Accelerated Carbonation of Recycled Aggregates Using the Pressurized Supercritical Carbon Dioxide Sparging Process
Abstract
1. Introduction
2. Material and Experimental Methods
2.1. Materials
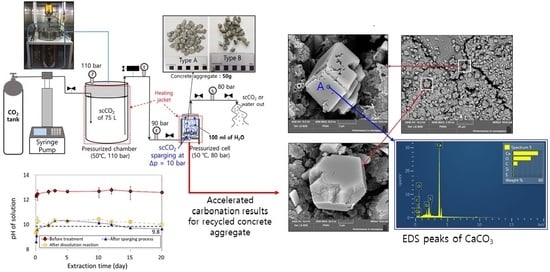
2.2. Accelerated Carbonation Using Pressurized scCO2 Sparging
2.3. Extraction Batch Experiment for Decrease of pH by Carbonation
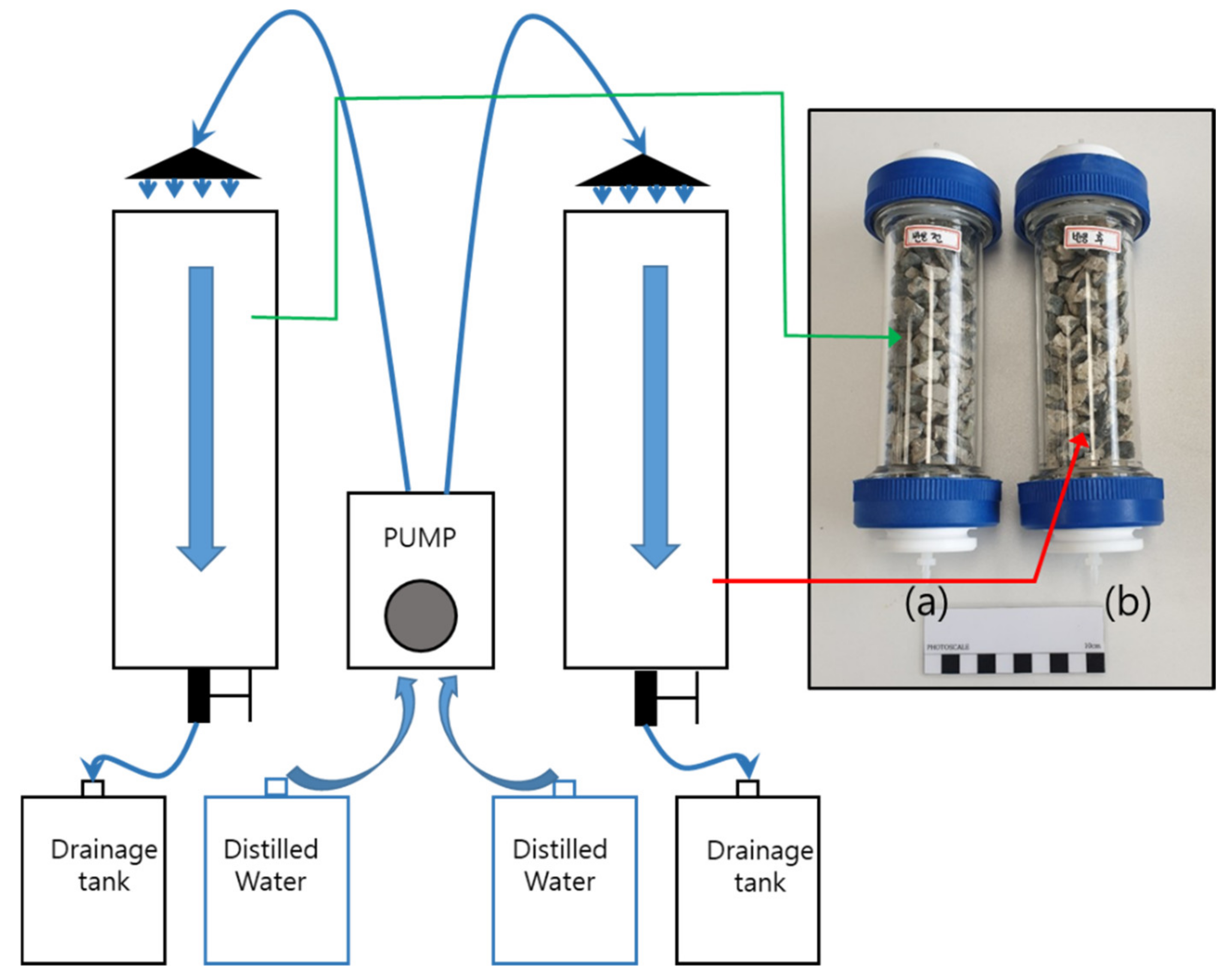
2.4. Continuous Column Experiment
3. Results and Discussion
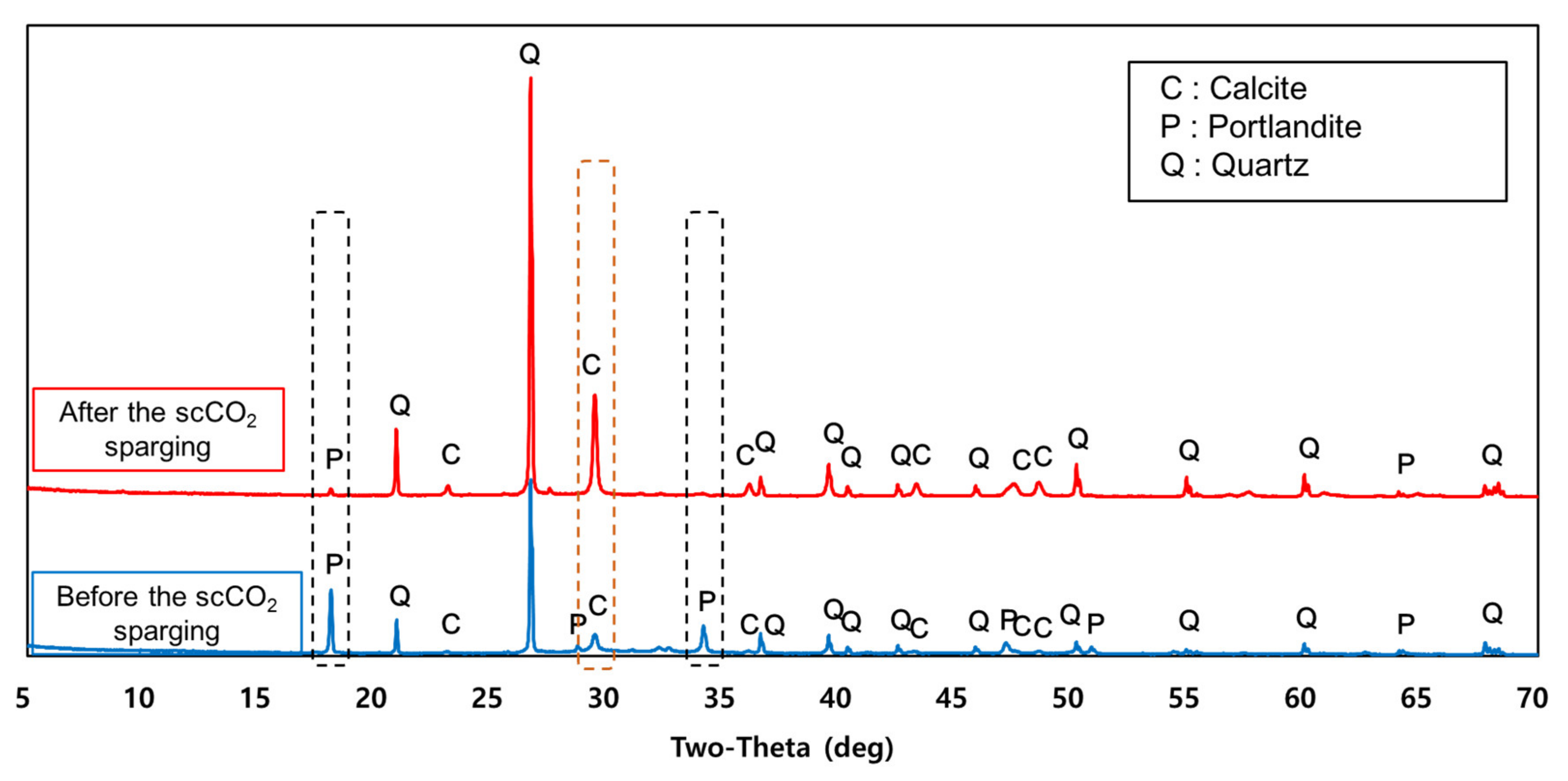
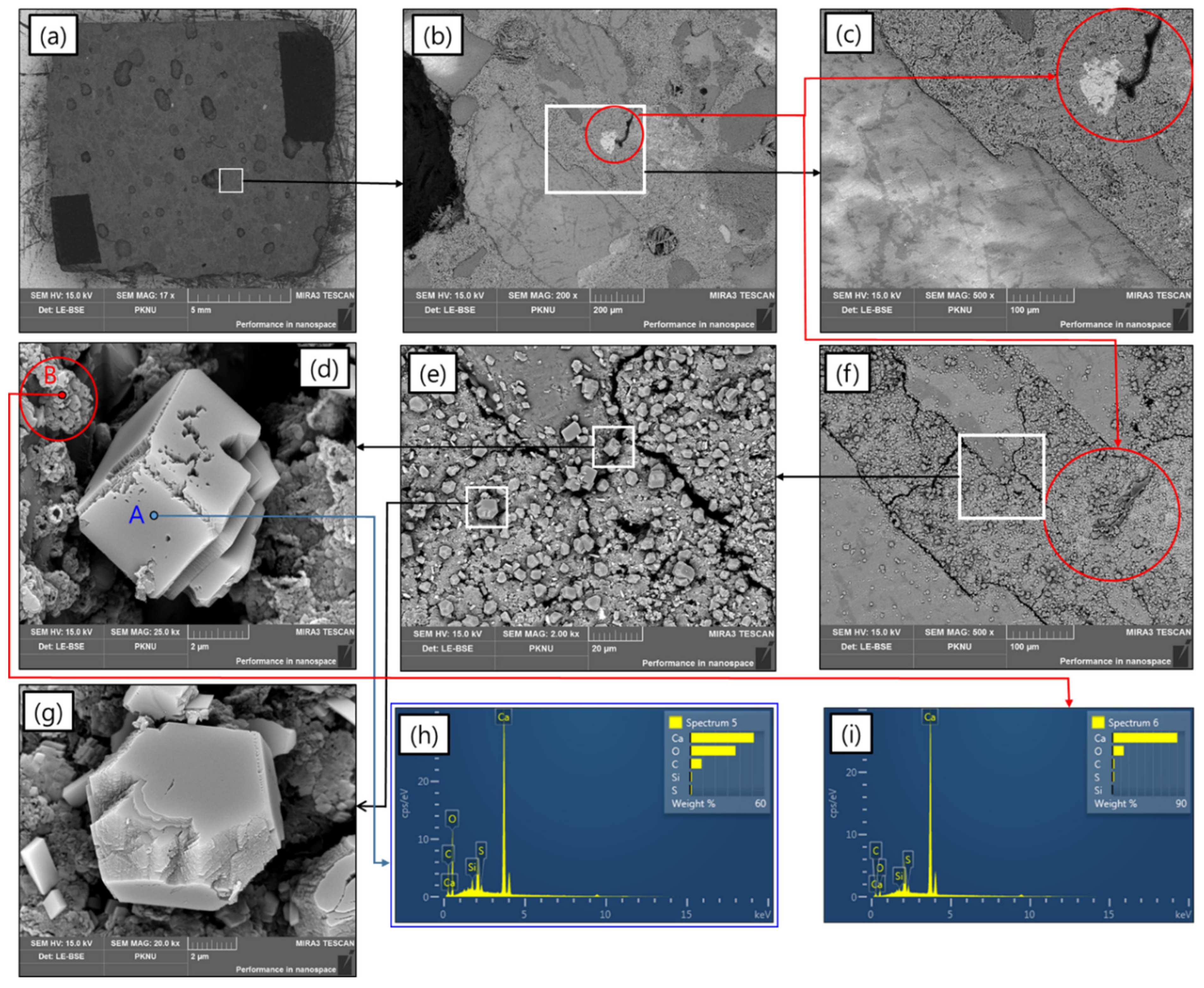
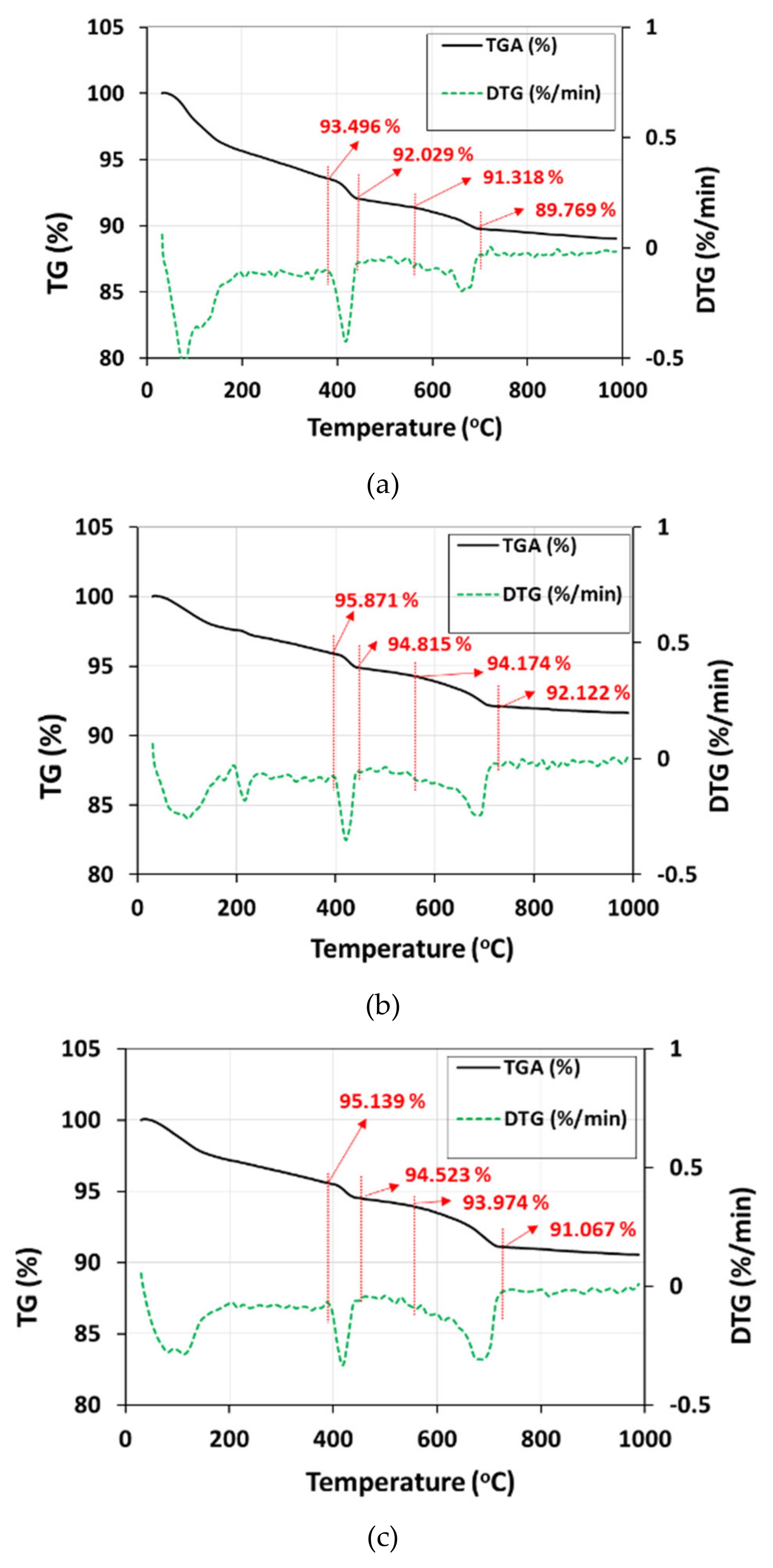
3.1. Accelerated Carbonation Using Pressurized scCO2 Sparging
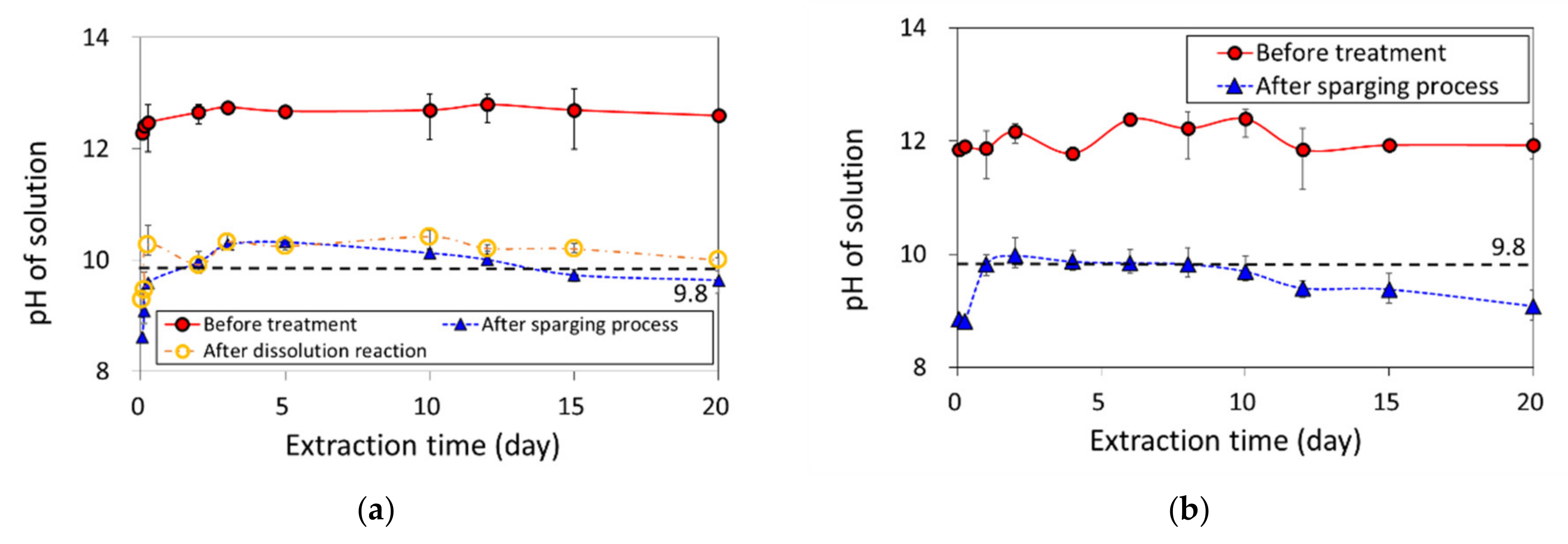
3.2. Batch Extraction Experiment for pH Neutralization by the Carbonation Process
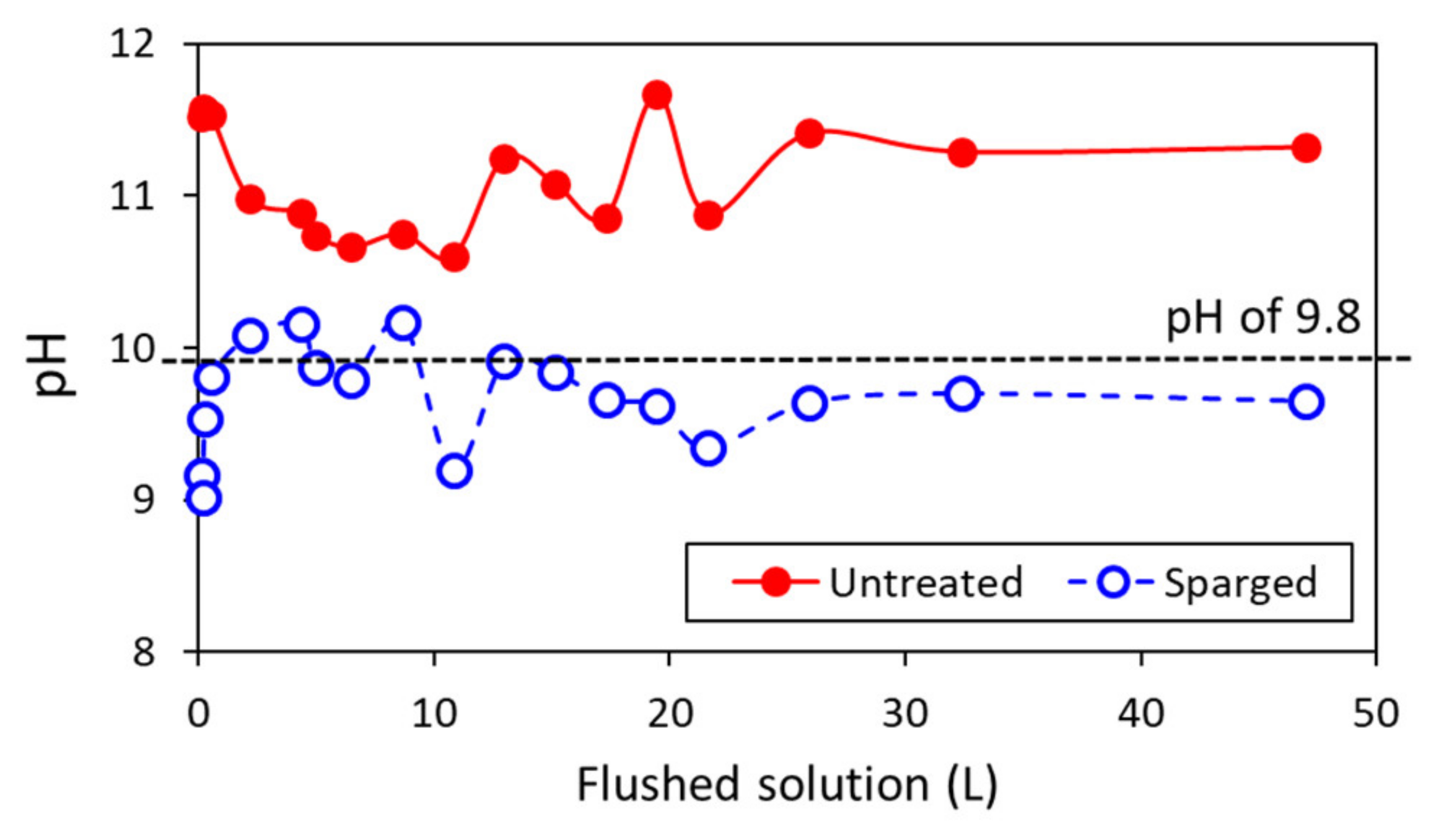
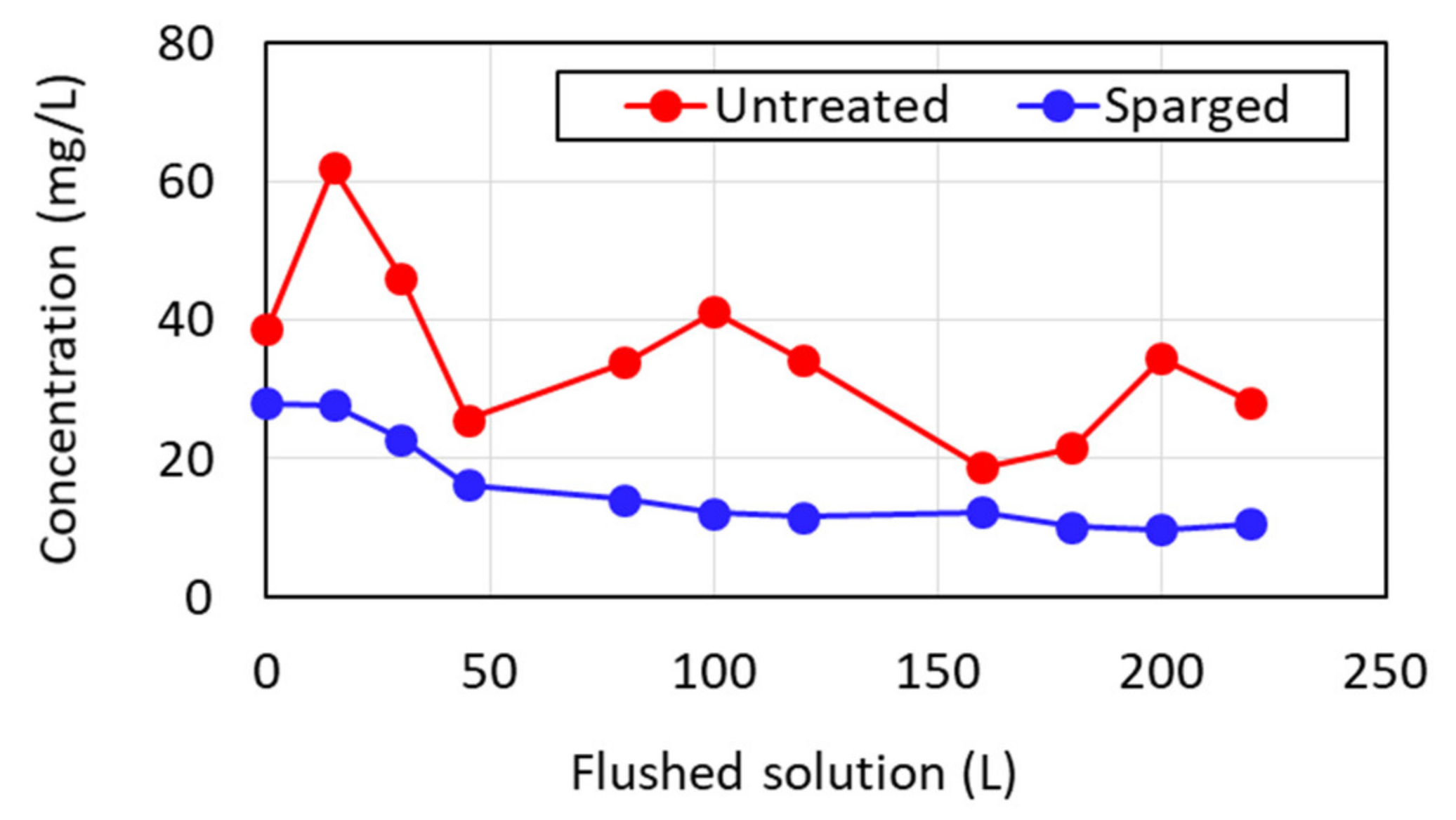
3.3. Continuous Column Experiment
3.4. Role of Carbonation in the Lowering of pH in Water Contacting scCO2-Treated Aggregate
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jo, H.; Ji, S.; Shin, H.; Jo, J.; Kim, J.; Bang, J.-H. Study on the Classification of Fine Particles by Using a Multi-step Hydrocyclone for the Recycling of Waste Concrete Slurry. J. Korean Soc. Miner. Energy Resour. Eng. 2019, 56, 73–78. [Google Scholar] [CrossRef]
- Kim, J.; Tae, S.; Kim, R. Theoretical study on the production of environment-friendly recycled cement using inorganic construction wastes as secondary materials in South Korea. Sustainability 2018, 10, 4449. [Google Scholar] [CrossRef]
- Ossa, A.; García, J.L.; Botero, E. Use of recycled construction and demolition waste (CDW) aggregates: A sustainable alternative for the pavement construction industry. J. Clean. Prod. 2016, 135, 379–386. [Google Scholar] [CrossRef]
- Rahman, M.T.; Mohajerani, A.; Giustozzi, F. Recycling of Waste Materials for Asphalt Concrete and Bitumen: A Review. Materials (Basel) 2020, 13, 1495. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Shi, C.; Shi, Z.; Lu, B.; Wan, S.; Zhang, Z.; Chang, J.; Zhang, T. Alkali-aggregate reaction in recycled aggregate concrete. J. Clean. Prod. 2020, 255. [Google Scholar] [CrossRef]
- Tam, V.W.Y.; Butera, A.; Le, K.N.; Li, W. Utilising CO2 technologies for recycled aggregate concrete: A critical review. Constr. Build. Mater. 2020, 250, 118903. [Google Scholar] [CrossRef]
- Tabsh, S.W.; Abdelfatah, A.S. Influence of recycled concrete aggregates on strength properties of concrete. Constr. Build. Mater. 2009, 23, 1163–1167. [Google Scholar] [CrossRef]
- Tu, T.Y.; Chen, Y.Y.; Hwang, C.L. Properties of HPC with recycled aggregates. Cem. Concr. Res. 2006, 36, 943–950. [Google Scholar] [CrossRef]
- Tam, V.W.Y.; Tam, C.M. A review on the viable technology for construction waste recycling. Resour. Conserv. Recycl. 2006, 47, 209–221. [Google Scholar] [CrossRef]
- Rao, A.; Jha, K.N.; Misra, S. Use of aggregates from recycled construction and demolition waste in concrete. Resour. Conserv. Recycl. 2007, 50, 71–81. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Academic Press: London, UK, 1997. [Google Scholar]
- Moreno-Pérez, E.; Hernández-Ávila, J.; Rangel-Martínez, Y.; Cerecedo-Sáenz, E.; Arenas-Flores, A.; Reyes-Valderrama, M.I.; Salinas-Rodríguez, E. Chemical and mineralogical characterization of recycled aggregates from construction and demolition waste from Mexico city. Minerals 2018, 8. [Google Scholar] [CrossRef]
- Lee, J.C.; Song, T.H.; Lee, S.H. Leaching behavior of toxic chemicals in recycled aggregates and their alkalinity. J. Mater. Cycles Waste Manag. 2012, 14, 193–201. [Google Scholar] [CrossRef]
- Galvín, A.P.; Ayuso, J.; Jiménez, J.R.; Agrela, F. Comparison of batch leaching tests and influence of pH on the release of metals from construction and demolition wastes. Waste Manag. 2012, 32, 88–95. [Google Scholar] [CrossRef] [PubMed]
- De Michelis, I.; Ferella, F.; Beolchini, F.; Olivieri, A.; Vegliò, F. Characterisation and classification of solid wastes coming from reductive acid leaching of low-grade manganiferous ore. J. Hazard. Mater. 2009, 162, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Gil, S.W. Long-term master planning for environment-friendly process and recycling promotion of Construction Wastes. J. Korean Recycl. Constr. Resour. Inst. 2006, 2, 8–12. [Google Scholar]
- Lee, W.P.; Lee, C.H. The Use of Recycled Aggregate and Recycled Products Revitalization. J. Korean Recycl. Constr. Resour. Inst. 2014, 9, 18–28. [Google Scholar] [CrossRef]
- Hong, S.W.; Park, S.; Ahn, Y.S. A Research on the Efficient Way and the Analysis of the Actual Condition for Recycling Waste Concrete Discharged as Construction Waste. J. Archit. Inst. Korea 2004, 20, 97–104. [Google Scholar]
- Martín, D.; Flores-Alés, V.; Aparicio, P. Proposed methodology to evaluate CO2 capture using construction and demolition waste. Minerals 2019, 9. [Google Scholar] [CrossRef]
- Atiş, C.D. Accelerated carbonation and testing of concrete made with fly ash. Constr. Build. Mater. 2003, 17, 147–152. [Google Scholar] [CrossRef]
- Turcry, P.; Oksri-Nelfia, L.; Younsi, A.; Aït-Mokhtar, A. Analysis of an accelerated carbonation test with severe preconditioning. Cem. Concr. Res. 2014, 57, 70–78. [Google Scholar] [CrossRef]
- Cui, H.; Tang, W.; Liu, W.; Dong, Z.; Xing, F. Experimental study on effects of CO2 concentrations on concrete carbonation and diffusion mechanisms. Constr. Build. Mater. 2015, 93, 522–527. [Google Scholar] [CrossRef]
- Silva, R.V.; Neves, R.; De Brito, J.; Dhir, R.K. Carbonation behaviour of recycled aggregate concrete. Cem. Concr. Compos. 2015, 62, 22–32. [Google Scholar] [CrossRef]
- Zhan, B.; Poon, C.S.; Liu, Q.; Kou, S.; Shi, C. Experimental study on CO2 curing for enhancement of recycled aggregate properties. Constr. Build. Mater. 2014, 67, 3–7. [Google Scholar] [CrossRef]
- Kou, S.C.; Zhan, B.J.; Poon, C.S. Use of a CO2 curing step to improve the properties of concrete prepared with recycled aggregates. Cem. Concr. Compos. 2014, 45, 22–28. [Google Scholar] [CrossRef]
- Duan, Z.; Sun, R. An improved model calculating CO2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2000 bar. Chem. Geol. 2003, 193, 257–271. [Google Scholar] [CrossRef]
- Wiebe, R.; Gaddy, V.L. The Solubility of Carbon Dioxide in Water at Various Temperatures from 12 to 40° and at Pressures to 500 Atmospheres. Critical Phenomena. J. Am. Chem. Soc. 1940, 62, 815–817. [Google Scholar] [CrossRef]
- Mathur, V. Precisely Sized Precipitated Calcium Carbonate (PCC) Crystals of Preselected Crystal Habit, Manufactured Using Pressure Carbonation. U.S. Patent Application NO 09/791,433, 24 January 2002. [Google Scholar]
- Declet, A.; Reyes, E.; Suárez, O.M. Calcium carbonate precipitation: A review of the carbonate crystallization process and applications in bioinspired composites. Rev. Adv. Mater. Sci. 2016, 44, 87–107. [Google Scholar]
- Korchef, A.; Touaibi, M. Effect of pH and temperature on calcium carbonate precipitation by CO2 removal from iron-rich water. Water Environ. J. 2019. [Google Scholar] [CrossRef]
- Chung, C.W.; Lee, M.; Kim, S.O.; Kim, J. The pH reduction of the recycled aggregate originated from the waste concrete by the scCO2 treatment. Econ. Environ. Geol. 2017, 50, 257–266. [Google Scholar] [CrossRef]
- De Sena Costa, B.L.; de Oliveira Freitas, J.C.; Santos, P.H.S.; de Araújo Melo, D.M.; da Silva Araujo, R.G.; de Oliveira, Y.H. Carbonation in oil well Portland cement: Influence of hydration time prior to contact with CO2. Constr. Build. Mater. 2018, 159, 252–260. [Google Scholar] [CrossRef]
- Hidalgo, A.; Domingo, C.; Garcia, C.; Petit, S.; Andrade, C.; Alonso, C. Microstructural changes induced in Portland cement-based materials due to natural and supercritical carbonation. J. Mater. Sci. 2008, 43, 3101–3111. [Google Scholar] [CrossRef]
- Ruiz-Agudo, E.; Kudłacz, K.; Putnis, C.V.; Putnis, A.; Rodriguez-Navarro, C. Dissolution and carbonation of portlandite [Ca(OH)2] single crystals. Environ. Sci. Technol. 2013, 47, 11342–11349. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Olek, J. Effects of sample preparation and interpretation of thermogravimetric curves on calcium hydroxide in hydrated pastes and mortars. Transp. Res. Rec. 2012, 10–18. [Google Scholar] [CrossRef]
- Moon, H.; Ramanathan, S.; Suraneni, P.; Shon, C.S.; Lee, C.J.; Chung, C.W. Revisiting the effect of slag in reducing heat of hydration in concrete in comparison to other supplementary cementitious materials. Materials (Basel) 2018, 11, 1847. [Google Scholar] [CrossRef]
- Visintin, P.; Xie, T.; Bennett, B. A large-scale life-cycle assessment of recycled aggregate concrete: The influence of functional unit, emissions allocation and carbon dioxide uptake. J. Clean. Prod. 2020, 248. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Lee, J.; Chung, C.-W.; Wang, S.; Lee, M. Accelerated Carbonation of Recycled Aggregates Using the Pressurized Supercritical Carbon Dioxide Sparging Process. Minerals 2020, 10, 486. https://doi.org/10.3390/min10060486
Park J, Lee J, Chung C-W, Wang S, Lee M. Accelerated Carbonation of Recycled Aggregates Using the Pressurized Supercritical Carbon Dioxide Sparging Process. Minerals. 2020; 10(6):486. https://doi.org/10.3390/min10060486
Chicago/Turabian StylePark, Jinyoung, Jinkyun Lee, Chul-Woo Chung, Sookyun Wang, and Minhee Lee. 2020. "Accelerated Carbonation of Recycled Aggregates Using the Pressurized Supercritical Carbon Dioxide Sparging Process" Minerals 10, no. 6: 486. https://doi.org/10.3390/min10060486
APA StylePark, J., Lee, J., Chung, C.-W., Wang, S., & Lee, M. (2020). Accelerated Carbonation of Recycled Aggregates Using the Pressurized Supercritical Carbon Dioxide Sparging Process. Minerals, 10(6), 486. https://doi.org/10.3390/min10060486