Sulphate Removal from Flotation Process Water Using Ion-Exchange Resin Column System
Abstract
1. Introduction
2. Materials and Methods
2.1. Ion Exchange Resin
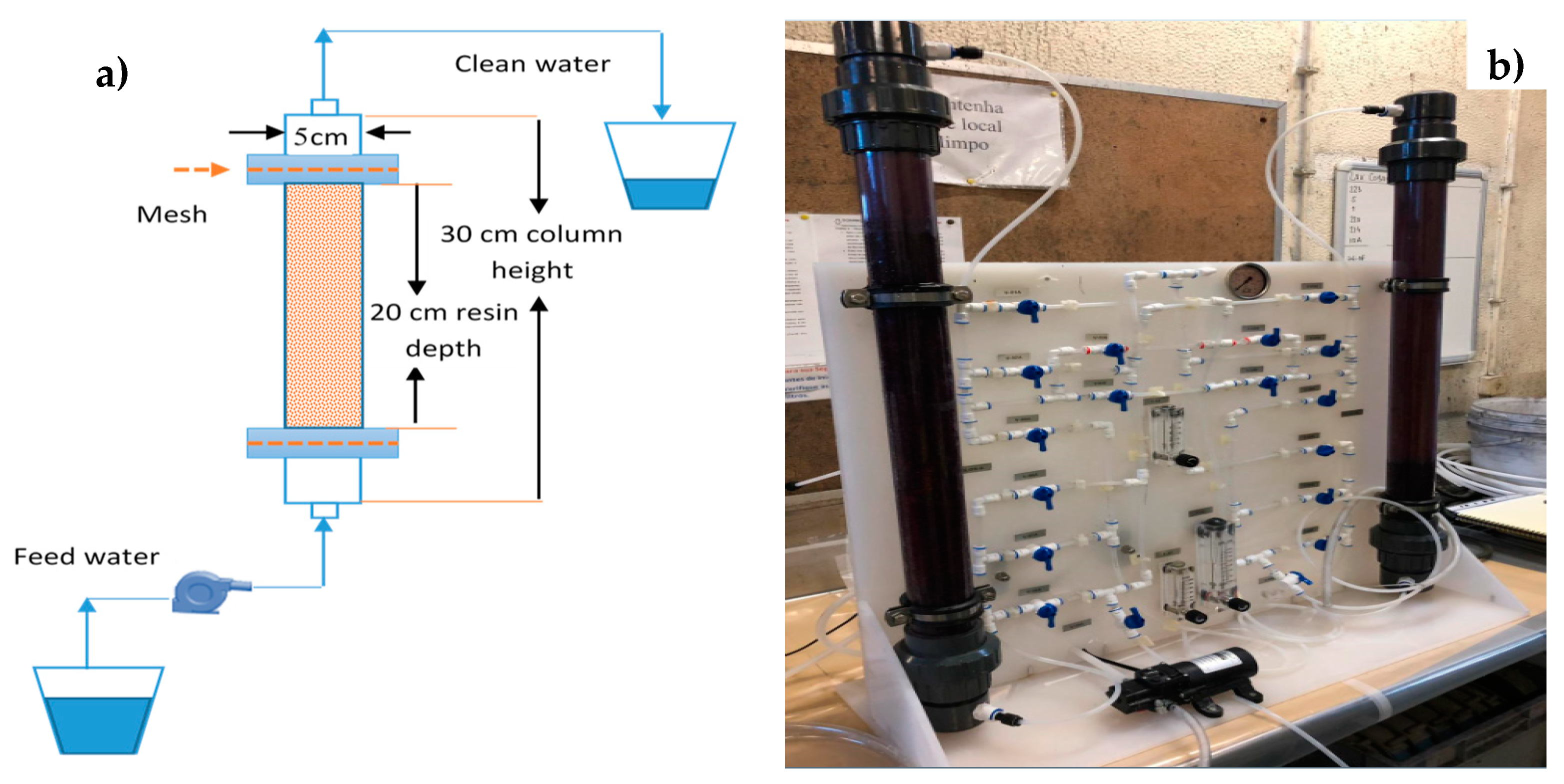
2.2. Column Systems
2.3. Conversion to Ionic Forms of Anion Resin
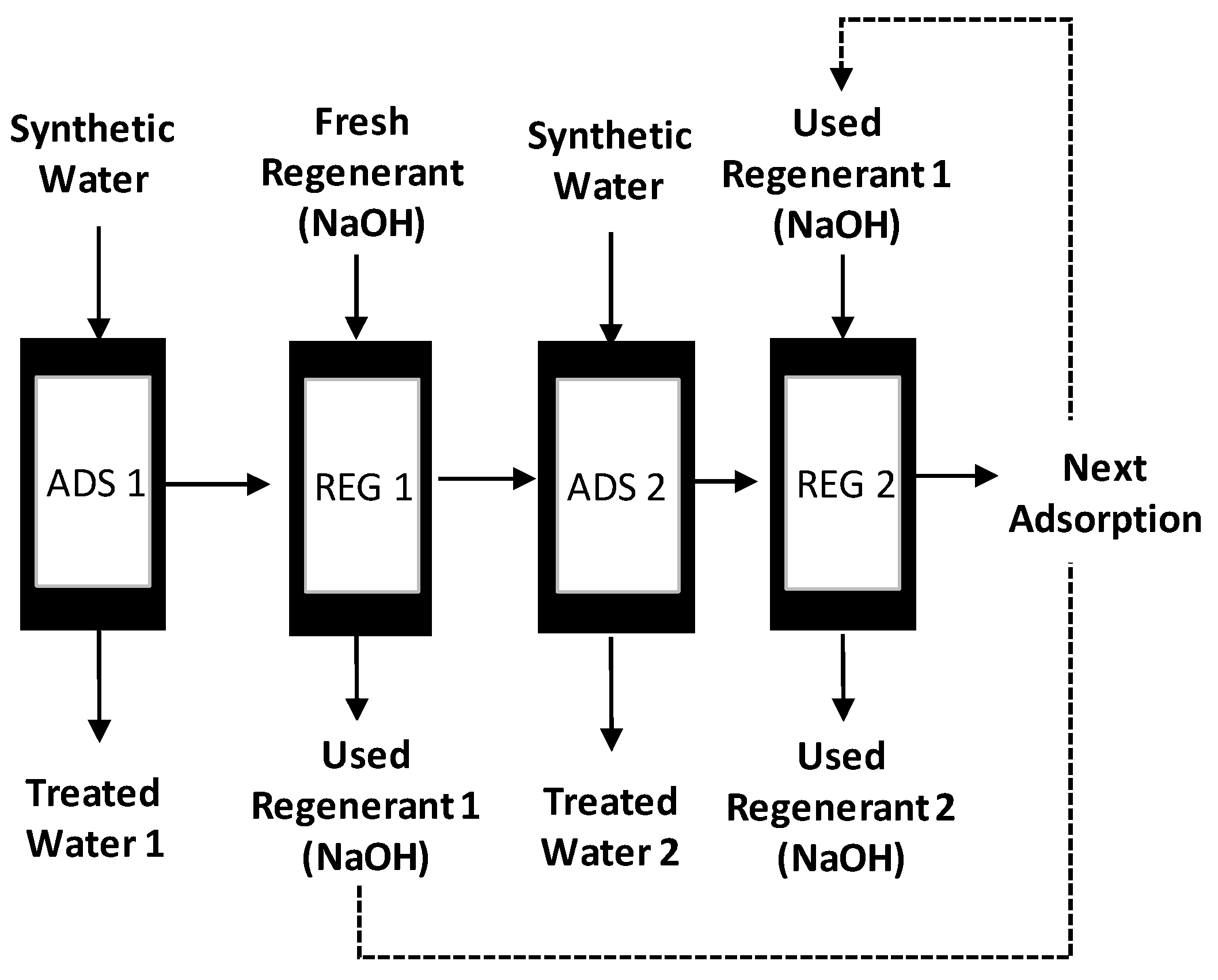
2.4. Adsorption and Regeneration Tests
3. Results and Discussion
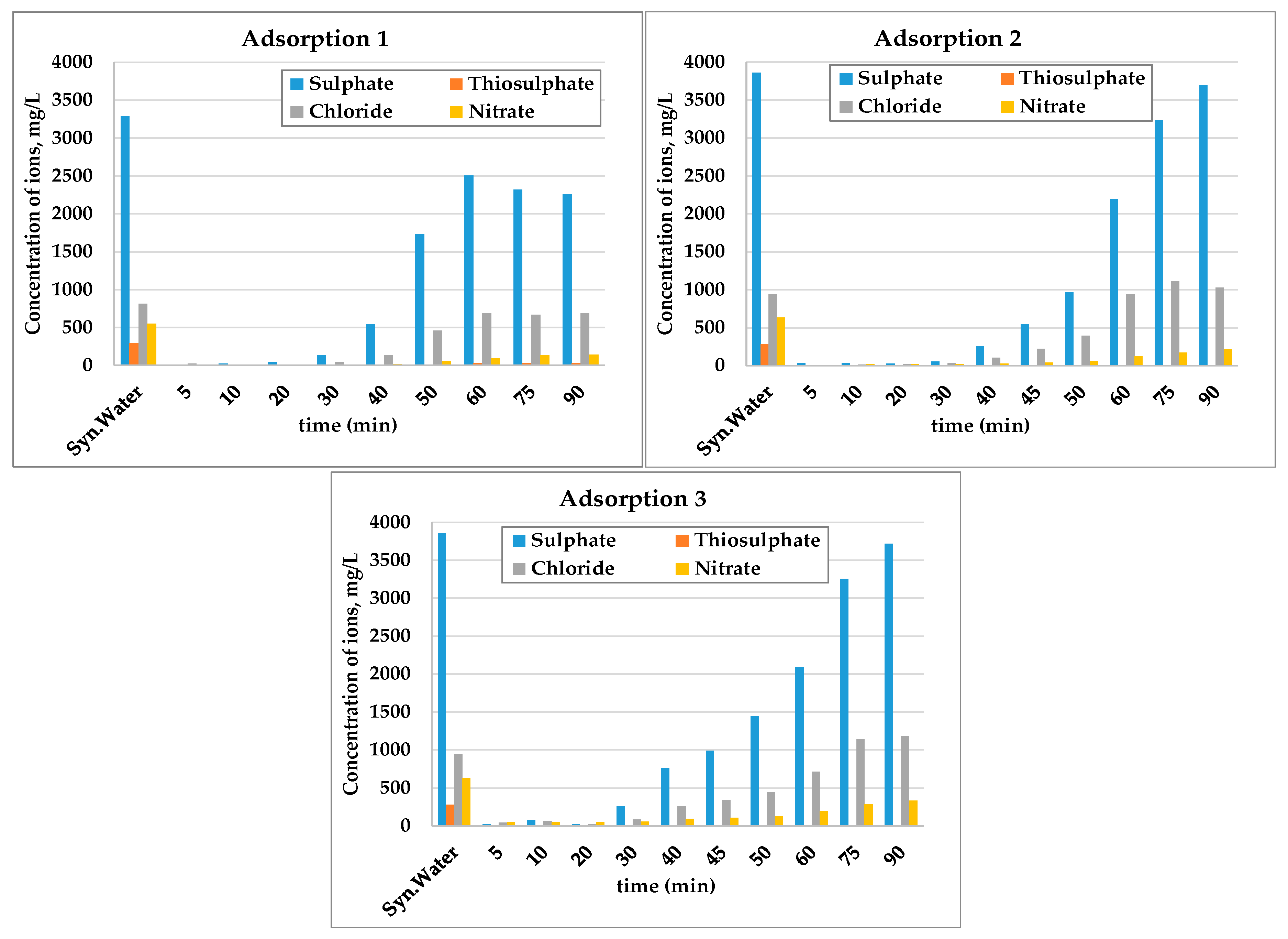
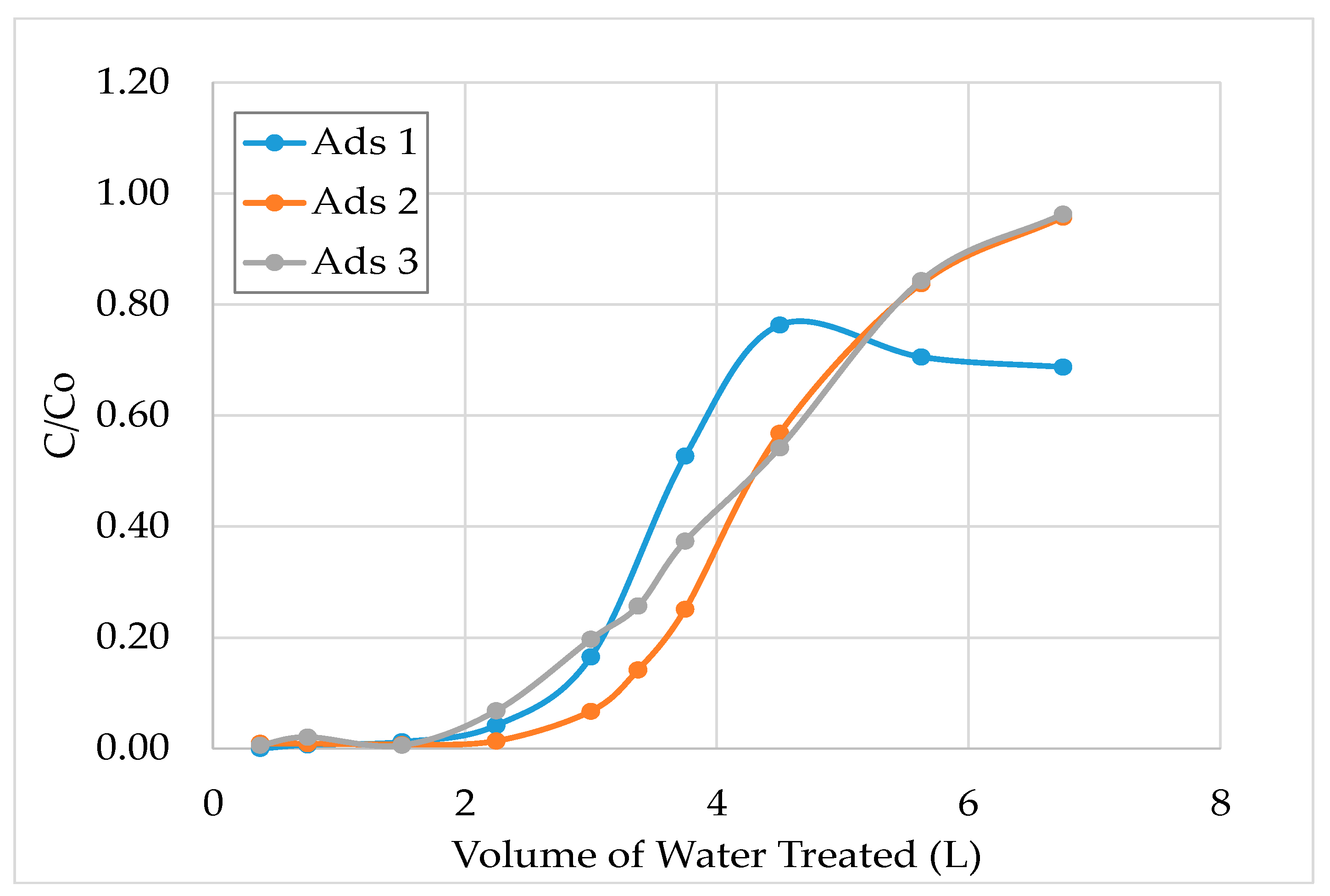
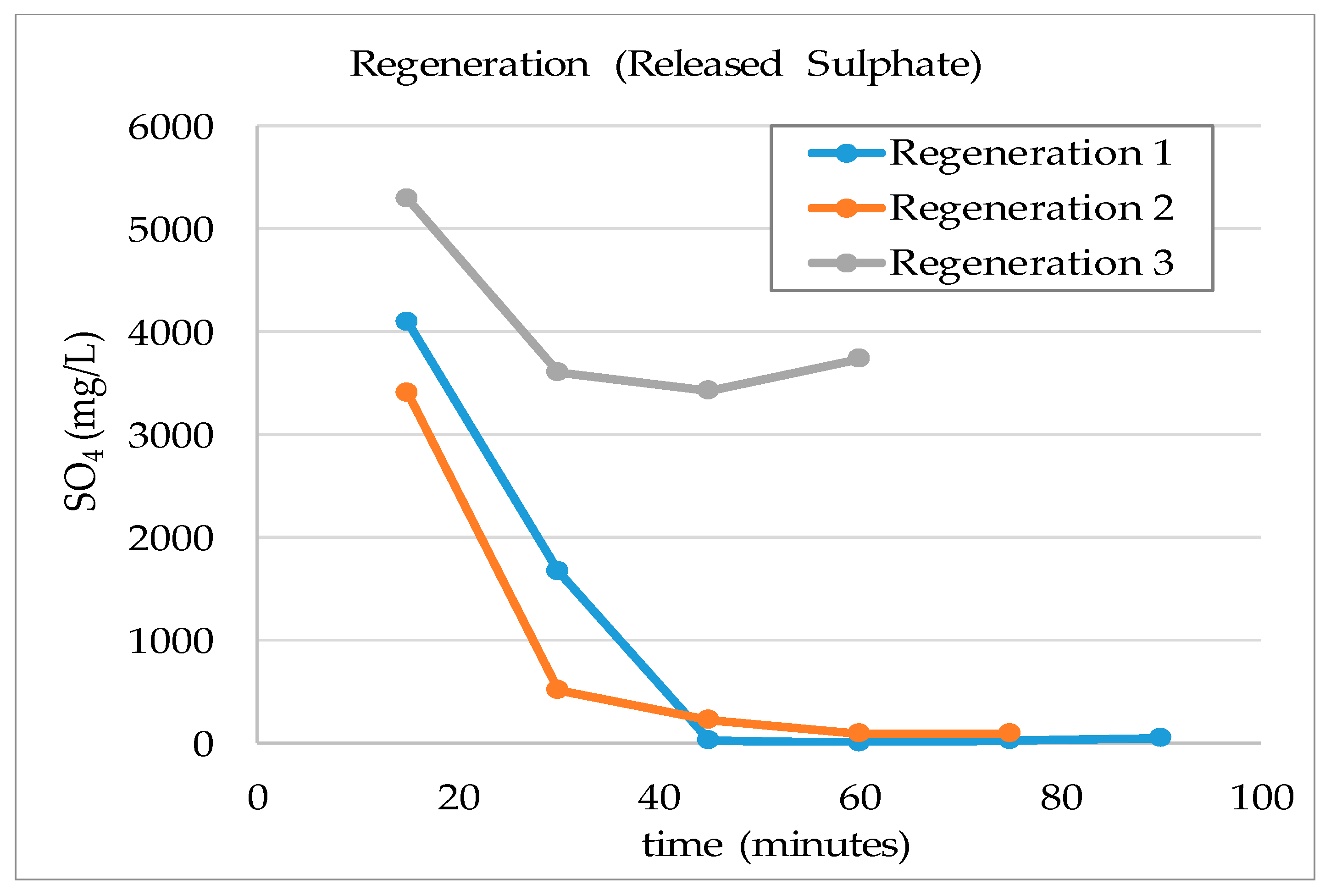
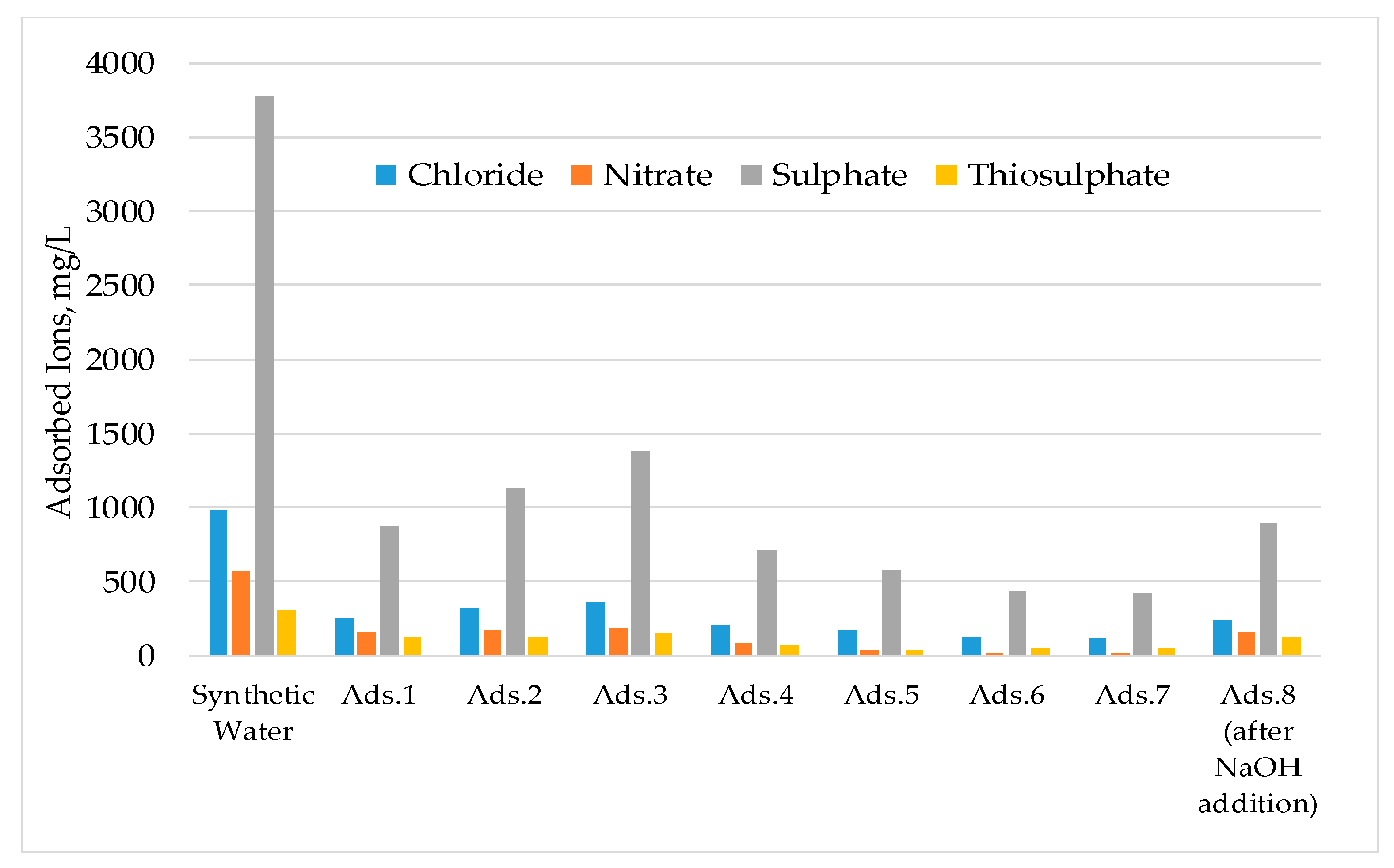
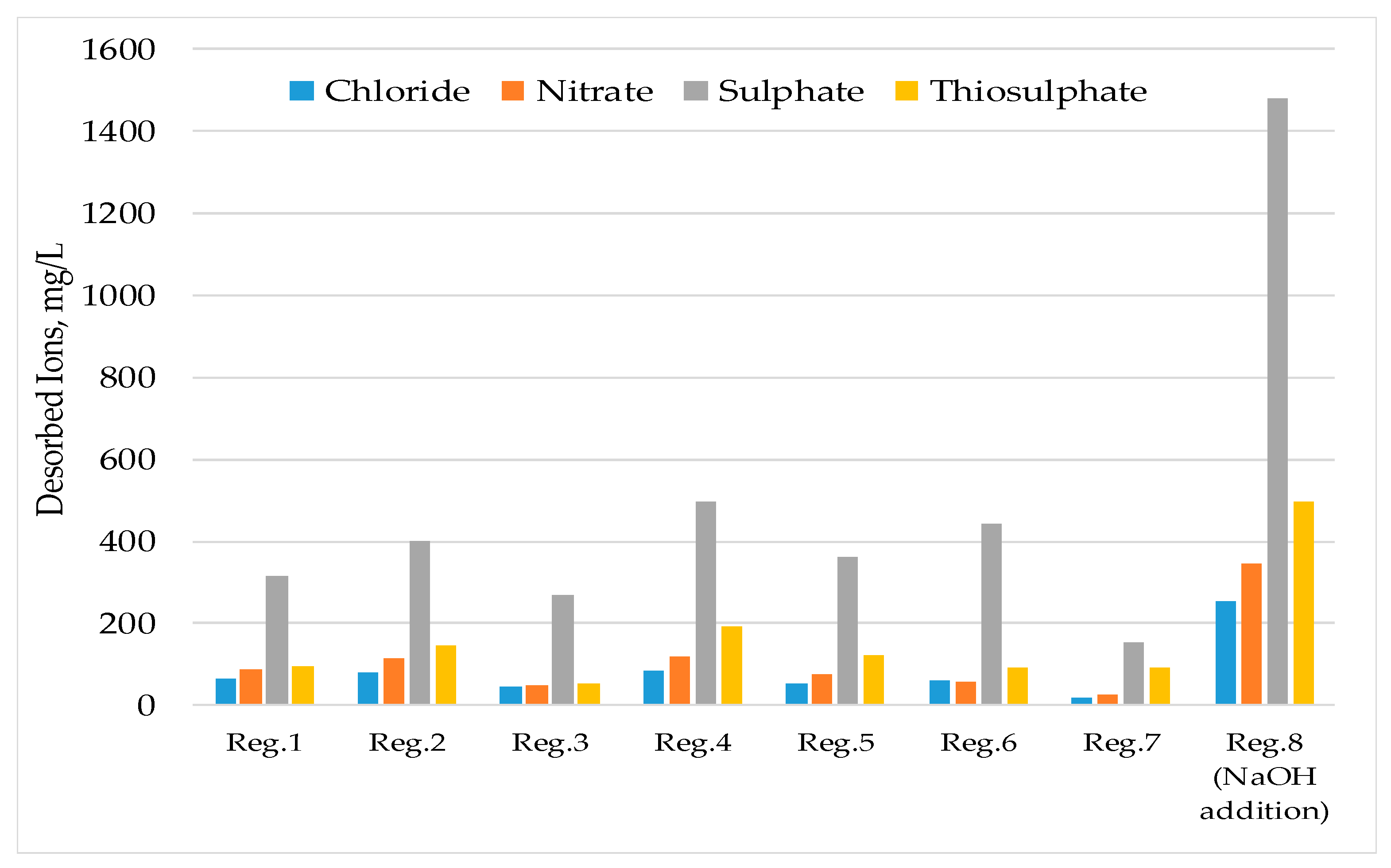
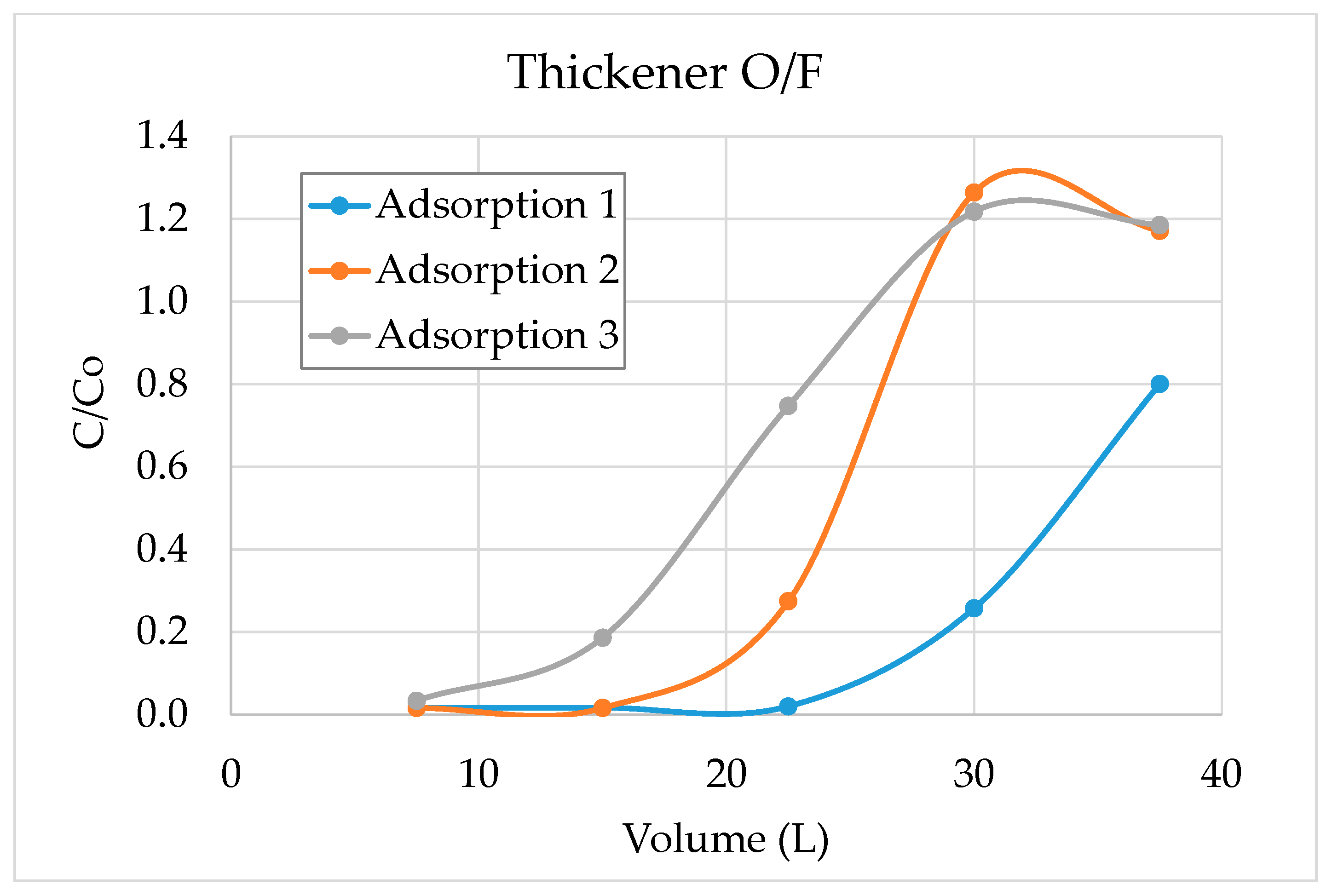
3.1. Laboratory Scale Studies
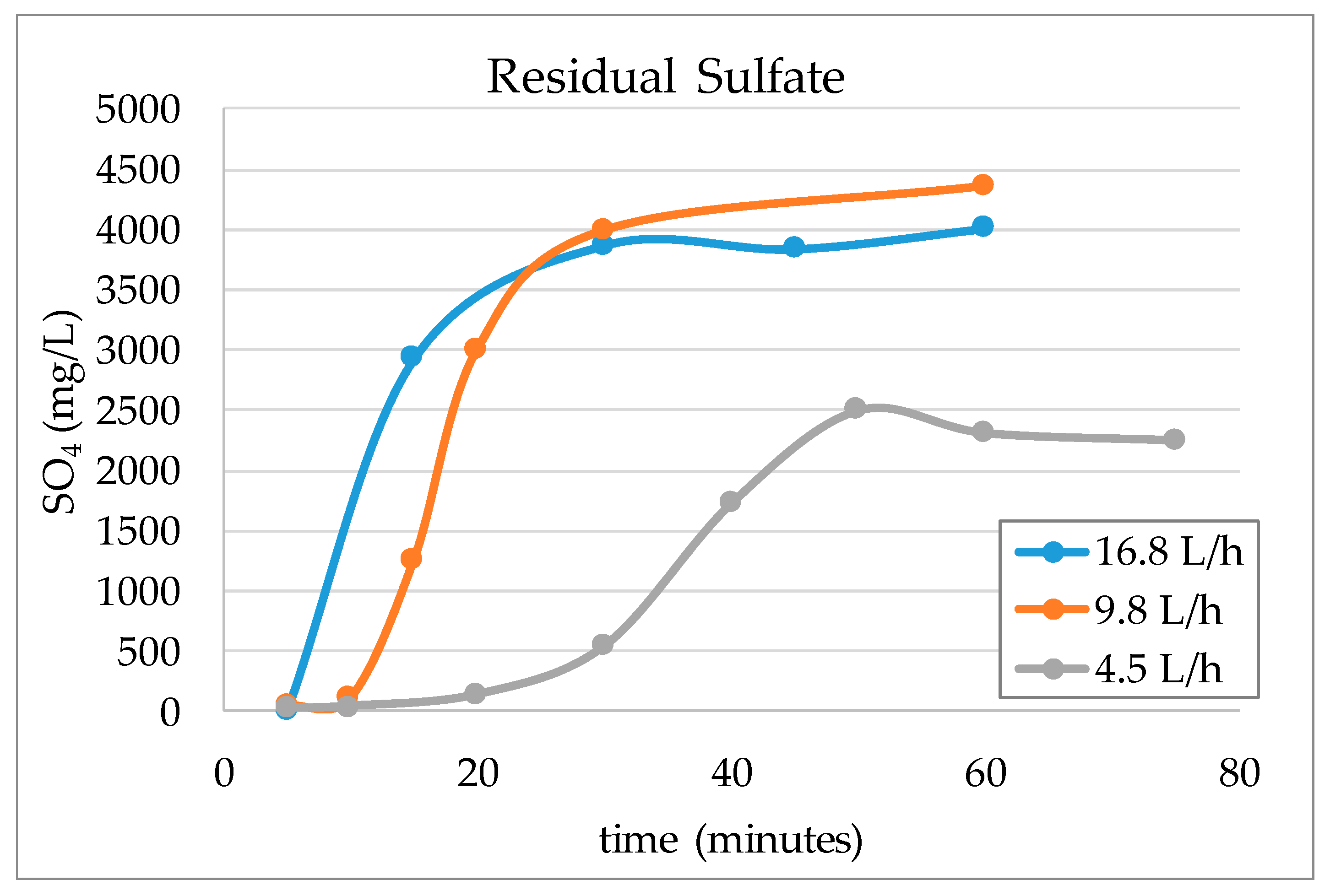
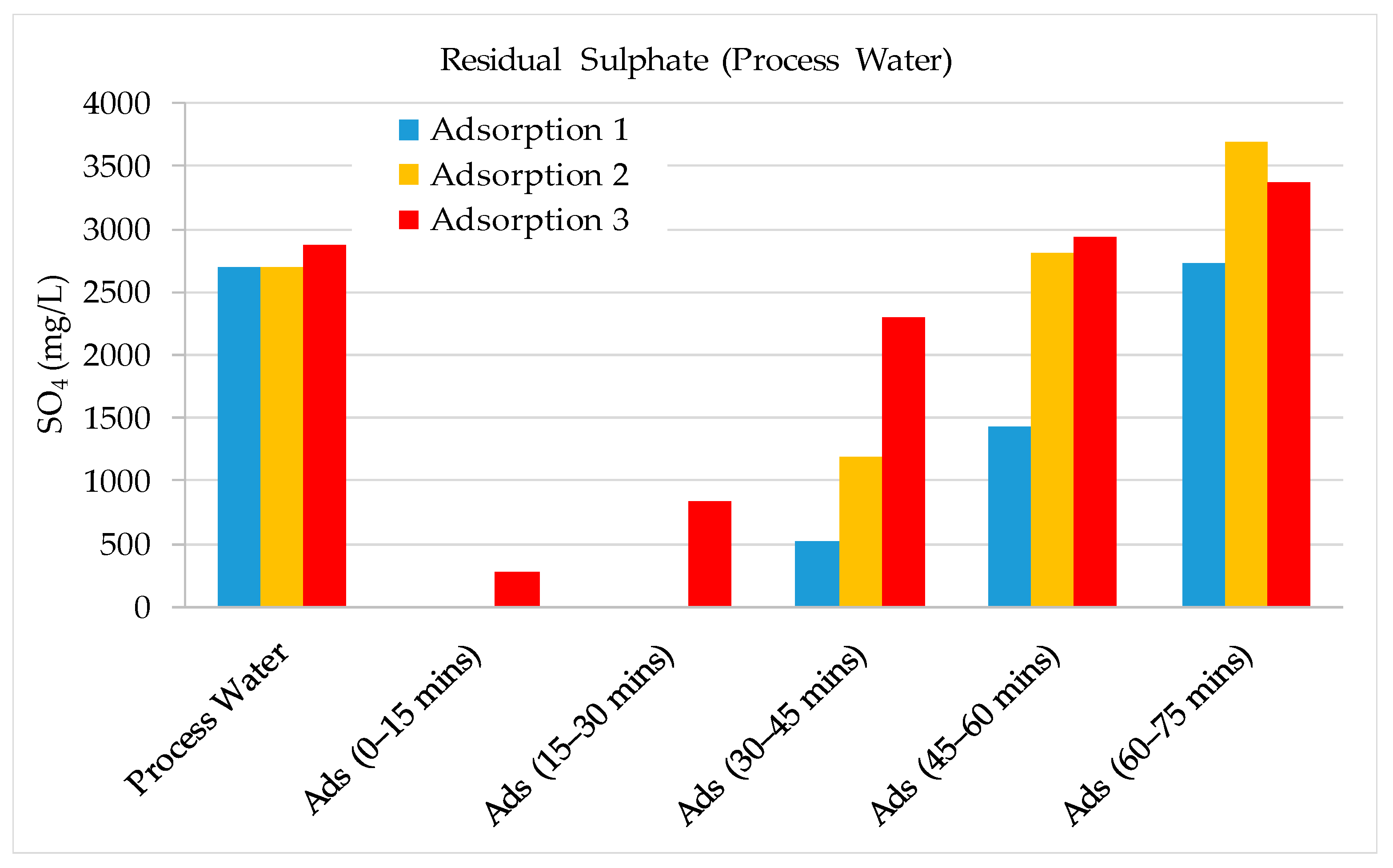
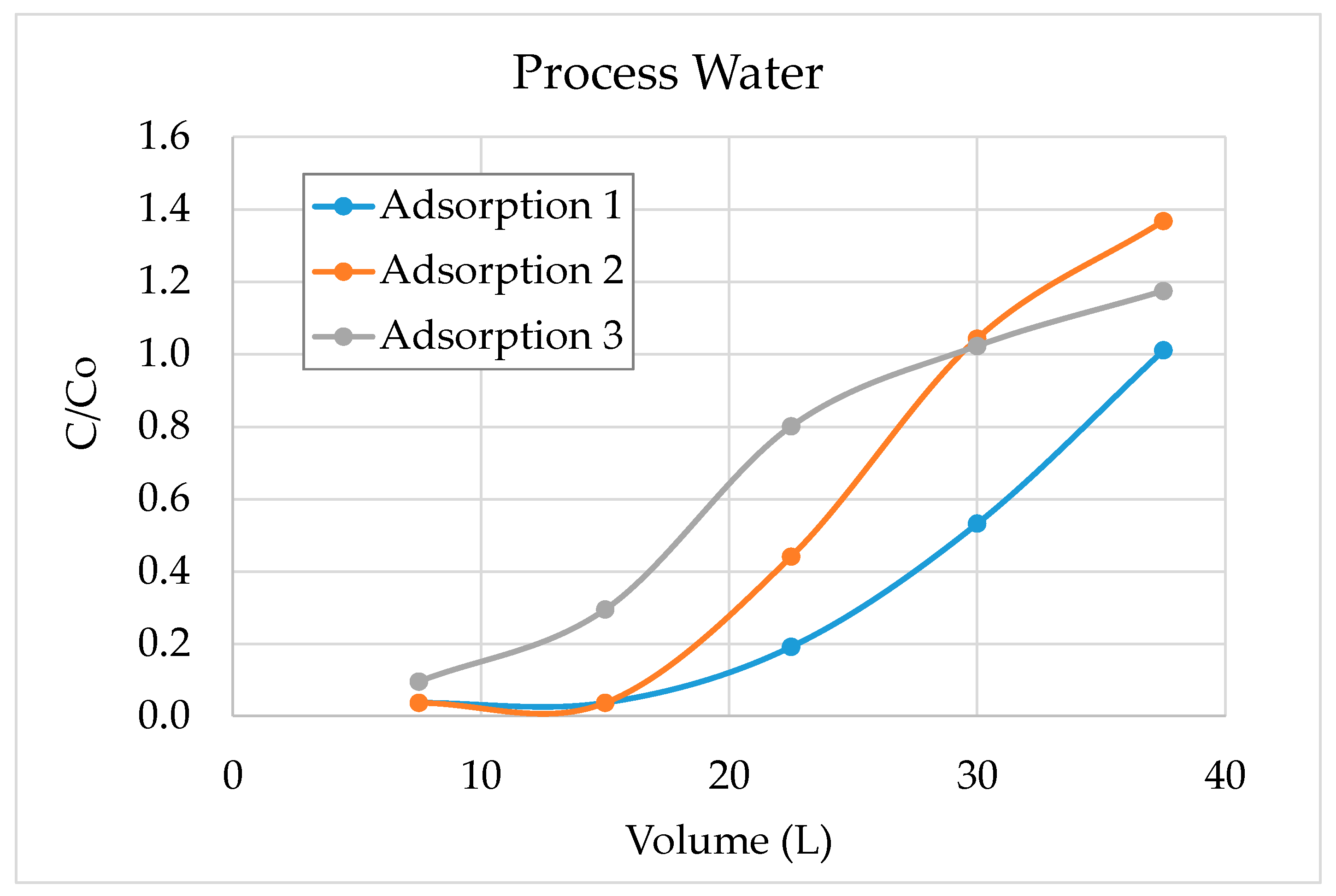
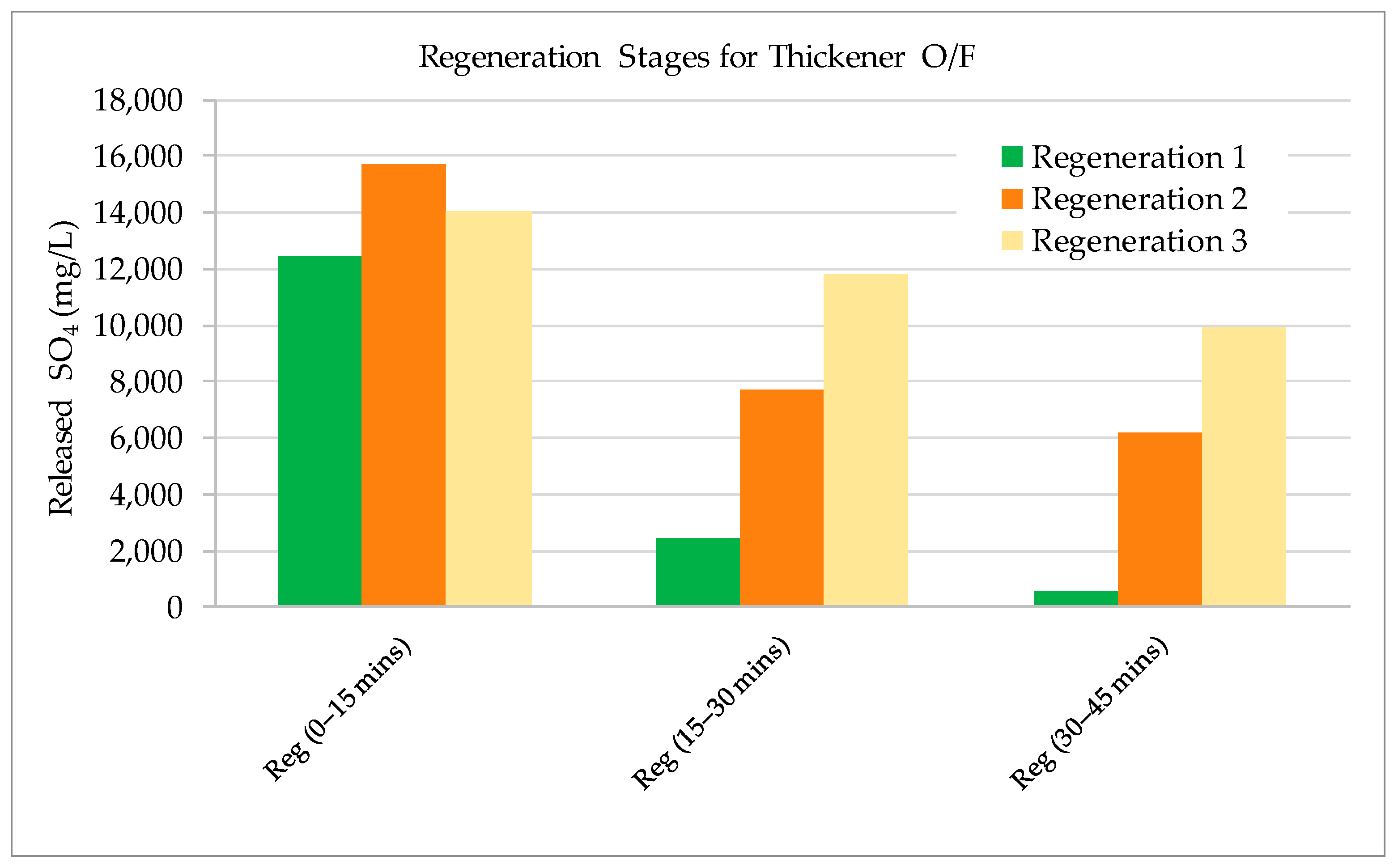
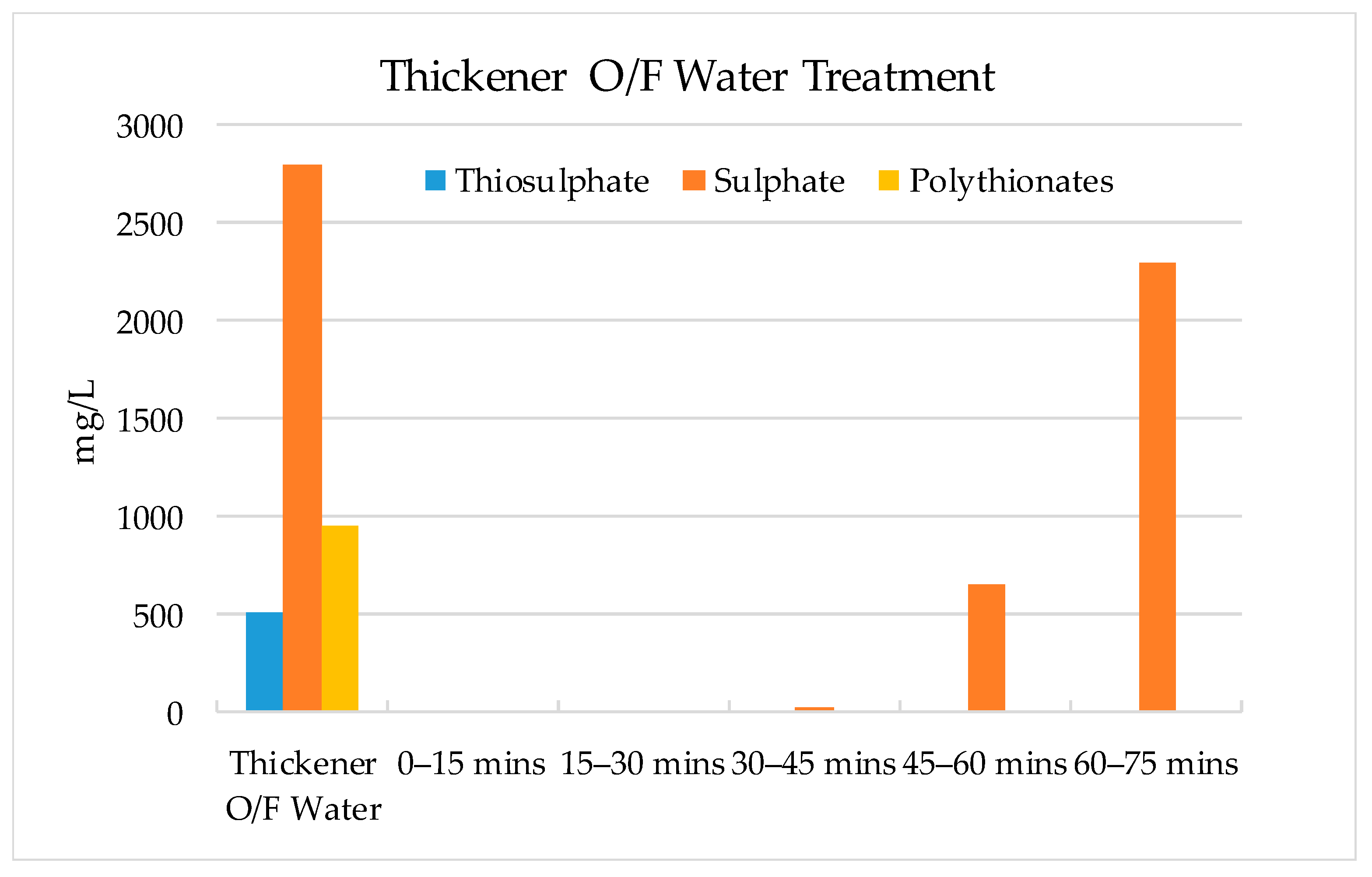
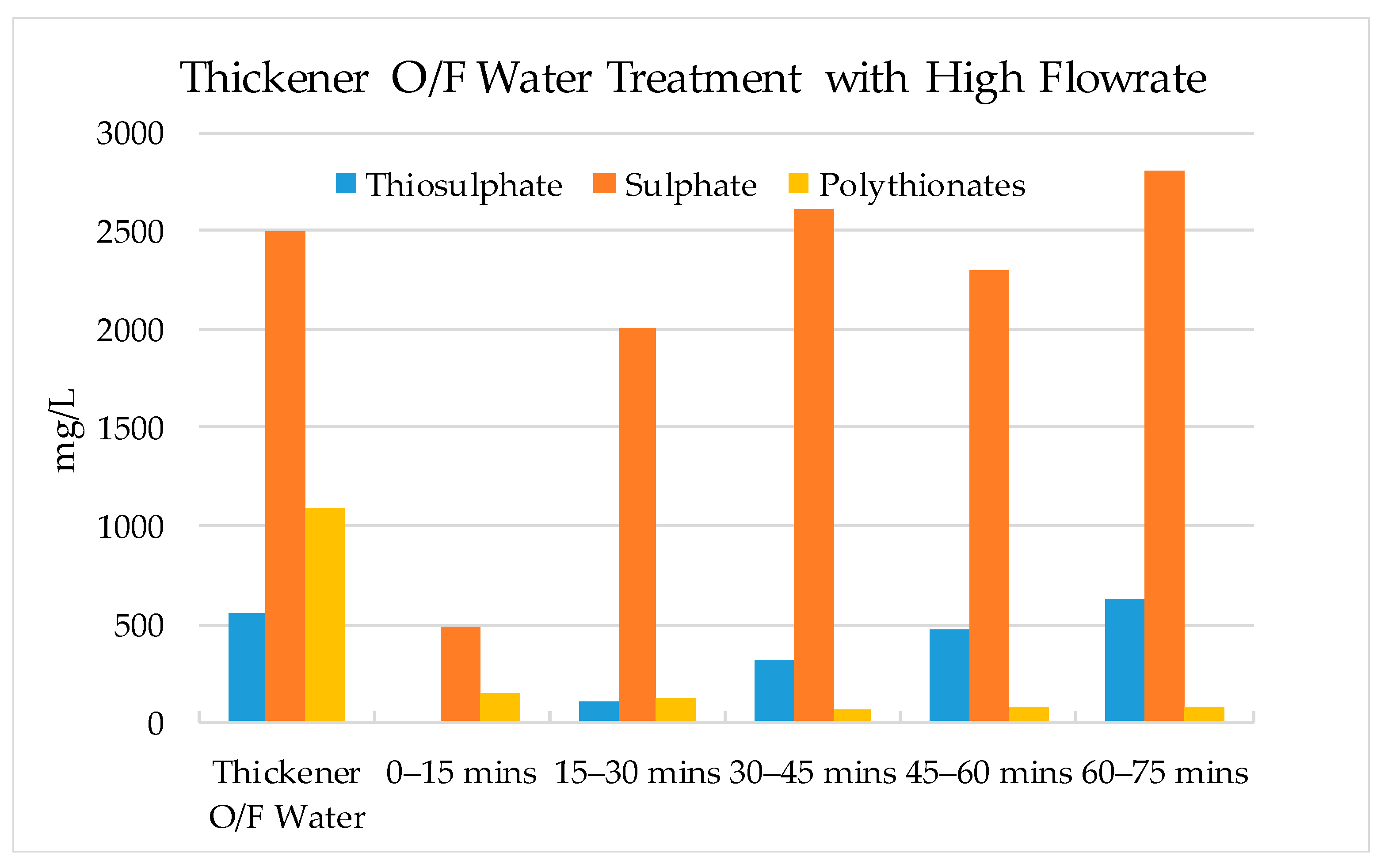
3.2. Validation Tests on Plant Site
4. Conclusions
- Water treatment with ion-exchange resin had a significant potential for successfully decreasing concentrations of sulfur-based problematic ions for flotation of sulfide ores.
- Partial cleaning of the process water was successfully applied using strong base type ion exchange resin after conversion from Cl– to OH− form.
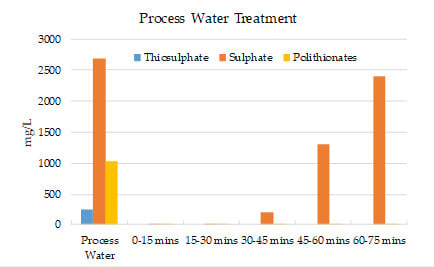
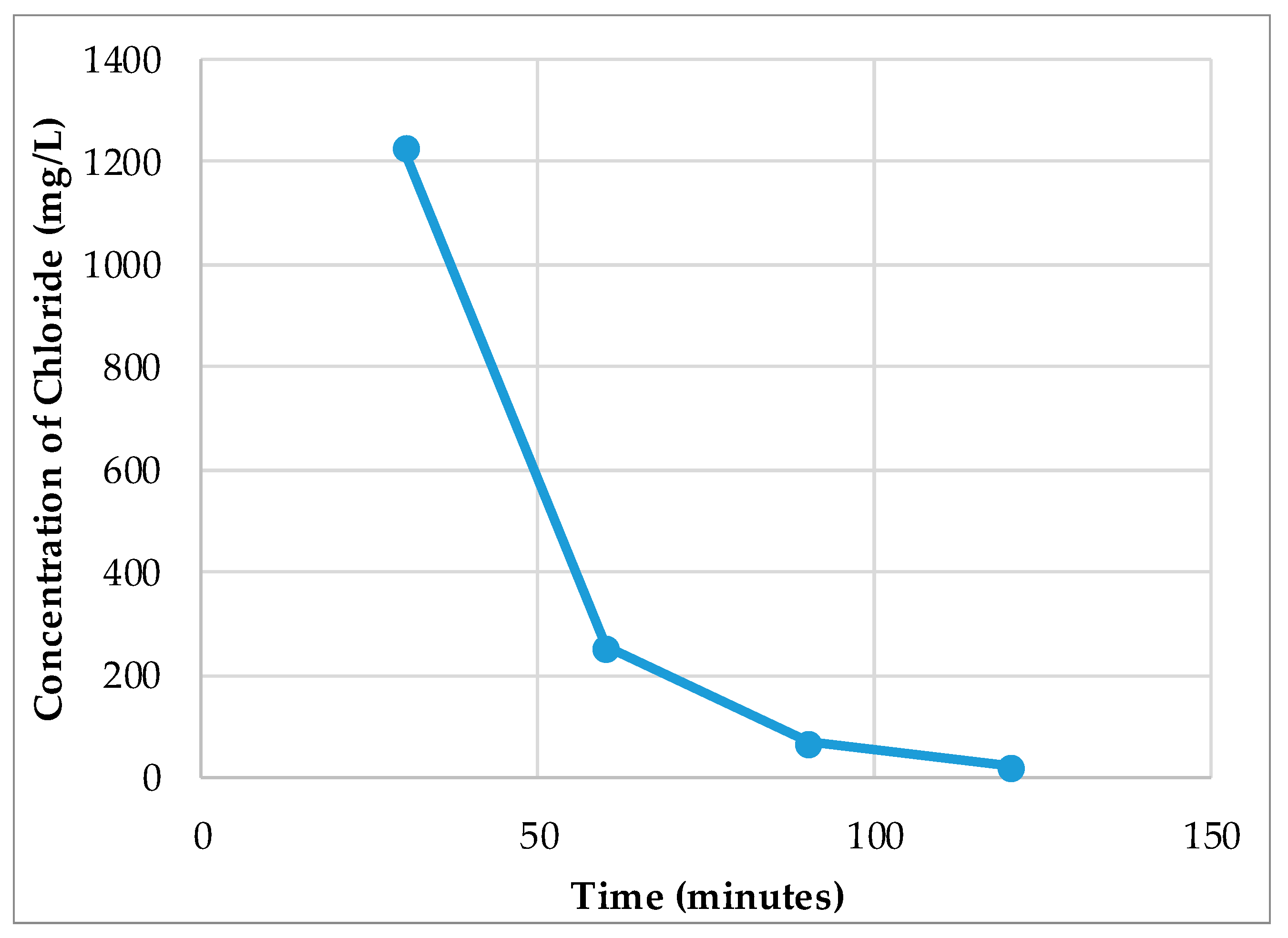
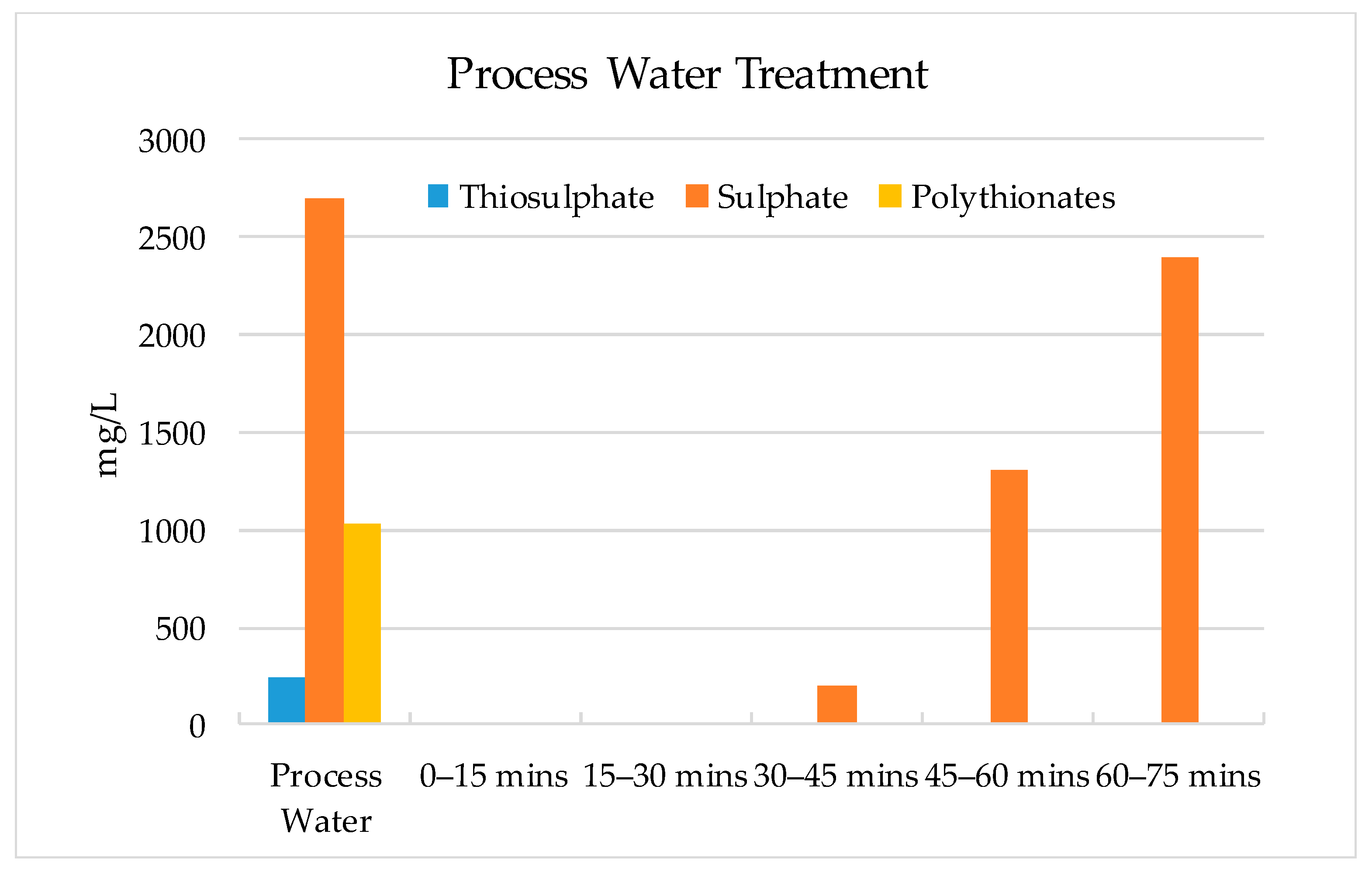
- The results show that 60–70% of sulphate could be removed by strong base-type resin from the process water. The concentration of sulphate ions in the process water could be reduced from 3000–3800 mg/L to 1000–1500 mg/L. The adsorption capacity of the resin was determined as 80.3 mg SO4/g of resin. In addition to the sulphate ion, the thiosulphates and polythionates could also be successfully removed from the process water.
- It should be noted that the flowrate of water had a significant effect on the performance of adsorption. Chemistry of process water is unique for each mine and hence, the required contact time between the resin and water components should be optimized for each case.
- The resin could be regenerated using a solution containing 2% (w/v) NaOH and reused for water treatment processes many times. The same regenerant solution can be used continuously if fresh NaOH solution was added to increase hydroxide concentration in the solution when required and the excess sulphate ion was removed from the solution by precipitation.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, W.; Moran, C.J.; Vink, S. A review of the effect of water quality on flotation. Miner. Eng. 2013, 53, 91–100. [Google Scholar] [CrossRef]
- Punkkinen, H.; Räsänen, L.; Mroueh, U.M.; Korkealaakso, J.; Luoma, S.; Kaipainen, T.; Backnäs, S.; Turunen, K.; Hentinen, K.; Pasanen, A.; et al. Guidelines for Mine Water Management; VTT Technology 266; VTT Technical Research Centre of Finland Ltd.: Espoo, Finland, 2016; p. 172. [Google Scholar]
- Bıçak, Ö.; Ekmekçi, Z.; Can, M.; Öztürk, Y. The effect of water chemistry on froth stability and surface chemistry of the flotation of a Cu–Zn sulfide ore. Int. J. Miner. Process. 2012, 102, 32–37. [Google Scholar] [CrossRef]
- Öztürk, Y.; Bıçak, Ö.; Özdemir, E.; Ekmekçi, Z. Mitigation negative effects of thiosulfate on flotation performance of a Cu-Pb-Zn sulfide ore. Miner. Eng. 2018, 122, 142–147. [Google Scholar] [CrossRef]
- Kirjavainen, V.; Schreithofer, N.; Heiskanen, K. Effect of calcium and thiosulfate ions on flotation selectivity of nickel–copper ores. Miner. Eng. 2002, 15, 1–5. [Google Scholar] [CrossRef]
- Forssberg, K.S.E.; Subrahmanyam, T.V.; Nilsson, L.K. Influence of grinding method on complex sulphide ore flotation: A pilot plant study. Int. J. Miner. Process. 1993, 38, 157–175. [Google Scholar] [CrossRef]
- Rao, S.R. Resource Recovery and Recycling from Metallurgical Wastes; Waste Management Series 7; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2011; Chapter 11; pp. 459–481. [Google Scholar]
- Shengo, L.M.; Gaydardzhiev, S.; Kalenga, N.M. Assessment of water quality effects on flotation of copper–cobalt oxide ore. Miner. Eng. 2014, 65, 145–148. [Google Scholar] [CrossRef]
- Rao, S.R.; Finch, J.A. A review of water re-use in flotation. Miner. Eng. 1989, 2, 65–85. [Google Scholar] [CrossRef]
- Corin, K.C.; Reddy, A.; Miyen, L.; Wiese, J.G.; Harris, P.J. The effect of ionic strength of plant water on valuable mineral and gangue recovery in a platinum bearing ore from the Merensky reef. Miner. Eng. 2011, 24, 131–137. [Google Scholar] [CrossRef]
- Levay, G.; Smart, R.S.C.; Skinner, W.M. The impact of water quality on flotation performance. J. South Afr. Ins. Min. Metall. 2001, 101, 69–75. [Google Scholar]
- Grano, S.R.; Wang, P.L.M.; Skinner, W.; Johnson, N.W.; Ralston, J. Detection and control of calcium sulphate precipitation in the lead circuit of the Hilton concentrator of Mount Isa Mines Limited, Australia. In Proceedings of the XIX International Mineral Processing Congress; S.M.E.: Littleton, CO, USA, 1995; pp. 171–179. [Google Scholar]
- Ikumapayi, F.; Makitalo, M.; Johansson, B.; Rao, K.H. Recycling of process water in sulphide flotation: Effect of calcium and sulphate ions on flotation of galena. Miner. Eng. 2012, 39, 77–88. [Google Scholar] [CrossRef]
- Bowell, R.J. A review of sulfate removal options for mine waters. In Mine Water 2004–Proceedings International Mine Water Association Symposium 2; Jarvis, A.P., Dudgeon, B.A., Younger, P.L., Eds.; University of Newcastle: Newcastle upon Tyne, UK, 2004; pp. 75–91. [Google Scholar]
- Silva, R.; Cadorin, L.; Rubio, J. Sulphate ions removal from an aqueous solution: I. Coprecipitation with hydrolysed aluminum-bearing salts. Miner. Eng. 2010, 23, 1220–1226. [Google Scholar] [CrossRef]
- Filho, J.A.; Azevedo, A.; Etchepare, R.; Rubio, J. Removal of sulfate ions by dissolved air flotation (DAF) following precipitation and flocculation. Int. J. Miner. Process. 2016, 149, 1–8. [Google Scholar] [CrossRef]
- Hekmatzadeh, A.A.; Karimi-Jashni, A.; Talebbeydokhti, N.; Kløve, B. Adsorption kinetics of nitrate ions on ion exchange resin. Desalination 2013, 326, 125–134. [Google Scholar] [CrossRef]
- Song, H.; Zhou, Y.; Li, A.; Mueller, S. Selective removal of nitrate from water by a macroporous strong basic anion exchange resin. Desalination 2012, 296, 53–60. [Google Scholar] [CrossRef]
- Tabassum, S. A combined treatment method of novel Mass Bio System and ion exchange for the removal of ammonia nitrogen from micro-polluted water bodies. Chem. Eng. J. 2019, 378, 122217. [Google Scholar] [CrossRef]
- Hussain, A.; Sharma, R.; Minier-Matar, J.; Hirani, Z.; Adham, S. Application of emerging ion exchange resin for boron removal from saline groundwater. J. Water Process Eng. 2019, 32, 100906. [Google Scholar] [CrossRef]
- Kalaitzidou, K.; Tzika, A.M.; Simeonidis, K.; Mitrakas, M. Evaluation of boron uptake by anion exchange resins in tap and geothermal water matrix. Mater. Today Proc. 2018, 5, 27599–27606. [Google Scholar] [CrossRef]
- Rahim, S.A.; Teo, K.L. Preliminary study of sulphide removal using ion exchange resin. Mater. Today Proc. 2018, 5, 22080–22084. [Google Scholar] [CrossRef]
- Arroyo, F.; Morillo, J.; Usero, J.; Rosado, D.; El Bakouri, H. Lithium recovery from desalination brines using specific ion-exchange resins. Desalination 2019, 468, 114073. [Google Scholar] [CrossRef]
- Martins, P.J.M.; Reis, P.M.; Martins, R.C.; Gando-Ferreira, L.M.; Quinta-Ferreira, R.M. Iron recovery from the Fenton’s treatment of winery effluent using an ion-exchange resin. J. Mol. Liq. 2017, 242, 505–511. [Google Scholar] [CrossRef]
- Lee, J.C.; Kurniawan; Honga, H.J.; Chung, K.W.; Kim, S. Separation of platinum, palladium and rhodium from aqueous solutions using ion exchange resin: A review. Sep. Purif. Technol. 2020, 246, 116896. [Google Scholar] [CrossRef]
- Fu, W.; Ji, G.; Chen, H.; Yang, S.; Yang, H.; Guo, B.; Huang, Z. Engineering anion resin based amorphous molybdenum sulphide composite for treatment of authentic acid mine drainage. J. Environ. Chem. Eng. 2020, 8, 104072. [Google Scholar] [CrossRef]
- Duan, S.; Tong, T.; Zheng, S.; Zhang, X.; Li, S. Achieving low-cost, highly selective nitrate removal with standard anion exchange resin by tuning recycled brine composition. Water Res. 2020, 173, 115571. [Google Scholar]
- Ozturk, Y. Development of Wastewater Treatment Methods for Flotation Plants. Ph.D. Thesis, Hacettepe University, Ankara, Turkey, 2018. [Google Scholar]
- Selion SBA2000 MSDS Sheet.
- Can, İ.B.; Bıçak, Ö.; Özçelik, S.; Can, N.M.; Ekmekçi, Z. Use of ion exchange resin for process water treatment. In Proceedings of the SME MineXChange Annual Conference & Expo, Phoenix, AZ, USA, 23–26 February 2020. Preprint 20-022. [Google Scholar]
- Hilal, N.; Kochkodan, V.; Abdulgader, H.A.; Mandale, S.; Al-Jlil, S.A. A combined ion exchange–nanofiltration process for water desalination: I. sulphate–chloride ion-exchange in saline solutions. Desalination 2015, 363, 44–50. [Google Scholar] [CrossRef]
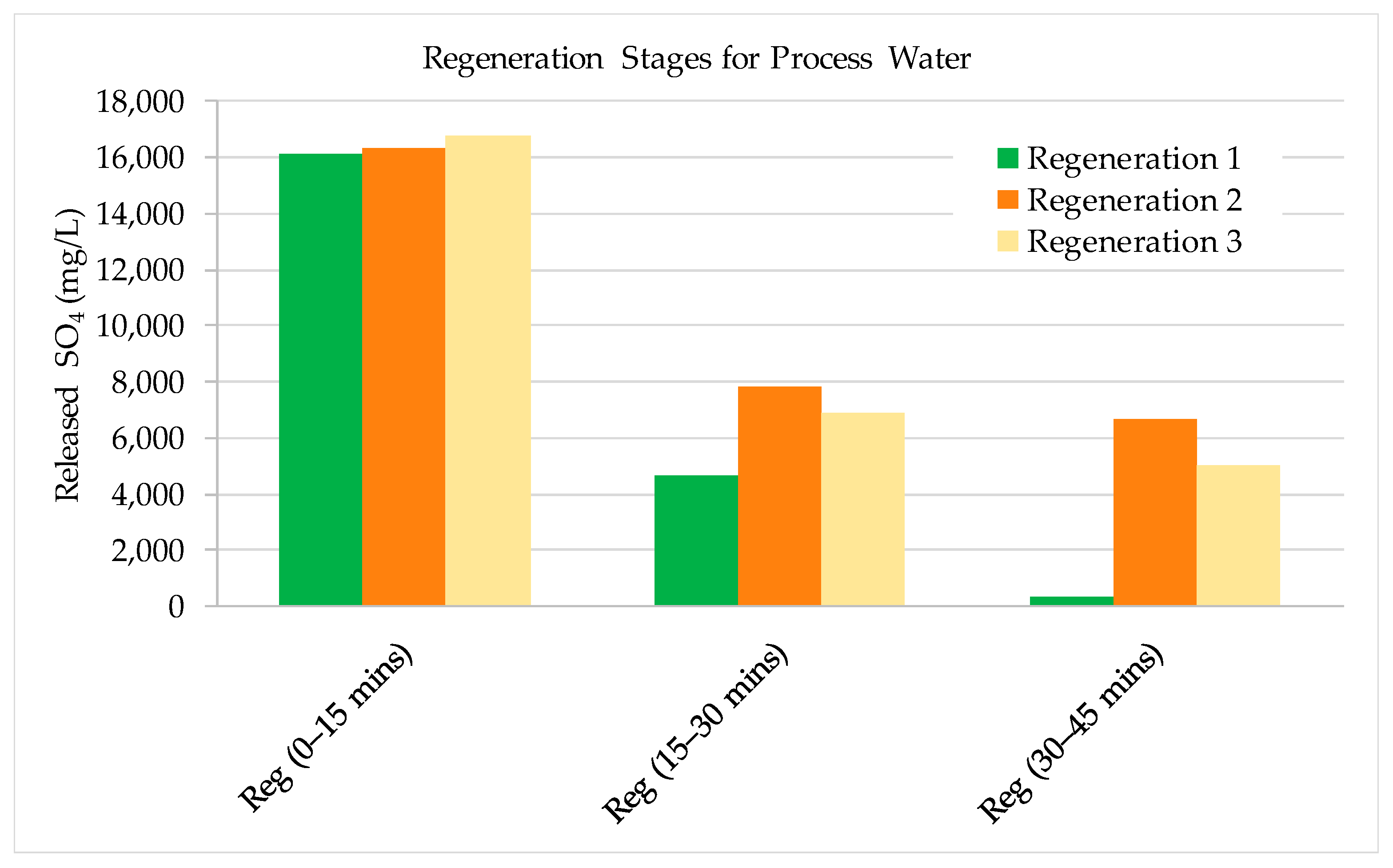
| Commercial Product Code | Selion SBA 2000 |
|---|---|
| Matrix | Crosslinked Polystyrene divinylbenzene (DVB) |
| Functional Group | Type II Quaternary Ammonium |
| Appearance | Spherical beads |
| Particle Size Range | 0.315–1.25 mm |
| Type | Anion Resin—Demineralization High Efficiency |
| Shipping Form | Cl− |
| Moisture Content | 45–51% (Cl− form) |
| Bulk Density | 680–750 g/L |
| Specific Gravity | 1.07–1.12 |
| Whole Beads | ≥95% |
| Total Exchange Capacity | ≥1.30 eq/L (min.) |
| Uniformity Coefficient | ≤1.6 (max.) |
| Max. Operating Temperature | 35–40 °C (OH– form) 85 °C (Cl– form) |
| Sulfate (SO42−) mg/L | Thiosulfate (S2O32−) mg/L | Chloride (Cl−) mg/L | Nitrate (NO3−) mg/L |
|---|---|---|---|
| 3500 | 288 | 870 | 593 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Can, İ.B.; Bıçak, Ö.; Özçelik, S.; Can, M.; Ekmekçi, Z. Sulphate Removal from Flotation Process Water Using Ion-Exchange Resin Column System. Minerals 2020, 10, 655. https://doi.org/10.3390/min10080655
Can İB, Bıçak Ö, Özçelik S, Can M, Ekmekçi Z. Sulphate Removal from Flotation Process Water Using Ion-Exchange Resin Column System. Minerals. 2020; 10(8):655. https://doi.org/10.3390/min10080655
Chicago/Turabian StyleCan, İlkay Bengü, Özlem Bıçak, Seda Özçelik, Metin Can, and Zafir Ekmekçi. 2020. "Sulphate Removal from Flotation Process Water Using Ion-Exchange Resin Column System" Minerals 10, no. 8: 655. https://doi.org/10.3390/min10080655
APA StyleCan, İ. B., Bıçak, Ö., Özçelik, S., Can, M., & Ekmekçi, Z. (2020). Sulphate Removal from Flotation Process Water Using Ion-Exchange Resin Column System. Minerals, 10(8), 655. https://doi.org/10.3390/min10080655