Formation of Spessartine and CO2 via Rhodochrosite Decarbonation along a Hot Subduction P-T Path
Abstract
1. Introduction
2. Materials and Methods
2.1. High-Pressure High-Temperature Experimental Methods
2.2. Analytical Methods
3. Results
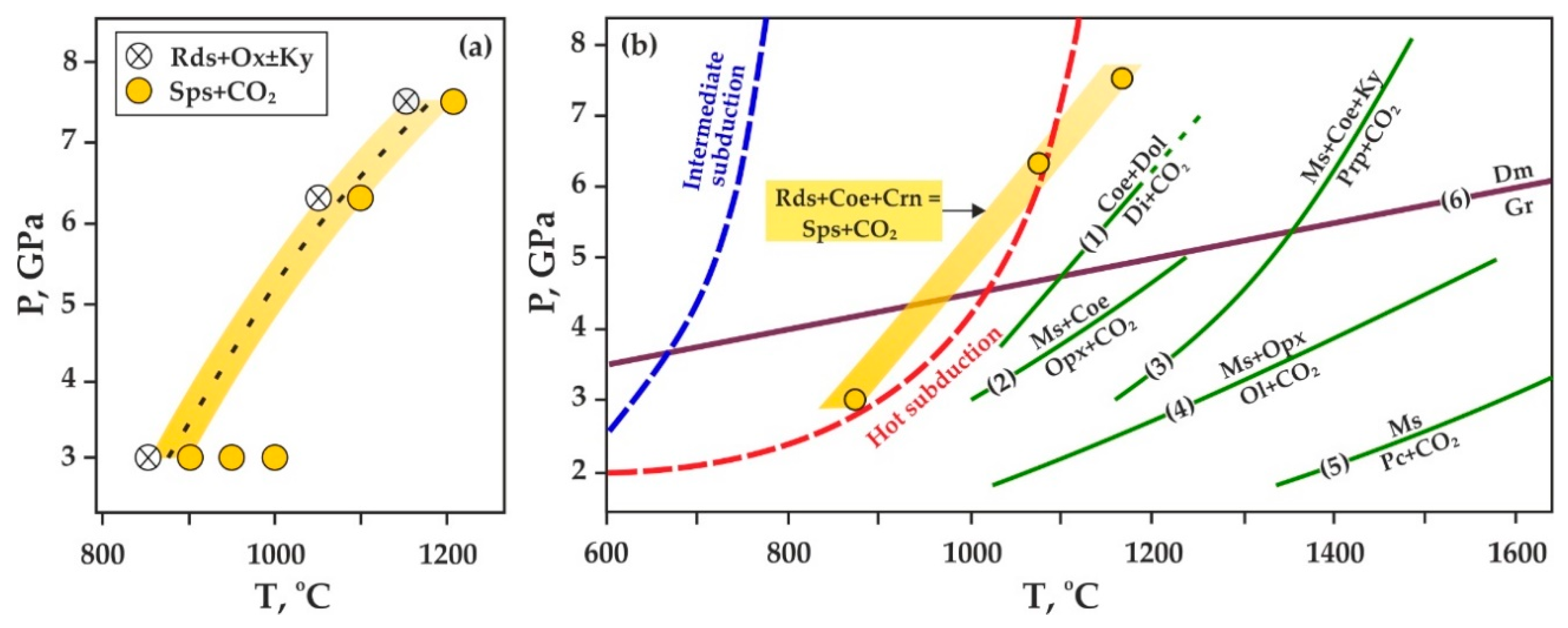
4. Discussion
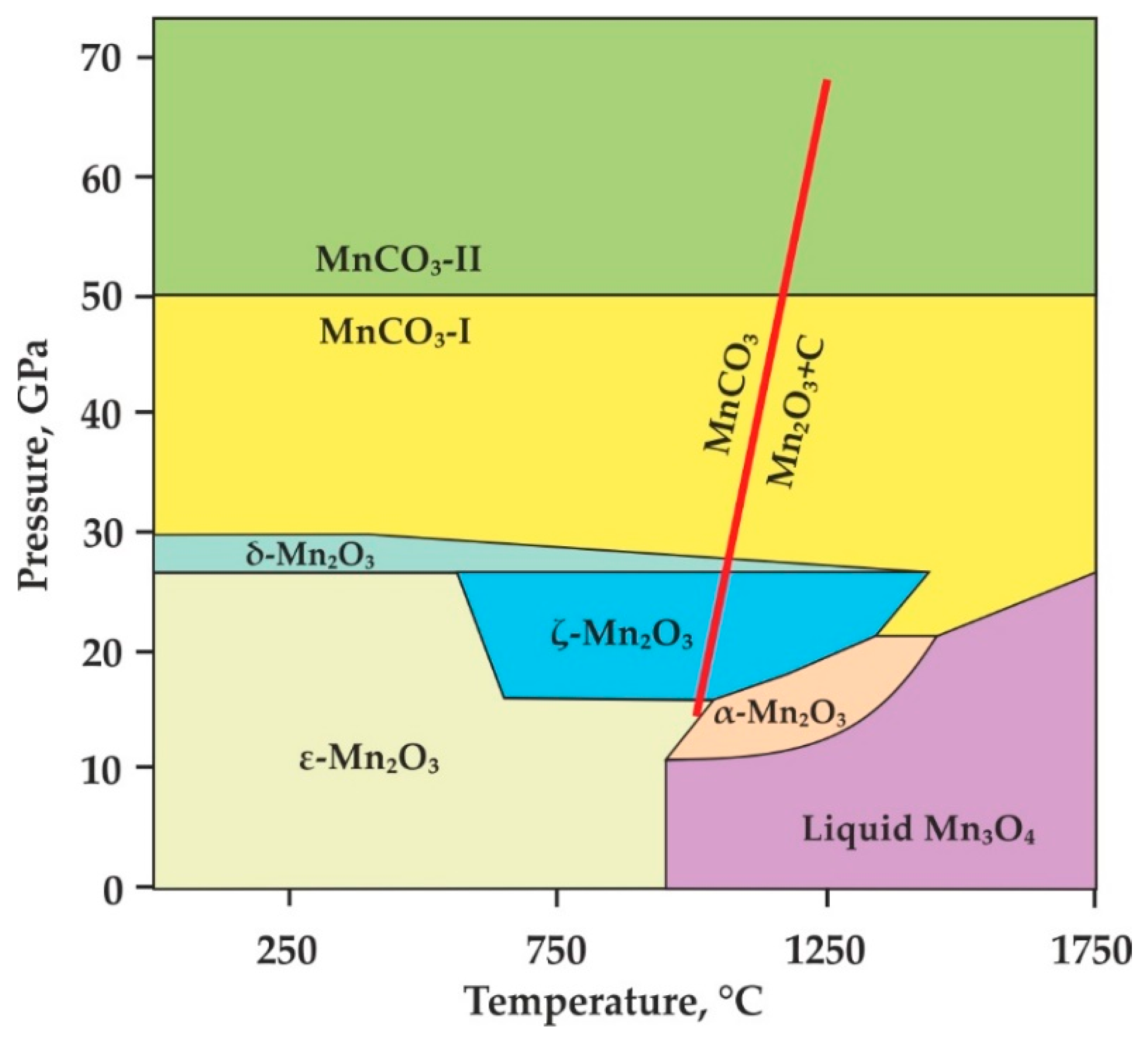
4.1. Conditions of Rhodochrosite-Involving Decarbonation Reactions and Comparison to the Stability of Other Carbonates
4.2. Spessartine + CO2 Formation and Possible Implications for Diamond or Graphite Genesis
5. Conclusions
- Our HP-HT experiments with a specially designed hematite-buffered high-pressure cell showed that decarbonation in the MnCO3-SiO2-Al2O3 system resulted in the formation of a CO2-fluid and spessartine at 870 ± 20 °C (3.0 GPa), 1070 ± 20 °C (6.3 GPa), and 1170 ± 20 °C (7.5 GPa) along a hot subduction P-T path.
- Using the mass spectrometry method (IRMS), the effectiveness of the hematite buffer was demonstrated, and it was shown that the composition of the fluid, liberated as a result of decarbonation, corresponded to pure CO2.
- An experimental reconstruction of the position of the decarbonation curve leading to the formation of a CO2-fluid in assemblage with spessartine was carried out in P-T space. It was found that the experimentally determined decarbonation curve for the formation of spessartine + CO2 was located 300–350 °C lower than that for pyrope + CO2. Our results indicate that the formation of spessartine + CO2 from the decarbonation of rhodochrosite under hot subduction settings would occur at a depth of ~90 km and 850–900 °C and at 190–225 km depth and 1070–1170 °C.
- We experimentally demonstrated that (1) the presence of rhodochrosite as solid solution with Mg,Ca-carbonates in the subducting slab can result in a significant decrease of the decarbonation temperatures, and (2) rhodochrosite decarbonation is an important reaction to explain the relationship between Mn-rich garnets and diamonds with subduction/crustal isotopic signature.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lyubetskaya, T.; Korenaga, J. Chemical composition of Earth’s primitive mantle and its variance: 1. Method and results. J. Geophys. Res. Space Phys. 2007, 112, 1–21. [Google Scholar] [CrossRef]
- McDonough, W.F. The Composition of the Earth. Int. Geophys. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Wang, H.S.; Lineweaver, C.H.; Ireland, T.R. The elemental abundances (with uncertainties) of the most Earth-like planet. Icarus 2018, 299, 460–474. [Google Scholar] [CrossRef]
- Allègre, C.J.; Manhes, G.; Lewin, É. Chemical composition of the Earth and the volatility control on planetary genetics. Earth Planet. Sci. Lett. 2001, 185, 49–69. [Google Scholar] [CrossRef]
- Margolis, S.V.; Burns, R.G. Pacific Deep-Sea Manganese Nodules: Their Distribution, Composition, and Origin. Annu. Rev. Earth Planet. Sci. 1976, 4, 229–263. [Google Scholar] [CrossRef]
- Santillán, J.; Williams, Q. A high-pressure infrared and X ray study of FeCO3 and MnCO3: Comparison with CaMg (CO3)2-dolomite. Phys. Earth Planet. Inter. 2004, 143–144, 291–304. [Google Scholar]
- Liu, L.-G.; Lin, C.-C.; Yang, Y.-J. Formation of diamond by decarbonation of MnCO3. Solid State Commun. 2001, 118, 195–198. [Google Scholar] [CrossRef]
- Ono, S. High-pressure phase transformation in MnCO3: A synchrotron XRD study. Miner. Mag. 2007, 71, 105–111. [Google Scholar] [CrossRef]
- Farfan, G.A.; Boulard, E.; Wang, S.; Mao, W.L. Bonding and electronic changes in rhodochrosite at high pressure. Am. Miner. 2013, 98, 1817–1823. [Google Scholar] [CrossRef]
- Merlini, M.; Hanfland, M.; Gemmi, M. The MnCO3-II high-pressure polymorph of rhodocrosite. Am. Miner. 2015, 100, 2625–2629. [Google Scholar] [CrossRef]
- Boulard, E.; Goncharov, A.F.; Blanchard, M.O.; Mao, W.L. Pressure-induced phase transition in MnCO3 and its implications on the deep carbon cycle. J. Geophys. Res. Solid Earth 2015, 120, 4069–4079. [Google Scholar] [CrossRef]
- Boulard, E.; Liu, Y.; Koh, A.L.; Reagan, M.M.; Stodolna, J.; Morard, G.; Mezouar, M.; Mao, W.L. Transformations and Decomposition of MnCO3 at Earth’s Lower Mantle Conditions. Front. Earth Sci. 2016, 4, 107. [Google Scholar] [CrossRef]
- Appleyard, C.; Viljoen, K.; Dobbe, R. A study of eclogitic diamonds and their inclusions from the Finsch kimberlite pipe, South Africa. Lithos 2004, 77, 317–332. [Google Scholar] [CrossRef]
- Deines, P.; Gurney, J.; Harris, J. Associated chemical and carbon isotopic composition variations in diamonds from Finsch and Premier kimberlite, South Africa. Geochim. Cosmochim. Acta 1984, 48, 325–342. [Google Scholar] [CrossRef]
- Deines, P. The carbon isotope geochemistry of mantle xenoliths. Earth-Sci. Rev. 2002, 58, 247–278. [Google Scholar] [CrossRef]
- Smith, C.; Gurney, J.; Harris, J.; Otter, M.; Kirkley, M.; Jagoutz, E. Neodymium and strontium isotope systematics of eclogite and websterite paragenesis inclusions from single diamonds, Finsch and Kimberley Pool, RSA. Geochim. Cosmochim. Acta 1991, 55, 2579–2590. [Google Scholar] [CrossRef]
- Smith, C.B.; Walter, M.J.; Bulanova, G.P.; Mikhail, S.; Burnham, A.D.; Gobbo, L.; Kohn, S.C. Diamonds from Dachine, French Guiana: A unique record of early Proterozoic subduction. Lithos 2016, 265, 82–95. [Google Scholar] [CrossRef]
- Frezzotti, M.L.; Selverstone, J.; Sharp, Z.D.; Compagnoni, R. Carbonate dissolution during subduction revealed by diamond-bearing rocks from the Alps. Nat. Geosci. 2011, 4, 703–706. [Google Scholar] [CrossRef]
- Groppo, C.; Beltrando, M.; Compagnoni, R. P-T path of the UHP Lago di Cignana and adjoining HP meta-ophiolitic units: Insights into the evolution of subducting Tethyan slab. J. Metamorph. Geol. 2009, 27, 207–231. [Google Scholar] [CrossRef]
- Glassley, W.E.; Korstgård, J.A.; Sørensen, K.; Platou, S.W. A new UHP metamorphic complex in the ~1.8 Ga Nagssugtoqidian Orogen of West Greenland. Am. Miner. 2014, 99, 1315–1334. [Google Scholar] [CrossRef]
- Sobolev, N.V.; Shatsky, V.S. Diamond inclusions in garnets from metamorphic rocks: A new environment for diamond formation. Nature 1990, 343, 742–746. [Google Scholar] [CrossRef]
- Kaminsky, F.; Belousova, E. Manganoan ilmenite as kimberlite/diamond indicator mineral. Russ. Geol. Geophys. 2009, 50, 1212–1220. [Google Scholar] [CrossRef]
- Knoche, R.; Sweeney, R.J.; Luth, R. Carbonation and decarbonation of eclogites: The role of garnet. Contrib. Miner. Pet. 1999, 135, 332–339. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Sokol, A.G.; Tomilenko, A.A.; Sobolev, N.V. Conditions of diamond formation through carbonate-silicate interaction. Eur. J. Miner. 2005, 17, 207–214. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Novoselov, I.D.; Kruk, A.N.; Furman, O.V.; Reutsky, V.N.; Palyanov, Y.N. Experimental modeling of decarbonation reactions resulting in the formation of Mg, Fe-garnets and CO2-fluid under mantle P,T-parameters. Russ. Geol. Geophys. 2020, 61, 650–662. [Google Scholar]
- Ogasawara, Y.; Liou, J.G.; Zhang, R.Y. Thermochemical calculation of logfO2-T-P stability relations of diamond-bearing assemblages in the model system CaO-MgO-SiO2-CO2-H2O. Russ. Geol. Geophys. 1997, 38, 546–557. [Google Scholar]
- Berman, R.G. Thermobarometry using multiequilibrium calculations: A new technique with petrologic applications. Can. Mineral. 1991, 29, 833–855. [Google Scholar]
- Ovsyannikov, S.V.; Abakumov, A.M.; Tsirlin, A.A.; Schnelle, W.; Egoavil, R.; Verbeeck, J.; Van Tendeloo, G.; Glazyrin, K.V.; Hanfland, M.; Dubrovinsky, L. Perovskite-like Mn2O3. A path to new manganites. Angew. Chem. Int. Ed. Eng. 2013, 52, 1494–1498. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Borzdov, Y.M.; Khokhryakov, A.F.; Kupriyanov, I.N.; Sokol, A.G. Effect of Nitrogen Impurity on Diamond Crystal Growth Processes. Cryst. Growth Des. 2010, 10, 3169–3175. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Kupriyanov, I.N.; Khokhryakov, A.F.; Borzdov, Y.M. High-pressure crystallization and properties of diamond from magnesium-based catalysts. CrystEngComm 2017, 19, 4459–4475. [Google Scholar] [CrossRef]
- Pal’Yanov, Y.N.; Sokol, A.G. The effect of composition of mantle fluids/melts on diamond formation processes. Lithos 2009, 112, 690–700. [Google Scholar] [CrossRef]
- Sokol, A.; Borzdov, Y.M.; Palyanov, Y.N.; Khokhryakov, A.F. High-temperature calibration of a multi-anvil high pressure apparatus. High Press. Res. 2015, 35, 139–147. [Google Scholar] [CrossRef]
- Sokol, A.; Khokhryakov, A.F.; Palyanov, Y.N. Composition of primary kimberlite magma: Constraints from melting and diamond dissolution experiments. Contrib. Miner. Pet. 2015, 170, 26. [Google Scholar] [CrossRef]
- Santamaria-Perez, D.; McGuire, C.; Makhluf, A.; Kavner, A.; Chuliá-Jordán, R.; Pellicer-Porres, J.; García, D.M.; Doran, A.; Kunz, M.; Rodriguez-Hernandez, P.; et al. Exploring the Chemical Reactivity between Carbon Dioxide and Three Transition Metals (Au, Pt, and Re) at High-Pressure, High-Temperature Conditions. Inorg. Chem. 2016, 55, 10793–10799. [Google Scholar] [CrossRef]
- Luth, R.W. Natural versus experimental control of oxidation state: Effects on the composition and speciation of C-O-H fluids. Am. Mineral. 1989, 74, 50–57. [Google Scholar]
- Boettcher, A.L.; Mysen, B.O.; Allen, J.C. Techniques for the control of water fugacity and oxygen fugacity for experimentation in solid-media high-pressure apparatus. J. Geophys. Res. 1973, 78, 5898–5901. [Google Scholar] [CrossRef]
- Reutsky, V.; Borzdov, Y.M.; Palyanov, Y.N. Carbon isotope fractionation associated with HPHT crystallization of diamond. Diam. Relat. Mater. 2008, 17, 1986–1989. [Google Scholar] [CrossRef]
- Reutsky, V.; Borzdov, Y.; Palyanov, Y.; Sokol, A.; Izokh, O. Carbon isotope fractionation during experimental crystallisation of diamond from carbonate fluid at mantle conditions. Contrib. Miner. Pet. 2015, 170, 41. [Google Scholar] [CrossRef]
- Luth, R.W. Experimental determination of the reaction dolomite + 2coesite = diopside + 2CO2 to 6GPa. Contrib. Miner. Pet. 1995, 122, 152–158. [Google Scholar] [CrossRef]
- Wyllie, P.; Huang, W.-L.; Otto, J.; Byrnes, A. Carbonation of peridotites and decarbonation of siliceous dolomites represented in the system CaO-MgO-SiO2-CO2 to 30 kbar. Tectonophys. 1983, 100, 359–388. [Google Scholar] [CrossRef]
- Eggler, D.H. The effect of CO2 upon partial melting of peridotite in the system Na2O-CaO-Al2O3-MgO-SiO2-CO2 to 35 kbar, with an analysis of melting in a peridotite-H2O-CO2 system. Am. J. Sci. 1978, 278, 305–343. [Google Scholar] [CrossRef]
- Newton, R.; Sharp, W. Stability of forsterite + CO2 and its bearing on the role of CO2 in the mantle. Earth Planet. Sci. Lett. 1975, 26, 239–244. [Google Scholar] [CrossRef]
- Koziol, A.M.; Newton, R.C. Experimental determination of the reaction; magnesite + enstatite = forsterite + CO2 in the ranges 6–25 kbar and 700–1100°. Am. Miner. 1998, 83, 213–219. [Google Scholar] [CrossRef]
- Shatskiy, A.; Litasov, K.D.; Palyanov, Y. Phase relations in carbonate systems at pressures and temperatures of lithospheric mantle: Review of experimental data. Russ. Geol. Geophys. 2015, 56, 113–142. [Google Scholar] [CrossRef]
- Kennedy, C.S.; Kennedy, G.C. The equilibrium boundary between graphite and diamond. J. Geophys. Res. 1976, 81, 2467–2470. [Google Scholar] [CrossRef]
- Kolesov, B.A.; Geiger, C.A. Raman spectra of silicate garnets. Phys. Chem. Miner. 1998, 25, 142–151. [Google Scholar] [CrossRef]
- Valenzano, L.; Meyer, A.; Demichelis, R.; Civalleri, B.; Dovesi, R. Quantum-mechanical ab initio simulation of the Raman and IR spectra of Mn3Al2Si3O12 spessartine. Phys. Chem. Miner. 2009, 36, 415–420. [Google Scholar] [CrossRef]
- Connolly, J.A. Computation of phase equilibria by linear programming: A tool for geodynamic modeling and its application to subduction zone decarbonation. Earth Planet. Sci. Lett. 2005, 236, 524–541. [Google Scholar] [CrossRef]
- Gorman, P.J.; Kerrick, D.M.; Connolly, J.A.D. Modeling open system metamorphic decarbonation of subducting slabs. Geochem. Geophys. Geosystems 2006, 7, 04007. [Google Scholar] [CrossRef]
- Molina, J.F. Carbonate stability and fluid composition in subducted oceanic crust: An experimental study on H2O–CO2-bearing basalts. Earth Planet. Sci. Lett. 2000, 176, 295–310. [Google Scholar] [CrossRef]
- Poli, S.; Franzolin, E.; Fumagalli, P.; Crottini, A. The transport of carbon and hydrogen in subducted oceanic crust: An experimental study to 5 GPa. Earth Planet. Sci. Lett. 2009, 278, 350–360. [Google Scholar] [CrossRef]
- Bulanova, G. The formation of diamond. J. Geochem. Explor. 1995, 53, 1–23. [Google Scholar] [CrossRef]
- Brenker, F.; Vollmer, C.; Vincze, L.; Vekemans, B.; Szymanski, A.; Janssens, K.; Szaloki, I.; Nasdala, L.; Joswig, W.; Kaminsky, F. Carbonates from the lower part of transition zone or even the lower mantle. Earth Planet. Sci. Lett. 2007, 260, 1–9. [Google Scholar] [CrossRef]
- Izraeli, E.S.; Harris, J.W.; Navon, O. Brine inclusions in diamonds: A new upper mantle fluid. Earth Planet. Sci. Lett. 2001, 187, 323–332. [Google Scholar] [CrossRef]
- Kaminsky, F.; Wirth, R.; Schreiber, A. Carbonatitic inclusions in deep mantle diamond from juina, Brazil: New minerals in the carbonate-halide association. Can. Miner. 2013, 51, 669–688. [Google Scholar] [CrossRef]
- Wang, A.; Pasteris, J.D.; Meyer, H.O.; Dele-Duboi, M.L. Magnesite-bearing inclusion assemblage in natural diamond. Earth Planet. Sci. Lett. 1996, 141, 293–306. [Google Scholar] [CrossRef]
- Navon, O.; Hutcheon, I.D.; Rossman, G.; Wasserburg, G.J. Mantle-derived fluids in diamond micro-inclusions. Nature 1988, 335, 784–789. [Google Scholar] [CrossRef]
- Schrauder, M.; Navon, O. Hydrous and carbonatitic mantle fluids in fibrous diamonds from Jwaneng, Botswana. Geochim. Cosmochim. Acta 1994, 58, 761–771. [Google Scholar] [CrossRef]
- Sobolev, N. Mineral inclusions in diamonds from the Sputnik kimberlite pipe, Yakutia. Lithos 1997, 39, 135–157. [Google Scholar] [CrossRef]
- Stachel, T.; Harris, J.W.; Brey, G.P. Rare and unusual mineral inclusions in diamonds from Mwadui, Tanzania. Contrib. Miner. Pet. 1998, 132, 34–47. [Google Scholar] [CrossRef]
- Abersteiner, A.; Kamenetsky, V.S.; Pearson, D.G.; Kamenetsky, M.; Goemann, K.; Courtney-Davies, L.; Rodemann, T. Monticellite in group-I kimberlites: Implications for evolution of parental melts and post-emplacement CO2 degassing. Chem. Geol. 2018, 478, 76–88. [Google Scholar] [CrossRef]
- Bussweiler, Y.; Stone, R.S.; Pearson, D.G.; Luth, R.W.; Stachel, T.; Kjarsgaard, B.A.; Menzies, A. The evolution of calcite-bearing kimberlites by melt-rock reaction: Evidence from polymineralic inclusions within clinopyroxene and garnet megacrysts from Lac de Gras kimberlites, Canada. Contrib. Miner. Pet. 2016, 171, 65. [Google Scholar] [CrossRef]
- Giuliani, A. Insights into kimberlite petrogenesis and mantle metasomatism from a review of the compositional zoning of olivine in kimberlites worldwide. Lithos 2018, 322–342. [Google Scholar] [CrossRef]
- Delaney, J.S.; Smith, J.V.; Dawson, J.B.; Nixon, P.H. Manganese thermometer for mantle peridotites. Contrib. Miner. Pet. 1979, 71, 157–169. [Google Scholar] [CrossRef]
- Creighton, S. A semi-empirical manganese-in-garnet single crystal thermometer. Lithos 2009, 112, 177–182. [Google Scholar] [CrossRef]
- Schulze, D.J.; Harte, B.; Valley, J.W.; Channer, D.M. Evidence of subduction and crust–mantle mixing from a single diamond. Lithos 2004, 77, 349–358. [Google Scholar] [CrossRef]
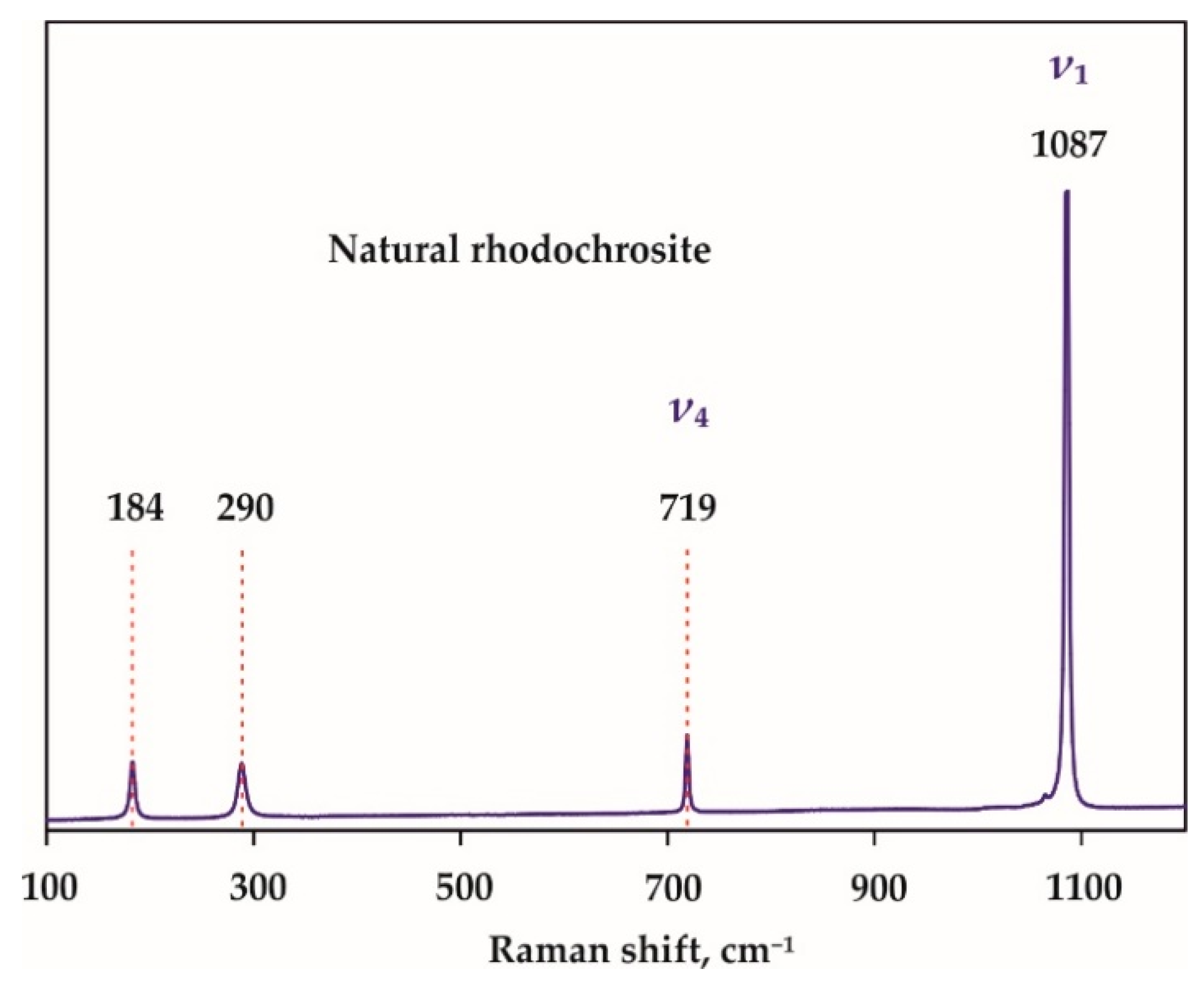
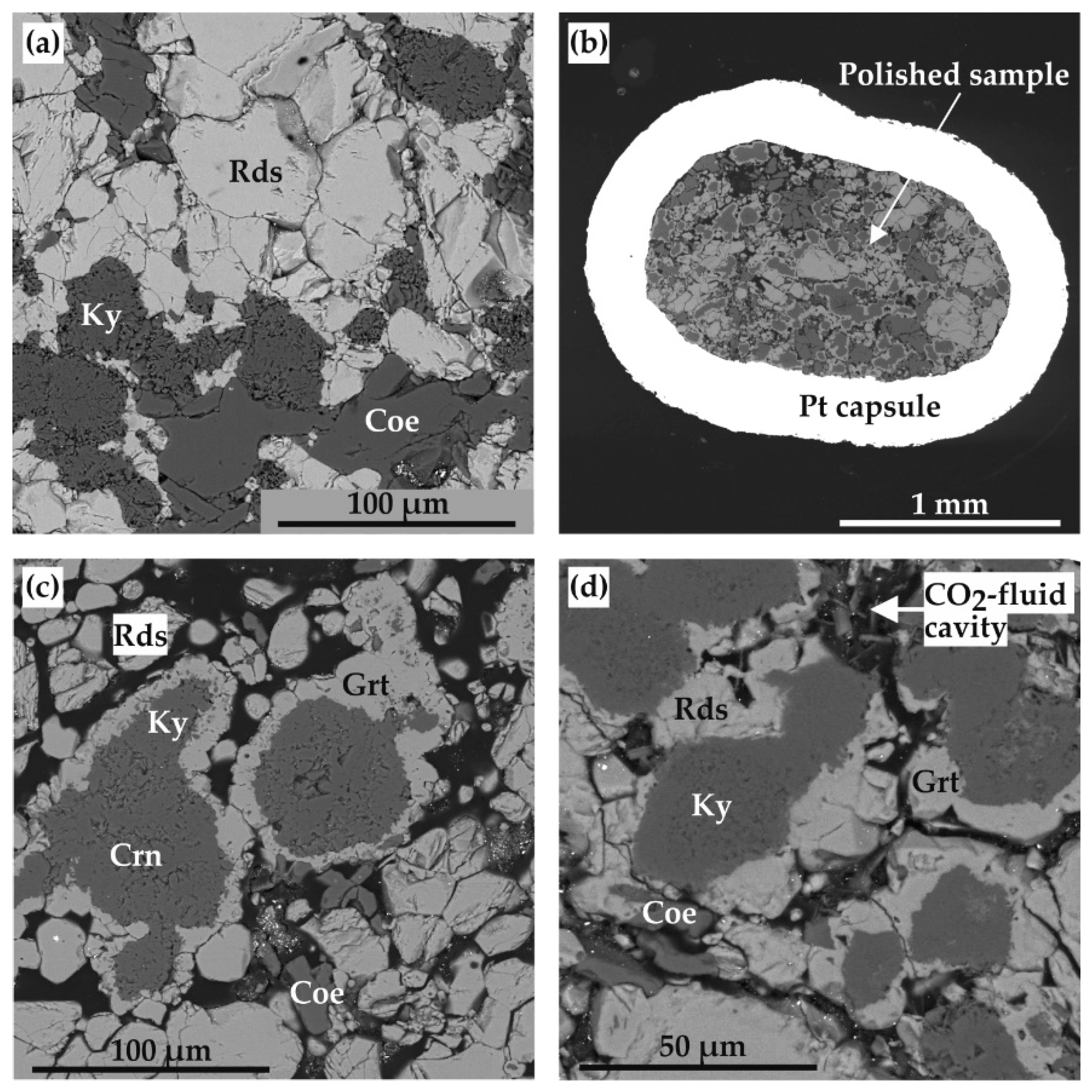
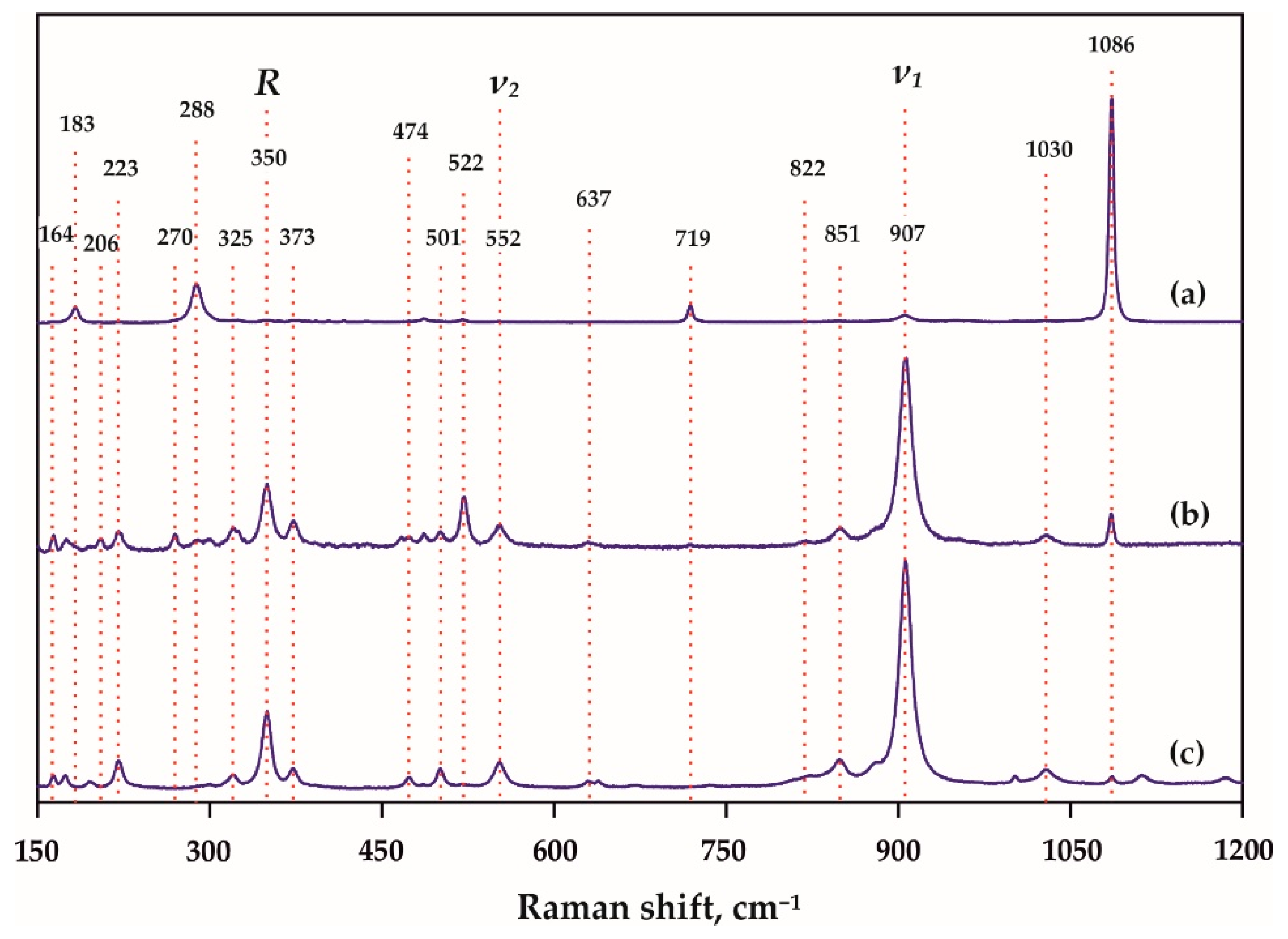
| Run N | P, GPa | T, °C | t, Hours | Final Mineral Phases |
|---|---|---|---|---|
| 2131-R | 3.0 | 850 | 100 | Ky, Crn, Rds, Coe |
| 2130-R | 3.0 | 900 | 100 | Sps, Ky, Crn, Rds |
| 1744-R | 3.0 | 950 | 60 | Sps, Ky, Crn, Rds, Coe |
| 1215-R | 3.0 | 1000 | 60 | Sps, Ky, Rds, Crn, Coe |
| 2129-R | 6.3 | 1050 | 60 | Ky, Rds, Coe |
| 2117-R | 6.3 | 1100 | 40 | Sps, Ky, Crn, Rds, Coe |
| 2143-R | 7.5 | 1150 | 60 | Ky, Rds, Coe |
| 2144-R | 7.5 | 1200 | 60 | Sps, Ky, Rds, Coe |
| Run N | P, GPa | T, °C | Phase | Mass Concentrations, wt.% | n(O) | Cations Per Formula Unit | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | FeO | MnO | CaO | CO2 * | Total | Si | Al | Fe | Mn | Ca | C * | Σ Cat | |||||
| 2131-R | 3.0 | 850 | Crn | - | 99.5(6) | - | 0.5(5) | - | - | 100.0 | 3 | - | 1.99(1) | - | 0.01(1) | - | - | 2.00 |
| Rds | - | - | 2.0(4) | 58.0(6) | - | 40(1) | 100.0 | 3 | - | - | 0.03(1) | 0.92(2) | - | 1.02(1) | 1.97 | |||
| Ky | 36.1(4) | 63.2(5) | - | 0.8(1) | - | - | 100.1 | 5 | 0.97(2) | 2.02(2) | - | 0.02(1) | - | - | 3.01 | |||
| Coe | 99.9(1) | - | - | 0.1(1) | - | - | 100.0 | 2 | 1.00(1) | - | - | 0.01(1) | - | - | 1.00 | |||
| 2130-R | 3.0 | 900 | Sps | 35.9(4) | 21.1(2) | 1.6(0) | 41.2(8) | 0.6(3) | - | 100.5 | 12 | 2.95(2) | 2.05(2) | 0.11(0) | 2.87(5) | 0.05(3) | - | 8.03 |
| Crn | - | 99.3(8) | - | 0.6(3) | - | - | 100.1 | 3 | - | 1.99(1) | - | 0.01(1) | - | - | 2.00 | |||
| Rds | - | - | 2.0(6) | 58.0(8) | - | 40(1) | 100.0 | 3 | - | - | 0.03(1) | 0.92(2) | - | 1.02(1) | 1.98 | |||
| Ky | 36.2(1) | 63.8(4) | - | 0.6(2) | - | - | 100.6 | 5 | 0.97(2) | 2.02(2) | - | 0.02(1) | - | - | 3.01 | |||
| Coe | 99.8(7) | - | - | 0.9(5) | - | - | 100.7 | 2 | 1.00(1) | - | - | 0.01(1) | - | - | 1.00 | |||
| 1743-R | 3.0 | 950 | Sps | 36.1(6) | 21(1) | 1.4(2) | 41(1) | 0.5(2) | - | 100.2 | 12 | 2.96(6) | 2.1(1) | 0.09(1) | 2.84(9) | 0.04(2) | - | 8.01 |
| Crn | - | 98.2(3) | - | 0.9(1) | - | - | 99.1 | 3 | - | 1.99(0) | - | 0.01(0) | - | - | 2.00 | |||
| Ky | 36.5(1) | 62.7(1) | - | 1.4(1) | - | - | 100.6 | 5 | 0.99(0) | 1.99(0) | - | 0.04(0) | - | - | 3.02 | |||
| Rds | - | - | 2.0(5) | 57.8(6) | 0.3(1) | 39.5(8) | 100.0 | 3 | - | - | 0.03(1) | 0.92(1) | - | 1.01(2) | 1.98 | |||
| Coe | 99.4(2) | - | - | 0.9(1) | - | - | 100.3 | 2 | 1.00(0) | - | - | 0.01(0) | - | - | 1.01 | |||
| 1215-R | 3.0 | 1000 | Sps | 36.4(2) | 20.6(1) | 1.6(2) | 41.3(6) | 0.5(1) | - | 100.2 | 12 | 2.99(2) | 1.99(1) | 0.11(2) | 2.88(3) | 0.04(1) | - | 8.01 |
| Rds | - | - | 1.9(1) | 58(1) | 0.1(3) | 40(1) | 100.0 | 3 | - | - | 0.03(0) | 0.93(4) | - | 1.02(1) | 1.98 | |||
| Crn | - | 99.8(1) | - | 0.4(1) | - | - | 100.1 | 3 | - | 2.00(0) | - | 0.00(1) | - | - | 2.00 | |||
| Ky | 36.4(9) | 62.5(4) | - | 1.1(5) | - | - | 100.0 | 5 | 0.99(0) | 1.99(0) | - | 0.04(0) | - | - | 3.02 | |||
| Coe | 99.7(1) | - | - | 0.7(4) | - | - | 100.4 | 2 | 1.00(0) | - | - | 0.01(1) | - | - | 1.01 | |||
| 2129-R | 6.3 | 1050 | Ky | 36.3(9) | 62.7(1) | - | 1.0(7) | - | - | 100.0 | 5 | 0.99(1) | 2.00(4) | - | 0.03(1) | - | - | 3.02 |
| Rds | - | - | 1.7(5) | 58.8(6) | - | 39.2(4) | 100.0 | 3 | - | - | 0.03(1) | 0.94(1) | - | 1.01(1) | 1.99 | |||
| Coe | 100.0(4) | - | - | - | - | - | 100.5 | 2 | 1.00(0) | - | - | - | - | - | 1.00 | |||
| 2117-R | 6.3 | 1100 | Sps | 36.1(3) | 20(1) | 1.3(1) | 41.9(4) | 0.5(3) | - | 100.4 | 12 | 3.01(5) | 1.92(8) | 0.09(1) | 2.96(5) | 0.05(3) | - | 8.04 |
| Ky | 36.0(5) | 63.4(8) | - | 0.8(3) | - | - | 100.2 | 5 | 0.97(2) | 2.02(2) | - | 0.02(1) | - | - | 3.01 | |||
| Crn | - | 100.0(7) | - | 0.4(1) | - | - | 100.3 | 3 | - | 2.00(0) | - | 0.01(1) | - | - | 2.01 | |||
| Rds | - | 0.5(2) | 1.9(3) | 58.2(7) | - | 39.8(6) | 100.0 | 3 | - | - | 0.03(1) | 0.93(2) | - | 1.02(1) | 1.98 | |||
| Coe | 99.8(4) | - | - | 0.8(3) | - | - | 100.6 | 2 | 1.00(0) | - | - | 0.01(1) | - | - | 1.01 | |||
| 2143-R | 7.5 | 1150 | Ky | 36(1) | 63.7(2) | - | 0.7(1) | - | - | 100.0 | 5 | 0.96(3) | 2.04(4) | - | 0.02(1) | - | - | 3.02 |
| Rds | - | - | 1.9(1) | 57.8(5) | - | 40.2(6) | 100.0 | 3 | - | - | 0.03(0) | 0.91(2) | - | 1.03(1) | 1.98 | |||
| Coe | 99.9(6) | - | - | - | - | - | 100.3 | 2 | 1.00(0) | - | - | - | - | - | 1.00 | |||
| 2144-R | 7.5 | 1200 | Sps | 36.6(2) | 21(1) | 1.0(9) | 41.7(4) | 0.5(1) | - | 100.8 | 12 | 3.02(2) | 1.94(8) | 0.05(1) | 2.96(1) | 0.05(1) | - | 8.04 |
| Ky | 36.1(1) | 63.2(3) | - | 0.8(3) | - | - | 100.1 | 5 | 0.97(3) | 2.03(1) | - | 0.02(1) | - | - | 3.02 | |||
| Rds | - | - | 2.0(1) | 58.1(3) | - | 39.9(5) | 100.0 | 3 | - | - | 0.03(0) | 0.91(1) | - | 1.03(1) | 1.98 | |||
| Coe | 99.9(3) | - | - | - | - | - | 99.9 | 2 | 1.00(0) | - | - | - | - | - | 1.00 | |||
| Run N | 1744-R | 2117-R | Sps1 | Sps2 | Sps2cal |
|---|---|---|---|---|---|
| P, GPa | 3.0 | 6.3 | |||
| T, °C | 950 | 1100 | |||
| Raman Shift, cm−1 | |||||
| 106 | - | - | - | 105 | |
| 163 | 164 | 162 | 162 | 163 | |
| 174 | 174 | 175 | 175 | - | |
| - | 195 | 196 | 196 | 195 | |
| 221 | 221 | 221 | 221 | 221 | |
| 270 | - | 269 | 269 | - | |
| 300 | 300 | 302 | 302 | 298 | |
| - | - | - | - | 314 | |
| 325 | 321 | 321 | 321 | 320 | |
| R(SiO4)4− | 349 | - | - | - | 347 |
| R(SiO4)4− | - | 350 | 350 | 350 | 365 |
| 376 | 371 | 372 | 372 | 375 | |
| 404 | 403 | - | - | - | |
| 415 | - | - | - | - | |
| 437 | 468 | - | - | - | |
| - | 473 | 475 | 475 | 475 | |
| 486 | 487 | - | - | - | |
| - | 500 | 500 | 500 | 505 | |
| 522 | - | 522 | 522 | 530 | |
| (Si-O)bend, υ2 | 553 | 553 | 552 | 552 | 560 |
| - | - | 573 | 573 | - | |
| - | - | 592 | 592 | 587 | |
| - | - | 630 | 630 | 639 | |
| - | 848 | 849 | 849 | 845 | |
| 852 | - | - | - | 852 | |
| - | - | 879 | 879 | 876 | |
| (Si-O)str, υ1 | 907 | 906 | 905 | 905 | 910 |
| (Si-O)str, υ1 | - | - | 913 | 913 | 913 |
| 954 | - | - | - | - | |
| - | 1031 | 1029 | 1029 | 1033 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bataleva, Y.V.; Kruk, A.N.; Novoselov, I.D.; Palyanov, Y.N. Formation of Spessartine and CO2 via Rhodochrosite Decarbonation along a Hot Subduction P-T Path. Minerals 2020, 10, 703. https://doi.org/10.3390/min10080703
Bataleva YV, Kruk AN, Novoselov ID, Palyanov YN. Formation of Spessartine and CO2 via Rhodochrosite Decarbonation along a Hot Subduction P-T Path. Minerals. 2020; 10(8):703. https://doi.org/10.3390/min10080703
Chicago/Turabian StyleBataleva, Yuliya V., Aleksei N. Kruk, Ivan D. Novoselov, and Yuri N. Palyanov. 2020. "Formation of Spessartine and CO2 via Rhodochrosite Decarbonation along a Hot Subduction P-T Path" Minerals 10, no. 8: 703. https://doi.org/10.3390/min10080703
APA StyleBataleva, Y. V., Kruk, A. N., Novoselov, I. D., & Palyanov, Y. N. (2020). Formation of Spessartine and CO2 via Rhodochrosite Decarbonation along a Hot Subduction P-T Path. Minerals, 10(8), 703. https://doi.org/10.3390/min10080703