Crystal Chemistry of an Erythrite-Köttigite Solid Solution (Co3–xZnx) (AsO4)2·8H2O
Abstract
1. Introduction
2. Sampling Site
3. Experimental Methods
3.1. Sample Description
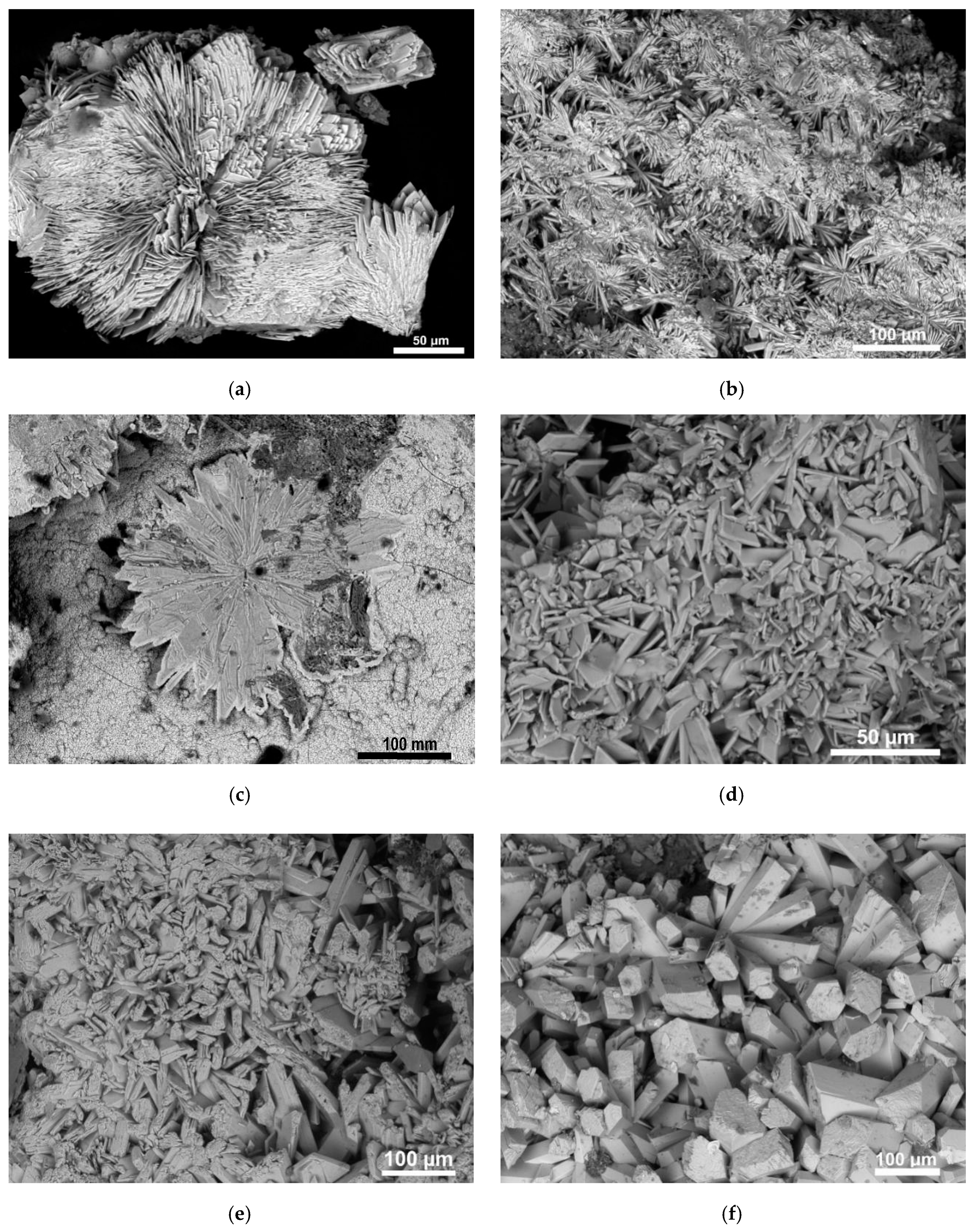
3.2. Scanning Electron Microscopy (SEM)
3.3. Electron Probe Microanalysis (EPMA)
3.4. Structure Refinement
3.5. X-ray Powder Diffraction (XRD)
3.6. Raman Spectroscopy
4. Results and Discussion
4.1. Paragenetic Sequence
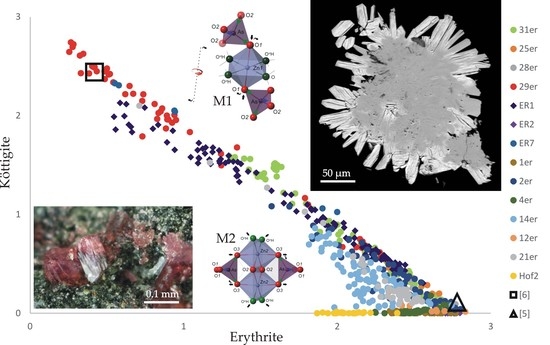
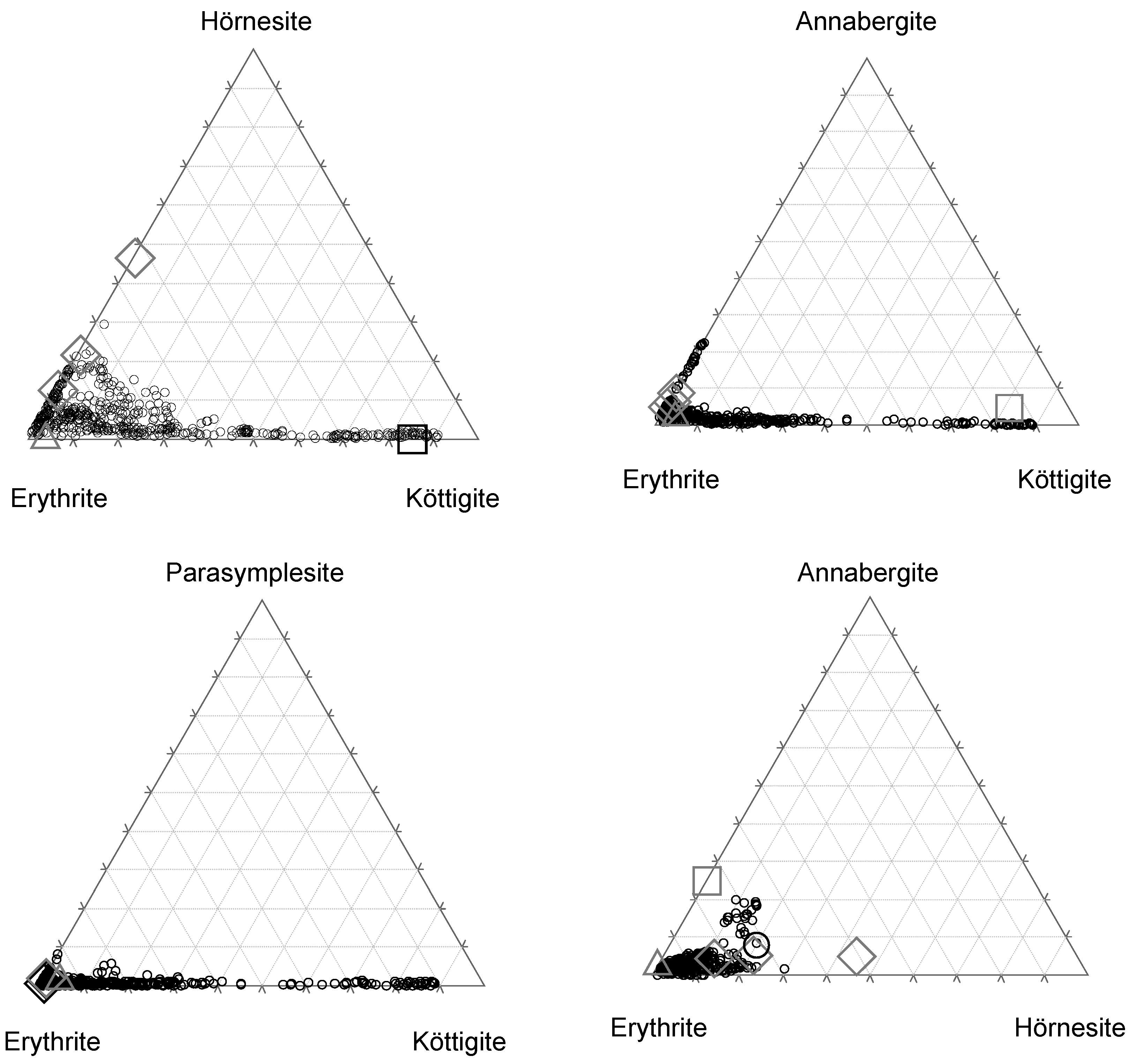
4.2. Chemical Composition
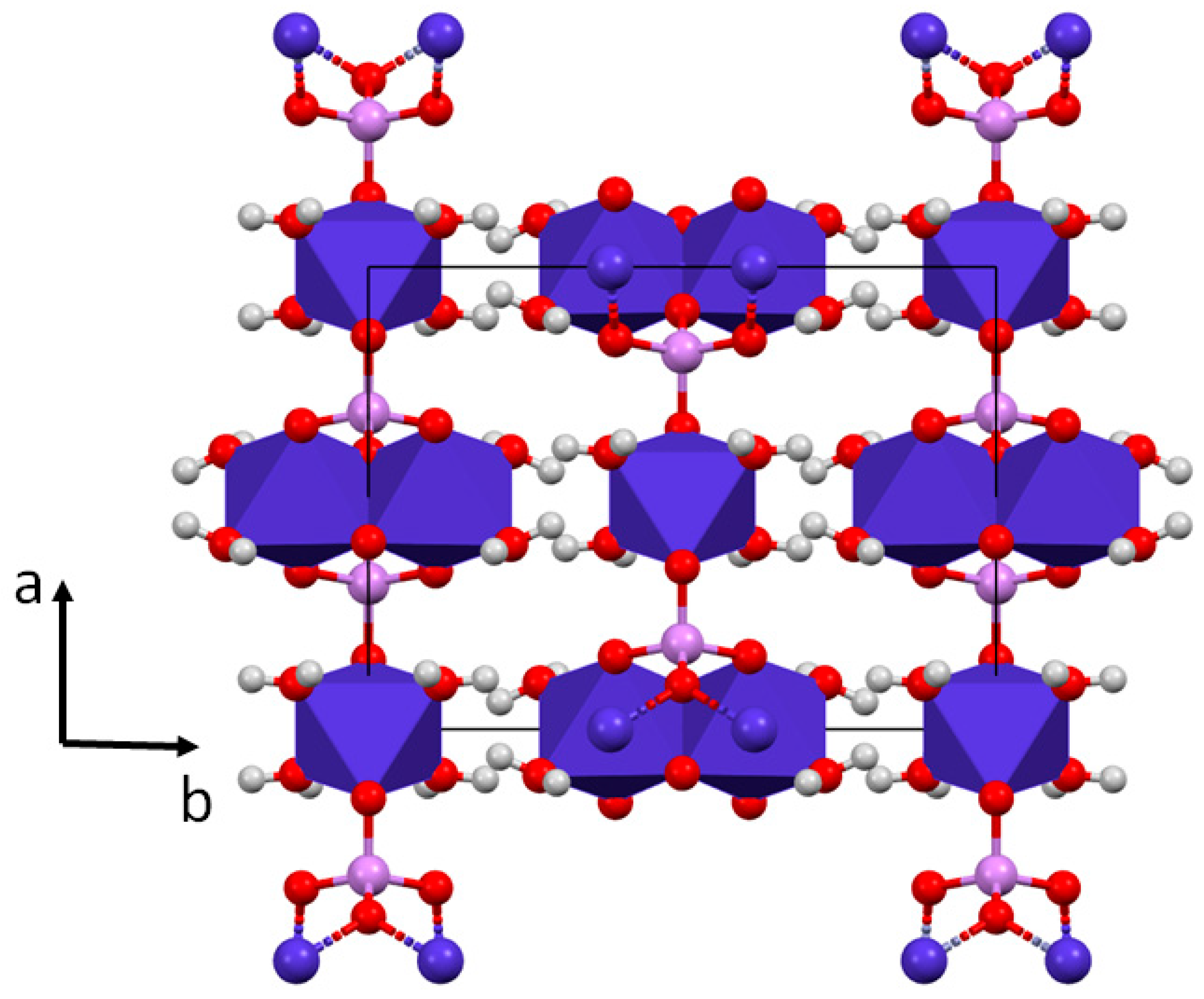
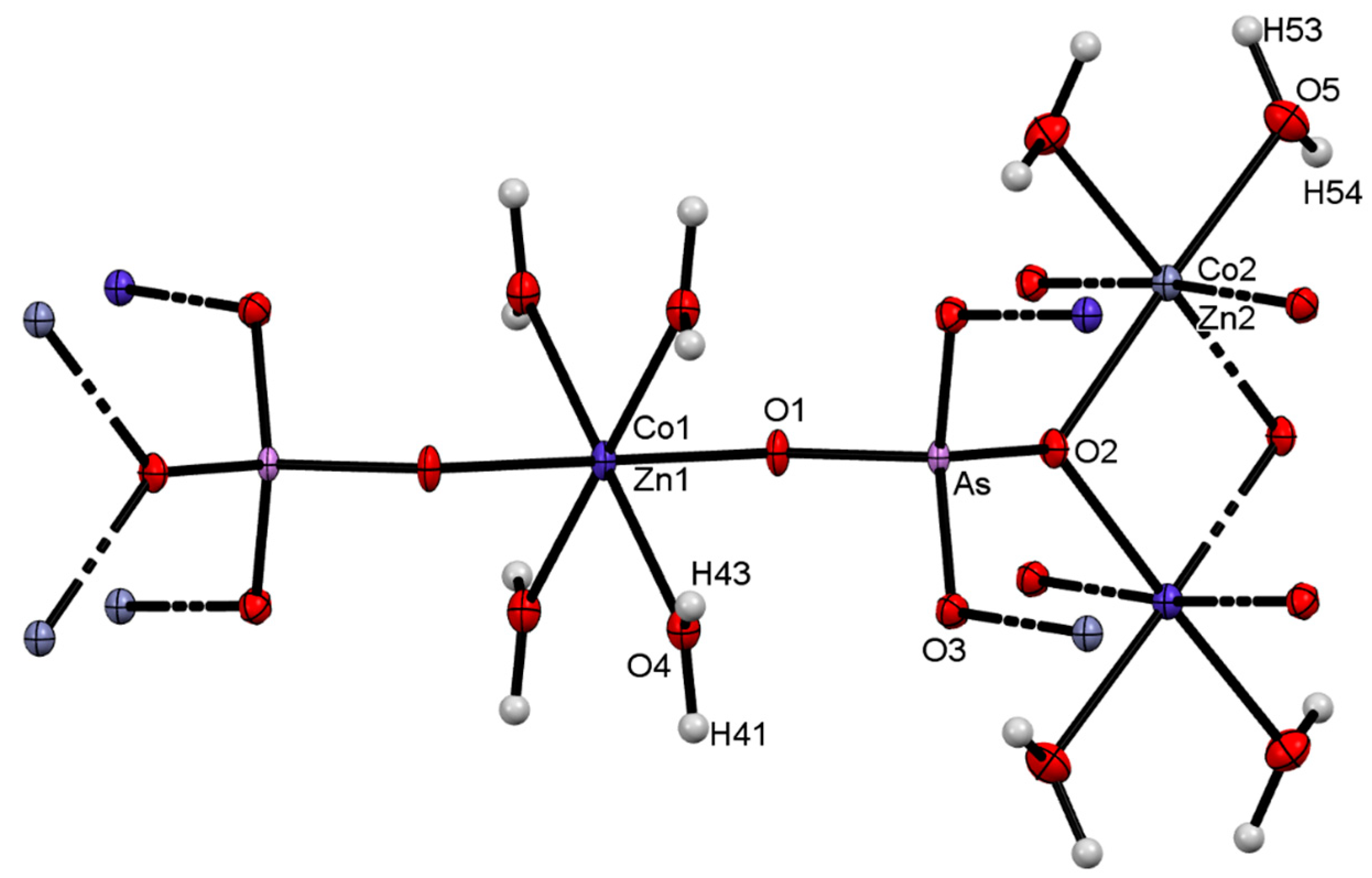
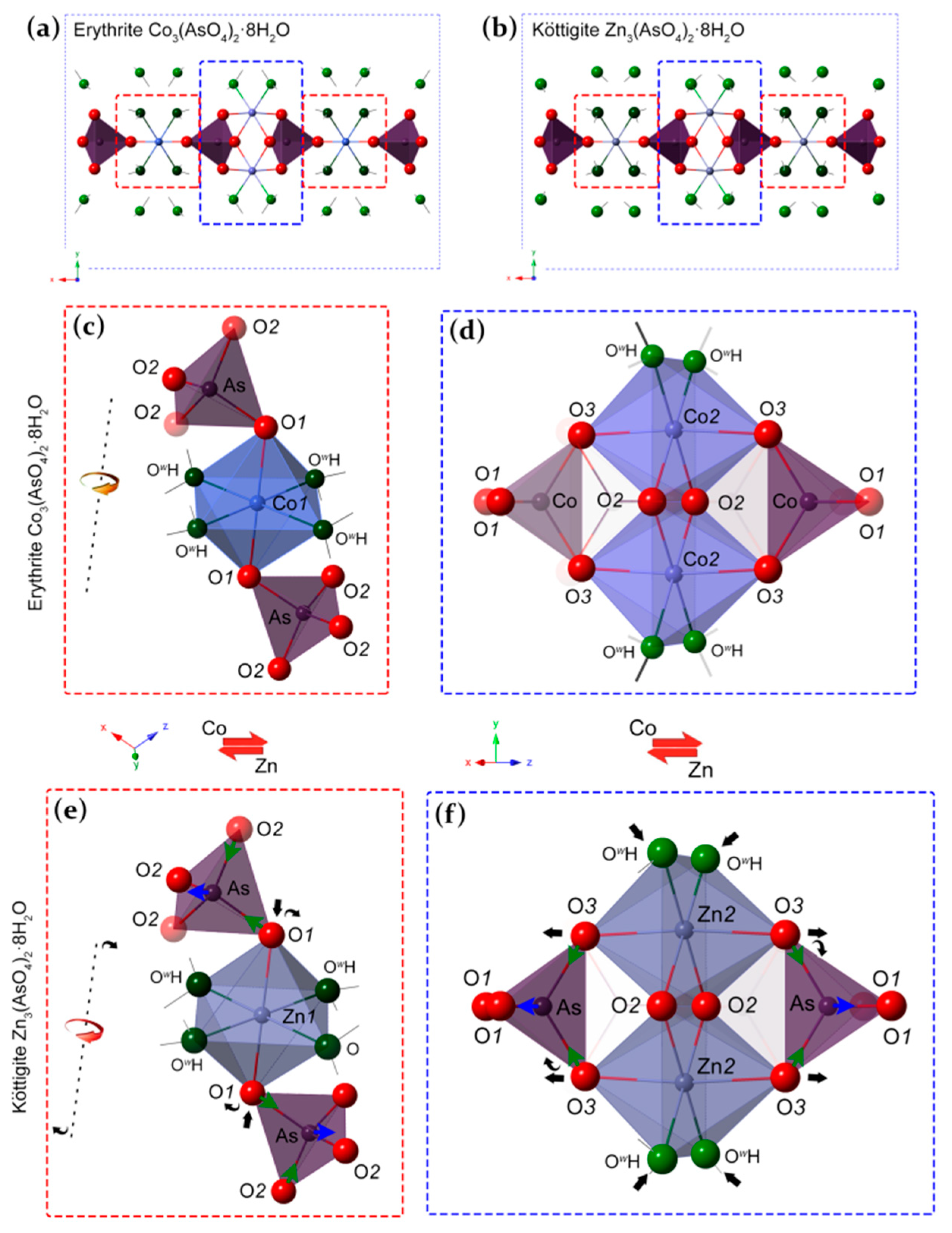
4.3. Structure Refinement
4.4. Unit-Cell Dimensions
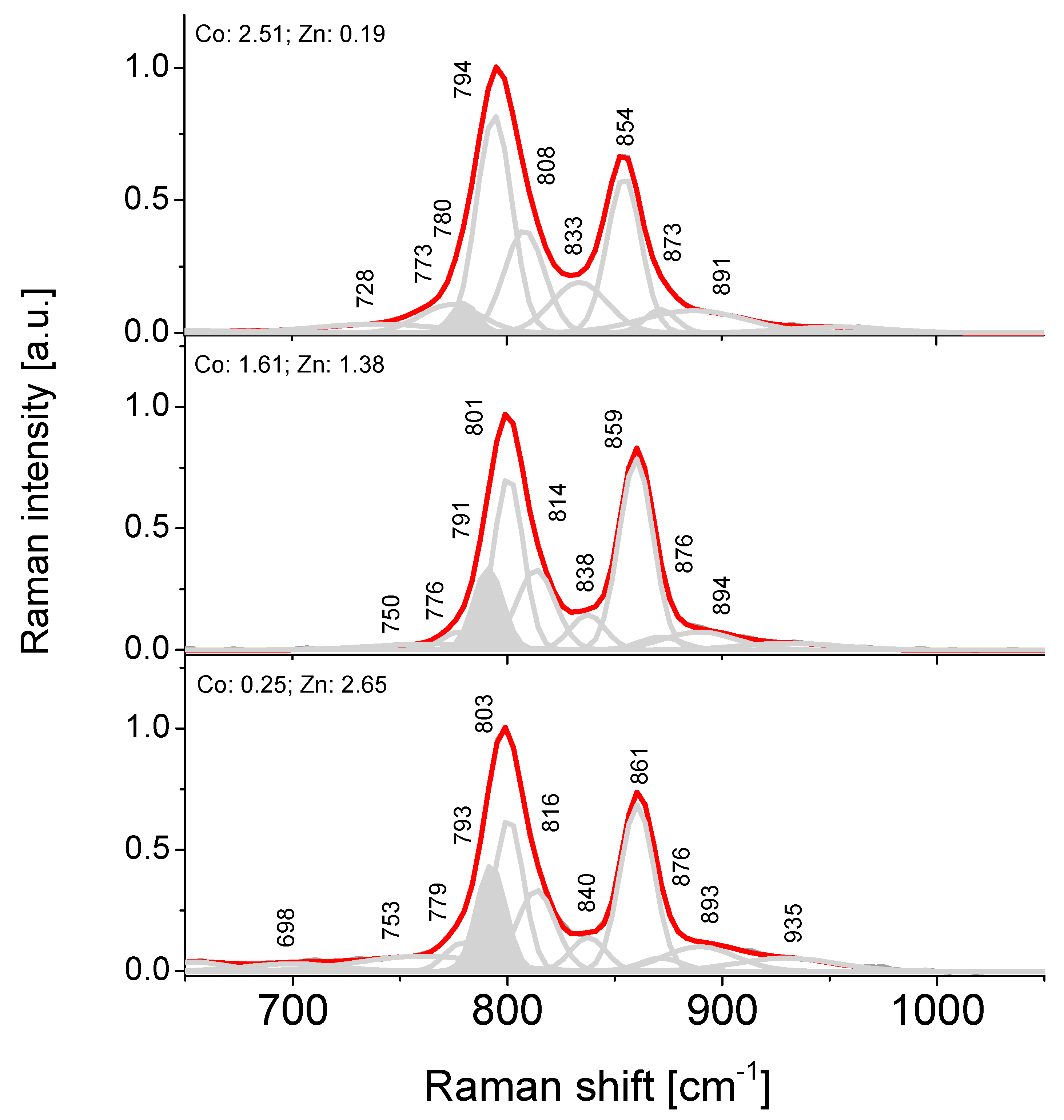
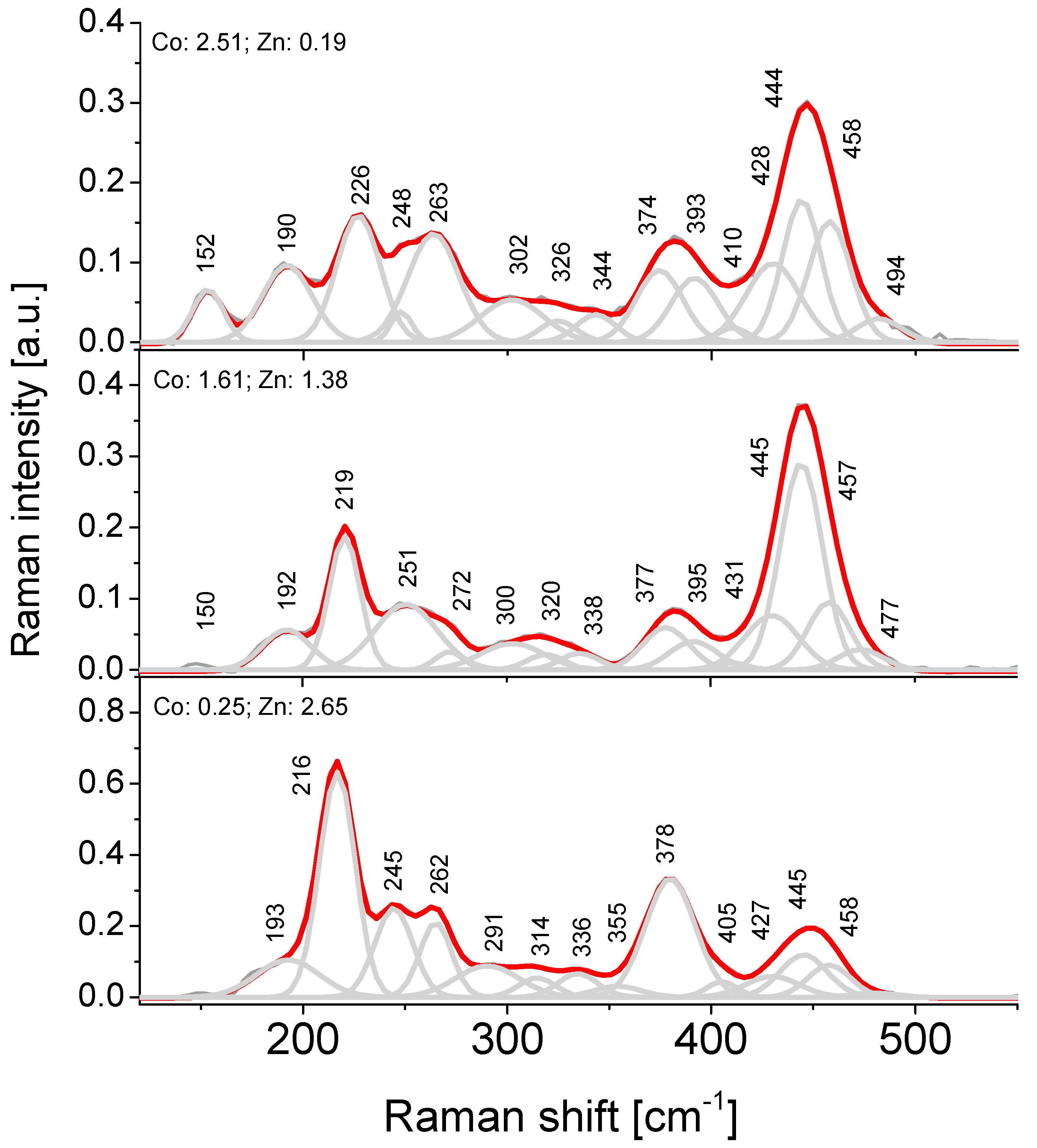
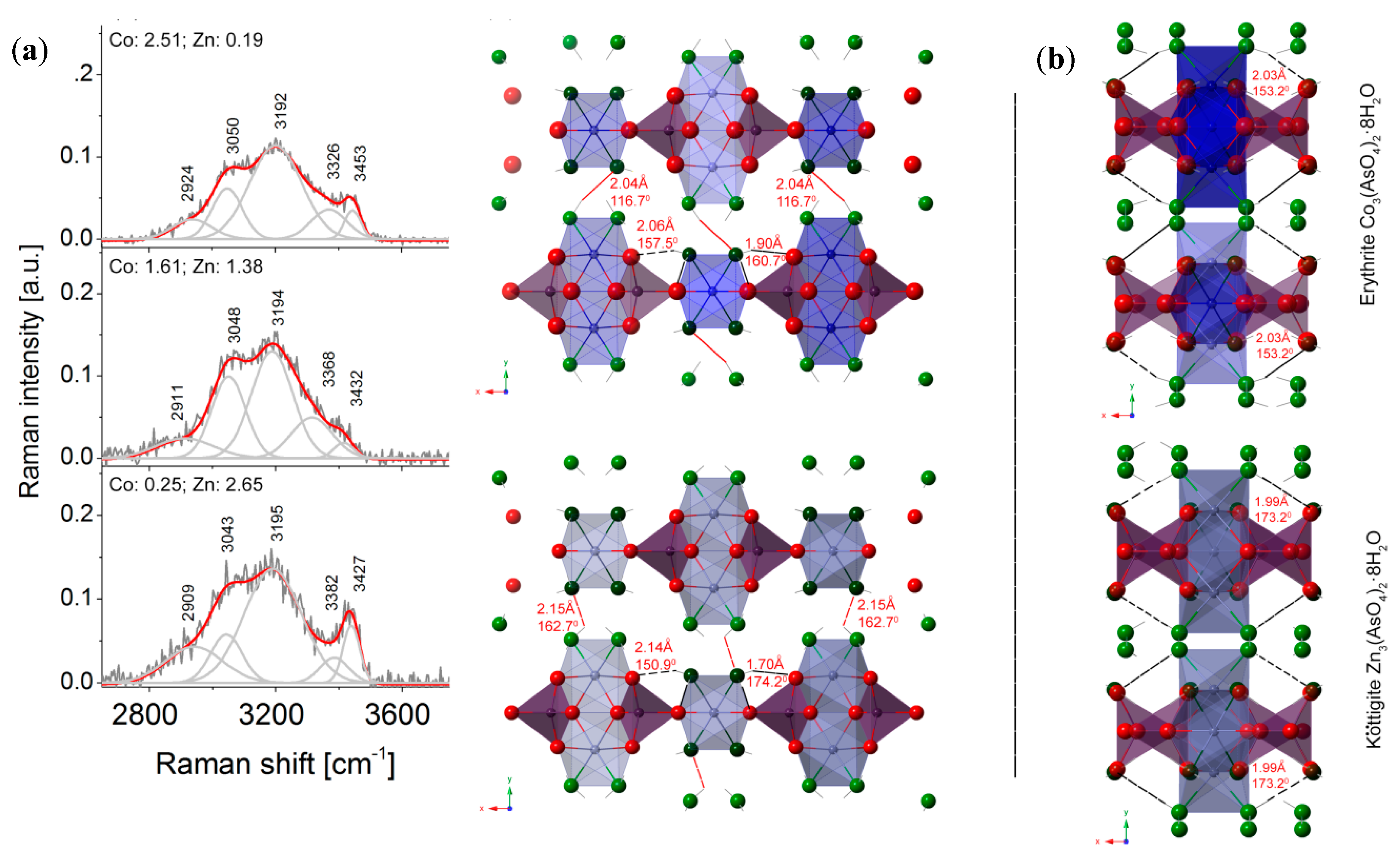
4.5. Raman Spectroscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wildner, M.; Giester, G.; Lengauer, C.; McCammon, C. Structure and crystal chemistry of vivianite-type compounds: Crystal structures of erythrite and annabergite with a Mössbauer study of erythrite. Eur. J. Miner. 1996, 8, 187–192. [Google Scholar] [CrossRef]
- Giuseppetti, G.; Tadini, C. The crystal structure of cabrerite, (Ni, Mg)3(AsO4)2·H2O, a variety of annabergite. Bull. Minéral. 1982, 105, 333–337. [Google Scholar] [CrossRef]
- Plášil, J.; Škácha, P.; Sejkora, J.; Škoda, R.; Novák, M.; Veselovský, F.; Hloušek, J. Babánekite, Cu3(AsO4)2 ∙8H2O, from Jáchymov, Czech Republic—A new member of the vivianite group. J. Geosci. 2017, 62, 261–270. [Google Scholar] [CrossRef]
- Capitelli, F.; Elaatmani, M.; Lalaoui, M.D.; Piniella, J.F. Crystal structure of a vivianite-type mineral: Mg-rich erythrite, (Co2.16Ni0.24Mg0.60)(AsO4)2·8H2O. Zeitschrift für Kristallographie -Cryst. Mater. 2007, 222, 676–679. [Google Scholar] [CrossRef]
- Antao, S.M.; Dhaliwal, I. Growth oscillatory zoning in erythrite, ideally Co3(AsO4)2·8H2O: Structural variations in vivianite-group minerals. Minerals 2017, 7, 136. [Google Scholar] [CrossRef]
- Hill, R.J. The crystal structure of köttigite. Am. Mineral. 1979, 64, 376–382. [Google Scholar]
- Yoshiasa, A.; Miyano, Y.; Isobe, H.; Sugiyama, K.; Arima, H.; Nakatsuka, A.; Momma, K.; Miyawaki, R. Structural refinement of köttigite–parasymplesite solid solution: Unique cation site occupancy and chemical bonding with water molecules. J. Miner. Pet. Sci. 2016, 111, 363–369. [Google Scholar] [CrossRef]
- Mori, H.; Ito, T. The structure of vivianite and symplesite. Acta Crystallogr. 1950, 3, 1–6. [Google Scholar] [CrossRef]
- Markl, G.; Marks, M.A.W.; Derrey, I.; Gühring, J.-E. Weathering of cobalt arsenides: Natural assemblages and calculated stability relations among secondary Ca-Mg-Co arsenates and carbonates. Am. Miner. 2014, 99, 44–56. [Google Scholar] [CrossRef]
- Dumańska-Słowik, M.; Pieczka, A.; Natkaniec-Nowak, L.; Kunecki, P.; Gaweł, A.; Heflik, W.; Smoliński, W.; Kozub-Budzyń, G. Mg-enriched erythrite from Bou Azzer, Anti-Atlas Mountains, Morocco: Geochemical and spectroscopic characteristics. Miner. Pet. 2018, 112, 381–392. [Google Scholar] [CrossRef]
- Siuda, R.; Macioch, A. Secondary arsenic minerals from the Złoty Stok As-Au abandoned mine (SW Poland). Geol. Quaterly 2018, 62, 925–940. [Google Scholar] [CrossRef]
- Anthony, J.W.; Bideaux, R.A.; Bladh, K.W.; Nichols, M.C. Handbook of Mineralogy: Arsenates, Phosphates, Vanadates; Mineral Data Publishing: Tucson, AZ, USA, 2000; Volume IV, p. 159. [Google Scholar]
- Jambor, J.L.; Dutrizac, J.E. Solid solutions in the annabergite-erythrite-hörnesite synthetic system. Can. Miner. 1995, 33, 1063–1071. [Google Scholar]
- Martens, W.N.; Kloprogge, J.T.; Frost, R.L.; Rintoul, L. Site occupancy of Co and Ni in erythrite-annabergite solid solutions deduced by vibrational spectroscopy. Can. Miner. 2005, 43, 1065–1075. [Google Scholar] [CrossRef]
- Wei, C.; Zhu, Y.; Zhang, X.; Wang, X.; Liu, J. Dissolution and solubility of the erythrite/annabergite solid solution [(CoxNi1-x)3(AsO4)2·8H2O] at 25 °C. Asian J. Chem. 2013, 25, 7687–7696. [Google Scholar] [CrossRef]
- Sturman, B.D. New data for köttigite and parasymplesite. Can. Miner. 1976, 14, 437–441. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies if interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Ciesielczuk, J.; Janeczek, J.; Dulski, M.; Krzykawski, T. Pseudomalachite–cornwallite and kipushite–philipsburgite solid solutions: Chemical composition and Raman spectroscopy. Eur. J. Miner. 2016, 28, 555–569. [Google Scholar] [CrossRef]
- Mochnacka, K.; Oberc-Dziedzic, T.; Mayer, W.; Pieczka, A. Ore mineralization related to geological evolution of the Karkonosze-Izera Massif (the Sudetes, Poland)–Towards a model. Ore Geol. Rev. 2015, 64, 215–238. [Google Scholar] [CrossRef]
- Parafiniuk, J.; Siuda, R.; Borkowski, A. Sulphate and arsenate minerals as environmental indicators in the weathering zones of selected ore deposits, Western Sudetes, Poland. Acta Geol. Pol. 2016, 66, 493–508. [Google Scholar] [CrossRef]
- Siuda, R.; Gołębiowska, B. New data on supergene minerals from Miedzianka-Ciechanowice deposit in the Rudawy Janowickie Mountains (Lower Silesia, Poland). Prz. Geol. 2011, 59, 226–234, (In Polish with English Abstract). [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar]
- Lee, J.S.; Nriagu, J.O. Stability constants for metal arsenates. Environ. Chem. 2007, 4, 123–133. [Google Scholar] [CrossRef]
- Charykova, M.V.; Krivovichev, O.S.; Yakovenko, O.S.; Depmeier, W. Thermodynamics of arsenates, selenites, and sulfates in the oxidation zone of sulfide ores: Part III: Eh–pH diagrams of the Me–As–H2O Systems (Me = Co, Ni, Fe, Cu, Zn, Pb) at 25°C. Geol. Ore Depos. 2011, 53, 501–513. [Google Scholar] [CrossRef]
- Magalhães, M.C.F.; Pedrosa de Jesus, J.D. The chemistry of formation of some secondary arsenate minerals of Cu(II), Zn(II) and Pb(II). Miner. Mag. 1988, 52, 679–690. [Google Scholar] [CrossRef]
- Frost, R.L.; Martens, W.N.; Williams, P.; Kloprogge, J.T. Raman spectroscopic study of the vivianite arsenate minerals. J. Raman Spectrosc. 2003, 34, 751–759. [Google Scholar] [CrossRef]
- Rojo, J.M.; Mesa, J.L.; Pizarro, J.L.; Lezama, L.; Arriotua, M.I.; Rojo, T. Spectroscopic and magnetic study of the (Mg,M)3(AsO4)2 8H2O (M = Ni2+, Co2+) arsenates. Mater. Res. Bull. 1996, 31, 925–934. [Google Scholar] [CrossRef]
- Frost, R.L.; Kloprogge, T.; Weier, M.L.; Martens, W.N.; Ding, Z.; Edwards, G.H. Raman spectroscopy of selected arsenates–implications for soil remediation. Spectrochim. Acta A 2003, 59, 2241–2246. [Google Scholar] [CrossRef]
- Martens, W.N.; Frost, R.L.; Kloprogge, T. Raman spectroscopy of synthetic erythrite, partially dehydrated erythrite and hydrothermally synthesized dehydrated erythrite. J. Raman Spectrosc. 2003, 34, 90–95. [Google Scholar] [CrossRef]
- Martens, W.N.; Kloprogge, T.; Frost, R.L.; Rintoul, L. Single-crystal Raman study of erythrite Co3(AsO4)2·H2O. J. Raman Spectrosc. 2004, 35, 208–216. [Google Scholar] [CrossRef][Green Version]
- Makreski, P.; Stefov, S.; Pejov, L.; Jovanovski, G. Theoretical and experimental study of the vibrational spectra of (para)symplesite and hornesite. Spectrochim. Acta A 2015, 144, 155–162. [Google Scholar] [CrossRef]
- Čejka, J.; Sejkora, J.; Bahfenne, S.; Palmer, S.J.; Plášil, J.; Frost, R.L. Raman spectroscopy of hydrogen-arsenate group (AsO3OH) in solid-state compounds: Cobalt mineral phase burgessite Co2(H2O)[AsO3OH]2·H2O. J. Raman Spectrosc. 2011, 42, 214–218. [Google Scholar] [CrossRef]
- Libowitzky, E. Correlation of the O-H stretching frequencies and the OH… H hydrogen bond lengths in minerals. Monatschefte für Chemie 1999, 130, 1047–1049. [Google Scholar]




| Erythrite Co3(AsO4)2·8H2O | Annabergite Ni3(AsO4)2·8H2O | Köttigite Zn3(AsO4)2·8H2O | Hörnesite Mg3(AsO4)2·8H2O | Parasymplesite Fe3(AsO4)2·8H2O | Ref. |
|---|---|---|---|---|---|
| 93 | 2 | 4 | - | 1 | [5] |
| 89 | - | - | - | 11 | [12] |
| 84 | 3 | - | 11 | 2 | [10] |
| 81 | 5 | - | 12 | 2 | [10] |
| 75 | 5 | 1 | 19 | 1 | [10] |
| 72 | 8 | - | 20 | - | [4] |
| 71 | 5 | 1 | 22 | 1 | [10] |
| 67 | 8 | - | - | 25 | [1] |
| 67 | 10 | - | 20 | - | [11] # |
| 50 | 5 | - | 45 | - | [10] |
| 49 | 5 | - | 46 | - | [10] |
| 46 | 8 | - | 42 | - | [11] # |
| 22 | 43 | - | 30 | - | [11] # |
| 19 | 51 | - | 27 | - | [11] |
| 19 | 5 | 76 | - | - | [16] |
| 14 | 5 | 81 | - | - | [16] |
| - | 83 | - | 17 | - | [1] |
| - | 74 | - | 23 | 3 | [2] |
| - | - | 46 | - | 54 | [7] |
| - | - | 44 | - | 56 | [7] |
| Crystal Data | |
|---|---|
| Chemical formula | As4Co2.16H32O32Zn3.84 |
| Mr | 1222.24 |
| Crystal system, space group | Monoclinic, C2/m |
| Temperature (K) | 293 |
| a, b, c (Å) | 10.2588 (3), 13.4200 (4), 4.76200 (14) |
| β (°) | 105.232 (3) |
| V (Å3) | 632.56 (3) |
| Z | 1 |
| F(000) | 594 |
| Radiation type | Mo Kα |
| µ (mm−1) | 10.29 |
| Crystal size (mm) | 0.18 × 0.09 × 0.07 |
| Data Collection | |
| Absorption correction | Multi-scan CrysAlis PRO 1.171.38.41q (Rigaku Oxford Diffraction, 2015) Empirical absorption correction using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm. |
| Tmin, Tmax | 0.528, 1.000 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 2474, 676, 661 |
| Rint | 0.014 |
| Θ values (°) | Θmax = 26.4, Θmin = 3.0 |
| (sin Θ/λ)max (Å−1) | 0.625 |
| Range of h, k, l | h = −12 → 12, k = −16 → 12, l = −5 → 5 |
| Refinement | |
| R[F2 > 2σ(F2)], wR(F2), S | 0.021, 0.060, 1.15 |
| No. of reflections | 676 |
| No. of parameters | 55 |
| No. of restraints | 6 |
| H-atom treatment | H-atom parameters constrained |
| Δ > max, Δ > min (e Å−3) | 0.77, −0.75 |
| Analysis Number | CoO | ZnO | MgO | NiO | FeO | As2O5 | P2O5 | SO2 | CaO | SiO2 | Total | Co | Zn | Mg | Ni | Fe | Mn | ΣX | As | P | ΣA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E | 37.54 | - | - | - | - | 38.39 | - | - | - | - | 75.93 | 3.00 | - | - | - | - | - | 3.00 | 2.00 | - | 2.00 |
| 1 | 34.17 | 0.73 | 0.87 | 0.41 | 0.13 | 37.86 | 0.02 | 0.01 | 0.21 | 0.05 | 74.46 | 2.77 | 0.05 | 0.13 | 0.03 | 0.01 | 0.00 | 3.00 | 2.00 | 0.00 | 2.00 |
| 2 | 33.85 | 1.19 | 0.75 | 1.12 | 0.00 | 38.75 | 0.09 | 0.05 | 0.37 | 0.06 | 76.22 | 2.69 | 0.09 | 0.11 | 0.09 | 0.00 | 0.00 | 2.98 | 2.01 | 0.01 | 2.02 |
| 3 | 33.36 | 1.71 | 0.82 | 0.63 | 0.11 | 38.49 | 0.00 | 0.00 | 0.25 | 0.01 | 75.50 | 2.67 | 0.13 | 0.12 | 0.05 | 0.01 | 0.01 | 2.99 | 2.01 | 0.00 | 2.01 |
| 4 | 32.58 | 0.62 | 1.24 | 1.10 | 0.07 | 37.72 | 0.03 | 0.10 | 0.25 | 0.02 | 73.75 | 2.66 | 0.05 | 0.19 | 0.09 | 0.01 | 0.00 | 2.99 | 2.01 | 0.00 | 2.01 |
| 5 | 31.77 | 2.35 | 0.60 | 1.06 | 0.05 | 37.79 | 0.00 | 0.16 | 0.13 | 0.27 | 74.22 | 2.61 | 0.18 | 0.09 | 0.09 | 0.00 | 0.00 | 2.98 | 2.02 | 0.00 | 2.02 |
| 6 | 31.72 | 0.22 | 1.05 | 2.00 | 0.27 | 37.66 | 0.06 | 0.27 | 0.64 | 0.23 | 74.19 | 2.61 | 0.02 | 0.16 | 0.16 | 0.02 | 0.01 | 2.98 | 2.02 | 0.01 | 2.02 |
| 7 | 31.44 | 2.54 | 1.03 | 0.41 | 0.19 | 37.42 | 0.00 | 0.21 | 0.47 | 0.07 | 73.85 | 2.59 | 0.19 | 0.16 | 0.03 | 0.02 | 0.01 | 2.99 | 2.01 | 0.00 | 2.01 |
| 8 | 30.23 | 1.53 | 1.25 | 1.20 | 0.03 | 36.75 | 0.02 | 0.05 | 0.46 | 0.45 | 72.01 | 2.55 | 0.12 | 0.20 | 0.10 | 0.00 | 0.00 | 2.98 | 2.02 | 0.00 | 2.02 |
| 9 | 30.79 | 0.11 | 1.74 | 1.39 | 0.10 | 37.14 | 0.00 | 0.19 | 0.52 | 0.02 | 72.74 | 2.54 | 0.01 | 0.27 | 0.12 | 0.01 | 0.06 | 3.00 | 2.00 | 0.00 | 2.00 |
| 10 | 30.81 | 0.38 | 2.08 | 1.51 | 0.05 | 37.12 | 0.04 | 0.13 | 0.57 | 0.01 | 72.80 | 2.53 | 0.03 | 0.32 | 0.12 | 0.00 | 0.01 | 3.01 | 1.99 | 0.00 | 1.99 |
| 11 | 30.14 | 2.00 | 1.27 | 1.07 | 0.00 | 36.80 | 0.00 | 0.01 | 0.59 | 0.00 | 72.11 | 2.53 | 0.15 | 0.20 | 0.09 | 0.00 | 0.02 | 2.99 | 2.01 | 0.00 | 2.01 |
| 12 | 30.59 | 0.00 | 1.58 | 2.00 | 0.28 | 37.04 | 0.00 | 0.08 | 0.82 | 0.26 | 73.30 | 2.52 | 0.00 | 0.24 | 0.17 | 0.02 | 0.06 | 3.01 | 1.99 | 0.00 | 1.99 |
| 13 | 29.83 | 4.61 | 1.11 | 0.24 | 0.18 | 37.08 | 0.00 | 0.21 | 0.19 | 0.15 | 73.59 | 2.46 | 0.35 | 0.17 | 0.02 | 0.02 | 0.00 | 3.01 | 1.99 | 0.00 | 1.99 |
| 14 | 29.83 | 3.21 | 1.25 | 0.96 | 0.19 | 37.95 | 0.01 | 0.12 | 0.63 | 0.11 | 74.51 | 2.43 | 0.24 | 0.19 | 0.08 | 0.02 | 0.02 | 2.98 | 2.02 | 0.00 | 2.02 |
| 15 | 29.62 | 0.00 | 2.59 | 1.57 | 0.10 | 37.59 | 0.04 | 0.25 | 0.70 | 0.00 | 72.70 | 2.43 | 0.00 | 0.39 | 0.13 | 0.01 | 0.02 | 2.98 | 2.01 | 0.00 | 2.02 |
| 16 | 28.48 | 5.15 | 1.49 | 0.46 | 0.17 | 38.26 | 0.00 | 0.04 | 0.43 | 0.13 | 74.72 | 2.31 | 0.38 | 0.22 | 0.04 | 0.01 | 0.01 | 2.98 | 2.02 | 0.00 | 2.02 |
| 17 | 28.05 | 8.25 | 0.37 | 0.31 | 0.00 | 37.84 | 0.20 | 0.04 | 0.58 | 0.05 | 75.69 | 2.27 | 0.62 | 0.06 | 0.04 | 0.00 | 0.00 | 2.98 | 2.00 | 0.02 | 2.02 |
| 18 | 27.68 | 7.94 | 0.91 | 0.44 | 0.05 | 37.57 | 0.06 | 0.02 | 0.38 | 0.40 | 75.45 | 2.24 | 0.59 | 0.14 | 0.04 | 0.00 | 0.00 | 3.01 | 1.98 | 0.01 | 1.99 |
| 19 | 26.61 | 5.37 | 1.36 | 0.55 | 0.75 | 36.83 | 0.01 | 0.06 | 0.12 | 0.03 | 71.72 | 2.24 | 0.42 | 0.21 | 0.05 | 0.07 | 0.00 | 2.98 | 2.02 | 0.00 | 2.02 |
| 20 | 27.66 | 1.70 | 3.67 | 0.99 | 0.24 | 37.59 | 0.00 | 0.03 | 0.53 | 0.00 | 72.60 | 2.23 | 0.13 | 0.55 | 0.08 | 0.02 | 0.02 | 3.02 | 1.98 | 0.00 | 1.98 |
| 21 | 26.64 | 4.44 | 2.68 | 0.40 | 0.25 | 37.22 | 0.00 | 0.13 | 0.25 | 0.76 | 72.98 | 2.19 | 0.34 | 0.41 | 0.03 | 0.02 | 0.02 | 3.01 | 1.99 | 0.00 | 1.99 |
| 22 | 28.24 | 9.94 | 0.46 | 0.78 | 0.00 | 39.45 | 0.20 | 0.03 | 0.38 | 0.20 | 79.74 | 2.18 | 0.71 | 0.07 | 0.03 | 0.00 | 0.01 | 2.99 | 1.99 | 0.02 | 2.01 |
| 23 | 27.44 | 2.90 | 3.56 | 0.70 | 0.32 | 38.46 | 0.00 | 0.08 | 0.28 | 0.09 | 74.10 | 2.17 | 0.21 | 0.52 | 0.06 | 0.03 | 0.02 | 3.01 | 1.99 | 0.00 | 1.99 |
| 24 | 27.29 | 9.54 | 0.33 | 0.49 | 0.07 | 38.45 | 0.22 | 0.06 | 0.37 | 0.08 | 77.04 | 2.17 | 0.70 | 0.05 | 0.05 | 0.01 | 0.01 | 2.99 | 1.99 | 0.02 | 2.01 |
| 25 | 26.54 | 9.19 | 0.34 | 0.64 | 0.38 | 37.18 | 0.10 | 0.09 | 0.26 | 0.38 | 75.14 | 2.17 | 0.69 | 0.05 | 0.06 | 0.03 | 0.00 | 3.01 | 1.98 | 0.01 | 1.99 |
| 26 | 26.11 | 9.41 | 0.49 | 0.46 | 0.14 | 37.03 | 0.00 | 0.06 | 0.30 | 0.17 | 74.17 | 2.16 | 0.72 | 0.07 | 0.04 | 0.01 | 0.00 | 3.00 | 2.00 | 0.00 | 2.00 |
| 27 | 27.74 | 2.32 | 4.04 | 0.88 | 0.05 | 39.69 | 0.02 | 0.16 | 0.27 | 0.00 | 75.38 | 2.15 | 0.17 | 0.58 | 0.07 | 0.00 | 0.02 | 2.99 | 2.01 | 0.00 | 2.01 |
| 28 | 27.42 | 6.94 | 2.09 | 0.58 | 0.35 | 40.19 | 0.01 | 0.06 | 0.25 | 0.19 | 78.21 | 2.11 | 0.49 | 0.30 | 0.04 | 0.03 | 0.01 | 2.98 | 2.02 | 0.00 | 2.02 |
| 29 | 25.64 | 6.79 | 1.70 | 0.86 | 0.32 | 37.41 | 0.00 | 0.11 | 0.32 | 0.04 | 73.40 | 2.11 | 0.51 | 0.26 | 0.07 | 0.03 | 0.02 | 3.00 | 2.00 | 0.00 | 2.00 |
| 30 | 24.88 | 6.15 | 3.00 | 0.53 | 0.14 | 37.33 | 0.03 | 0.00 | 0.25 | 2.20 | 74.72 | 2.03 | 0.46 | 0.45 | 0.04 | 0.01 | 0.02 | 3.02 | 1.98 | 0.00 | 1.98 |
| 31 | 25.46 | 13.26 | 0.36 | 0.50 | 0.06 | 39.42 | 0.04 | 0.28 | 0.64 | 0.21 | 80.35 | 1.96 | 0.94 | 0.05 | 0.04 | 0.00 | 0.01 | 3.01 | 1.98 | 0.00 | 1.99 |
| 32 | 23.66 | 12.93 | 0.38 | 0.37 | 0.35 | 37.41 | 0.11 | 0.06 | 0.29 | 0.35 | 75.99 | 1.92 | 0.97 | 0.06 | 0.03 | 0.03 | 0.01 | 3.01 | 1.98 | 0.01 | 1.99 |
| 33 | 24.04 | 12.52 | 0.53 | 0.70 | 0.09 | 39.09 | 0.00 | 0.61 | 0.18 | 0.14 | 78.01 | 1.91 | 0.92 | 0.08 | 0.06 | 0.01 | 0.01 | 2.98 | 2.02 | 0.00 | 2.02 |
| 34 | 17.18 | 18.19 | 0.15 | 0.47 | 0.09 | 36.13 | 0.00 | 0.16 | 0.08 | 0.08 | 72.53 | 1.47 | 1.44 | 0.02 | 0.04 | 0.01 | 0.00 | 2.98 | 2.02 | 0.00 | 2.02 |
| 35 | 16.10 | 22.50 | 0.23 | 0.06 | 0.12 | 38.82 | 0.03 | 0.00 | 0.09 | 0.09 | 78.03 | 1.27 | 1.64 | 0.03 | 0.04 | 0.01 | 0.00 | 3.00 | 2.00 | 0.00 | 2.00 |
| 36 | 15.92 | 25.63 | 0.11 | 0.25 | 0.45 | 41.89 | 0.05 | 0.35 | 0.12 | 0.18 | 84.94 | 1.17 | 1.73 | 0.01 | 0.03 | 0.03 | 0.00 | 2.99 | 2.01 | 0.00 | 2.01 |
| 37 | 12.60 | 25.21 | 0.36 | 0.19 | 0.05 | 38.45 | 0.07 | 0.00 | 0.10 | 0.14 | 77.17 | 1.01 | 1.87 | 0.05 | 0.04 | 0.00 | 0.00 | 2.98 | 2.02 | 0.01 | 2.02 |
| 38 | 12.31 | 30.00 | 0.17 | 0.14 | 0.25 | 41.70 | 0.06 | 0.01 | 0.02 | 0.16 | 84.84 | 0.91 | 2.03 | 0.02 | 0.01 | 0.02 | 0.00 | 2.99 | 2.00 | 0.00 | 2.01 |
| 39 | 11.76 | 32.19 | 0.23 | 0.13 | 0.42 | 43.68 | 0.00 | 0.00 | 0.11 | 0.20 | 88.72 | 0.83 | 2.09 | 0.03 | 0.01 | 0.03 | 0.00 | 2.99 | 2.01 | 0.00 | 2.01 |
| 40 | 11.21 | 32.42 | 0.12 | 0.29 | 0.36 | 43.53 | 0.00 | 0.21 | 0.08 | 0.25 | 88.48 | 0.80 | 2.12 | 0.02 | 0.02 | 0.03 | 0.00 | 2.98 | 2.02 | 0.00 | 2.02 |
| 41 | 11.22 | 33.86 | 0.22 | 0.14 | 0.07 | 43.34 | 0.11 | 0.12 | 0.09 | 0.46 | 89.62 | 0.79 | 2.18 | 0.03 | 0.01 | 0.01 | 0.00 | 3.01 | 1.98 | 0.01 | 1.99 |
| 42 | 10.15 | 32.03 | 0.19 | 0.08 | 0.08 | 41.21 | 0.11 | 0.21 | 0.15 | 1.00 | 85.31 | 0.76 | 2.19 | 0.03 | 0.00 | 0.01 | 0.01 | 2.99 | 2.00 | 0.01 | 2.01 |
| 43 | 9.94 | 33.69 | 0.27 | 0.48 | 0.09 | 42.83 | 0.03 | 0.00 | 0.00 | 0.40 | 87.73 | 0.71 | 2.22 | 0.04 | 0.03 | 0.01 | 0.00 | 3.00 | 1.99 | 0.00 | 2.00 |
| 44 | 8.18 | 36.04 | 0.24 | 0.16 | 0.45 | 43.88 | 0.03 | 0.15 | 0.00 | 0.15 | 89.28 | 0.58 | 2.33 | 0.03 | 0.01 | 0.03 | 0.00 | 2.99 | 2.01 | 0.00 | 2.01 |
| 45 | 7.21 | 36.62 | 0.23 | 0.18 | 0.03 | 42.60 | 0.00 | 0.04 | 0.00 | 0.31 | 87.23 | 0.52 | 2.43 | 0.03 | 0.01 | 0.00 | 0.00 | 3.00 | 2.00 | 0.00 | 2.00 |
| 46 | 7.03 | 37.11 | 0.37 | 0.00 | 0.20 | 43.01 | 0.00 | 0.05 | 0.15 | 0.42 | 88.34 | 0.50 | 2.44 | 0.05 | 0.00 | 0.02 | 0.00 | 3.00 | 2.00 | 0.00 | 2.00 |
| 47 | 6.95 | 37.54 | 0.42 | 0.00 | 0.40 | 43.37 | 0.08 | 0.07 | 0.24 | 0.35 | 89.44 | 0.49 | 2.43 | 0.06 | 0.00 | 0.03 | 0.00 | 3.01 | 1.99 | 0.01 | 1.99 |
| 48 | 6.78 | 38.47 | 0.22 | 0.03 | 0.00 | 43.55 | 0.17 | 0.00 | 0.17 | 0.54 | 89.91 | 0.48 | 2.49 | 0.03 | 0.00 | 0.00 | 0.00 | 2.99 | 1.99 | 0.01 | 2.01 |
| 49 | 5.30 | 39.13 | 0.22 | 0.00 | 0.25 | 43.29 | 0.35 | 0.02 | 0.13 | 0.21 | 88.96 | 0.38 | 2.55 | 0.03 | 0.00 | 0.02 | 0.00 | 2.98 | 2.00 | 0.03 | 2.02 |
| 50 | 4.54 | 39.85 | 0.20 | 0.00 | 0.24 | 42.98 | 0.00 | 0.07 | 0.12 | 0.28 | 88.26 | 0.32 | 2.63 | 0.03 | 0.00 | 0.02 | 0.00 | 3.00 | 2.01 | 0.00 | 2.01 |
| K | - | 39.50 | - | - | - | 37.18 | - | - | - | - | 76.68 | - | 3.00 | - | - | - | - | 3.00 | 2.00 | - | 2.00 |
| x | y | z | Ueq (Å2) | |
|---|---|---|---|---|
| As | 0.31553(3) | 0 | 0.37387(7) | 0.0080(1) |
| (Zn,Co)1 | 0 | 0 | 0 | 0.0106(2) |
| (Zn,Co)2 | 0 | 0.38524(3) | 0 | 0.0104(2) |
| O1 | 0.1490(2) | 0 | 0.3751(5) | 0.0124(7) |
| O2 | 0.4051(3) | 0 | 0.7254(5) | 0.0119(7) |
| O3 | 0.34275(18) | 0.10662(13) | 0.2115(4) | 0.0121(5) |
| O4 | 0.09853(18) | 0.11482(13) | 0.8081(4) | 0.0144(5) |
| O5 | 0.39981(19) | 0.22666(15) | 0.7149(4) | 0.0186(5) |
| As–O1 | 1.710(3) | O3–As–O2(x2) | 110.08(7) | |
| As–O2 | 1.685(2) | O3i–As–O3(x2) | 116.33(12) | |
| As–O3(x2) | 1.6843(18) | O3–As–O1(x2) | 106.73(7) | |
| <As–O> | 1.691(2) | O2–As–O1 | 106.31(12) | |
| (Zn,Co)1–O1(x2) | 2.023(2) | O1– (Zn,Co)1–O1ii | 180.0 | |
| (Zn,Co)1–O4ii(x4) | 2.1718(18) | O1– (Zn,Co)1–O4ii(x4) | 87.46(7) | |
| <(Zn,Co)1–O> | 2.122(2) | O1– (Zn,Co)1–O4iv(x4) | 92.54(7) | |
| O4ii– (Zn,Co)1–O4iv(x2) | 180.00(11) | |||
| O4ii– (Zn,Co)1–O4v(x2) | 89.62(10) | |||
| O4iv– (Zn,Co)1–O4v(x2) | 90.38(10) | |||
| (Zn,Co)2–O2vi(x2) | 2.0883(17) | O2vi– (Zn,Co)2–O2ix | 84.97(10) | |
| (Zn,Co)2–O5vii(x2) | 2.1027(19) | O2vi– (Zn,Co)2–O5vii (x2) | 178.02(7) | |
| (Zn,Co)2–O3viii(x2) | 2.1171(18) | O2ix– (Zn,Co)2–O5vii (x2) | 93.10(8) | |
| <(Zn,Co)2–O> | 2.1027(18) | O5vii– (Zn,Co)2–O5vi | 88.84(11) | |
| O2vi– (Zn,Co)2–O3viii (x2) | 88.37(9) | |||
| O2ix– (Zn,Co)2–O3viii (x2) | 87.27(9) | |||
| O5vii– (Zn,Co)2–O3viii (x2) | 91.95(7) | |||
| O5vi– (Zn,Co)2–O3viii (x2) | 92.27(7) | |||
| O3viii– (Zn,Co)2–O3x | 174.08(10) | |||
| D—H···A | D—H | H···A | D···A | D—H···A |
|---|---|---|---|---|
| O4—H41···O5 i | 0.96 | 2.56 | 3.109 (3) | 116.1 |
| O4—H41···O5 ii | 0.96 | 2.38 | 2.899 (3) | 113.7 |
| O4—H43···O1 | 0.96 | 1.84 | 2.730 (3) | 153.4 |
| O5—H53···O4 iii | 0.96 | 2.01 | 2.899 (3) | 152.3 |
| O5—H54···O3 | 0.96 | 1.90 | 2.819 (3) | 159.4 |
| Member of the Solid Solution | Köttigite mol% | M1-O | M2-O | As-O | O-As-O |
|---|---|---|---|---|---|
| Erythrite [4] | 4 | 2.122(1) | 2.088(1) | 1.710(1) | 109.36(3) |
| Co-köttigite | 64 | 2.122(2) | 2.103(2) | 1.691(2) | 109.41(8) |
| Köttigite [5] | 81 | 2.115(5) | 2.100(5) | 1.682(5) | 109.4(2) |
| ER2 | Erythrite [5] | Hof | Na31 | Na27 | ER1 | Na29 | Köttigite [6] | |
|---|---|---|---|---|---|---|---|---|
| a, Å | 10.2591(6) | 10.2480 | 10.2657(9) | 10.2657(1) | 10.2669(2) | 10.2458(1) | 10.2567(1) | 10.241(3) |
| b, Å | 13.4091(6) | 13.4249 | 13.4355(8) | 13.4264(2) | 13.4240(2) | 13.4196(1) | 13.4247(2) | 13.405(3) |
| c, Å | 4.7635(6) | 4.7559 | 4.7640(6) | 4.7616(1) | 4.7626(1) | 4.7580(9) | 4.7575(1) | 4.757(2) |
| βo | 105.077(9) | 105.1116 | 105.126(2) | 105.1379(3) | 105.1372(4) | 105.162(2) | 105.1658(4) | 105.21(2) |
| V, Å3 | 632.739(7) | 631.680 | 634.305(7) | 633.519 | 633.627(3) | 631.439(2) | 632.265(2) | 630.168 |
| Co/(Co + Zn) | 1.00 | 0.96 | - | 0.71 | - | 0.60 | 0.35 | 0.15 |
| Co (apfu) | 2.72 (n = 28) | 2.78 | Co >> Zn | 2.04 (n = 75) | Co>Zn | 1.69 (n = 117) | 1.00 (n = 52) | 0.42 |
| Zn (apfu] | 0.01 (n = 28) | 0.11 | 0.83 (n = 75) | 1.11 (n = 117) | 1.87 (n = 52) | 2.44 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciesielczuk, J.; Dulski, M.; Janeczek, J.; Krzykawski, T.; Kusz, J.; Szełęg, E. Crystal Chemistry of an Erythrite-Köttigite Solid Solution (Co3–xZnx) (AsO4)2·8H2O. Minerals 2020, 10, 548. https://doi.org/10.3390/min10060548
Ciesielczuk J, Dulski M, Janeczek J, Krzykawski T, Kusz J, Szełęg E. Crystal Chemistry of an Erythrite-Köttigite Solid Solution (Co3–xZnx) (AsO4)2·8H2O. Minerals. 2020; 10(6):548. https://doi.org/10.3390/min10060548
Chicago/Turabian StyleCiesielczuk, Justyna, Mateusz Dulski, Janusz Janeczek, Tomasz Krzykawski, Joachim Kusz, and Eligiusz Szełęg. 2020. "Crystal Chemistry of an Erythrite-Köttigite Solid Solution (Co3–xZnx) (AsO4)2·8H2O" Minerals 10, no. 6: 548. https://doi.org/10.3390/min10060548
APA StyleCiesielczuk, J., Dulski, M., Janeczek, J., Krzykawski, T., Kusz, J., & Szełęg, E. (2020). Crystal Chemistry of an Erythrite-Köttigite Solid Solution (Co3–xZnx) (AsO4)2·8H2O. Minerals, 10(6), 548. https://doi.org/10.3390/min10060548