Hydrogeochemical Behavior of Reclaimed Highly Reactive Tailings, Part 2: Laboratory and Field Results of Covers Made with Mine Waste Materials
Abstract
1. Introduction
2. Summary of the Material Characterization Results
3. Materials and Methods
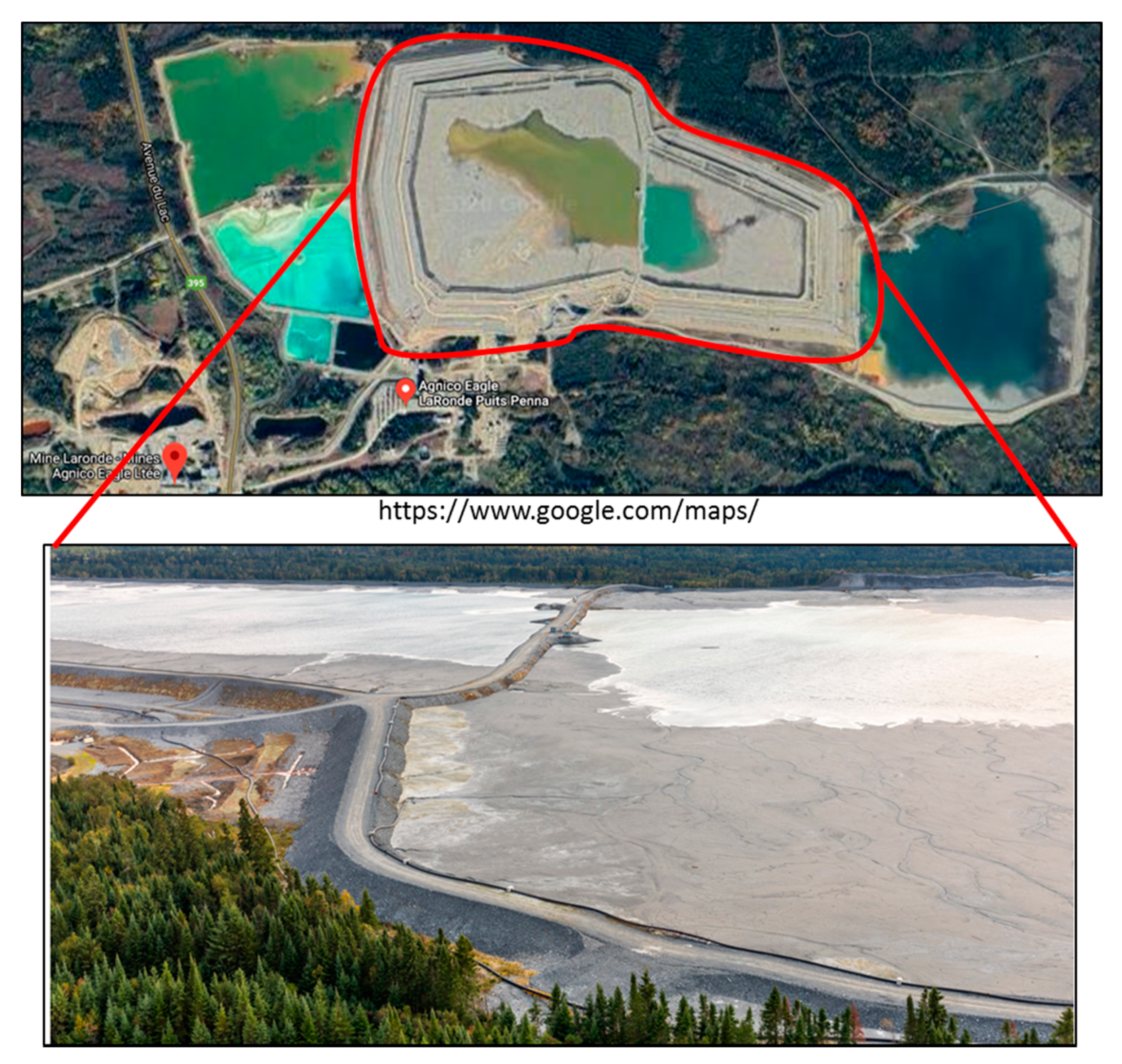
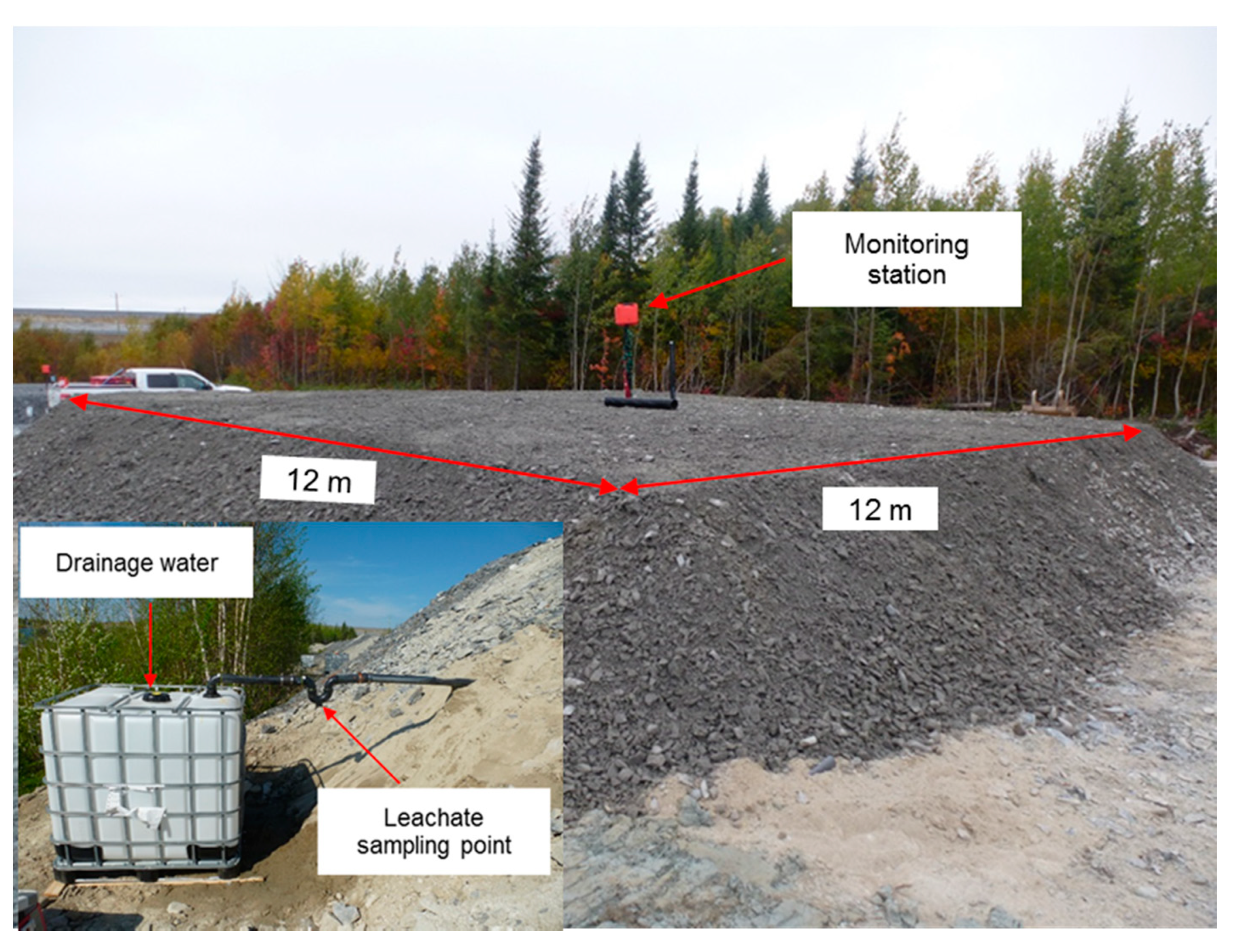
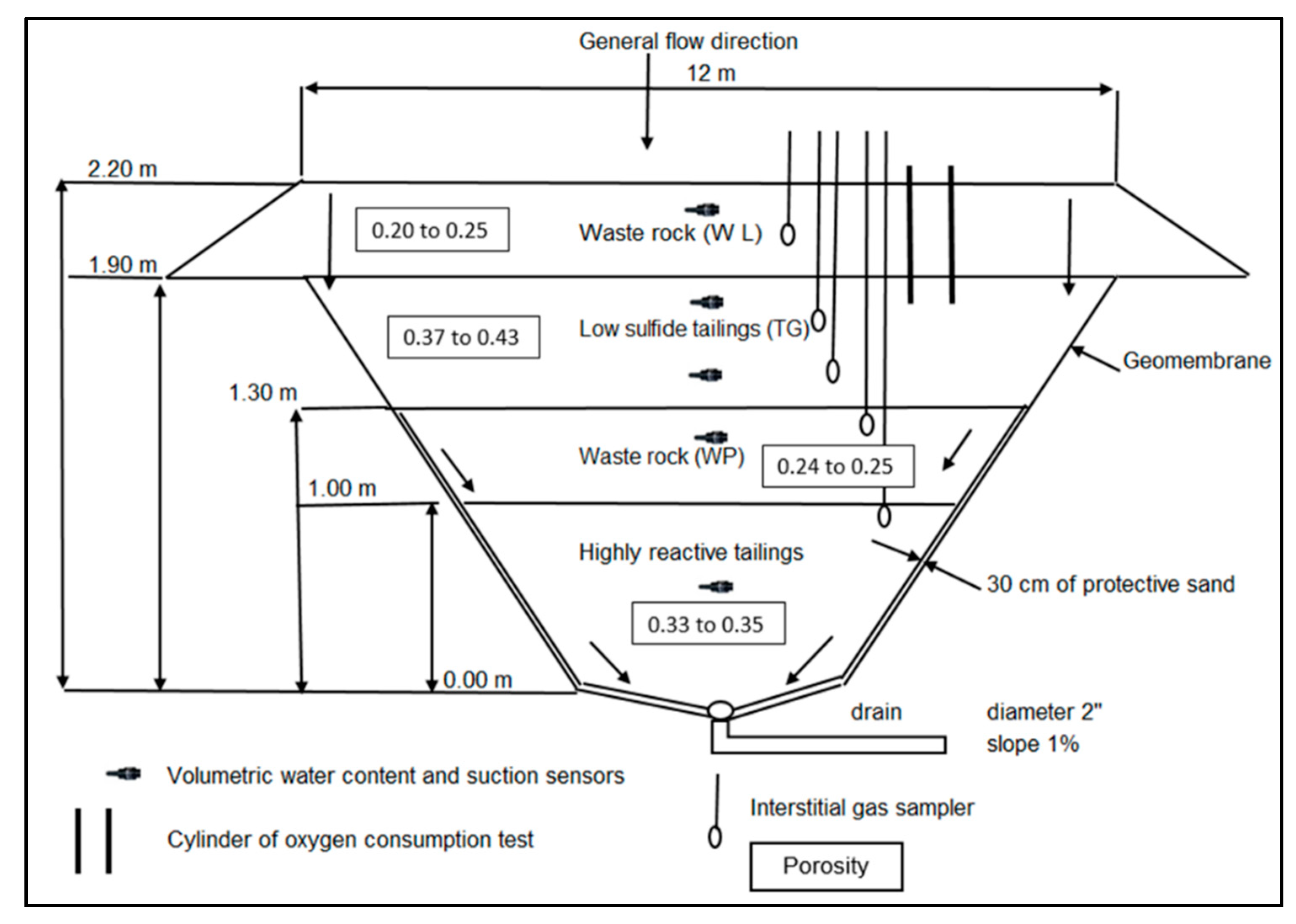
3.1. Construction and Instrumentation of the CCBE Column and Field Cell
3.2. Oxygen Flux Measurements
3.2.1. Oxygen Consumption Tests Method
3.2.2. Oxygen Gradient Method
3.2.3. Sulfate-Release Method
4. Results and Discussion
4.1. Hydrogeological Behavior
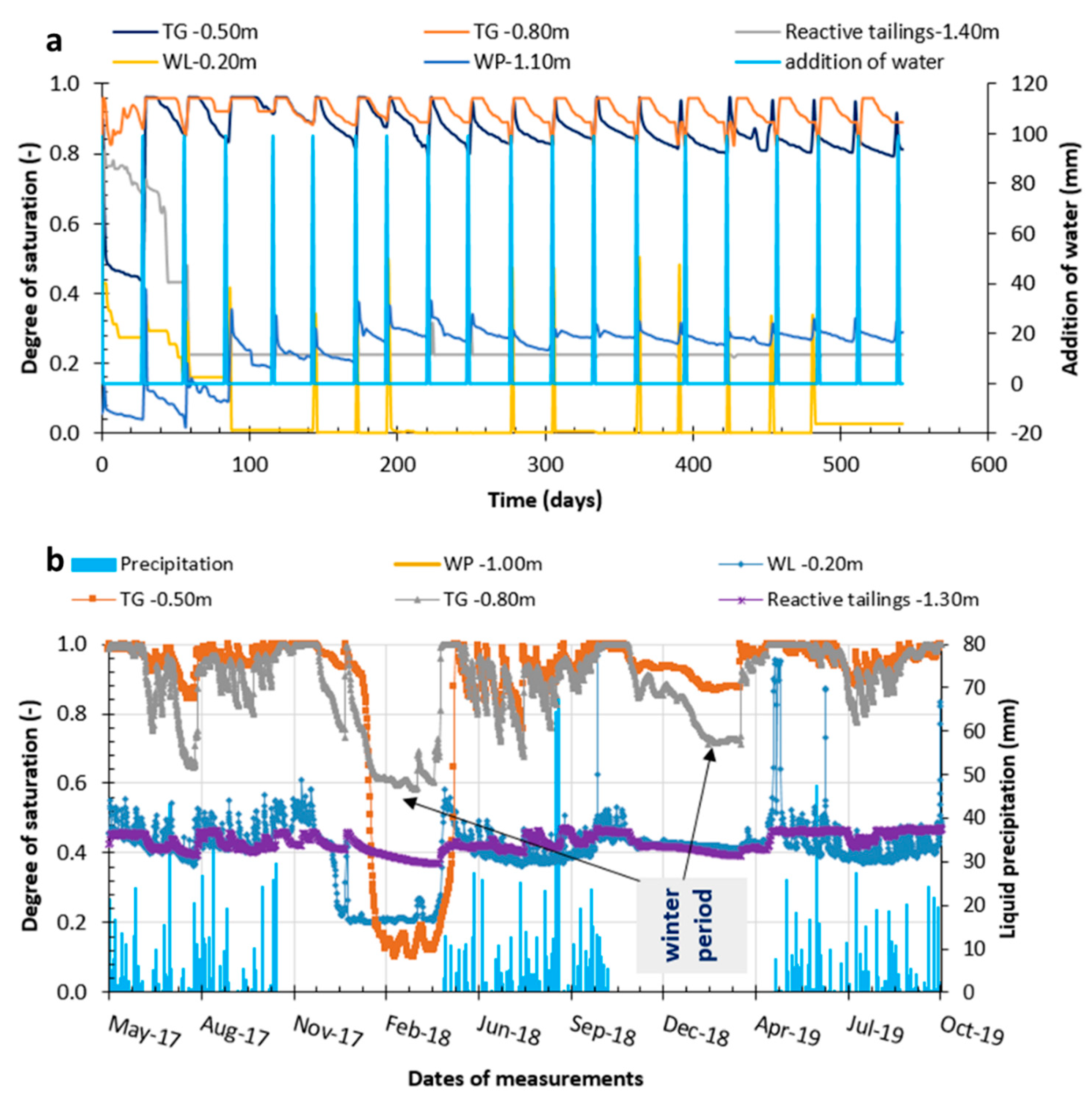
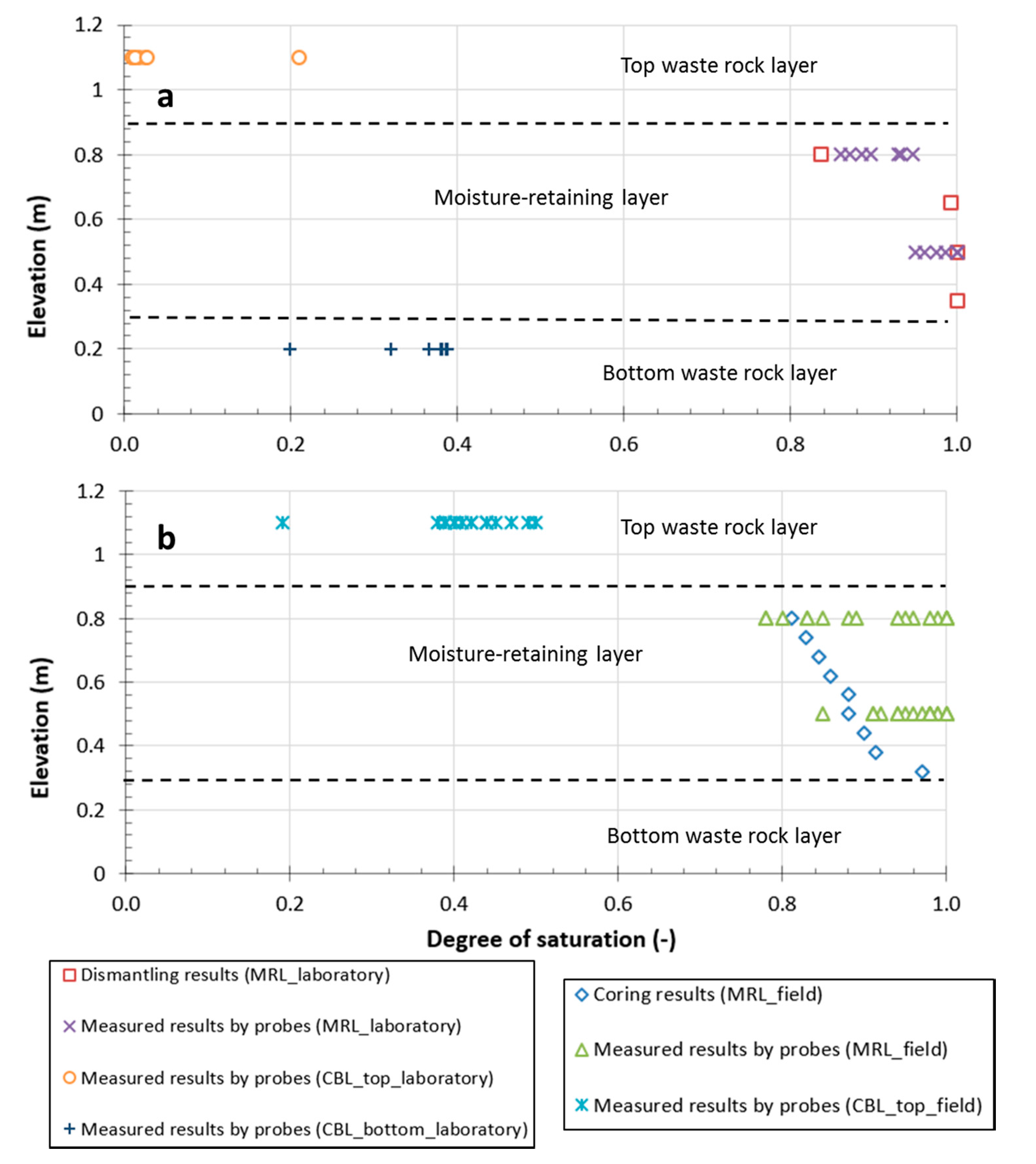
4.1.1. Degree of Saturation
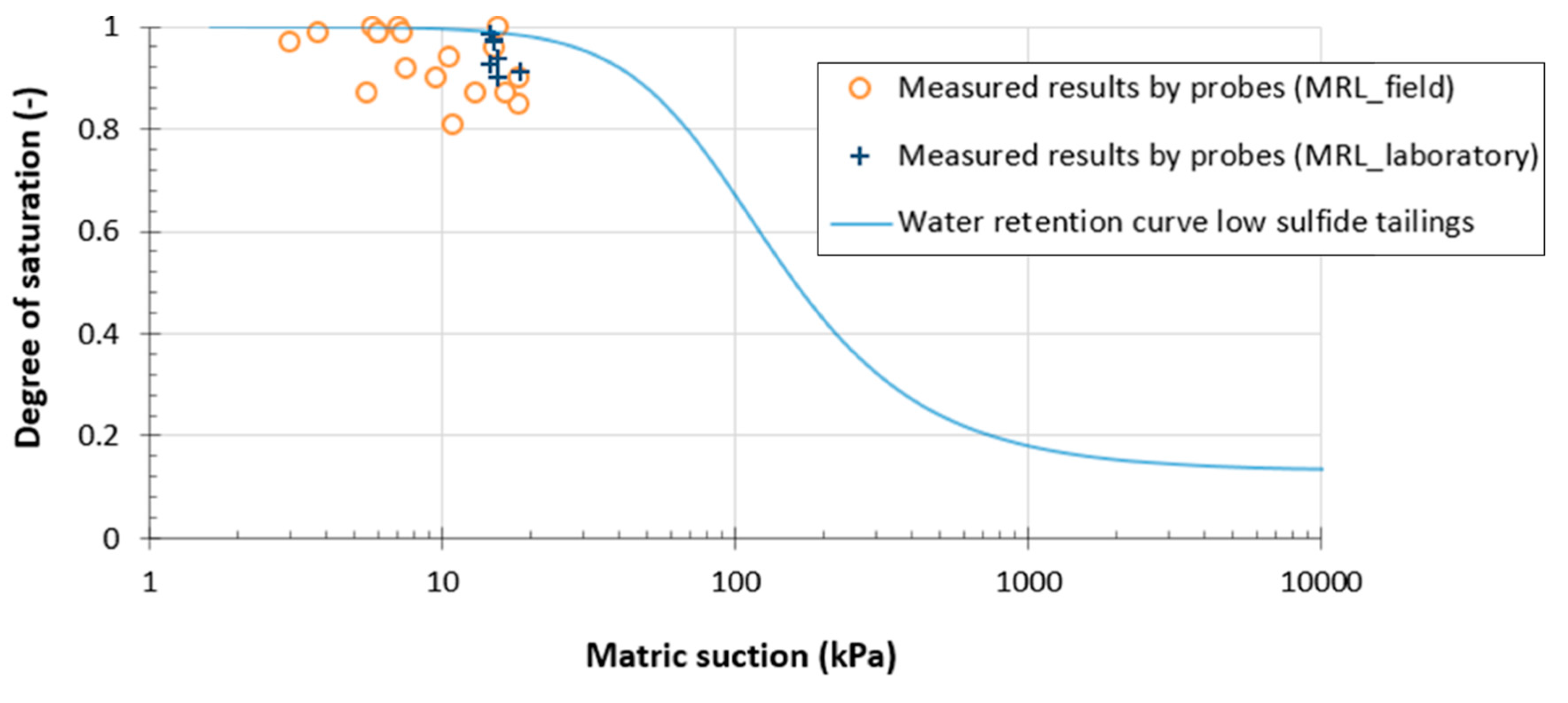
4.1.2. Suction Values
4.1.3. Post-Testing Measurements and in Situ Water Retention Curves (WRCs)
4.2. Water Quality
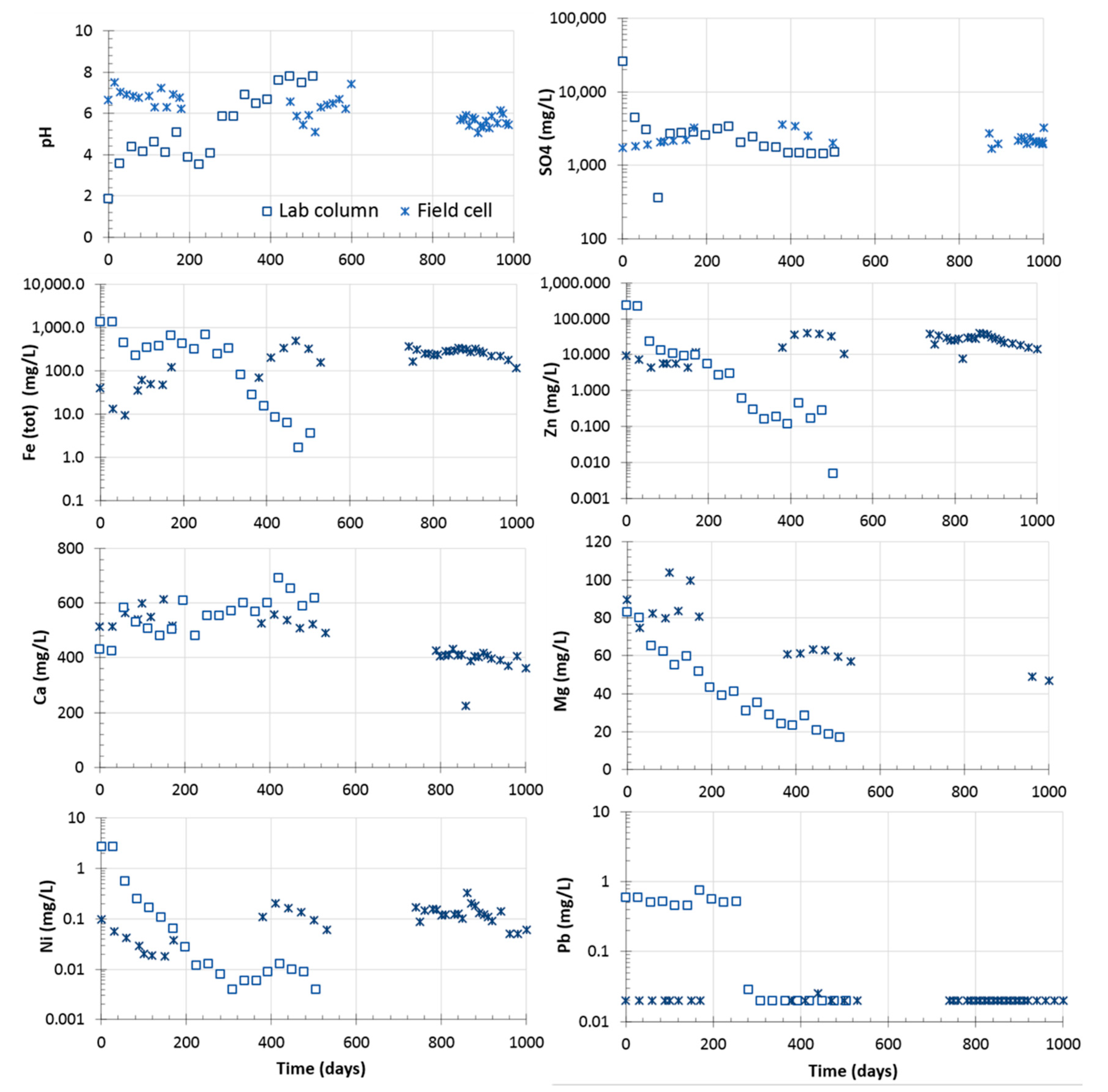
4.2.1. Laboratory Columns
4.2.2. Field Experimental Cells
4.3. Oxygen Fluxes
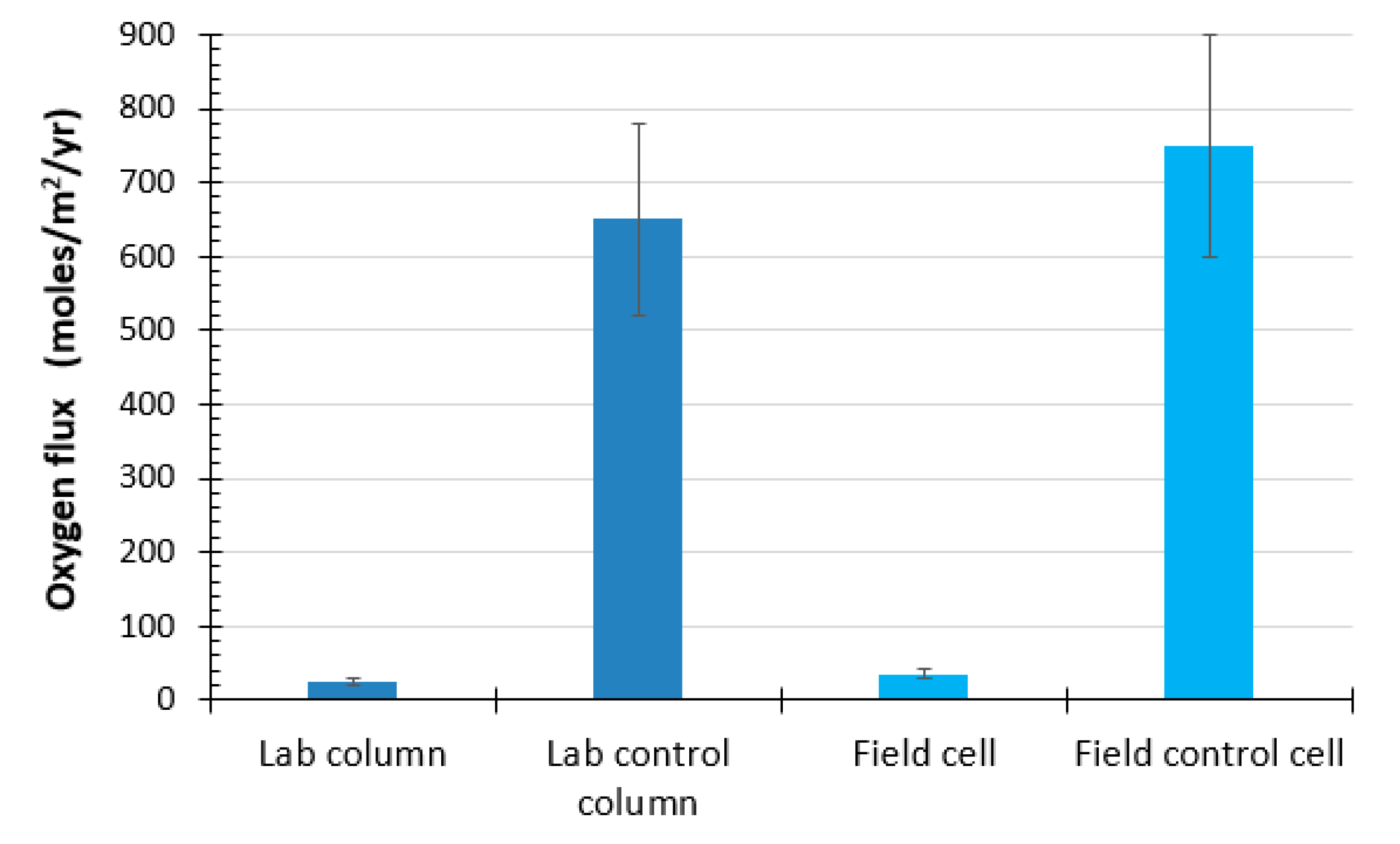
4.3.1. Oxygen Consumption Test
4.3.2. Oxygen Gradient Method
4.3.3. Sulfate-Release Method
5. Cover Efficiency
5.1. Efficiency with Respect to Controlling Contaminant Generation
5.2. Efficiency with Respect to Controlling Oxygen Fluxes at the Base of the CCBE
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aubertin, M.; Chapuis, R.P.; Aachib, M.; Bussière, B.; Ricard, J.F.; Tremblay, L. Evaluation en laboratoire de barrières sèches construites à partir de résidus miniers. In Ecole Polytechnique de Montréal; Rapport NEDEM/MEND: Ottawa, ON, Canada, 1995. [Google Scholar]
- Ricard, J.F.; Aubertin, M.; Firlotte, F.W.; Knapp, R.; McMullen, J. Design and construction of a dry cover made of tailings for the closure of Les Terrains Aurifères site. In Proceedings of the 4th International Conference on Acid Rock Drainage, Vancouver, BC, Canada, 31 May–6 June 1997; pp. 1515–1530. [Google Scholar]
- Ricard, J.F.; Aubertin, M.; Garand, P. Performance d’un recouvrement multicouche au site Barrick-Bousquet de Les Terrains Aurifères. In the 20th Symposium on Wastewater; Delisle, C.E., Bouchard, M.A., Eds.; Collection Environnement de l’Université de Montréal: Montréal, Canada, 1997; Volume 10, pp. 291–305. [Google Scholar]
- Aachib, M.; Aubertin, M.; Chapuis, R.P. Essais en colonne sur des couvertures avec effets de barrière capillaire. In Proceedings of the 51st Canadian Geotechnical Conference, Edmonton, AB, Canada, 4–7 October 1998; Volume 2, pp. 837–844. [Google Scholar]
- Bussière, B.; Benzaazoua, M.; Aubertin, M.; Mbonimpa, M. A laboratory study of covers made of low sulphide tailings to prevent acid mine drainage. Environ. Geol. 2004, 45, 609–622. [Google Scholar] [CrossRef]
- Kalonji-Kabambi, A.; Bussière, B.; Demers, I. Hydrogeological Behaviour of cover with Capillary Barrier Effect made of Mining Materials. Geotech. Geol. Eng. J. 2017, 35, 1199–1220. [Google Scholar] [CrossRef]
- Pabst, T.; Bussière, B.; Aubertin, M.; Molson, J. Comparative performance of cover systems to prevent acid mine drainage from pre-oxidized tailings: A numerical hydro geochemical assessment. J. Contam. Hydrol. 2018, 214, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Maqsoud, A.; Bussière, B.; Turcotte, S.; Roy, M. Performance Evaluation of Covers with Capillary Barrier Effects under Deep Groundwater Conditions Using Experimental Cells. In Proceedings of the Géo Ottawa Conference, Ottawa, ON, Canada, 1–4 October 2017; p. 256. [Google Scholar]
- Larochelle, C.G.; Bussière, B.; Pabst, T. Acid-generating waste rocks as capillary break layers in covers with capillary barrier effects for mine site reclamation. Water Air Soil Pollut. 2019, 230, 57. [Google Scholar] [CrossRef]
- Yanful, E.K. Engineering soil covers for reactive tailings management: Theoretical concepts and laboratory development. In Proceedings of the Second International Conference on the Abatement of Acidic Drainage, Montréal, QC, Canada, 16–18 September 1991; pp. 461–485. [Google Scholar]
- Dagenais, A.-M.; Aubertin, M.; Bussière, B.; Martin, V. Large scale applications of covers with capillary barrier effects to control the production of acid mine drainage. In Proceedings of the Post-Mining, Nancy, France, 16–17 November 2005. [Google Scholar]
- Demers, I.; Bussière, B.; Benzaazoua, M.; Mbonimpa, M.; Blier, A. Column test investigation on the performance of monolayer covers made of desulphurized tailings to prevent acid mine drainage. Miner. Eng. 2008, 21, 317–329. [Google Scholar] [CrossRef]
- Pabst, T.; Aubertin, M.; Bussière, B.; Molson, J. Column Tests to Characterise the Hydrogeochemical Response of Pre-oxidised Acid-Generating Tailings with a Monolayer Cover. Water Air Soil Pollut. 2014, 225, 1841. [Google Scholar] [CrossRef]
- Pabst, T.; Molson, J.; Aubertin, M.; Bussière, B. Reactive transport modelling of the hydro-geochemical behaviour of partially oxidized acid-generating mine tailings with a monolayer Cover. Appl. Geochem. 2017, 78, 219–233. [Google Scholar] [CrossRef]
- Aubertin, M.; Bussière, B.; Chapuis, R.D.; Barbera, J.M. Construction of experimental cells with covers on acid producing tailings. In Proceedings of the 49th Canadian Geotechnical Conference, St-John’s, NL, Canada, 23–25 September 1996; pp. 655–662. [Google Scholar]
- Aubertin, M.; Bussière, B.; Barbera, J.M.; Chapuis, R.P.; Monzon, M.; Aachib, M. Construction and instrumentation of in situ test plots to evaluate covers built with clean tailings. In Proceedings of the 4th International Conference on Acid Rock Drainage (ICARD), Vancouver, BC, Canada, 31 May–6 June 1997; Volume 2, pp. 715–730. [Google Scholar]
- Adu-Wusu, C.; Yanful, E.K. Performance of engineered test covers on acidgenerating waste rock at Whistle mine, Ontario. Can. Geotech. J. 2006, 43, 1–18. [Google Scholar] [CrossRef]
- Bussière, B.; Aubertin, M.; Mbonimpa, M.; Molson, J.; Chapuis, R. Field experimental cells to evaluate the hydrogeological behaviour of oxygen barriers made of silty materials. Can. Geotech. J. 2007, 44, 245–265. [Google Scholar] [CrossRef]
- Demers, I.; Bussière, B.; Benzaazoua, M.; Mbonimpa, M. Preliminary optimization of a single-layer cover made of desulfurized tailings: Application to the Doyon Mine tailings impoundment. SME Trans. 2009, 326, 21–33. [Google Scholar]
- Demers, I.M.; Bouda, M.; Mbonimpa, M.; Benzaazoua, D.; Bois, M.; Gagnon, M. Valorization of acid mine drainage treatment sludge as remediation component to control acid generation from mine wastes, part 2: Field experimentation. Minerais Eng. 2015, 76, 117–125. [Google Scholar] [CrossRef]
- Plante, B.; Bussière, B.; Benzaazoua, M. Lab to field scale effects on contaminated neutral drainage prediction from the Tio mine waste rocks. J. Geochem. Explor. 2014, 137, 37–47. [Google Scholar] [CrossRef]
- Kalonji-Kabambi, A.; Bussière, B.; Demers, I. Hydrogeochemical behavior of reclaimed highly reactive tailings, Part 1: Characterization of reclamation materials. Miner. J. 2020, 10, 596. [Google Scholar] [CrossRef]
- Bussière, B.; NicholsonI, R.V.; Aubertin, M.; Servant, S. Effectiveness of covers built with desulphurized tailings: Column tests investigation. In Proceedings of the 4th International Conference on Acid Rock Drainage, Vancouver, BC, Canada, 31 May–6 June 1997; Volume 2, pp. 763–778. [Google Scholar]
- Aachib, M. Étude en Laboratoire de la Performance de Barrières de Recouvrement Constituées de Rejets Miniers Pour Limiter le DMA. Ph.D. Thesis, École Polytechnique de Montréal, Montréal, QC, Canada, 1997. [Google Scholar]
- Dagenais, A.M. Techniques de Contrôle du Drainage Minier Acide Basées sur les Effets Capillaires. Ph.D. Thesis, École Polytechnique de Montréal, Montréal, QC, Canada, 2005. [Google Scholar]
- Kalonji-Kabambi, A. Évaluation de la Performance des Recouvrements en Materiaux Miniers Pour la Restauration d’un parc à Résidus Miniers Hautement Reactifs. Ph.D. Thesis, École Polytechnique de Montréal (en extension à l’Université du Québec en Abitibi-Témiscarningue), Rouyn-Noranda, QC, Canada, 2020. [Google Scholar]
- Kalonji-Kabambi, A.; Demers, I.; Bussière, B. Short term in situ performance of a CCBE made entirely of mining materials to control acid mine drainage. In Proceedings of the 12th International Confrence on Mine Closure, Leipzig, Germany, 3–7 September 2018. [Google Scholar]
- Elberling, B.; Nicholson, R.V.; Reardon, E.J.; Tibble, P. Evaluation of sulphide oxidation rates: A laboratory study comparing oxygen fluxes and rates of oxidation product release. Can. Geotech. J. 1994, 31, 375–383. [Google Scholar] [CrossRef]
- Elberling, B.; Nicholson, R.V. Field Determination of Sulphide Oxidation Rates in Mine Tailings. Water Resour. Res. 1996, 32, 1773–1784. [Google Scholar] [CrossRef]
- Elberling, B.; Langdahl, B.R. Natural heavy-metal release by sulphide oxidation in the High Arctic. Can. Geotech. J. 1998, 35, 895–901. [Google Scholar] [CrossRef]
- Elberling, B. Environmental controls of the seasonal variation in oxygen uptake in sulfidic tailings deposited in a permafrostaffected area. Water Resour. Res. 2001, 37, 99–107. [Google Scholar] [CrossRef]
- Dagenais, A.-M.; Mbonimpa, M.; Bussière, B.; Aubertin, M. A modified oxygen consumption test to evaluate gas flux through oxygen barrier cover systems. Geotech. Test. J. 2012, 35. [Google Scholar] [CrossRef]
- Yanful, E.K. Development of Laboratory Methodology for Evaluating the Effectiveness of Reactive Tailings Covers; Final report; Centre de Technologie Noranda: Pointe-Claire, QC, Canada, 1991. [Google Scholar]
- Millington, R.J.; Shearer, R.C. Diffusion in aggregated porous media. Sail Sci. 1971, 111, 372–378. [Google Scholar] [CrossRef]
- Aachib, M.; Aubertin, M.; Mbonimpa, M. Laboratory Measurements and Predictive Equations for Gas Diffusion Coefficient of Unsaturated Soils. In Proceedings of the 55th Canadian Geotechnical Conference and 3rd joint IAH-CNC and CGS Groundwater Specialty Conference, Niagara Falls, ON, Canada, 20–23 October 2002; pp. 163–172. [Google Scholar]
- Aachib, M.; Mbonimpa, M.; Aubertin, M. Measurement and prediction of the oxygen diffusion coefficient in the unsaturated media, with applications to soil covers. Water Air Soil Pollut. 2004, 156, 163–193. [Google Scholar] [CrossRef]
- Mbonimpa, M.; Aubertin, M.; Dagenais, A.-M.; Bussière, B.; Julien, M.; Kissiova, M. Interpretation of field tests to determine the oxygen diffusion and reaction rate coefficients of tailings and soil covers. In Proceedings of the 55th Canadian geotechnical conference and 3rd joint IAH-CNC and CGS groundwater specialty conference, Niagara Falls, ON, Canada, 20–23 October 2002; pp. 147–154. [Google Scholar]
- Mbonimpa, M.; Aubertin, M.; Aachib, M.; Bussière, B. Diffusion and consumption of oxygen in unsaturated cover materials. Can. Geotech. J. 2003, 40, 916–932. [Google Scholar] [CrossRef]
- Mbonimpa, M.; Aubertin, M.; Bussière, B. Oxygen consumption test to evaluate the diffusive flux into reactive tailings: Interpretation and numerical assessment. Can. Geotech. J. 2011, 48, 878–890. [Google Scholar] [CrossRef]
- Ullom, W.L. Soil gas sampling. In Handbook of Vadose Zone Characterization & Monitoring; Wilson, L.G., Everett, L.G., Cullen, S.J., Eds.; CRC Press: Boca Raton, FL, USA, 1995; pp. 555–567. [Google Scholar]
- Bussière, B.; Nicholson, R.V.; Aubertin, M.; Benzaazoua, M. Evaluation of the effectiveness of covers built with desulfurized tailings for preventing Acid Mine Drainage. In Proceedings of the 50th Canadian Geotechnical Conference, Ottawa, ON, Canada, 20–22 October 1997; pp. 17–25. [Google Scholar]
- Aubertin, M.; Bussière, B.; Monzon, M.; Joanes, A.M.; Gagnon, D.; Barbera, J.M.; Aachib, M.; Bédard, C.; Chapuis, R.P.; Bernier, L. Étude sur les barrières sèches construites à partir des résidus miniers. Phase II, Essais en place; Rapport de Recherche; Projet CDT P1899.NEDEM/MEND 2.22.2c.: Ottawa, ON, Canada, 1999. [Google Scholar]
- Nicholson, R.V.; Gillham, R.W.; Reardon, E.J. Pyrite oxidation in carbonate-buffered solution: 1. Experimental kinetics. Geochim. Cosmochim. Acta 1988, 52, 1077–1085. [Google Scholar] [CrossRef]
- Nicholson, R.V.; Gillham, R.W.; Cherry, J.A.; Reardon, E.J. Reduction of acid generation in mine tailings through the use of moisture-retaining layers as oxygen barriers. Can. Geotech. J. 1989, 26, 1–8. [Google Scholar] [CrossRef]
- Akindunni, F.F.; Gillham, R.W.; Nicholson, R.V. Numerical simulations to investigate moisture-retention characteristics in the design of oxygen-limiting covers for reactive mine tailings. Can. Geotech. J. 1991, 28, 446–451. [Google Scholar] [CrossRef]
- Morel-Seytoux, H.J. L’effet de barrière capillaire à l’interface de deux couches de sol aux propriétés fort contrastées. Hydrol. Cont. 1992, 7, 117–128. [Google Scholar]
- Aubertin, M.; Bussière, B.; Aachib, M.; Chapuis, R.P.; Crespo, J.R. Une modélisation numérique des écoulements non saturés dans des couvertures multicouches en sols. Hydrogéologie 1996, 1, 3–13. [Google Scholar]
- Tibble, P.A.; Nicholson, R.V. Oxygen consumption on sulphide tailings and covers: Measured rates and applications. In Proceedings of the 4th International Confrence on Acid Rock Drainage, Vancouver, BC, Canada, 31 May–6 June 1997; Volume 2, pp. 647–661. [Google Scholar]
- Nastev, M.; Aubertin, M. Hydrogeological modelling for the reclamation work at the Lorraine mine site Québec. In Proceedings of the 1st Joint IAH-CNC-CGS Groundwater Specialty Conference, Montréal, QC, Canada, 15–18 October 2000; pp. 311–318. [Google Scholar]
- Neculita, C.M.; Zagury, G.J.; Bussière, B. Passive treatment of AMD in the bioreactors using sulfate-reducing bacteria: Critical review and research needs. J. Environ. Qual. 2007, 36, 1–16. [Google Scholar] [CrossRef]
- Genty, T.; Bussière, B.; Zagury, G.J.; Benzaazoua, M. Passivetreatment ofhighiron acid mine drainage using sulphate reducing bacteria: Comparison between eight biofilter mixtures. In Wolkersdorfer & Freud. In Proceedings of the IMW A, Sydney, NS, Canada, 5–9 September 2010. [Google Scholar]
- Skousen, J.; Zipper, C.E.; Rose, A.; Ziemkiewicz, P.F.; Naim, R.; McDonald, L.M.; Kleinmann, R.L. Review of passive systems for acid mine drainage treatment. Mine Waterandthe Environ. 2017, 36, 133–153. [Google Scholar] [CrossRef]
- Amos, R.T.; Blowes, D.W.; Bailey, B.L.; Sego, D.C.; Smith, L.; Ritchie, A.I.M. Waste-rock hydrogeology and geochemistry. Appl. Geochem. 2015, 57, 140–156. [Google Scholar] [CrossRef]
- RoyChowdhury, A.; Sarkar, D.; Datta, R. Remediation of acid mine drainage impacted water. Curr. Pollut. Rep. 2015, 1, 131–141. [Google Scholar] [CrossRef]


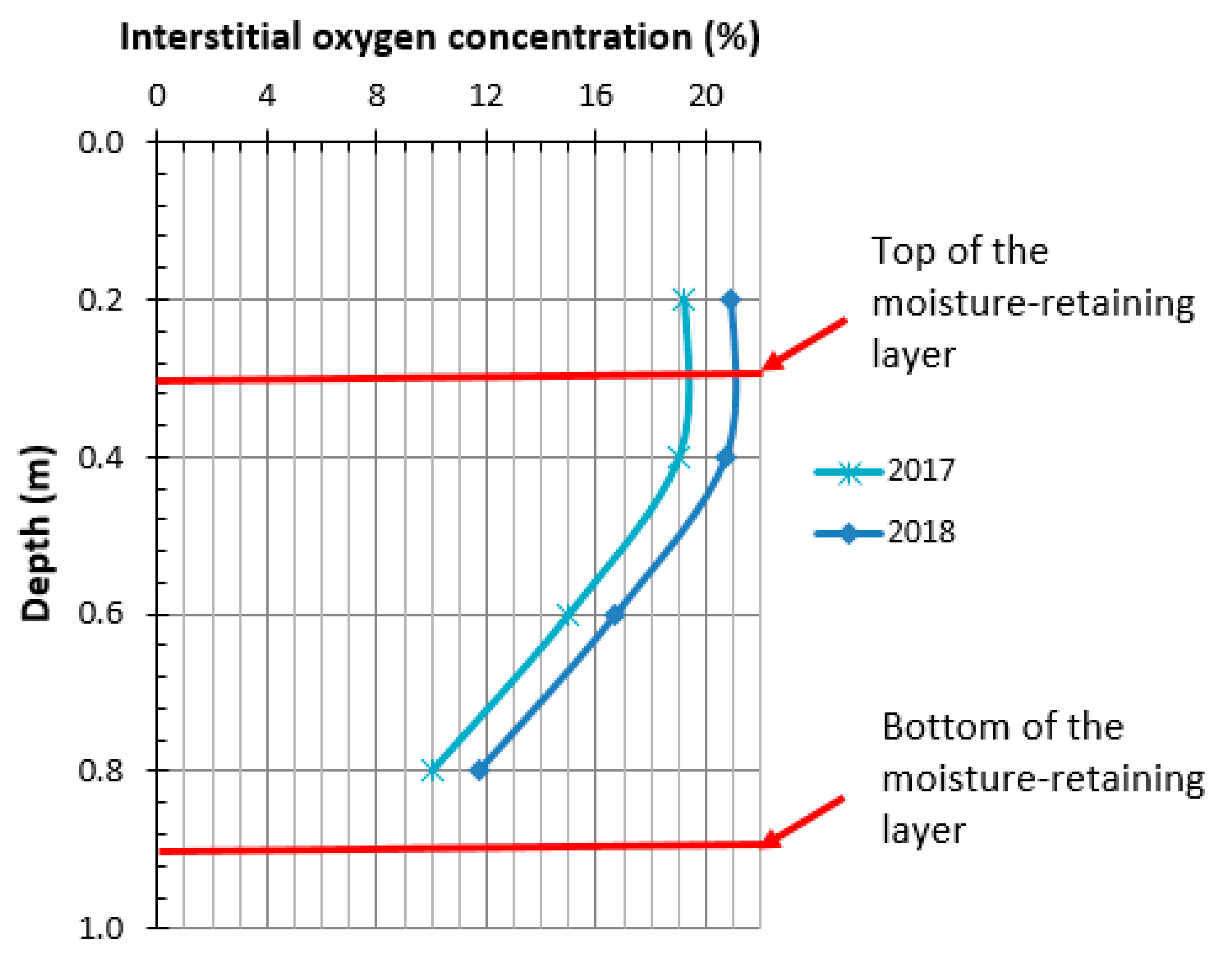
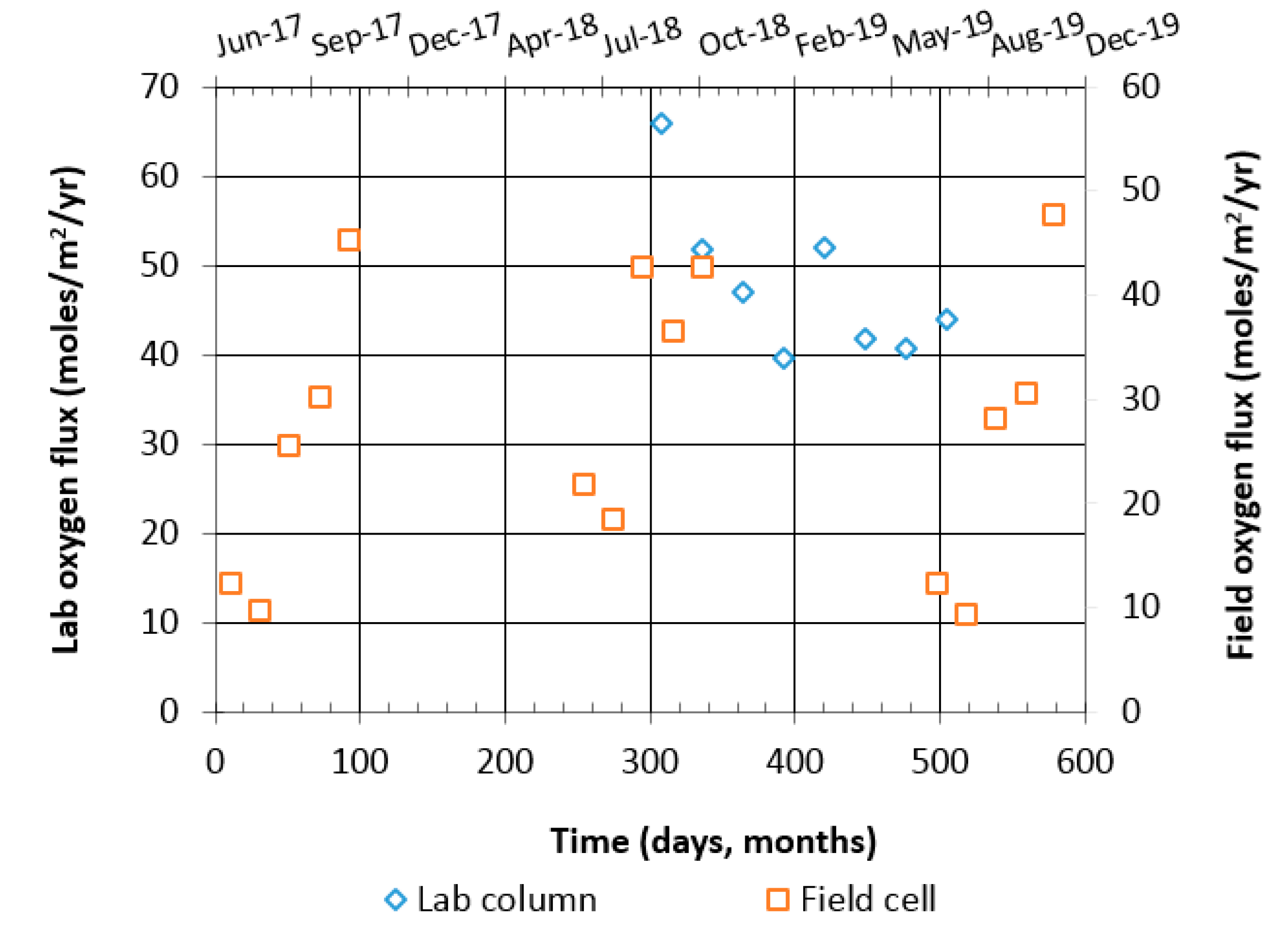
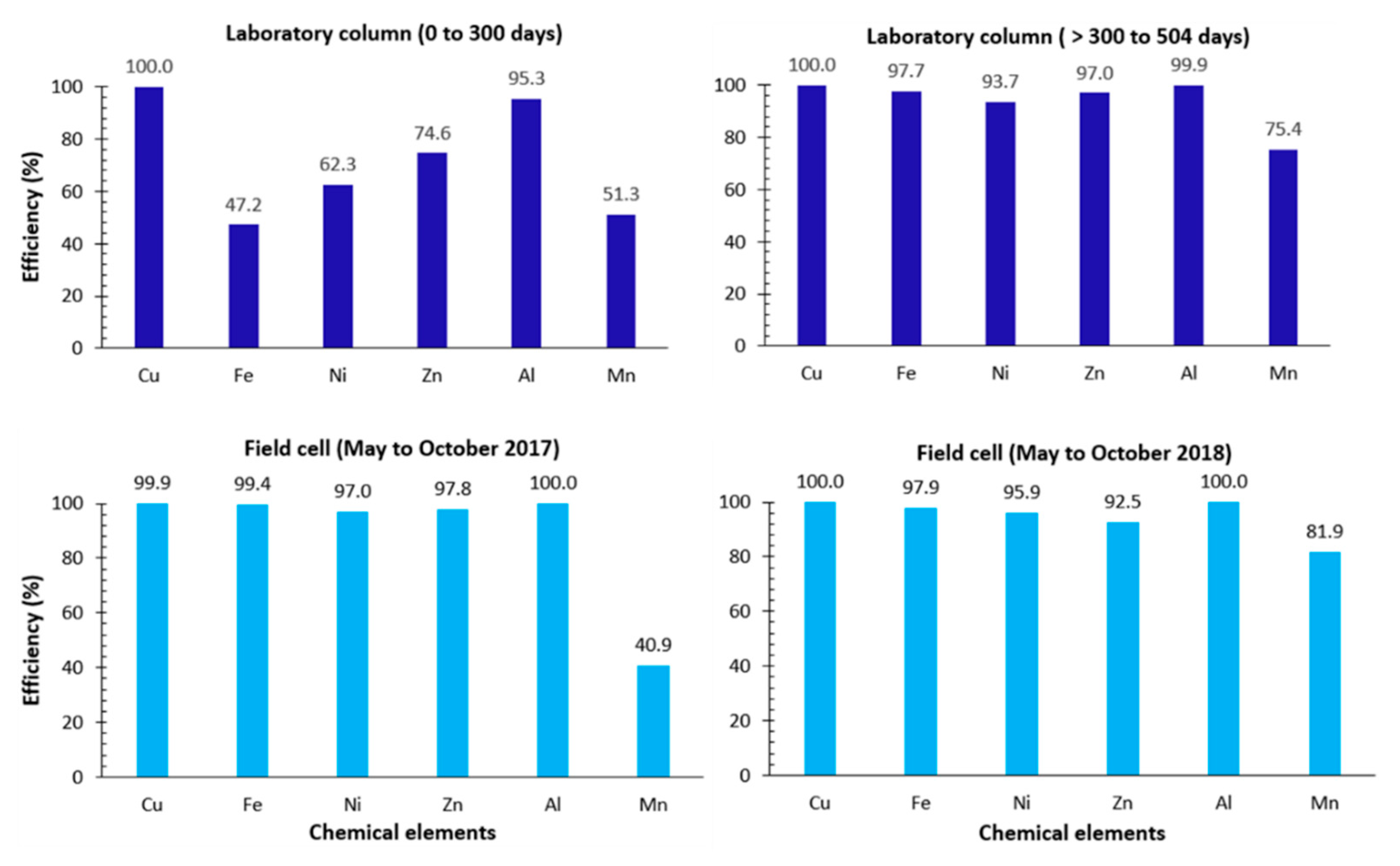
| Parameter | Reactive Tailings (TR) | Low-Sulfide Tailings (TG) | Waste Rock (WP) | Waste Rock (WL) |
|---|---|---|---|---|
| Mineralogy | Quartz 53% | Albite 52% | Quartz 68% | Albite 38% |
| Pyrite 24% | Quartz 22% | Albite 27% | Quartz 34% | |
| Albite 8.2% | Chlorite 13% | Muscovite 3.4% | Chlorite 14% | |
| Pyrrhotite 4.5% | Calcite 8.7% | Pyrite 1.4% | Actinolite 5% | |
| Chlorite 4.4% | Muscovite 2.8% | Calcite 4% | ||
| Sphalerite 2% | Dolomite 1.2% | Pyrite 0.5% | ||
| Other 4.5% | Other 1.2% | |||
| Stotal | 17% | 0.13% | 0.61% | 0.21% |
| Ctotal | 0.03% | 0.85% | 0.14% | 0.26% |
| NNP | −528 | 67 | −7 | 15 |
| Kr | 3.2 × 10−4/s | 4.2 × 10−6/s | - | - |
| USCS classification | Plastic silt (ML) | ML | well-graded sand (SW) | SW |
| GS (-) | 3.22 | 2.68 | 2.72 | 2.76 |
| ksat (cm/s) | 2 × 10−4 | 5 × 10−5 | 3 × 10−2 | 2 × 10−2 |
| Monitoring Settings | Laboratory Column | Field Cell | ||
|---|---|---|---|---|
| Equipment | Details | Equipment | Details | |
| Hydrogeological parameters | ||||
| θ | EC5 and GS3 | Accuracy: ±0.03; specific calibration curves | 5TM and GS3 | Accuracy: ±0.03; specific calibration curves |
| ψ | WATERMARK sensors | Accuracy: ±1 kPa; measurement range: 0–200 kPa | WATERMARK sensors | Accuracy: ±1 kPa; measurement range: 0–200 kPa |
| O2 consumption test (OCT) | 10 cm void above column and an apogee SO-110 oxygen sensor | Precision (good for oxygen flux >10 moles of O2/m2/yr). Allow to measure a nearly instantaneous oxygen flux | 15 cm cylinder and an apogee SO-110 oxygen sensor | Precision (good for oxygen flux >10 moles of O2/m2/yr). Allow to measure a nearly instantaneous oxygen flux |
| O2 gradient method | Not measured | Sampling tubes, optical sensor, and OXY 10 system | ||
| Exfiltration water | Bucket | Tote tank | ||
| Water quality parameters | ||||
| pH, EC, and temperature | Oakton pHTestr®30 tester | Accuracy: ±0.01 (pH) and ±0.5% (EC). | Oakton pHTestr®30 tester | Accuracy: ±0.01 (pH) and ±0.5% (EC). |
| Eh | Oakton ORPTestr®30 tester | Range: −999 mV to 1000 mV Resolution: 1 mV Accuracy: ±2 mV | Oakton ORPTestr®30 tester | Range: −999 mV to 1000 mV Resolution: 1 mV Accuracy: ±2 mV |
| Alkalinity and acidity | Metrohm 848 Titrino plus automatic titrator | High precision and accuracy (±15%.) | Metrohm 848 Titrino plus automatic titrator | High precision and accuracy (±15%). |
| Sulfate anion | 850 Professional IC Anion–MCS | Detection limit: 1 mg/L | 850 Professional IC Anion–MCS | Detection limit: 1 mg/L |
| Metals concentration | ICP-AES analysis | Detection limit depending of the chemical elements (0.001 to 0.1 mg/L) | ICP-AES analysis | Detection limit depending of the chemical elements (0.001–0.1 mg/L) |
| Overview of Suction | Laboratory Column | ||||
|---|---|---|---|---|---|
| Suctions (kPa) | TG—0.50 m | TG—0.80 m | TR | WL | WP |
| Min/Max | 12/18 | 7/16 | 0/27 | 5/80 | 1/10 |
| Mean | 16 | 14 | 6 | 32 | 8 |
| Field experimental cell | |||||
| Min/Max | 2/26 | 1/23 | 2/18 | 3/27 | 3/19 |
| Mean | 13 | 9 | 9 | 15 | 10 |
| Physicochemical Parameters | Lab Control Column | Lab Column with CCBE | Field Control Cell | Field Cell with CCBE | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min/Max | Mean | STD | Min/Max | Mean | STD | Min/Max | Mean | STD | Min/Max | Mean | STD | |
| pH | 1.2/2.0 | 1.6 | 0.23 | 1.9/7.8 | 5.4 | 1.7 | 1.1/5.1 | 2.3 | 0.77 | 5.1/7.5 | 6.5 | 0.56 |
| Fe (mg/L) | 65/3210 | 1560 | 1142 | 1.7/1400 | 373 | 424 | 495/14700 | 4325 | 4050 | 9.5/491 | 140 | 148 |
| Zn (mg/L) | 7/2940 | 230 | 662 | 0/232 | 29 | 73 | 57/368 | 163 | 100 | 4.3/40 | 16 | 14 |
| Sulfates (mg/L) | 1533/22,000 | 6300 | 4424 | 364/25,951 | 3523 | 5511 | 4660/47,644 | 18,022 | 10,083 | 58/3559 | 2219 | 896 |
| Ca (mg/L) | 23/660 | 300 | 214 | 427/693 | 557 | 71.4 | 307/447 | 369 | 41 | 491/614 | 540 | 35 |
| Mg (mg/L) | 0.8/400 | 50 | 87 | 17/80 | 43 | 20 | 69/621 | 298 | 150 | 57/104 | 76 | 15 |
| As (mg/L) | 0.06/0.48 | 0.14 | 0.12 | <DLM | N.D. | N.D. | 0.06/34 | 3.9 | 7.5 | <DLM | N.D. | N.D. |
| Cu (mg/L) | 8.53/72 | 32 | 19 | <DLM | N.D. | N.D. | 0.3/73 | 15 | 15 | <DLM | N.D. | N.D. |
| Ni (mg/L) | 0.028/30 | 2.0 | 7.0 | 0.004/2.75 | 0.36 | 0.86 | 0.7/3.0 | 2.0 | 0.6 | 0.02/0.33 | 0.11 | 0.06 |
| Pb (mg/L) | 0.13/2.0 | 0.5 | 0.40 | 0.020/0.76 | 0.30 | 0.28 | 0.34/35 | 7.0 | 0.79 | <DLM | N.D. | N.D. |
| S (mg/L) | 650/7600 | 2100 | 1527 | 516/1660 | 855 | 14 | 0.1/11,900 | 5618 | 3615 | 0.1/933 | 674 | 223 |
| EC (mS/cm) | 2.5/11 | 5 | 2.3 | 1.2/14 | 2.8 | 2.7 | 4.2/20 | 10 | 4.3 | 1.4/7.7 | 2.8 | 1.2 |
| Alkalinity | <DLM | N.D. | N.D. | 0/147 | 44 | 58 | <DLM | N.D. | N.D. | 1/219 | 95 | 85 |
| Acidity | 900/14,000 | 1500 | 3542 | 21/1470 | 553 | 481 | 447/34,020 | 15864 | 11128 | 23/1136 | 360 | 375 |
| Parameters | Analytical Solution | Oxygen Gradient Method | Sulfate-Release Method | Oxygen Consumption Test Method | ||||
|---|---|---|---|---|---|---|---|---|
| Lab column | Field cell | Lab column | Field cell | Lab column | Field cell | Lab column | Field cell | |
| Porosity (n) | 0.39 | 0.40 | ||||||
| Effective diffusion coefficients (De, m2/s) | 2 × 10−12 | 2 × 10−11 | ||||||
| Pyrite content over mass of dry tailings (Cp, kg/kg) | 2.5 × 10−3 | 2.5 × 10−3 | ||||||
| Fcontrol (moles/m2/yr) | 650 | 750 | 650 | 750 | 650 | 750 | 650 | 750 |
| Fbase cover (moles/m2/yr) | 1 × 10−3 | 6 × 10−3 | N.C. | 4.5 × 10−1 | 49 | 28 | 25 | 35 |
| Efficiency (%) | 99.9 | 99.9 | N.C. | 99.9 | 92.5 | 96.3 | 96.2 | 95.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalonji-Kabambi, A.; Bussière, B.; Demers, I. Hydrogeochemical Behavior of Reclaimed Highly Reactive Tailings, Part 2: Laboratory and Field Results of Covers Made with Mine Waste Materials. Minerals 2020, 10, 589. https://doi.org/10.3390/min10070589
Kalonji-Kabambi A, Bussière B, Demers I. Hydrogeochemical Behavior of Reclaimed Highly Reactive Tailings, Part 2: Laboratory and Field Results of Covers Made with Mine Waste Materials. Minerals. 2020; 10(7):589. https://doi.org/10.3390/min10070589
Chicago/Turabian StyleKalonji-Kabambi, Alex, Bruno Bussière, and Isabelle Demers. 2020. "Hydrogeochemical Behavior of Reclaimed Highly Reactive Tailings, Part 2: Laboratory and Field Results of Covers Made with Mine Waste Materials" Minerals 10, no. 7: 589. https://doi.org/10.3390/min10070589
APA StyleKalonji-Kabambi, A., Bussière, B., & Demers, I. (2020). Hydrogeochemical Behavior of Reclaimed Highly Reactive Tailings, Part 2: Laboratory and Field Results of Covers Made with Mine Waste Materials. Minerals, 10(7), 589. https://doi.org/10.3390/min10070589






