Bioinformatics and Transcriptional Study of the Nramp Gene in the Extreme Acidophile Acidithiobacillus ferrooxidans Strain DC
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Growth Curves
2.3. Cell Collection
2.4. General DNA Techniques
2.5. RNA Extraction and Reverse Transcription Quantitative Real-Time PCR (RT-qPCR)
2.6. Bioinformatic Analysis
2.7. Expression Stability of Candidate Reference Genes
2.8. Nucleotide Sequence Accession Number
3. Results
3.1. Identification and Sequencing of AFE_2126 Gene from At. Ferrooxidans DC
3.2. Bioinformatic Analysis of the AFE_2126 from At. Ferrooxidans DC
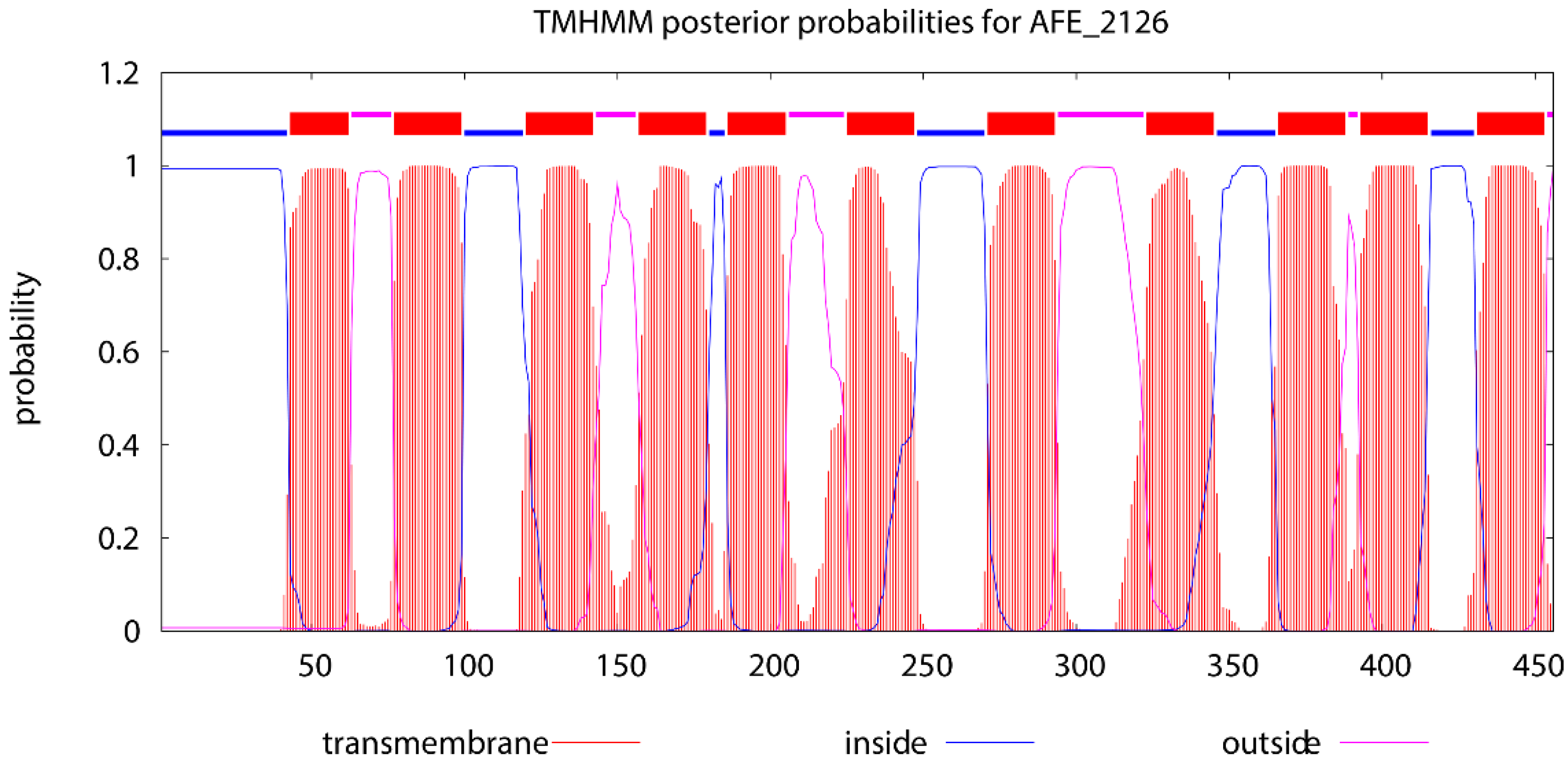
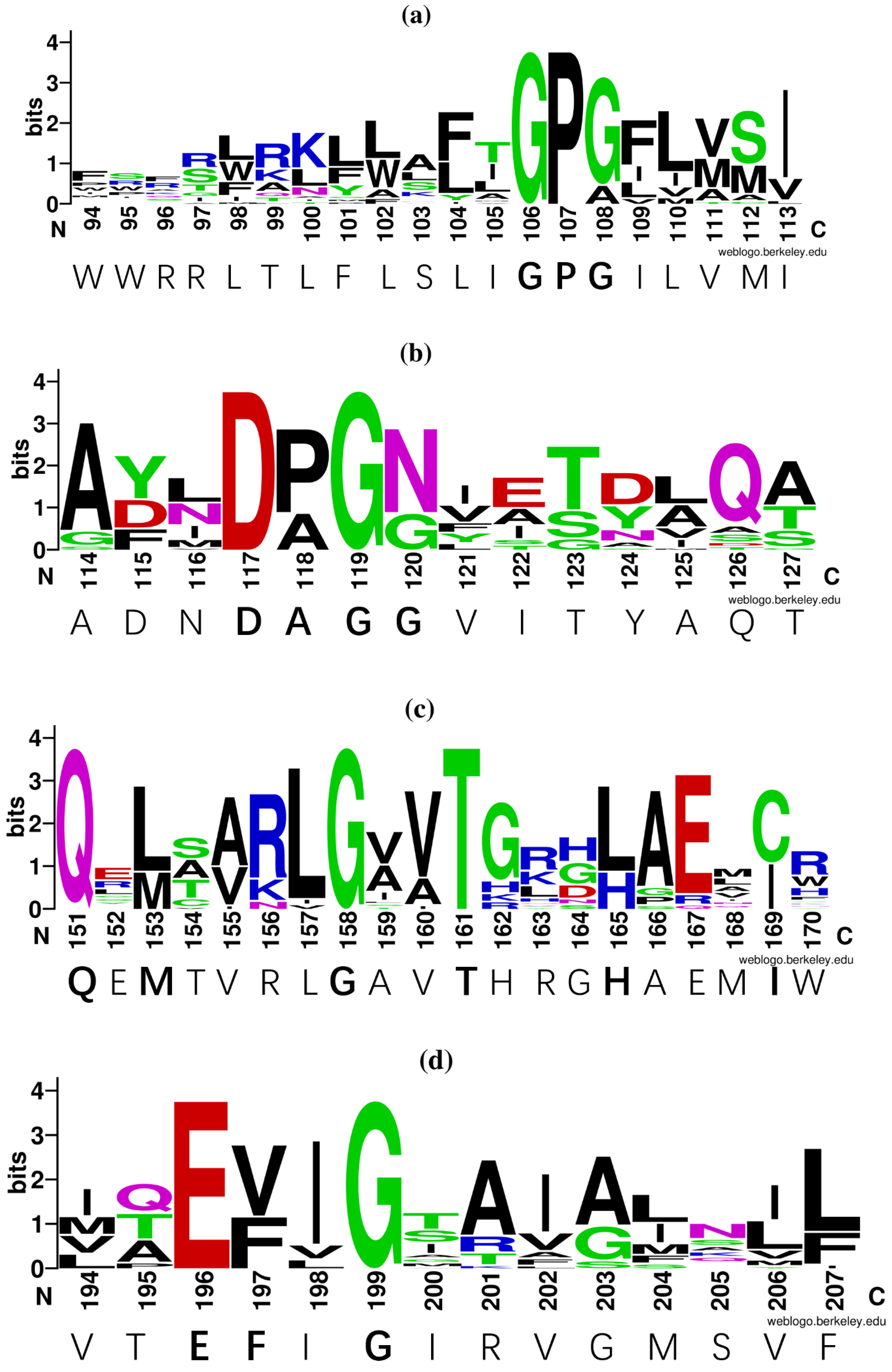
3.3. Prediction of Conserved Sites and Domains of the NRAMP Homolog
3.4. Selection and Evaluation of Reference Genes
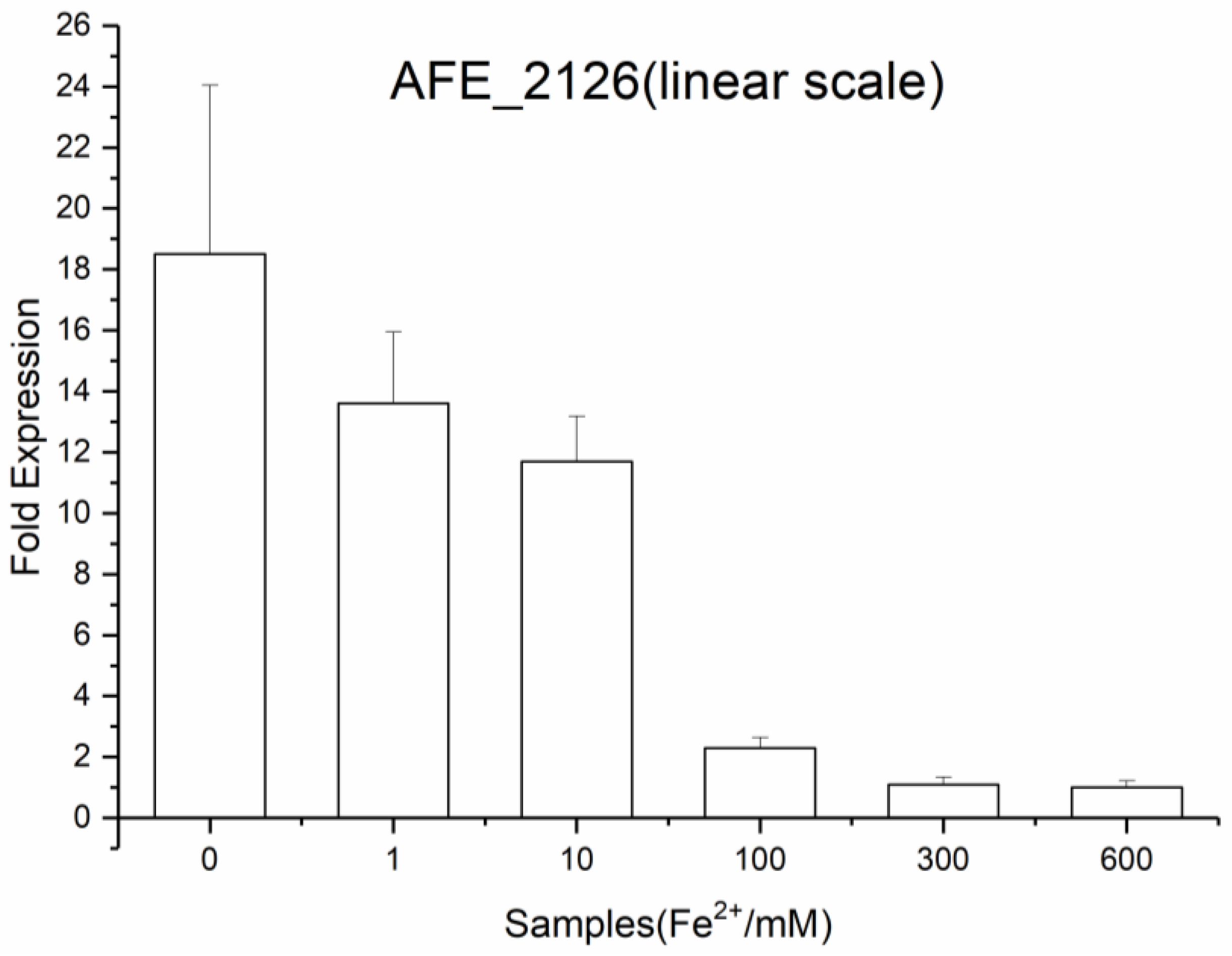
3.5. Expression of NRAMP in At. Ferrooxidans DC in Different Growth Conditions
4. Discussion
4.1. The AFE_2126 Protein Belongs to the NRAMP Family of Membrane Transporters
4.2. AFE_2126 Protein has Many Conserved Motifs Associated with Metal Ion Transport
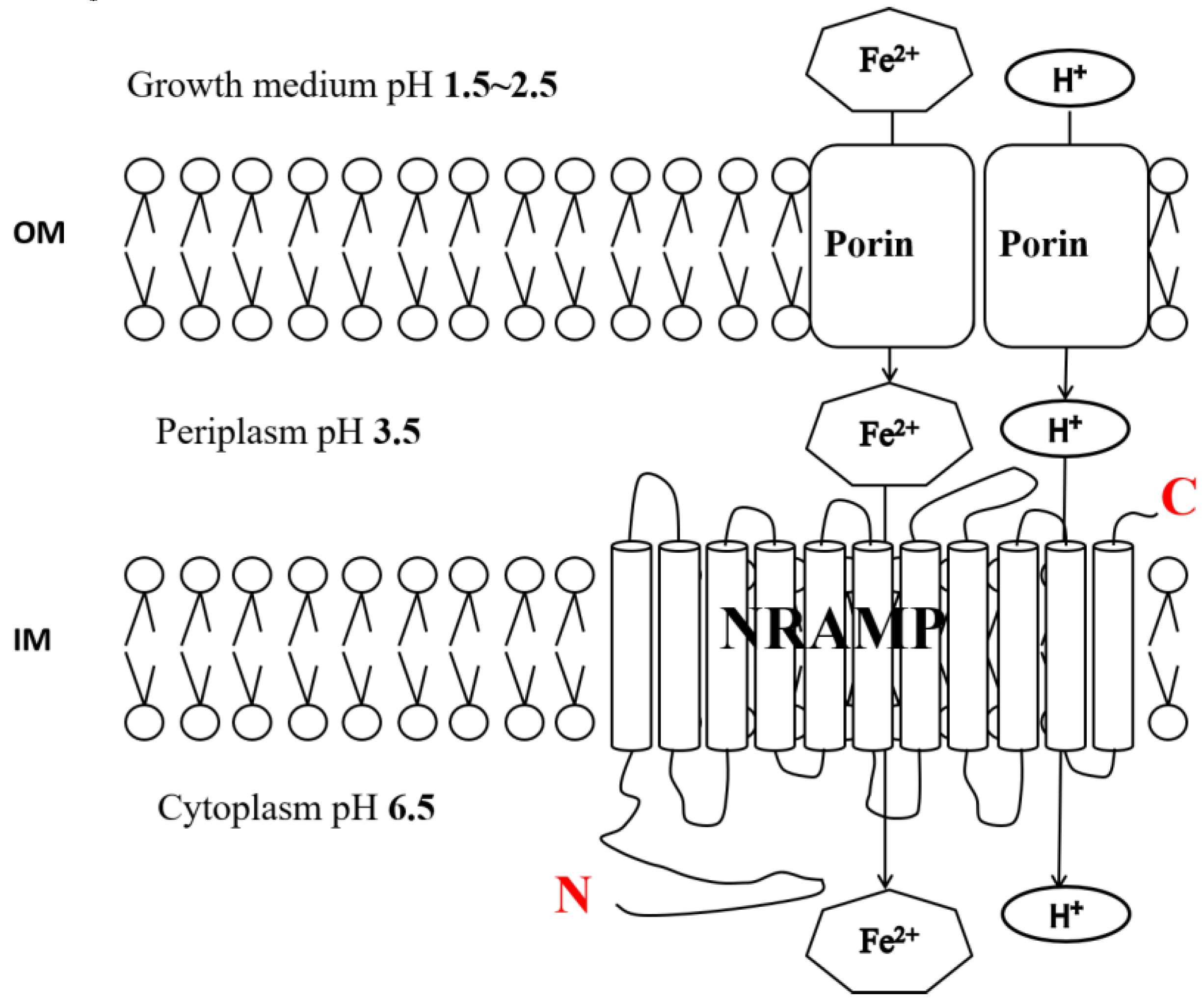
4.3. AFE_2126 Protein Mediates the Uptake of Fe2+
4.4. AFE_2126 Protein is a Symporter of H+ and Iron (Fe2+)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gruenheid, S.; Cellier, M.; Vidal, S.; Gros, P. Identification and characterization of a second mouse Nramp gene. Genomics 1995, 25, 514–525. [Google Scholar] [CrossRef]
- Cellier, M.F.; Bergevin, I.; Boyer, E.; Richer, E. Polyphyletic origins of bacterial Nramp transporters. Trends Genet. 2001, 17, 365–370. [Google Scholar] [CrossRef]
- Williams, L.E.; Pittman, J.K.; Hall, J.L. Emerging mechanisms for heavy metal transport in plants. Biochim. et Biophys. Acta 2000, 1465, 104–126. [Google Scholar] [CrossRef]
- Vidal, S.; Belouchi, A.M.; Cellier, M.; Beatty, B.; Gros, P. Cloning and characterization of a second human NRAMP gene on chromosome 12q13. Mamm. Genome 1995, 6, 224–230. [Google Scholar] [CrossRef]
- Bozzi, A.T.; Bane, L.B.; Weihofen, W.A.; Singharoy, A.; Guillen, E.R.; Ploegh, H.L.; Schulten, K.; Gaudet, R. Crystal structure and conformational change mechanism of a bacterial Nramp-family divalent metal transporter. Structure 2016, 24, 2102–2114. [Google Scholar] [CrossRef] [PubMed]
- Yanatori, I.; Kishi, F. DMT1 and iron transport. Free. Radic. Biol. Med. 2019, 133, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Courville, P.; Chaloupka, R.; Cellier, M.F. Recent progress in structure-function analyses of Nramp proton-dependent metal-ion transporters. Biochem. Cell Biol. 2006, 84, 960–978. [Google Scholar] [CrossRef]
- Peracino, B.; Wagner, C.; Balest, A.; Balbo, A.; Pergolizzi, B.; Noegel, A.A.; Steinert, M.; Bozzaro, S. Function and mechanism of action of Dictyostelium Nramp1 (Slc11a1) in bacterial infection. Traffic 2006, 7, 22–38. [Google Scholar] [CrossRef]
- Lam-Yuk-Tseung, S.; Govoni, G.; Forbes, J.; Gros, P. Iron transport by Nramp2/DMT1: pH regulation of transport by 2 histidines in transmembrane domain 6. Blood 2003, 101, 3699–3707. [Google Scholar] [CrossRef]
- Cohen, A.; Nelson, H.; Nelson, N. The family of SMF metal ion transporters in yeast cells. J. Biol. Chem. 2000, 275, 33388–33394. [Google Scholar] [CrossRef] [PubMed]
- Vidal, S.M.; Malo, D.; Vogan, K.; Skamene, E.; Gros, P. Natural resistance to infection with intracellular parasites: Isolation of a candidate for Bcg. Cell 1993, 73, 469–485. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Zheng, L.; Wang, Y.; Chen, X.; Zhang, W. A functional study identifying critical residues involving metal transport activity and selectivity in natural resistance-associated macrophage protein 3 in Arabidopsis thaliana. Int. J. Mol. Sci. 2018, 19, 1430. [Google Scholar] [CrossRef] [PubMed]
- Curie, C.; Alonso, J.M.; Le Jean, M.; Ecker, J.R.; Briat, J.F. Involvement of NRAMP1 from Arabidopsis thaliana in iron transport. Biochem. J. 2000, 347 Pt 3, 749–755. [Google Scholar] [CrossRef]
- Thomine, S.; Wang, R.; Ward, J.; Crawford, N.; Schroeder, J. Cadmium and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. Proc. Natl. Acad. Sci. USA 2000, 97, 4991–4996. [Google Scholar] [CrossRef]
- Agranoff, D.; Monahan, I.M.; Mangan, J.A.; Butcher, P.D.; Krishna, S. Mycobacterium tuberculosis expresses a novel pH-dependent divalent cation transporter belonging to the Nramp family. J. Exp. Med. 1999, 190, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Osorio, H.; Martinez, V.; Veloso, F.A.; Pedroso, I.; Valdes, J.; Jedlicki, E.; Holmes, D.S.; Quatrini, R. Iron homeostasis strategies in acidophilic iron oxidizers: Studies in Acidithiobacillus and Leptospirillum. Hydrometallurgy 2008, 94, 175–179. [Google Scholar] [CrossRef]
- Mangold, S.; Potrykus, J.; Bjorn, E.; Lovgren, L.; Dopson, M. Extreme zinc tolerance in acidophilic microorganisms from the bacterial and archaeal domains. Extremophiles 2013, 17, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Potrykus, J.; Jonna, V.R.; Dopson, M. Iron homeostasis and responses to iron limitation in extreme acidophiles from the Ferroplasma genus. Proteomics 2011, 11, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Ai, C.B.; Yan, Z.; Chai, H.S.; Gu, T.Y.; Wang, J.J.; Chai, L.Y.; Qiu, G.Z.; Zeng, W.M. Increased chalcopyrite bioleaching capabilities of extremely thermoacidophilic Metallosphaera sedula inocula by mixotrophic propagation. J. Ind. Microbiol. Biot. 2019, 46, 1113–1127. [Google Scholar] [CrossRef]
- Rawlings, D.E. Characteristics and adaptability of iron- and sulfur-oxidizing microorganisms used for the recovery of metals from minerals and their concentrates. Microb. Cell Factories 2005, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, H.; Zhang, Y.; Liu, H.; Yin, H.; Deng, J.; Qiu, G. Interaction mechanism between marmatite and chalcocite in acidic (microbial) environments. Hydrometallurgy 2020, 191, 105217. [Google Scholar] [CrossRef]
- Ma, L.; Wang, X.; Feng, X.; Liang, Y.; Xiao, Y.; Hao, X.; Yin, H.; Liu, H.; Liu, X. Co-culture microorganisms with different initial proportions reveal the mechanism of chalcopyrite bioleaching coupling with microbial community succession. Bioresour. Technol. 2017, 223, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Y.; Wang, X.J.; Tao, J.M.; Feng, X.; Zou, K.; Xiao, Y.H.; Liang, Y.L.; Yin, H.Q.; Liu, X.D. Bioleaching of the mixed oxide-sulfide copper ore by artificial indigenous and exogenous microbial community. Hydrometallurgy 2017, 169, 41–46. [Google Scholar] [CrossRef]
- Gao, X.Y.; Liu, X.J.; Fu, C.A.; Gu, X.F.; Lin, J.Q.; Liu, X.M.; Pang, X.; Lin, J.Q.; Chen, L.X. Novel strategy for improvement of the bioleaching efficiency of Acidithiobacillus ferrooxidans based on the AfeI/R quorum sensing system. Minerals-Basel 2020, 10, 222. [Google Scholar] [CrossRef]
- Ai, C.; Liang, Y.; Miao, B.; Chen, M.; Zeng, W.; Qiu, G. Identification and analysis of a novel gene cluster involves in Fe2+ oxidation in Acidithiobacillus ferrooxidans ATCC 23270, a typical biomining acidophile. Curr. Microbiol. 2018, 75, 818–826. [Google Scholar] [CrossRef]
- Liu, H.C.; Xia, J.L.; Nie, Z.Y.; Zheng, L.; Ma, C.Y.; Zhao, Y.D. Differential utilization and speciation transformation of orthorhombic alpha-S-8 and amorphous mu-S by substrate-acclimated mesophilic Acidithiobacillus ferrooxidans. Trans. Nonferrous Met. Soc. China 2015, 25, 3096–3102. [Google Scholar] [CrossRef]
- Ma, L.; Wang, X.; Tao, J.; Feng, X.; Liu, X.; Qin, W. Differential fluoride tolerance between sulfur- and ferrous iron-grown Acidithiobacillus ferrooxidans and its mechanism analysis. Biochem. Eng. J. 2017, 119, 59–66. [Google Scholar] [CrossRef]
- Braun, V.; Killmann, H. Bacterial solutions to the iron-supply problem. Trends Biochem. Sci. 1999, 24, 104–109. [Google Scholar] [CrossRef]
- Touati, D. Iron and oxidative stress in bacteria. Arch. Biochem. Biophys. 2000, 373, 1–6. [Google Scholar] [CrossRef]
- Velayudhan, J.; Hughes, N.J.; McColm, A.A.; Bagshaw, J.; Clayton, C.L.; Andrews, S.C.; Kelly, D.J. Iron acquisition and virulence in Helicobacter pylori: A major role for FeoB, a high-affinity ferrous iron transporter. Mol. Microbiol. 2000, 37, 274–286. [Google Scholar] [CrossRef]
- Schalk, I.J.; Yue, W.W.; Buchanan, S.K. Recognition of iron-free siderophores by TonB-dependent iron transporters. Mol. Microbiol. 2004, 54, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Hao, X.; Xiao, Y.; Zou, K.; Liu, H.; Liu, X.; Yin, H.; Qiu, G.; Liang, Y. Responses of Acidithiobacillus thiooxidans A01 to Individual and Joint Nickel (Ni2+) and Ferric (Fe3+). Minerals-Basel 2019, 9, 82. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, L.; Li, H.Y.; Liu, H.T. Optimal conditions for growth and magnetosome formation of Acidithiobacillus ferrooxidans. Afr. J. Microbiol. Res. 2012, 6, 6142–6151. [Google Scholar] [CrossRef]
- Liu, W.B.; Wu, H.Y.; Liu, X.D.; Liu, X.X. Influence of different Fe sources and concentrations on formation of magnetosomes in Acidithiobacillus ferrooxidans. T. Nonferr. Metal Soc. 2008, 18, 1379–1385. [Google Scholar] [CrossRef]
- Lin, W.; Pan, Y.; Bazylinski, D.A. Diversity and ecology of and biomineralization by magnetotactic bacteria. Environ. Microbiol. Rep. 2017, 9, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.B.; Wang, F.X.; Zhang, J.; Tong, R.A.; Chong, S.K.; Wang, W.; Wang, C.A.; Chen, C.F. A monocrystal Fe3O4@ultrathin N-doped carbon core/shell structure: From magnetotactic bacteria to Li storage. J. Mater. Chem. A 2019, 7, 20899–20904. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, X.; Liu, D.; Duan, H.; Fan, H. Sequencing and bioinformatics analysis of the metal-related genes in Acidithiobacillus ferrooxidans strain DC. Folia Microbiol. (Praha) 2013, 58, 551–560. [Google Scholar] [CrossRef]
- Silverman, M.P.; Lundgren, D.G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans: I. An improved medium and a harvesting procedure for securing high cell yields. J. Bacteriol. 1959, 77, 642–647. [Google Scholar] [CrossRef]
- Nolan, T.; Hands, R.; Bustin, S. Quantification of mRNA using real-time RT-PCR. Nat. Protoc. 2006, 1, 1559–1582. [Google Scholar] [CrossRef]
- Pamela, N.; Paulo, C.; Eugenia, J.; David, H.; Raquel, Q. Selection and evaluation of reference genes for improved interrogation of microbial transcriptomes: Case study with the extremophile Acidithiobacillus ferrooxidans. BMC Mol. Biol. 2009, 10, 1–11. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome. Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef] [PubMed]
- Gil, R.; Silva, F.J.; Pereto, J.; Moya, A. Determination of the core of a minimal bacterial gene set. Microbiol. Mol. Biol. Rev. 2004, 68, 518–537. [Google Scholar] [CrossRef] [PubMed]
- Cellier, M.; Prive, G.; Belouchi, A.; Kwan, T.; Rodrigues, V.; Chia, W.; Gros, P. Nramp defines a family of membrane proteins. Proc. Natl. Acad. Sci. USA 1995, 92, 10089–10093. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.Y.; Wagner, J.R.; Laird, M.R.; Melli, G.; Rey, S.; Lo, R.; Dao, P.; Sahinalp, S.C.; Ester, M.; Foster, L.J.; et al. PSORTb 3.0: Improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 2010, 26, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Moller, S.; Croning, M.D.R.; Apweiler, R. Evaluation of methods for the prediction of membrane spanning regions. Bioinformatics 2001, 17, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Nevo, Y.; Nelson, N. The NRAMP family of metal-ion transporters. Biochim. et Biophys. Acta 2006, 1763, 609–620. [Google Scholar] [CrossRef]
- Cohen, A.; Nevo, Y.; Nelson, N. The first external loop of the metal ion transporter DCT1 is involved in metal ion binding and specificity. Proc. Natl. Acad. Sci. USA 2003, 100, 10694–10699. [Google Scholar] [CrossRef]
- Bozzi, A.T.; Bane, L.B.; Weihofen, W.A.; McCabe, A.L.; Singharoy, A.; Chipot, C.J.; Schulten, K.; Gaudet, R. Conserved methionine dictates substrate preference in Nramp-family divalent metal transporters. Proc. Natl. Acad. Sci. USA 2016, 113, 10310–10315. [Google Scholar] [CrossRef]
- Navarro, C.A.; Orellana, L.H.; Mauriaca, C.; Jerez, C.A. Transcriptional and functional studies of Acidithiobacillus ferrooxidans genes related to survival in the presence of copper. Appl. Environ. Microbiol. 2009, 75, 6102–6109. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Chai, T.; Zhang, Y.; Han, L.; Xu, J.; Guan, Z. The Thlaspi caerulescens NRAMP homologue TcNRAMP3 is capable of divalent cation transport. Mol. Biotechnol. 2009, 41, 15–21. [Google Scholar] [CrossRef]
- van Zyl, L.J.; van Munster, J.M.; Rawlings, D.E. Construction of arsB and tetH mutants of the sulfur-oxidizing bacterium Acidithiobacillus caldus by marker exchange. Appl. Environ. Microbiol. 2008, 74, 5686–5694. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Liu, X.M.; Liu, S.S.; Yu, Y.Y.; Lin, J.Q.; Lin, J.Q.; Pang, X.; Zhao, J. Development of a markerless gene replacement system for Acidithiobacillus ferrooxidans and construction of a pfkB mutant. Appl. Environ. Microbiol. 2012, 78, 1826–1835. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yu, Z.J.; Yu, R.L.; Liu, A.J.; Liu, J.; Zeng, W.M.; Liu, X.D.; Qiu, G.Z. Effect of pH values on extracellular protein and polysaccharide secretions of Acidithiobacillus ferrooxidans during chalcopyrite bioleaching. Trans. Nonferr Metal Soc China 2017, 27, 406–412. [Google Scholar] [CrossRef]
- Kai, M.; Yano, T.; Fukumori, Y.; Yamanaka, T. Cytochrome oxidase of an acidophilic iron-oxidizing bacterium, Thiobacillus ferrooxidans, functions at pH 3.5. Biochem. Biophys. Res Commun. 1989, 160, 839–843. [Google Scholar] [CrossRef]
- Gunshin, H.; Mackenzie, B.; Berger, U.V.; Gunshin, Y.; Romero, M.F.; Boron, W.F.; Nussberger, S.; Gollan, J.L.; Hediger, M.A. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 1997, 388, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Goswami, T.; Bhattacharjee, A.; Babal, P.; Searle, S.; Moore, E.; Li, M.; Blackwell, J.M. Natural-resistance-associated macrophage protein 1 is an H+/bivalent cation antiporter. Biochem. J. 2001, 354, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Garrick, M.D.; Gniecko, K.; Liu, Y.; Cohan, D.S.; Garrick, L.M. Transferrin and the transferrin cycle in Belgrade rat reticulocytes. J. Biol. Chem. 1993, 268, 14867–14874. [Google Scholar]
- Nelson, N. Energizing porters by proton-motive force. J. Exp. Biol. 1994, 196, 7–13. [Google Scholar]
- Ehrnstorfer, I.A.; Manatschal, C.; Arnold, F.M.; Laederach, J.; Dutzler, R. Structural and mechanistic basis of proton-coupled metal ion transport in the SLC11/NRAMP family. Nat. Commun. 2017, 8, 14033. [Google Scholar] [CrossRef]
- Nelson, N.; Sacher, A.; Nelson, H. The significance of molecular slips in transport systems. Nat. Rev. Mol. Cell Biol. 2002, 3, 876–881. [Google Scholar] [CrossRef]
- Wu, L.B.; Yang, B.J.; Wang, X.X.; Wu, B.Q.; He, W.L.; Gan, M.; Qiu, G.Z.; Wang, J. Effects of single and mixed energy sources on intracellular nanoparticles synthesized by Acidithiobacillus ferrooxidans. Minerals-Basel 2019, 9, 163. [Google Scholar] [CrossRef]

| Loci* | Forward Primer (5’ > 3’) | Reverse Primer (5’ > 3’) | Amplicon Size (bp) | Q-PCR Linear Correlation Coefficient |
|---|---|---|---|---|
| AFE_2126 (NRAMP) | CTCTGGTGGTGCTGATTC | ATGAATATGGAAGCGATTACCT | 76 | 1.000 |
| AFE_2121 (map) | CTTTCCCAAATCCGTCTG | GATAGCCATCCTTGATAACC | 122 | 0.995 |
| AFE_2475 (gmk) | GATGCGTTAGCCGACTTG | ACTGTTAATACCGAGGAGATTC | 108 | 1.000 |
| AFE_2714 (rplL) | TGGAAGAACAGACCGAAT | AGACCAAGACCAGTGATG | 97 | 0.999 |
| AFE_2150 (gyrA) | CGTATCGCCATTGAACTC | TTGATATTGAACACGCTCTG | 95 | 0.997 |
| AFE_2114 (recA) | GCTCACCGCCAATATCTC | GGACTGCCATACATCACC | 87 | 0.998 |
| AFE_1680 (era) | CAATCTGCGGTTTATGGC | CGTGTCGTAGGGTAGTTC | 79 | 0.990 |
| AFE_2711 (rpoC) | GGTGAACGCTATAACAAG | TACACGGTATCCTTACTG | 95 | 0.999 |
| TIGR Locus | Gene Symbol | Common Name TIGR | Biological Process | Functional Category |
|---|---|---|---|---|
| AFE_2121 | map | methionine aminopeptidase, type I | Protein processing | Protein posttranslational modification |
| AFE_2475 | gmk | guanylate kinase | Energetic and intermediary metabolism | Biosynthesis of nucleotides |
| AFE_2714 | rplL | ribosomal protein L7/L12 | Protein processing | Synthesis and modification of ribosomal proteins: |
| AFE_2150 | gyrA | DNA gyrAse, A subunit | DNA Metabolism | Basic replication machinery |
| AFE_2114 | recA | recA protein | DNA Metabolism | DNA replication, recombination, and repair |
| AFE_1680 | era | GTP-binding protein Era | RNA Metabolism | Translation: ribosome function, maturation, and modification |
| AFE_2711 | rpoC | DNA-directed RNA polymerase, β subunit | RNA Metabolism | Basic transcription machinery |
| GI Number | Organisms | Protein Symbol and Protein Description | Similarity |
|---|---|---|---|
| gi:218667514 (456 aa) | Acidithiobacillus ferrooxidans ATCC 23270 | NRAMP family | 100 (100) |
| gi:198282304 (456 aa) | Acidithiobacillus ferrooxidans ATCC 53993 | NRAMP family | 95 (88) |
| gi:218288940 (448 aa) | Alicyclobacillus acidocaldarius LAA1 | NRAMP family | 75 (60) |
| gi:148244067 (457 aa) | Acidiphilium cryptum JF5 | NRAMP family | 72 (52) |
| gi:219850775 (425 aa) | Candidatus Methanosphaerula palustris E19c | NRAMP family | 64 (43) |
| gi:260869095 (412 aa) | Escherichia coli O111:H-str. 11128 | manganese/divalent cation transporter MntH | 40 (20) |
| gi: 6468012 (509 aa) | Arabidopsis thaliana | metal transporter Nramp3 | 36 (17) |
| gi:6321841 (549 aa) | Saccharomyces cerevisiae S288c | Smf2 (probable NRAMP divalent cation transporter) | 35 (18) |
| gi:295293167 (590 aa) | Homo sapiens | Nramp2 (multiple divalent cation transporter for Fe2+, Mn2+, and Zn2+) | 34 (18) |
| gi:730193 (548 aa) | Mouse | Nramp1, divalent transition metal (iron and manganese) transporter | 32 (17) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, B.; Shen, L.; Liu, X.; Zeng, W.; Wu, X. Bioinformatics and Transcriptional Study of the Nramp Gene in the Extreme Acidophile Acidithiobacillus ferrooxidans Strain DC. Minerals 2020, 10, 544. https://doi.org/10.3390/min10060544
Miao B, Shen L, Liu X, Zeng W, Wu X. Bioinformatics and Transcriptional Study of the Nramp Gene in the Extreme Acidophile Acidithiobacillus ferrooxidans Strain DC. Minerals. 2020; 10(6):544. https://doi.org/10.3390/min10060544
Chicago/Turabian StyleMiao, Bo, Li Shen, Xueduan Liu, Weimin Zeng, and Xueling Wu. 2020. "Bioinformatics and Transcriptional Study of the Nramp Gene in the Extreme Acidophile Acidithiobacillus ferrooxidans Strain DC" Minerals 10, no. 6: 544. https://doi.org/10.3390/min10060544
APA StyleMiao, B., Shen, L., Liu, X., Zeng, W., & Wu, X. (2020). Bioinformatics and Transcriptional Study of the Nramp Gene in the Extreme Acidophile Acidithiobacillus ferrooxidans Strain DC. Minerals, 10(6), 544. https://doi.org/10.3390/min10060544




