First Direct Dating of Alteration of Paleo-Oil Pools Using Rubidium-Strontium Pyrite Geochronology
Abstract
1. Introduction
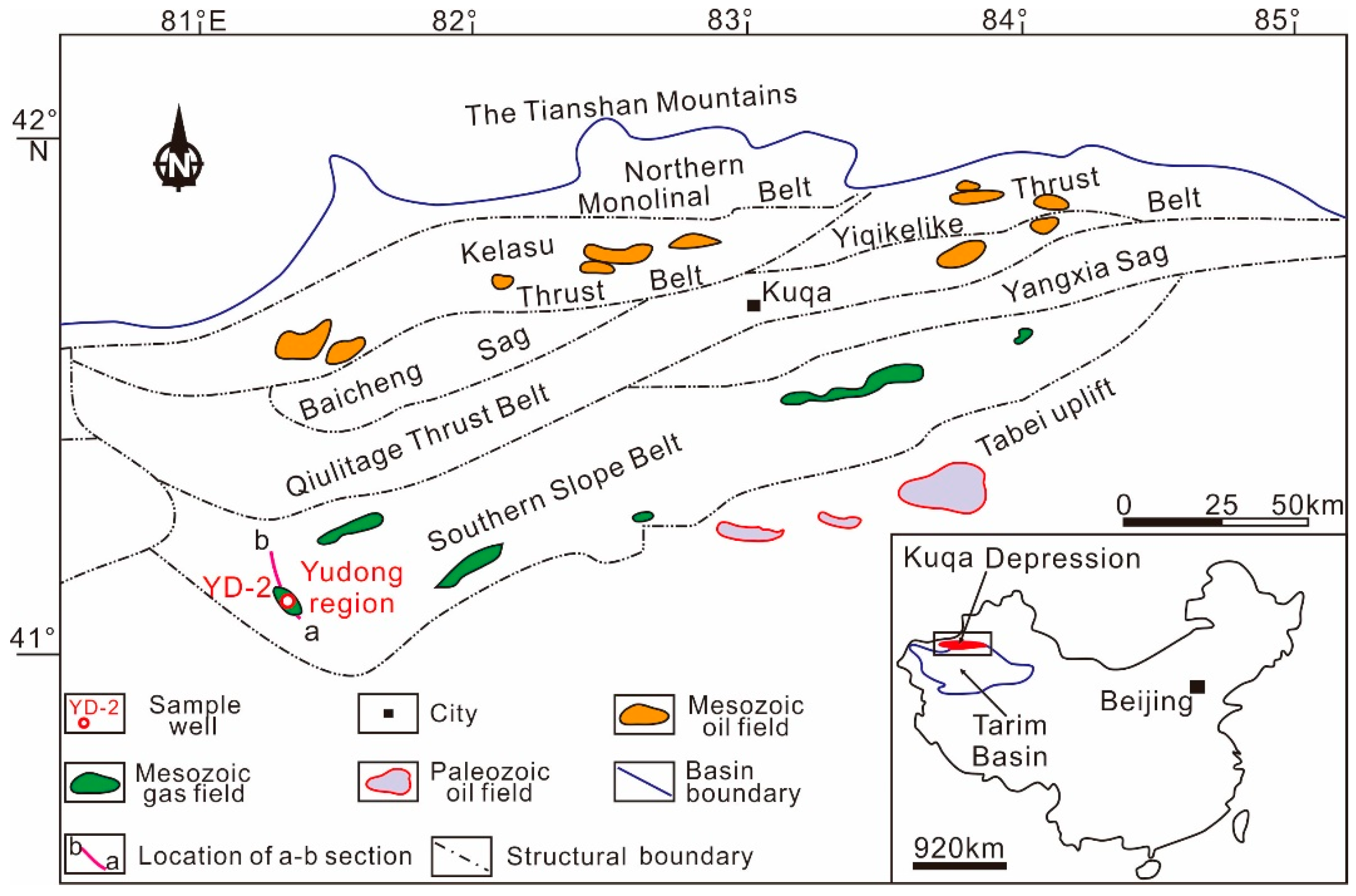
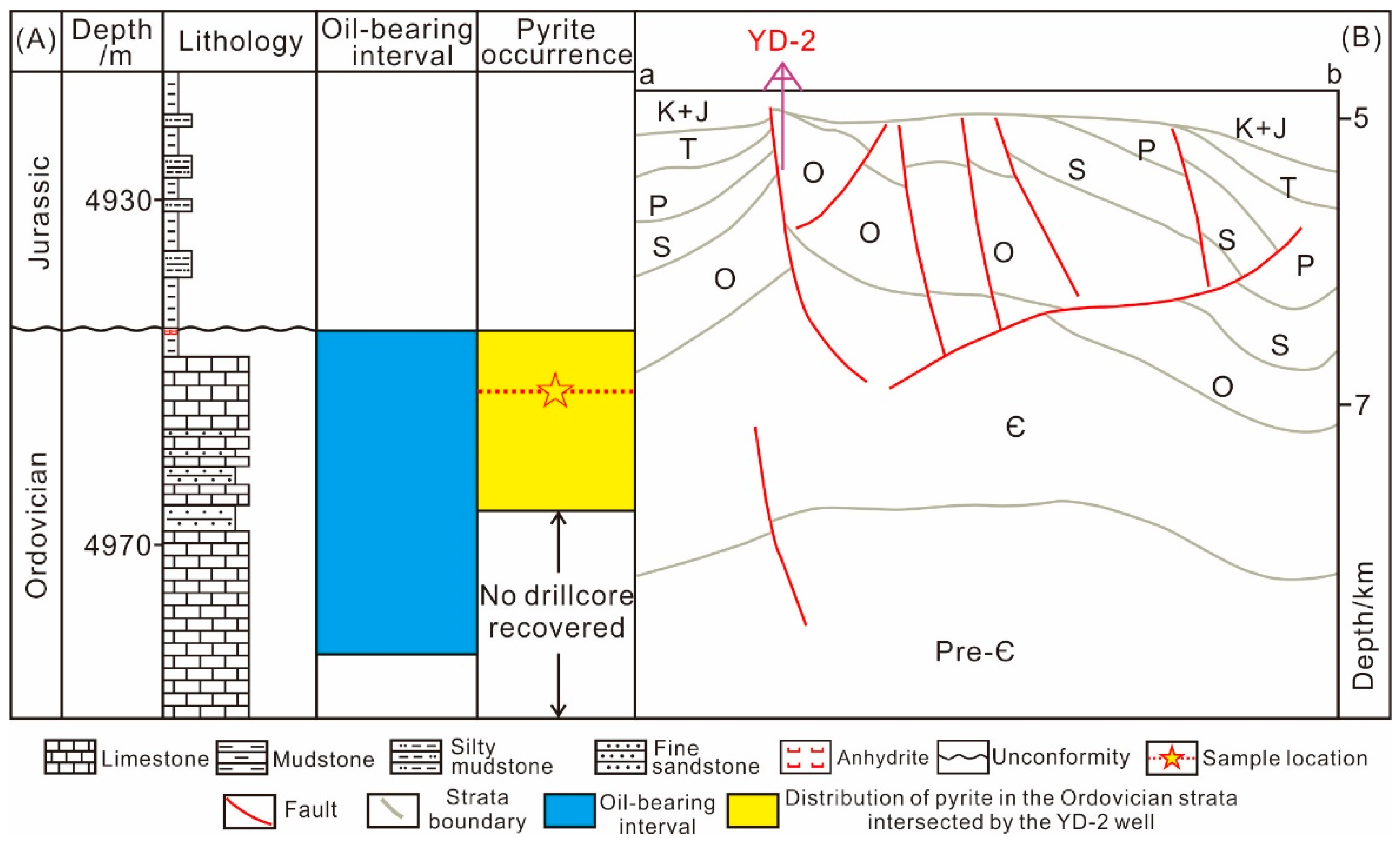
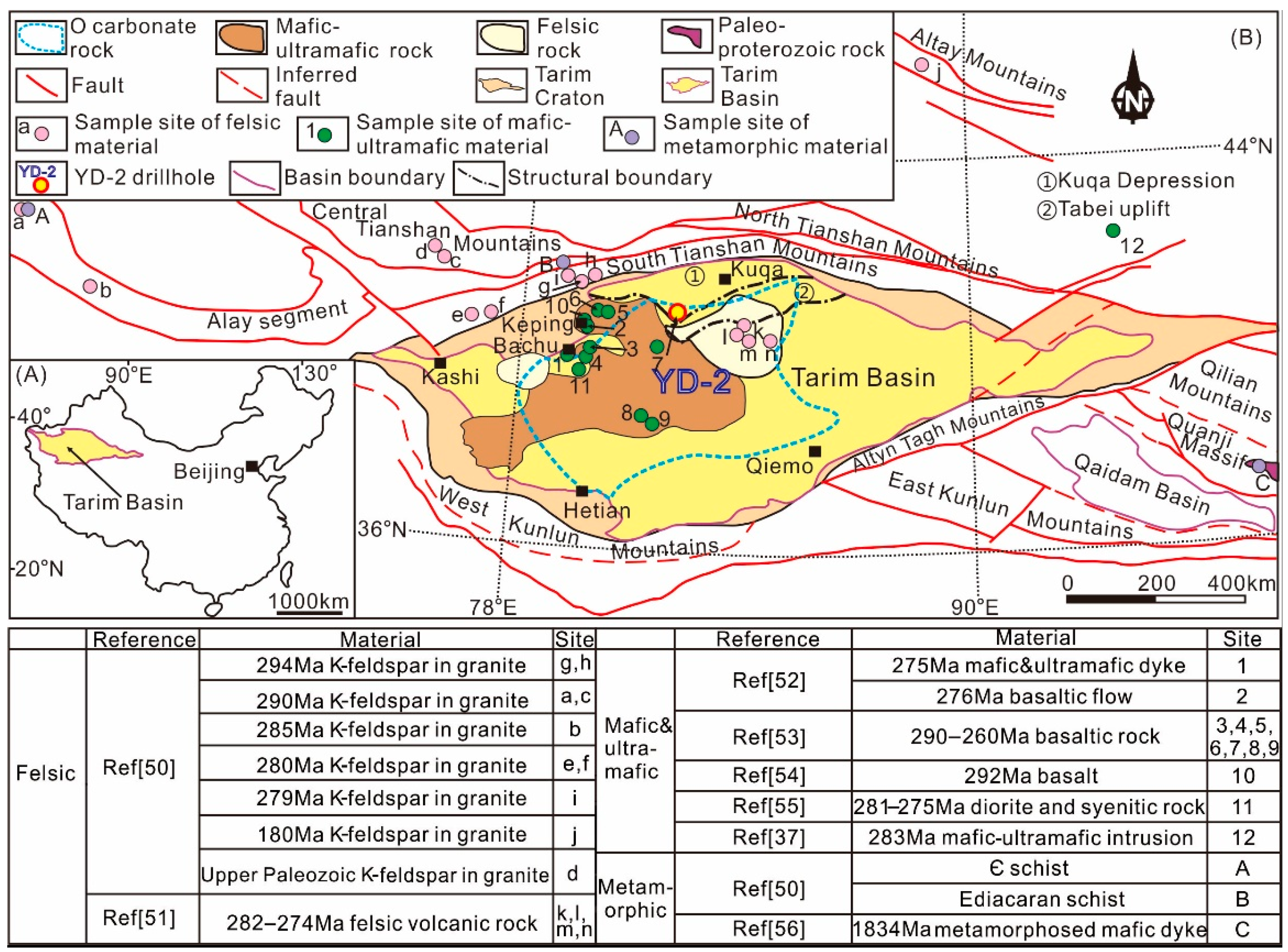
2. Geological Background
3. Samples and Analytical Method
4. Results
4.1. Petrographic Observations
4.2. Rb-Sr-Pb-S Isotope Data and Isochron Ages
5. Discussion
5.1. Pyrite Rb-Sr Geochronology: A Robust Dating Technique for Petroleum Systems
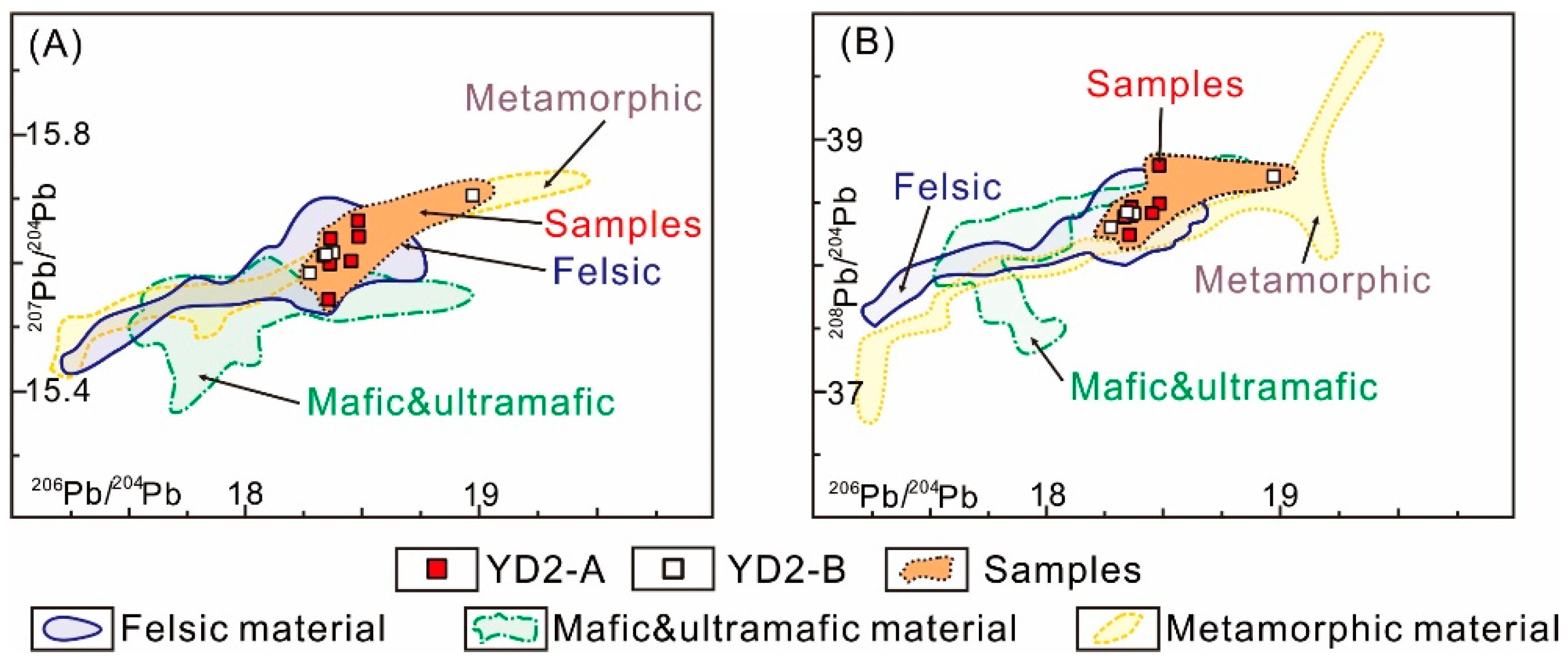
5.2. Source of Metals in Hydrothermal Fluid
5.3. Source of Sulfur
5.4. Timing of Alteration of the Paleo-Oil Pool
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Georgiev, S.V.; Stein, H.J.; Hannah, J.L.; Galimberti, R.; Nali, M.; Yang, G.; Zimmerman, A. Re–Os dating of maltenes and asphaltenes within single samples of crude oil. Geochim. Cosmochim. Acta 2016, 179, 53–75. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.-C.; Li, C.-F.; Wilde, S.A.; Zhang, Y.; Golding, S.D.; Liu, K.; Zhang, Y. Direct Rubidium-Strontium Dating of Hydrocarbon Charge Using Small Authigenic Illitic Clay Aliquots from the Silurian Bituminous Sandstone in the Tarim Basin, NW China. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhou, X. The Rb-Sr isochron of ore and pyrite sub-samples from Linglong gold deposit, Jiaodong Peninsula, eastern China and their geological significance. Chin. Sci. Bull. 2000, 45, 2272–2277. [Google Scholar] [CrossRef]
- Berner, R.A. Sedimentary pyrite formation: An update. Geochim. Cosmochim. Acta 1984, 48, 605–615. [Google Scholar] [CrossRef]
- Rasmussen, B. Evidence for pervasive petroleum generation and migration in 3.2 and 2.63 Ga shales. Geology 2005, 33, 497–500. [Google Scholar] [CrossRef]
- Ellis, G. Hydrocarbon Entrapment in Triassic to Late Jurassic Reservoirs in the Timor Sea, Australia—New Insights. APPEA J. 2007, 47, 39–53. [Google Scholar] [CrossRef]
- Machel, H.G.; Krouse, H.R.; Sassen, R. Products and distinguishing criteria of bacterial and thermochemical sulfate reduction. Appl. Geochem. 1995, 10, 373–389. [Google Scholar] [CrossRef]
- Large, R.R.; Danyushevsky, L.; Hollitt, C.; Maslennikov, V.; Meffre, S.; Gilbert, S.; Bull, S.; Scott, R.; Emsbo, P.; Thomas, H.; et al. Gold and Trace Element Zonation in Pyrite Using a Laser Imaging Technique: Implications for the Timing of Gold in Orogenic and Carlin-Style Sediment-Hosted Deposits. Econ. Geol. 2009, 104, 635–668. [Google Scholar] [CrossRef]
- Large, R.R.; Halpin, J.A.; Danyushevsky, L.; Maslennikov, V.V.; Bull, S.W.; Long, J.A.; Gregory, D.D.; Lounejeva, E.; Lyons, T.W.; Sack, P.J.; et al. Trace element content of sedimentary pyrite as a new proxy for deep-time ocean–atmosphere evolution. Earth Planet. Sci. Lett. 2014, 389, 209–220. [Google Scholar] [CrossRef]
- Reich, M.; Deditius, A.; Chryssoulis, S.; Li, J.-W.; Ma, C.-Q.; Parada, M.-A.; Barra, F.; Mittermayr, F. Pyrite as a record of hydrothermal fluid evolution in a porphyry copper system: A SIMS/EMPA trace element study. Geochim. Cosmochim. Acta 2013, 104, 42–62. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.-C.; Xu, J.-F.; Xu, Y.-G.; Tang, G.-J.; Wang, Q. Disequilibrium-induced initial Os isotopic heterogeneity in gram aliquots of single basaltic rock powders: Implications for dating and source tracing. Chem. Geol. 2015, 406, 10–17. [Google Scholar] [CrossRef]
- Li, C.; Chu, Z.-Y.; Guo, J.-H.; Li, Y.-L.; Yang, Y.-H.; Li, X.-H. A rapid single column separation scheme for high-precision Sr–Nd–Pb isotopic analysis in geological samples using thermal ionization mass spectrometry. Anal. Methods 2015, 7, 4793–4802. [Google Scholar] [CrossRef]
- Cai, C.; Hu, W.; Worden, R.H. Thermochemical sulphate reduction in Cambro–Ordovician carbonates in Central Tarim. Mar. Pet. Geol. 2001, 18, 729–741. [Google Scholar] [CrossRef]
- Cai, C.; Worden, R.H.; Wang, Q.; Xiang, T.; Zhu, J.; Chu, X. Chemical and isotopic evidence for secondary alteration of natural gases in the Hetianhe Field, Bachu Uplift of the Tarim Basin. Org. Geochem. 2002, 33, 1415–1427. [Google Scholar] [CrossRef]
- Cai, C.; Li, K.; Anlai, M.; Zhang, C.; Xu, Z.; Worden, R.H.; Wu, G.; Zhang, B.; Chen, L. Distinguishing Cambrian from Upper Ordovician source rocks: Evidence from sulfur isotopes and biomarkers in the Tarim Basin. Org. Geochem. 2009, 40, 755–768. [Google Scholar] [CrossRef]
- Li, S.; Shi, Q.; Pang, X.; Zhang, B.; Zhang, H. Origin of the unusually high dibenzothiophene oils in Tazhong-4 Oilfield of Tarim Basin and its implication in deep petroleum exploration. Org. Geochem. 2012, 48, 56–80. [Google Scholar] [CrossRef]
- Lü, X.; Xie, Q.; Yang, N.; Li, J. Hydrocarbon accumulation in deep fluid modified carbonate rock in the Tarim Basin. Chin. Sci. Bull. 2007, 52, 184–192. [Google Scholar] [CrossRef]
- Jia, L.; Cai, C.; Yang, H.; Li, H.; Wang, T.; Zhang, B.; Jiang, L.; Tao, X. Thermochemical and bacterial sulfate reduction in the Cambrian and Lower Ordovician carbonates in the Tazhong Area, Tarim Basin, NW China: Evidence from fluid inclusions, C, S, and Sr isotopic data. Geofluids 2014, 15, 421–437. [Google Scholar] [CrossRef]
- Zhu, D.-Y.; Meng, Q.-Q. Genesis of pyrite in Ordovician carbonate of the Tarim Basin. Acta Petrol. Mineral. 2010, 29, 516–524. [Google Scholar]
- Li, K.; Cai, C.; He, H.; Jiang, L.; Cai, L.; Xiang, L.; Huang, S.; Zhang, C. Origin of palaeo-waters in the Ordovician carbonates in Tahe oilfield, Tarim Basin: Constraints from fluid inclusions and Sr, C and O isotopes. Geofluids 2010, 11, 71–86. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, S.; Su, J. Palaeozoic oil–source correlation in the Tarim Basin, NW China: A review. Org. Geochem. 2016, 94, 32–46. [Google Scholar] [CrossRef]
- Zhou, J.-F.; Zhang, H.-Q.; Yu, J.-P.; Zhu, Y.-F.; Yao, Q.-Z.; Yuan, Y.-C. Analysis of the Hydrocarbon Accumulation Conditions and Exploration Potential in the Ordovician in Yudong Area of West Tabei Uplift. Nat. Gas Geosci. 2015, 26, 121–129. [Google Scholar]
- Liu, J.; Jiang, Z.; Liu, K.; Gui, L.; Xing, J. Hydrocarbon sources and charge history in the Southern Slope Region, Kuqa Foreland Basin, northwestern China. Mar. Pet. Geol. 2016, 74, 26–46. [Google Scholar] [CrossRef]
- Jia, C.; Wei, G. Structural characteristics and petroliferous features of Tarim Basin. Chin. Sci. Bull. 2002, 47, 1–11. [Google Scholar] [CrossRef]
- Li, C.-F.; Guo, J.-H.; Chu, Z.-Y.; Feng, L.-J.; Wang, X.-C. Direct High-Precision Measurements of the 87Sr/86Sr Isotope Ratio in Natural Water without Chemical Separation Using Thermal Ionization Mass Spectrometry Equipped with 1012 Ω Resistors. Anal. Chem. 2015, 87, 7426–7432. [Google Scholar] [CrossRef]
- Pin, C.; Gannoun, A.; Dupont, A. Rapid, simultaneous separation of Sr, Pb, and Nd by extraction chromatography prior to isotope ratios determination by TIMS and MC-ICP-MS. J. Anal. At. Spectrom. 2014, 29, 1858–1870. [Google Scholar] [CrossRef]
- Ludwig, K. User’s Manual for Isoplot 3.00: A Geochronological Toolkit for Microsoft Excel; Geochronology Center: Berkeley, CA, USA, 2003. [Google Scholar]
- Steiger, R.H.; Jäger, E. Subcommission on geochronology: Convention on the use of decay constants in geo- and cosmochronology. Earth Planet. Sci. Lett. 1977, 36, 359–362. [Google Scholar] [CrossRef]
- Li, Q.-L.; Chen, F.; Yang, J.-H.; Fan, H.-R. Single grain pyrite Rb–Sr dating of the Linglong gold deposit, eastern China. Ore Geol. Rev. 2008, 34, 263–270. [Google Scholar] [CrossRef]
- Yang, J.-H.; Zhou, X.-H. Rb-Sr, Sm-Nd, and Pb isotope systematics of pyrite: Implications for the age and genesis of lode gold deposits. Geology 2001, 29, 711–714. [Google Scholar] [CrossRef]
- Cao, H.-W.; Zhang, S.-T.; Santosh, M.; Zheng, L.; Tang, L.; Li, D.; Zhang, X.-H.; Zhang, Y.-H. The Luanchuan Mo–W–Pb–Zn–Ag magmatic–hydrothermal system in the East Qinling metallogenic belt, China: Constrains on metallogenesis from C–H–O–S–Pb isotope compositions and Rb–Sr isochron ages. J. Asian Earth Sci. 2015, 111, 751–780. [Google Scholar] [CrossRef]
- Dong, L.; Wan, B.; Yang, W.; Deng, C.; Chen, Z.; Yang, L.; Cai, K.; Xiao, W. Rb-Sr geochronology of single gold-bearing pyrite grains from the Katbasu gold deposit in the South Tianshan, China and its geological significance. Ore Geol. Rev. 2018, 100, 99–110. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Zhang, S.; Zhang, Y.; Chen, F. Single grain Rb-Sr dating of euhedral and cataclastic pyrite from the Qiyugou gold deposit in western Henan, central China. Chin. Sci. Bull. 2007, 52, 1820–1826. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, Y.; Mao, J.; Wei, R.; Liu, S.; Ye, D.; Yuan, Q.; Dou, P.; Qiaoqing, H.; Yitian, W.; et al. Timing of the formation of the Changba–Lijiagou Pb–Zn ore deposit, Gansu Province, China: Evidence from Rb–Sr isotopic dating of sulfides. J. Asian Earth Sci. 2015, 103, 350–359. [Google Scholar] [CrossRef]
- Ni, Z.-Y.; Chen, Y.-J.; Li, N.; Zhang, H. Pb–Sr–Nd isotope constraints on the fluid source of the Dahu Au–Mo deposit in Qinling Orogen, central China, and implication for Triassic tectonic setting. Ore Geol. Rev. 2012, 46, 60–67. [Google Scholar] [CrossRef]
- Vadlamani, R.; Golani, P.R. Rb-Sr direct dating of pyrite from the Pipela VMS Zn-Cu prospect, Rajasthan, NW India. J. Geol. Soc. India 2011, 77, 149–159. [Google Scholar]
- Sun, W.-Y.; Li, S.-R.; Santosh, M.; Wang, X.; Zhang, L.-J. Isotope geochemistry and geochronology of the Qiubudong silver deposit, central North China Craton: Implications for ore genesis and lithospheric dynamics. Ore Geol. Rev. 2014, 57, 229–242. [Google Scholar] [CrossRef]
- Wang, C.; Deng, J.; Santosh, M.; Carranza, E.J.M.; Gong, Q.; Guo, C.; Xia, R.; Lai, X. Timing, tectonic implications and genesis of gold mineralization in the Xincheng gold deposit, China: C–H–O isotopes, pyrite Rb–Sr and zircon fission track thermochronometry. Ore Geol. Rev. 2015, 65, 659–673. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, Q.; Liu, J. Rb-Sr Dating of Gold-bearing Pyrites from Wulaga Gold Deposit and its Geological Significance. Resour. Geol. 2014, 64, 262–270. [Google Scholar] [CrossRef]
- Yao, J.; Hua, R.; Lin, J. REE, Pb-S isotope geochemistry, and Rb-Sr isochron age of pyrites in the Baoshan deposit, south Hunan province, China. Acta Geol. Sin. 2006, 80, 1045–1054. [Google Scholar]
- Zhang, L. 40Ar/39Ar and Rb-Sr isochron dating of the gold deposits on northern margin of the Jiaolai Basin, Shandong, China. Sci. China Ser. D: Earth Sci. 2003, 46, 708–718. [Google Scholar] [CrossRef]
- Zhao, X.-F.; Zhou, M.-F.; Li, J.-W.; Selby, D.; Li, X.-H.; Qi, L. Sulfide Re-os and Rb-sr isotope dating of the kangdian IOCG metallogenic province, southwest china: Implications for regional metallogenesis. Econ. Geol. 2013, 108, 1489–1498. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, Z.; Zhou, M.; Li, X.; Jin, Z. Constraints of C–O–S–Pb isotope compositions and Rb–Sr isotopic age on the origin of the Tianqiao carbonate-hosted Pb–Zn deposit, SW China. Ore Geol. Rev. 2013, 53, 77–92. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, H. Geochemistry of Palaeozoic marine petroleum from the Tarim Basin, NW China: Part 1. Oil family classification. Org. Geochem. 2005, 36, 1204–1214. [Google Scholar] [CrossRef]
- Riciputi, L.R.; Cole, D.R.; Machel, H.G. Sulfide formation in reservoir carbonates of the Devonian Nisku Formation, Alberta, Canada: An ion microprobe study. Geochim. Cosmochim. Acta 1996, 60, 325–336. [Google Scholar] [CrossRef]
- Hedenquist, J.W.; Lowenstern, J.B. The role of magmas in the formation of hydrothermal ore deposits. Nature 1994, 370, 519–527. [Google Scholar] [CrossRef]
- Worden, R.; Smalley, P. H2S-producing reactions in deep carbonate gas reservoirs: Khuff Formation, Abu Dhabi. Chem. Geol. 1996, 133, 157–171. [Google Scholar] [CrossRef]
- Cai, C.; Li, K.; Li, H.; Zhang, B. Evidence for cross formational hot brine flow from integrated 87Sr/86Sr, REE and fluid inclusions of the Ordovician veins in Central Tarim, China. Appl. Geochem. 2008, 23, 2226–2235. [Google Scholar] [CrossRef]
- Xu, Y.-G.; Wei, X.; Luo, Z.-Y.; Liu, H.-Q.; Cao, J. The Early Permian Tarim Large Igneous Province: Main characteristics and a plume incubation model. Lithos 2014, 204, 20–35. [Google Scholar] [CrossRef]
- Chiaradia, M.; Konopelko, D.; Seltmann, R.; Cliff, R.A. Lead isotope variations across terrane boundaries of the Tien Shan and Chinese Altay. Miner. Deposita 2006, 41, 411–428. [Google Scholar] [CrossRef]
- Yu, J.; Mo, X.; Dong, G.; Yu, X.; Xing, F.; Li, Y.; Huang, X. Felsic volcanic rocks from northern Tarim, NW China: Zircon U-Pb dating and geochemical characteristics. Acta Petrol. Sin. 2011, 27, 2184–2194. [Google Scholar]
- Zhou, M.-F.; Zhao, J.-H.; Jiang, C.-Y.; Gao, J.-F.; Wang, W.; Yang, S.-H. OIB-like, heterogeneous mantle sources of Permian basaltic magmatism in the western Tarim Basin, NW China: Implications for a possible Permian large igneous province. Lithos 2009, 113, 583–594. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Guo, Z. Permian basaltic rocks in the Tarim basin, NW China: Implications for plume–lithosphere interaction. Gondwana Res. 2010, 18, 596–610. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, T.; Yuan, F.; Jowitt, S.M.; Fan, Y.; Liu, S. Source, evolution and emplacement of Permian Tarim Basalts: Evidence from U–Pb dating, Sr–Nd–Pb–Hf isotope systematics and whole rock geochemistry of basalts from the Keping area, Xinjiang Uygur Autonomous region, northwest China. J. Asian Earth Sci. 2012, 49, 175–190. [Google Scholar] [CrossRef]
- Zou, S.-Y.; Li, Z.-L.; Song, B.; Ernst, R.E.; Li, Y.-Q.; Ren, Z.-Y.; Yang, S.-F.; Chen, H.-L.; Xu, Y.-G.; Song, X.-Y. Zircon U–Pb dating, geochemistry and Sr–Nd–Pb–Hf isotopes of the Wajilitag alkali mafic dikes, and associated diorite and syenitic rocks: Implications for magmatic evolution of the Tarim large igneous province. Lithos 2015, 212, 428–442. [Google Scholar] [CrossRef]
- Liao, F.; Zhang, L.; Chen, N.; Sun, M.; Santosh, M.; Wang, Q.; Mustafa, H.A. Geochronology and geochemistry of meta-mafic dykes in the Quanji Massif, NW China: Paleoproterozoic evolution of the Tarim Craton and implications for the assembly of the Columbia supercontinent. Precambrian Res. 2014, 249, 33–56. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, B.; Yang, H.; Su, J.; Han, J. Origin of deep strata gas of Tazhong in Tarim Basin, China. Org. Geochem. 2014, 74, 85–97. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, C.; Cai, L.; Wu, G.; Jiang, L.; Xu, Z.; Li, K.; Ma, A.; Chen, L. Origins of Palaeozoic oils in the Tarim Basin: Evidence from sulfur isotopes and biomarkers. Chem. Geol. 2009, 268, 197–210. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, Z.; Zhang, S.; Liang, D. Geochemical features and classification of crude oil in the Kuqa Depression, Tarim Basin. Bulletin of Mineralogy. Petrol. Geochem. 2005, 24, 369. [Google Scholar]


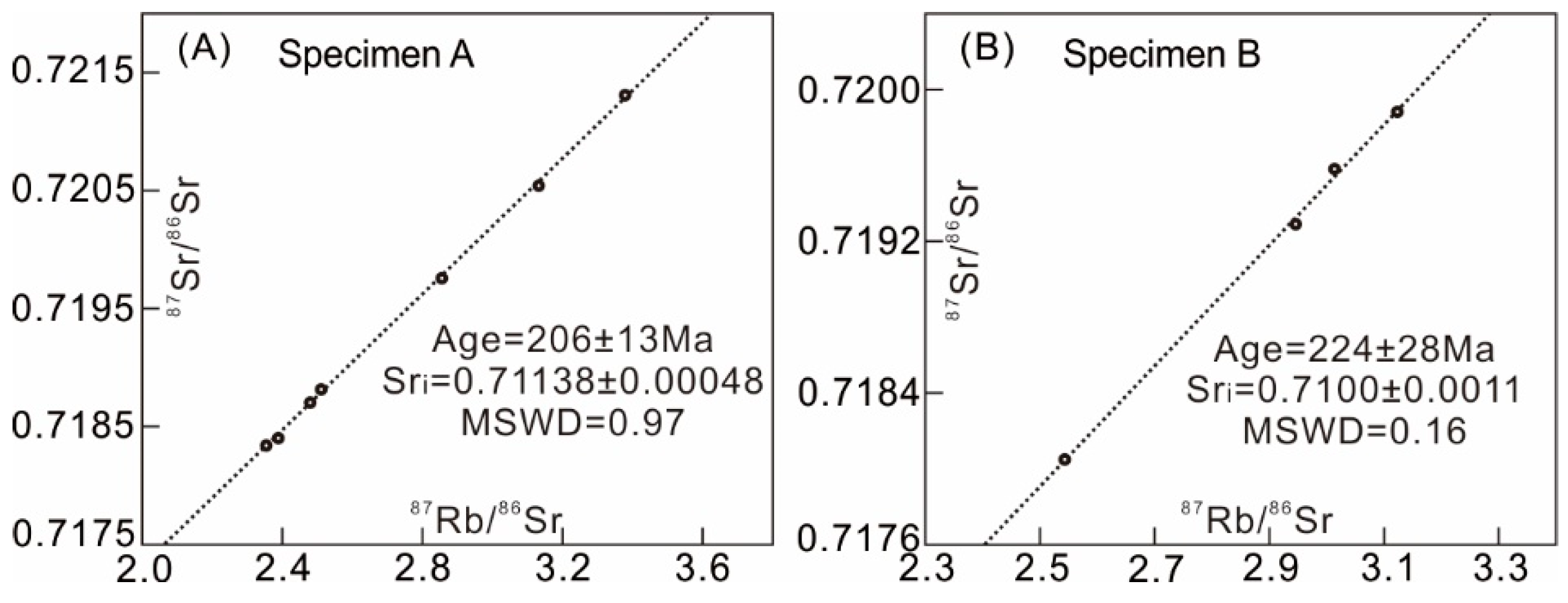


| Sample | Subsample Analysis | Rb [ppm] | Sr [ppm] | 87Rb/86Sr | 87Sr/86Sr | ±2σ | * Sri |
|---|---|---|---|---|---|---|---|
| A | A-1 | 0.1644 | 0.1420 | 3.379 | 0.721307 | 0.000031 | 0.711409 |
| A-2 | 0.1353 | 0.1373 | 2.856 | 0.719757 | 0.000033 | 0.711392 | |
| A-3 | 0.1459 | 0.1358 | 3.133 | 0.720542 | 0.000036 | 0.711365 | |
| A-4 | 0.1637 | 0.1890 | 2.511 | 0.718813 | 0.000019 | 0.711456 | |
| A-5 | 0.1655 | 0.1938 | 2.480 | 0.718702 | 0.000029 | 0.711436 | |
| A-6 | 0.1580 | 0.1947 | 2.355 | 0.718340 | 0.000030 | 0.711442 | |
| A-7 | 0.1647 | 0.1958 | 2.438 | 0.718401 | 0.000039 | 0.711261 | |
| B | B-1 | 0.2251 | 0.2214 | 2.946 | 0.719290 | 0.000026 | 0.709903 |
| B-2 | 0.2611 | 0.2511 | 3.014 | 0.719580 | 0.000026 | 0.709978 | |
| B-3 | 0.1891 | 0.2154 | 2.543 | 0.718049 | 0.000024 | 0.709946 | |
| B-4 | 0.2383 | 0.2211 | 3.124 | 0.719882 | 0.000024 | 0.709931 |
| Sample | Sub-Sample Analysis | 206Pb/204Pb | ±2σ | 207Pb/204Pb | ±2σ | 208Pb/204Pb | ±2σ |
|---|---|---|---|---|---|---|---|
| A | A-1 | 18.355 | 0.006 | 15.546 | 0.005 | 38.245 | 0.013 |
| A-2 | 18.482 | 0.002 | 15.672 | 0.002 | 38.797 | 0.005 | |
| A-3 | 18.361 | 0.002 | 15.603 | 0.001 | 38.398 | 0.004 | |
| A-4 | 18.484 | 0.003 | 15.646 | 0.002 | 38.493 | 0.006 | |
| A-5 | 18.334 | 0.004 | 15.616 | 0.004 | 38.390 | 0.008 | |
| A-6 | 18.363 | 0.003 | 15.643 | 0.003 | 38.465 | 0.007 | |
| A-7 | 18.451 | 0.003 | 15.607 | 0.003 | 38.418 | 0.007 | |
| B | B-1 | 18.374 | 0.003 | 15.621 | 0.002 | 38.413 | 0.006 |
| B-2 | 18.343 | 0.004 | 15.618 | 0.003 | 38.427 | 0.008 | |
| B-3 | 18.275 | 0.004 | 15.588 | 0.003 | 38.306 | 0.008 | |
| B-4 | 18.972 | 0.004 | 15.712 | 0.003 | 38.711 | 0.007 |
| Sample ID | No. | δ34S (‰) |
|---|---|---|
| Specimen A | A | 31.624 |
| Specimen A | A-rpt * | 31.799 |
| Specimen B | B | 31.310 |
| Specimen B | B-rpt | 31.032 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Wang, X.-C.; Li, C.-F.; Liu, K.; Wilde, S.A.; Hu, S.-Y.; Gui, L.; Liu, J.; Wu, L. First Direct Dating of Alteration of Paleo-Oil Pools Using Rubidium-Strontium Pyrite Geochronology. Minerals 2020, 10, 606. https://doi.org/10.3390/min10070606
Li S, Wang X-C, Li C-F, Liu K, Wilde SA, Hu S-Y, Gui L, Liu J, Wu L. First Direct Dating of Alteration of Paleo-Oil Pools Using Rubidium-Strontium Pyrite Geochronology. Minerals. 2020; 10(7):606. https://doi.org/10.3390/min10070606
Chicago/Turabian StyleLi, Shaojie, Xuan-Ce Wang, Chao-Feng Li, Keyu Liu, Simon A. Wilde, Si-Yu Hu, Lili Gui, Jianliang Liu, and Luya Wu. 2020. "First Direct Dating of Alteration of Paleo-Oil Pools Using Rubidium-Strontium Pyrite Geochronology" Minerals 10, no. 7: 606. https://doi.org/10.3390/min10070606
APA StyleLi, S., Wang, X.-C., Li, C.-F., Liu, K., Wilde, S. A., Hu, S.-Y., Gui, L., Liu, J., & Wu, L. (2020). First Direct Dating of Alteration of Paleo-Oil Pools Using Rubidium-Strontium Pyrite Geochronology. Minerals, 10(7), 606. https://doi.org/10.3390/min10070606






