Abstract
Sulfide hemimorphite can be depressed by Fe3+ during flotation. In this study, the depression mechanism was studied by microflotation, inductively-coupled plasma mass spectrometry, local electrochemical impedance spectroscopy (LEIS), and X-ray photoelectron spectroscopy (XPS). Flotation test results suggested that sulfated hemimorphite can be depressed by Fe3+ across the entire pH range. LEIS, adsorption analysis, and XPS indicated that S species were adsorbed on hemimorphite as ZnS. The sulfide film was attenuated and no adsorbed Fe species were found after treatment with Fe3+. The results indicate that Fe3+ reacts with the ZnS film, which decreases the number S species, and this leads to hemimorphite depression.
1. Introduction
Zinc is the fourth most used metal after aluminum, iron, and copper. It is commonly used in alloys and batteries [1]. Zinc sulfide minerals, which are the main sources for zinc production, are commonly concentrated and recovered by froth flotation. Recently, zinc sulfide minerals have become depleted because of increasing exploitation [2]. Hemimorphite is a typical zinc oxide mineral, and its use as an alternative source of zinc metal has been developed to meet future demands [3]. Many techniques such as pyrometallurgy, hydrometallurgy, flotation, and gravity separation combined with flotation have been used to treat zinc oxide ores [4]. The sulfidization-xanthate flotation method is the most commonly used commercial method for the concentration and pretreatment of zinc oxide minerals [5,6].
Generally, sulfidization flotation performs well in the commercial processing of lead, copper, and zinc oxide minerals. Sulfidization-xanthate flotation is usually used for the separation and concentration of hemimorphite from other minerals. A key step in the sulfidization-xanthate flotation separation process is sulfation by Na2S [7]. Adjustment and control of sulfation is important for achieving good flotation indexes. Jia et al. [8]. studied the sulfation of hemimorphite. They reported that the formation of ZnS can be described by the following chemical reaction:
Zn4Si2O7(OH)2H2O(s) + xHS− → Zn4−xSi2O7(OH)2−2xH2O(ZnS)x(s) + xHO− + xH2O
S(II) enters the hemimorphite crystal lattice and forms a new zinc sulfide phase on the hemimorphite surface, but the freshly generated zinc sulfide film differs from natural sphalerite. Flotation and sulfation are inevitably affected by ions in solution. Previous research has shown that ions have important effects on the flotation of oxide minerals. Shen et al. [9]. reported that NH4+ causes dissolution of the malachite surface; it acts as a sulfidization promoter and prevents depression of excess S2− during sulfidization. Feng et al. [10] suggested that more lead sulfide species form on the cerussite surface when chloride ions are added prior to sulfidization. The addition of chloride ions prior to sulfidization significantly improves cerussite sulfidization, and this improves the flotation performance. Fe3+ is highly oxidized and can react with sulfide ores and films. The hydrophilic compound ferric hydroxide can be adsorbed on mineral surfaces. Grano et al. [11] reported that Fe3+ depresses chalcopyrite when Fe3+ in the form of iron oxyhydroxide is adsorbed on the chalcopyrite surface and that the extent of flotation depression by iron oxyhydroxide depended on pH, conditioning gas, and the presence of adsorbed collector on chalcopyrite. Yu et al. [12] reported that Fe3+ exists in various forms in solution and its adsorption on an arsenopyrite surface leads to oxidation of Fe, As, and S on the mineral surface, and oxidation increases with increasing pH. The oxide residues on the arsenopyrite surface decrease its floatability. This report shows that water interface and the mineral surface following treatment with Fe3+ have a big influence in sulfide ore flotation, and the experiments also should pay attention to the iron hydroxyl complexes which exist in aqueous solutions. Additionally, for another sulfide mineral pyrite, Jiang et al. [13] suggested that the presence of Fe2+ and Fe3+ ions in solution significantly depresses pyrite ore flotation in a weakly alkaline environment. They found that the complete or partial depression of pyrite is mainly attributed to the reactions between xanthate and ferrous/ferric ions to form the poorly hydrophobic ferric dihydroxy xanthate on the surfaces and in solution. The flotation results showed that ferrous ions depress the pyrite near this pH range more remarkably than ferric ions. Deng et al. [3] reported that Fe3+ can react with a sulfated smithsonite surface. This reaction accelerates the dissolution of smithsonite. This dissolution and the generated carbon dioxide weaken the linked surface layer of sulfide (ZnS) film. This results in easy removal of the film from the surface under high-speed stirring. This process not only decreases the hydrophobicity of the mineral surface but also removes large quantities of the mineral surface layers.
The amount of information available in the literature on the sulfidization and flotation of hemimorphite is much lower than that available on other oxidized ores. In particular, there are few reports on the mode of action of iron ions on hemimorphite surfaces and the implications for sulfidization and flotation. But the Fe3+ is unavoidable ions in flotation process. The Fe3+ may go into pulp from grinding process, stirring process even though the flotation process. These Fe3+ may have some influence on the hemimorphite flotation. In this study, the depression of sulfated hemimorphite by Fe3+ and the depression mechanism were studied by microflotation, time-of-flight secondary-ion mass spectrometry (TOF-SIMS), local electrochemical impedance spectroscopy (LEIS), and X-ray photoelectron spectroscopy (XPS). The changes in the surface properties caused by Fe3+ addition and the subsequent effects on hemimorphite flotation and sulfidization were studied in detail.
2. Materials and Methods
2.1. Materials
The pure hemimorphite samples used in all experiments were obtained from Guangxi Province, China. The samples were dry ground with an agate pestle. The ground hemimorphite was sieved with a standard screen to achieve a particle fraction of −74–+38 µm for the flotation tests and inductively coupled plasma mass spectrometry (ICP-MS). The rest of the hemimorphite sample was ground to a particle fraction finer than 5 µm for XPS. The X-ray diffraction (XRD) pattern in Figure 1 shows that only the diffraction peaks from hemimorphite were detected. This confirms that the hemimorphite samples used in the experiments were of high purity.
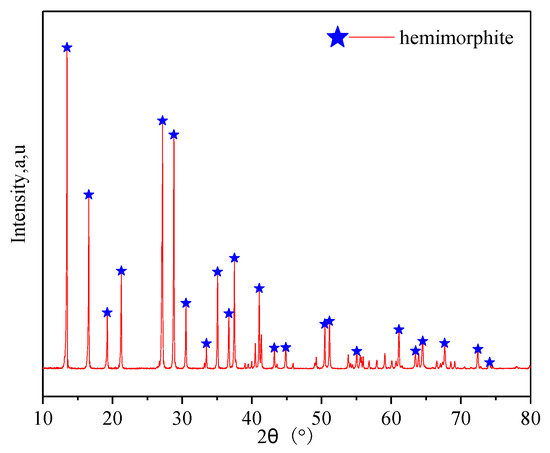
Figure 1.
XRD pattern of pure hemimorphite samples.
Isopentyl sodium xanthate (NaIX) was used as the collector and FeCl3 was used as the Fe3+ source. Sodium sulfide (Na2S·9H2O) was used as the sulfurizing agent. The pulp pH was adjusted with aqueous NaOH and H2SO4. All reagents were analytical grade. Deionized water was produced by using a Milli-Q5O system (Millipore, Burlington, MA, USA).
2.2. Flotation Tests
A small-scale flotation apparatus (XFGCⅡ) with mechanical agitation was used for the flotation experiments. The hemimorphite samples (2 g) were mixed with deionized water (40 mL) and the pH was adjusted to the desired value by adding NaOH or H2SO4; the pH was determined with a pH meter (PHS-3C, Nanjing T-Bota Scietech Instruments & Equipment Co., Ltd., Nanjing, China). The pulp was then sequentially conditioned with S2− (5 min), NaIX (2 min), and terpenic oil (1 min) at an agitation rate of 1700 r/min. A flotation time of 2 min was used in all single-mineral experiments. After flotation, the solid was collected by filtration, dried, and weighed to determine the flotation recovery. All experiments were repeated in triplicate, and average flotation recoveries were recorded.
2.3. LEIS
A small layer of the hemimorphite sample was adhered to epoxy resin to form a substrate. The substrate was polished by hand with wet silicon carbide paper in the grit sequence 240, 320, 400, 600, 800, and 1200. The freshly polished hemimorphite substrate was washed by ultrasonication in ethanol and deionized water for 5 min. The freshly prepared substrate was immediately transferred to a 1 L electrochemical cell for LEIS. Pt wire was used as the counter electrode and an Ag/AgCl electrode was used as the reference electrode. LEIS was performed with a scanning electrochemical workstation (VersaSCAN, AMETEK SI, PA, USA).
The probe was fixed approximately 50 μm above the sample surface and was moved across a designated 0.2 × 0.2 mm2 surface area in step sizes of 10 μm in the X and Y directions. Similar experiments have been described in the literature. After LEIS, imaging of the hemimorphite substrate was performed in 5 mM Na2S solution, and 5 mM Na2S + 1 mM Fe3+ solution. The hemimorphite substrate was ultrasonically washed in ethanol and deionized water for 15 min after use. KNO3 was added to the solution to improve the electrical conductivity.
2.4. XPS
A beaker containing deionized water (40 mL) was charged with a hemimorphite sample (2 g). The pulp (deionized water, 5 mM Na2S solution, or 5 mM Na2S + 2mM Fe3+ solution) was magnetically stirred for 5 min at 25 °C; the temperature was controlled with a water bath. The solid was collected by filtration and dried at 25 °C in a vacuum oven.
XPS was performed with a PHI 5000 Versaprobe-II (PHI5000, ULVAC-PHI, Kanagawa, Japan) scanning XPS microprobe system, with a monochromatic Al X-ray source (200 W). Each analysis started with a survey scan from 0 to 1100 eV. The acquired data were processed using MultiPak software and 284.8 eV was used as the standard C 1s binding energy
2.5. ICP-MS
Pure hemimorphite (0.5 g) of particle size 38–74 μm was conditioned in a beaker with deionized water. Then 0.01 M Na2S was added and the pulp was conditioned for 5 min. FeCl3 solution (if needed) was added and the mixture was conditioned for 2 min. After solid-liquid separation, the solids were dissolved in 0.1 M HNO3 solution. The liquid samples were centrifuged (TL-4.7W, SCI, Jiangsu, China) for 20 min at a rotation speed of 4000 r/min. The S concentration in the supernatant was determined by ICP-MS (7700x, Agilent Technologies, Santa Clara, CA, USA).
2.6. TOF-SIMS
Bulk hemimorphite samples were acquired by slicing and polishing by artificial methods. The bulk samples were added to a 100 mL beaker containing the desired solutions. Dilute NaOH solution was used to adjust the pulp pH to 11. After reaction for 5 min, the samples were air dried for analysis by TOF-SIMS (ION-TOF GmbH, Münster, Germany). All samples were placed in the middle of an electron gun and evacuation was performed for 12 h to give a vacuum of at least 10−7 Pa during the analysis. The following surface analysis conditions were used: primary ion source Bi3+; ion energy 15 keV; analysis current 0.45 pA; analysis area 250,000 μm2; and measurement time 60 s. The following depth-profiling analysis conditions were used: sputter ion Cs+; sputter energy 2 keV; sputter current 34.23 nA; analysis area 100 × 100 μm2; and sputter time 52 s. Negative secondary-ion mass spectra were calibrated. C−, O2−, OH−, and C2−; positive secondary-ion mass spectra were calibrated using H+, C+, C2H3+, C3H5+, and C4H7+.
3. Results and Discussion
3.1. Flotation Test Results
Flotation tests were performed at various pH values. The results (Figure 2a) show that the hemimorphite recovery increased significantly across the pH range on addition of Na2S to solutions with 5 × 10−4 mol/L NaIX as the collector; The results (Figure 2b) show that the hemimorphite recovery increased with the concentration of Na2S but decline when use Na2S over 5mM, we speculation that too much Na2S may contend with NaIX as the collector. This shows that sulfurization considerably improved the floatability of hemimorphite. But in experiment results shown in Figure 2c, when we add Fe3+, the recovery of hemimorphite is in decline. This is consistent with our conjecture. Kai et al. [8] observed the formation of amorphous or poorly crystalline ZnS on hemimorphite surfaces after treatment with Na2S, and the ZnS film on the hemimorphite surface enabled easy adsorption of xanthate species on the mineral surface. However, after Fe3+ addition to the pulp, the flotation recovery of hemimorphite decreased at the same Na2S concentration, i.e., between that for hemimorphite before and after treatment with Na2S. Zhang et al. [14] reported that sphalerite (ZnS) floated readily at pH 8–11 in the presence of Fe2+, but not in the presence of Fe3+. This suggests that Fe3+ can modify the Zn–S surface and make it more hydrophilic. We conclude that hemimorphite treated with Na2S can be depressed by Fe3+. There are two reasons for this: (1) Generation of a hydrotropic substance such as Fe(OH)3 on the hemimorphite surface; and (2) A decrease in the amount of sulfide film. More research was needed to clarify the mechanism of depression by Fe3+ during hemimorphite flotation.
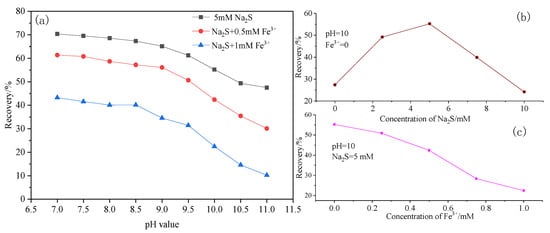
Figure 2.
(a) Hemimorphite flotation recovery as a function of pH value in the absence and presence of Fe3+, (b) Hemimorphite flotation recovery as a function of Na2S without Fe3+, (c) Hemimorphite flotation recovery as a function of Fe3+.
3.2. LEIS
Electrochemical reactions can affect the surface conductivity of hemimorphite. LEIS maps of the untreated hemimorphite substrate and those treated with Na2S or Na2S + Fe3+ at pH 11 are shown in Figure 3a–c. The LEIS map of the untreated hemimorphite is predominantly red-orange, which indicates that the average impedance of the untreated hemimorphite sample is 250 846.3 Ω. The LEIS map of the hemimorphite sample after treatment with Na2S is mainly blue, which indicates that the average impedance of the hemimorphite sample is 199 946.3 Ω. The decrease in the average impedance suggests that the material has good electrical conductivity because of the generation of species such as sulfide on the hemimorphite sample surface by treatment with Na2S. However, the chemical component could not be identified by LEIS analysis. When Fe3+ is present in the solution during sulfuration of hemimorphite, the LEIS map of the hemimorphite sample is predominantly green-yellow. This implies that the average impedance of this sample is 231 002.4 Ω. The average impedance of the hemimorphite samples was lower than that of the sample in the solution without Fe3+. These results suggest that the good electrical conductivity generated in the hemimorphite samples decreased when Fe3+ was present in the solution or species with poor electrical conductivity (e.g., iron oxide hydroxide) were generated on the surface of the hemimorphite sample. These results, which show that a decrease in the amount of sulfide species or an increase in the amount of iron oxide hydroxide on the hemimorphite surface decreases the floatability of hemimorphite samples compared with that in a solution without Fe3+, are in good agreement with the flotation test results.
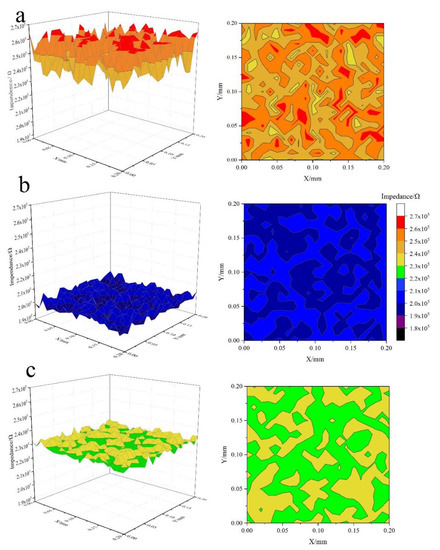
Figure 3.
LEIS maps of hemimorphite surfaces treated with (a) deionized water, (b) 5 mM Na2S, and (c) 5 mM Na2S and 1mM Fe3+.
3.3. Adsorption Test Results
LEIS analysis (Section 3.2) identified the changes in the surface properties of hemimorphite and showed that the average impedance of a hemimorphite sample decreases in a solution containing Fe3+. This section focuses on variations in the amount of S adsorbed on the surface. Adsorption amounts were determined by ICP-MS; the results are shown in Figure 4. The amount of S adsorbed for the pulp without Fe3+ was 4.2 × 10−6 mol/m2. However, with addition of Fe3+, the amount of adsorbed S decreased significantly. The amount of S adsorbed on the hemimorphite surface decreased with increasing Fe3+ concentration. This is because Fe3+ ions react with S species adsorbed on the hemimorphite surface, which decreases the amount of S, and the effect of sulfidization is therefore attenuated with increasing Fe3+ concentration. The amount of adsorbed Fe species detected was low, which suggests that this process does not lead to adsorption of Fe species on the hemimorphite surface.
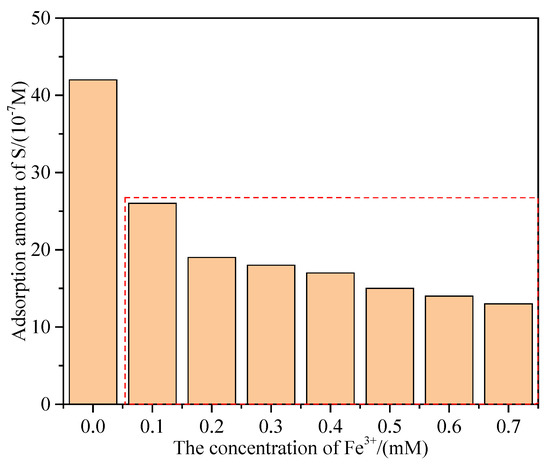
Figure 4.
Adsorption amount of S on the hemimorphite surface.
More sensitive information regarding adsorption of iron and sulfide species on the hemimorphite surface was obtained by using TOF-SIMS to perform surface analysis and depth-profile analysis. TOF-SIMS is a sensitive and reliable method for identifying and understanding interactions between minerals and various pulp components.
The normalized TOF-SIMS peak intensities for the hemimorphite samples treated with Na2S in solutions with and without Fe3+ are shown in Figure 5. Reconstructed 3D negative-depth profiles for the hemimorphite samples after S adsorption are shown in Figure 6. Figure 5a shows that the range of the normalized ZnS−, Zn2S, S−, and S2− peak relative intensities was 10−4 to 10−1. This implies that sulfide species are adsorbed on the hemimorphite surface and mineral surface in the form Zn–S. These results are in agreement with those of previous research. After treatment with Fe3+, the normalized TOF-SIMS intensities of the peaks for ZnS−, Zn2S, S−, and S2− decreased gradually with increasing Fe3+ concentration. This indicates that the amount of sulfide film on the hemimorphite surface decreased when Fe3+ was added to the solution.
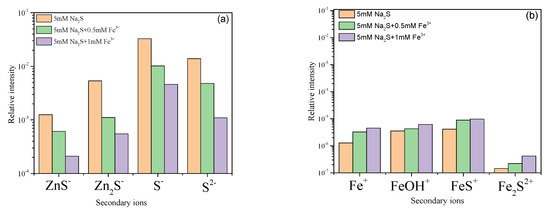
Figure 5.
Relative intensities of (a) positive secondary ions and (b) negative secondary ions on the hemimorphite surface layer.
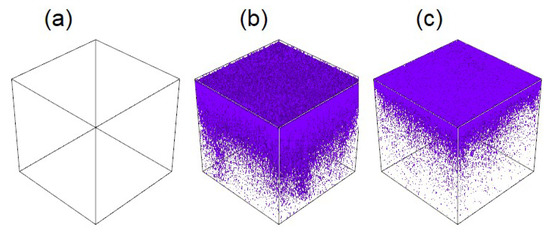
Figure 6.
Reconstructed 3D negative-ion (ZnS−) depth-profiling images of hemimorphite samples treated with (a) deionized water, (b) 5 mM Na2S, and (c) 5 mM Na2S and 1mM Fe3+.
TOF-SIMS positive-ion analysis was used to detect iron species adsorbed on the surface. The normalized peak intensities for Fe+, FeOH, FeS+, and Fe2S2+ are shown in Figure 5b. The range of the normalized Fe+, FeOH, FeS+, and Fe2S2+ peak relative intensities was 10−7 to 10−5. The positive-ion peak relative intensities increased with increasing concentration of Fe3+ in the pulp. However, the relative intensity of the peaks was too low to show that Fe species were adsorbed on the hemimorphite surface. The results differ from those obtained in a study of Fe3+ adsorption on smithsonite surfaces. Deng et al. found that Fe(III) greatly decreased adsorption of S2− on the smithsonite surface and Fe(III) species were adsorbed on the smithsonite surface via both electrostatic and chemical interactions. This difference shows that the mechanism of depression by iron ions of zinc oxide ores varies. The difference show that the depression mechanism of iron ions to zinc oxide ores is various. Meanwhile, the implication for minerals sulfide process is also different.
Figure 6 shows the reconstructed 3D negative-depth profile for the hemimorphite sample after S− adsorption. A Zn-S film was generated on the hemimorphite surface by treatment with Na2S. However, the Zn-S film was attenuated in a solution containing 1 mM Fe3+. This shows that Fe3+ can decrease the adsorption of S species on the hemimorphite surface. These results are in good agreement with the ICP-MS results. The results show that the Zn-S film on the hemimorphite surface was attenuated in a solution containing Fe3+ because hemimorphite was depressed, not because Fe species were adsorbed on the hemimorphite surface. The chemical reaction at the mineral surface is unknown and needs to be studied further.
3.4. XPS Results
XPS is a surface-sensitive technique and can be used to characterize a surface to a depth of less than 10 nm. This technique was used to identify the chemical states and contents of the elements on the surfaces of the untreated and treated hemimorphite samples, on the basis of the distinctive binding energies of the inner electrons of each element [15,16]. In this work, XPS was used to identify the differences between untreated hemimorphite samples and those treated with Na2S or Na2S + Fe3+, and to clarify the chemical reactions on the mineral surface.
The full spectra of the treated and untreated hemimorphite samples over the binding energy range 1100–0 eV are shown in Figure 7. The spectra show the presence of the expected elements from hemimorphite, such as Si, O, and Zn, but no other peaks were detected in the XP spectrum of the untreated mineral. This implies that the hemimorphite was pure. A new, weak peak appeared at 161.86 eV in the XP spectrum of hemimorphite treated with Na2S. In the solution containing Fe3+, the S 2p peak was clearly attenuated. The iron species sign was not identified by XPS; the same results were obtained for the Fe 2p high-resolution XP spectrum. This is in agreement with the adsorption test and TOF-SIMS results. The results suggest that Fe3+ cannot be adsorbed on the hemimorphite surface. However, Fe3+ can decrease the adsorption of S species on the hemimorphite surface. The high-resolution S 2p XP spectra are shown in Figure 8. The spectrum of the sample with Fe3+ shows no S signals, whereas the latter (sulfated without Fe3+) spectrum features S 2p3/2 (161.56 eV) and S 2p1/2 (162.74 eV) peaks. These peaks are ascribable to S2−; no other S species were detected on the hemimorphite surface. The spectrum of the sample with the solution containing Fe3+ shows S 2p3/2 (161.64 eV) and S 2p1/2 (162.82 eV) peaks, which are also ascribable to S2− [17,18]; no other S species were found on the hemimorphite surface. The S 2p peak was clearly less intense than that for the sample with no added Fe3+. Table 1 summarizes the concentrations of the various Si, Zn, O, and S species on the treated and untreated hemimorphite surfaces. The data in Table 1 show that the levels of O, Si, and S in the untreated hemimorphite were 49.59, 10.67, and 39.74 at%, respectively. The concentration of S was determined to be 8.20 at% from the S signals detected after treatment with 0.1 mM Na2S. Compared to that of the hemimorphite sample in the solution with Fe3+, the concentration of S in the hemimorphite sample was lower, namely 5.18 at%. These results are in agreement with those reported above. We can conclude that S species were adsorbed on the hemimorphite surface in the form of S2−, and the addition of Fe3+ to the solution decreased sulfide adsorption.
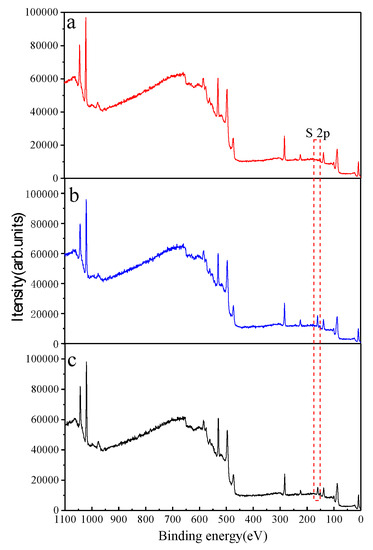
Figure 7.
XPS survey spectra of hemimorphite treated with (a) deionized water, (b) 5 mM Na2S (c) 5 mM Na2S + 1mM Fe3+.
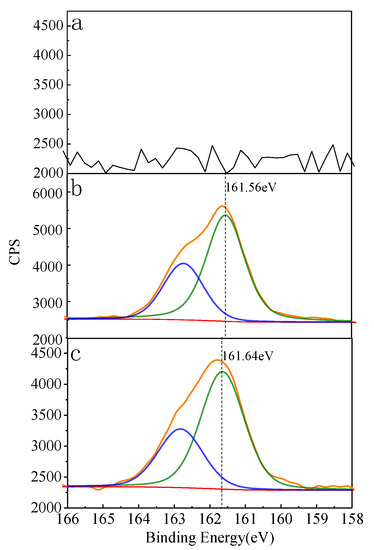
Figure 8.
S2p XPS spectra of hemimorphite treated with (a) deionized water, (b) 5 mM Na2S, (c) 5 mM Na2S + 1mM Fe3+.

Table 1.
Concentrations of various elements on the hemimorphite surfaces.
4. Conclusions
The mechanism of sulfated hemimorphite depression by Fe3+ is a complicated process. Use of a combination of techniques is needed to understand the mechanism. This study examined changes caused by S adsorption at the hemimorphite/water interface and on the mineral surface and assessed the implications of these changes for hemimorphite flotation. The following conclusions can be drawn from our analysis of the results of this study.
(1) High hemimorphite floatability can be achieved by treatment with Na2S, but the flotation recovery decreases with increasing concentration of Fe3+ in solution. The addition of Fe3+ to the solution leads to hemimorphite sample depression at all pH values.
(2) The electrochemical impedance of the hemimorphite sample decreased after treatment with Na2S. The sulfuration of hemimorphite in a solution containing Fe3+ improved the average impedance of the hemimorphite sample. This implies that Fe3+ attenuates the adsorption of high-conductivity materials or leads to the generation of low-conductivity materials on the mineral surface.
(3) Adsorption tests showed that Fe species were not adsorbed on the hemimorphite surface but treatment with Fe3+ attenuated the adsorption of S species. Reconstructed 3D negative-depth profiles for the hemimorphite samples showed that the amount of sulfide film decreased significantly after treatment with Fe3+.
(4) The results of XPS analysis were similar to those of the adsorption tests and suggested that S was adsorbed on the hemimorphite surface in the form S2− and Fe3+ addition to the pulp led to a decrease in sulfide film formation. The hemimorphite floatability, therefore, decreases after treatment with Fe3+.
Author Contributions
Conceptualization, J.L. and Q.F.; Methodology, J.L.; Software, Q.Z.; Validation, S.W., Q.F. and J.L.; Formal Analysis, Y.Z.; Investigation, Y.W.; Resources, J.L.; Data Curation, W.N.; Writing—original draft preparation, J.L.; Writing—review and editing, J.L.; Visualization, Q.F.; Supervision, Q.F.; Project Administration, S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (No. 51804144), and Ten Thousand Talent Plans for Young Top-notch Talents of Yunnan Province (No. YNWR-QNBJ-2018-051).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, C.; Feng, Q.M.; Zhang, G.F.; Ma, W.K.; Meng, Q.Y.; Chen, Y.F. Effects of lead ions on the flotation of hemimorphite using sodium oleate. Miner. Eng. 2016, 89, 163–167. [Google Scholar] [CrossRef]
- Qin, J.Q.; Liu, G.Y.; Fan, H.L.; Tan, W. The hydrophobic mechanism of di(2-ethylhexyl) phosphoric acid to hemimorphite flotation. Colloid Surf. A 2018, 545, 68–77. [Google Scholar] [CrossRef]
- Deng, R.D.; Hu, Y.; Ku, J.G.; Zuo, W.R.; Yang, Z.G. Adsorption of Fe(III) on smithsonite surfaces and implications for flotation. Colloid Surf. A 2017, 533, 308–315. [Google Scholar] [CrossRef]
- Zhao, W.J.; Liu, D.W.; Feng, Q.C. Enhancement of salicylhydroxamic acid adsorption by Pb(II) modified hemimorphite surfaces and its effect on floatability. Miner. Eng. 2020, 152, 106373. [Google Scholar] [CrossRef]
- Barker, G.J.; Gerson, A.R.; Menuge, J.F. The impact of iron sulfide on lead recovery at the giant Navan Zn-Pb orebody, Ireland. Int. J. Miner. Process. 2014, 128, 16–24. [Google Scholar] [CrossRef]
- Moradi, S.; Monhemius, A.J. Mixed sulphide-oxide lead and zinc ores: Problems and solutions. Miner. Eng. 2011, 24, 1062–1076. [Google Scholar] [CrossRef]
- Liu, W.G.; Zhao, L.; Liu, W.B.; Yang, T.; Duan, H. Synthesis and utilization of a gemini surfactant as a collector for the flotation of hemimorphite from quartz. Miner. Eng. 2019, 134, 394–401. [Google Scholar] [CrossRef]
- Jia, K.; Feng, Q.M.; Zhang, G.F.; Shi, Q.; Chang, Z.Y. Understanding the roles of Na2S and Pb(II)in the flotation of hemimorphite. Miner. Eng. 2017, 111, 167–173. [Google Scholar] [CrossRef]
- Shen, P.L.; Liu, D.W.; Zhang, X.L.; Jia, X.D.; Song, K.W.; Liu, D. Effect of (NH4)2SO4 on eliminating the depression of excess sulfide ions in the sulfidization flotation of malachite. Miner. Eng. 2019, 137, 43–52. [Google Scholar] [CrossRef]
- Feng, Q.C.; Wen, S.M.; Zhao, W.J.; Cao, Q.B.; Lu, C. A novel method for improving cerussite sulfidization. Int. J. Min. Met. Mater. 2016, 23, 609–617. [Google Scholar] [CrossRef]
- Grano, S.R.; Cnossen, H.; Skinner, W.; Prestidge, C.A.; Ralston, J. Surface modifications in the chalcopyrite-sulphite ion system, II. Dithiophosphate collector adsorption study. Int. J. Miner. Process. 1997, 50, 27–45. [Google Scholar] [CrossRef]
- Yu, L.; Liu, Q.J.; Li, S.M.; Deng, J.S.; Luo, B.; Lai, H. Depression mechanism involving Fe3+ during arsenopyrite flotation. Sep. Purif. Technol. 2019, 222, 109–116. [Google Scholar] [CrossRef]
- Jiang, C.L.; Wang, X.H.; Parekh, B.K.; Leonard, J.W. The surface and solution chemistry of pyrite flotation with xanthate in the presence of iron ions. Colloids Surf. A Physicochem. Eng. Asp. 1998, 136, 51–62. [Google Scholar] [CrossRef]
- Zhang, Q.; Rao, S.R.; Finch, J.A. Flotation of sphalerite in the presence of iron ions. Colloids Surf. 1992, 66, 81–89. [Google Scholar] [CrossRef]
- Han, G.; Wen, S.M.; Wang, H.; Feng, Q.C. Selective adsorption mechanism of salicylic acid on pyrite surfaces and its application in flotation separation of chalcopyrite from pyrite. Sep. Purif. Technol. 2020, 240, 116650. [Google Scholar] [CrossRef]
- Wang, H.; Wen, S.M.; Han, G.; Xu, L.; Feng, Q.C. Activation mechanism of lead ions in the flotation of sphalerite depressed with zinc sulfate. Miner. Eng. 2020, 146, 106132. [Google Scholar] [CrossRef]
- Yu, L.; Liu, Q.; Li, S.M.; Deng, J.S.; Luo, B.; Lai, H. Adsorption performance of copper ions on arsenopyrite surfaces and implications for flotation. Appl. Surf. Sci. 2019, 488, 185–193. [Google Scholar] [CrossRef]
- Li, Y.; Quanjun, L.; Shimei, L.; Chao, S.; Yang, G.; Jianwen, S. The synergetic depression effect of KMnO4 and CMC on the depression of galena flotation. Chem. Eng. Commun. 2018, 1–11. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).