Elemental and Mineral Composition of the Barents Sea Recent and Late Pleistocene−Holocene Sediments: A Correlation with Environmental Conditions
Abstract
:1. Introduction
2. Geologic Setting and Sedimentation Features
3. Materials and Methods
3.1. Sediment Core’s Location and Processing
3.2. Methods
3.2.1. Lithology
3.2.2. Geochemistry
Elemental Analysis
Chemical Speciation
3.2.3. Radionuclides
3.2.4. Mineralogy
3.3. Data Processing
4. Results
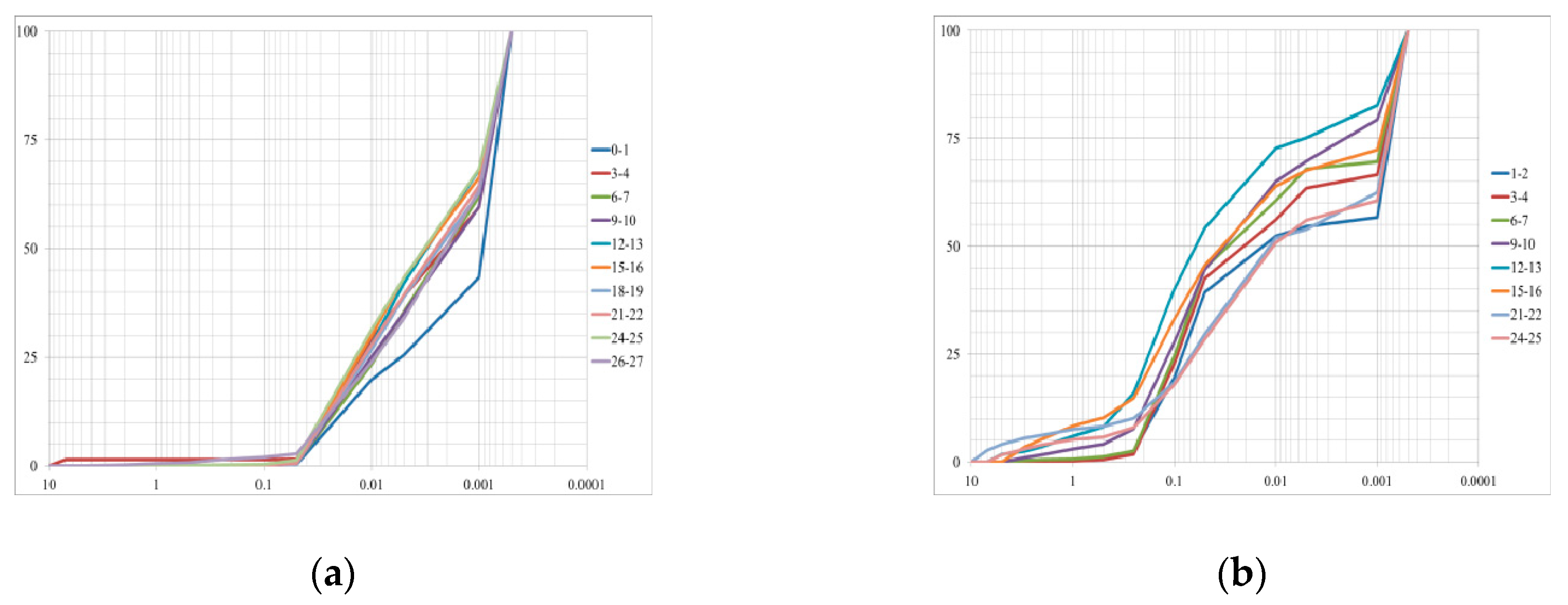
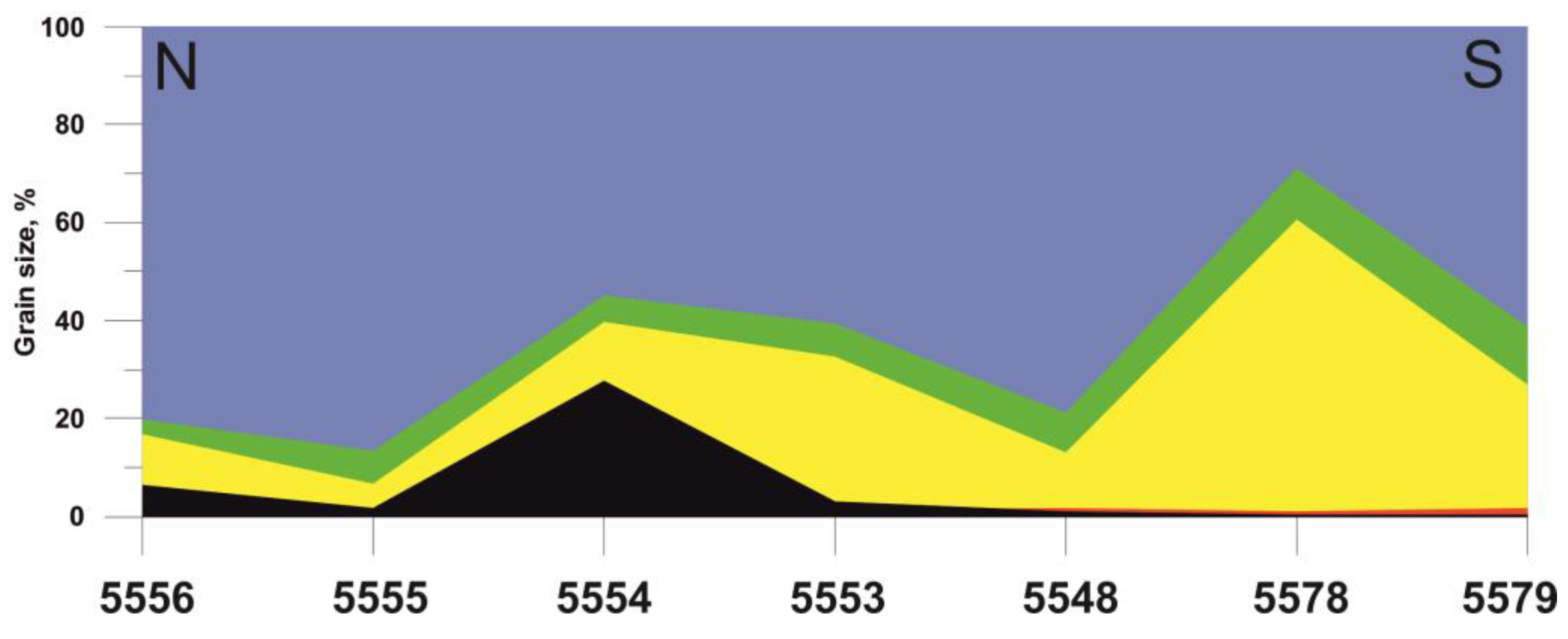
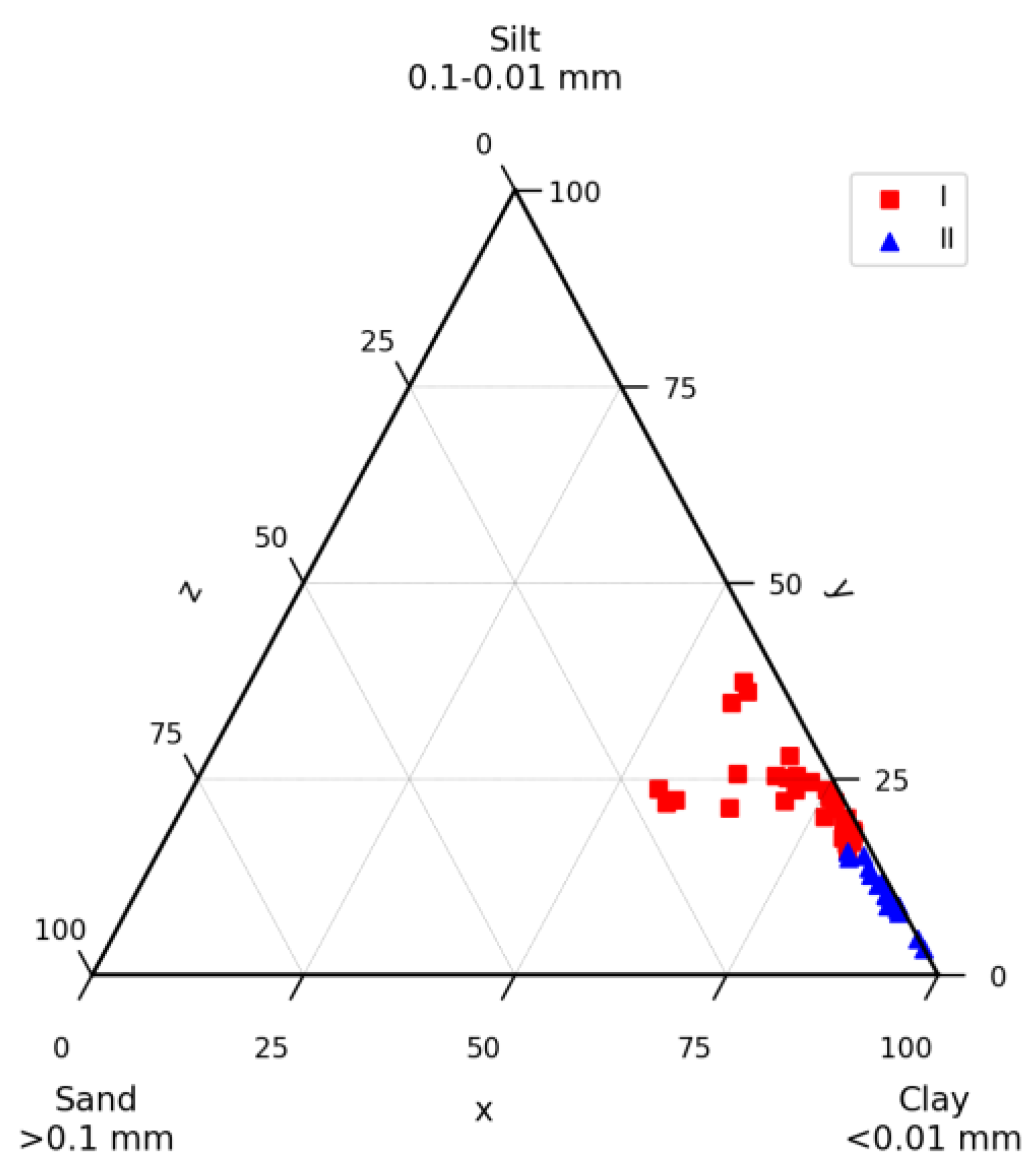
4.1. Lithology and Grain Size Distribution
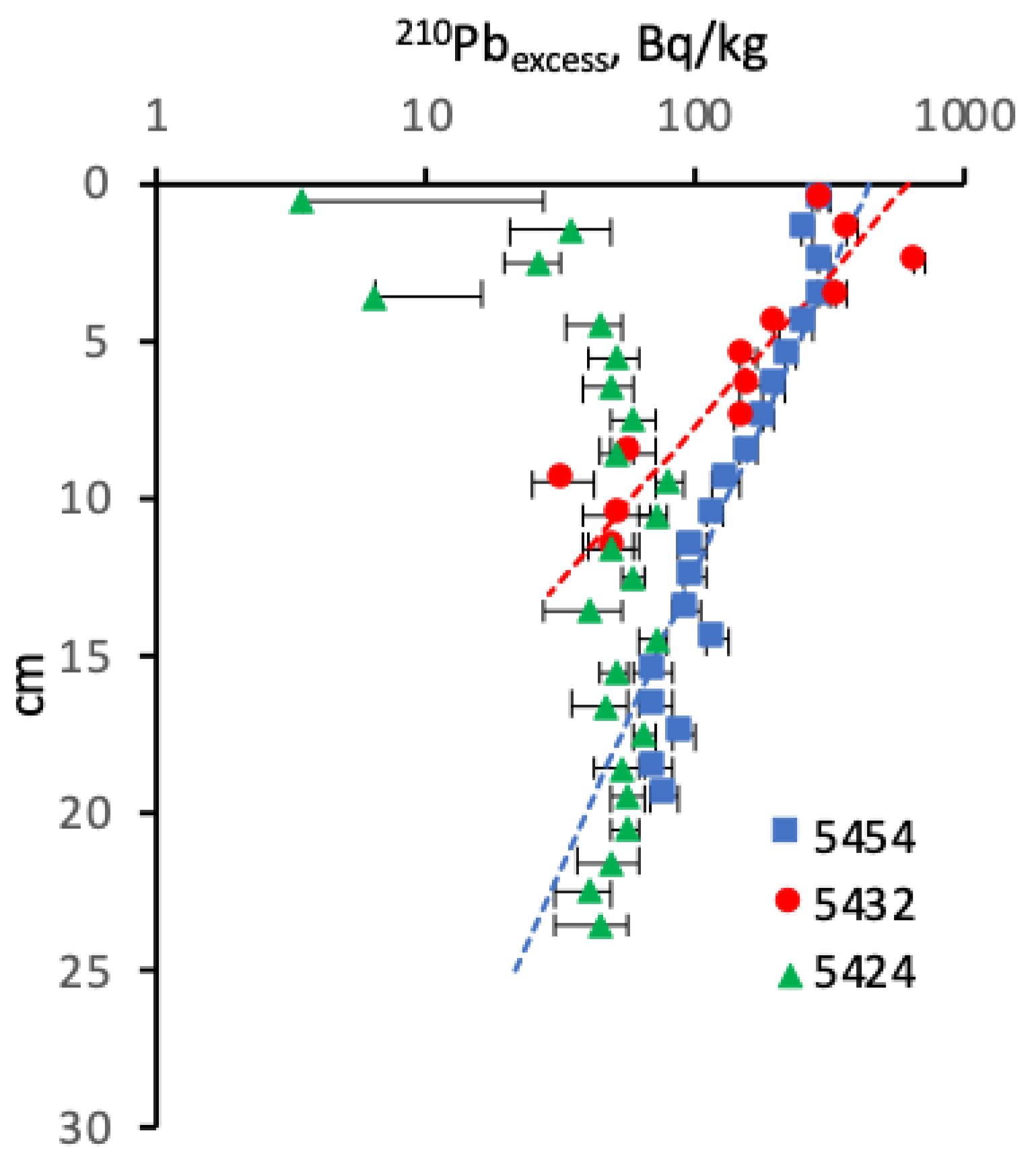
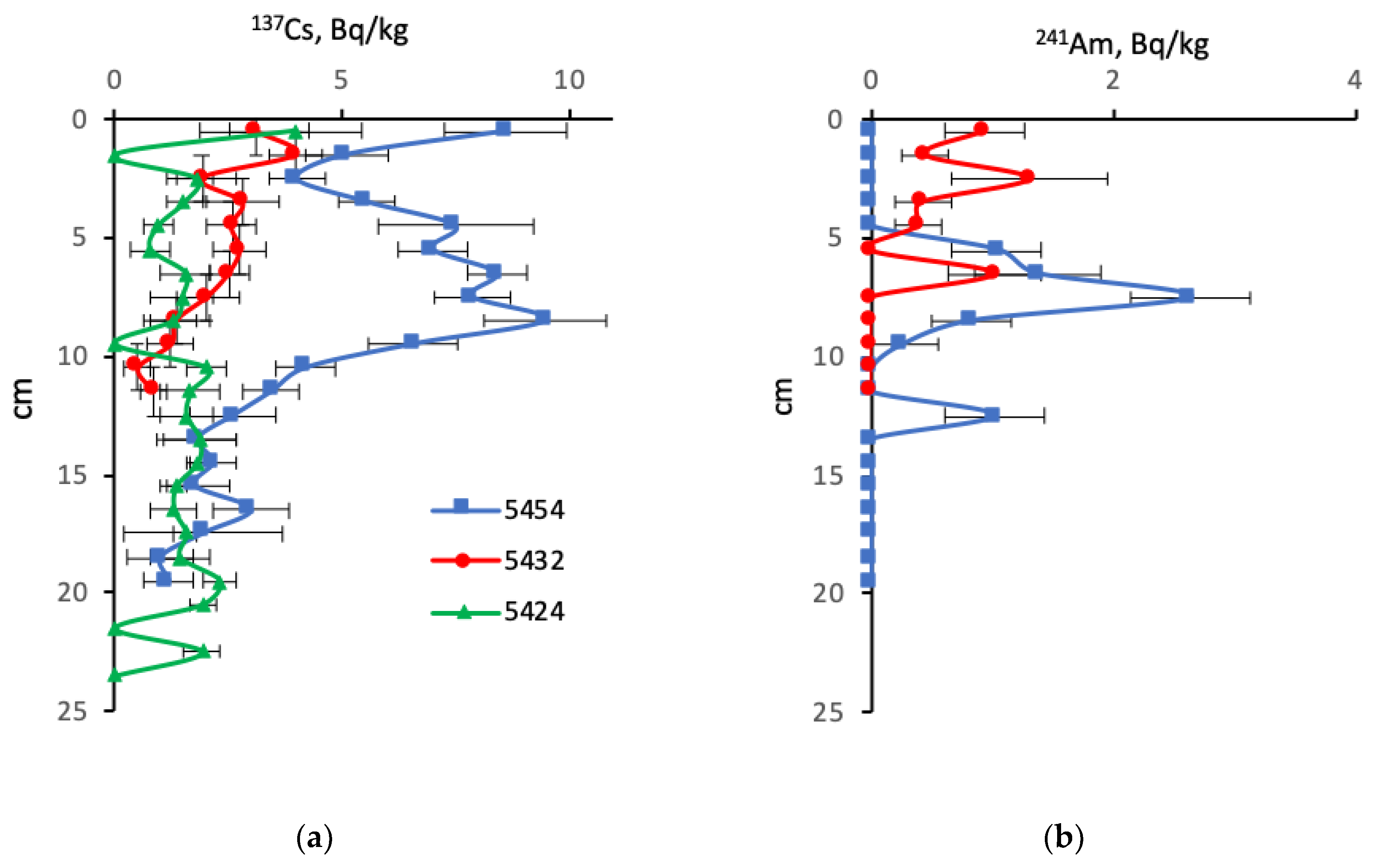
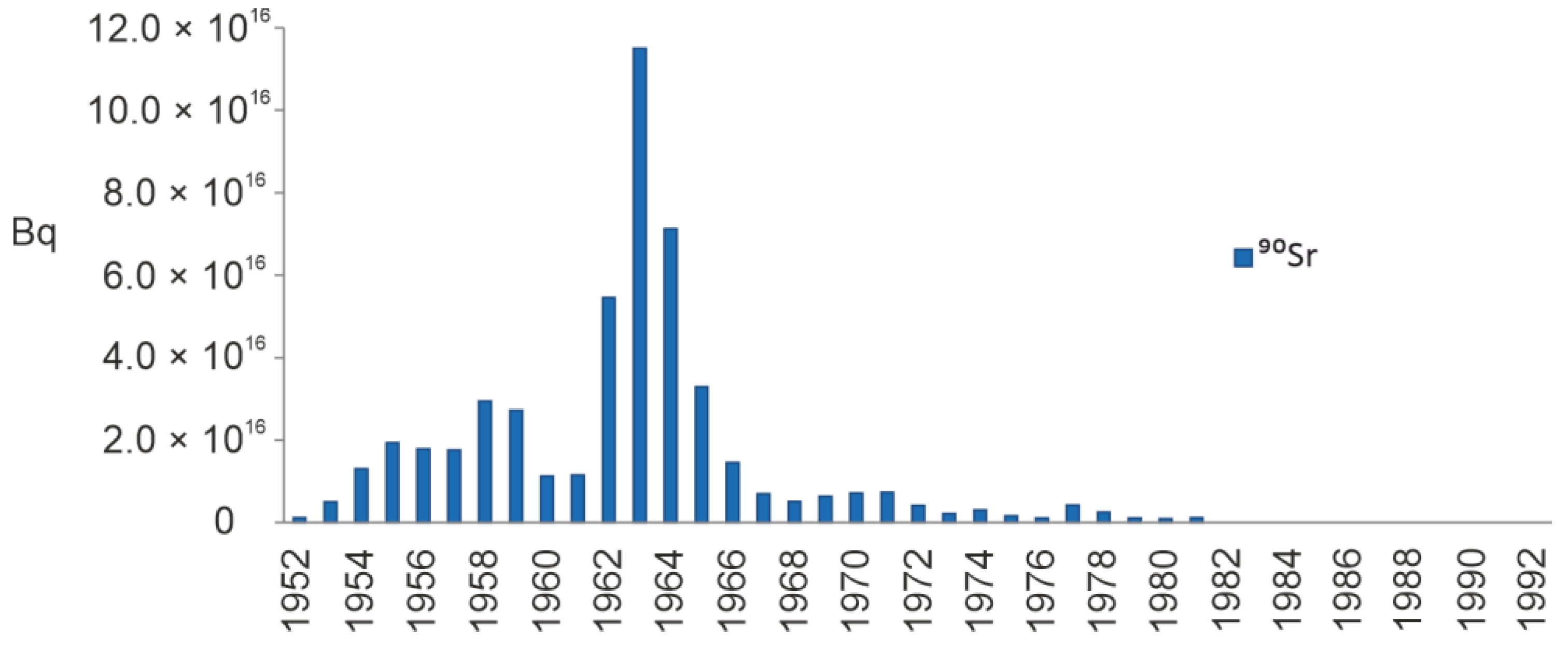
4.2. Radionuclides
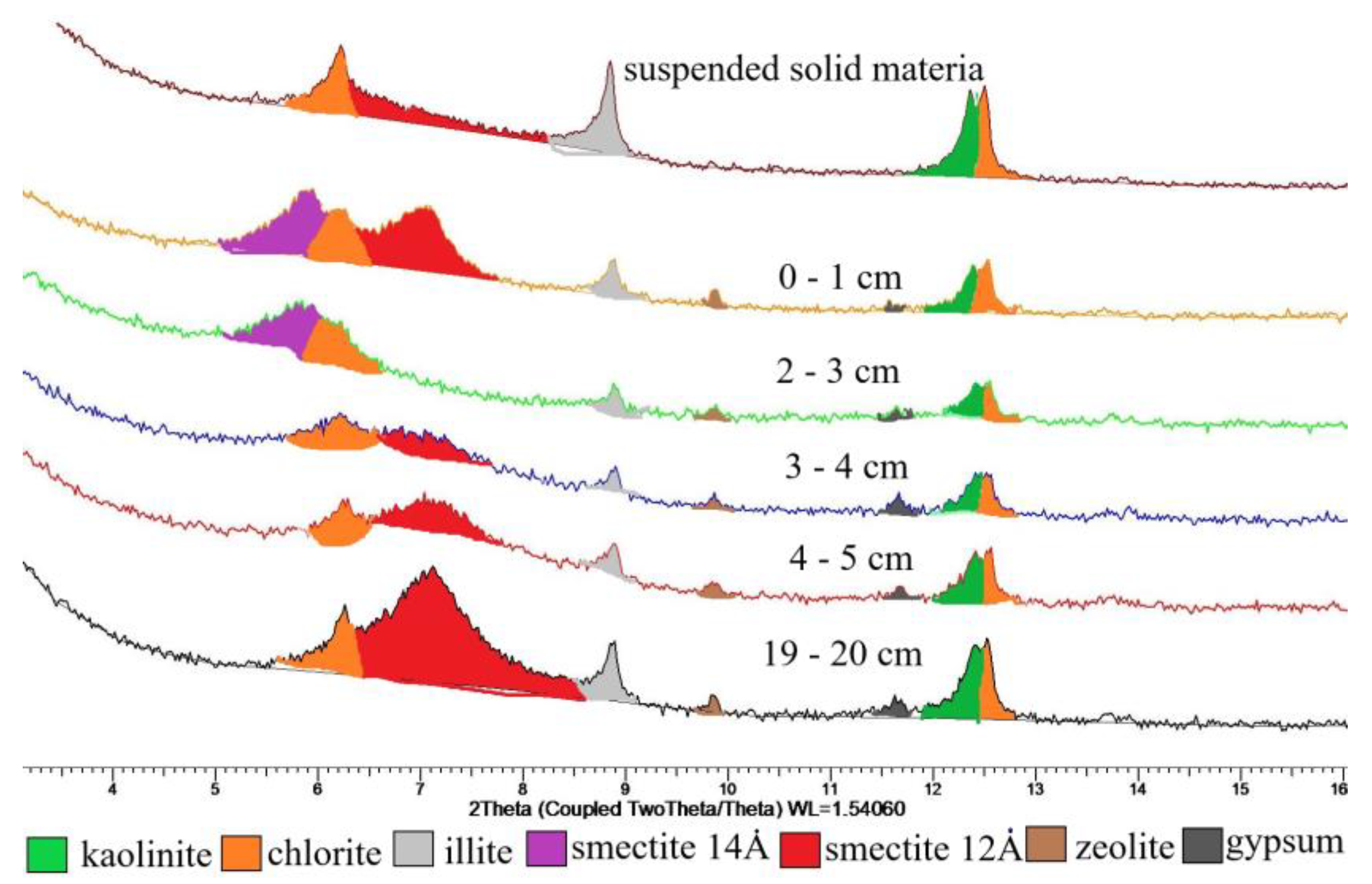
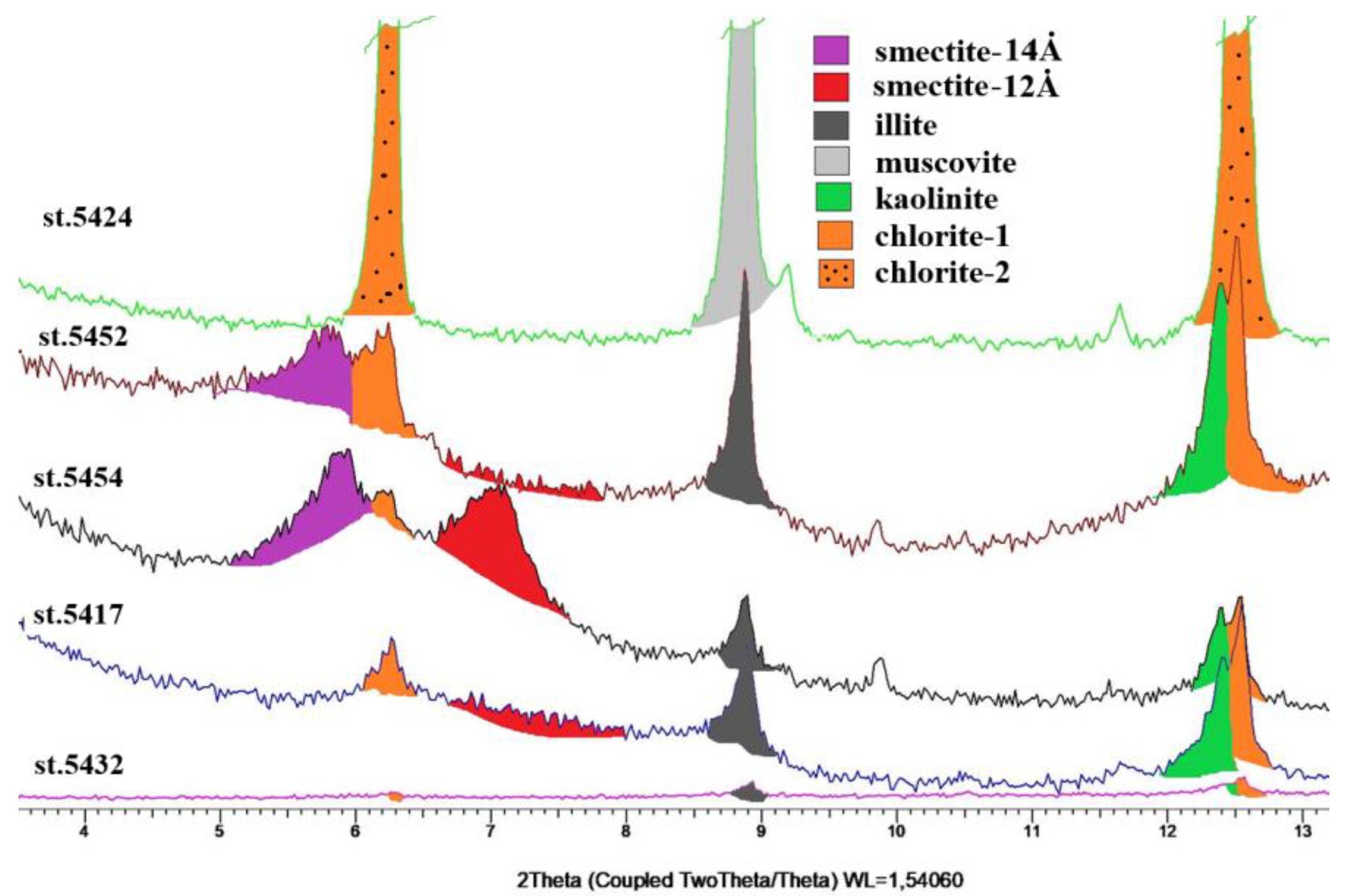
4.3. Mineralogy
4.4. Geochemistry
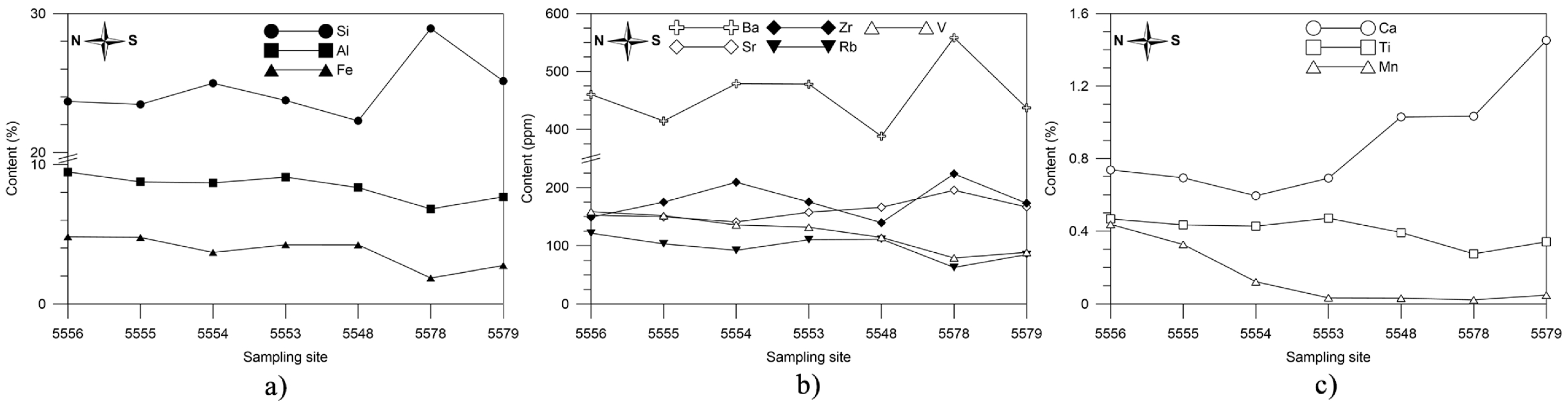
4.4.1. Elemental Composition of the Surface Sediments
4.4.2. Major and Trace Elements’ Distribution in Sediment Cores (Sampled with GC and MUC)
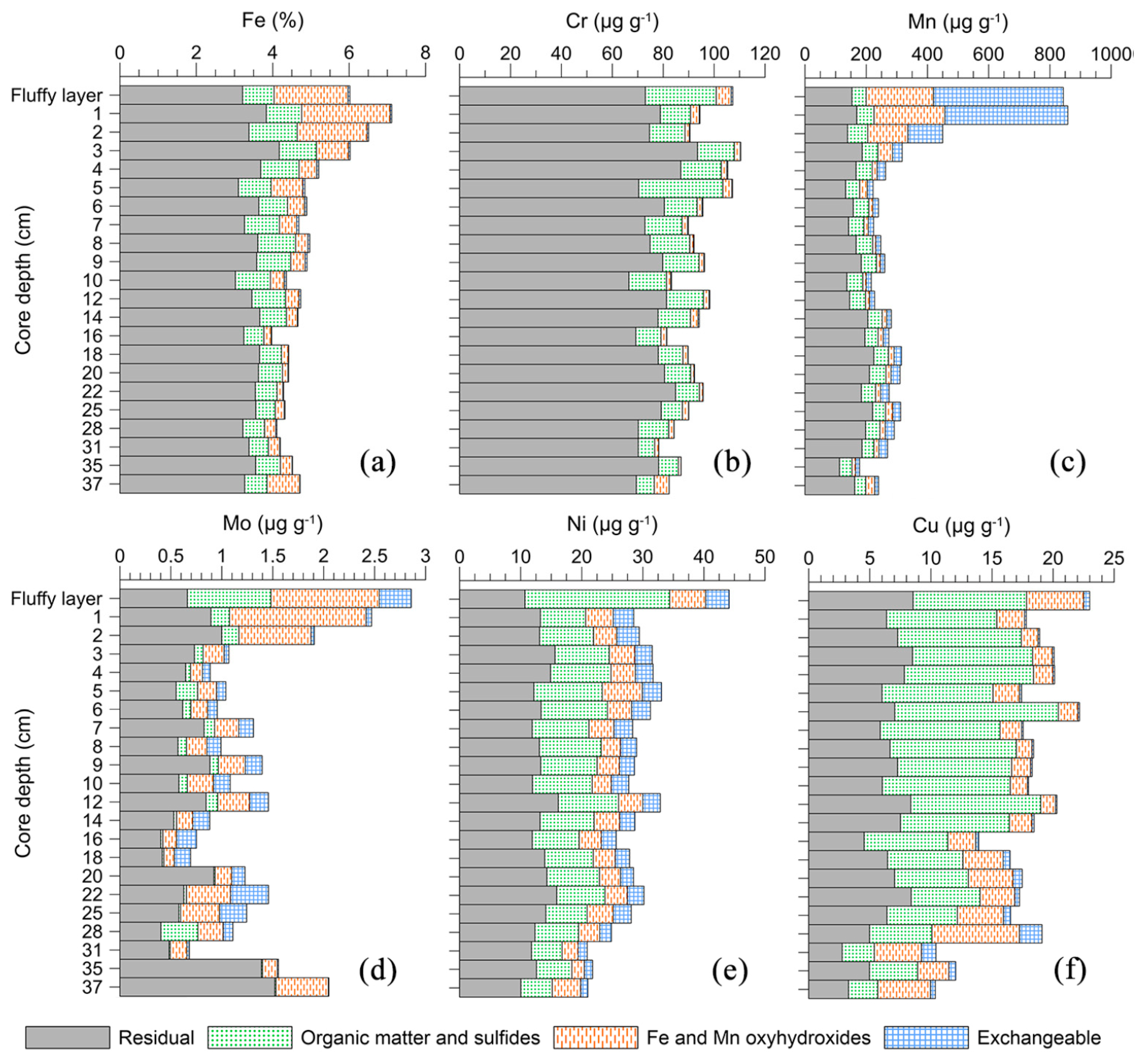
4.4.3. Trace Metal Speciation
5. Discussion
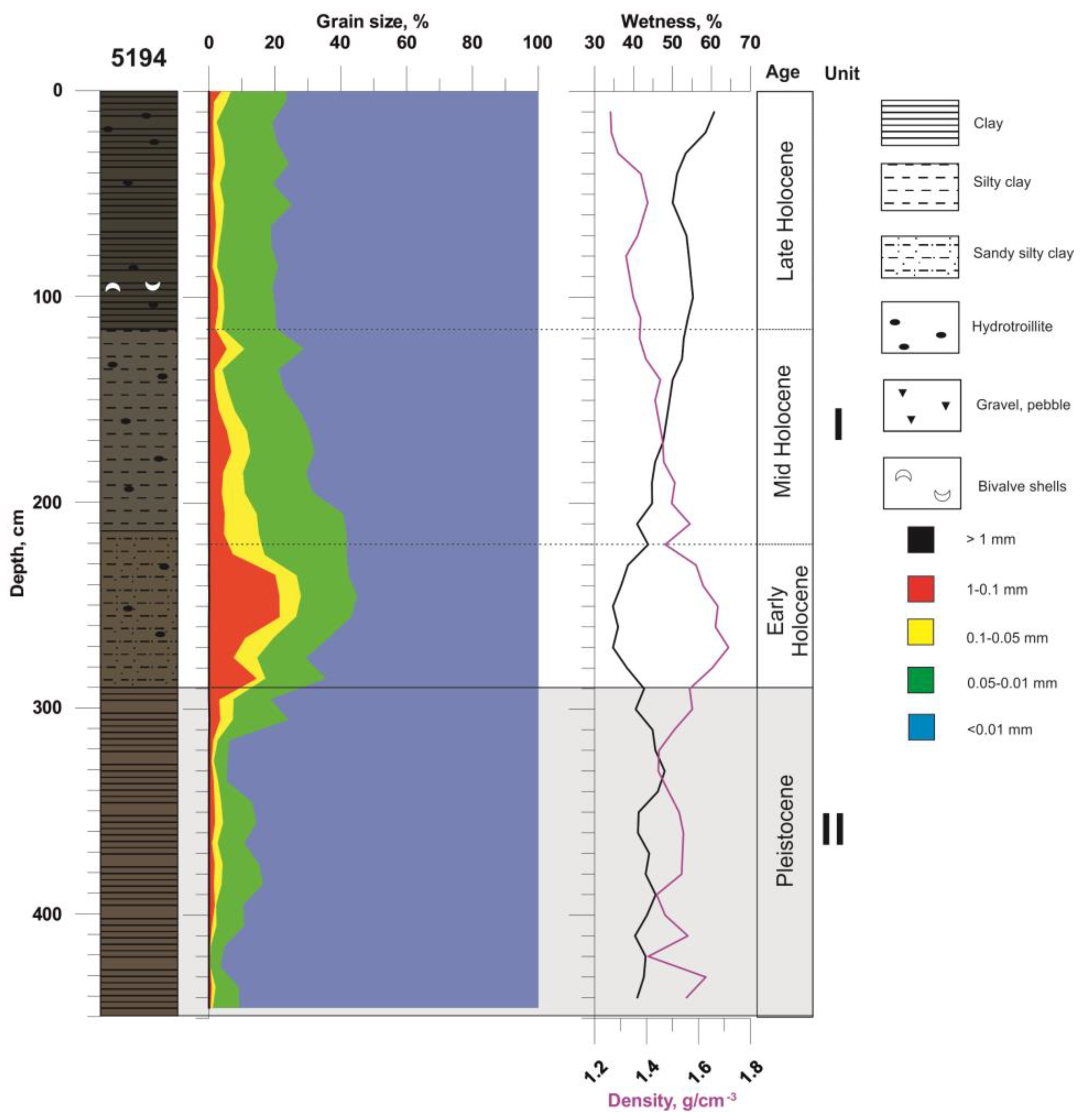
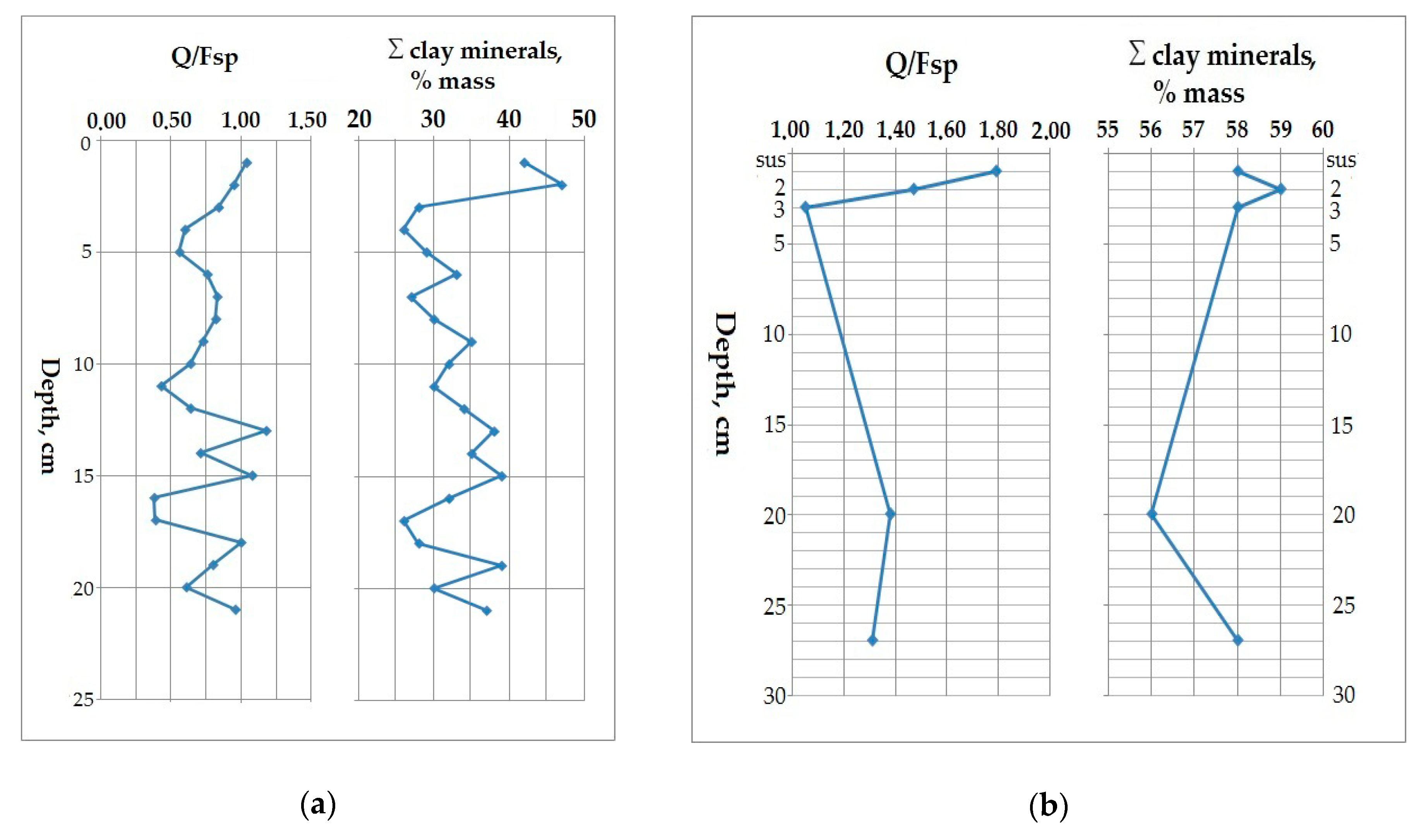
5.1. Lithology of the Sediment Core AMK-5194, the Central Deep of the Barents Sea
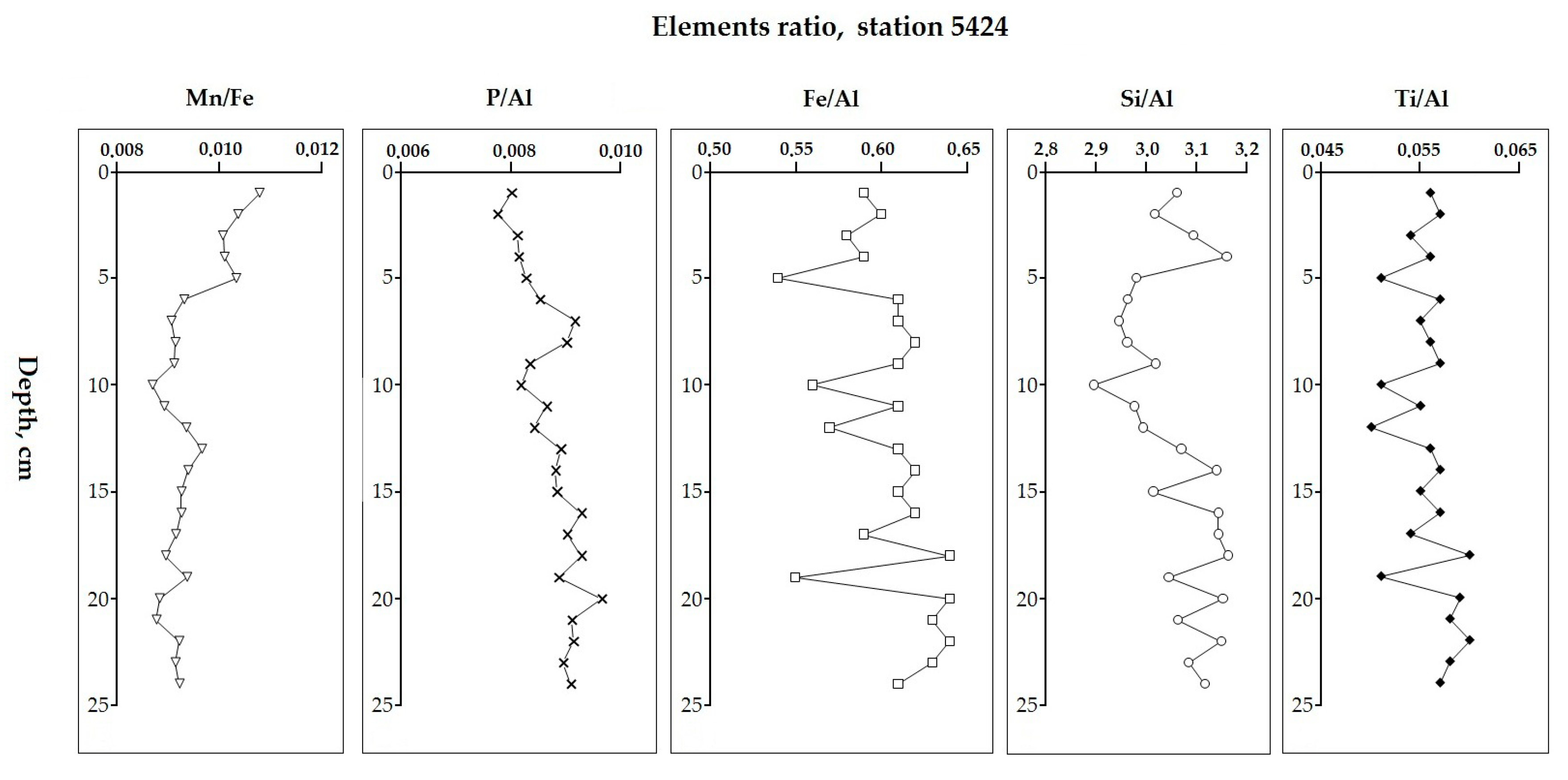
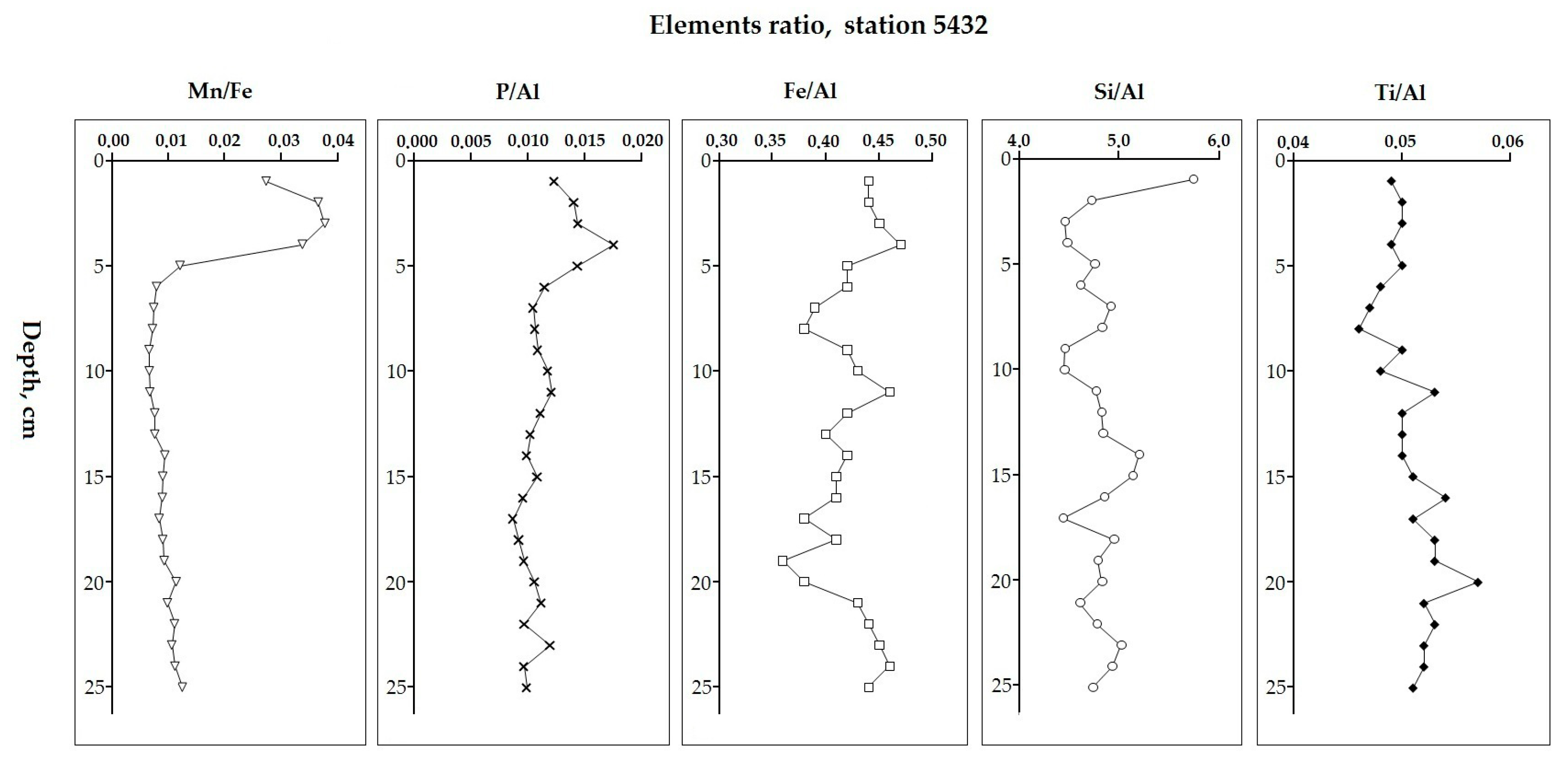
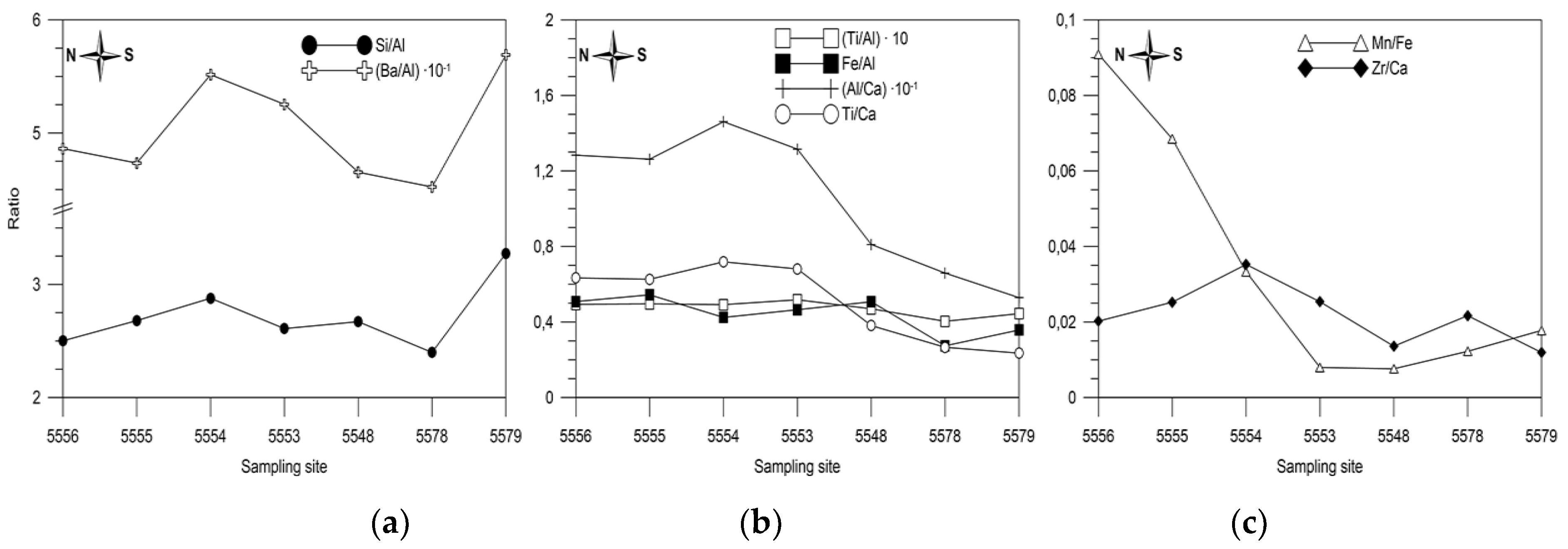
5.2. Variation in Elemental Ratios in the AMK-5194 Core (Central Deep) over the Late Glacial to Holocene Periods: Relation to Sedimentation Environment
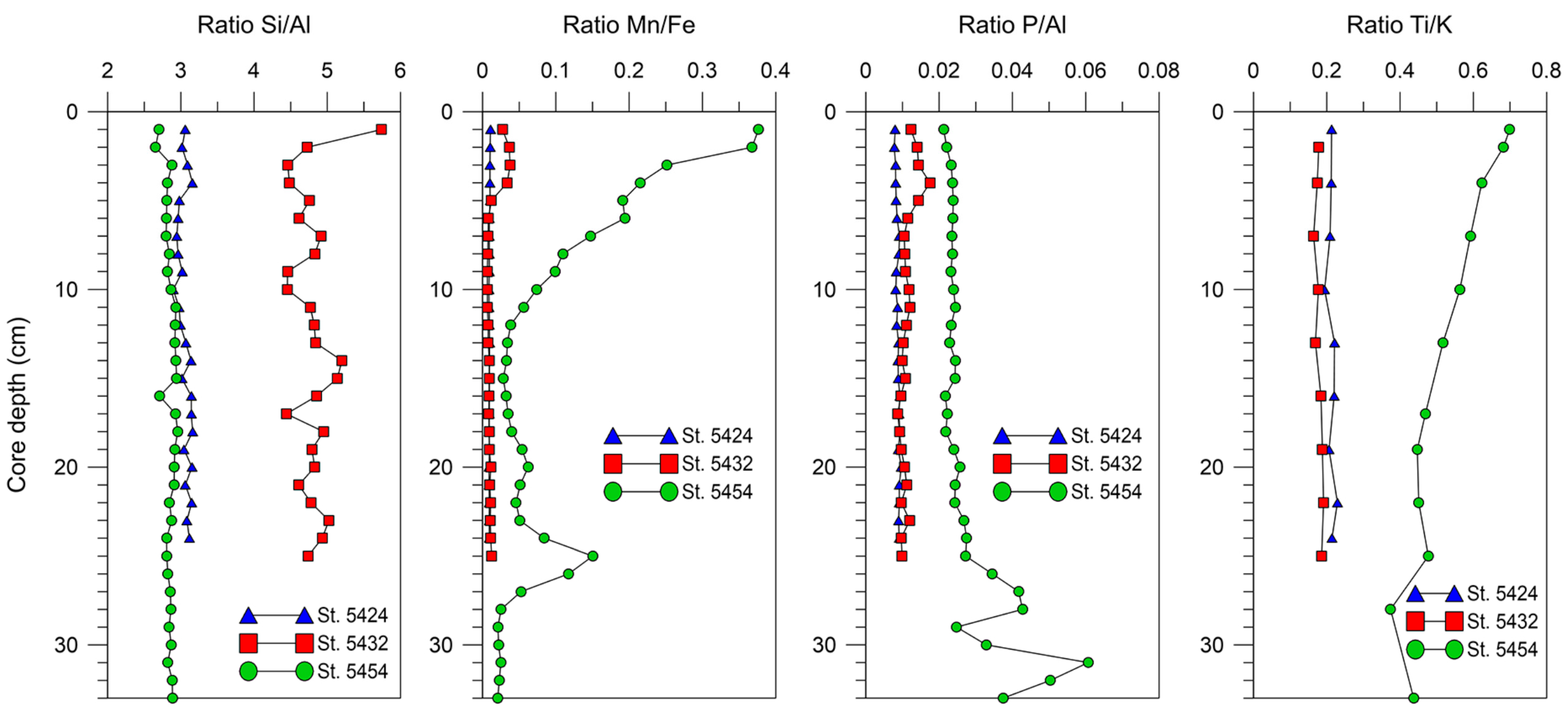
5.3. Elemental Distribution in the Surface Sediments
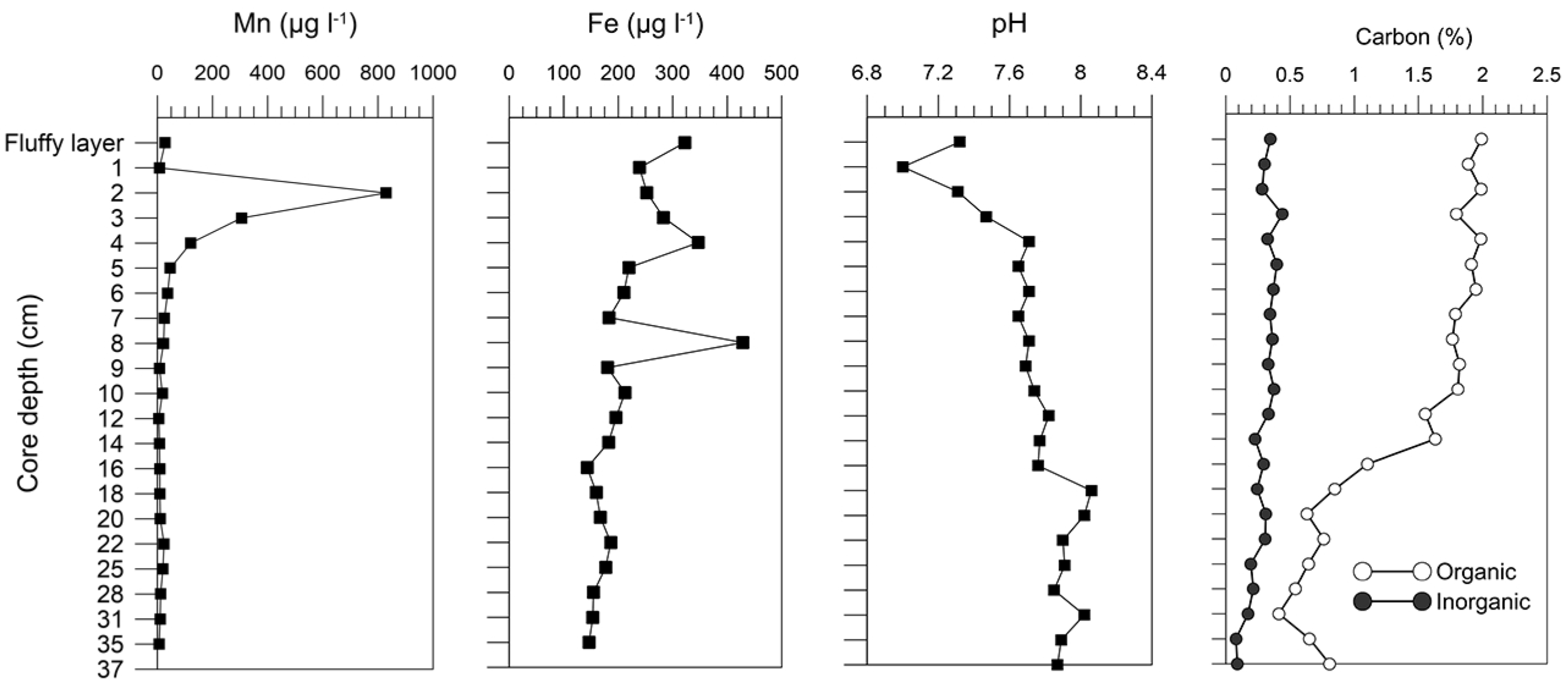
5.4. Radionuclides’ Distribution in the Modern Sediments
5.4.1. Sources of Artificial Radioactivity in the Barents Sea
5.4.2. The 210Pb and Sedimentation Rates in the Barents Sea
5.4.3. The Artificial Radionuclides in the Barents Sea
5.5. Mineral Composition of the Modern Sediments in the Barents Sea
5.6. Comparison of Elemental Ratios’ Distribution in Different Sedimentation Environments
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lubinski, D.J.; Polyak, L.; Forman, S.L. Freshwater and Atlantic water inflows to the deep northern Barents and Kara seas since ca 13 14C ka: Foraminifera and stable isotopes. Quat. Sci. Rev. 2001, 20, 1851–1879. [Google Scholar] [CrossRef]
- Ivanova, E.; Murdmaa, I.; Duplessy, J.-C.; Paterne, M. Late Weichselian to Holocene paleoenvironments in the Barents Sea. Glob. Planet. Chang. 2002, 34, 209–218. [Google Scholar] [CrossRef]
- Murdmaa, I.O.; Ivanova, E.V. Postglacial sedimentation history in shelf depressions of the Barents Sea. Lithol. Miner. Resour. 1999, 6, 576–595. [Google Scholar]
- Murdmaa, I.; Ivanova, E.; Duplessy, J.-C.; Levitan, M.; Khusid, T.; Bourtmann, M.; Alekhina, G.; Alekseeva, T.; Belousov, M.; Serova, V. Facies system of the central and Eastern Barents Sea since the last glaciation to present. Mar. Geol. 2006, 230, 275–303. [Google Scholar] [CrossRef]
- Gurvich, E.G.; Vlasova, I.E.; Gordeev, V.Y.; Isaeva, A.B. Chemical composiyion of bottom sediments of the Barents Sea. In Experience of System Oceanologic Studies in the Arctic; Lisitsyn, A.P., Vinogradov, M.E., Romankevich, E.A., Eds.; Science World: Moscow, Russia, 2001; pp. 616–635. (In Russian) [Google Scholar]
- Chase, Z.; Ellword, M.J.; Van de Flierdt, T. Discovering the Ocean’s Past through Geochemistry. Elements 2018, 14, 397–402. [Google Scholar] [CrossRef]
- Calvert, S.E.; Pedersen, T.F. Elemental proxies for palaeoclimatic and palaeoceanographic variability in marine sediments: Interpretation and application. In Developments in Marine Geology, Proxies in Late Cenozoic Paleoceanography; Hillaire-Marcel, C., De Vernal, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 568–644. [Google Scholar]
- Spofforth, D.J.A.; Pälike, H.; Green, D.R.H. Paleogene record of elemental concentrations in sediments from the Arctic Ocean obtained by XRF analyses. Paleoceanography 2008, 3, PA1S09. [Google Scholar] [CrossRef] [Green Version]
- Polyak, L.; Bischof, J.; Ortiz, J.D.; Darby, D.A.; Channell, J.E.T.; Xuand, C.; Kaufmane, D.S.; Løvlie, R.; Schneider, D.A.; Eberl, D.D.; et al. Late quaternary stratigraphy and sedimentation patterns in the western Arctic Ocean. Glob. Planet. Chang. 2009, 68, 5–17. [Google Scholar] [CrossRef]
- Jonkers, L.; Moros, M.; Prince, M.A.; Dokken, T.; Anderssen Dahl, K.; Dijkstra, N.; Perner, K.; Brummer, G.-J.A. A reconstraction of sea surface warming in the North Atlantic during MIS 3 ice-rafting events. Quat. Sci. Rev. 2012, 29, 1791–1800. [Google Scholar] [CrossRef]
- Krylov, A.A. The Clay minerals as indicators of Late-Quaternary sedimentation conditions in the area of the Mendeleev Ridge. Lithol. Miner. Resour. 2013, 6, 507–518. [Google Scholar]
- Schoster, F.; Behrends, M.; Muller, C.; Stein, R.; Wahsner, M. Modern river discharge and pathways of supplied material in the Eurasian Arctic Ocean: Evidence from mineral assemblages and major and minor element distribution. Int. J. Earth Sci. 2000, 89, 486–495. [Google Scholar] [CrossRef]
- Maslov, A.V.; Kuznetsov, A.B.; Politova, N.V.; Kozina, N.V.; Novigatsky, A.N.; Schevchenko, V.P. The isotope composition of Nd, Pb and Sr in modern bottom sediments of the Barents Sea. Dokl. Earth Sci. 2019, 485, 71–75. [Google Scholar] [CrossRef]
- Gurevich, V.I. The Modern Sedimentation and Geologic Ecology of the Western Arctic Eurazian Shelf; Science World: Moscow, Russia, 2002; p. 135. (In Russian) [Google Scholar]
- Romankevich, E.A.; Danyushevskaya, A.I.; Belyaeva, A.N.; Rusanov, V.P. Biogeochemistry of Organic Matter in the Arctic Seas; Nauka: Moscow, Russia, 1982; p. 256. (In Russian) [Google Scholar]
- Gurevich, V.I. Recent Sedimentogenesis and Environment on the Arctic Shelf of Western Eurasia; Norsk Polar Inst.: Oslo, Norway, 1995; Volume Voulme 13, p. 92. [Google Scholar]
- Nurnberg, D. Biogenic barium and opal in shallow Eurasian shelf sediments in relation to the pelagic Arctic Ocean environment. In Surface Sediment Composition and Sedimentary Processe in Central Arctic Ocean and along the Eurasian Continental Margin; AWI: Bremerhaven, Germany, 1996; pp. 96–118. [Google Scholar]
- Bogdanov, Y.A.; Murdmaa, I.O.; Gurvich, E.G.; Pimenov, N.Y.; Pavlova, G.A.; Karpenko, A.A.; Vlasova, I.E.; Plishkin, A.N. The study of upper part of Barents Sea sedimentary layer for sedimentation history outline and paleoceanographic reconstructions. In Experience of System Oceanologic Studies in the Arctic; Lisitsyn, A.P., Vinogradov, M.E., Romankevich, E.A., Eds.; Science World: Moscow, Russia, 2001; pp. 598–615. (In Russian) [Google Scholar]
- Strekopytov, S.V. The iron and sulfur occurence forms in bottom sediments of the Barents Sea. In Experience of System Oceanologic Studies in the Arctic; Lisitsyn, A.P., Vinogradov, M.E., Romankevich, E.A., Eds.; Science World: Moscow, Russia, 2001; pp. 586–597. [Google Scholar]
- Ivanov, G.I. Geology and Ecology of the Western Arctic Shelf of Russia: Lithological and Geochemical Aspects; Kamensky, A.N.A., Ed.; Nauka: Saint Peterburg, Russia, 2006; p. 303. [Google Scholar]
- Novikov, M.A.; Zhilin, A.Y. Distribution type of heavy metals in bottom sediments of the Barents Sea (based on statistical analysis). Vestnik KRAUNZ. Earth Sci. 2016, 1, 78–88. [Google Scholar]
- Poulton, S.W.; Raiswell, R. The low-temperature geochemical cycle of iron: From continental fluxes to marine sediment deposition. Am. J. Sci. 2002, 302, 774–805. [Google Scholar] [CrossRef]
- Raiswell, R.; Canfield, D.E. The iron biogeochemical cycle: Past and present. Geochem. Perspect. Lett. 2012, 1, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Demina, L.L.; Levitan, M.A.; Politova, N.V. Speciation of some heavy metals in bottom sediments of the Ob and Yenisei estuarine zones. Geochem. Int. 2006, 44, 182–195. [Google Scholar] [CrossRef]
- Demina, L.L.; Bud’ko, D.F.; Alekseeva, T.N.; Novigatsky, A.N.; Filippov, A.S.; Kochenkova, A.I. Partitioning of trace elements in the process of early diagenesis of bottom sediments in the White Sea. Geochem. Int. 2017, 55, 144–149. [Google Scholar] [CrossRef]
- Koukina, S.E.; Vetrov, A.A. Metal forms in sediments from Arctic coastal environments in Kandalaksha Bay, White Sea, under separation processes. Estuar. Coast. Shelf Sci. 2013, 130, 21–29. [Google Scholar] [CrossRef]
- Grotti, M.; Soggia, F.; Ianni, C.; Magi, E.; Udisti, R. Bioavailability of trace elements in surface sediments from Kongsfjorden, Svalbard. Mar. Pollut. Bull. 2013, 77, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Budko, D.F.; Demina, L.L.; Lisitzin, A.P.; Kravchishina, M.D.; Politova, N.V. Occurrence forms of trace metals in recent bottom sediments from the White and Barents Seas. Dokl. Earth Sci. 2017, 474, 552–556. [Google Scholar] [CrossRef]
- Budko, D.F.; Demina, L.L.; Novichkova, E.A.; Polyakova, Y.I.; Kravchishina, M.D.; Melenevsky, V.N. Postglacial sedimentation in the White Sea (Northwestern Russia) reconstructed by integrated microfossils’ and geochemical data. Quat. Res. 2020, 93, 110–123. [Google Scholar] [CrossRef]
- Vasiliev, V.V.; Viskunova, K.G.; Kiiko, O.A.; Kozlov, S.A.; Kostin, D.A.; Lopatin, B.G.; Markina, N.V.; Orgo, V.V.; Pavlov, S.P.; Penedyuk, E.V.; et al. State geological map of the Russian Federationtion. Scale 1:1,000,000, 3rd ed.; Northern-Kara-Barents Seas. L T-41–44—Cape Zhelaniya; Kartfabrika VSEGEI: Saint Petersburg, Russia, 2013; p. 200. (In Russian) [Google Scholar]
- Vorren, T.O.; Laberg, J.S. Late glacial air temperature, oceanographic and ice sheet interactions in the southern Barents Sea region. In Late Quaternary Paleoceanography of the North Atlantic Margins; Andrews, J.T., Austin, W.E., Bergsten, W.E.N., Jennings, A.E., Eds.; Geological Society Special Publications: London, UK, 1996; Volume 111, pp. 303–321. [Google Scholar]
- Thiede, J.; Mangerud, J. New Map Revises Extent of Last Ice Sheet Over Barents and Kara Seas. EOS Trans. 1999, 80, 493–494. [Google Scholar] [CrossRef]
- Polyak, L.; Gataullin, V.; Okuneva, O.; Stelle, V. New constrains on the limits of the Barents-Kara ice sheet during the Last Glacial Maximum based on borehole stratigraphy from the Pechora Sea. Geology 2000, 28, 611–614. [Google Scholar] [CrossRef] [Green Version]
- Svendsen, J.I.; Gataullin, V.; Mangerud, J.; Poljak, L. The glacial history of the Barents and Kara Sea region. In Quaternary Glaciations—Extent and Chronology; Ehlers, J., Gibbard, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; Volume 1, pp. 369–378. [Google Scholar]
- Barash, M.S. Quaternary Paleooceanology of the Atlantic Ocean; Nauka: Moscow, Russia, 1988; p. 254. (In Russian) [Google Scholar]
- Ivanova, E.V.; Duplessy, J.C.; Paterne, M.; Murdmaa, I.O.; Levitan, M.A.; Vetrov, A.A.; Romankevich, E.A.; Khusid, T.A. The Influence of Climate and Thermohaline Circulatiopn on the Productivity of the Eastern Barents Sea during the Holocene; ICP VII Program and Abstracts: Sapporo, Japan, 2001; p. 125. [Google Scholar]
- Murdmaa, I.O.; Polyak, L.; Ivanova, E.V.; Khromova, N.V. Paleoenvironments in the Russkaya Gavan’ Fjord (NW Novaya Zemlya) during the Last Millenium. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004, 209, 141–154. [Google Scholar] [CrossRef]
- Hald, M.; Kolstad, V.; Polyak, L.; Forman, S.L.; Herlihy, F.A.; Ivanov, G.; Nescheretov, A. Late glacial and Holocene paleoceanography and sedimentary environments in the Saint Anna Trough, Eurasian Arctic Ocean margin. Palaeogeogr. Palaeoclim. Palaeoecol. 1999, 146, 229–249. [Google Scholar] [CrossRef]
- Polyak, L.; Mikhailov, V. Post-glacial environments of the southeastern Barents Sea: Foraminiferal evidence. In Late Quaternary Paleoceanography of the North Atlantic Margins; Andrews, J.T., Austin, W.E.N., Bergsten, H., Jennings, A.E., Eds.; Geological Society Special Publications: London, UK, 1996; Volume 111, pp. 323–337. [Google Scholar]
- Elverhoi, A.; Pfirman, S.L.; Solheim, A.; Larsen, B.B. Glaciomarine sedimentation in epicontinental seas exemplified by the Northern Barents Sea. Mar. Geol. 1989, 85, 225–250. [Google Scholar] [CrossRef]
- Aybulatov, N.M.; Matyushenko, V.A.; Shevchenko, V.P.; Politova, N.V. New data on structure of lateral suspended matter fluxes in the periphery of the Barents Sea. Geoecology 1999, 6, 526–540. [Google Scholar]
- Lisitzin, A.P. Sea-Ice and Iceberg Sedimentation in the World Ocean: Present and Past; Springer Verlag: Berlin, Germany, 2002; p. 563. [Google Scholar]
- AMAP Assessment 2007: Oil and Gas Activities in the Arctic—Effects and Potential Effects. Arctic Monitoring and Assessment Programme. V.1. Published by: AMAP. 2010. Available online: http://www.amap.no/documents/download/1015 (accessed on 1 June 2010).
- Pavlidis, Y.A.; Polyakova, E.I. Late Pleistocene and Holocene depositional environments and paleoceanography of the Barents Sea: Evidence from seismic and biostratigraphic data. Mar. Geol. 1997, 143, 189–205. [Google Scholar] [CrossRef]
- Shevchenko, V.P. The influence of aerosols on the oceanic sedimentation and environmental conditions in the Arctic. Ber. Polar-und Meeresforsch (Rep. Polar Mar. Res.) 2003, 464, 153. [Google Scholar]
- Djenynk, S.L. Non-periodical and summarized currents. In Hydrometeorology and hydrochemistry of USSR. 1.Barents Sea; Issue 1. Hydrologic Meteorologic Conditions; Gidrometizdat: Leningrad, Russia, 1990; pp. 33–40. (In Russian) [Google Scholar]
- Byshev, V.I.; Galerkin, L.I.; Gorotov, A.S. Thermohaline structure of the Barents Sea waters in September 1998. In Experience of System Oceanologic Studies in the Arctic; Lisitzin, A.P., Vinogradov, M.E., Romankevich, E.A., Eds.; Science World: Moscow, Russia, 2001; pp. 102–109. (In Russian) [Google Scholar]
- Zubakin, G.K. Sea-ice conditions and location of the ice margin. In Hydrometeorology and Hydrochemistry of USSR: 1.Barents Sea; Issue 1. Hydrometeorological Conditions; Gidrometizdat: Leningrad, Russia, 1990; pp. 250–254. (In Russian) [Google Scholar]
- Wassmann, P.; Slagstad, D. Annual dynamics of carbon flux in the Barents Sea: Preliminary results. Norsk Geol. Tidskr. 1991, 71, 231–234. [Google Scholar]
- Romankevich, E.A.; Vetrov, A.A. The Carbon Cycle in Russian the Arctic Seas; Nauka: Moscow, Russia, 2001; p. 302. [Google Scholar]
- Lisitzin, A.P.; Demina, L.L. Introduction. In Sedimentation Processes in the White Sea: The White Sea Environment Part II; Lisitsyn, A.P., Demina, L.L., Eds.; Handbook of Environmental Chemistry; Springer-Nature: Berlin, Germany, 2018; Volume 82, pp. 1–12. [Google Scholar] [CrossRef]
- Politova, N.V.; Novigatsky, A.N.; Kozina, N.V.; Terpugova, S.A. Multidisciplinary research in the Barents Sea on cruise 67 of the R/V Akademik Mstislav Keldysh. Oceanology 2018, 58, 499–501. [Google Scholar] [CrossRef]
- Kravchishina, M.D.; Novigatskii, A.N.; Savvichev, A.S.; Pautova, L.A.; Lisitsyn, A.P. Studies on sedimentary systems in the Barents Sea and Norwegian–Greenland basin during cruise 68 of the R/V Akademik Mstislav Keldysh. Oceanology 2019, 59, 158–160. [Google Scholar] [CrossRef]
- Petelin, V.P. Granulometrical Analysis of Marine Sediments; Nauka: Moscow, Russia, 1967; p. 218. (In Russian) [Google Scholar]
- Bezrukov, P.L.; Lisitzyn, A.P. Classification of bottom sediments in modern basins. Proceed. Inst. Oceanol. USSR Acad. Sci. 1960, 32, 179. [Google Scholar]
- Ure, A.M.; Quevauviller, P.; Muntau, H.; Griepink, B. Speciation of heavy metals in soils and sediments. An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the Commission of the European Community. Int. J. Environ. Anal. Chem. 1993, 51, 135–151. [Google Scholar] [CrossRef]
- Luoma, S.N.; Bryan, G.W. A statistical assessment of the forms of trace metals in oxidized estuarine sediments employing chemical extractants. Sci. Total Environ. 1981, 17, 165–196. [Google Scholar] [CrossRef]
- Chester, R.; Hughes, M.J. A chemical technique for separation of ferromanganese minerals and adsorbed trace metals from pelagic sediments. Chem. Geol. 1967, 3, 249–262. [Google Scholar] [CrossRef]
- Kitano, Y.; Fujiyoshi, R. Selective chemical leaching of Cd, Cu, Mn and Fe in marine sediments. Geochem. J. 1980, 14, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Quevauviller, P.; Ure, A.; Muntau, H.; Griepink, B. Improvement of analytical measurements within the BCR programme: Single and sequential extraction procedures applied to soil and sediment analysis. Int. J. Environ. Anal. Chem. 1993, 51, 129–134. [Google Scholar] [CrossRef]
- Pueyo, M.; Rauret, G.; Luck, D. Certification of the extractable contents of Cd, Cr, Cu, Ni, Pb and Zn in a freshwater sediment following a collaboratively tested and optimized three-step sequential extraction procedure. J. Environ. Monit. 2001, 3, 243–250. [Google Scholar] [CrossRef]
- Sutherland, R.A.; Tack, F.M.G. Fractionation of Cu, Pb and Zn in certified reference soils SRM 2710 and SRM 2711 using the optimized BCR sequential. Adv. Environ. Res. 2003, 8, 37–50. [Google Scholar] [CrossRef]
- Moore, D.M.; Reynolds, R.C. X-ray Diffraction and the Identification and Analysis of Clay Minerals, 2nd ed.; Oxford university Press: Oxford, UK; New York, NY, USA, 1997; p. 378. [Google Scholar]
- Frank-Kamenetsky, V.A. (Ed.) X-ray Analysis of the Main Types of Rock-Forming Minerals; Nedra: Leningrad, Russia, 1983; p. 360. (In Russian) [Google Scholar]
- Krishnaswamy, S.; Lal, D.; Martin, J.M.; Meybeck, M. Geochronology of lake sediments. Earth Planet. Sci. Lett. 1971, 11, 407–414. [Google Scholar] [CrossRef]
- Robbins, J.A.; Edgington, D.N. Determination of recent sedimentation rates in Lake Michigan using Pb-210 and Cs-137. Geochim. Cosmochim. Acta 1975, 39, 285–304. [Google Scholar] [CrossRef] [Green Version]
- Wedepohl, H. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1239. [Google Scholar] [CrossRef]
- Moros, M.; Kuijpers, A.; Snowball, I.; Lassen, S.; Backstrom, D.; Gingele, P.; McManus, J. Were glacial iceberg surges in the North Atlantic triggered by climatic warming? Mar. Geol. 2002, 192, 393–417. [Google Scholar] [CrossRef]
- Erbs-Hansen, D.R.; Knudsen, K.L.; Olsen, J.; Lykke-Andersen, H.; Underbjerg, J.A.; Sha, L. Paleoceanographycal development of Sisimiut, West Greenland, during the mid- and late Holocene: A multiproxy study. Mar. Micropaleontol. 2013, 102, 79–97. [Google Scholar] [CrossRef]
- Romankevich, E.A.; Vetrov, A.A.; Vinogradov, M.E.; Vedernikov, V.I. Some Elements of the Carbon Cycle in the Russian Arctic Seas. Fluxes of Carbon from Land, Carbon in Bottom Sediments, Elements of Budget. Oceanology 2000, 40, 363–372. [Google Scholar]
- Sayago-Gil, M.; López-González, N.; Long, D.; Fernández-Salas, L.M.; Durán-Muñoz, P. Multi-proxy aproach for identifying Heinrich Events in sediment cores from Hatton Bank (NE Atlantic Ocean). Geosciences 2020, 10, 14. [Google Scholar] [CrossRef] [Green Version]
- Migdisov, A.A. The ratio of titanium and aluminum in sedimentary rocks. Geochem. Int. 1960, 2, 149–163. [Google Scholar]
- Demina, L.; Novichkova, E.; Lisitzin, A.; Kozina, N. Geochemical signatures of paleoclimate changes in the sediment cores from the Gloria and Snorri Drifts (Northwest Atlantic) over the Holocene-Mid Pleistocene. Geosciences 2019, 9, 432. [Google Scholar] [CrossRef] [Green Version]
- Jakobsson, M.; Løvlie, R.; Al-Hanbali, H.; Arnold, E.; Backman, J.; Mörth, M. Manganese and color cycles in Arctic Ocean sediments constrain Pleistocene chronology. Geology 2000, 28, 23–26. [Google Scholar] [CrossRef]
- Chester, R. Marine Geochemistry; Blackwell: London, UK, 2000; p. 506. [Google Scholar]
- Tribovillard, N.; Algeo, T.J.; Lyons, T.; Ribouleau, A. Trace metals as paleoredox and paleoproductivity proxies: An update. Chem. Geol. 2006, 232, 12–32. [Google Scholar] [CrossRef]
- Naeher, S.; Gilli, A.; North, R.P.; Hamann, Y.; Schubert, C.J. Tracing bottom water oxygenation with sedimentary Mn/Fe ratios in Lake Zurich, Switzerland. Chem. Geol. 2013, 352, 125–133. [Google Scholar] [CrossRef]
- Zaborska, A.; Carroll, J.; Papucci, C.; Torricelli, L.; Carroll, M.L.; Walkusz-Miotk, J.; Pempkowiak, J. Recent sediment accumulation rates for the Western margin of the Barents Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2008, 55, 2352–2360. [Google Scholar] [CrossRef]
- Lisitzin, A.P. Sedimentation in the World Ocean. International Society of Ecology, Paleoceanology and Mineralogy. Spec. Publ. 1972, 17, 218. [Google Scholar]
- Lisitsyn, A.P. The up-to-date insights to sediment formation in marine basins: An oacean as a natural recorder of the Earth geospheres’ interaction. In The World Ocean; v. 2; Nigmatulin, R.I., Lobkovskii, L.I., Eds.; Science World: Moscow, Russia, 2015; pp. 331–553. (In Russian) [Google Scholar]
- Bakke, T.; Breedveld, G.; Kællgvist, T. Veileder for Klassifisering av Miljøkvalitæt I Fjorder og Kystfarvann – Revisjon av Lassifisering av Metaller og Organiske Miljøgifter I van nog Sedimenter; SFT Veiledming: Oslo, Norway, 2007; p. 12. (In Norwegian) [Google Scholar]
- Maslov, A.V.; Kozina, N.V.; Politova, N.V.; Shevchenko, V.P. About analysis of distribution of a number of trace and rare elements in modern bottom sediments of the Barents sea (based on data of 67 cruise R/V “Akademik Mstislav Keldysh”. Annual Report-2017. Proc. Inst. Geol. Ural Branch RAS 2018, 165, 53–59. [Google Scholar]
- Imai, N.; Ando, A.; Takeda, E. Minor elements in Japanese coal (II). Bull. Geol. Surv. Jpn. 1984, 35, 287–314. [Google Scholar]
- Yudovich, Y.E.; Ketris, M.P. Arsenic in coal: A review. Int. J. Coal Geol. 2005, 61, 141–196. [Google Scholar] [CrossRef]
- Ohta, A.; Imai, N.; Terashima, S.; Tachibana, Y.; Ikehara, K.; Katayama, H.; Noda, A. Factors controlling regional spatial distribution of 53 elements in coastal sea sediments in northern Japan: Comparison of geochemical data derived from stream and marine sediments. Appl. Geochem. 2010, 25, 357–376. [Google Scholar] [CrossRef]
- Bergan, T.D. Radioactive fallout in Norway from atmospheric nuclear weapons tests. J. Environ. Radioact. 2002, 60, 189–208. [Google Scholar] [CrossRef]
- Unscear 2000 Report, Vol. I: Sources and Effects of Ionizing Radiation. United Nations Scientific Committee on the Effects of Atomic Radiation UNSCEAR 2000 Report to the General Assembly, with Scientific Annexes United Nations, NY. 2000. Available online: http://www.unscear.org/unscear/en/publications/2000_1.html (accessed on 1 May 2016).
- Gray, J.; Jones, S.R.; Smith, A.D. Discharges to the environment from the Sellafield Site, 1951–1992. J. Radiol. Prot. 1995, 15, 99–131. [Google Scholar] [CrossRef]
- Kershaw, P.; Baxter, A. The transfer of reprocessing wastes from north-west Europe to the Arctic. Deep Sea Res. Part II Top. Stud. Oceanogr. 1995, 42, 1413–1448. [Google Scholar] [CrossRef]
- Brown, J.E.; Iospje, M.; Kolstad, K.E.; Lind, B.; Rudjord, A.L.; Strand, P. Temporal trends for 99Tc in Norwegian coastal environments and spatial distribution in the Barents Sea. J. Environ. Radioact. 2002, 60, 49–60. [Google Scholar] [CrossRef]
- Orlandini, K.A.; Penrose, W.R.; Nelson, D.M. Pu(V) as the stable form of oxidized plutonium in natural waters. Mar. Chem. 1986, 18, 49–57. [Google Scholar] [CrossRef]
- Sanchez, A.L.; Gastaud, J.; Noshkin, V.; Buesseler, K.O. Plutonium oxidation states in the southwestern Black Sea: Evidence regarding the origin of the cold intermediate layer. Deep Sea Res. Part A. Oceanogr. Res. Pap. 1991, 38, S845–S853. [Google Scholar] [CrossRef]
- Zaborska, A.; Mietelski, J.W.; Carroll, J.; Papucci, C.; Pempkowiak, J. Sources and distributions of 137Cs, 238Pu, 239,240Pu radionuclides in the north-western Barents Sea. J. Environ. Radioact. 2010, 101, 323–331. [Google Scholar] [CrossRef]
- Aarkrog, A. Radioactivity in polar regions—Main sources. J. Environ. Radioact. 1994, 25, 21–35. [Google Scholar] [CrossRef]
- Leppänen, A.-P.; Kasatkina, N.; Vaaramaa, K.; Matishov, G.G.; Solatie, D. Selected anthropogenic and natural radioisotopes in the Barents Sea and off the western coast of Svalbard. J. Environ. Radioact. 2013, 126, 196–208. [Google Scholar] [CrossRef]
- Gwynn, J.P.; Heldal, H.E.; Gäfvert, T.; Blinova, O.; Eriksson, M.; Sværen, I.; Brungot, A.L.; Strålberg, E.; Møller, B.; Rudjord, A.L. Radiological status of the marine environment in the Barents Sea. J. Environ. Radioact. 2012, 113, 155–162. [Google Scholar] [CrossRef]
- Matishov, G.G.; Matishov, D.G.; Solatie, D.; Kasatkina, N.E.; Leppanen, A. Natural decrease of the intensity level of artificial radioactive isotopes in the Barents Sea. Dokl. Earth Sci. 2009, 427, 1006–1011. [Google Scholar] [CrossRef]
- Bogdanov, A.Y.; Gorshkov, A.I.; Gurvich, E.G. Ferromanganese nodules of the Kara Sea. Oceanology 1994, 34, 789–800. [Google Scholar]
- Baturin, G.N. Variations in the composition of ferromanganese nodules in the Kara Sea. Oceanology 2011, 51, 153–161. [Google Scholar] [CrossRef]
- Baturin, G.N.; Dubinchuk, V.T. On the composition of ferromanganese nodules of the Chukotka and East Siberian Seas. Dokl. Earth. Sci. 2011, 440, 93–99. [Google Scholar] [CrossRef]
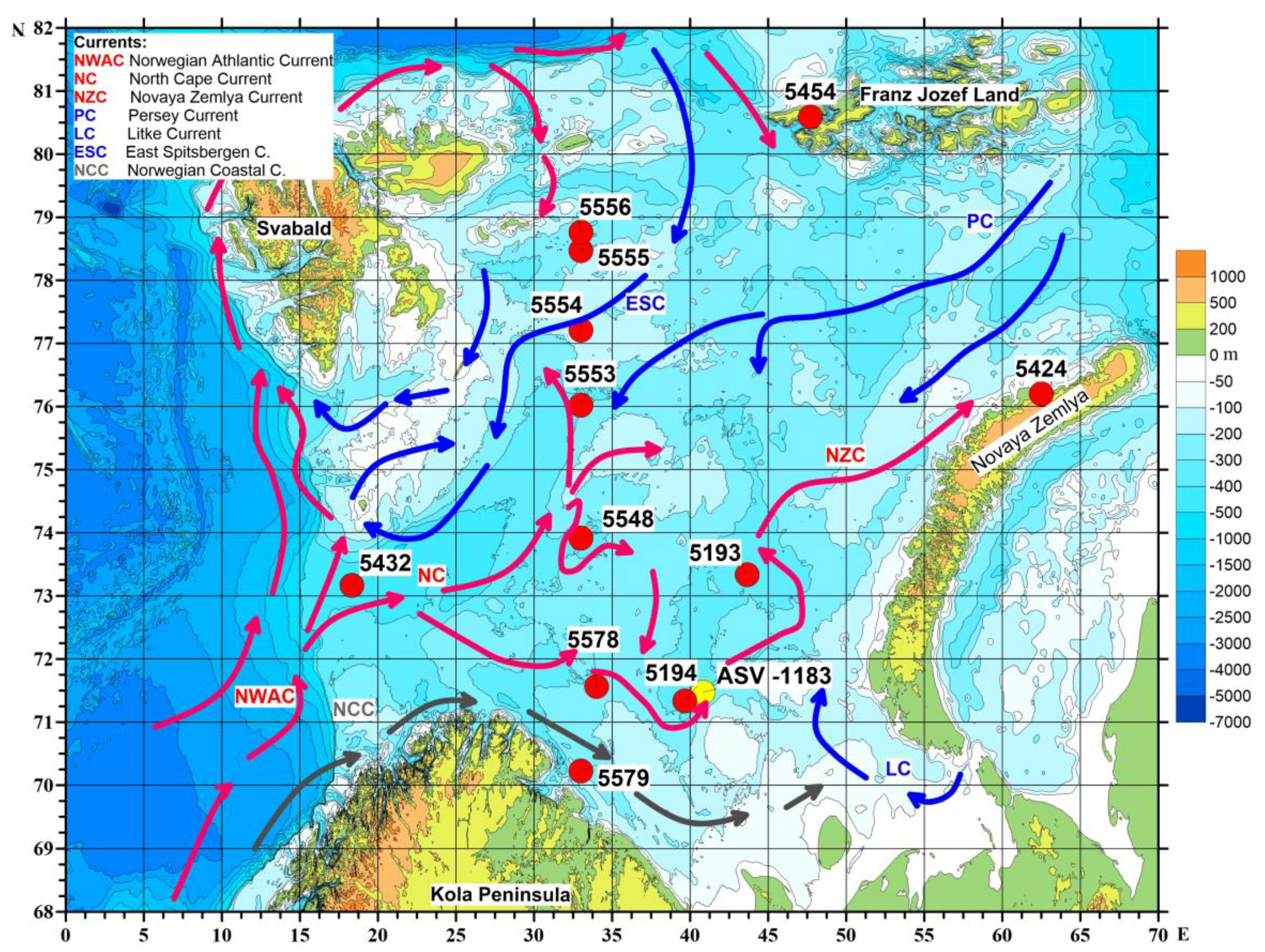







| Core | Sampler | Latitude (°N) | Longitude (°E) | Cruise | Water Depth, m | Length, cm |
|---|---|---|---|---|---|---|
| 5193 | Niemisto | 73.333 | 43.667 | 62AMK | 328 | 37 |
| 5194 | Gravity core | 71.333 | 39.667 | 62AMK | 353 | 450 |
| 5424 | MUC | 76.204 | 62.487 | 67AMK | 176 | 24 |
| 5432 | MUC | 73.166 | 18.333 | 67AMK | 462 | 25 |
| 5454 | MUC | 80.593 | 47.702 | 67AMK | 638 | 33 |
| Kola Transect (along ~33° N) | ||||||
| 5556 | MUC | 78.763 | 33.004 | 68AMK | 360 | 32 |
| 5555 | MUC | 78.472 | 33.009 | 68AMK | 122 | 29 |
| 5554 | Grab | 77.213 | 33.011 | 68AMK | 160 | 5 |
| 5553 | Grab | 76.021 | 33.008 | 68AMK | 297 | 14 |
| 5548 | MUC | 73.916 | 33.007 | 68AMK | 322 | 28 |
| 5578 | Grab | 71.568 | 34.000 | 68AMK | 235 | 17 |
| 5579 | MUC | 70.224 | 33.006 | 68AMK | 248 | 30 |
| Depth | Qz | K-Fsp | Ab | Ep | Cal | Dol | Ilt + Ms | Chl 2 | Gp |
|---|---|---|---|---|---|---|---|---|---|
| (cm) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) |
| Suspension | 25 | 3 | 11 | n.d. | 1 | 1 | 15 | 43 | n.d. |
| 0–1 | 22 | 3 | 12 | 1 | 1 | 1 | 11 | 48 | n.d. |
| 2–3 | 18 | 3 | 14 | n.d. | n.d. | n.d. | 26 | 32 | 6 |
| 19–20 | 22 | 3 | 13 | n.d. | 1 | 1 | 11 | 45 | 3 |
| 26–27 | 21 | 3 | 13 | n.d. | 1 | 2 | 12 | 46 | 1 |
| Depth | Qz | Ab | An | Ans | K-Fsp | Amp | Aug | Sme 12 Å | Sme 14 Å | Chl | Cln | Ilt | Gp | Zeo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (cm) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) |
| Suspension | 27 | 12 | 8 | n.d. | 6 | 2 | 2 | 7 | n.d. | 12 | 10 | 13 | n.d. | n.d. |
| 0–1 | 20 | n.d. | 9 | 12 | n.d. | n.d. | 6 | 8 | 10 | 10 | 8 | 11 | n.d. | 5 |
| 1–2 | 26 | n.d. | 31 | n.d. | n.d. | n.d. | 9 | n.d. | 8 | 6 | 8 | 6 | 2 | 4 |
| 2–3 | 21 | n.d. | 35 | n.d. | n.d. | n.d. | 10 | n.d. | 10 | 5 | 5 | 6 | 1 | 6 |
| 3–4 | 22 | n.d. | 17 | 22 | n.d. | n.d. | 7 | 9 | n.d. | 6 | 6 | 8 | 2 | 1 |
| 4–5 | 25 | n.d. | 33 | n.d. | n.d. | n.d. | 6 | 10 | n.d. | 6 | 7 | 10 | 1 | 1 |
| 5–6 | 29 | n.d. | 35 | n.d. | n.d. | n.d. | 7 | 10 | n.d. | 4 | 5 | 8 | 1 | 1 |
| 6–7 | 27 | n.d. | 31 | n.d. | n.d. | n.d. | 7 | 9 | n.d. | 5 | 6 | 10 | 1 | 3 |
| 7–8 | 22 | n.d. | 30 | n.d. | n.d. | n.d. | 6 | 10 | n.d. | 7 | 7 | 11 | 1 | 6 |
| 8–9 | 21 | n.d. | 33 | n.d. | n.d. | n.d. | 10 | 10 | n.d. | 7 | 6 | 9 | 2 | 2 |
| 9–10 | 18 | n.d. | 42 | n.d. | n.d. | n.d. | 7 | 10 | n.d. | 5 | 6 | 9 | 2 | 1 |
| 10–11 | 21 | n.d. | 33 | n.d. | n.d. | n.d. | 9 | 11 | n.d. | 5 | 8 | 10 | 2 | 2 |
| 11–12 | 26 | n.d. | 22 | n.d. | n.d. | n.d. | 9 | 11 | n.d. | 6 | 8 | 13 | 2 | 3 |
| 12–13 | 22 | n.d. | 18 | 13 | n.d. | n.d. | 11 | 13 | n.d. | 6 | 7 | 9 | n.d. | 1 |
| 13–14 | 27 | n.d. | 25 | n.d. | n.d. | n.d. | 9 | 16 | n.d. | 6 | 7 | 10 | n.d. | n.d. |
| 14–15 | 15 | n.d. | 30 | 10 | n.d. | n.d. | 7 | 8 | n.d. | 6 | 7 | 11 | 1 | 5 |
| 15–16 | 16 | n.d. | 31 | 10 | n.d. | n.d. | 9 | 6 | n.d. | 5 | 5 | 10 | n.d. | 7 |
| 16–17 | 30 | n.d. | 16 | 14 | n.d. | n.d. | 9 | 9 | n.d. | 4 | 6 | 9 | n.d. | 2 |
| 17–18 | 24 | n.d. | 30 | n.d. | n.d. | n.d. | 6 | 14 | n.d. | 5 | 7 | 13 | n.d. | 1 |
| 18–19 | 22 | n.d. | 36 | n.d. | n.d. | n.d. | 5 | 9 | n.d. | 4 | 6 | 11 | 1 | 6 |
| 19–20 | 25 | n.d. | 26 | n.d. | n.d. | n.d. | 8 | 16 | n.d. | 7 | 8 | 6 | 2 | 2 |
| Core | Depth | Smectite | Illite | Kaolinite | Chlorite |
|---|---|---|---|---|---|
| (cm) | (%) | (%) | (%) | (%) | |
| 5432 | 1–4 | 8 | 54 | 19 | 19 |
| 5432 | 24 | 1 | 49 | 25 | 25 |
| 5454 | 1–4 | 39 | 32 | 15 | 14 |
| 5454 | 24 | 28 | 38 | 19 | 15 |
| Depth | Qz | K-Fsp | Ab | Amp | Aug | Ms | Ep | Cal | Dol | Sme | Ilt | Chl | Kln | MLC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (cm) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) |
| Suspension | 25 | 6 | 13 | 2 | n.d. | 8 | 3 | 7 | 1 | 7 | 10 | 11 | 7 | n.d. |
| 0–1 | 26 | 9 | 19 | 3 | 4 | 13 | n.d. | 4 | 1 | n.d. | 14 | 3 | 3 | 1 |
| Element | C min | C max | C aver | UCC [67] | Regional Background [21] |
|---|---|---|---|---|---|
| C org, % | 0.49 | 1.76 | 0.98 | - | - |
| Al, % | 7.12 | 9.52 | 8.30 | 7.96 | - |
| Si, % | 24.21 | 29.58 | 27.28 | 28.8 | - |
| Fe, % | 3.92 | 9.35 | 4.43 | 4.32 | - |
| Ca, % | 0.60 | 1.45 | 0.84 | 3.85 | - |
| Ti, % | 0.34 | 0.50 | 0.43 | 0.40 | - |
| Mg, % | 1.53 | 1.99 | 1.79 | 2.20 | - |
| Mn, % | 0.03 | 0.89 | 0.27 | 0.07 | - |
| V, mg/kg | 89 | 202 | 140 | 98 | - |
| Sr, mg/kg | 141 | 168 | 157 | 333 | - |
| Zr, mg/kg | 132 | 209 | 165 | 203 | - |
| Ba, mg/kg | 188 | 479 | 439 | 584 | - |
| As, mg/kg | 2 | 28 | 19 | 8 | - |
| Cr, mg/kg | 70 | 128 | 85 | 126 | 70 |
| Zn, mg/kg | 37 | 97 | 69 | 65 | 150 |
| Pb, mg/kg | 15 | 31 | 18 | 15 | 30 |
| Cu, mg/kg | 24 | 47 | 31 | 25 | 35 |
| Ni, mg/kg | 29 | 51 | 39 | 56 | 30 |
| Co, mg/kg | 6 | 33 | 20 | 24 | 12 |
| Element | Central Deep 5194 (n = 42) | Russkaya Gavan’ Bay 5424 (n = 24) | Bear Island Trough 5432 (n = 25) | Cambridge Strait 5454 (n = 33) | UCC [67] |
|---|---|---|---|---|---|
| Si, % | 24.41 | 25.61 | 30.37 | 21.12 | 28.8 |
| Al, % | 8.29 | 8.39 | 6.33 | 7.40 | 7.96 |
| Fe, % | 5.07 | 5.06 | 2.67 | 8.87 | 4.32 |
| Ca, % | 1.02 | n.d. | n.d. | n.d. | 3.85 |
| Mg, % | 2.15 | n.d. | n.d. | n.d. | 2.20 |
| K, % | 2.80 | 2.18 | 1.78 | 1.42 | 2.14 |
| Ti, % | 0.48 | 0.47 | 0.32 | 0.71 | 0.40 |
| P, % | 0.09 | 0.07 | 0.07 | 0.21 | 0.08 |
| Mn, % | 0.05 | 0.05 | 0.04 | 0.79 | 0.07 |
| Zr, ppm | 193 | 189 | 230 | 104 | 203 |
| Sr, ppm | 165 | 140 | 170 | 215 | 333 |
| Ba, ppm | 449 | n.d. | n.d. | n.d. | 584 |
| Zn, ppm | 107 | 91 | 47 | 108 | 65 |
| Cr, ppm | 75 | 53 | 44 | 51 | 126 |
| Co, ppm | 19 | n.d. | 10 | n.d. | 24 |
| Ni, ppm | 50 | n.d. | 33 | n.d. | 56 |
| Cu, ppm | 31 | 34 | 27 | 199 | 25 |
| Pb, ppm | 16 | n.d. | 13 | n.d. | 15 |
| Si/Al | 2.96 | 3.06 | 4.81 | 2.82 | 3.61 |
| Si/Fe | 4.81 | 5.06 | 11.37 | 2.38 | 6.67 |
| Fe/Al | 0.61 | 0.60 | 0.42 | 1.20 | 0.54 |
| Ti/Al | 0.06 | 0.06 | 0.05 | 0.10 | 0.06 |
| Ti/K | 0.17 | 0.22 | 0.18 | 0.51 | 0.19 |
| Mn/Fe | 0.009 | 0.009 | 0.013 | 0.090 | 0.016 |
| P/Al | 0.010 | 0.009 | 0.011 | 0.030 | 0.010 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demina, L.L.; Dara, O.; Aliev, R.; Alekseeva, T.; Budko, D.; Novichkova, E.; Politova, N.; Solomatina, A.; Bulokhov, A. Elemental and Mineral Composition of the Barents Sea Recent and Late Pleistocene−Holocene Sediments: A Correlation with Environmental Conditions. Minerals 2020, 10, 593. https://doi.org/10.3390/min10070593
Demina LL, Dara O, Aliev R, Alekseeva T, Budko D, Novichkova E, Politova N, Solomatina A, Bulokhov A. Elemental and Mineral Composition of the Barents Sea Recent and Late Pleistocene−Holocene Sediments: A Correlation with Environmental Conditions. Minerals. 2020; 10(7):593. https://doi.org/10.3390/min10070593
Chicago/Turabian StyleDemina, Liudmila L., Olga Dara, Ramiz Aliev, Tatiana Alekseeva, Dmitry Budko, Ekaterina Novichkova, Nadezhda Politova, Aleksandra Solomatina, and Anton Bulokhov. 2020. "Elemental and Mineral Composition of the Barents Sea Recent and Late Pleistocene−Holocene Sediments: A Correlation with Environmental Conditions" Minerals 10, no. 7: 593. https://doi.org/10.3390/min10070593
APA StyleDemina, L. L., Dara, O., Aliev, R., Alekseeva, T., Budko, D., Novichkova, E., Politova, N., Solomatina, A., & Bulokhov, A. (2020). Elemental and Mineral Composition of the Barents Sea Recent and Late Pleistocene−Holocene Sediments: A Correlation with Environmental Conditions. Minerals, 10(7), 593. https://doi.org/10.3390/min10070593







