Atomic Structure, Electronic and Mechanical Properties of Pyrophyllite under Pressure: A First-Principles Study
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussions
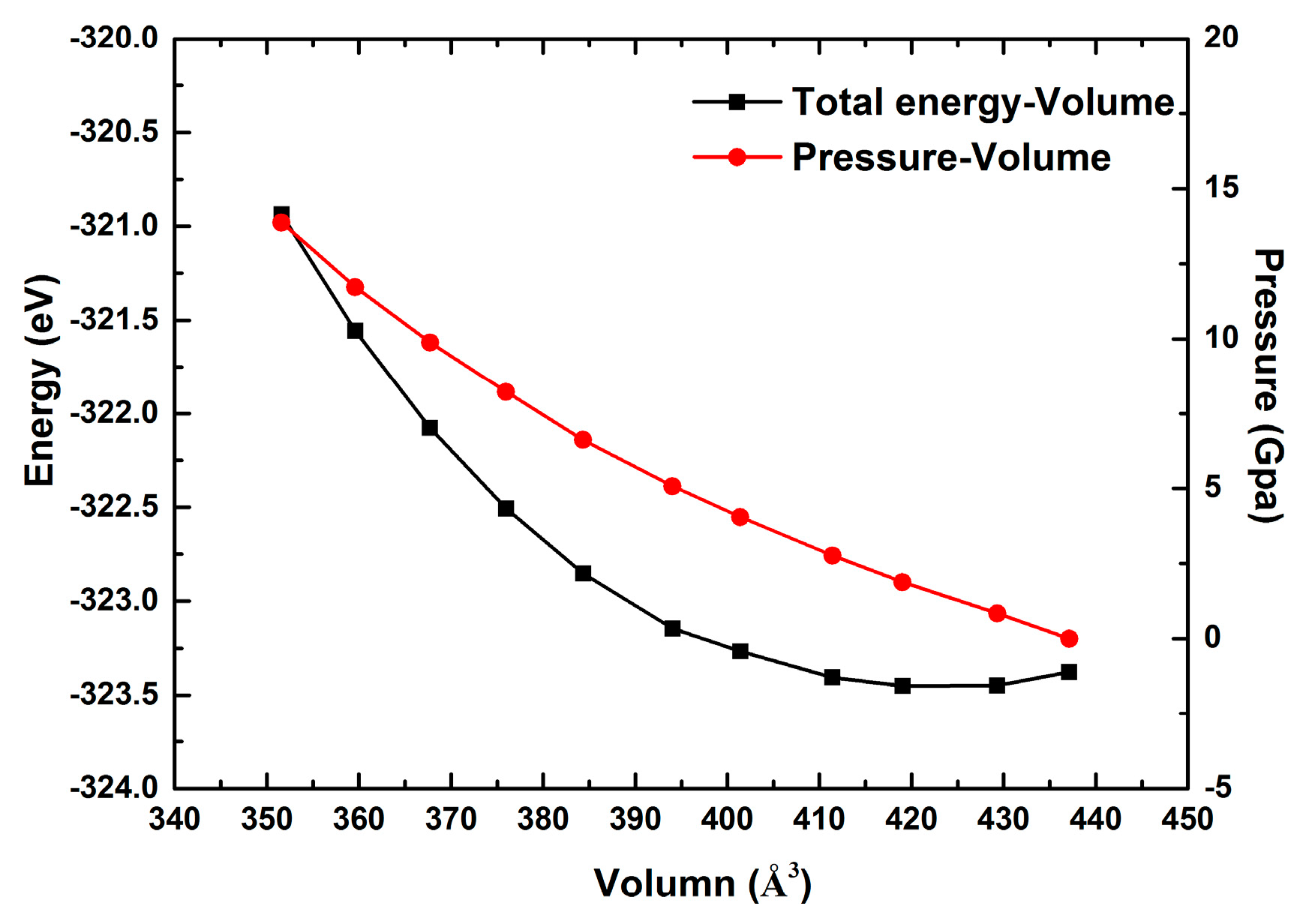
3.1. Effect of Pressure on the Atomic Structure of Pyrophyllite
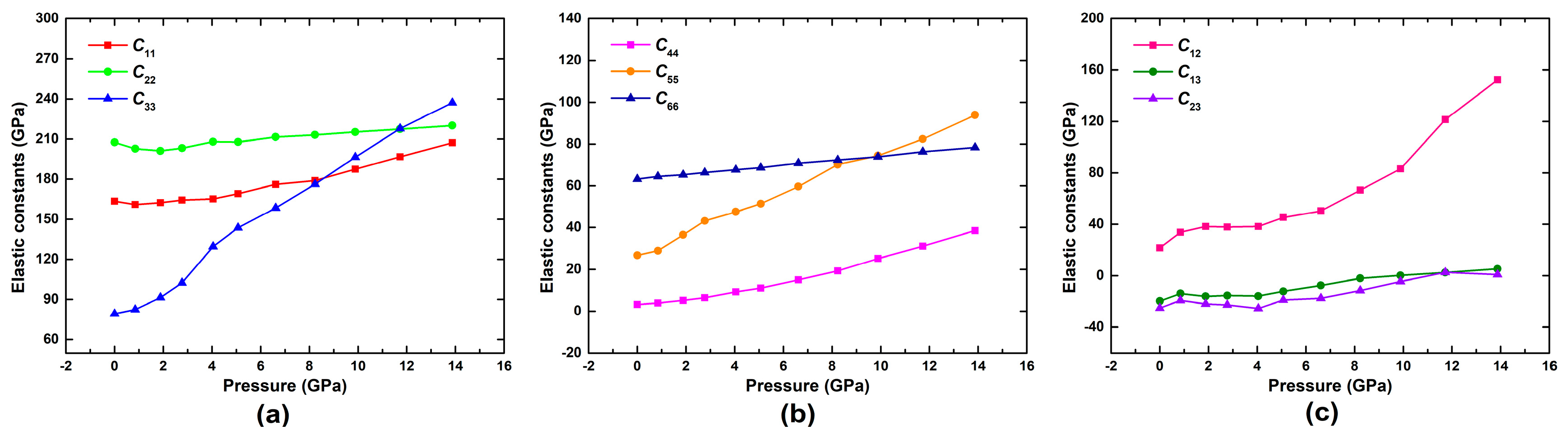
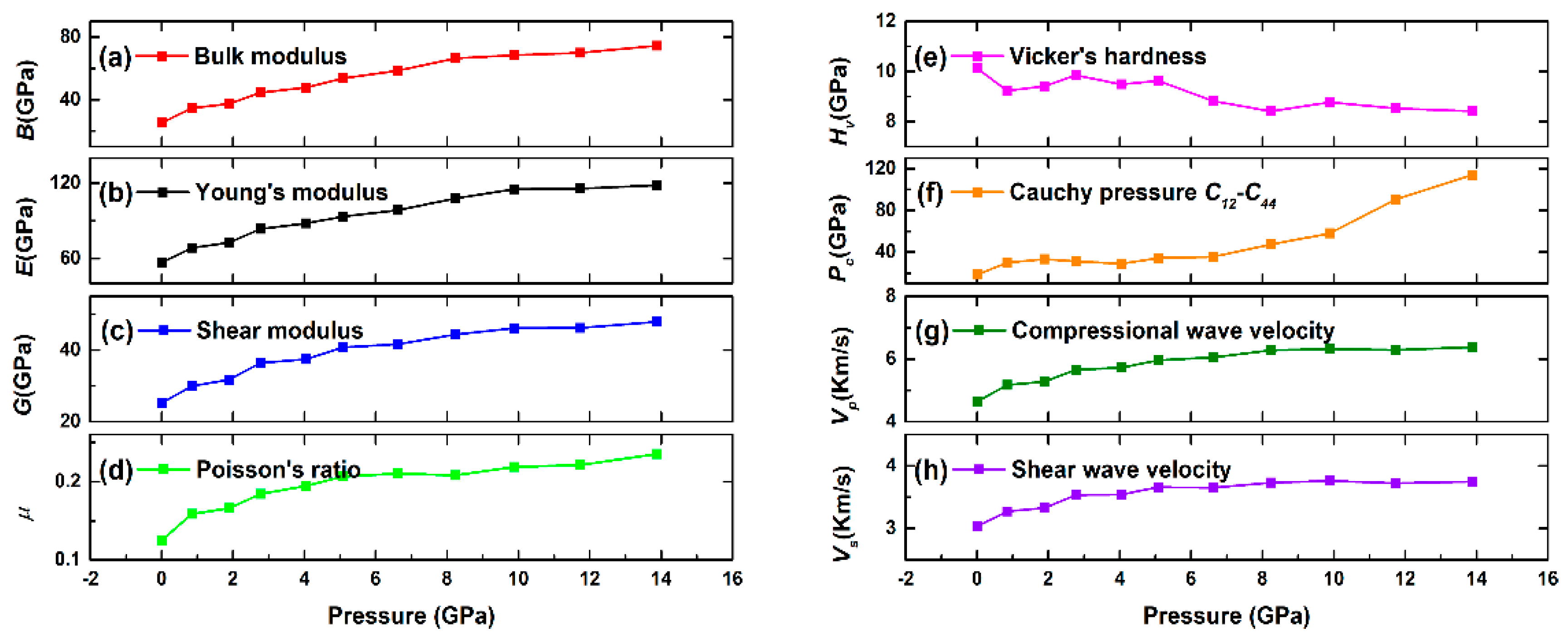
3.2. Effect of Pressure on the Mechanical Properties of Pyrophyllite
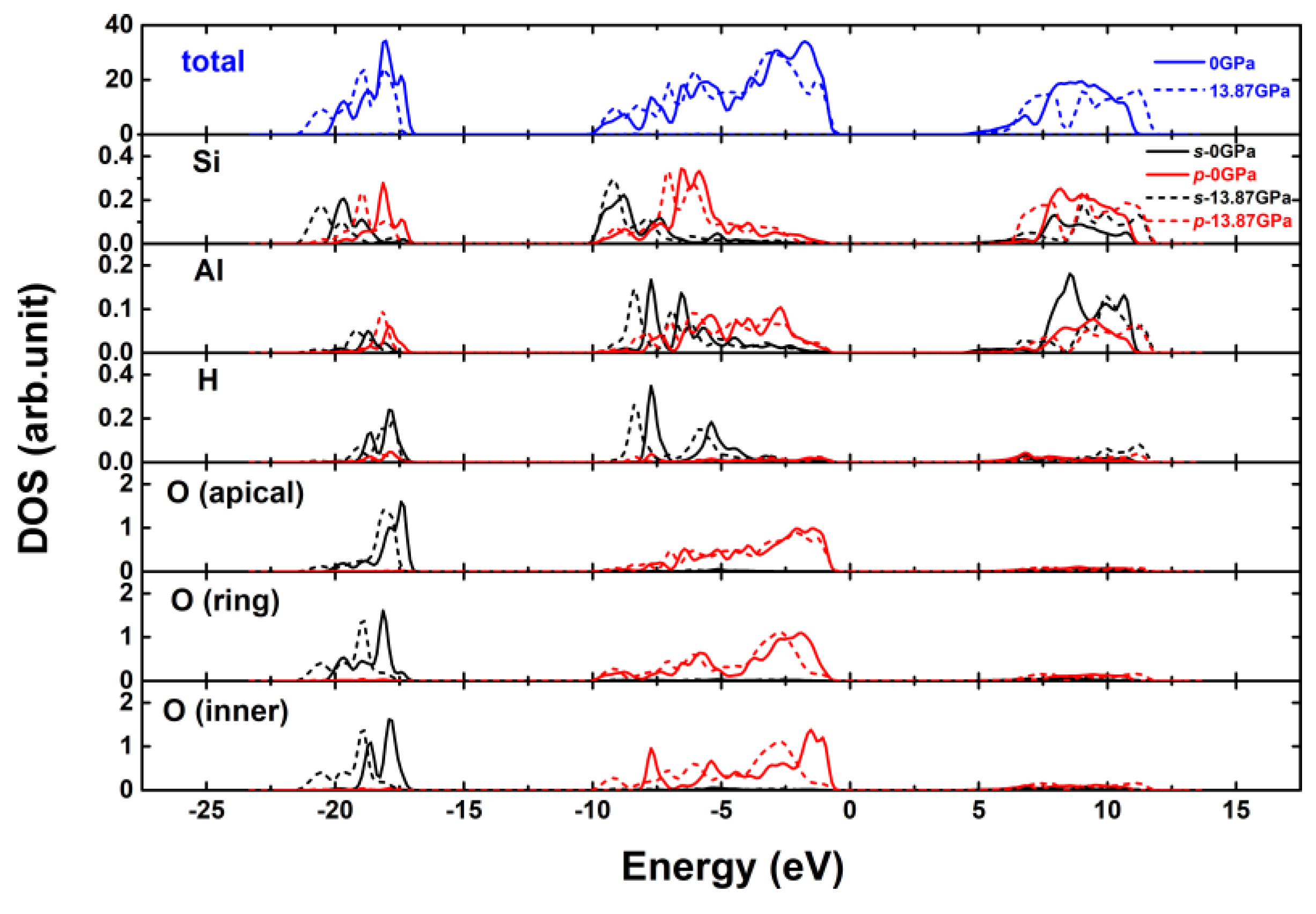
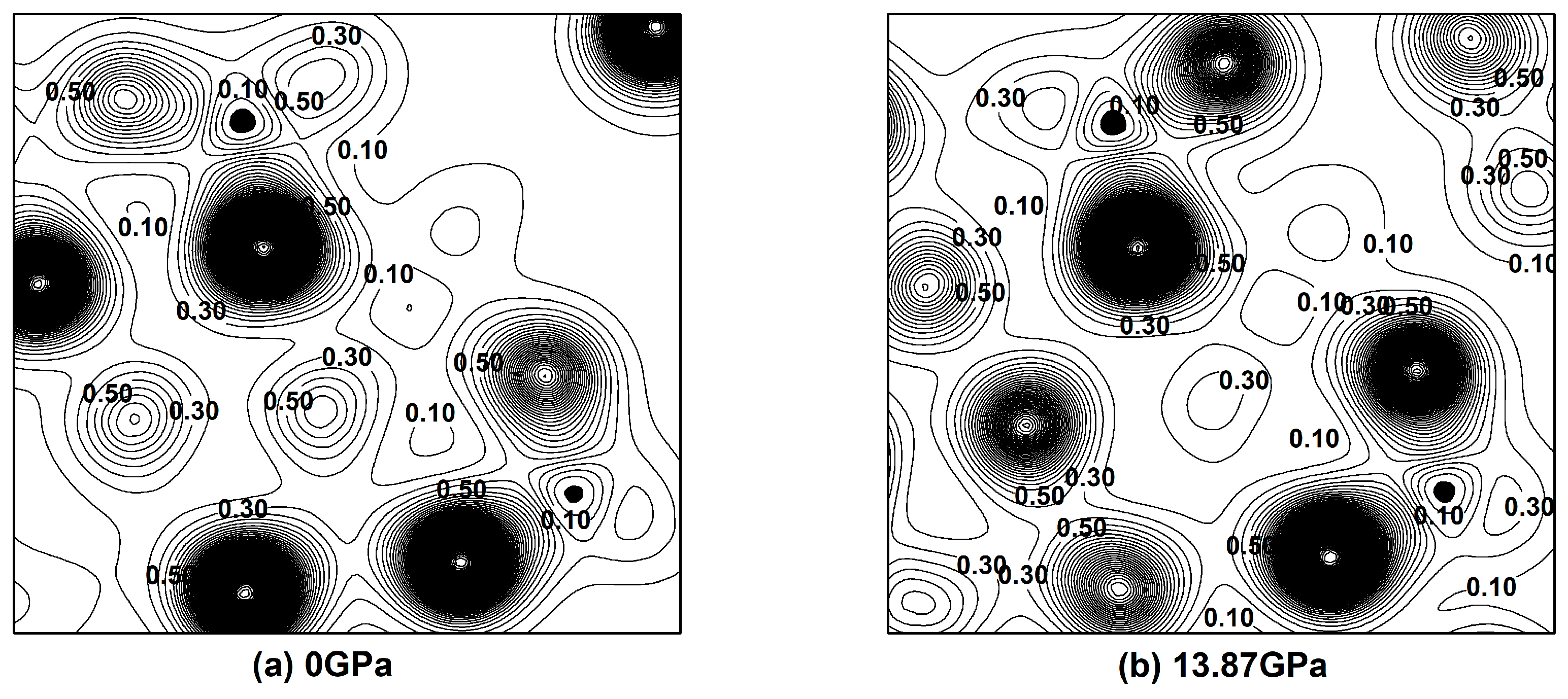
3.3. Effect of Pressure on the Electronic Properties of Pyrophyllite
4. Conclusions
- (1)
- At zero pressure, the calculated structural parameters of pyrophyllite in this paper are in good accord with the existing experimental results. With increasing pressure, the volume, bond lengths, interlayer thickness, and layer thickness of pyrophyllite decrease gradually. In particular, the cation-anion bond along the c-axis in pyrophyllite is weaker than the cation-anion bond along the a-b plane, which indicates the deformation observed in pyrophyllite is mostly along the c-axis.
- (2)
- At different pressures, the calculated elastic constants of pyrophyllite satisfy with Born-Huang criteria, indicating that triclinic pyrophyllite is mechanical stability. The elastic constants C11, C22, C33, C44, C55, C66, C12, C13, C14, C15, C16, C23, C24, C25, C34, and C35 increase with increasing pressure, while the other elastic stiffness constants decrease with increasing pressure. Meanwhile, the calculated bulk modulus, shear modulus, Young modulus, Poisson’s ratio, Pugh’s modulus, Cauchy pressure increase with increasing pressure, indicating the elastic mechanics and the ductility of pyrophyllite is significantly improved.
- (3)
- The DOS, charge density distribution and band structure results show that pyrophyllite has covalent feature and is an insulator at zero pressure. With the increase of pressure from 0 GPa to 13.87 GPa, the calculated electronic quantity changes slightly, indicating that the pressure has a little effect on the electronic properties of pyrophyllite. At 13.87 GPa, pyrophyllite still maintains the typical insulation, which partly proves the reliability of pyrophyllite as a sealed pressure-transmission medium from the perspective of electronic structure., and pyrophyllite still has good stability under high pressure
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, W. Application progress of pyrophyllite in China. Miner. Rock 2016, 36, 15–28. (In Chinese) [Google Scholar]
- Greathouse, J.A.; Cygan, R.T. Chapter 3—Molecular Simulation of Clay Minerals. In Developments in Clay Science, 3rd ed.; Bergaya, F., Lagaly, G., Eds.; Handbook of Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; pp. 405–423. [Google Scholar]
- Li, D.M.; Liu, G.Z.; Zhou, J.X. Water promoted phase transition of pyrophyllite under ultra high pressure and high temperature and its influence on technical application. Sci. Bull. 1978, 481–485. (In Chinese) [Google Scholar]
- Du, P.X.; Yuan, P. Studies and Applications of Pyrophyllite in Key Mineral Material Areas Such as Superhard Materials. Conserv. Util. Miner. Resour. 2019, 39, 87–92. (In Chinese) [Google Scholar]
- Deng, W.L.; Deng, F.M.; Ma, X.D.; Xu, C.Y.; Zhao, J. Synchroteon radiation X-ray diffraction analysis of pyrophyllite under high-pressure. J. Min. Sci. Technol. 2019, 4, 254–260. [Google Scholar]
- Ulian, G.; Valdrè, G. Structural, vibrational and thermophysical properties of pyrophyllite by semi-empirical density functional modelling. Phys. Chem. Miner. 2015, 42, 609–627. [Google Scholar] [CrossRef]
- Berthonneau, J.; Hoover, C.G.; Granby, O.; Baronnet, M.; Pellenq, R.J.M.; Ulm, F.J. Crystal-chemistry control of the mechanical properties of 2:1 clay minerals. Appl. Clay Sci. 2017, 143, 387–398. [Google Scholar] [CrossRef]
- Sachse, W.; Ruoff, A.L. Elastic moduli of precompressed pyrophyllite used in ultrahigh, ressure research. J. Appl. Phys. 1975, 46, 3725–3730. [Google Scholar] [CrossRef]
- Zhang, G.P.; Wei, Z.G.; Ferrell, R.E.; Guggenheim, S.; Cygan, R.T.; Luo, J. Evaluation of the elasticity normal to the basal plane of non-expandable 2:1 phyllosilicate minerals by nanoindentation. Am. Mineral. 2010, 95, 863–869. [Google Scholar] [CrossRef]
- Cheng, Q.L.; Sondergeld, C.; Rai, C. Experimental study of rock strength anisotropy and elastic modulus anisotropy. In SEG Technical Program Expanded Abstracts 2013; SEG: Houston, TX, USA, 2013; pp. 362–367. [Google Scholar]
- LiBalan, E.; Pietrucci, F.; Gervais, C.; Blanchard, M.; Schott, J.; Gaillardet, J. First-principles study of boron speciation in kaolinite and aragonite. Geochim. Cosmochim. Acta 2016, 193, 119–131. [Google Scholar]
- Weck, P.F.; Kim, E.; Jové-Colón, C.F. Relationship between crystal structure and thermo-mechanical properties of kaolinite clay: Beyond standard density functional theory. Dalton Trans. 2015, 44, 12550–12560. [Google Scholar] [CrossRef]
- Fang, Z.J.; Zhai, X.S.; Li, Z.L.; Pan, R.J.; Mo, M. Pressure dependence of the electronic structure in kaolinite: A first-principles study. Mod. Phys. Lett. B 2017, 31, 1750194. [Google Scholar] [CrossRef]
- Molina-Montes, E.; Donadio, D.; Herna, N.L.A.; Sainz, C.I.; Parrinello, M. DFT research on the dehydroxylation reaction of pyrophyllite1. first-principle molecular dynamics simulations. J. Phys. Chem. B 2008, 112, 7051–7060. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; He, M.C.; Hu, X.X.; Gao, W. Density functional theory investigation of carbon monoxide adsorption on the kaolinite (001) surface. Chin. Phys. B 2017, 26, 079101. [Google Scholar] [CrossRef]
- Gatta, G.D.; Lotti, P.; Merlini, M.; Liermann, H.P.; Lausi, A.; Valdrè, G.; Pavese, A. Elastic behaviour and phase stability of pyrophyllite and talc at high pressure and temperature. Phys. Chem. Miner. 2014, 42, 309–318. [Google Scholar] [CrossRef]
- Bridgeman, C.H. Ab-initio total energy study of uncharged 2:1 clays and their interaction with water. Mol. Phys. 1996, 89, 879–888. [Google Scholar] [CrossRef]
- Refson, K.; Park, S.H.; Sposito, G. Ab Initio Computational Crystallography of 2:1 Clay Minerals: 1. Pyrophyllite-1Tc. J. Phys. Chem. B 2003, 107, 13376–13383. [Google Scholar] [CrossRef]
- Katti, D.R.; Schmidt, S.R.; Ghosh, P.; Katti, K.S. Modeling the response of pyrophyllite interlayer to applied stress using steered molecular dynamics. Clays Clay Miner. 2005, 53, 171–178. [Google Scholar] [CrossRef]
- Militzer, B.; Wenk, H.R.; Stackhouse, S.; Stixrude, L. First-principles calculation of the elastic moduli of sheet silicates and their application to shale anisotropy. Am. Mineral. 2011, 96, 125–137. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W.T. Density-Functional Theory of Atoms and Molecules; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Bucko, T.; Hafner, J.; Lebegue, S.; Angyan, J.G. Improved description of the structure of molecular and layered crystals: Ab initio DFT calculations with van der Waals corrections. J. Phys. Chem. A 2010, 114, 11814. [Google Scholar] [CrossRef]
- Blchl, P.E. Projector Agmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Monkhorst, H.J. Special points for Brillouin-zone integrations. Phys. Rev. B Condens. Matter 1976, 16, 1748–1749. [Google Scholar] [CrossRef]
- Pawley, A.; Clark, S.; Chinnery, N. Equation of state measurements of chlorite, pyrophyllite, and talc. Am. Mineral. 2002, 87, 1172–1182. [Google Scholar] [CrossRef]
- Bruno, M.; Prencipe, M.; Giovanni, V. Ab initio quantum mechanical modeling of pyrophyllite [Al2Si4O10(OH)2] and talc [Mg3Si4O10(OH)2] surfaces. Phys. Chem. Miner. 2006, 33, 63–71. [Google Scholar] [CrossRef]
- Aleksandrov, K.; Prodaivoda, G. Elastic properties of minerals. Crystallogr. Rep. 1993, 38, 698–709. [Google Scholar]
- Zhao, J.; He, M.C. Theoretical study of heavy metal Cd, Cu, Hg, and Ni(II) adsorption on the kaolinite(001) surface. Appl. Surf. Sci. 2014, 317, 718–723. [Google Scholar] [CrossRef]
- Voigt, W. Lehrbuch der Kristallphysik; Teubner: Leipzig, Germany, 1928. [Google Scholar]
- Reuss, A. Berechnung der Fließgrenze von Mischkristallen auf Grund der Plastizitätsbedingung für Einkristalle. ZAMM J. Appl. Math. Mech. 1929, 9, 49–58. [Google Scholar] [CrossRef]
- Hill, R. The Elastic Behaviour of a Crystalline Aggregate. Proc. Phys. Soc. Sect. A 1952, 65, 349–354. [Google Scholar] [CrossRef]
- Brindley, G.W. Phyllosilicate. In Mineralogy; Springer: New York, NY, USA, 1981. [Google Scholar]
- Kogure, T. Stacking structures in pyrophyllite revealed by high-resolution transmission electron microscopy (hrtem). Am. Mineral. 2006, 91, 1293–1299. [Google Scholar] [CrossRef]
- Hendricks, S.B. Lattice Structure of Clay Minerals and Some Properties of Clays. J. Geol. 1942, 50, 276–290. [Google Scholar] [CrossRef]
- Rayner, J.; Brown, G. Structure of pyrophyllite. Clays Clay Miner. 1966, 25, 73–84. [Google Scholar] [CrossRef]
- Giese, R. Hydroxyl orientation in pyrophyllite. Nature 1973, 241, 151. [Google Scholar] [CrossRef]
- Churakov, S.V. Ab Initio Study of Sorption on Pyrophyllite: Structure and Acidity of the Edge Sites. J. Phys. Chem. B 2006, 110, 4135–4146. [Google Scholar] [CrossRef] [PubMed]
- Drits, V.A.; Guggenheim, S.; Zviagina, B.B.; Kogure, T. Structures of the 2:1 Layers of Pyrophyllite and Talc. Clays Clay Miner. 2012, 60, 574–587. [Google Scholar] [CrossRef]
- Lee, J.H.; Guggenheim, S. Single crystal X-ray refinement of pyrophyllite-1Tc. Am. Mineral. 1981, 66, 350–357. [Google Scholar]
- Benazzouz, B.K.; Zaoui, A. Phase diagram of kaolinite from Molecular Dynamics calculations. Phys. B Condens. Matter 2012, 407, 2462–2470. [Google Scholar] [CrossRef]
- Zhao, J.; Qin, X.Z.; Wang, J.M.; He, M.C. Effect of Mg(II) and Na(I) Doping on the Electronic Structure and Mechanical Properties of Kaolinite. Minerals 2020, 10, 368. [Google Scholar] [CrossRef]
- Zartman, G.D.; Liu, H.; Akdim, B.; Pachter, R.; Heinz, H. Nanoscale Tensile, Shear, and Failure Properties of Layered Silicates as a Function of Cation Density and Stress. J. Phys. Chem. C 2010, 114, 1763–1772. [Google Scholar] [CrossRef]
- Cowley, R.A. Critical Scattering from Structural Phase Transitions Associated with a Homogeneous Deformation of the Crystal. In Proceedings of the Conference on Neutron Scattering, Gatlinburg, TN, USA, 6–10 June 1976. [Google Scholar]


| Parameters | Experiment [39] | This Work | Difference |
|---|---|---|---|
| a | 5.160Å | 5.180Å | 0.387% |
| b | 8.966Å | 8.970Å | 0.414% |
| c | 9.347Å | 9.950Å | 6.451% |
| α | 91.18° | 90.00° | −1.129% |
| β | 100.46° | 99.50° | −0.955% |
| γ | 89.64° | 90.00° | 0.401% |
| V | 425.16Å3 | 437.13Å3 | 2.815% |
| Pressure (GPa) | Experiment [39] | This Work | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.00 | 0.00 | 0.85 | 1.88 | 2.77 | 4.05 | 5.07 | 6.62 | 8.23 | 9.89 | 11.73 | 13.87 | |
| Si–Oa(Å) | 1.633 | 1.632 | 1.631 | 1.630 | 1.629 | 1.628 | 1.627 | 1.626 | 1.625 | 1.625 | 1.622 | 1.622 |
| Si–Or(Å) | 1.612 | 1.613 | 1.612 | 1.611 | 1.610 | 1.609 | 1.609 | 1.608 | 1.607 | 1.606 | 1.602 | 1.600 |
| Al–Oa(Å) | 1.924 | 1.914 | 1.910 | 1.903 | 1.898 | 1.892 | 1.889 | 1.884 | 1.880 | 1.875 | 1.875 | 1.873 |
| Al–Oi(Å) | 1.889 | 1.885 | 1.879 | 1.871 | 1.865 | 1.858 | 1.852 | 1.844 | 1.838 | 1.832 | 1.832 | 1.828 |
| Pressure (GPa) | Experiment [39] | This Work | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.00 | 0.00 | 0.85 | 1.88 | 2.77 | 4.05 | 5.07 | 6.62 | 8.23 | 9.89 | 11.73 | 13.87 | |
| dI (Å) | 2.759 | 3.462 | 3.377 | 3.268 | 3.185 | 2.811 | 2.740 | 2.638 | 2.552 | 2.461 | 2.359 | 2.500 |
| dT1 (Å) | 2.153 | 2.296 | 2.282 | 2.263 | 2.249 | 2.364 | 2.346 | 2.326 | 2.308 | 2.293 | 2.284 | 2.158 |
| dO (Å) | 2.079 | 1.945 | 1.998 | 2.066 | 2.117 | 2.180 | 2.227 | 2.289 | 2.341 | 2.393 | 2.440 | 2.485 |
| dT2 (Å) | 2.153 | 2.296 | 2.282 | 2.263 | 2.249 | 2.364 | 2.346 | 2.326 | 2.308 | 2.293 | 2.284 | 2.158 |
| Pressure (GPa) | Other Calculation [42] | This Work | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.00 | 0.00 | 0.85 | 1.88 | 2.77 | 4.05 | 5.07 | 6.62 | 8.23 | 9.89 | 11.73 | 13.87 | |
| C11 | 198.70 | 163.42 | 160.91 | 162.36 | 164.37 | 165.21 | 169.04 | 176.04 | 178.95 | 187.50 | 196.68 | 207.13 |
| C22 | 186.00 | 207.47 | 202.72 | 201.10 | 203.14 | 208.01 | 207.80 | 211.56 | 213.11 | 215.30 | 217.42 | 220.09 |
| C33 | 70.10 | 79.19 | 82.42 | 91.45 | 102.34 | 129.50 | 143.48 | 158.48 | 176.22 | 196.33 | 217.80 | 237.13 |
| C44 | 33.40 | 3.18 | 3.91 | 5.14 | 6.42 | 9.24 | 10.97 | 14.90 | 19.28 | 25.13 | 31.10 | 38.48 |
| C55 | 21.60 | 26.71 | 28.91 | 36.48 | 43.21 | 47.52 | 51.44 | 59.62 | 70.19 | 74.41 | 82.43 | 93.86 |
| C66 | 80.10 | 63.17 | 64.42 | 65.28 | 66.33 | 67.71 | 68.71 | 70.84 | 72.34 | 73.87 | 76.29 | 78.31 |
| C12 | 60.50 | 21.66 | 33.79 | 38.33 | 37.78 | 38.31 | 45.35 | 50.50 | 66.60 | 83.17 | 121.51 | 152.40 |
| C13 | 10.40 | −19.64 | −13.83 | −15.99 | −15.35 | −15.82 | −12.12 | −7.57 | −2.04 | 0.25 | 2.51 | 5.31 |
| C14 | / | 0.01 | −0.17 | −0.37 | 0.26 | 2.11 | 4.05 | 4.87 | 7.11 | 7.19 | 7.12 | 4.91 |
| C15 | / | −23.40 | −22.23 | −18.14 | −16.28 | −9.12 | −8.01 | −9.05 | −12.71 | −8.95 | −4.83 | −2.74 |
| C16 | / | 0.86 | 0.75 | 1.06 | 1.55 | 2.74 | 3.13 | 0.93 | −0.03 | −2.99 | −7.74 | 8.97 |
| C23 | 10.50 | −25.09 | −19.17 | −21.81 | −22.57 | −25.32 | −18.80 | −17.47 | −11.55 | −4.55 | 2.57 | 0.85 |
| C24 | / | 0.27 | −0.38 | −0.59 | −1.17 | −1.48 | 0.20 | 1.24 | 2.16 | 3.01 | 4.53 | 5.30 |
| C25 | / | −4.84 | −1.75 | 0.37 | −2.49 | 0.43 | −1.66 | −0.09 | 2.99 | 7.72 | 15.14 | 21.70 |
| C26 | / | −1.10 | −1.52 | −1.11 | −1.64 | −2.00 | −1.57 | −5.75 | −5.73 | −8.56 | −11.56 | −15.06 |
| C34 | / | 5.99 | 7.41 | 12.04 | 16.08 | 28.62 | 35.75 | 44.74 | 52.76 | 59.46 | 64.59 | 63.53 |
| C35 | / | 18.38 | 22.74 | 22.17 | 20.46 | 20.66 | 30.90 | 30.96 | 30.07 | 29.58 | 27.91 | 23.98 |
| C36 | / | −11.84 | −8.45 | −9.60 | −9.94 | −12.94 | −11.61 | −13.26 | −14.07 | −16.65 | −21.21 | −24.22 |
| C45 | / | −2.92 | −4.76 | −7.67 | −8.06 | −8.72 | −10.86 | −7.53 | −21.48 | −15.89 | −22.54 | −25.49 |
| C46 | / | −10.71 | −10.90 | −11.26 | −11.42 | −11.90 | −11.97 | −12.11 | −12.22 | −12.58 | −12.38 | −11.80 |
| C56 | / | −5.74 | −5.85 | −8.16 | −10.42 | −11.35 | −10.91 | −12.13 | −16.39 | −16.72 | −19.50 | −24.00 |
| Pressure (GPa) | Experiment [28] | This Work | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.00 | 0.00 | 0.85 | 1.88 | 2.77 | 4.05 | 5.07 | 6.62 | 8.23 | 9.89 | 11.73 | 13.87 | |
| B (GPa) | 14.8–37.5 | 25.20 | 34.62 | 37.21 | 44.53 | 47.61 | 53.50 | 58.41 | 66.29 | 68.42 | 69.84 | 74.48 |
| E (GPa) | 38.6–53.6 | 56.70 | 68.46 | 72.71 | 83.56 | 88.02 | 93.43 | 98.36 | 107.81 | 115.29 | 115.77 | 118.33 |
| G (GPa) | 18.2–21.3 | 25.20 | 29.91 | 31.64 | 36.39 | 37.42 | 40.72 | 41.58 | 44.33 | 46.09 | 46.17 | 47.90 |
| μ | 0.06–0.26 | 0.12 | 0.16 | 0.17 | 0.18 | 0.19 | 0.21 | 0.21 | 0.21 | 0.22 | 0.22 | 0.24 |
| Hv (GPa) | / | 10.12 | 9.23 | 9.40 | 9.85 | 9.48 | 9.63 | 8.82 | 8.41 | 8.77 | 8.53 | 8.41 |
| Pc (GPa) | / | 18.48 | 29.88 | 33.19 | 31.36 | 29.07 | 34.38 | 35.60 | 47.32 | 58.04 | 90.41 | 113.92 |
| Vp (km/s) | 3.78~4.91 | 4.63 | 5.17 | 5.27 | 5.66 | 5.72 | 5.96 | 6.05 | 6.28 | 6.32 | 6.28 | 6.37 |
| Vs (km/s) | 2.58~2.79 | 3.03 | 3.28 | 3.33 | 3.54 | 3.54 | 3.66 | 3.65 | 3.73 | 3.76 | 3.72 | 3.75 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, X.; Zhao, J.; Wang, J.; He, M. Atomic Structure, Electronic and Mechanical Properties of Pyrophyllite under Pressure: A First-Principles Study. Minerals 2020, 10, 778. https://doi.org/10.3390/min10090778
Qin X, Zhao J, Wang J, He M. Atomic Structure, Electronic and Mechanical Properties of Pyrophyllite under Pressure: A First-Principles Study. Minerals. 2020; 10(9):778. https://doi.org/10.3390/min10090778
Chicago/Turabian StyleQin, Xinzhan, Jian Zhao, Jiamin Wang, and Manchao He. 2020. "Atomic Structure, Electronic and Mechanical Properties of Pyrophyllite under Pressure: A First-Principles Study" Minerals 10, no. 9: 778. https://doi.org/10.3390/min10090778
APA StyleQin, X., Zhao, J., Wang, J., & He, M. (2020). Atomic Structure, Electronic and Mechanical Properties of Pyrophyllite under Pressure: A First-Principles Study. Minerals, 10(9), 778. https://doi.org/10.3390/min10090778