar] [CrossRef]
- Overall, R.A.; Parry, D.L. The uptake of uranium by Eleocharis dulcis (Chinese water chestnut) in the Ranger Uranium Mine constructed wetland filter. Environ. Pollut. 2004, 132, 307–320. [Google Scholar] [CrossRef]
- Yang, M.; Her, N. Perchlorate in Soybean Sprouts (Glycine max L. Merr.), Water Dropwort (Oenanthe stolonifera DC.), and Lotus (Nelumbo nucifera Gaertn.) Root in South Korea. J. Agric. Food Chem. 2011, 59, 7490–7495. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).