Effect of Sulfuric Acid Baking and Caustic Digestion on Enhancing the Recovery of Rare Earth Elements from a Refractory Ore
Abstract
1. Introduction
2. Materials and Methods
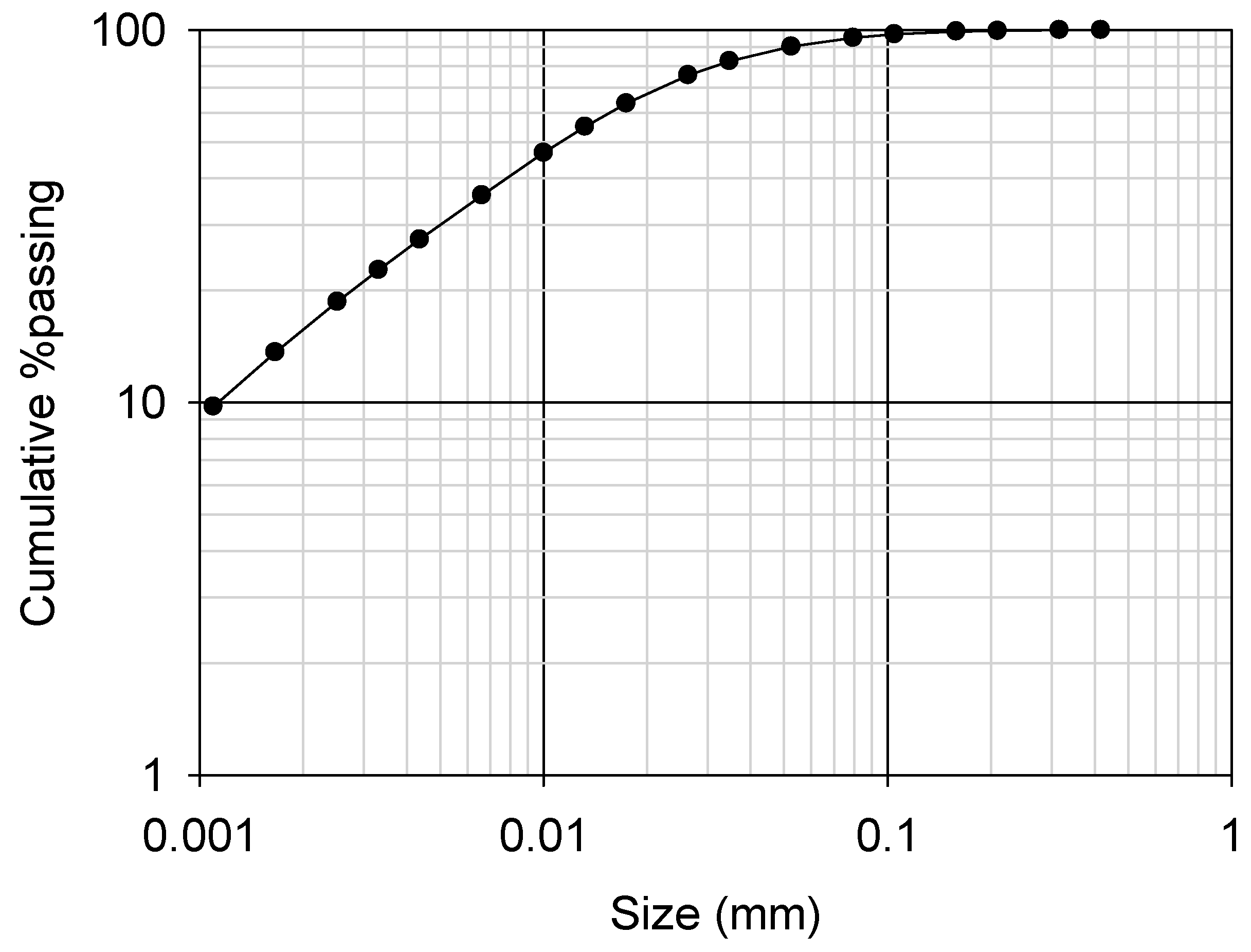
2.1. Ore Characteristics
2.2. Preliminary Leaching
2.3. Sulfuric Acid Baking–Water-Leaching
2.4. Caustic Digestion–Acid-Leaching
3. Results and Discussion
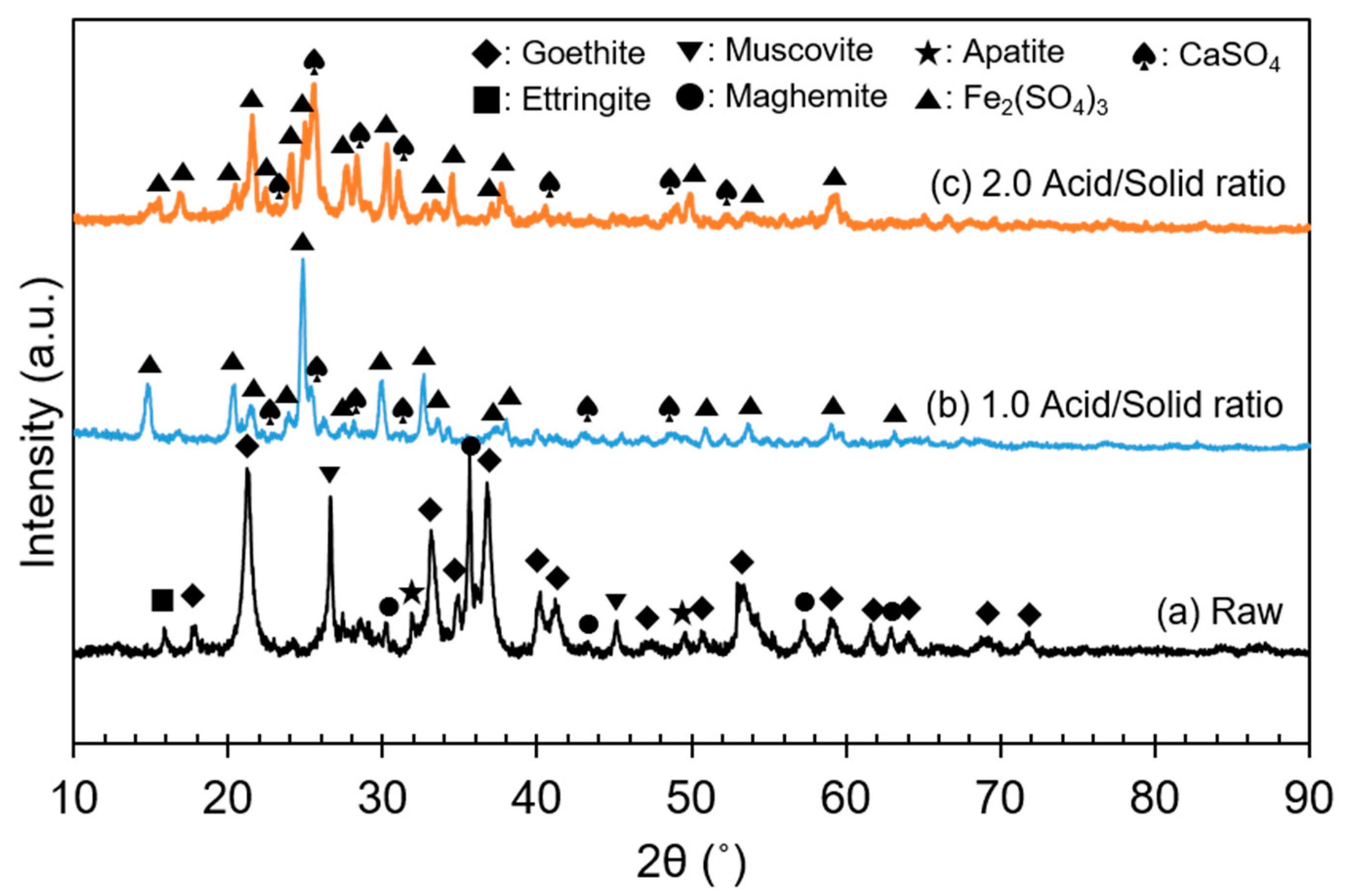
3.1. Preliminary Leaching Results
3.2. Sulfuric Acid Baking–Water-Leaching
3.2.1. Effect of Acid/Solid Ratio
3.2.2. Effect of Leaching Temperature
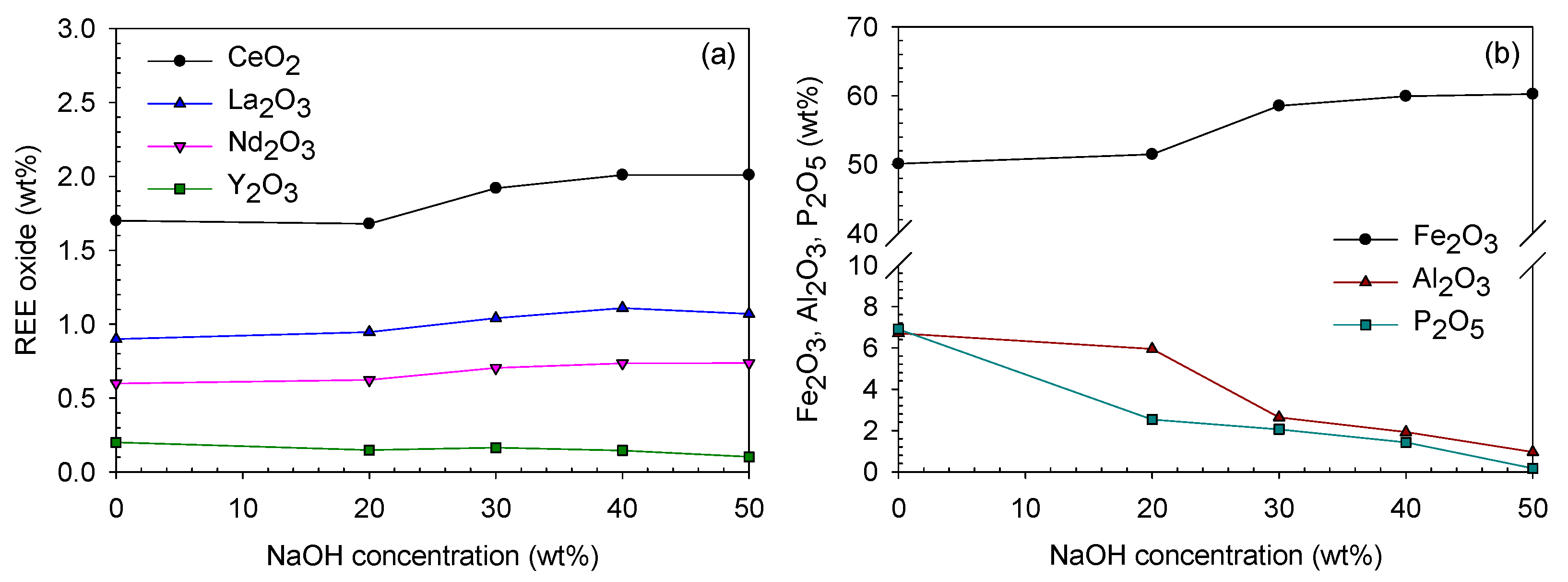
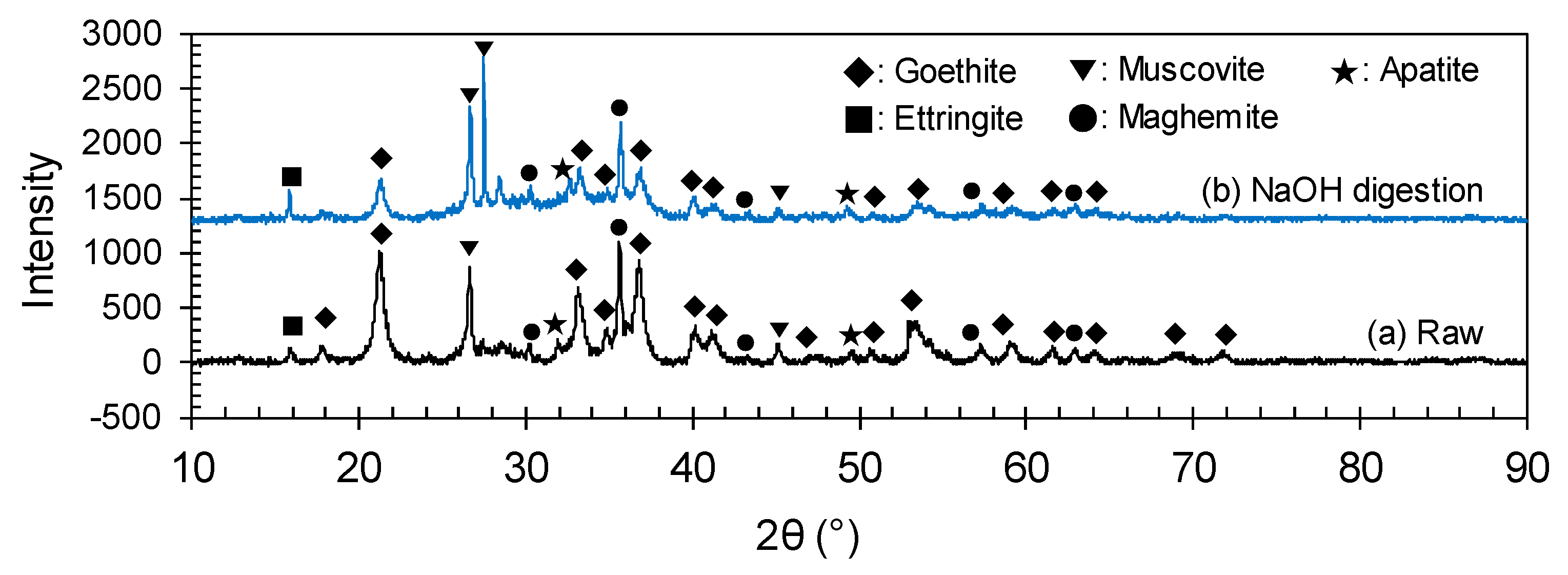
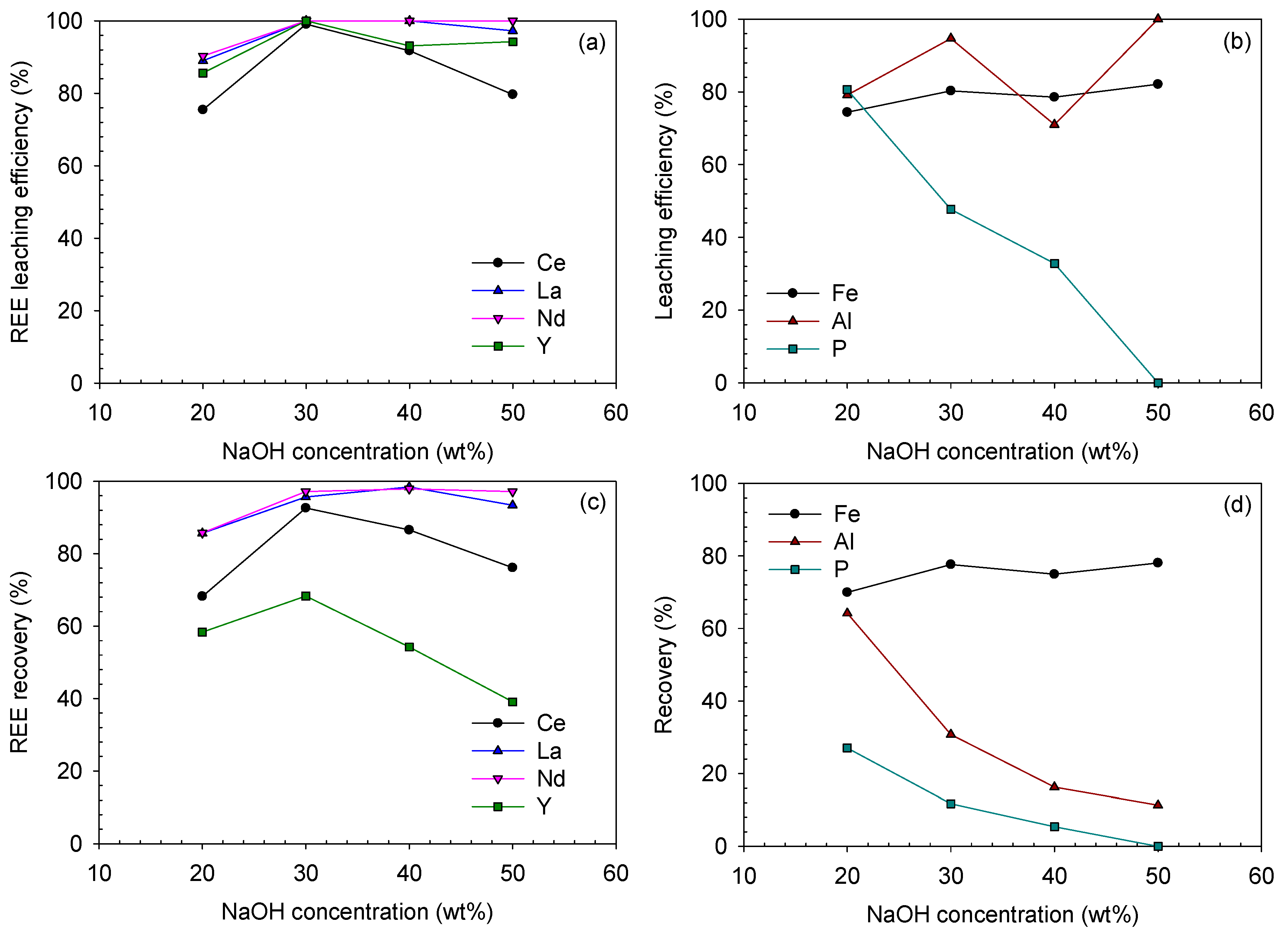
3.3. Caustic Digestion–Acid-Leaching
3.3.1. Caustic Digestion Behavior
3.3.2. Acid-Leaching Behavior
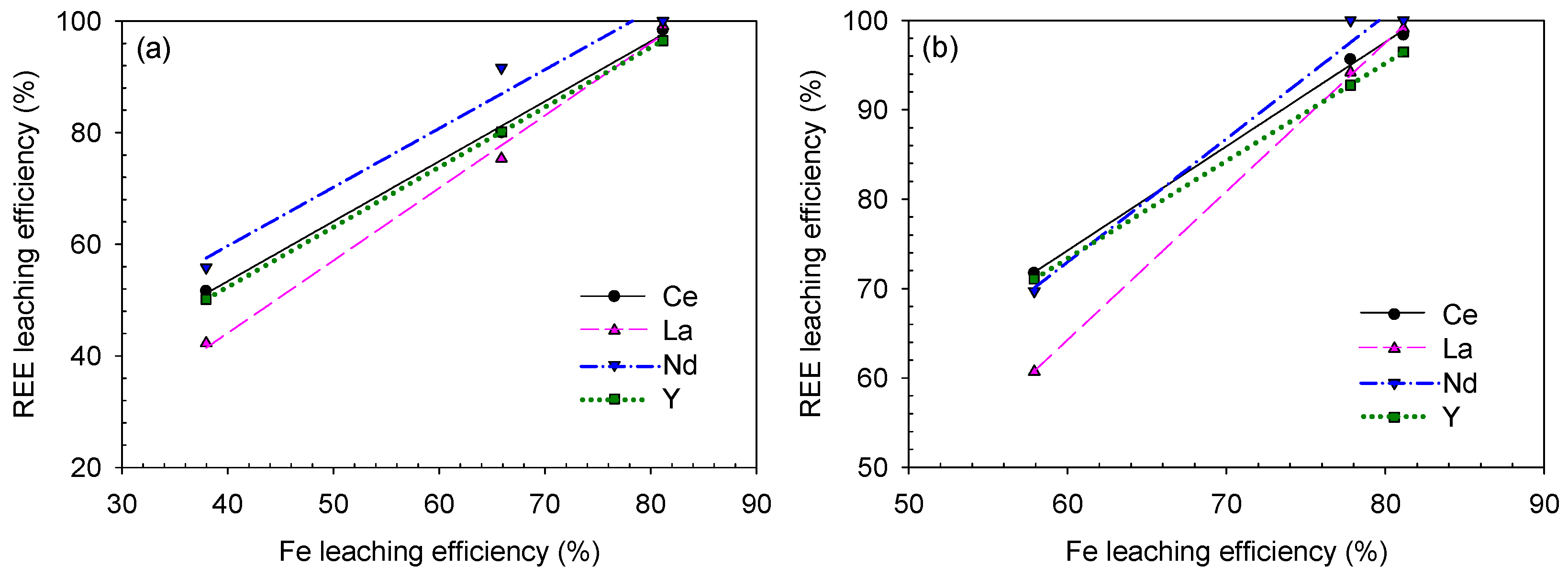
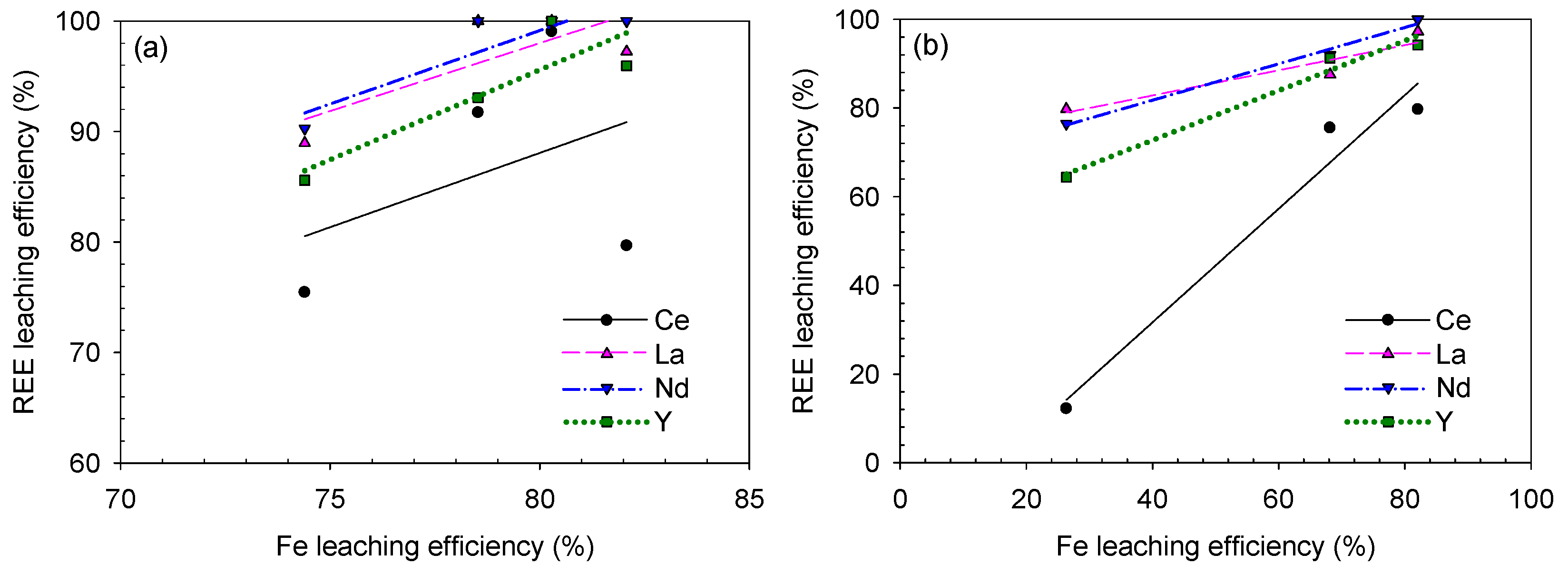
3.4. Determination of the Optimum Condition for Analyzing the REE–Fe-Leaching Efficiency Correlation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jordens, A.; Cheng, Y.P.; Waters, K.E. A review of the beneficiation of rare earth element bearing minerals. Miner. Eng. 2013, 41, 97–114. [Google Scholar] [CrossRef]
- Massari, S.; Ruberti, M. Rare earth elements as critical raw materials: Focus on international markets and future stategies. Resour. Policy 2013, 38, 36–43. [Google Scholar] [CrossRef]
- Golev, A.; Scott, M.; Erskine, P.D.; Ali, S.H.; Ballantyne, G.R. Rare earths supply chains: Current status, constraints and opportunities. Resour. Policy 2014, 41, 52–59. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, T.A.; Dreisinger, D.; Doyle, F. A critical review on solvent extraction of rare earths from aqueous solutions. Miner. Eng. 2014, 56, 10–28. [Google Scholar] [CrossRef]
- Tian, J.; Chi, R.; Yin, J. Leaching process of rare earths from weathered crust elution-deposited rare earth ore. Trans. Nonferrous Met. Soc. China 2010, 20, 892–896. [Google Scholar] [CrossRef]
- Chen, Z. Global rare earth resources and scenarios of future rare earth industry. J. Rare Earths 2011, 29, 1–6. [Google Scholar] [CrossRef]
- Moldoveanu, G.A.; Papangelakis, V.G. Recovery of rare earth elements adsorbed on clay minerals: II. Leaching with ammonium sulfate. Hydrometallurgy 2013, 131–132, 158–166. [Google Scholar] [CrossRef]
- Sadri, F.; Nazari, A.M.; Ghahreman, A. A review on the cracking, baking and leaching processes of rare earth element concentrates. J. Rare Earths 2017, 35, 739–752. [Google Scholar] [CrossRef]
- Berry, L.; Agarwal, V.; Galvin, J.; Safarzadeh, M.S. Decomposition of monazite concentrate in sulphuric acid. Can. Metall. Quart. 2018, 57, 422–433. [Google Scholar] [CrossRef]
- Demol, J.; Ho, E.; Senanayake, G. Sulfuric acid baking and leaching of rare earth elements, thorium and phosphate from a monazite concentrate: Effect of bake temperature from 200 to 800 °C. Hydrometallurgy 2018, 179, 254–267. [Google Scholar] [CrossRef]
- Borra, C.R.; Mermans, J.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Gerven, T.V. Selective recovery of rare earths from bauxite residue by combination of sulfation, roasting and leaching. Miner. Eng. 2016, 92, 151–159. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumari, A.; Panda, R.; Kumar, J.R.; Yoo, K.; Lee, J.Y. Review on hydrometallurgical recovery of rare earth metals. Hydrometallurgy 2016, 165, 2–26. [Google Scholar] [CrossRef]
- Soltani, F.; Abdollahy, M.; Petersen, J.; Ram, R.; Javad Koleini, S.M.; Moradkhani, D. Leaching and recovery of phosphate and rare earth elements from an iron-rich fluorapatite concentrate: Part II: Selective leaching of calcium and phosphate and acid baking of the residue. Hydrometallurgy 2019, 184, 29–38. [Google Scholar] [CrossRef]
- Huang, Y.; Dou, Z.; Zhang, T.; Liu, J. Leaching kinetics of rare earth elements and fluoride from mixed rare earth concentrate after roasting with calcium hydroxide and sodium hydroxide. Hydrometallurgy 2017, 173, 15–21. [Google Scholar] [CrossRef]
- Buchanan, J.; Reveley, S.; Forrester, K.; Cummings, A. Review of current rare earth processing practice. In Proceedings of the ALTA 2014, Perth, Australia, 24–31 May 2014. [Google Scholar]
- Habashi, F. The recovery of the lanthanides from phosphate rock. J. Chem. Technol. Biotechnol. Chem. Technol. 1985, 35, 5–14. [Google Scholar] [CrossRef]
- Jorjani, E.; Bagherieh, A.H.; Chelgani, S.C. Rare Earth Elements Leaching from Chadormalu Apatite Concentrate: Laboratory Studies and Regression Predictions. Korean J. Chem. Eng. 2011, 28, 557–562. [Google Scholar] [CrossRef]
- Preston, J.S.; Cole, P.M.; Craig, W.M.; Feather, A.M. the recovery of rare earth oxides from a phosphoric acid by-product. Part 1: Leaching of rare earth values and recovery of a mixed rare earth oxide by solvent extraction. Hydrometallurgy 1996, 41, 1–19. [Google Scholar] [CrossRef]
- Wang, L.; Long, Z.; Huang, X.; Yu, Y.; Cui, D.; Zhang, G. Recovery of rare earths from wet-process phosphoric acid. Hydrometallurgy 2010, 101, 41–47. [Google Scholar] [CrossRef]
- Kim, R.; Cho, H.; Han, K.N.; Kim, K.; Mun, M. Optimization of acid leaching of rare-earth elements from mongolian apatite-based ore. Minerals 2016, 6, 63. [Google Scholar] [CrossRef]
- Neumann, R.; Medeiros, E.B. Comprehensive mineralogical and technological characterisation of the Araxá (SE Brazil) complex REE (Nb-P) ore, and the fate of its processing. Int. J. Miner. Process. 2015, 144, 1–10. [Google Scholar] [CrossRef]
- Kuzmin, V.I.; Pashkov, G.L.; Lomaev, V.G.; Voskresenskaya, E.N.; Kuzmina, V.N. Combined approaches for comprehensive processing of rare earth metal ores. Hydrometallurgy 2012, 129–130, 1–6. [Google Scholar] [CrossRef]
- Kim, R.; Cho, H.; Han, K.N. Behavior of anions in association with metal ions under hydrometallurgical environments: Part I–OH- effect on various cations. Min. Metall. Explor. 2014, 31, 34–39. [Google Scholar] [CrossRef]
- Guo, X.; Li, D.; Park, K.; Tian, Q.; Wu, Z. Leaching behavior of metals from a limonitic nickel laterite using a sulfation–roasting–leaching process. Hydrometallurgy 2009, 99, 144–150. [Google Scholar] [CrossRef]
- Sadri, F.; Kim, R.; Yang, Z.; Ghahreman, A. The effect of calcium sulfate crystallization and the crystal modification on aqueous REE stability in Ca saturated REE-Ca-SO4-H2O systems. Hydrometallurgy 2018, 182, 82–96. [Google Scholar] [CrossRef]
- Dutrizac, J.E. Calcium sulphate solubilities in simulated zinc processing solutions. Hydrometallurgy 2002, 65, 109–135. [Google Scholar] [CrossRef]
- Azimi, G.; Papangelakis, V.G.; Dutrizac, J.E. Modelling of calcium sulphate solubility in concentrated multi-component sulphate solutions. Fluid Phase Equilibr. 2007, 260, 300–315. [Google Scholar] [CrossRef]
- Sadri, F.; Kim, R.; Ghahreman, A. Substitution of Calcium with Ce, Nd, Er, and Tb in the Structure of Microcrystals of Calcium Sulfates with Controlled Hydration Water: A Proposed Mechanism. Cryst. Growth Des. 2019, 19, 2621–2631. [Google Scholar] [CrossRef]
- Firsching, F.H.; Brune, S.N. solubility products of the trivalent rare-earth phosphates. J. Chem. Eng. Data 1991, 36, 93–95. [Google Scholar] [CrossRef]
- Poitrasson, F.; Oelkers, E.; Schott, J.; Montel, J. Experimental determination of synthetic NdPO4 monazite end-member solubility in water from 21 °C to 300 °C: Implications for rare earth element mobility in crustal fluids. Geochim. Cosmochim. Acta 2004, 68, 2207–2221. [Google Scholar] [CrossRef]
- Cetiner, Z.S.; Wood, S.A.; Gammons, C.H. The aqueous geochemistry of the rare earth elements. Part XIV. The solubility of rare earth element phosphates from 23 to 150 °C. Chem. Geol. 2005, 217, 147–169. [Google Scholar] [CrossRef]
- Das, G.; Lencka, M.M.; Eslamimanesh, A.; Wang, P.; Anderko, A.; Riman, R.E.; Mavrotsky, A. Rare earth sulfates in aqueous systems: Thermodynamic modeling of binary and multicomponent systems over wide concentration and temperature ranges. J. Chem. Thermodyn. 2019, 131, 49–79. [Google Scholar] [CrossRef]
- Ivano-Emie, B.N.; Nisel’son, L.A.; Ivolgina, A.T. Solubility of yttrium hydroxide in sodium hydroxide solution. Zhur. Neorg. Khim. 1961, 6, 1483–1484. [Google Scholar]
- Joshi, S.; Kulp, E.A.; Fahrenholtz, W.G.; O’Keefe, M.J. Dissolution of cerium from cerium-based conversion coatings on Al 7075-T6 in 0.1 M NaCl solutions. Corros. Sci. 2012, 60, 290–295. [Google Scholar] [CrossRef]


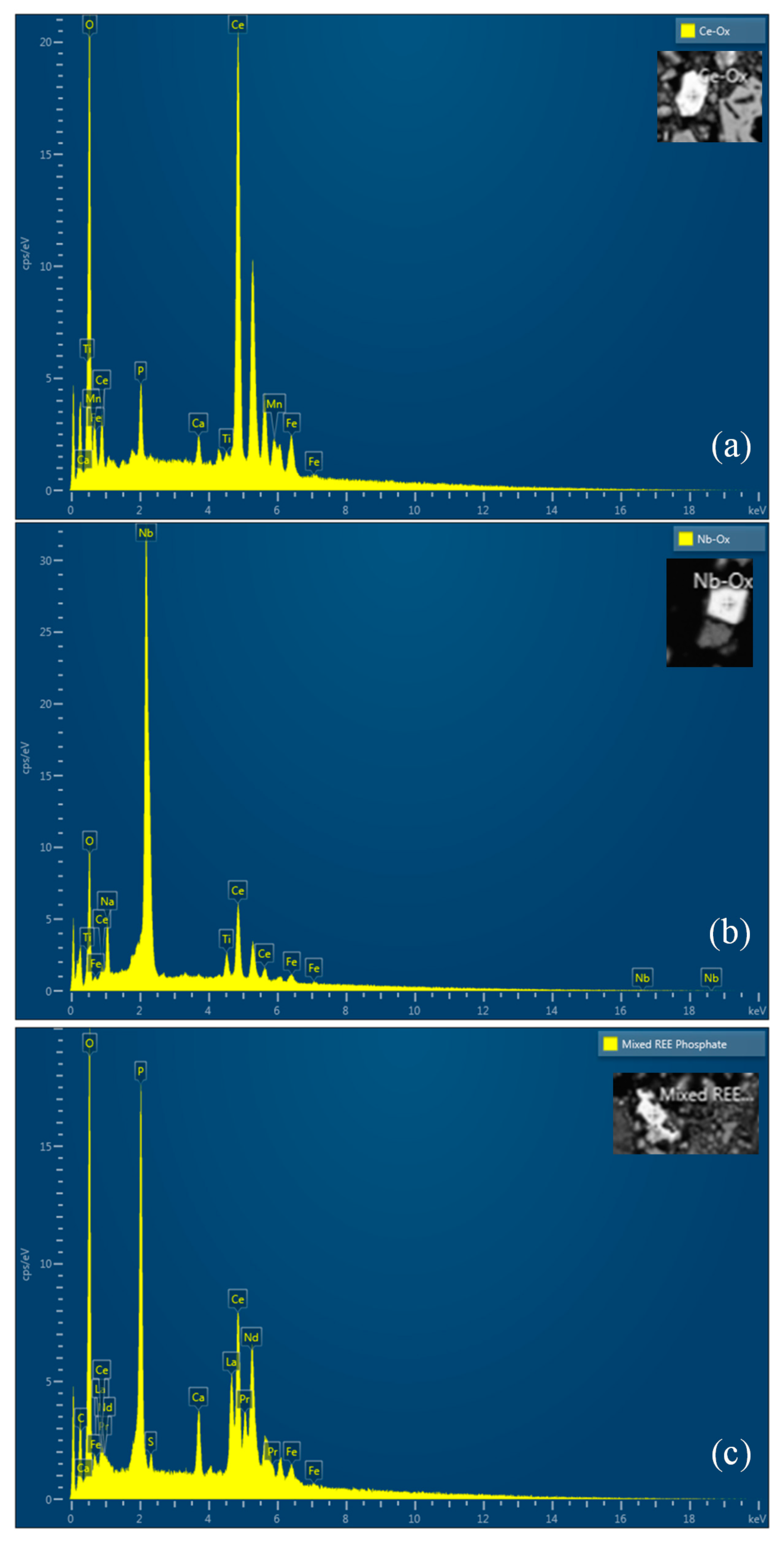


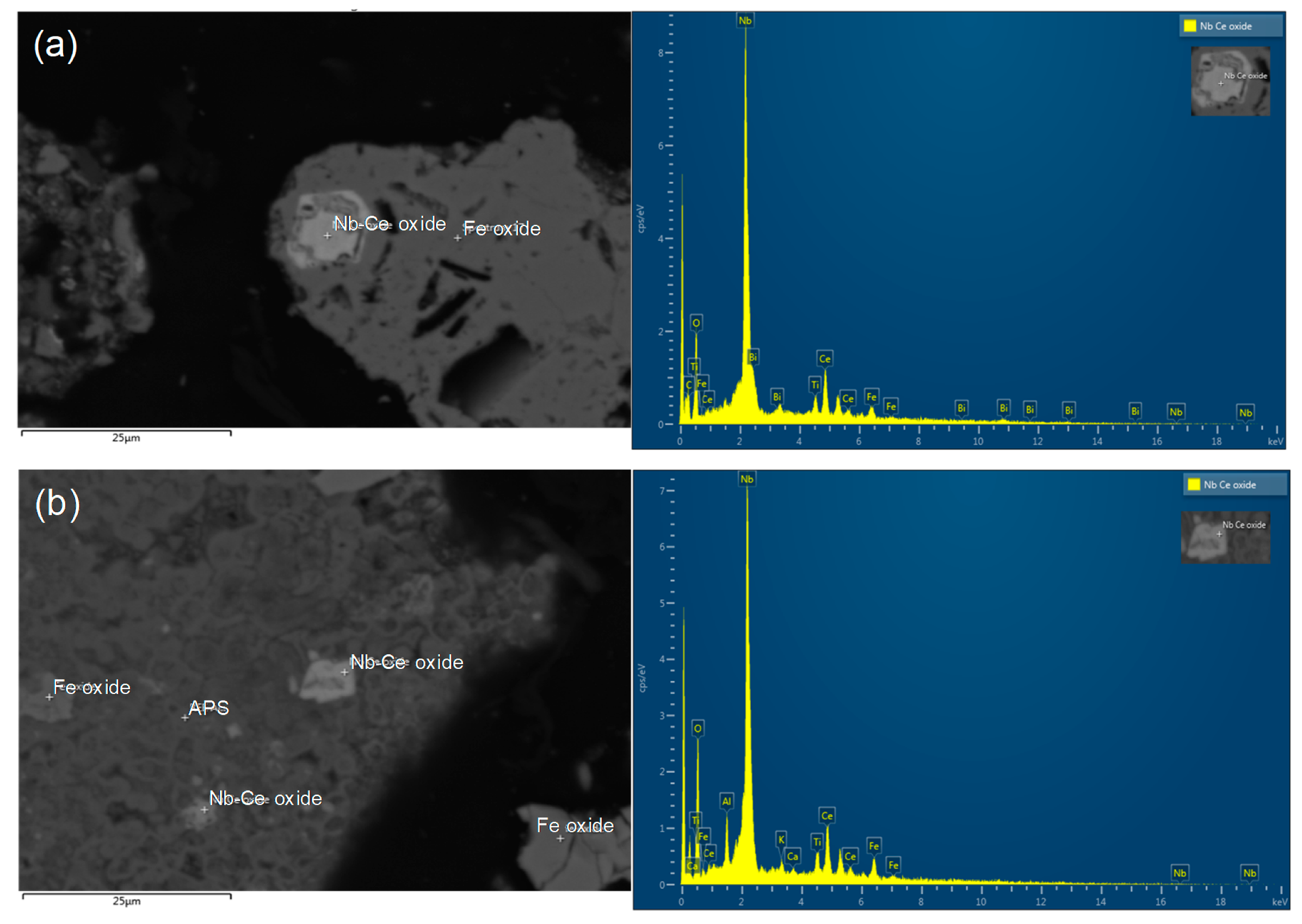





| REE Mineral Type | Origin | Method | REE-Leaching Efficiency | Reference |
|---|---|---|---|---|
| Monazite | Unknown | H2SO4 baking (180–250 °C)/ Water-leaching (70 °C) | 85–100% | Berry et al. [9] |
| Monazite | Australia | H2SO4 baking (250 °C)/ Acid-leaching (0.9 M H2SO4, 20–25 °C) | 96% | Demol et al. [10] |
| Red mud | Greece | H2SO4 baking (700 °C)/ Water-leaching (room temperature) | >80% (Sc 60%) | Borra et al. [11] |
| Apatite residue | Iran | H2SO4 baking (270 °C)/ Water-leaching | 95% | Soltani et al. [13] |
| Bastnasite, monazite | China | Caustic digestion (NaOH + Ca(OH)2 roasting, 700 °C)/ Acid washing (0.5 M HCl + 0.05 M citric acid, 40 °C)/ Acid-leaching (3 M HCl + 0.5 M AlCl3, 70 °C) | 86% | Huang et al. [14] |
| Monazite, xenotime, bastnasite | Kyrgyz Republic | Caustic digestion (Na2CO3 fusion)/Acid-leaching (HNO3) | – | Buchanan et al. [15] |
| Apatite, allanite, chevkinite, titanite | Turkey | Caustic digestion (600 °C)/ Water–HCl-leaching | – | Buchanan et al. [15] |
| Ion-adsorbed clays | Unknown | Acidic-leaching (0.5 M (NH4)2SO4, pH 3 by H2SO4, 25 °C) | 80–90% | Moldoveanu and Papangelakis [7] |
| Apatite | Iran | Acid-leaching (60% HNO3, 60 °C) | 59–74% | Jorjani et al. [17] |
| Apatite | South Africa | Acid-leaching (1.0 M HNO3, 0.5 M Ca(NO3)2) | 85% | Preston et al. [18] |
| Apatite | Mongolia | Two-step acid-leaching (1st: 1.0 M HCl/2nd: 2.0 M HCl) | 92–94% | Kim et al. [20] |
| Formula | Fe2O3 | MnO | SiO2 | P2O5 | Al2O3 | TiO2 | CaO | BaO | CeO2 | MgO | La2O3 |
| wt% | 50.1 | 9.9 | 7.8 | 6.9 | 6.7 | 4.4 | 3.6 | 2.2 | 1.7 | 1.2 | 0.9 |
| Formula | K2O | SrO | Na2O | Nd2O3 | SO3 | Nb2O5 | ZnO | Cl | ZrO2 | Y2O3 | MoO3 |
| wt% | 0.8 | 0.7 | 0.6 | 0.6 | 0.5 | 0.4 | 0.3 | 0.2 | 0.2 | 0.2 | 0.07 |
| HNO3 Concentration (M) | Temperature (°C) | Initial pH | Leaching Efficiency (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ce | La | Nd | Y | Fe | Ca | P | |||
| 1.0 | 25 | 0.25 | 8.4 | 10.2 | 13.8 | 23.2 | 0.9 | 100.0 | 18.0 |
| 2.0 | 25 | −0.37 | 17.3 | 18.6 | 26.3 | 31.3 | 1.6 | 94.6 | 18.9 |
| 2.0 | 50 | −0.24 | 22.5 | 24.7 | 27.8 | 30.5 | 4.5 | 98.1 | 22.2 |
| 2.0 | 80 | −0.13 | 43.6 | 49.9 | 57.3 | 48.2 | 18.8 | 98.2 | 32.3 |
| 3.0 | 80 | −0.36 | 63.0 | 61.4 | 72.3 | 55.9 | 19.9 | 94.1 | 41.2 |
| Compound | Solubility (g/L) | ||
|---|---|---|---|
| 25 °C | 50 °C | 80 °C | |
| CaSO4·2H2O 1 | 2.68 (0.62) | 2.59 (0.60) | 2.30 (0.54) |
| Ce2(SO4)3 2 | 85.2 (42.0) | 46.9 (23.1) | 32.2 (15.9) |
| La2(SO4)3 2 | 20.9 (10.3) | 14.7 (7.22) | 9.53 (4.68) |
| Nd2(SO4)3 2 | 48.3 (24.2) | 28.8 (14.4) | 19.7 (9.87) |
| Y2(SO4)3 2 | 61.4 (23.4) | 43.1 (16.5) | 29.0 (11.1) |
| Correlated Metals | Sulfuric Acid Baking-Water-Leaching | Caustic Digestion–Acid-Leaching | ||
|---|---|---|---|---|
| Acid/Solid Ratio | Leaching Temperature | NaOH Concentration | HCl Concentration | |
| Ce–Fe | 0.9980 | 0.9984 | 0.1657 | 0.9652 |
| La–Fe | 0.9946 | 0.9997 | 0.5990 | 0.8849 |
| Nd–Fe | 0.9692 | 0.9824 | 0.8062 | 0.9899 |
| Y–Fe | 1.0000 | 1.0000 | 0.7706 | 0.9772 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, R.; Cho, H.; Jeong, J.; Kim, J.; Lee, S.; Chung, K.W.; Yoon, H.-S.; Kim, C.-J. Effect of Sulfuric Acid Baking and Caustic Digestion on Enhancing the Recovery of Rare Earth Elements from a Refractory Ore. Minerals 2020, 10, 532. https://doi.org/10.3390/min10060532
Kim R, Cho H, Jeong J, Kim J, Lee S, Chung KW, Yoon H-S, Kim C-J. Effect of Sulfuric Acid Baking and Caustic Digestion on Enhancing the Recovery of Rare Earth Elements from a Refractory Ore. Minerals. 2020; 10(6):532. https://doi.org/10.3390/min10060532
Chicago/Turabian StyleKim, Rina, Heechan Cho, Jinan Jeong, Jihye Kim, Sugyeong Lee, Kyeong Woo Chung, Ho-Sung Yoon, and Chul-Joo Kim. 2020. "Effect of Sulfuric Acid Baking and Caustic Digestion on Enhancing the Recovery of Rare Earth Elements from a Refractory Ore" Minerals 10, no. 6: 532. https://doi.org/10.3390/min10060532
APA StyleKim, R., Cho, H., Jeong, J., Kim, J., Lee, S., Chung, K. W., Yoon, H.-S., & Kim, C.-J. (2020). Effect of Sulfuric Acid Baking and Caustic Digestion on Enhancing the Recovery of Rare Earth Elements from a Refractory Ore. Minerals, 10(6), 532. https://doi.org/10.3390/min10060532






