Gemological Characteristics and Origin of the Zhanguohong Agate from Beipiao, Liaoning Province, China: A Combined Microscopic, X-ray Diffraction, and Raman Spectroscopic Study
Abstract
1. Introduction
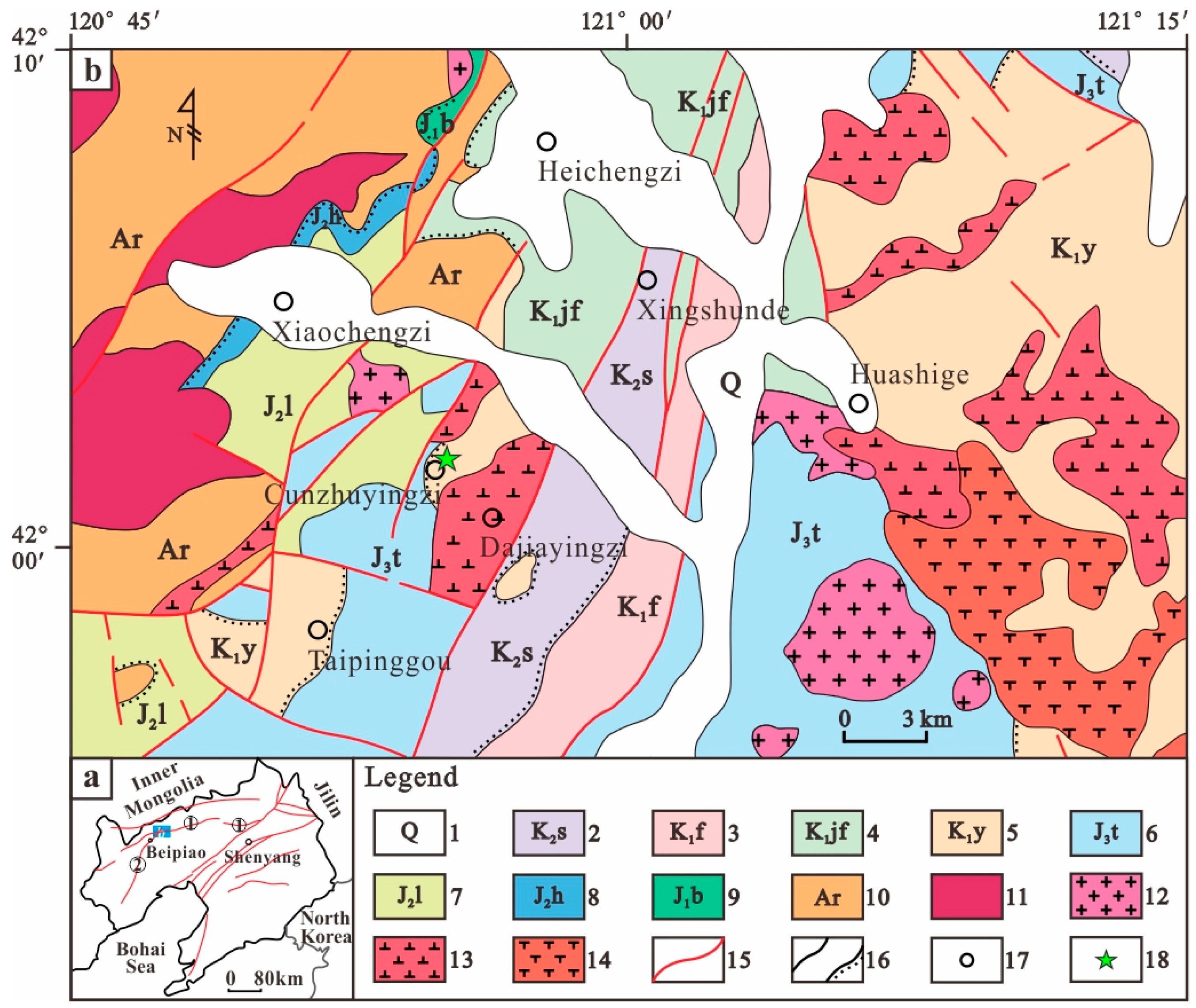
2. Geological Setting
3. Materials and Methods
4. Results
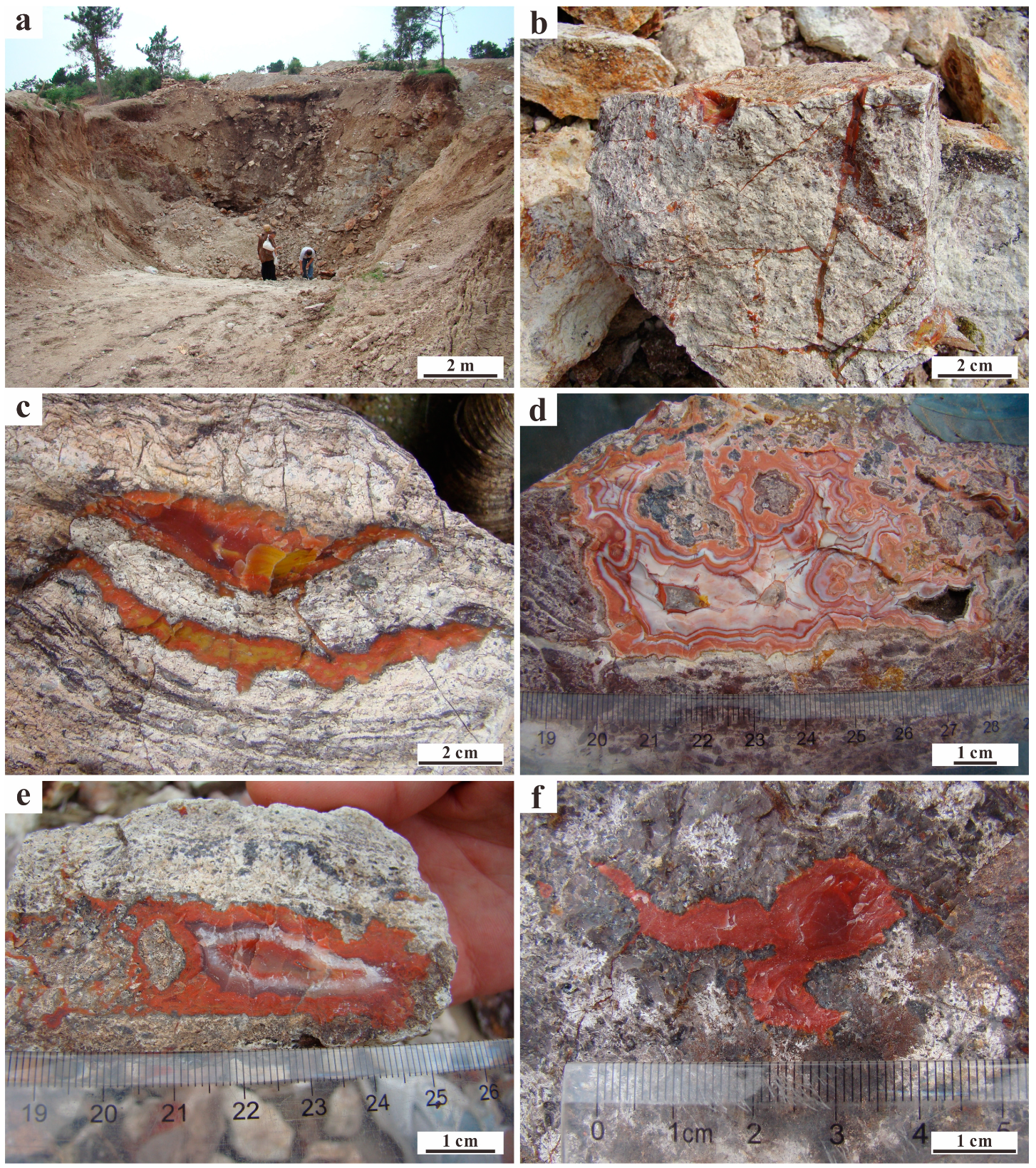
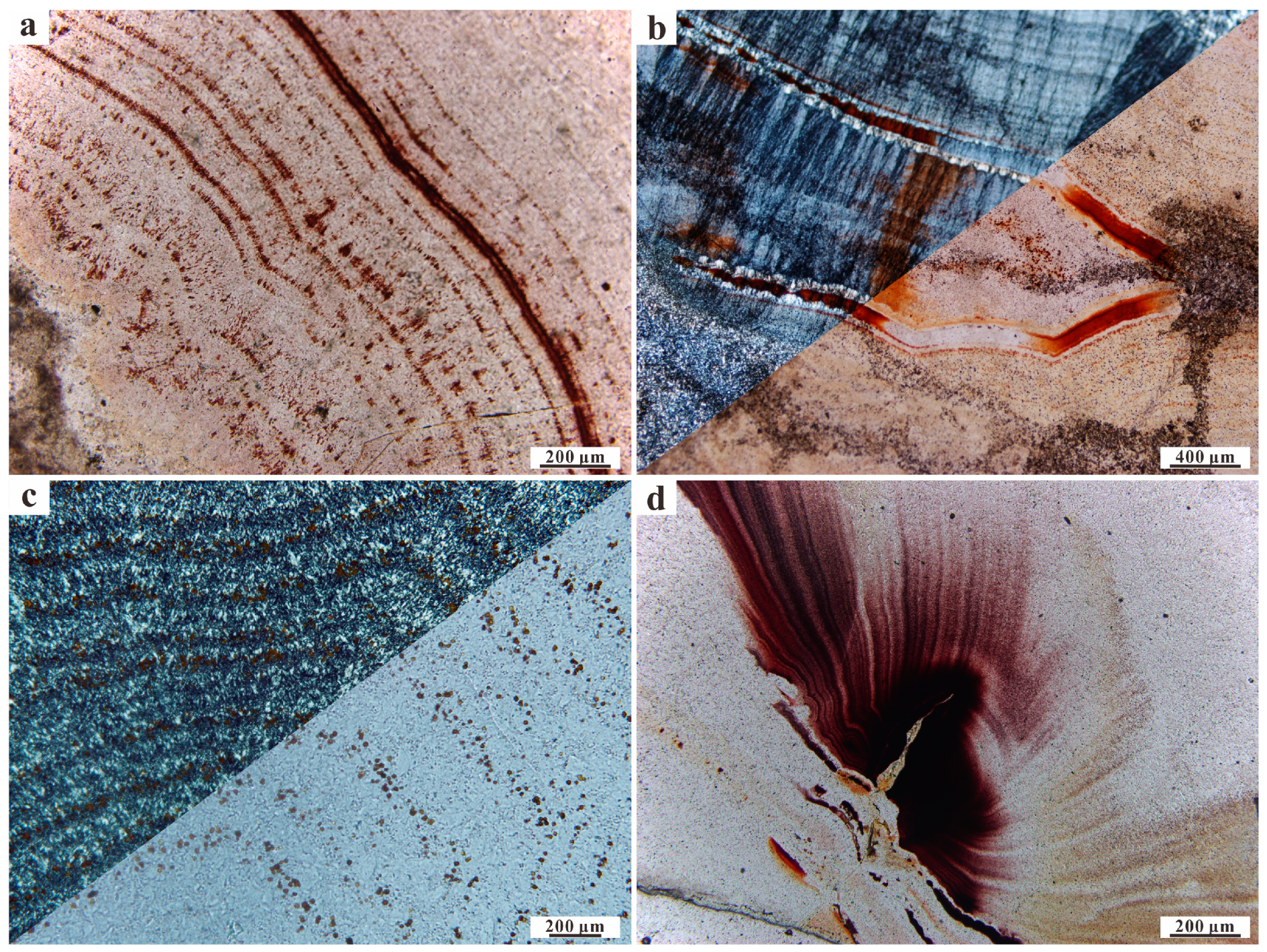
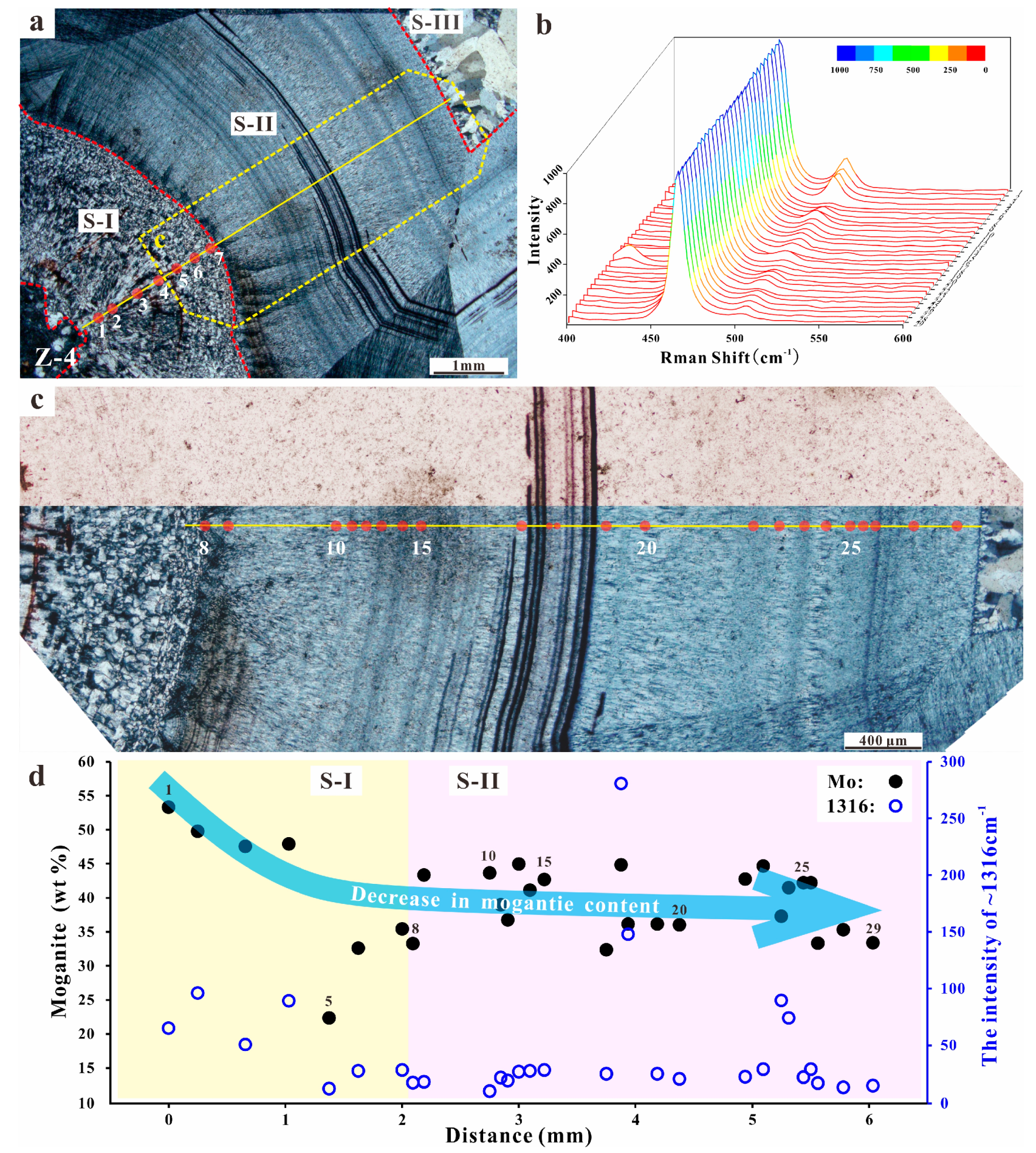
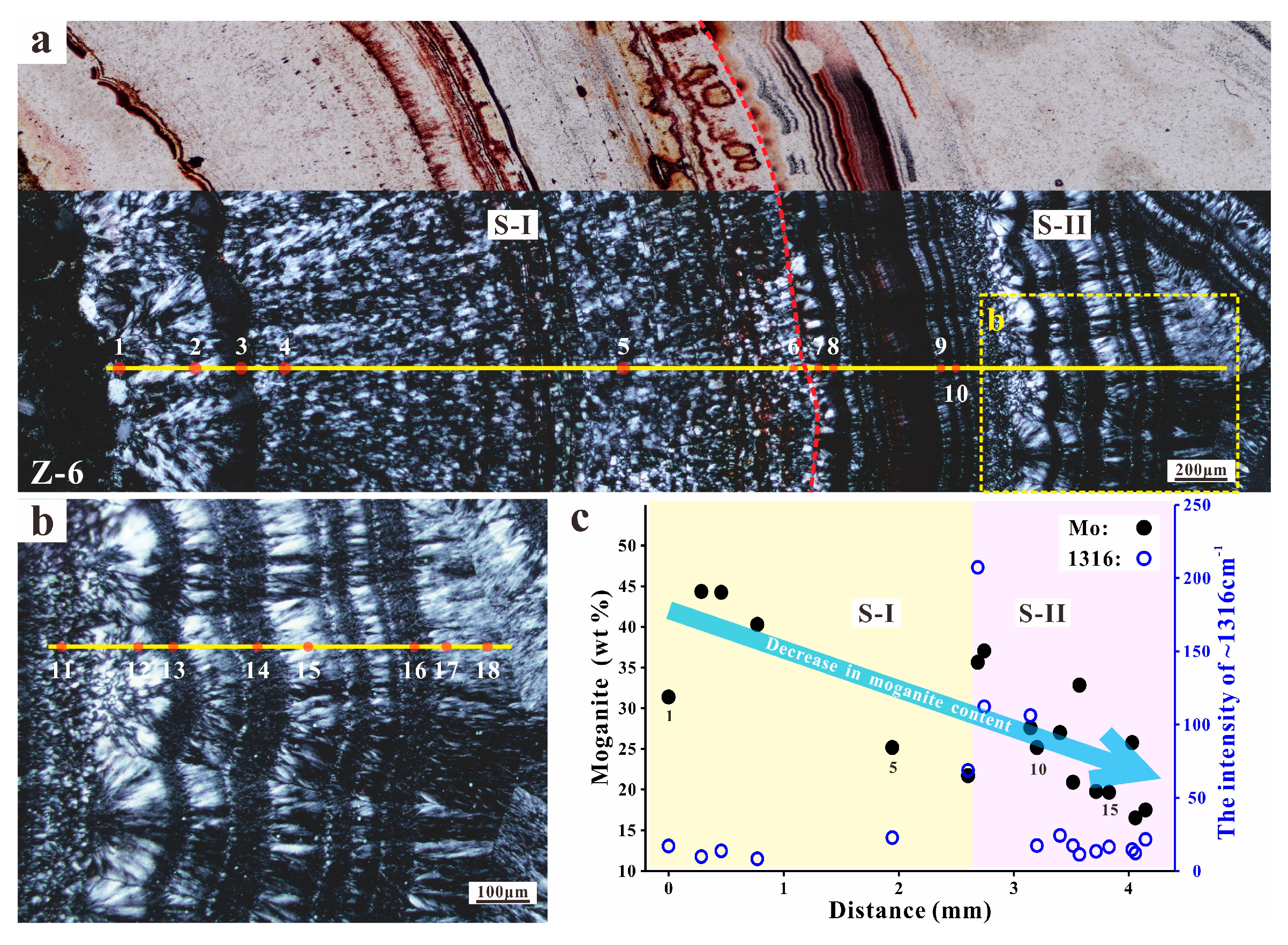
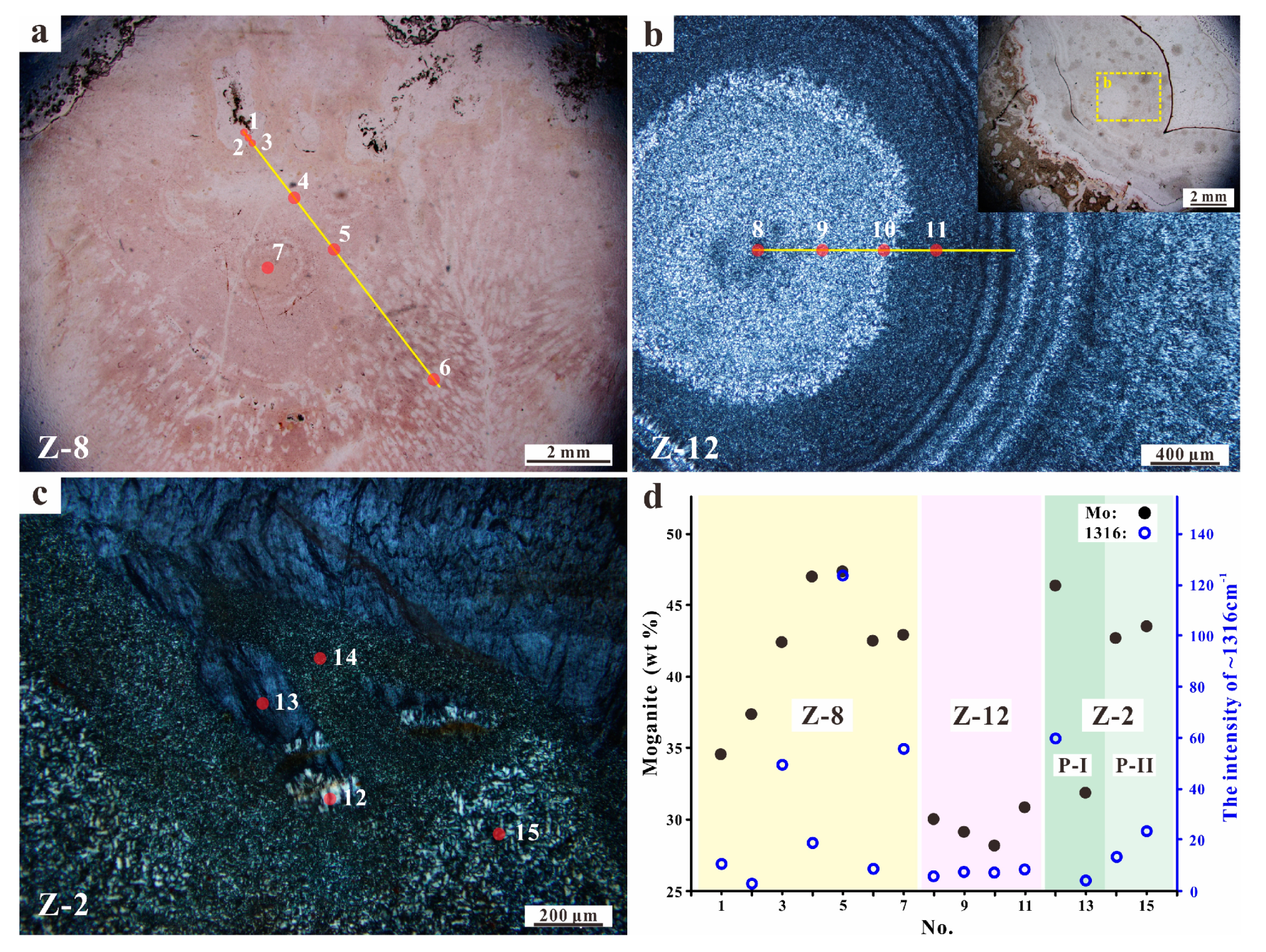
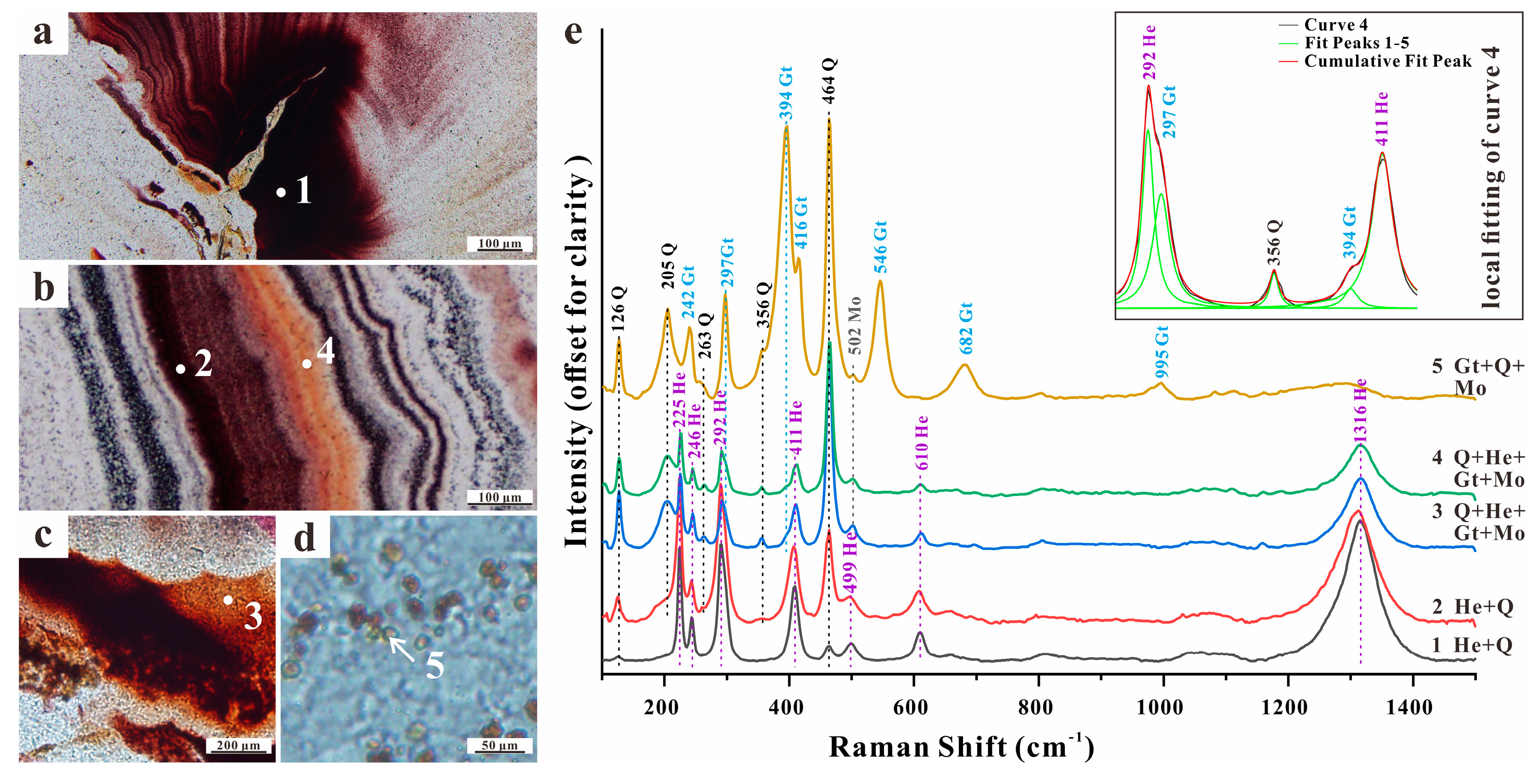
4.1. Microscopic Observations
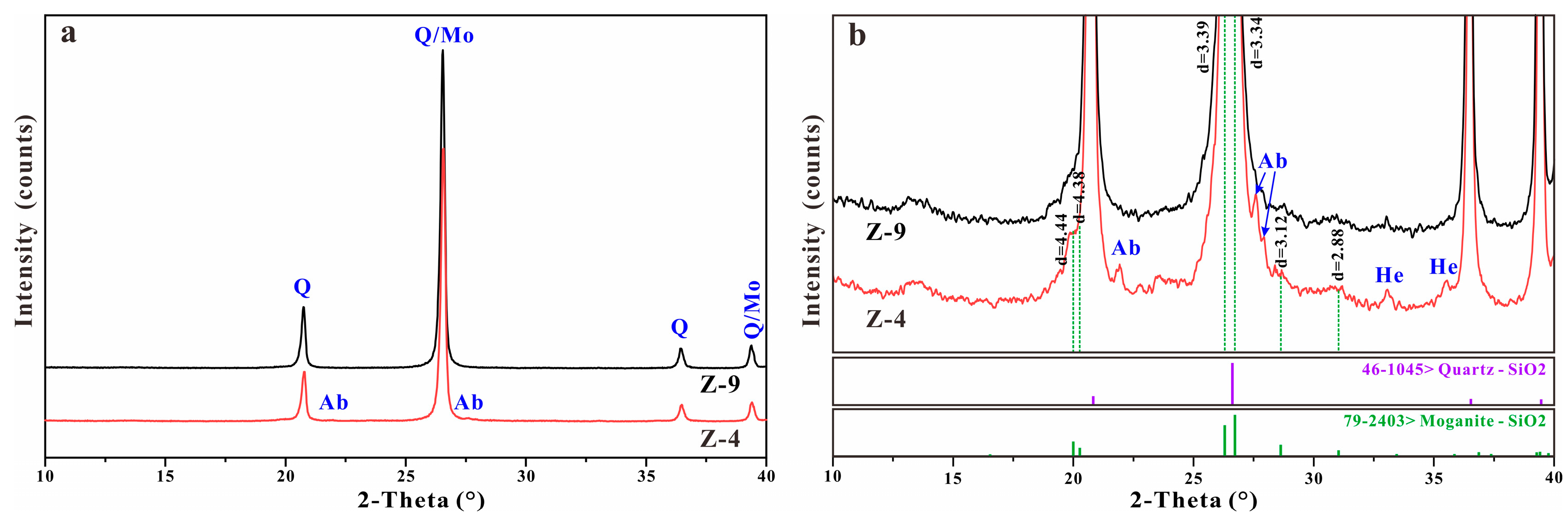
4.2. XRD Analysis
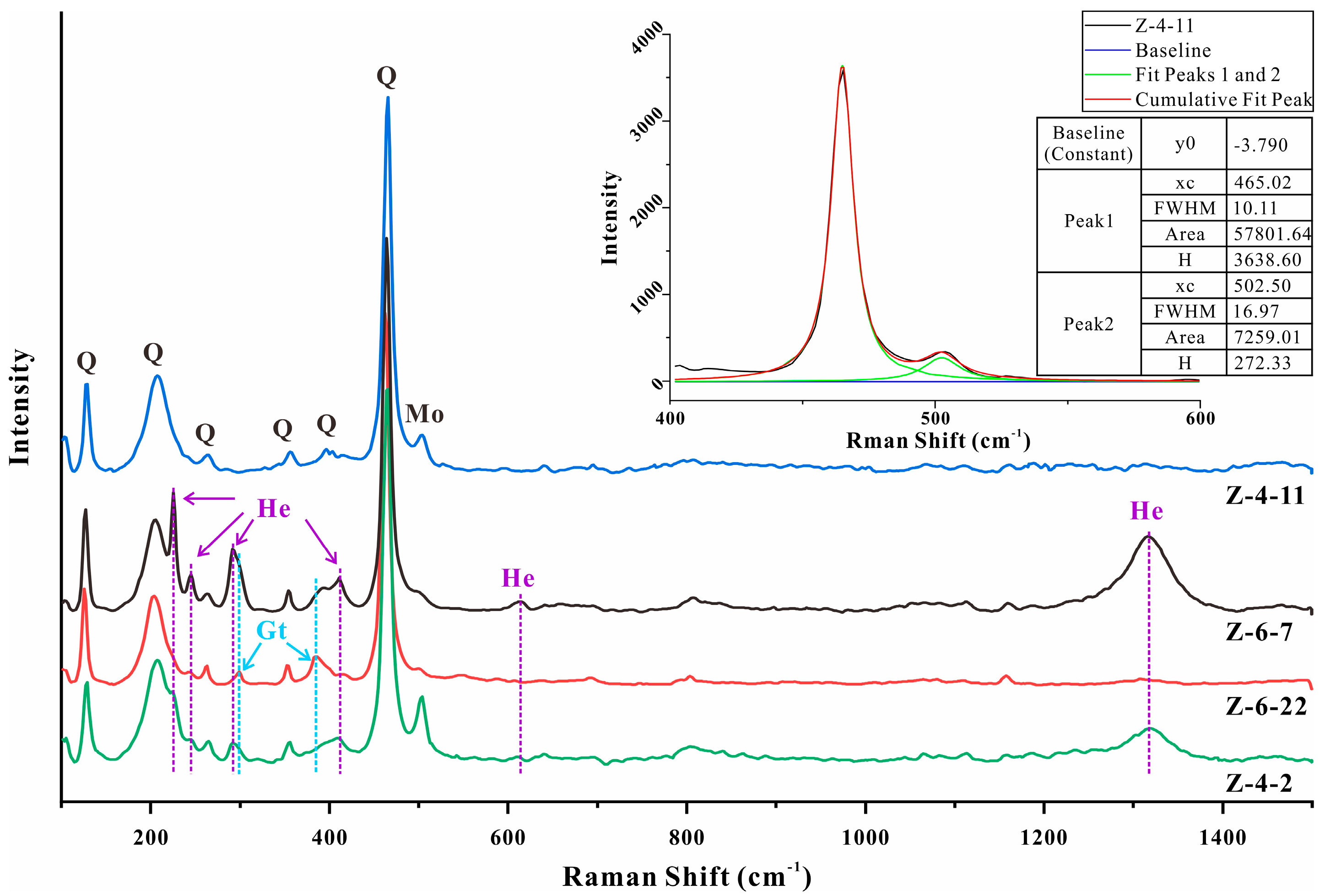
4.3. Raman Spectroscopic Analysis
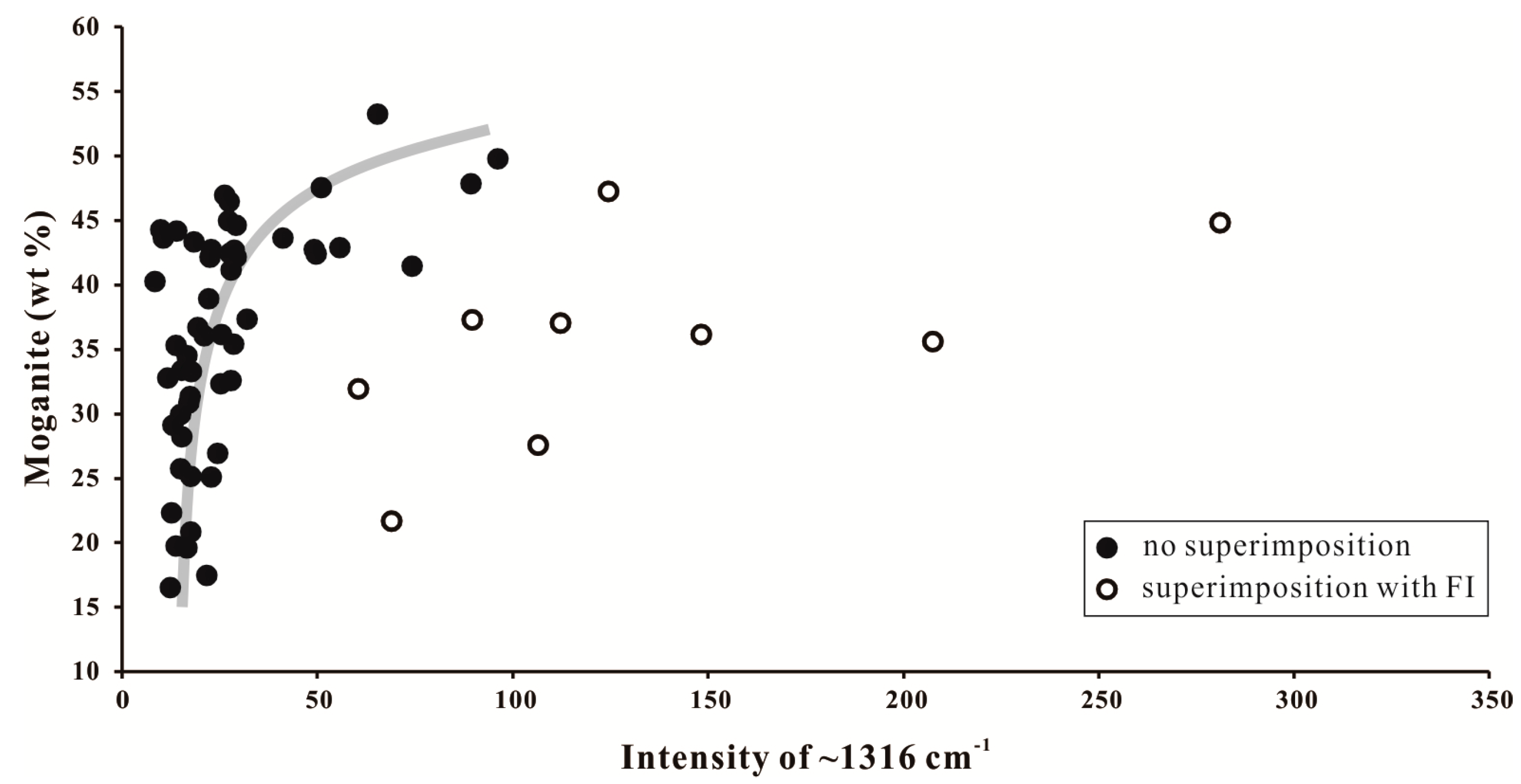
4.3.1. Silica Matrix
4.3.2. Ferruginous Inclusions
5. Discussion
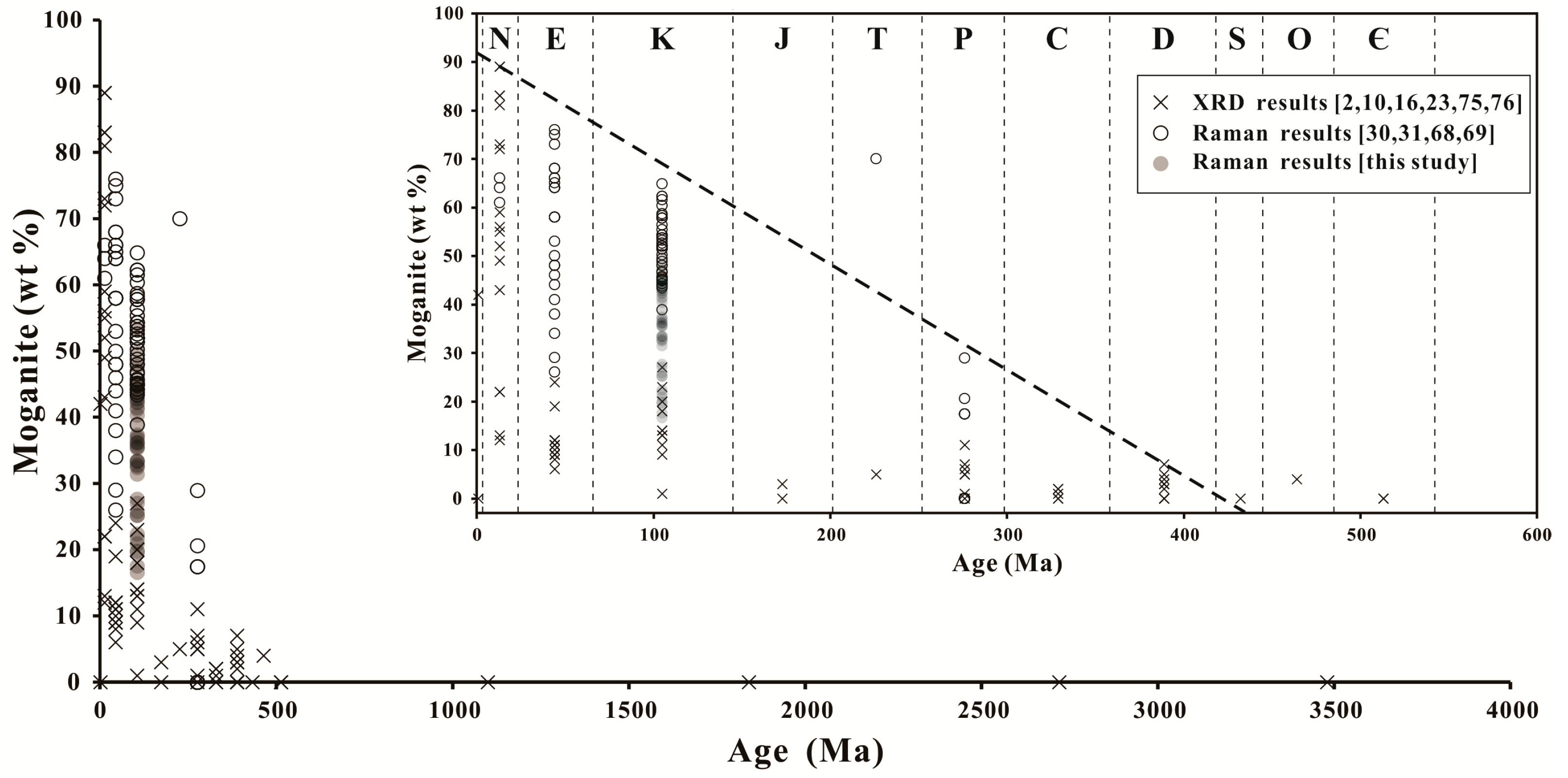
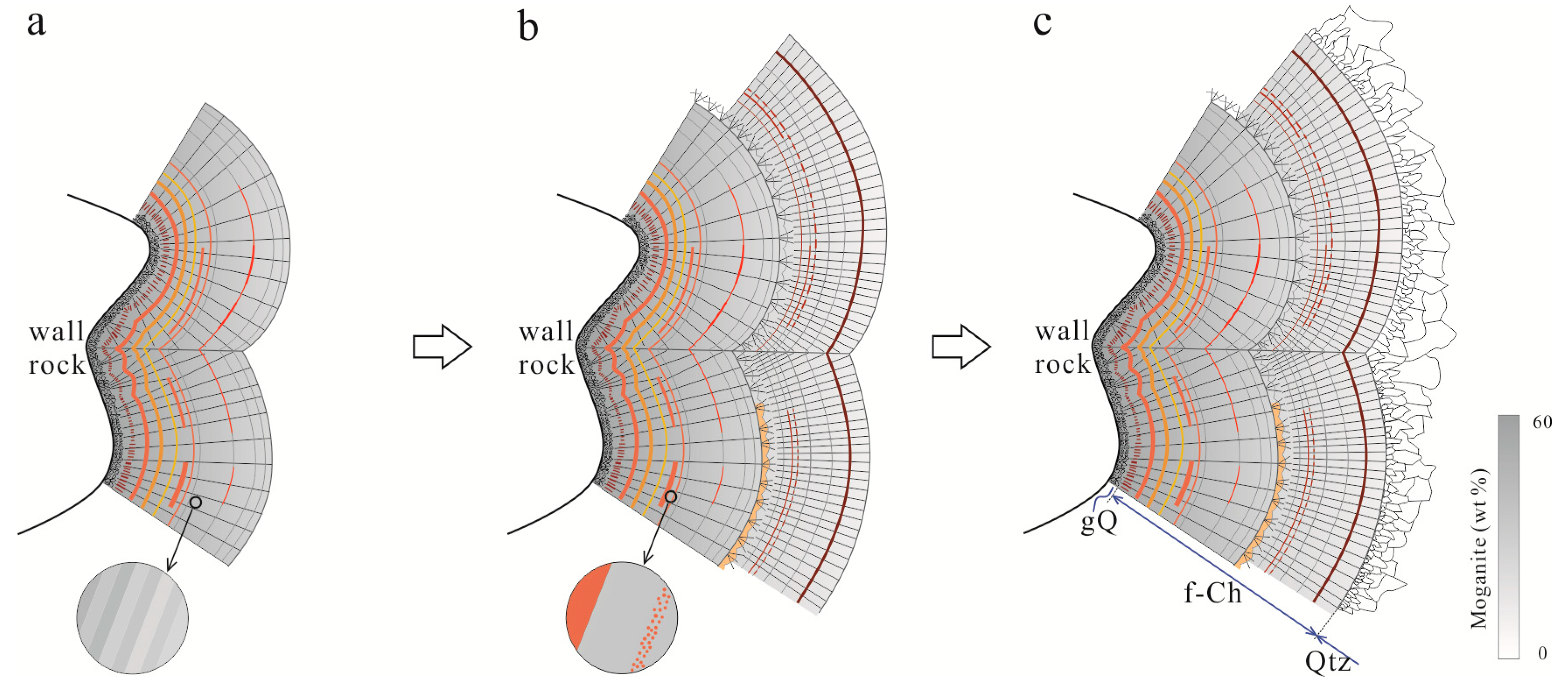
5.1. Moganite in the Zhanguohong Agate
5.2. Coloration Mechanism
5.3. Origin of the Zhanguohong Agates
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Flörke, O.W.; Köhler-Herbertz, B.; Langer, K.; Tönges, I. Water in microcrystalline quartz of volcanic origin: Agates. Contrib. Mineral. Petrol. 1982, 80, 324–333. [Google Scholar] [CrossRef]
- Heaney, P.J. Moganite as an indicator for vanished evaporites: A testament reborn? J. Sediment. Res. 1995, A65, 633–638. [Google Scholar]
- Götze, J.; Nasdala, L.; Kleeberg, R.; Wenzel, M. Occurrence and distribution of “moganite” in agate/chalcedony: A combined micro-Raman, Rietveld, and cathodoluminescence study. Contrib. Mineral. Petrol. 1998, 133, 96–105. [Google Scholar] [CrossRef]
- Dumańska-Słowik, M.; Natkaniec-Nowak, L.; Wesełucha-Birczyńska, A.; Gaweł, A.; Lankosz, M.; Wróbel, P. Agates from Sidi Rahal, in the Atlas Mountains of Morocco: Gemological characteristics and proposed origin. Gems Gemol. 2013, 49, 148–159. [Google Scholar] [CrossRef]
- Dumańska-Słowik, M.; Powolny, T.; Sikorska-Jaworowska, M.; Gaweł, A.; Kogut, L.; Poloński, K. Characteristics and origin of agates from Płóczki Górne (Lower Silesia, Poland): A combined microscopic, micro-Raman, and cathodoluminescence study. Spectrochim. Acta, Part A 2018, 192, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Powolny, T.; Dumańska-Słowik, M.; Sikorska-Jaworowska, M.; Wójcik-Bania, M. Agate mineralization in spilitized Permian volcanics from “Borówno” quarry (Lower Silesia, Poland)—microtextural, mineralogical, and geochemical constraints. Ore Geol. Rev. 2019, 114, 103130. [Google Scholar] [CrossRef]
- Götze, J.; Möckel, R.; Kempe, U.; Kapitonov, I.; Vennemann, T. Characteristics and origin of agates in sedimentary rocks from the Dryhead area, Montana, USA. Mineral. Mag. 2009, 73, 673–690. [Google Scholar] [CrossRef]
- Heaney, P.J. A proposed mechanism for the growth of chalcedony. Contrib. Mineral. Petrol. 1993, 115, 66–74. [Google Scholar] [CrossRef]
- Moxon, T. Agate: A study of ageing. Eur. J. Mineral. 2002, 14, 1109–1118. [Google Scholar] [CrossRef]
- Moxon, T.; Nelson, D.R.; Zhang, M. Agate recrystallisation: Evidence from samples found in Archaean and Proterozoic host rocks, western Australia. Aust. J. Earth Sci. 2006, 53, 235–248. [Google Scholar] [CrossRef]
- Götze, J.; Schrön, W.; Möckel, R.; Heide, K. The role of fluids in the formation of agates. Geochemistry 2012, 72, 283–286. [Google Scholar] [CrossRef]
- Saunders, J.A. Silica and gold textures in bonanza ores of the Sleeper deposit, Humboldt county, Nevada: Evidence for colloids and implications for epithermal ore-forming processes. Econ. Geol. 1994, 89, 628–638. [Google Scholar] [CrossRef]
- Miehe, G.; Graetsch, H.; Flörke, O.W. Crystal structure and growth fabric of length-fast chalcedony. Phys. Chem. Miner. 1984, 10, 197–199. [Google Scholar] [CrossRef]
- Miehe, G.; Graetsch, H. Crystal structure of moganite: A new structure type for silica. Eur. J. Mineral. 1992, 4, 693–706. [Google Scholar] [CrossRef]
- Gíslason, S.R.; Heaney, P.J.; Oelkers, E.H.; Schott, J. Kinetic and thermodynamic properties of moganite, a novel silica polymorph. Geochim. Cosmochim. Acta 1997, 61, 1193–1204. [Google Scholar] [CrossRef]
- Moxon, T.; Ríos, S. Moganite and water content as a function of age in agate: An XRD and thermogravimetric study. Eur. J. Mineral. 2004, 16, 269–278. [Google Scholar] [CrossRef]
- Rodgers, K.A.; Cressey, G. The occurrence, detection and significance of moganite (SiO2) among some silica sinters. Mineral. Mag. 2001, 65, 157–167. [Google Scholar] [CrossRef]
- Rodgers, K.A.; Browne, P.R.L.; Buddle, T.F.; Cook, K.L.; Greatrex, R.A.; Hampton, W.A.; Herdianita, N.R.; Holland, G.R.; Lynne, B.Y.; Martin, R.; et al. Silica phases in sinters and residues from geothermal fields of New Zealand. Earth-Sci. Rev. 2004, 66, 1–61. [Google Scholar] [CrossRef]
- Bourli, N.; Kokkaliari, M.; Iliopoulos, I.; Pe-Piper, G.; Piper, D.J.W.; Maravelis, A.G.; Zelilidis, A. Mineralogy of siliceous concretions, Cretaceous of Ionian zone, western Greece: Implication for diagenesis and porosity. Mar. Petrol. Geol. 2019, 105, 45–63. [Google Scholar] [CrossRef]
- Heaney, P.J.; Post, J.E. The widespread distribution of a novel silica polymorph in microcrystalline quartz varieties. Science 1992, 255, 441–443. [Google Scholar] [CrossRef]
- Heaney, P.J.; McKeown, D.A.; Post, J.E. Anomalous behavior at the I2/a to Imab phase transition in SiO2-moganite: An analysis using hard-mode Raman spectroscopy. Am. Mineral. 2007, 92, 631–639. [Google Scholar] [CrossRef]
- Saminpanya, S.; Sutherland, F.L. Silica phase-transformations during diagenesis within petrified woods found in fluvial deposits from Thailand–Myanmar. Sediment. Geol. 2013, 290, 15–26. [Google Scholar] [CrossRef]
- Schmidt, P.; Bellot-Gurlet, L.; Leá, V.; Sciau, P. Moganite detection in silica rocks using Raman and infrared spectroscopy. Eur. J. Mineral. 2014, 25, 797–805. [Google Scholar] [CrossRef]
- Kayama, M.; Tomioka, N.; Ohtani, E.; Seto, Y.; Nagaoka, H.; Götze, J.; Miyake, A.; Ozawa, S.; Sekine, T.; Miyahara, M.; et al. Discovery of moganite in a lunar meteorite as a trace of H2O ice in the Moon’s regolith. Sci. Adv. 2018, 4, eaar4378. [Google Scholar] [CrossRef]
- Chen, Q.L.; Yuan, X.Q.; Jia, L. Study on the vibrational spectra characters of Taiwan blue chalcedony. Spectrosc. Spect. Anal. 2011, 31, 1549–1551, (In Chinese with English abstract). [Google Scholar]
- Meng, G.Q.; Chen, M.H.; Jiang, J.L.; Chen, S. Structural characteristic and cause of colour of “Zhanguohong” agate from Xuanhua, Hebei province. J. Gems Gemmol. 2016, 18, 28–34, (In Chinese with English abstract). [Google Scholar]
- Xu, W.H.; Xu, X.C.; Yang, L.L.; Wu, H.Y. Ore-geology and metallogenesis of “Zhanguohong” agate from Xuanhua, Hebei province. J. Gems Gemmol. 2017, 19, 1–11, (In Chinese with English abstract). [Google Scholar]
- Gao, Y.Q. Geological features and metallogenetic conditions of agate deposits in Cunzhuyingzi village. Jilin Agric. 2011, 12, 265. (In Chinese) [Google Scholar]
- Wang, Y.L.; Zhong, M.S.; Jia, C.; Yan, N.; Wang, Z.L.; Wang, J.; Zhou, C.S. Metallogeneic regularity and prospecting direction of agate in volcanic rocks of Fuxin area, western Liaoning. Geol. China 2011, 38, 1179–1187, (In Chinese with English abstract). [Google Scholar]
- Zhou, D.Y.; Chen, H.; Lu, T.J.; Ke, J.; He, M.Y. Study on the relationship between the relative content of moganite and the crystallinity of quartzite jade by Raman scattering spectroscopy, infrared absorption spectroscopy and X-ray diffraction techniques. Rock Miner. Anal. 2015, 34, 652–658, (In Chinese with English abstract). [Google Scholar]
- Lu, Z.Y.; He, X.M.; Lin, C.L.; Jin, X.Y.; Pan, Y.M. Identification of Beihong agate and Nanhong agate from China based on chromaticity and Raman spectra. Spectrosc. Spect. Anal. 2019, 39, 2153–2159, (In Chinese with English abstract). [Google Scholar]
- Xu, C.; Liang, R.; Yang, H.X.; Zhao, J. Prospecting of agate deposit in Zhangjiakou, Hebei province. J. Gems Gemmol. 2017, 19, 17–24, (In Chinese with English abstract). [Google Scholar]
- Qi, J.Y. Metallogenic characteristics and prospecting orientation of the agate deposits in western Liaoning, China. Geol. Resour. 2014, 23, 135–137, (In Chinese with English abstract). [Google Scholar]
- Fourth Bureau of Geology and Mineral Resources of the Liaoning Province (FBGMRLP). Prospecting Report of Agate Deposits Near Cunzhuyingzi Village, Beipiao, Liaoning Province, 2011; unpublished report in Chinese.
- Bureau of Geology and Mineral Resources of the Liaoning Province (BGMRLP). Regional Geology of Liaoning Province; Geological Publishing House: Beijing, China, 1989; pp. 1–856, (In Chinese with English summary). [Google Scholar]
- Yang, X.D.; Li, X.Y. Stratigraphy of Liaoning Province; China University of Geosciences Press: Wuhan, China, 1997; pp. 1–247. (In Chinese) [Google Scholar]
- Mi, J.R.; Xu, K.Z.; Zhang, C.B.; Chang, J.P.; Yao, P.Y. Mosozic strata in Beipiao, Liaoning province. J. Jilin Univ. Earth Sci. Ed. 1980, 4, 18–37. (In Chinese) [Google Scholar]
- Wong, W.H. Geological structures in Beipiao, Jehol. Bull. Geol. Surv. China 1928, 11, 1–23. (In Chinese) [Google Scholar]
- Wong, W.H. Crustal movement in eastern China. Proccedings of the 3th Pan-Pacific Scientific Congress, Tokyo, Japan, 30 October–11 November 1926. [Google Scholar]
- Zhao, Y.; Zhang, S.H.; Xu, G.; Yang, Z.Y.; Hu, J.M. The Jurassic major tectonic events of the Yanshanian intraplate deformation belt. Geol. Bull. China 2004, 23, 854–863, (In Chinese with English abstract). [Google Scholar]
- Zhang, C.H.; Li, C.M.; Deng, H.L.; Liu, Y.; Liu, L.; Wei, B.; Li, H.B.; Liu, Z. Mesozoic contraction deformation in the Yanshan and northern Taihang mountains and its implications to the destruction of the North China Craton. Sci. China Earth Sci. 2011, 54, 798–822. [Google Scholar] [CrossRef]
- Davis, G.A.; Zheng, Y.D.; Wang, C.; Darby, B.J.; Zhang, C.H.; Gehrels, G. Mesozoic tectonic evolution of the Yanshan fold and thrust belt, with emphasis on Hebei and Liaoning provinces, northern China. In Paleozoic and Mesozoic Tectonic Evolution of Central Asia: From Continental Assembly to Intracontinental Deformation; Hendrix, M.S., Davis, G.A., Eds.; Geological Society of America Memoir: Boulder, CO, USA, 2001; Volume 194, pp. 171–197. [Google Scholar]
- Liu, S.F.; Su, S.; Zhang, G.W. Early Mesozoic basin development in north China: Indications of cratonic deformation. J. Asian Earth Sci. 2013, 62, 221–236. [Google Scholar] [CrossRef]
- Meng, Q.R.; Wei, H.H.; Wu, G.L.; Duan, L. Early Mesozoic tectonic settings of the northern North China Craton. Tectonophysics 2014, 611, 155–166. [Google Scholar] [CrossRef]
- Grabau, A.W. Stratigraphy of China, Part II: Mesozoic; Geological Survey of China: Beijing, China, 1928; pp. 1–774.
- Chen, P.J.; Jin, F. The Jehol Biota; Press of University of Science and Technology of China: Hefei, China, 1999; pp. 1–342, (In Chinese with English summary). [Google Scholar]
- Zhou, Z.H.; Barrett, P.M.; Hilton, J. An exceptionally preserved Lower Cretaceous ecosystem. Nature 2003, 421, 807–814. [Google Scholar] [CrossRef]
- Chen, J.S.; Li, B.; Yao, Y.L.; Liu, M.; Yang, F.; Xing, D.H.; Li, W.; Wang, Y. Comparison between Mesozoic volcanic rock strata in northeast of Liaoning–South of Jilin and Yixian Formation in west of Liaoning. Acta Geol. Sin. 2016, 90, 2733–2746, (In Chinese with English abstract). [Google Scholar]
- Xu, D.B.; Li, B.F.; Chang, Z.L.; Zhang, J.B.; Cai, H. A study of the U-Pb isotope age and the sequence of the Cretaceous volcanics and coal-searching in Fuxin-Zhangwu-Heishan area, west Liaoning province. Earth Sci. Front. 2012, 19, 155–166, (In Chinese with English abstract). [Google Scholar]
- Zhang, H.; Liu, X.M.; Zhang, Y.Q.; Yuan, H.L.; Hu, Z.C. Zircon U-Pb ages of bottom and top parts of the Zhangjiakou Formation in Lingyuan area (west Liaoning province) and Luanping area (north Hebei province) and their significance. J. China Univ. Geosci. 2005, 16, 115–129. [Google Scholar]
- Fan, W.M.; Zhang, H.F.; Baker, J.; Jarvis, K.E.; Mason, P.R.D.; Menzies, M.A. On and off the North China Craton: Where is the Archaean keel? J. Petrol. 2000, 41, 933–950. [Google Scholar] [CrossRef]
- Gao, S.; Rudnick, R.L.; Yuan, H.L.; Liu, X.M.; Liu, Y.S.; Xu, W.L.; Ling, W.L.; Ayers, J.; Wang, X.C.; Wang, Q.H. Recycling lower continental crust in the North China Craton. Nature 2004, 432, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhang, J.F.; Xu, W.L.; Liu, Y.S. Delamination and destruction of the North China Craton. Chin. Sci. Bull. 2009, 54, 3367–3378. [Google Scholar] [CrossRef]
- Griffin, W.L.; Andi, Z.; O’Reilly, S.Y.; Ryan, C.G. Phanerozoic evolution of the lithosphere beneath the Sino-Korean Craton. In Mantle Dynamics and Plate Interactions in East Asia; Flower, M.F.J., Chung, S.L., Lo, C.H., Lee, T.Y., Eds.; American Geophysical Union: Washington, DC, USA, 1998; Volume 27, pp. 107–126. [Google Scholar]
- Liu, J.L.; Davis, G.A.; Ji, M.; Guan, H.M.; Bai, X.D. Crustal detachment and destruction of the keel of North China Craton: Constraints from Late Mesozoic extensional structures. Earth Sci. Front. 2008, 15, 72–81. [Google Scholar] [CrossRef]
- Menzies, M.A.; Fan, W.M.; Zhang, M. Palaeozoic and Cenozoic lithoprobes and the loss of > 120 km of Archaean lithosphere, Sino-Korean Craton, China. Geol. Soc. Lond. Spec. Publ. 1993, 76, 71–81. [Google Scholar] [CrossRef]
- Menzies, M.A.; Xu, Y.G. Geodynamics of the North China Craton. In Mantle Dynamics and Plate Interactions in East Asia; Flower, M.F.J., Chung, S.L., Lo, C.H., Lee, T.Y., Eds.; American Geophysical Union: Washington, DC, USA, 1998; Volume 27, pp. 155–165. [Google Scholar]
- Lin, J.Q.; Tan, D.J.; Chi, X.G.; Bi, L.J.; Xie, C.F.; Xu, W.L. Mesozoic Granites in Jiao–Liao Peninsula; Science Press: Beijing, China, 1992; pp. 1–208. (In Chinese) [Google Scholar]
- Sun, J.F.; Yang, J.H. Early Cretaceous A-type granites in the eastern North China Block with relation to destruction of the craton. J. China Univ. Geosci. Chin. Ed. 2009, 34, 137–147, (In Chinese with English abstract). [Google Scholar]
- Wu, F.Y.; Lin, J.Q.; Wilde, S.A.; Zhang, X.O.; Yang, J.H. Nature and significance of the Early Cretaceous giant igneous event in eastern China. Earth Planet. Sci. Lett. 2005, 233, 103–119. [Google Scholar] [CrossRef]
- Cooper, J.B. Chemometric analysis of Raman spectroscopic data for process control applications. Chemometr. Intell. Lab. 1999, 46, 231–247. [Google Scholar] [CrossRef]
- Kingma, K.J.; Hemley, R.J. Raman spectroscopic study of microcrystalline silica. Am. Mineral. 1994, 79, 269–273. [Google Scholar]
- Ilieva, A.; Mihailova, B.; Tsintsov, Z.; Petrov, O. Structural state of microcrystalline opals: A Raman spectroscopic study. Am. Mineral. 2007, 92, 1325–1333. [Google Scholar] [CrossRef]
- Ciobotă, V.; Salama, W.; Jentzsch, P.V.; Tarcea, N.; Rösch, P.; El Kammar, A.; Morsy, R.S.; Popp, J. Raman investigations of Upper Cretaceous phosphorite and black shale from Safaga district, Red Sea, Egypt. Spectrochim. Acta Part A 2014, 118, 42–47. [Google Scholar] [CrossRef]
- De Faria, D.L.A.; Venâncio Silva, S.; De Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Oh, S.J.; Cook, D.C.; Townsend, H.E. Characterization of iron oxides commonly formed as corrosion products on steel. Hyperfine Interact. 1998, 112, 59–66. [Google Scholar] [CrossRef]
- Legodi, M.A.; de Waal, D. The preparation of magnetite, goethite, hematite and maghemite of pigment quality from mill scale iron waste. Dyes Pigments 2007, 74, 161–168. [Google Scholar] [CrossRef]
- Natkaniec-Nowak, L.; Dumańska-Słowik, M.; Pršek, J.; Lankosz, M.; Wróbel, P.; Gaweł, A.; Kowalczyk, J.; Kocemba, J. Agates from Kerrouchen (the Atlas mountains, Morocco): Textural types and their gemmological characteristics. Minerals 2016, 6, 77. [Google Scholar] [CrossRef]
- Pop, D.; Constantina, C.; Tătar, D.; Kiefer, W. Raman spectroscopy on gem-quality microcrystalline and amorphous silica varieties from Romania. Stud. UBB Geol. 2004, 49, 41–52. [Google Scholar] [CrossRef]
- French, M.W.; Worden, R.H.; Lee, D.R. Electron backscatter diffraction investigation of length-fast chalcedony in agate: Implications for agate genesis and growth mechanisms. Geofluids 2013, 13, 32–44. [Google Scholar] [CrossRef]
- Götze, J.; Plötze, M.; Fuchs, H.; Habermann, D. Defect structure and luminescence behaviour of agate—Results of electron paramagnetic resonance (EPR) and cathodoluminescence (CL) studies. Mineral. Mag. 1999, 63, 149–163. [Google Scholar] [CrossRef]
- Hatipoğlu, M.; Ajò, D.; Kırıkoğlu, M.S. Cathodoluminescence (CL) features of the Anatolian agates, hydrothermally deposited in different volcanic hosts from Turkey. J. Lumin. 2011, 131, 1131–1139. [Google Scholar] [CrossRef]
- Frondel, C. Systematic compositional zoning in the quartz fibers of agates. Am. Mineral. 1985, 70, 975–979. [Google Scholar]
- Heaney, P.J.; Davis, A.M. Observation and origin of self-organized textures in agates. Science 1995, 269, 1562–1565. [Google Scholar] [CrossRef] [PubMed]
- Moxon, T.; Carpenter, M.A. Crystallite growth kinetics in nanocrystalline quartz (agate and chalcedony). Mineral. Mag. 2009, 73, 551–568. [Google Scholar] [CrossRef]
- Zhang, M.; Moxon, T. Infrared absorption spectroscopy of SiO2-moganite. Am. Mineral. 2014, 99, 671–680. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurences and Uses, 2nd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, German, 2003; pp. 1–663. [Google Scholar]
- Götze, J.; Plötze, M.; Tichomirowa, M.; Fuchs, H.; Pilot, J. Aluminium in quartz as an indicator of the temperature of formation of agate. Mineral. Mag. 2001, 65, 407–413. [Google Scholar] [CrossRef]
- Götze, J.; Möckel, R.; Vennemann, T.; Müller, A. Origin and geochemistry of agates in Permian volcanic rocks of the Sub-Erzgebirge basin, Saxony (Germany). Chem. Geol. 2016, 428, 77–91. [Google Scholar] [CrossRef]
- Harder, H.; Flehmig, W. Quarzsynthese bei tiefen temperaturen. Geochim. Cosmochim. Acta 1970, 34, 295–305, (In German with English abstract). [Google Scholar] [CrossRef]
- Harder, H. Nontronite synthesis at low temperatures. Chem. Geol. 1976, 18, 169–180. [Google Scholar] [CrossRef]
- Dekov, V.M.; Kamenov, G.D.; Stummeyer, J.; Thiry, M.; Savelli, C.; Shanks, W.C.; Fortin, D.; Kuzmann, E.; Vértes, A. Hydrothermal nontronite formation at Eolo Seamount (Aeolian volcanic arc, Tyrrhenian Sea). Chem. Geol. 2007, 245, 103–119. [Google Scholar] [CrossRef]
- Christensen, A.N. Hydrothermal preparation of goethite and haematite from amorphous iron (III) hydroxide. Acta Chem. Scand. 1968, 22, 1487–1490. [Google Scholar] [CrossRef]
- Das, S.; Hendry, M.J.; Essilfie-Dughan, J. Transformation of Two-Line Ferrihydrite to Goethite and Hematite as a Function of pH and Temperature. Environ. Sci. Technol. 2011, 45, 268–275. [Google Scholar] [CrossRef] [PubMed]


| Hematite [64,65,66] | Goethite [65,66] | Moganite [62] | α-Quartz [62] | |
|---|---|---|---|---|
| ν1 (cm−1) | ν1 (cm−1) | ν1 (cm−1) | ν1 (cm−1) | Mode Symmetry |
| 129 | 128 | E(LO+TO) | ||
| 141 | ||||
| 227 | 220 | 206 | A1 | |
| 246 | 243 | |||
| 265 | 265 | E(LO+TO) | ||
| 293 | 299 | |||
| 317 | ||||
| 355 | A1 | |||
| 370 | ||||
| 377 | ||||
| 394 | 398 | 394 | E(TO) | |
| 411 | 418 | 401 | E(LO) | |
| 432 | ||||
| 449 | 450 | E(TO) | ||
| 483 | 463 | 464 | A1 | |
| 498 | 501 | |||
| 511 | E(LO) | |||
| 550 | ||||
| 610 | ||||
| 685 | 693 | 696 | E(LO+TO) | |
| 792 | ||||
| 796 | E(TO) | |||
| 808 | E(LO) | |||
| 833 | ||||
| 950 | ||||
| 993 | 978 | |||
| 1058 | ||||
| 1069 | E(TO) | |||
| 1084 | 1085 | A1 | ||
| 1171 | 1162 | E(LO+TO) | ||
| 1177 | ||||
| 1230 | E(LO) | |||
| 1320 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Ji, L.; He, X. Gemological Characteristics and Origin of the Zhanguohong Agate from Beipiao, Liaoning Province, China: A Combined Microscopic, X-ray Diffraction, and Raman Spectroscopic Study. Minerals 2020, 10, 401. https://doi.org/10.3390/min10050401
Zhang X, Ji L, He X. Gemological Characteristics and Origin of the Zhanguohong Agate from Beipiao, Liaoning Province, China: A Combined Microscopic, X-ray Diffraction, and Raman Spectroscopic Study. Minerals. 2020; 10(5):401. https://doi.org/10.3390/min10050401
Chicago/Turabian StyleZhang, Xuemei, Lei Ji, and Xuemei He. 2020. "Gemological Characteristics and Origin of the Zhanguohong Agate from Beipiao, Liaoning Province, China: A Combined Microscopic, X-ray Diffraction, and Raman Spectroscopic Study" Minerals 10, no. 5: 401. https://doi.org/10.3390/min10050401
APA StyleZhang, X., Ji, L., & He, X. (2020). Gemological Characteristics and Origin of the Zhanguohong Agate from Beipiao, Liaoning Province, China: A Combined Microscopic, X-ray Diffraction, and Raman Spectroscopic Study. Minerals, 10(5), 401. https://doi.org/10.3390/min10050401




