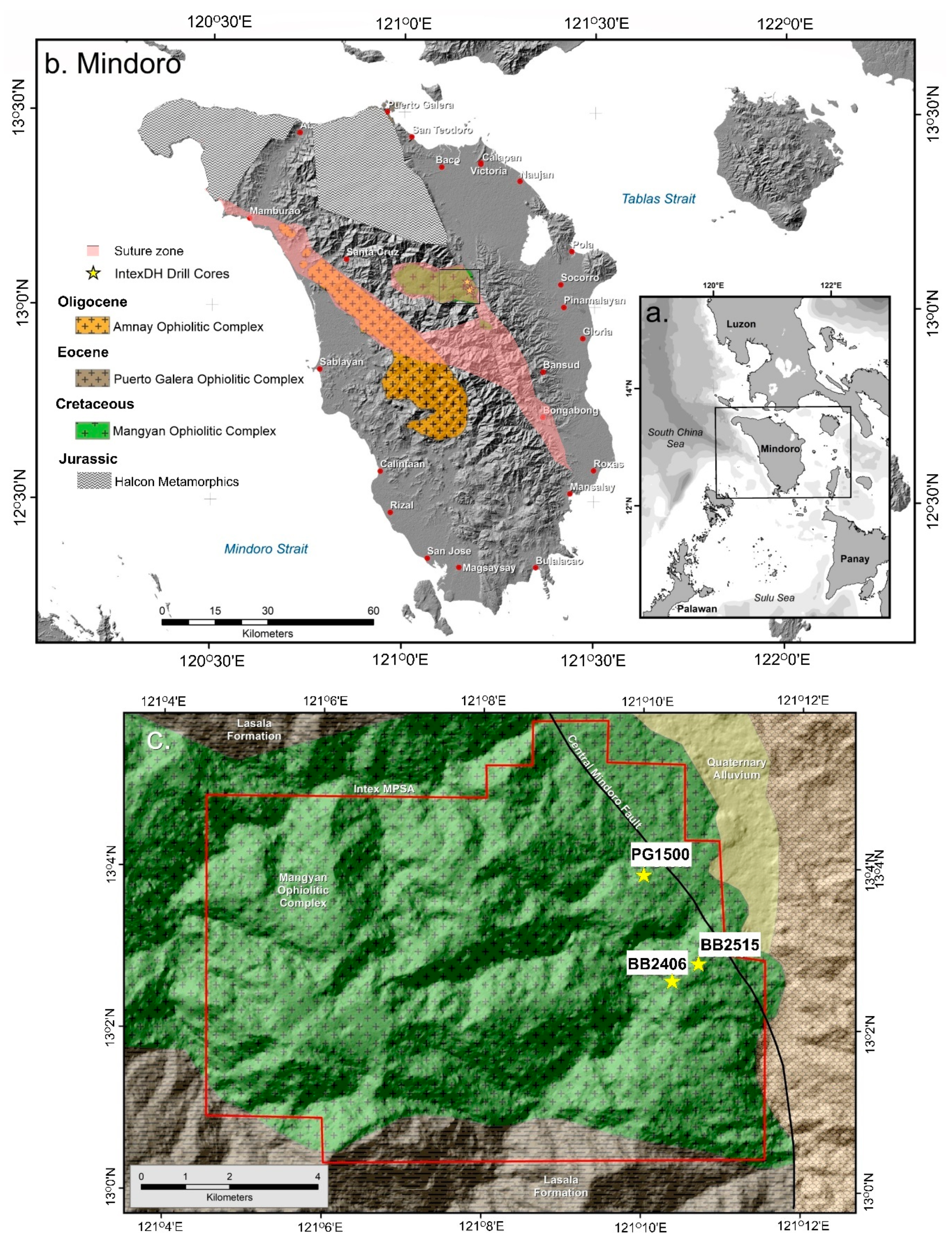
Figure 1.
Location map of (a) Mindoro (b) ophiolitic complexes on the island and (c) studied drill cores.
Figure 1.
Location map of (a) Mindoro (b) ophiolitic complexes on the island and (c) studied drill cores.
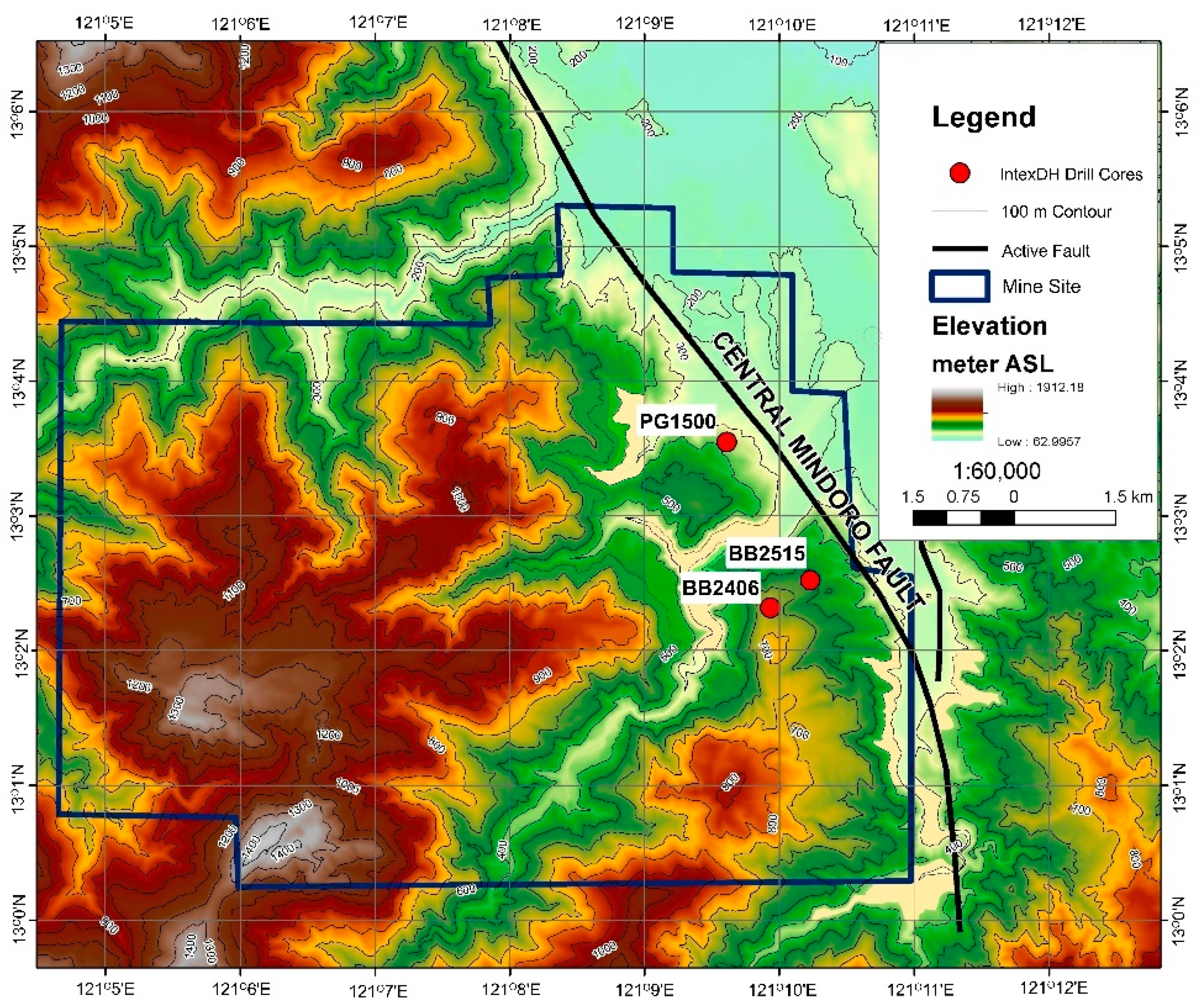
Figure 2.
Relief map showing the studied drill cores within the Intex’s exploration project area.
Figure 2.
Relief map showing the studied drill cores within the Intex’s exploration project area.
Figure 3.
Photos of (a) serpentinized lherzolite (BB2515 core), (b) harzburgite-dunite (BB2406 core), and (c) clinopyroxenite (PG1500 core), (d) earthy material including pebble-sized rock remnants (e) silica-bearing limonite sample from the limonite horizon, and (f) dark orange highly weathered materials.
Figure 3.
Photos of (a) serpentinized lherzolite (BB2515 core), (b) harzburgite-dunite (BB2406 core), and (c) clinopyroxenite (PG1500 core), (d) earthy material including pebble-sized rock remnants (e) silica-bearing limonite sample from the limonite horizon, and (f) dark orange highly weathered materials.
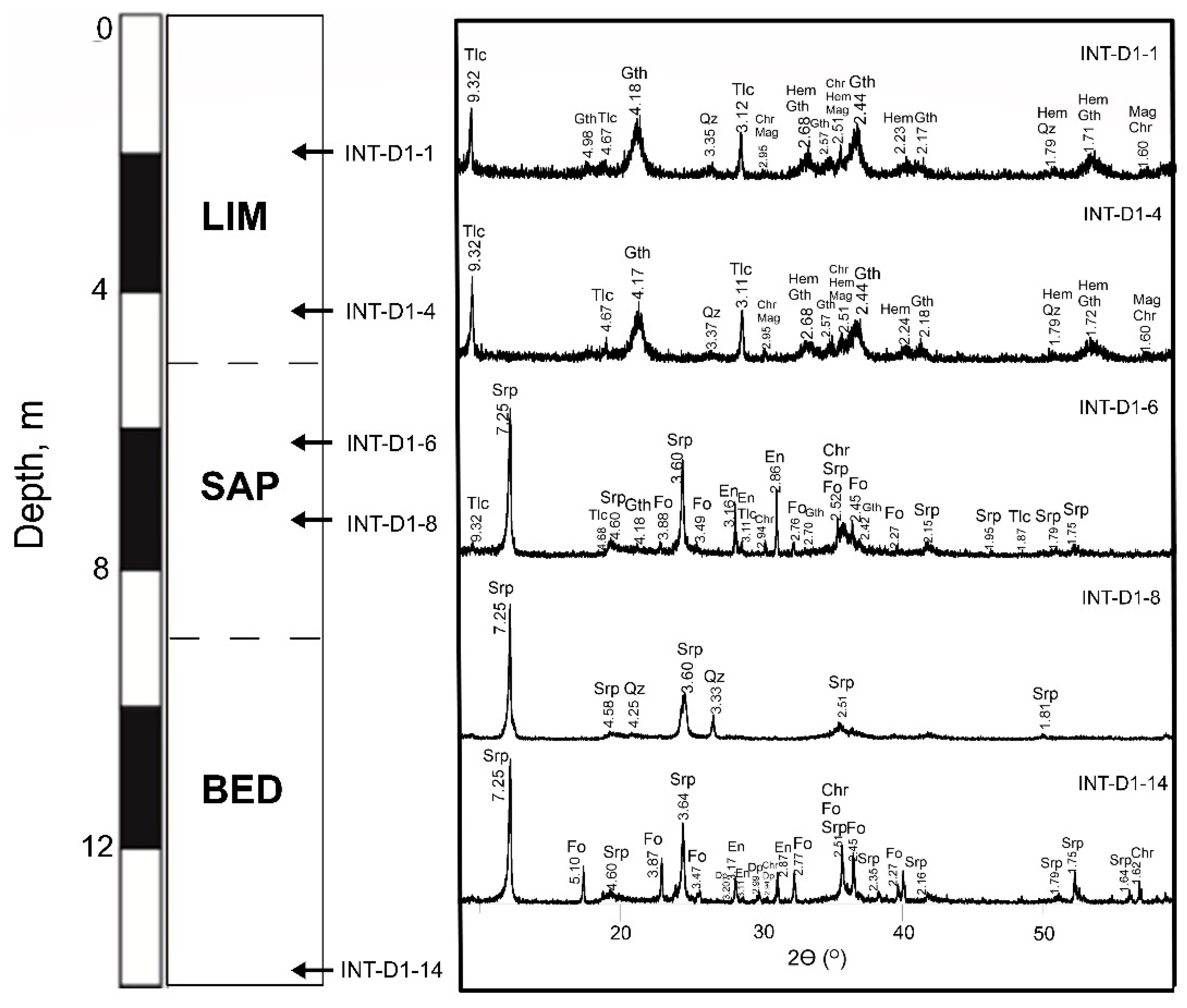
Figure 4.
Stacked XRPD patterns of bedrock, saprolite, and limonite representative samples from BB2515 drill core. Fo—forsterite, En—enstatite, Srp—serpentine, Tlc—talc, Qz—quartz, Chr—chromite, Mag—magnetite, Gth—goethite, Hem—hematite.
Figure 4.
Stacked XRPD patterns of bedrock, saprolite, and limonite representative samples from BB2515 drill core. Fo—forsterite, En—enstatite, Srp—serpentine, Tlc—talc, Qz—quartz, Chr—chromite, Mag—magnetite, Gth—goethite, Hem—hematite.
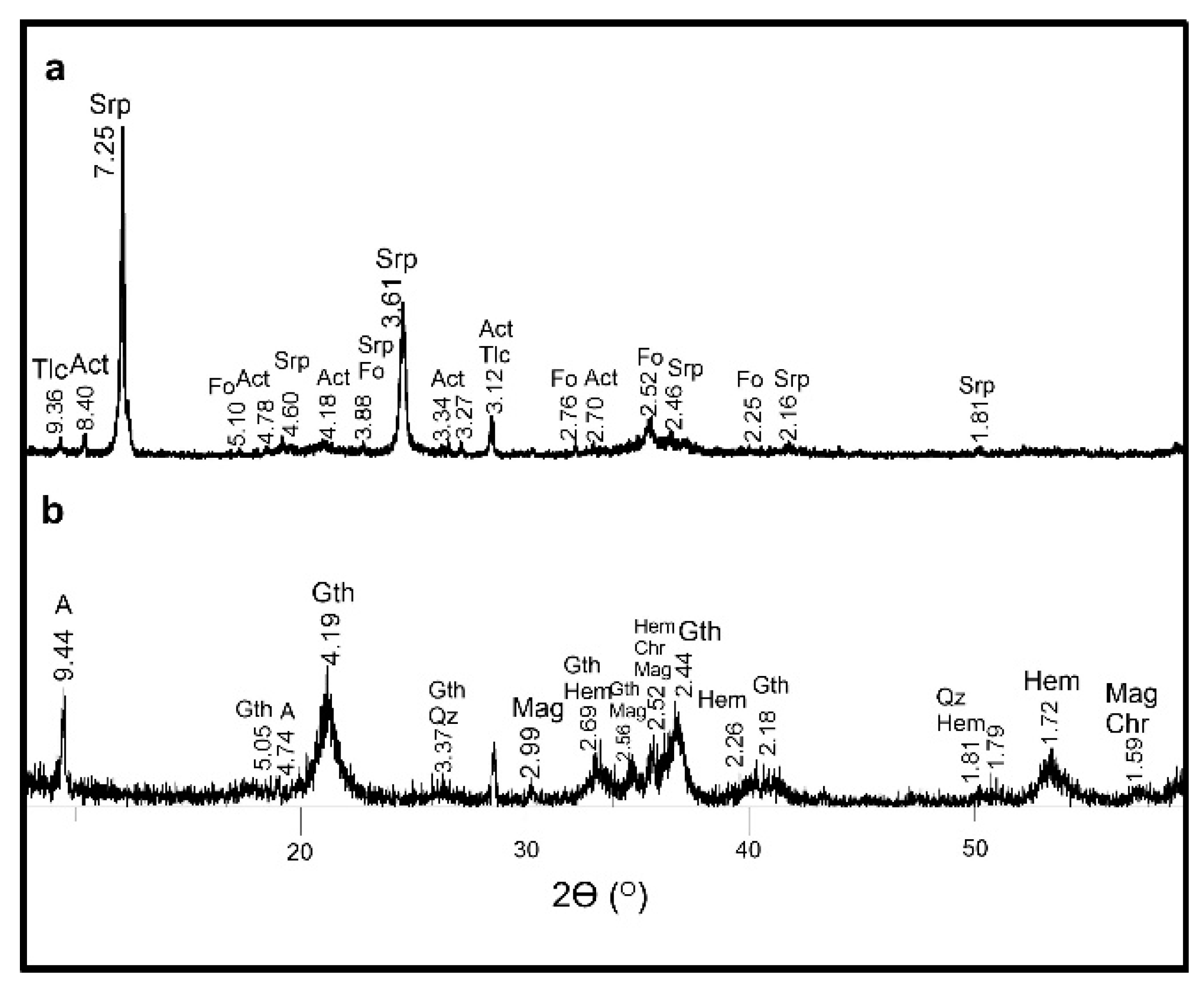
Figure 5.
XRPD patterns of samples showing the (a) occurrence of serpentine, actinolite, talc and relict olivine, and (b) presence of asbolane with goethite, hematite, magnetite, chromite, and quartz. Srp—serpentine, Act—actinolite, Fo—forsterite, Tlc—talc, A—asbolane, Gth—goethite, Mag—magnetite, Hem—hematite, Chr—chromite, Qz—quartz.
Figure 5.
XRPD patterns of samples showing the (a) occurrence of serpentine, actinolite, talc and relict olivine, and (b) presence of asbolane with goethite, hematite, magnetite, chromite, and quartz. Srp—serpentine, Act—actinolite, Fo—forsterite, Tlc—talc, A—asbolane, Gth—goethite, Mag—magnetite, Hem—hematite, Chr—chromite, Qz—quartz.
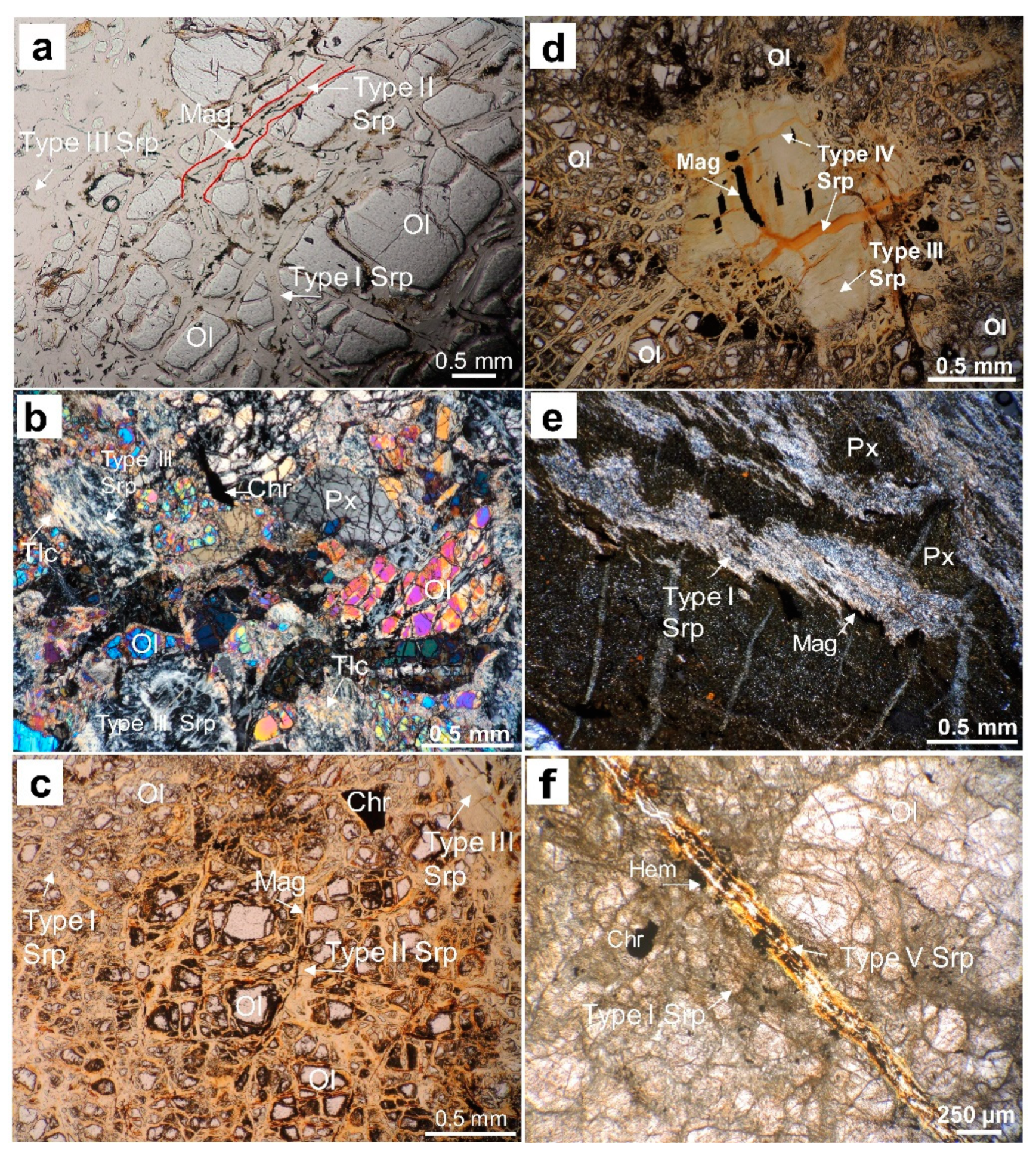
Figure 6.
Photomicrographs of (a,b) type I, II and III serpentine veins cutting primary olivine, pyroxene, chromite, and Fe-oxide in lherzolite (BB2515 core); serpentine veins in the saprock developed above (c) lherzolite (BB2515 core), (d) harzburgite-dunite (BB2406 core), and (e,f) clinopyroxenite (PG1500 core). Ol—olivine, Px—pyroxene, Srp—serpentine, Tlc—talc, Chr—chromite, Gth—goethite.
Figure 6.
Photomicrographs of (a,b) type I, II and III serpentine veins cutting primary olivine, pyroxene, chromite, and Fe-oxide in lherzolite (BB2515 core); serpentine veins in the saprock developed above (c) lherzolite (BB2515 core), (d) harzburgite-dunite (BB2406 core), and (e,f) clinopyroxenite (PG1500 core). Ol—olivine, Px—pyroxene, Srp—serpentine, Tlc—talc, Chr—chromite, Gth—goethite.
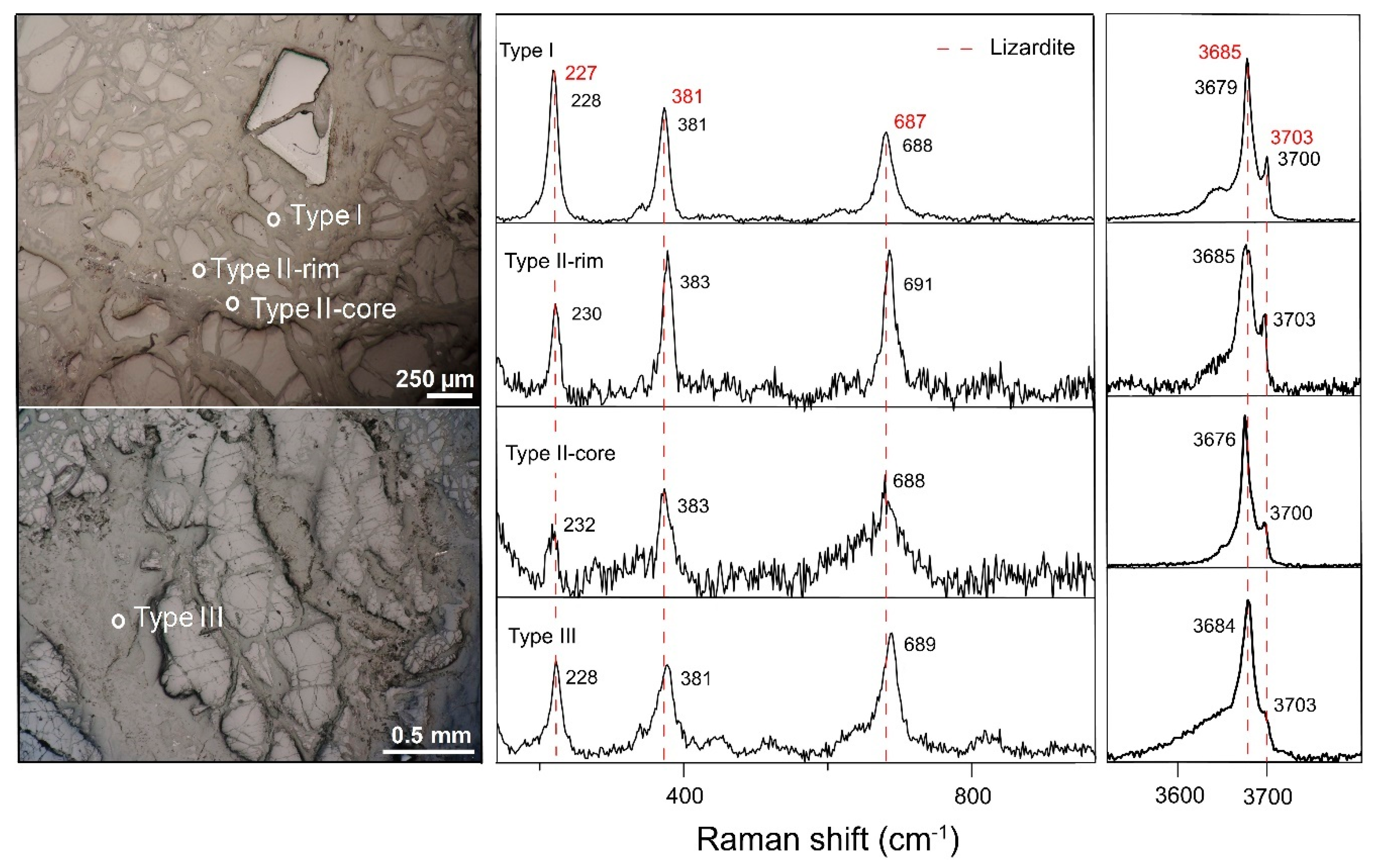
Figure 7.
Raman signatures of type I, type II and type III vein serpentines identified in the lherzolite bedrock. Red dashed lines indicate the Raman bands from previous work [
21].
Figure 7.
Raman signatures of type I, type II and type III vein serpentines identified in the lherzolite bedrock. Red dashed lines indicate the Raman bands from previous work [
21].
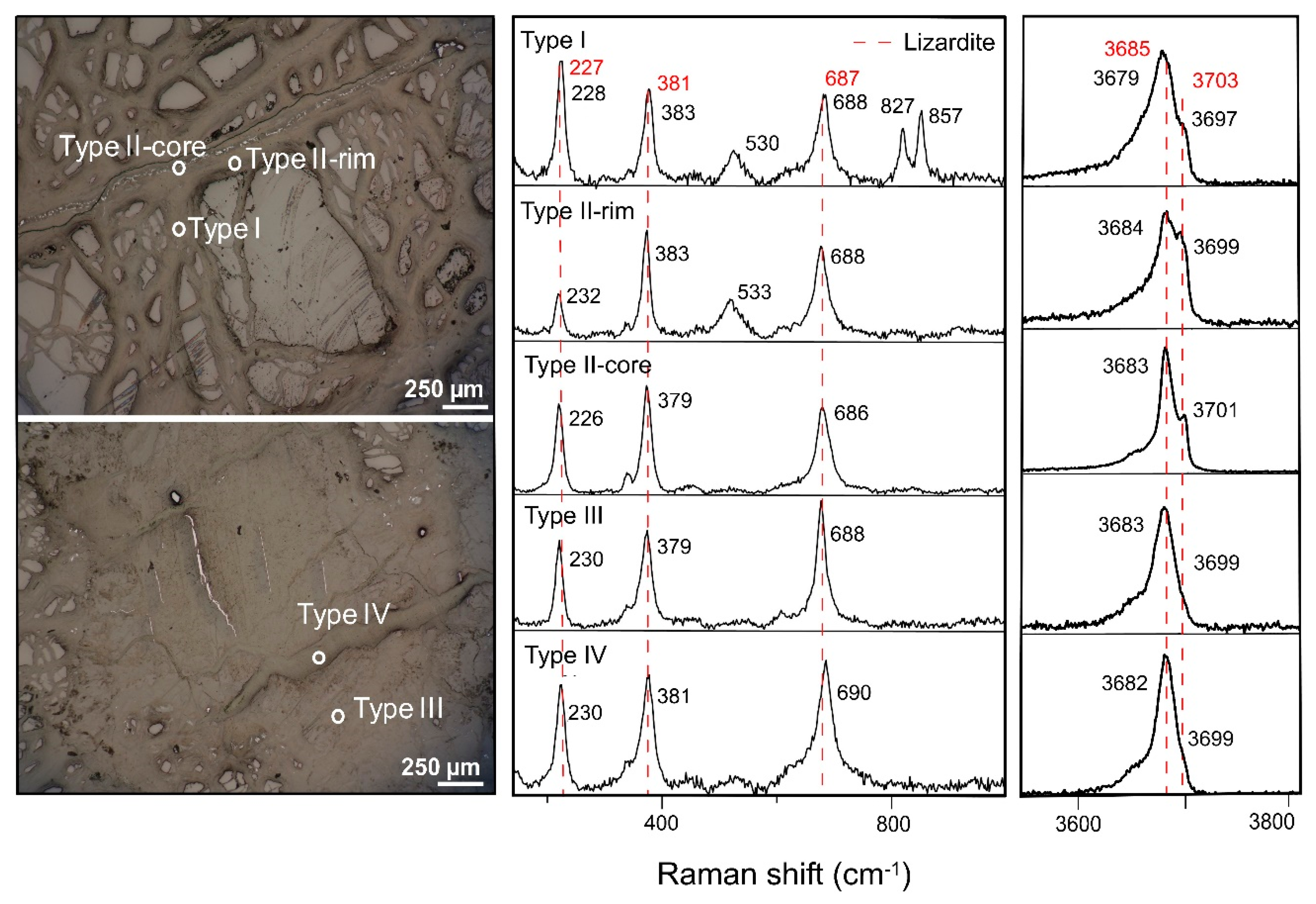
Figure 8.
Raman signatures of type I, type II, type III and type IV vein serpentines identified in the saprock derived from harzburgite-dunite (BB2406 core). Red dashed lines indicate the Raman bands from previous work [
21].
Figure 8.
Raman signatures of type I, type II, type III and type IV vein serpentines identified in the saprock derived from harzburgite-dunite (BB2406 core). Red dashed lines indicate the Raman bands from previous work [
21].
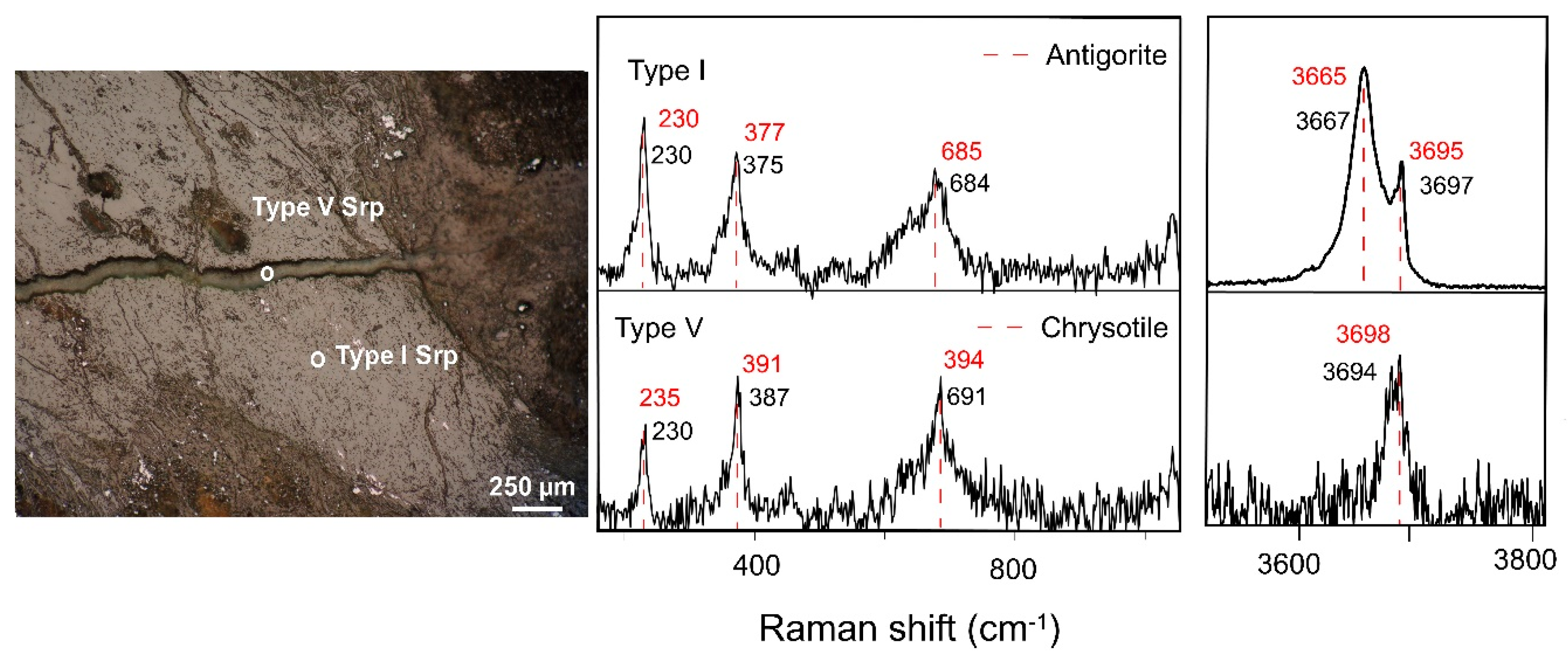
Figure 9.
Raman signatures of type I and type V vein serpentines identified in the saprock developed above clinopyroxenite bedrock (PG1500 core). Red dashed lines indicate the Raman bands from previous work [
21,
39].
Figure 9.
Raman signatures of type I and type V vein serpentines identified in the saprock developed above clinopyroxenite bedrock (PG1500 core). Red dashed lines indicate the Raman bands from previous work [
21,
39].
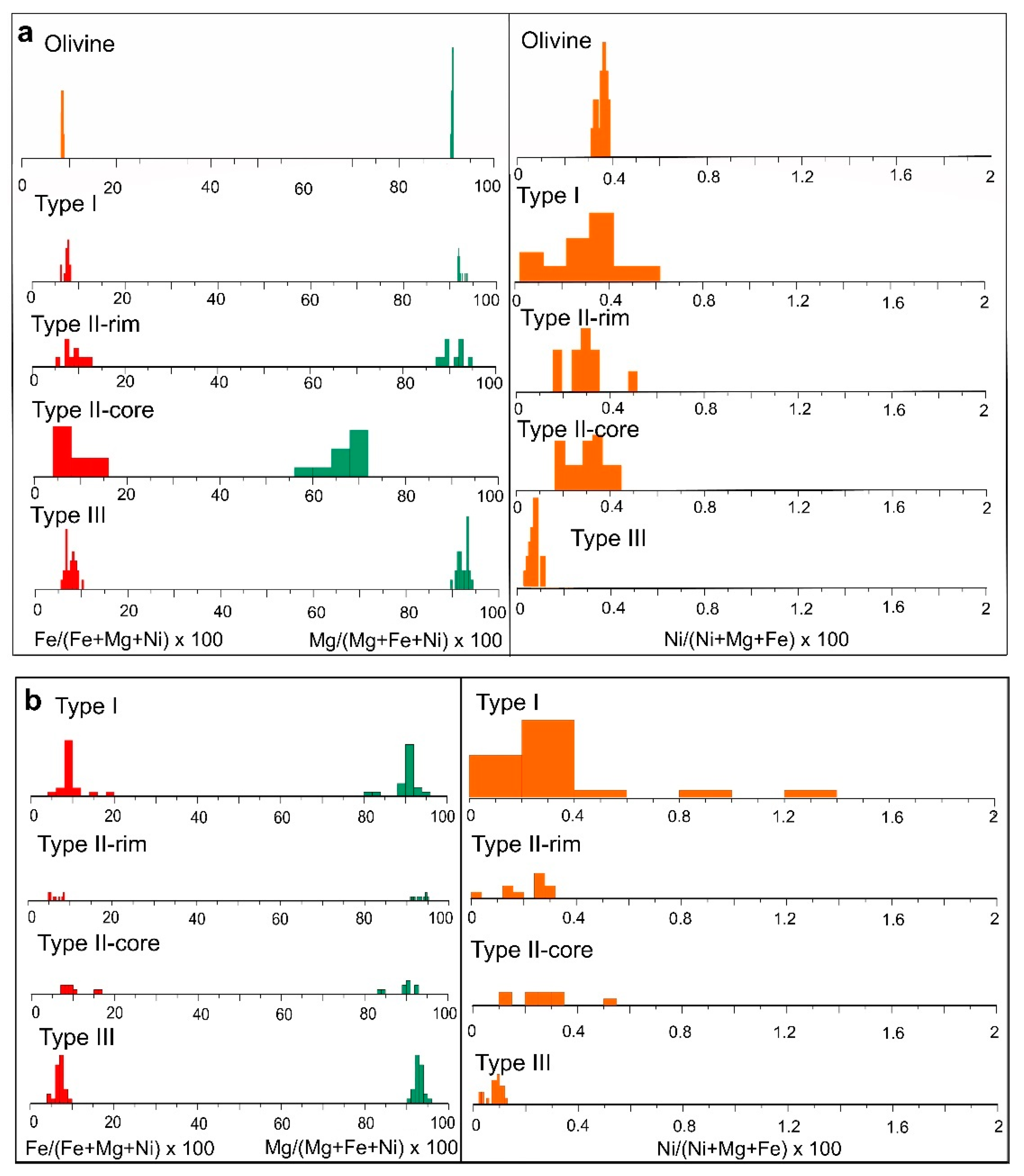
Figure 10.
Histogram of Mg (green), Fe (red) and Ni (orange) mol ratio in olivine and the different types of serpentine identified in the (a) lherzolite and (b) harzburgite-dunite bedrock.
Figure 10.
Histogram of Mg (green), Fe (red) and Ni (orange) mol ratio in olivine and the different types of serpentine identified in the (a) lherzolite and (b) harzburgite-dunite bedrock.
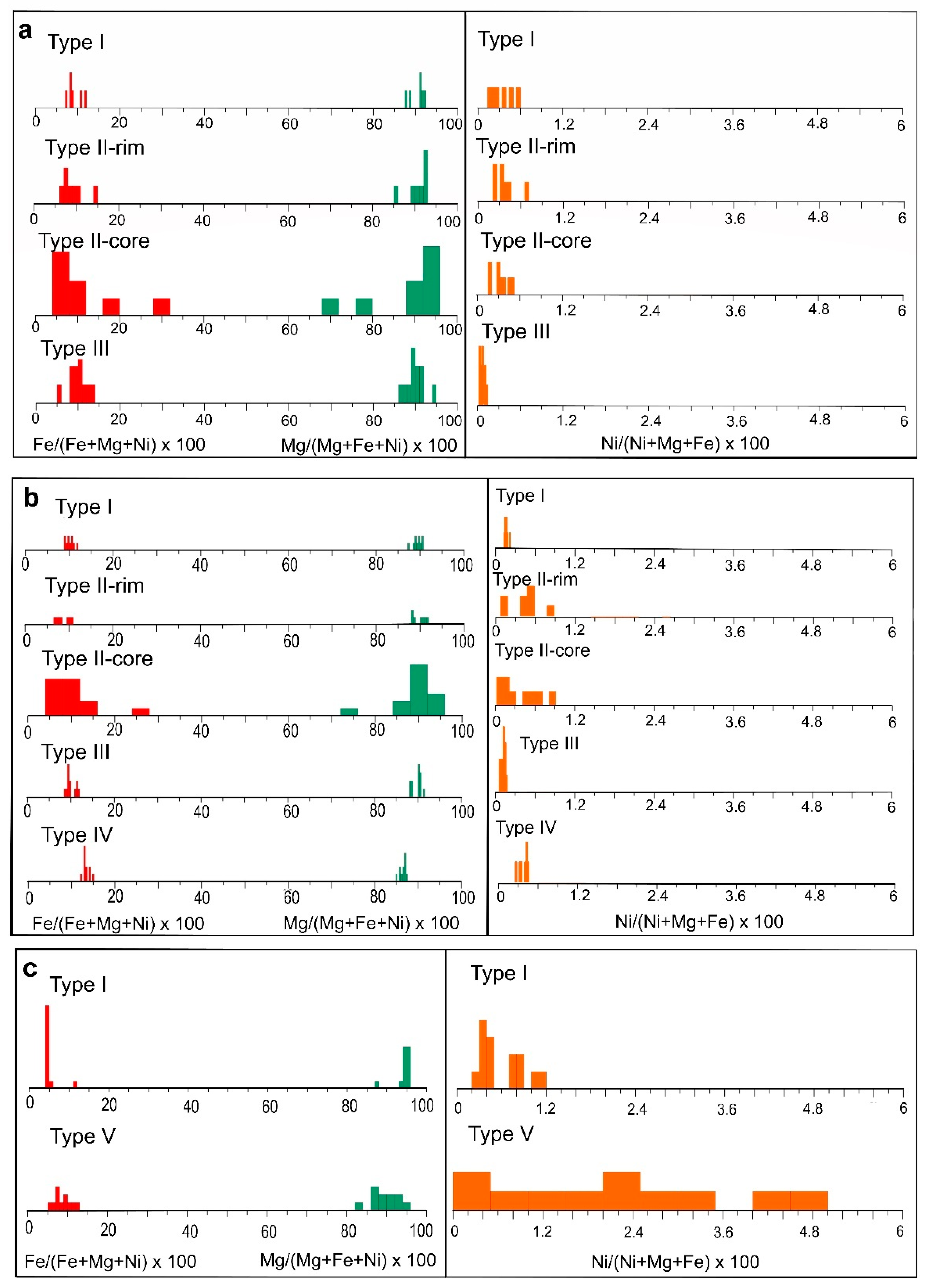
Figure 11.
Histogram of Mg (green), Fe (red) and Ni (orange) mol ratio in the different types of serpentine identified in the saprock derived from (a) lherzolite (b) harzburgite-dunite and (c) clinopyroxenite bedrock.
Figure 11.
Histogram of Mg (green), Fe (red) and Ni (orange) mol ratio in the different types of serpentine identified in the saprock derived from (a) lherzolite (b) harzburgite-dunite and (c) clinopyroxenite bedrock.
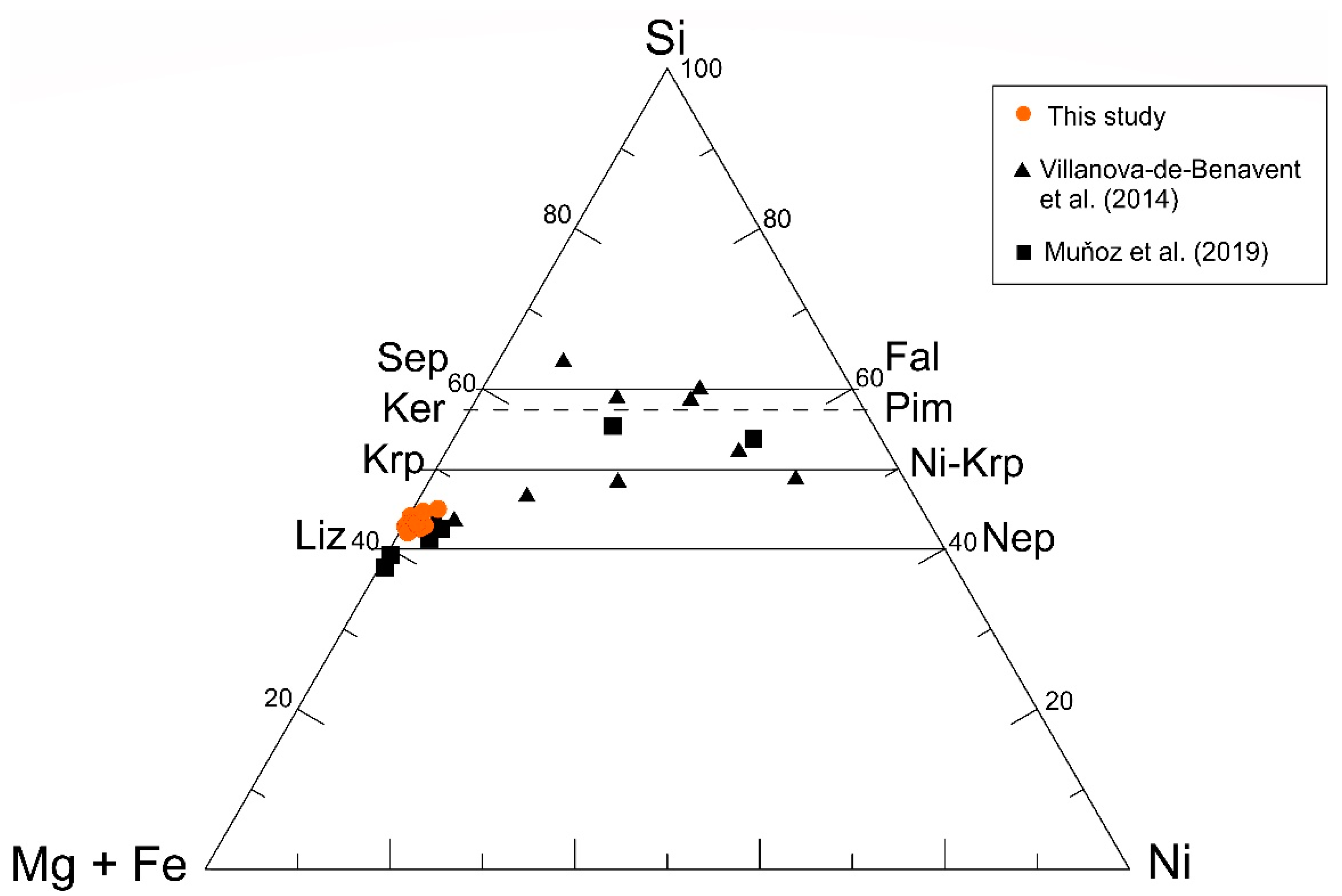
Figure 12.
Si-Mg-(+Fe)-Ni plot showing the composition of serpentine-like garnierite in this study and previous works. Lz—lizardite, Nep—népouite, Krp—karpinskite, Ni-Krp—Ni-karpinskite, Ker—kerolite, Pim—pimelite, Sep—sepiolite, Fal—falcondoite.
Figure 12.
Si-Mg-(+Fe)-Ni plot showing the composition of serpentine-like garnierite in this study and previous works. Lz—lizardite, Nep—népouite, Krp—karpinskite, Ni-Krp—Ni-karpinskite, Ker—kerolite, Pim—pimelite, Sep—sepiolite, Fal—falcondoite.
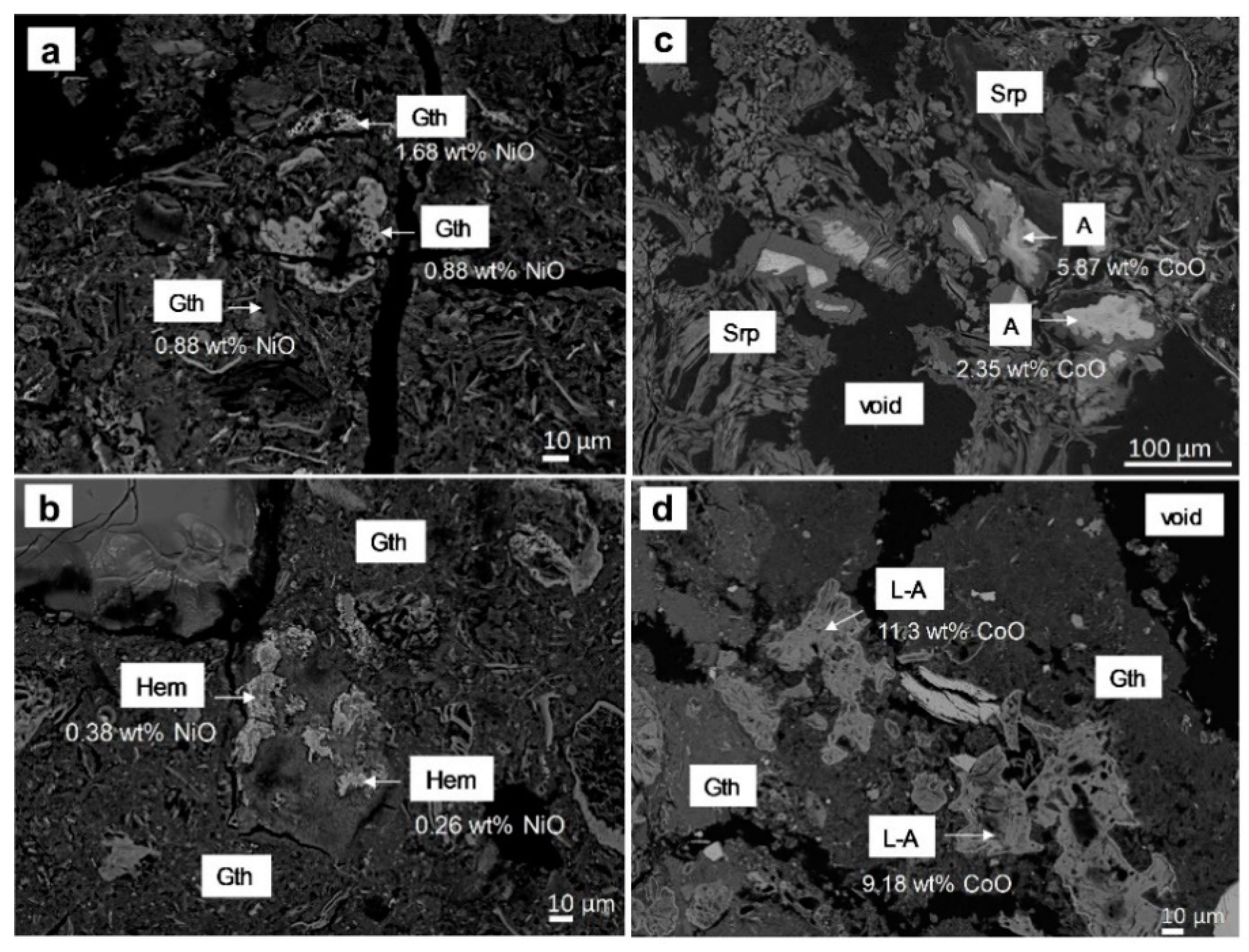
Figure 13.
Backscattered electron images of (a) massive goethite (Gth) irregular aggregates of (b) hematite (Hem), (c) asbolane (A) in a serpentine (Srp) matrix and (d) lithiophorite-asbolane intermediate (L-A) in a goethite matrix.
Figure 13.
Backscattered electron images of (a) massive goethite (Gth) irregular aggregates of (b) hematite (Hem), (c) asbolane (A) in a serpentine (Srp) matrix and (d) lithiophorite-asbolane intermediate (L-A) in a goethite matrix.
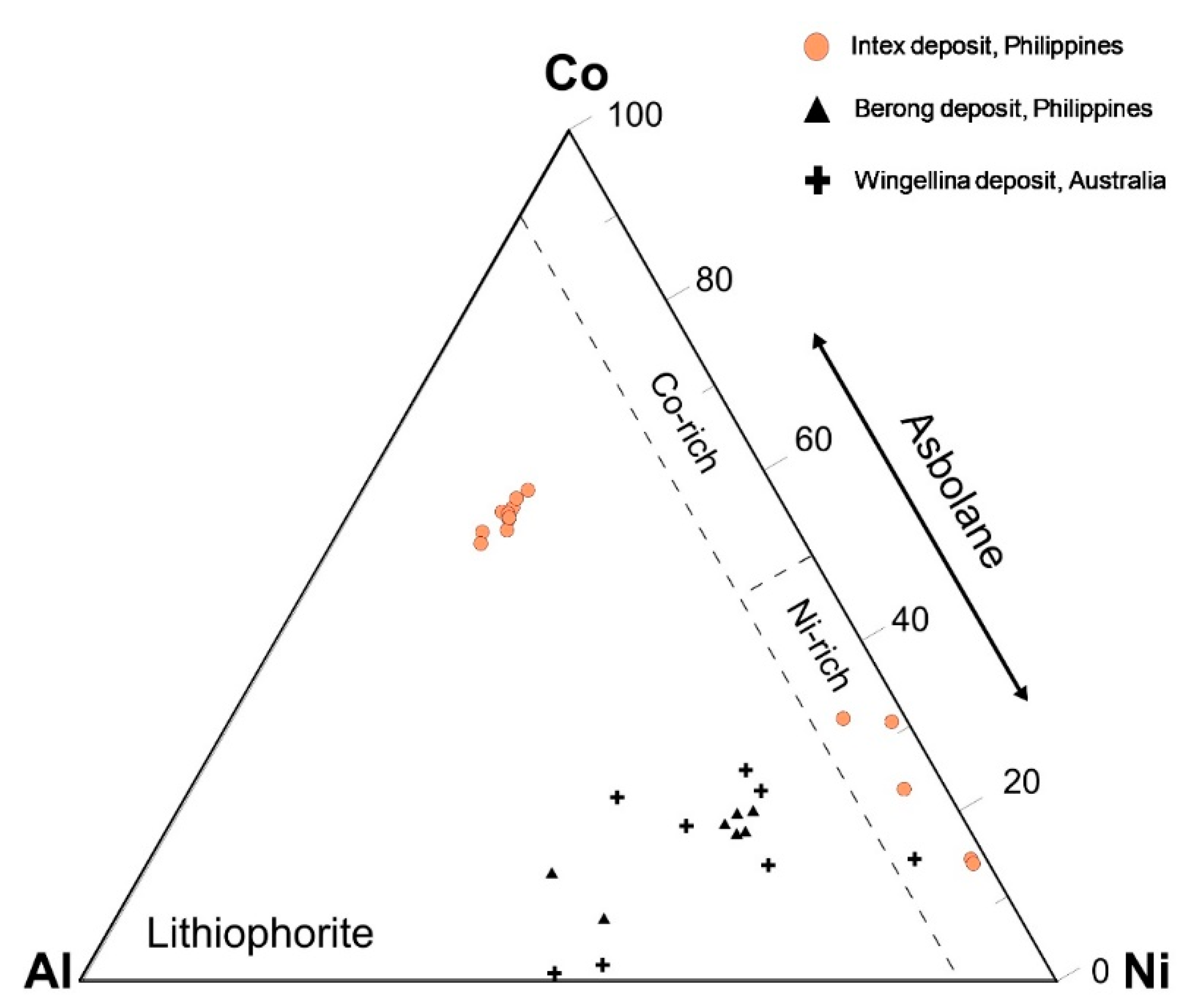
Figure 14.
Co-Ni-Al ternary diagram (wt%) displaying the mineralogy and composition of asbolane and lithiophorite-asbolane intermediate in the limonite horizon of the Intex deposit.
Figure 14.
Co-Ni-Al ternary diagram (wt%) displaying the mineralogy and composition of asbolane and lithiophorite-asbolane intermediate in the limonite horizon of the Intex deposit.
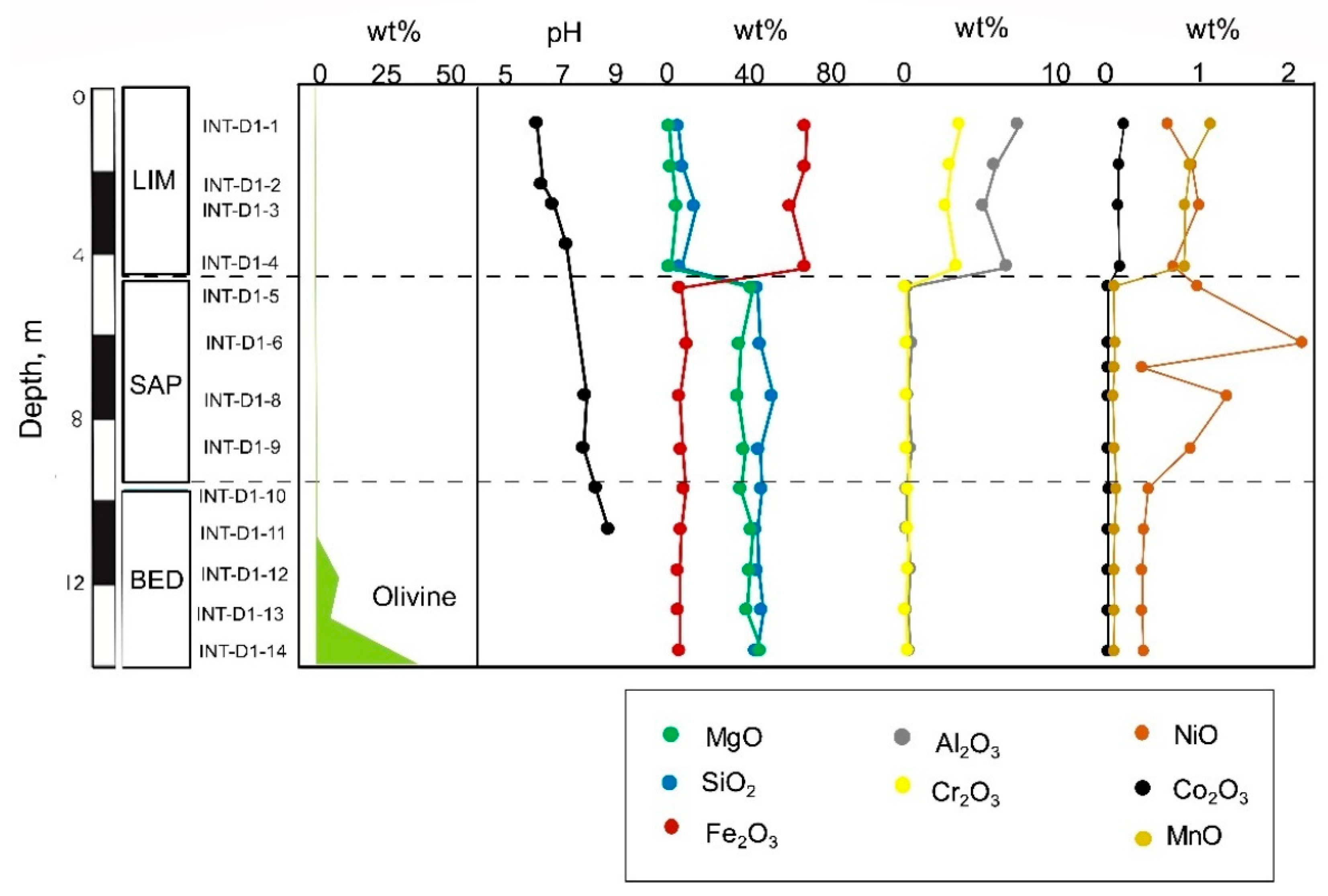
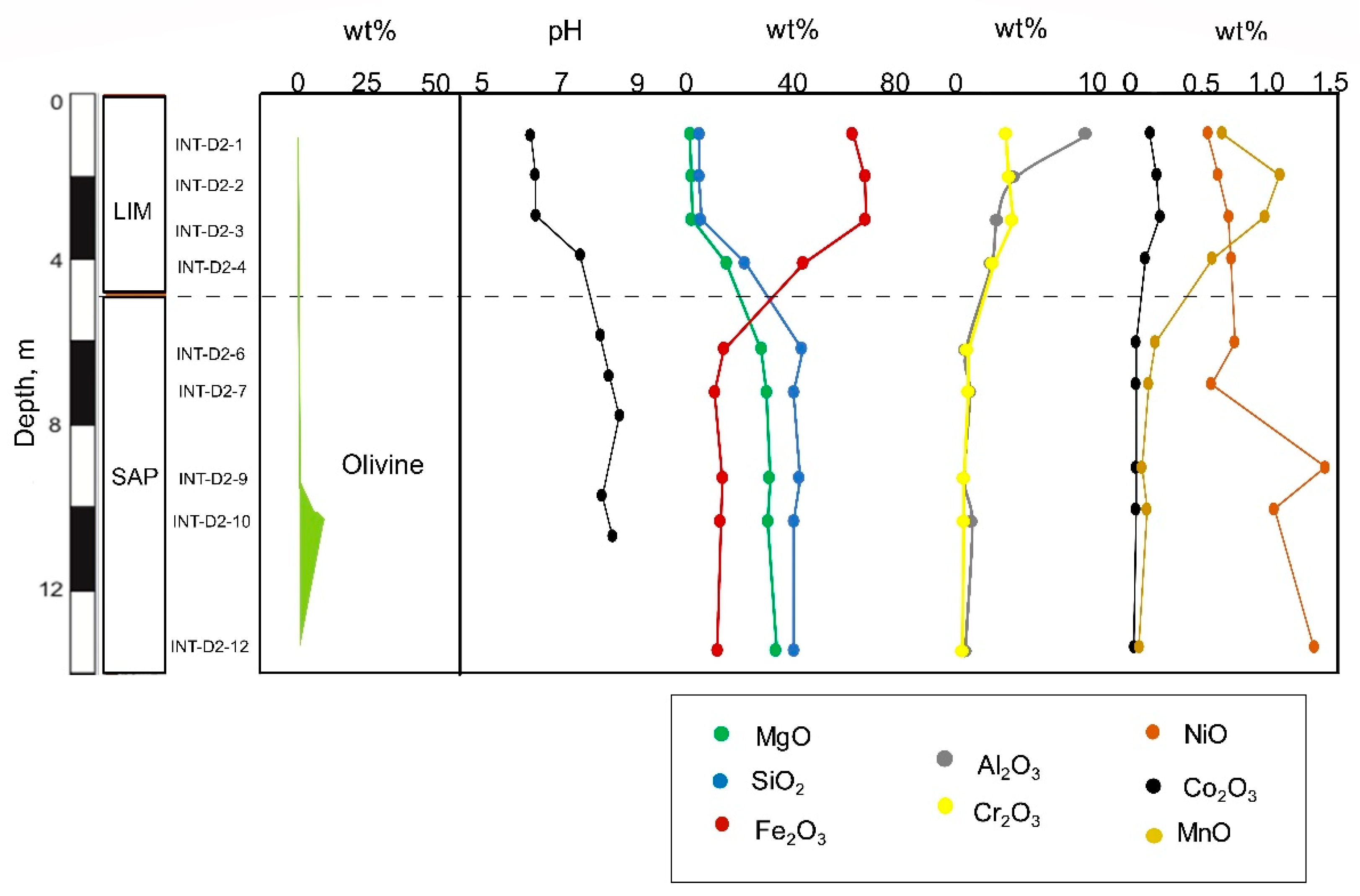
Figure 15.
Distribution of olivine, pH and elements with depth in the laterite derived from lherzolite bedrock (BB2515 core).
Figure 15.
Distribution of olivine, pH and elements with depth in the laterite derived from lherzolite bedrock (BB2515 core).
Figure 16.
Distribution of olivine, pH and elements with depth in the laterite derived from clinopyroxenite bedrock (PG1500 core).
Figure 16.
Distribution of olivine, pH and elements with depth in the laterite derived from clinopyroxenite bedrock (PG1500 core).
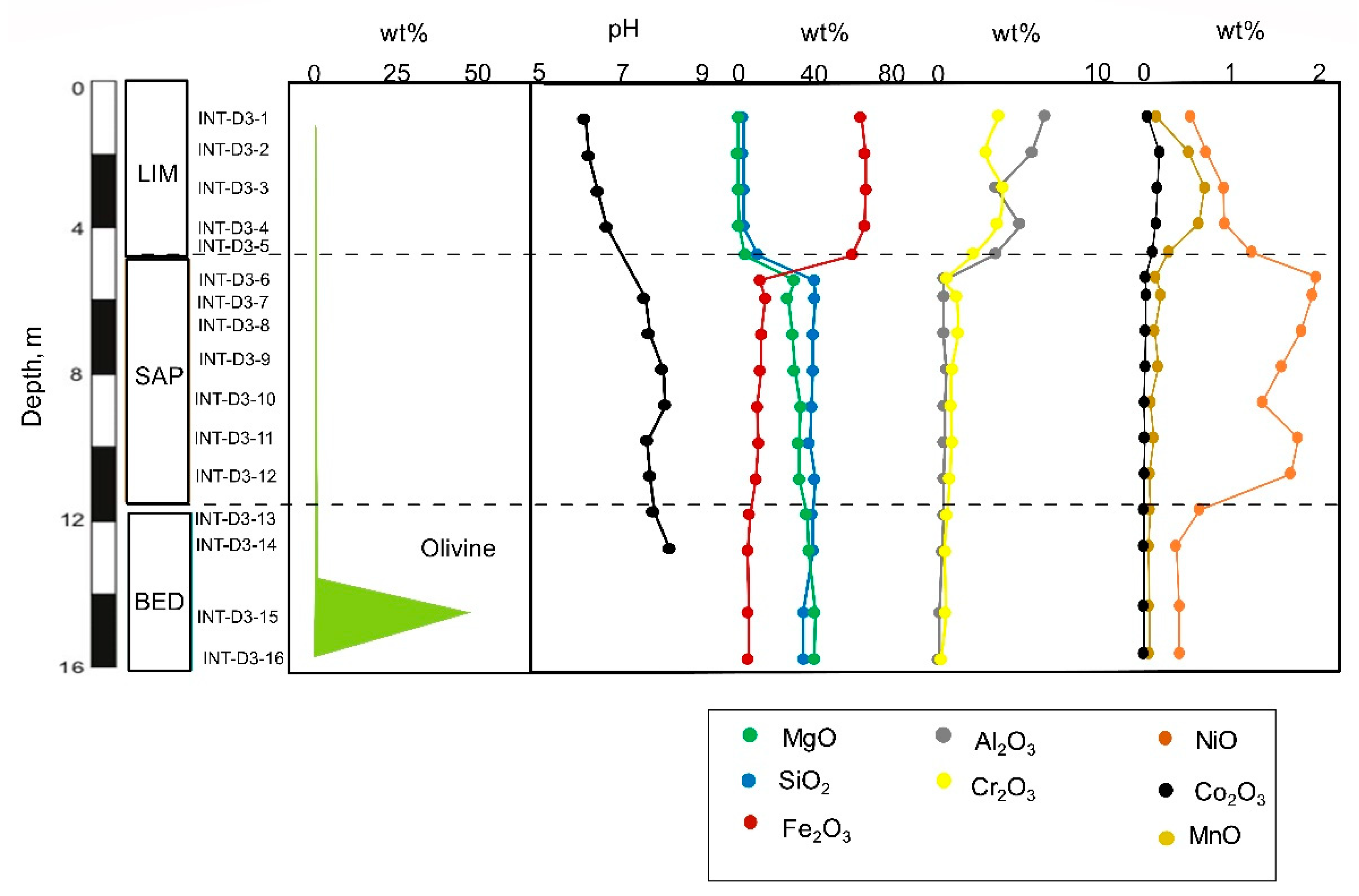
Figure 17.
Distribution of olivine, pH and elements with depth in the laterite derived from harzburgite-dunite bedrock (BB2406 core).
Figure 17.
Distribution of olivine, pH and elements with depth in the laterite derived from harzburgite-dunite bedrock (BB2406 core).
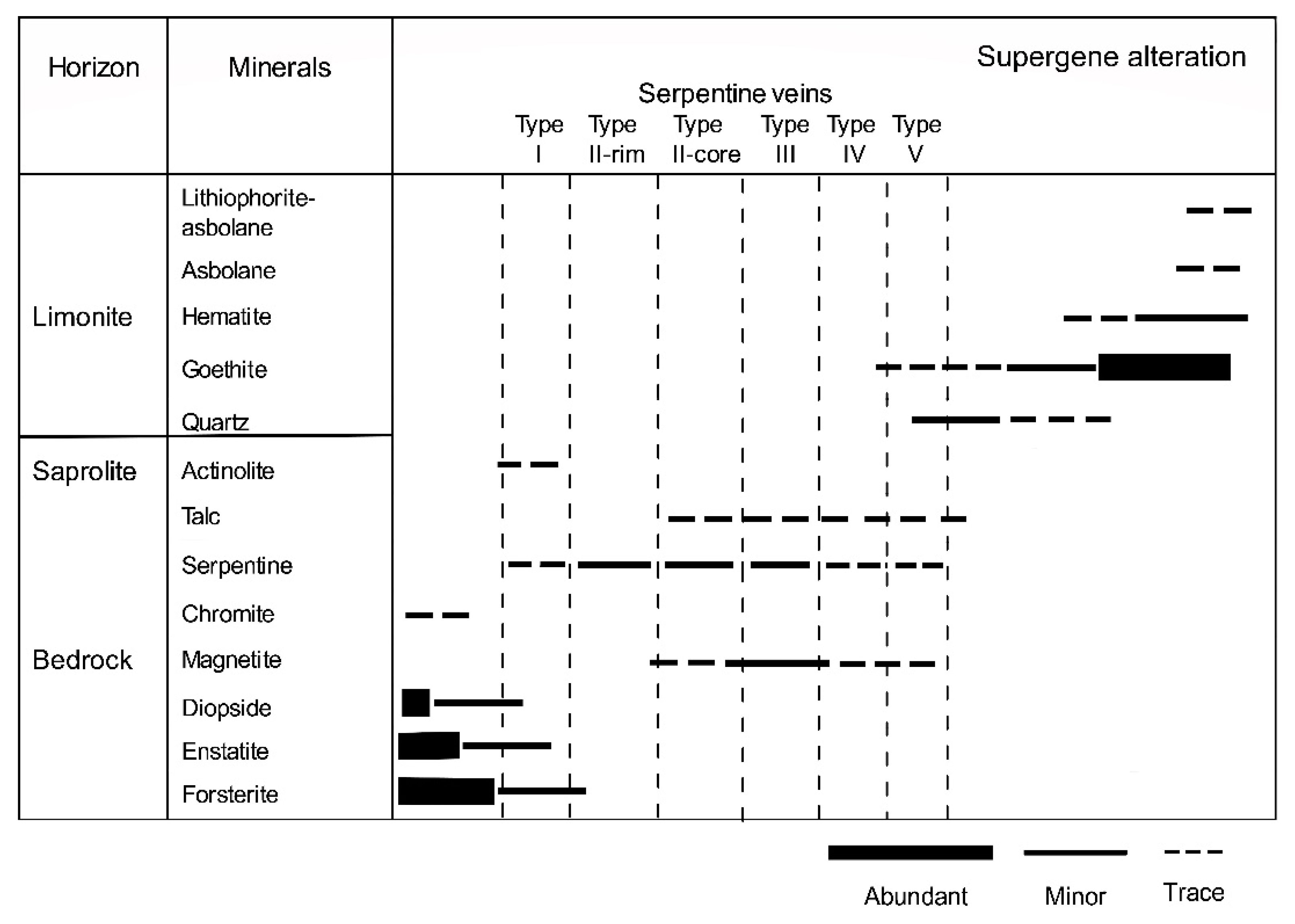
Figure 18.
Mineral paragenesis and abundance of primary and alteration minerals with Ni-bearing serpentine veins in the bedrock and saprock of the Intex laterite deposit in Mindoro, Philippines.
Figure 18.
Mineral paragenesis and abundance of primary and alteration minerals with Ni-bearing serpentine veins in the bedrock and saprock of the Intex laterite deposit in Mindoro, Philippines.
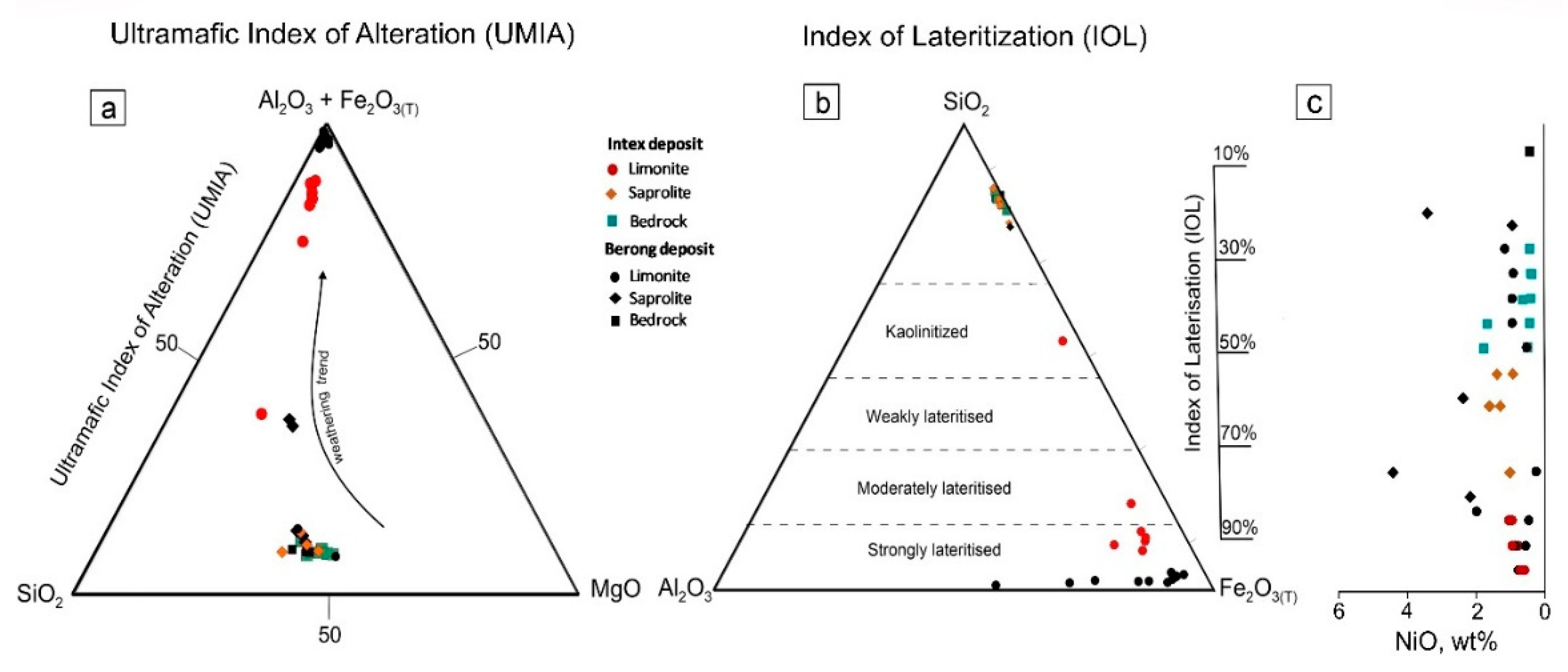
Figure 19.
Molar ternary plot of (a) Al2O3+Fe2O3+-SiO2-MgO showing the weathering of ultramafic bedrock from Intex deposit with the depletion of SiO2 and MgO and relative enrichment of Al2O3 and Fe2O3. (b) Mass ternary plot of SiO2-Al2O3-Fe2O3 displaying the Index of Lateritization (IOL) for Intex deposit as well as the Berong deposit in Palawan, Philippines. (c) Bulk Ni contents in the bedrock and weathered horizons of the Intex and Berong deposits.
Figure 19.
Molar ternary plot of (a) Al2O3+Fe2O3+-SiO2-MgO showing the weathering of ultramafic bedrock from Intex deposit with the depletion of SiO2 and MgO and relative enrichment of Al2O3 and Fe2O3. (b) Mass ternary plot of SiO2-Al2O3-Fe2O3 displaying the Index of Lateritization (IOL) for Intex deposit as well as the Berong deposit in Palawan, Philippines. (c) Bulk Ni contents in the bedrock and weathered horizons of the Intex and Berong deposits.
Table 1.
Average chemical compositions (wt%) and structural formula (apfu) of olivine, orthopyroxene, clinopyroxene, and talc in the peridotite bedrock.
Table 1.
Average chemical compositions (wt%) and structural formula (apfu) of olivine, orthopyroxene, clinopyroxene, and talc in the peridotite bedrock.
| Mineral | Olivine | Orthopyroxene | Clinopyroxene | Talc |
|---|
| | Σ | | σ | | σ | | σ |
|---|
| n | 18 | | 12 | | 6 | | 4 | |
| SiO2 | 39.99 | 0.44 | 55.75 | 0.48 | 53.22 | 1.37 | 58.28 | 2.17 |
| Al2O3 | 0.01 | 0.02 | 1.69 | 0.22 | 0.97 | 0.41 | 1.24 | 0.78 |
| FeO | 8.42 | 0.37 | 5.56 | 0.09 | 1.78 | 0.15 | 1.75 | 0.33 |
| MgO | 50.70 | 2.18 | 35.39 | 0.38 | 20.03 | 2.89 | 28.87 | 0.60 |
| NiO | 0.37 | 0.03 | 0.07 | 0.01 | 0.04 | 0.02 | 0.07 | 0.01 |
| CoO | 0.02 | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 | 0.01 | 0.02 |
| CaO | 0.01 | 0.03 | 0.54 | 0.23 | 24.41 | 1.37 | 0.02 | 0.01 |
| Cr2O3 | 0.00 | 0.00 | 0.48 | 0.09 | 0.30 | 0.21 | 0.30 | 0.16 |
| MnO | 0.12 | 0.01 | 0.13 | 0.01 | 0.07 | 0.02 | 0.02 | 0.01 |
| Total | 99.66 | 2.73 | 99.66 | 0.39 | 100.85 | 0.31 | 90.56 | 2.09 |
| Si | 0.981 | 0.021 | 1.931 | 0.010 | 1.922 | 0.048 | 3.915 | 0.080 |
| Al | 0.000 | 0.001 | 0.069 | 0.009 | 0.041 | 0.017 | 0.098 | 0.062 |
| Fe | 0.173 | 0.004 | 0.161 | 0.003 | 0.054 | 0.005 | 0.099 | 0.019 |
| ∑ Tetra. | 1.154 | 0.018 | 2.161 | 0.005 | 2.017 | 0.054 | 4.113 | 0.039 |
| Mg | 1.854 | 0.040 | 1.827 | 0.017 | 1.079 | 0.157 | 2.893 | 0.077 |
| Ni | 0.007 | 0.001 | 0.002 | 0.000 | 0.001 | 0.000 | 0.004 | 0.001 |
| Co | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 |
| Cr | 0.000 | 0.000 | 0.013 | 0.002 | 0.009 | 0.006 | 0.016 | 0.008 |
| Ca | 0.000 | 0.001 | 0.020 | 0.009 | 0.945 | 0.052 | 0.001 | 0.001 |
| Mn | 0.003 | 0.000 | 0.004 | 0.000 | 0.002 | 0.001 | 0.001 | 0.001 |
| ∑ Oct. | 1.864 | 0.039 | 1.867 | 0.011 | 2.035 | 0.105 | 2.915 | 0.082 |
| Tetra./Oct. | 0.620 | 0.025 | 1.158 | 0.009 | 0.994 | 0.072 | 1.412 | 0.052 |
| Ni/Mg | 0.004 | 0.000 | 0.001 | 0.000 | 0.001 | 0.000 | 0.001 | 0.000 |
Table 2.
Average chemical compositions (wt%) and structural formula (apfu) of the different vein serpentines in the bedrock.
Table 2.
Average chemical compositions (wt%) and structural formula (apfu) of the different vein serpentines in the bedrock.
| Drill Core | BB2515 | | BB2406 | | BB2515 | | BB2406 | | BB2515 | | BB2406 | | BB2515 | | BB2406 | |
|---|
| Type I | σ | Type I | σ | Type II-rim | σ | Type II-rim | σ | Type II-core | σ | Type II-core | σ | Type III | σ | Type III | σ |
|---|
| n | 14 | | 20 | | 10 | | 10 | | 11 | | 9 | | 26 | | 25 | |
| SiO2 | 38.65 | 1.58 | 37.27 | 2.60 | 37.14 | 2.98 | 39.43 | 1.33 | 35.51 | 4.95 | 36.67 | 1.85 | 39.55 | 0.63 | 40.40 | 1.47 |
| Al2O3 | 0.32 | 0.37 | 0.11 | 0.10 | 0.05 | 0.05 | 0.03 | 0.02 | 0.04 | 0.03 | 0.02 | 0.02 | 1.93 | 0.62 | 2.34 | 1.66 |
| FeO | 5.43 | 0.47 | 7.16 | 2.28 | 6.91 | 2.01 | 4.79 | 1.05 | 8.74 | 7.28 | 7.59 | 2.41 | 5.35 | 0.79 | 4.88 | 0.90 |
| MgO | 37.40 | 1.94 | 37.71 | 1.99 | 38.95 | 1.94 | 37.79 | 0.75 | 36.45 | 5.10 | 36.50 | 1.71 | 36.59 | 0.86 | 35.92 | 0.99 |
| NiO | 0.22 | 0.10 | 0.27 | 0.25 | 0.24 | 0.06 | 0.16 | 0.07 | 0.23 | 0.06 | 0.20 | 0.09 | 0.05 | 0.01 | 0.06 | 0.02 |
| CoO | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.04 | 0.04 | 0.01 | 0.01 | 0.01 | 0.01 |
| CaO | 0.22 | 0.19 | 0.10 | 0.05 | 0.14 | 0.07 | 0.04 | 0.02 | 0.12 | 0.04 | 0.05 | 0.02 | 0.11 | 0.13 | 0.04 | 0.03 |
| Cr2O3 | 0.13 | 0.24 | 0.01 | 0.01 | 0.00 | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.47 | 0.13 | 0.73 | 0.65 |
| MnO | 0.09 | 0.04 | 0.11 | 0.04 | 0.10 | 0.03 | 0.09 | 0.04 | 0.08 | 0.02 | 0.13 | 0.11 | 0.08 | 0.03 | 0.09 | 0.03 |
| Total | 82.48 | 1.34 | 82.76 | 1.24 | 83.53 | 1.09 | 82.36 | 1.01 | 81.19 | 2.61 | 81.20 | 1.78 | 84.17 | 1.02 | 84.51 | 1.16 |
| Si | 1.949 | 0.062 | 1.898 | 0.098 | 1.874 | 0.119 | 1.978 | 0.036 | 1.858 | 0.144 | 1.908 | 0.073 | 1.945 | 0.021 | 1.969 | 0.070 |
| Al | 0.019 | 0.022 | 0.006 | 0.006 | 0.003 | 0.003 | 0.002 | 0.001 | 0.002 | 0.002 | 0.001 | 0.001 | 0.112 | 0.036 | 0.135 | 0.095 |
| Fe | 0.229 | 0.021 | 0.307 | 0.106 | 0.293 | 0.090 | 0.202 | 0.047 | 0.416 | 0.436 | 0.331 | 0.105 | 0.220 | 0.034 | 0.199 | 0.037 |
| ∑ Tetra. | 2.197 | 0.069 | 2.211 | 0.092 | 2.170 | 0.067 | 2.181 | 0.028 | 2.276 | 0.311 | 2.240 | 0.106 | 2.277 | 0.037 | 2.303 | 0.036 |
| Mg | 2.812 | 0.148 | 2.866 | 0.158 | 2.934 | 0.171 | 2.826 | 0.043 | 2.844 | 0.213 | 2.833 | 0.148 | 2.683 | 0.045 | 2.610 | 0.050 |
| Ni | 0.009 | 0.004 | 0.011 | 0.010 | 0.010 | 0.003 | 0.007 | 0.003 | 0.010 | 0.002 | 0.008 | 0.004 | 0.002 | 0.001 | 0.002 | 0.001 |
| Co | 0.001 | 0.000 | 0.001 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 | 0.002 | 0.002 | 0.001 | 0.000 | 0.000 | 0.000 |
| Cr | 0.005 | 0.009 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.018 | 0.005 | 0.028 | 0.025 |
| Ca | 0.012 | 0.011 | 0.006 | 0.003 | 0.007 | 0.004 | 0.002 | 0.001 | 0.007 | 0.002 | 0.003 | 0.001 | 0.006 | 0.007 | 0.002 | 0.001 |
| Mn | 0.004 | 0.002 | 0.005 | 0.002 | 0.004 | 0.001 | 0.004 | 0.002 | 0.004 | 0.001 | 0.006 | 0.005 | 0.003 | 0.001 | 0.004 | 0.001 |
| ∑ Oct. | 2.843 | 0.138 | 2.888 | 0.161 | 2.955 | 0.171 | 2.840 | 0.043 | 2.865 | 0.212 | 2.852 | 0.150 | 2.713 | 0.046 | 2.647 | 0.042 |
| Tetra./Oct. | 0.776 | 0.061 | 0.769 | 0.072 | 0.737 | 0.063 | 0.768 | 0.019 | 0.806 | 0.187 | 0.789 | 0.080 | 0.840 | 0.028 | 0.871 | 0.027 |
| Ni/Mg | 0.003 | 0.001 | 0.004 | 0.003 | 0.003 | 0.001 | 0.002 | 0.001 | 0.003 | 0.001 | 0.003 | 0.001 | 0.001 | 0.000 | 0.001 | 0.000 |
Table 3.
Average chemical compositions (wt%) and structural formula (apfu) of the different vein serpentines in the saprock.
Table 3.
Average chemical compositions (wt%) and structural formula (apfu) of the different vein serpentines in the saprock.
| Drill Core | BB2515 | | PG1500 | | BB2515 | | BB2515 | | BB2515 | | PG1500 | |
|---|
| Sample Name | INT-D1-7 | | INT-D2-8 | | INT-D1-7 | | INT-D1-7 | | INT-D1-7 | | INT-D2-8 | |
|---|
| | Type I | σ | Type I | σ | Type II-rim | σ | Type II-core | σ | Type III | σ | Type V | σ |
|---|
| n | 6 | | 14 | | 7 | | 8 | | 11 | | 11 | |
| SiO2 | 39.49 | 2.44 | 42.08 | 1.67 | 39.41 | 2.79 | 39.84 | 3.31 | 44.28 | 4.75 | 40.92 | 1.25 |
| Al2O3 | 0.69 | 1.37 | 1.40 | 1.09 | 0.01 | 0.02 | 0.01 | 0.01 | 0.89 | 0.38 | 0.04 | 0.03 |
| FeO | 5.90 | 1.46 | 3.19 | 1.32 | 6.23 | 1.78 | 8.06 | 5.86 | 5.71 | 1.28 | 5.47 | 1.16 |
| MgO | 32.21 | 1.63 | 32.86 | 0.95 | 34.27 | 1.87 | 32.77 | 2.63 | 28.56 | 1.52 | 31.81 | 2.21 |
| NiO | 0.23 | 0.12 | 0.39 | 0.19 | 0.26 | 0.12 | 0.23 | 0.09 | 0.05 | 0.02 | 1.42 | 0.95 |
| CoO | 0.02 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.03 | 0.03 | 0.01 | 0.01 | 0.02 | 0.02 |
| CaO | 0.02 | 0.01 | 0.01 | 0.00 | 0.01 | 0.01 | 0.03 | 0.03 | 0.05 | 0.02 | 0.03 | 0.02 |
| Cr2O3 | 0.03 | 0.04 | 0.21 | 0.28 | 0.00 | 0.01 | 0.01 | 0.01 | 0.29 | 0.13 | 0.02 | 0.01 |
| MnO | 0.08 | 0.02 | 0.03 | 0.01 | 0.10 | 0.03 | 0.16 | 0.19 | 0.09 | 0.05 | 0.10 | 0.03 |
| Total | 78.69 | 1.03 | 80.18 | 1.76 | 80.32 | 3.43 | 81.14 | 2.08 | 79.97 | 3.99 | 79.84 | 1.55 |
| Si | 2.070 | 0.112 | 2.121 | 0.045 | 2.035 | 0.072 | 2.052 | 0.093 | 2.240 | 0.114 | 2.119 | 0.030 |
| Al | 0.043 | 0.085 | 0.083 | 0.064 | 0.001 | 0.001 | 0.001 | 0.001 | 0.054 | 0.025 | 0.003 | 0.002 |
| Fe | 0.259 | 0.067 | 0.135 | 0.060 | 0.272 | 0.088 | 0.356 | 0.275 | 0.243 | 0.055 | 0.238 | 0.054 |
| ∑ Tetra. | 2.372 | 0.055 | 2.339 | 0.038 | 2.307 | 0.040 | 2.409 | 0.191 | 2.538 | 0.090 | 2.359 | 0.074 |
| Mg | 2.519 | 0.149 | 2.469 | 0.062 | 2.640 | 0.071 | 2.518 | 0.130 | 2.168 | 0.189 | 2.453 | 0.128 |
| Ni | 0.010 | 0.005 | 0.016 | 0.008 | 0.011 | 0.005 | 0.010 | 0.004 | 0.002 | 0.001 | 0.060 | 0.041 |
| Co | 0.001 | 0.001 | 0.000 | 0.000 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.000 | 0.001 | 0.001 |
| Cr | 0.001 | 0.002 | 0.008 | 0.011 | 0.000 | 0.000 | 0.000 | 0.000 | 0.012 | 0.006 | 0.001 | 0.001 |
| Ca | 0.001 | 0.001 | 0.000 | 0.000 | 0.001 | 0.000 | 0.002 | 0.002 | 0.003 | 0.001 | 0.002 | 0.001 |
| Mn | 0.004 | 0.001 | 0.001 | 0.000 | 0.004 | 0.002 | 0.007 | 0.009 | 0.004 | 0.002 | 0.005 | 0.001 |
| ∑ Oct. | 2.536 | 0.151 | 2.495 | 0.052 | 2.657 | 0.077 | 2.538 | 0.118 | 2.189 | 0.192 | 2.521 | 0.098 |
| Tetra./Oct. | 0.939 | 0.080 | 0.938 | 0.032 | 0.869 | 0.034 | 0.954 | 0.122 | 1.171 | 0.143 | 0.938 | 0.067 |
Table 4.
Average chemical compositions and structural formula (apfu) of the different veins serpentines in the saprock derived from harzburgite-dunite (wt%).
Table 4.
Average chemical compositions and structural formula (apfu) of the different veins serpentines in the saprock derived from harzburgite-dunite (wt%).
| Drill Core | BB2406 | | BB2406 | | BB2406 | | BB2406 | | BB2406 | |
|---|
| Sample Name | INT-D3-9 | | INT-D3-9 | | INT-D3-9 | | INT-D3-9 | | INT-D3-9 | |
|---|
| | Type I | σ | Type II-rim | σ | Type II-core | σ | Type III | σ | Type IV | σ |
|---|
| n | 10 | | 7 | | 13 | | 12 | | 11 | |
| SiO2 | 36.92 | 1.27 | 39.92 | 1.44 | 37.15 | 5.86 | 38.94 | 0.93 | 39.12 | 0.52 |
| Al2O3 | 0.00 | 0.00 | 0.04 | 0.05 | 0.06 | 0.13 | 0.42 | 0.13 | 0.13 | 0.04 |
| FeO | 7.52 | 0.82 | 5.37 | 1.18 | 7.91 | 5.24 | 7.13 | 0.81 | 9.65 | 0.66 |
| MgO | 37.22 | 0.72 | 38.58 | 0.75 | 37.78 | 1.41 | 35.83 | 1.22 | 34.94 | 0.80 |
| NiO | 0.32 | 0.06 | 0.24 | 0.05 | 0.28 | 0.13 | 0.09 | 0.02 | 0.11 | 0.02 |
| CoO | 0.03 | 0.01 | 0.01 | 0.01 | 0.03 | 0.03 | 0.02 | 0.01 | 0.03 | 0.02 |
| CaO | 0.02 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 |
| Cr2O3 | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.47 | 0.12 | 0.01 | 0.00 |
| MnO | 0.10 | 0.02 | 0.07 | 0.02 | 0.14 | 0.15 | 0.16 | 0.02 | 0.24 | 0.05 |
| Total | 82.13 | 1.69 | 84.26 | 1.06 | 83.37 | 1.74 | 83.09 | 1.90 | 84.24 | 1.02 |
| Si | 1.900 | 0.040 | 1.964 | 0.045 | 1.877 | 0.232 | 1.964 | 0.024 | 1.971 | 0.028 |
| Al | 0.000 | 0.000 | 0.002 | 0.003 | 0.004 | 0.007 | 0.025 | 0.008 | 0.008 | 0.002 |
| Fe | 0.324 | 0.033 | 0.222 | 0.051 | 0.350 | 0.283 | 0.301 | 0.034 | 0.407 | 0.028 |
| ∑ Tetra. | 2.224 | 0.031 | 2.188 | 0.024 | 2.231 | 0.074 | 2.290 | 0.035 | 2.385 | 0.019 |
| Mg | 2.856 | 0.063 | 2.832 | 0.051 | 2.871 | 0.189 | 2.694 | 0.055 | 2.624 | 0.040 |
| Ni | 0.013 | 0.002 | 0.010 | 0.002 | 0.012 | 0.007 | 0.004 | 0.001 | 0.005 | 0.001 |
| Co | 0.001 | 0.000 | 0.001 | 0.000 | 0.001 | 0.001 | 0.001 | 0.000 | 0.001 | 0.001 |
| Cr | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.019 | 0.005 | 0.000 | 0.000 |
| Ca | 0.001 | 0.000 | 0.001 | 0.000 | 0.001 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 |
| Mn | 0.004 | 0.001 | 0.003 | 0.001 | 0.006 | 0.007 | 0.007 | 0.001 | 0.010 | 0.002 |
| ∑ Oct. | 2.876 | 0.064 | 2.846 | 0.052 | 2.891 | 0.198 | 2.724 | 0.054 | 2.640 | 0.040 |
| Tetra./Oct. | 0.774 | 0.026 | 0.769 | 0.020 | 0.774 | 0.050 | 0.841 | 0.028 | 0.904 | 0.020 |
Table 5.
Average compositions (wt%) and structural formula (apfu) of goethite, hematite, and talc in the limonite samples.
Table 5.
Average compositions (wt%) and structural formula (apfu) of goethite, hematite, and talc in the limonite samples.
| | Goethite | Hematite | Talc |
|---|
| Drill Core | BB2515 | | PG1500 | | BB2515 | | PG1500 | | BB2406 | | PG1500 |
|---|
| Sample Name | INT-D1-1 | | INT-D2-1 | | INT-D1-1 | | INT-D2-1 | | INT-D3-2 | | INT-D1-1 |
|---|
| Depth, m | 1 | σ | 1 | σ | 1 | σ | 1 | σ | 2.4 | σ | 1 |
|---|
| n | 8 | | 10 | | 12 | | 5 | | 11 | | 1 |
| SiO2 | 2.81 | 0.42 | 3.30 | 0.90 | 1.32 | 0.64 | 0.89 | 0.52 | 0.41 | 0.65 | 55.04 |
| MgO | 0.37 | 0.24 | 0.62 | 0.50 | 0.50 | 0.25 | 0.56 | 0.60 | 0.27 | 0.09 | 24.07 |
| Al2O3 | 6.47 | 2.48 | 6.79 | 2.30 | 1.46 | 0.60 | 0.10 | 0.12 | 0.08 | 0.12 | 1.53 |
| FeO | 62.58 | 3.70 | 62.66 | 1.89 | 77.61 | 3.49 | 80.72 | 2.32 | 82.50 | 3.92 | 6.55 |
| NiO | 0.79 | 0.38 | 0.76 | 0.35 | 0.24 | 0.08 | 0.41 | 0.76 | 0.13 | 0.08 | 0.10 |
| CoO | 0.43 | 0.28 | 0.10 | 0.04 | 0.14 | 0.02 | 0.64 | 0.68 | 0.16 | 0.02 | 0.02 |
| Cr2O3 | 0.34 | 0.24 | 0.96 | 0.68 | 0.89 | 0.35 | 0.08 | 0.05 | 0.10 | 0.09 | 0.17 |
| CaO | 0.07 | 0.06 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 | 0.03 | 0.00 |
| MnO | 1.78 | 1.22 | 0.39 | 0.30 | 0.15 | 0.06 | 1.04 | 0.95 | 0.19 | 0.24 | 0.04 |
| Total | 75.69 | 3.22 | 75.81 | 3.16 | 82.34 | 3.45 | 84.46 | 2.52 | 83.88 | 3.86 | 87.56 |
| Fe | 0.72 | 0.06 | 0.71 | 0.06 | 2.69 | 0.09 | 2.81 | 0.11 | 2.92 | 0.06 | |
| Al | 0.10 | 0.04 | 0.11 | 0.03 | 0.07 | 0.03 | 0.01 | 0.01 | 0.00 | 0.01 | |
| Mn | 0.02 | 0.01 | 0.00 | 0.00 | 0.01 | 0.00 | 0.04 | 0.03 | 0.01 | 0.01 | |
| Ni | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | 0.02 | 0.00 | 0.00 | |
| Co | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.02 | 0.01 | 0.00 | |
Table 6.
Average chemical compositions (wt%) and structural formula (apfu) of the representative. asbolane and lithiophorite-asbolane intermediate in the limonite horizon.
Table 6.
Average chemical compositions (wt%) and structural formula (apfu) of the representative. asbolane and lithiophorite-asbolane intermediate in the limonite horizon.
| | Asbolane | | Lithiophorite-Asbolane Intermediate |
|---|
| Sample Name | INT-D1-4 | | INT-D2-1 | | INT-D3-2 | |
|---|
| Depth, m | 4 | σ | 1 | σ | 2.4 | σ |
|---|
| n | 5 | | 8 | | 3 | |
| SiO2 | 5.95 | 8.92 | 0.03 | 0.02 | 0.04 | 0.05 |
| MgO | 6.68 | 3.15 | 0.33 | 0.07 | 0.43 | 0.07 |
| Al2O3 | 0.50 | 0.31 | 5.60 | 0.40 | 4.88 | 0.34 |
| FeO | 9.64 | 1.97 | 8.66 | 2.36 | 12.79 | 11.00 |
| NiO | 12.89 | 3.22 | 3.14 | 0.34 | 2.85 | 0.61 |
| CoO | 3.82 | 1.53 | 10.43 | 1.07 | 9.72 | 2.20 |
| Cr2O3 | 0.11 | 0.09 | 0.02 | 0.02 | 0.01 | 0.01 |
| CaO | 0.95 | 1.69 | 0.06 | 0.02 | 0.08 | 0.03 |
| MnO | 32.17 | 5.82 | 39.41 | 4.07 | 39.01 | 9.65 |
| Total | 72.70 | 4.45 | 67.68 | 3.85 | 69.82 | 1.83 |
| Ni | 0.305 | 0.108 | 0.081 | 0.005 | 0.072 | 0.014 |
| Al | 0.016 | 0.010 | 0.213 | 0.009 | 0.182 | 0.008 |
| Co | 0.089 | 0.046 | 0.269 | 0.014 | 0.245 | 0.050 |
| Mn | 0.796 | 0.251 | 1.074 | 0.061 | 1.040 | 0.235 |
| Fe | 0.230 | 0.063 | 0.237 | 0.079 | 0.343 | 0.304 |
| Mg | 0.265 | 0.056 | 0.016 | 0.003 | 0.020 | 0.003 |
| Co/Ni | 0.316 | 0.152 | 3.317 | 0.161 | 3.388 | 0.062 |
Table 7.
Whole-rock chemical analyses (wt%) of the bedrock (BED), saprolite (SAP), and limonite (LIM) samples through BB2515 core.
Table 7.
Whole-rock chemical analyses (wt%) of the bedrock (BED), saprolite (SAP), and limonite (LIM) samples through BB2515 core.
| Drill Core | BB2515 | BB2515 | BB2515 | BB2515 | BB2515 | BB2515 | BB2515 | BB2515 | BB2515 | BB2515 | BB2515 | BB2515 | BB2515 |
|---|
| Horizon | 1 | 2 | 3 | 5 | 5 | 6 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|
| Depth, m | LIM | LIM | LIM | LIM-SAP | SAP | SAP | SAP | SAP | BED | BED | BED | BED | BED |
|---|
| Na2O | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| MgO | 2.63 | 3.72 | 5.86 | 3.03 | 38.14 | 33.15 | 32.00 | 35.13 | 33.56 | 38.25 | 37.21 | 35.85 | 41.96 |
| SiO2 | 6.41 | 8.54 | 13.76 | 7.56 | 40.59 | 41.82 | 47.07 | 41.57 | 42.39 | 40.13 | 40.72 | 42.73 | 39.99 |
| Al2O3 | 7.363 | 5.906 | 5.202 | 6.631 | 0.488 | 0.783 | 0.487 | 0.719 | 0.410 | 0.468 | 0.678 | 0.472 | 0.636 |
| P2O5 | 0.010 | 0.005 | 0.006 | 0.007 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| K2O | 0.011 | 0.007 | 0.009 | 0.010 | 0.003 | 0.003 | 0.007 | 0.004 | 0.003 | 0.006 | 0.002 | 0.003 | 0.002 |
| CaO | 0.027 | 0.054 | 1.154 | 0.067 | 0.365 | 0.498 | 0.089 | 0.241 | 0.126 | 0.319 | 0.271 | 0.122 | 0.623 |
| TiO2 | 0.115 | 0.087 | 0.077 | 0.115 | bdl | bdl | 0.005 | 0.008 | 0.006 | 0.005 | bdl | bdl | bdl |
| Cr2O3 | 3.729 | 3.153 | 2.870 | 3.540 | 0.375 | 0.455 | 0.443 | 0.458 | 0.553 | 0.553 | 0.512 | 0.372 | 0.490 |
| MnO | 1.148 | 0.917 | 0.863 | 0.859 | 0.080 | 0.091 | 0.072 | 0.085 | 0.109 | 0.084 | 0.078 | 0.084 | 0.081 |
| Fe2O3 | 61.277 | 60.966 | 54.806 | 61.325 | 7.290 | 10.416 | 7.037 | 8.050 | 9.274 | 7.854 | 6.876 | 6.676 | 6.998 |
| Co2O3 | 0.191 | 0.133 | 0.129 | 0.142 | 0.013 | 0.014 | 0.012 | 0.014 | 0.018 | 0.013 | 0.011 | 0.013 | 0.013 |
| NiO | 0.675 | 0.938 | 1.014 | 0.730 | 1.000 | 2.153 | 1.327 | 0.919 | 0.461 | 0.410 | 0.388 | 0.390 | 0.405 |
| LOI | 12.55 | 11.48 | 10.61 | 12.18 | 10.55 | 9.37 | 10.32 | 11.58 | 11.96 | 11.31 | 12.20 | 12.05 | 7.059 |
| Total | 96.10 | 95.83 | 96.37 | 96.18 | 98.86 | 98.73 | 98.83 | 98.75 | 98.84 | 99.42 | 98.90 | 98.76 | 98.21 |
Table 8.
Whole-rock chemical analyses (wt%) of the bedrock (BED), saprolite (SAP), and limonite (LIM) samples through PG1500 core.
Table 8.
Whole-rock chemical analyses (wt%) of the bedrock (BED), saprolite (SAP), and limonite (LIM) samples through PG1500 core.
| Drill Core | PG1500 | PG1500 | PG1500 | PG1500 | PG1500 | PG1500 | PG1500 | PG1500 | PG1500 | PG1500 |
|---|
| Horizon | 1 | 2 | 3.0 | 3.8 | 4 | 6 | 7 | 9 | 10 | 13.3 |
|---|
| Depth, m | LIM | LIM | LIM | LIM | LIM-SAP | SAP | SAP | SAP | SAP | SAP |
|---|
| Na2O | 0.049 | bdl | bdl | bdl | 0.051 | bdl | 0.04 | 0.08 | bdl | bdl |
| MgO | 1.348 | 2.016 | 2.093 | 24.02 | 14.92 | 28.10 | 29.92 | 31.11 | 30.47 | 32.94 |
| SiO2 | 4.879 | 4.861 | 5.486 | 29.86 | 21.96 | 43.27 | 40.01 | 42.23 | 40.07 | 40.06 |
| Al2O3 | 9.286 | 4.214 | 2.972 | 1.601 | 2.585 | 0.763 | 1.045 | 0.648 | 1.220 | 0.706 |
| P2O5 | 0.015 | 0.005 | bdl | 0.003 | 0.009 | bdl | bdl | bdl | bdl | 0.003 |
| K2O | 0.043 | 0.012 | 0.009 | 0.005 | 0.042 | 0.006 | 0.010 | 0.018 | 0.006 | 0.002 |
| CaO | 0.040 | bdl | bdl | 0.078 | 0.122 | 0.317 | 0.339 | 0.906 | 1.293 | 0.153 |
| TiO2 | 0.345 | 0.081 | 0.058 | 0.021 | 0.047 | 0.010 | 0.011 | 0.011 | 0.009 | 0.007 |
| Cr2O3 | 3.597 | 3.812 | 4.077 | 1.552 | 2.700 | 0.872 | 0.919 | 0.600 | 0.614 | 0.693 |
| MnO | 0.694 | 1.139 | 1.022 | 0.430 | 0.618 | 0.177 | 0.126 | 0.075 | 0.114 | 0.050 |
| Fe2O3 | 62.08 | 66.60 | 66.98 | 30.11 | 43.81 | 14.07 | 10.71 | 13.43 | 12.78 | 12.22 |
| Co2O3 | 0.135 | 0.189 | 0.212 | 0.067 | 0.101 | 0.030 | 0.030 | 0.030 | 0.030 | 0.011 |
| NiO | 0.584 | 0.664 | 0.743 | 0.696 | 0.765 | 0.793 | 0.613 | 1.492 | 1.098 | 1.406 |
| LOI | 13.66 | 11.89 | 11.94 | 11.29 | 10.89 | 10.12 | 18.05 | 9.23 | 8.77 | 11.46 |
| Total | 96.76 | 95.51 | 95.61 | 99.76 | 98.62 | 98.54 | 101.8 | 99.86 | 96.50 | 99.66 |
Table 9.
Whole-rock chemical analyses (wt%) of the bedrock (BED), saprolite (SAP), and limonite (LIM) samples through BB2406 core.
Table 9.
Whole-rock chemical analyses (wt%) of the bedrock (BED), saprolite (SAP), and limonite (LIM) samples through BB2406 core.
| Drill Core | BB2406 | BB2406 | BB2406 | BB2406 | BB2406 | BB2406 | BB2406 | BB2406 | BB2406 | BB2406 | BB2406 | BB2406 | BB2406 | BB2406 | BB2406 | BB2406 |
|---|
| Sample Name | 1 | 2 | 3 | 4 | 4.8 | 5.5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 15 | 16 |
|---|
| Depth, m | LIM | LIM | LIM | LIM | LIM-SAP | SAP | SAP | SAP | SAP | SAP | SAP | SAP | SAP | BED | BED | BED |
|---|
| Na2O | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| MgO, % | 1.655 | 1.404 | 1.708 | 1.849 | 5.183 | 30.75 | 27.17 | 29.58 | 30.73 | 33.97 | 32.81 | 33.33 | 37.13 | 38.06 | 41.39 | 40.78 |
| SiO2, % | 4.055 | 4.129 | 4.263 | 4.355 | 11.91 | 40.76 | 41.22 | 40.12 | 40.07 | 39.35 | 38.47 | 41.34 | 40.02 | 40.25 | 35.33 | 35.31 |
| K2O,% | 0.013 | bdl | bdl | bdl | 0.014 | 0.009 | 0.005 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| CaO,% | 0.022 | 0.017 | 0.014 | 0.019 | 0.374 | -0.001 | 0.037 | 0.007 | 0.155 | 0.181 | 0.085 | 0.233 | 0.150 | 0.350 | 0.060 | 0.049 |
| Al2O3, % | 6.773 | 6.000 | 3.752 | 5.264 | 3.692 | 0.457 | 0.479 | 0.465 | 0.627 | 0.491 | 0.521 | 0.441 | 0.472 | 0.370 | 0.203 | 0.139 |
| P2O5, % | 0.013 | 0.005 | 0.003 | 0.005 | 0.007 | 0.007 | 0.003 | 0.002 | 0.003 | 0.001 | 0.003 | 0.004 | 0.000 | 0.001 | 0.000 | 0.001 |
| TiO2, % | 0.129 | 0.083 | 0.076 | 0.067 | 0.105 | 0.006 | 0.008 | 0.006 | 0.006 | 0.006 | bdl | 0.007 | bdl | bdl | bdl | bdl |
| Cr2O3 | 3.925 | 3.136 | 4.133 | 3.838 | 2.366 | 0.675 | 1.280 | 1.364 | 0.972 | 0.951 | 0.967 | 0.857 | 0.691 | 0.529 | 0.605 | 0.290 |
| MnO, % | 0.151 | 0.522 | 0.710 | 0.644 | 0.295 | 0.146 | 0.207 | 0.139 | 0.176 | 0.092 | 0.125 | 0.078 | 0.086 | 0.069 | 0.077 | 0.075 |
| Fe2O3, % | 65.21 | 67.09 | 67.58 | 66.79 | 60.43 | 12.92 | 15.69 | 13.69 | 13.11 | 11.52 | 12.10 | 10.77 | 7.694 | 6.625 | 7.026 | 6.827 |
| Co2O3, % | 0.053 | 0.192 | 0.170 | 0.155 | 0.112 | 0.034 | 0.037 | 0.032 | 0.029 | 0.021 | 0.024 | 0.016 | 0.014 | 0.012 | 0.015 | 0.015 |
| NiO, % | 0.547 | 0.726 | 0.925 | 0.938 | 1.246 | 1.985 | 1.940 | 1.810 | 1.584 | 1.367 | 1.776 | 1.693 | 0.655 | 0.383 | 0.421 | 0.421 |
| LOI | 13.83 | 13.31 | 11.77 | 11.49 | 10.99 | 11.49 | 11.06 | 11.79 | 11.45 | 11.13 | 12.43 | 10.38 | 12.45 | 12.49 | 13.89 | 15.38 |
| Total | 96.35 | 96.59 | 95.08 | 95.38 | 96.71 | 99.15 | 99.05 | 98.99 | 98.88 | 99.08 | 99.31 | 99.12 | 99.30 | 99.10 | 98.99 | 99.26 |