Nephrite-Bearing Mining Waste As a Promising Mineral Additive in the Production of New Cement Types
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
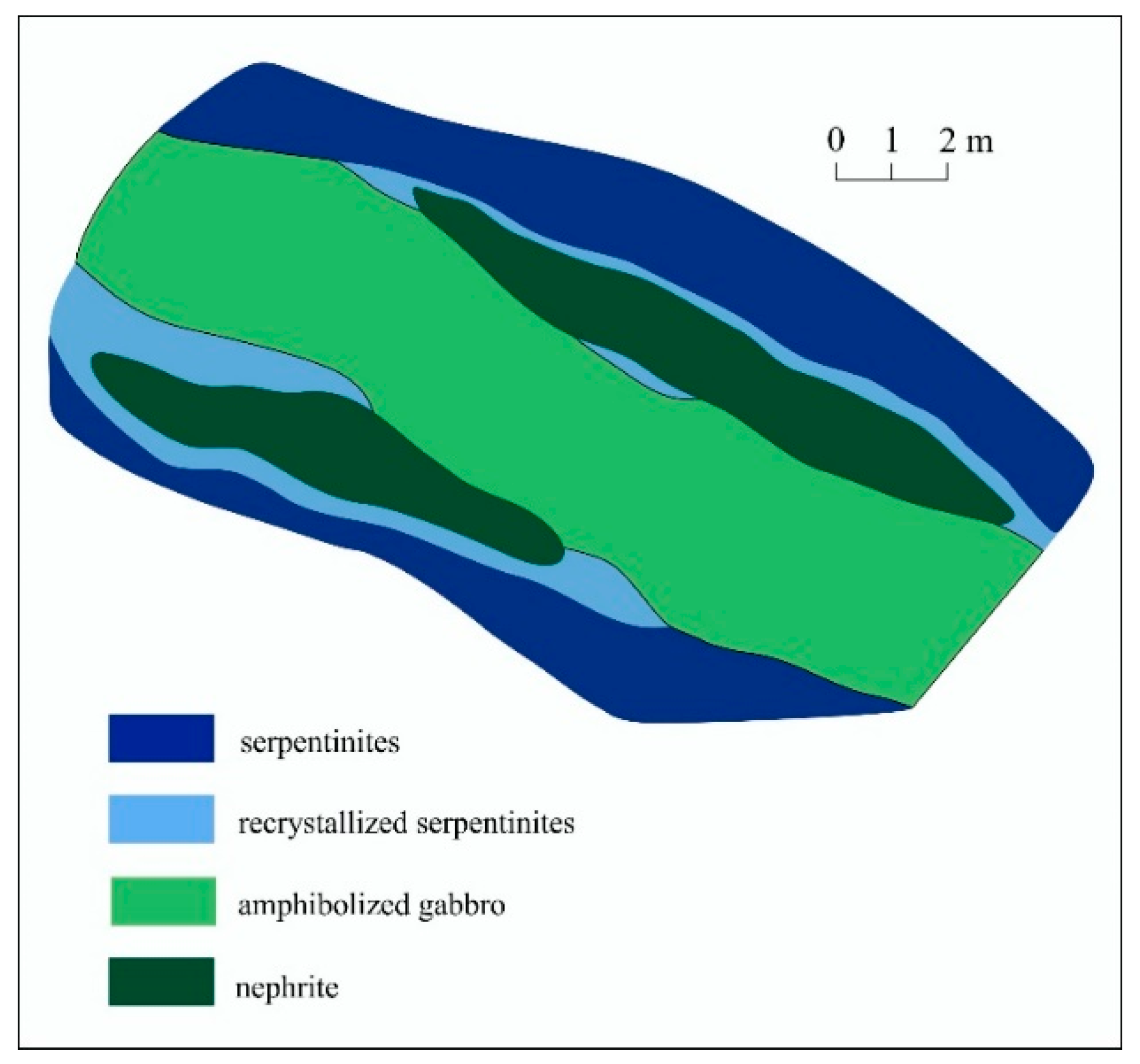
2.1.1. Low Grade Nephrite of the Ulankhodinskoye Deposit
2.1.2. Portland Cement Clinker
2.1.3. Additional Materials
2.2. Methods
2.2.1. Sample Preparation of Cement Binding Compositions
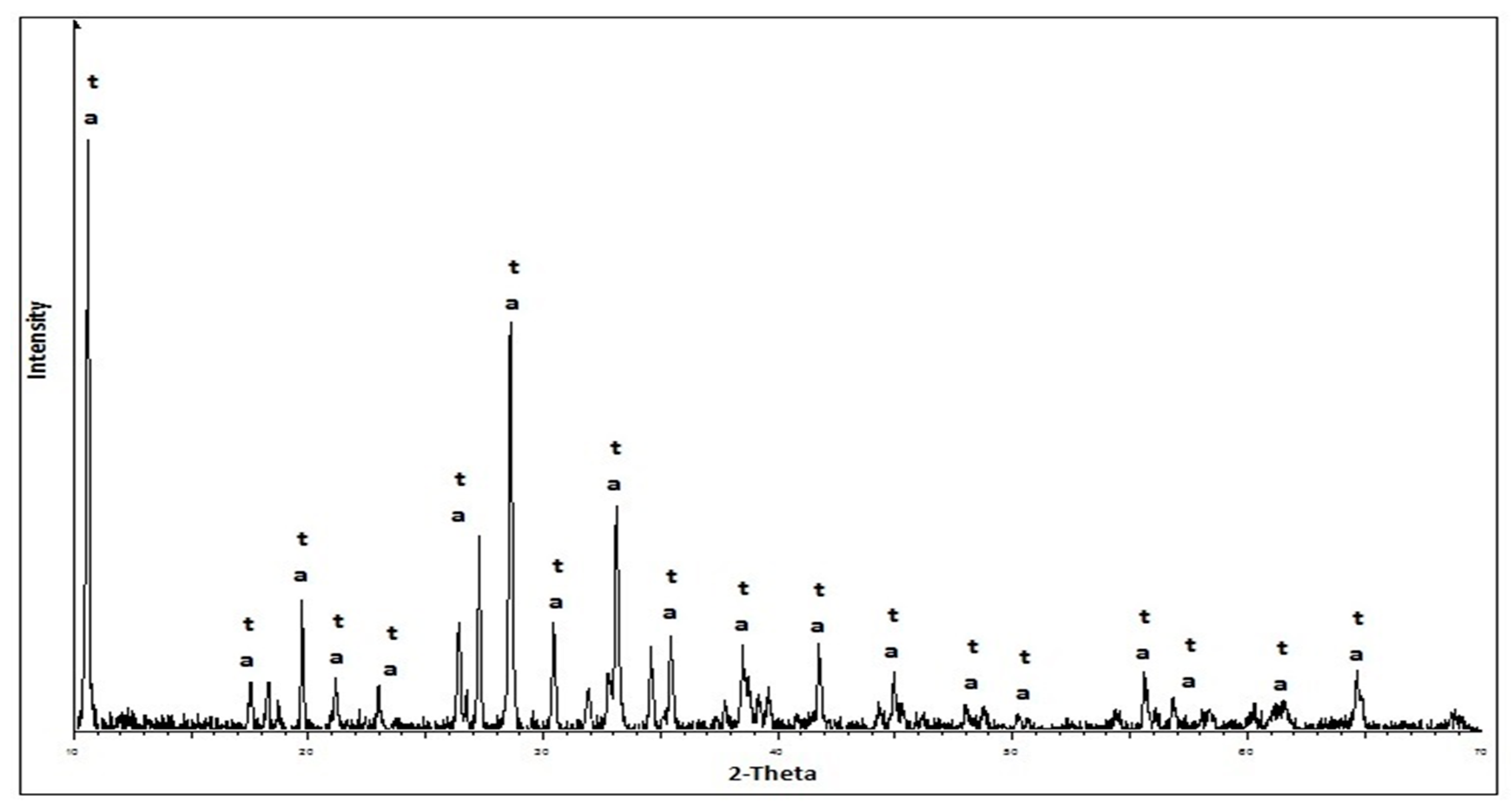
2.2.2. Methods of the Research of Cement Compositions
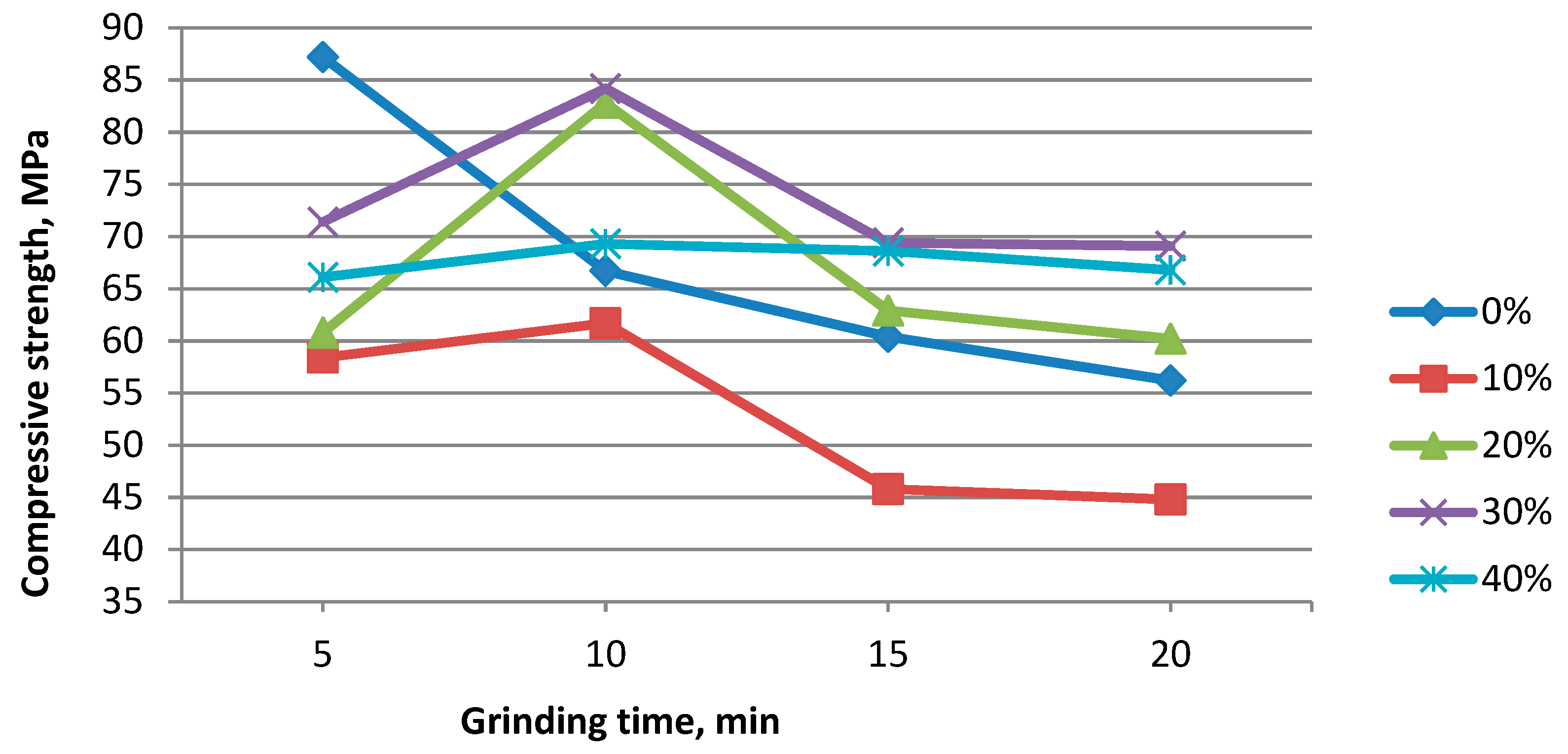
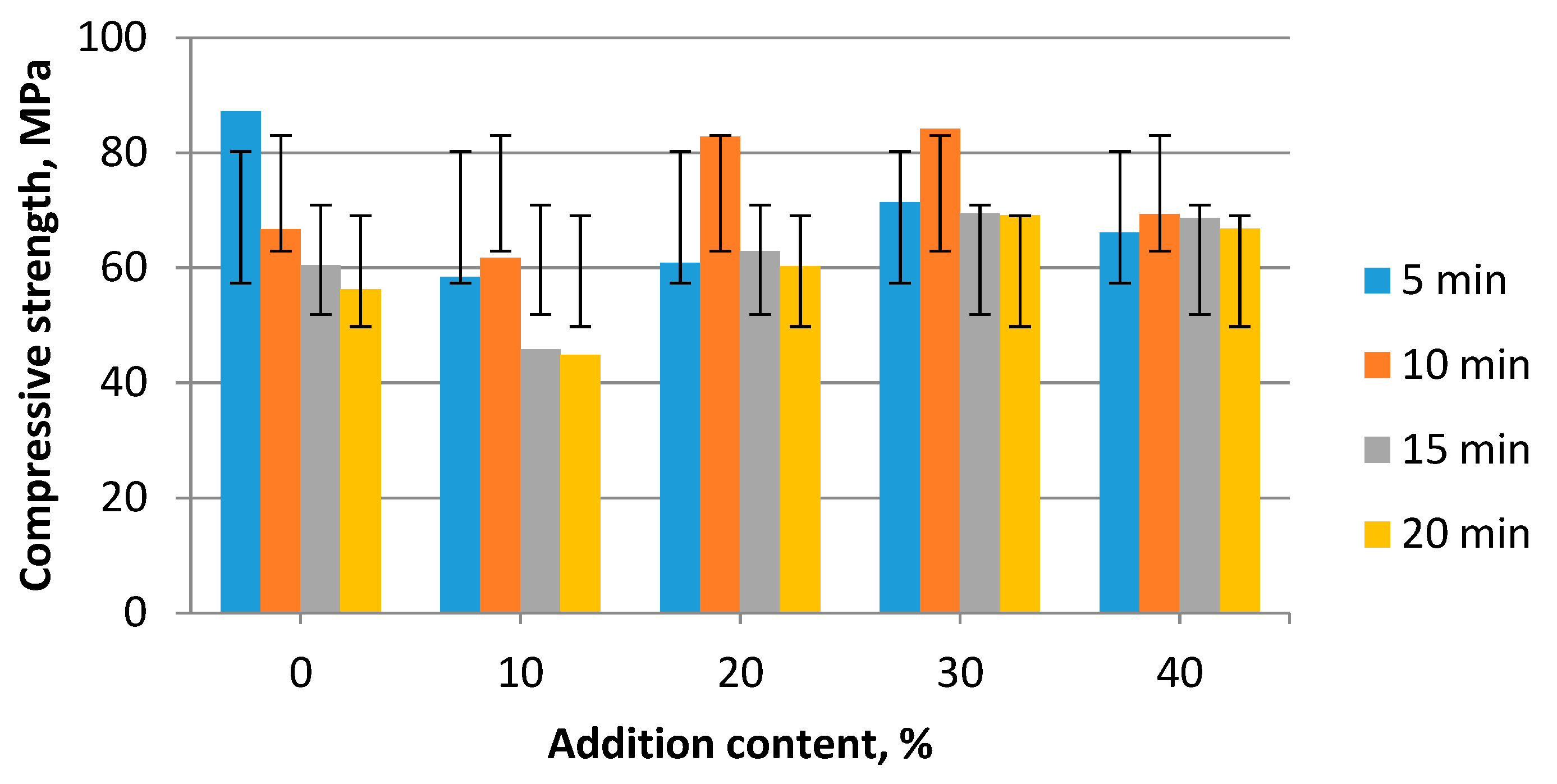
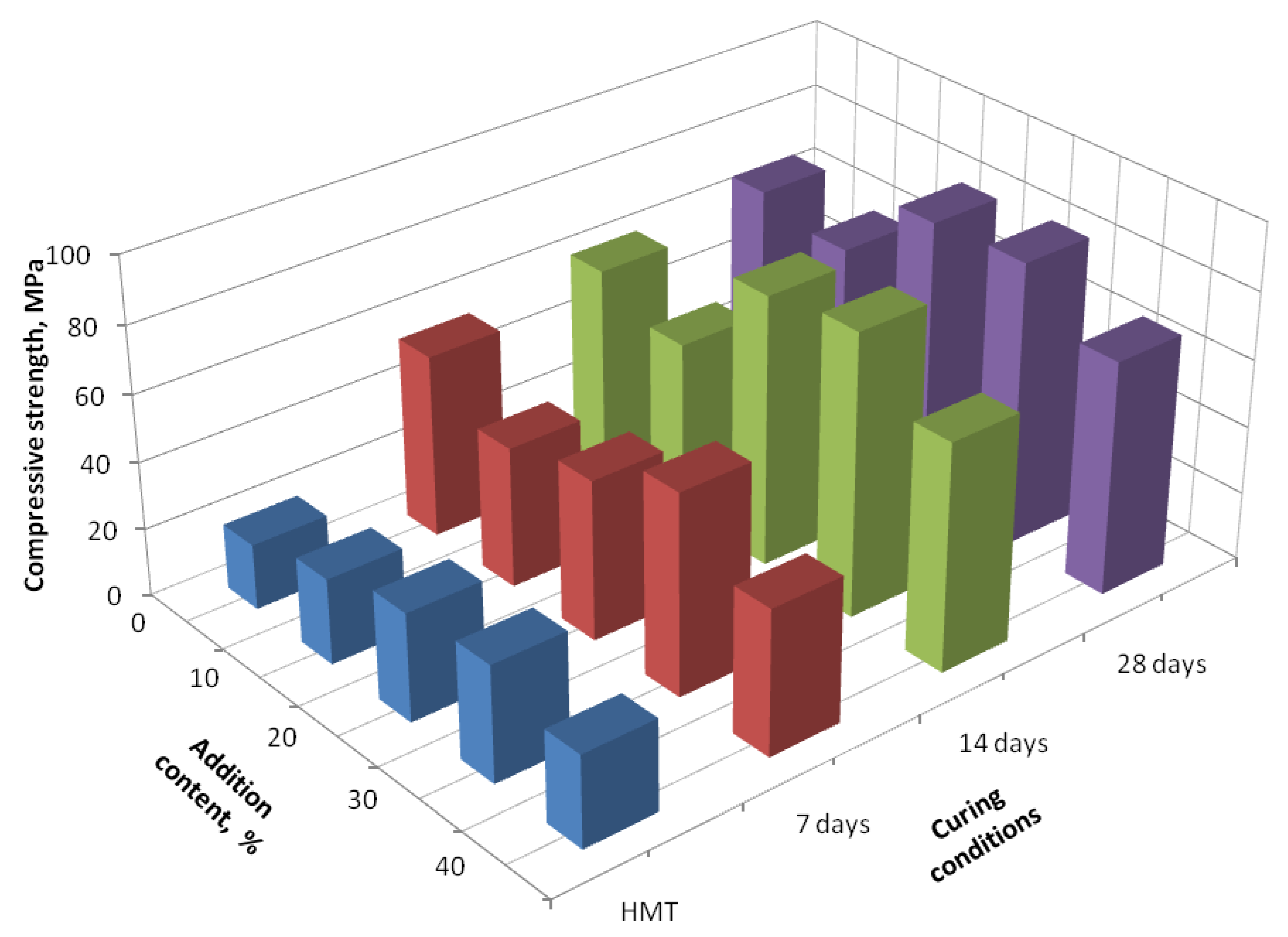
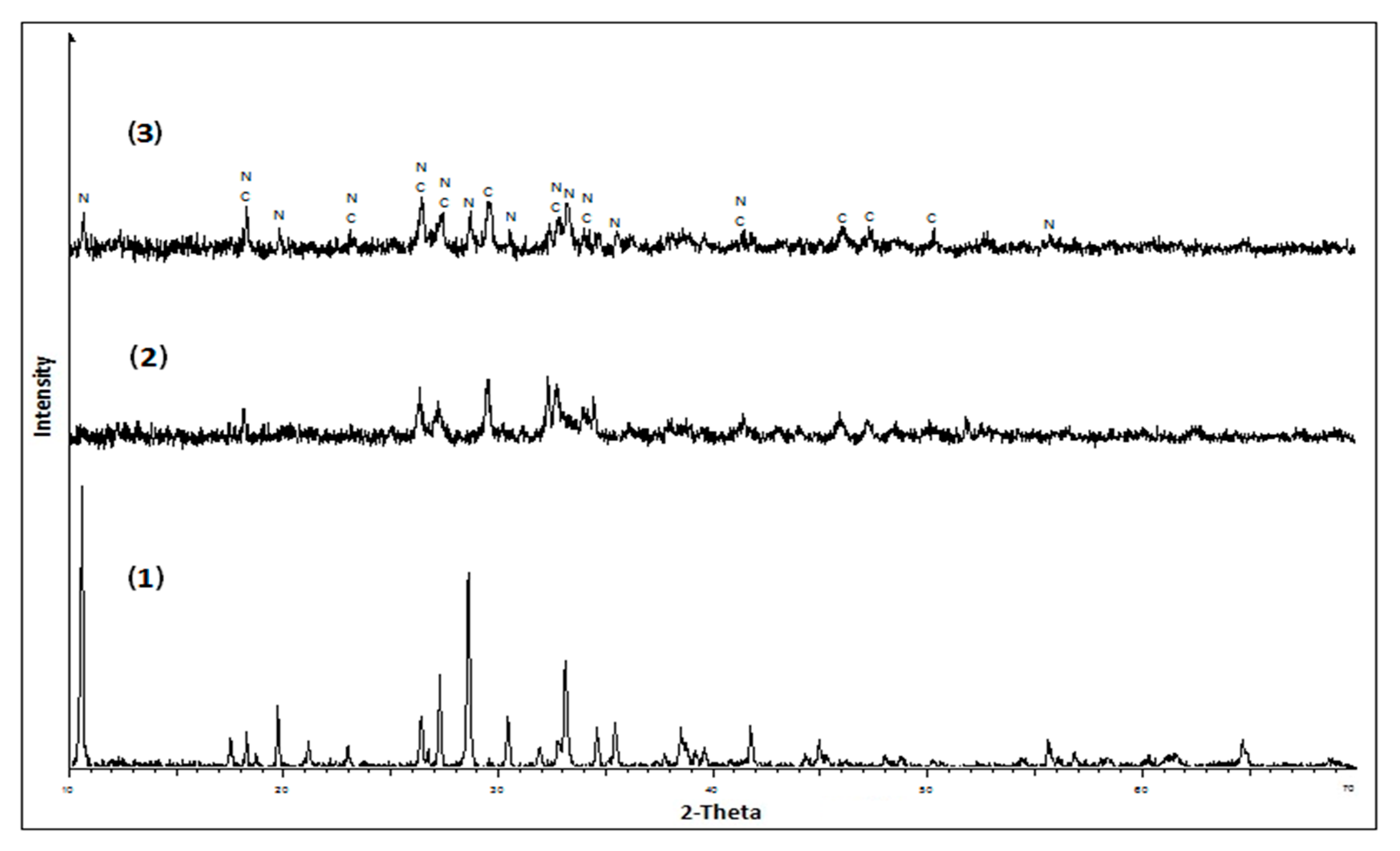
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Přikryl, R. Constructional geomaterials: Versatile Earth resources in the service of humankind -introduction to the thematic set of papers on: Challenges to supply and quality of geomaterials used in construction. Bull. Eng. Geol. Environ. 2017, 76, 1–9. [Google Scholar] [CrossRef]
- Přikryl, R.; Török, Á.; Gómez-Heras, M.; Miskovsky, K.; Theodoridou, M. Geomaterials in construction and their sustainability: Understanding their role in modern society. In Sustainable Use of Traditional Geomaterials in Construction Practice; Přikryl, R., Török, Á., Gómez-Heras, M., Miskovsky, K., Theodoridou, M., Eds.; The Geological Society of London: London, UK, 2016; Volume 416, pp. 1–22. [Google Scholar]
- Blezard, R.G. The history of calcareous cements. In Lea’s Chemistry of Cement and Concrete, 4th ed.; Hewlett, P.C., Ed.; Elsevier: Oxford, UK, 1998; pp. 1–23. [Google Scholar]
- Kurdowski, W. Cement and Concrete Chemistry; Springer: Dordrecht, The Netherlands, 2014; 699p. [Google Scholar]
- Worrell, E.; Price, L.; Martin, N.; Hendriks, C.; Meida, L.O. Carbon dioxide emissions from the global cement industry. Annu. Rev. Energy Environ. 2001, 26, 303–329. [Google Scholar] [CrossRef]
- Biernacki, J.J.; Bullard, J.W.; Sant, G.; Brown, K.; Glasser, F.P.; Jones, S.; Ley, T.; Livingston, R.; Nicoleau, L.; Olek, J.; et al. Cements in the 21st century: Challenges, perspectives, and opportunities. J. Am. Ceram. Soc. 2017, 100, 2746–2773. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.P.; Gupta, D. Assessing environmental impacts of a cement industry during its operation phase. IJRSI 2017, IV, 65–68. [Google Scholar]
- Mishra, S.; Siddigue, N.A. A review on environmental and health impacts of cement manufacturing emissions. IJGAES 2012, 2, 26–31. [Google Scholar]
- Naqi, A.; Jang, J.G. Recent progress in green cement technology utilizing low-carbon emission fuels and raw materials: A review. Sustainability 2019, 11, 537. [Google Scholar] [CrossRef] [Green Version]
- Juenger, M.C.G.; Winnefeld, F.; Provis, J.L.; Ideker, J.H. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Fernández, Á.; Alonso, M.C.; García-Calvo, J.L.; Lothenbach, B. Influence of the synergy between mineral additions and Portland cement in the physical-mechanical properties of ternary binders. Mater. Construcc. 2016, 66, e097. [Google Scholar] [CrossRef] [Green Version]
- Erdogmus, E. Combined effect of waste colemanite and silica fume on properties of cement mortar. Sci. Eng. Compos. Mater. 2014, 21, 369–375. [Google Scholar] [CrossRef]
- Mejia-Ballesteros, J.E.; Savastano, H., Jr.; Fiorelli, J.; Rojas, M.F. Effect of mineral additions on the microstructure and properties of blended cement matrices for fibre-cement applications. Cem. Concr. Compos. 2019, 98, 49–60. [Google Scholar] [CrossRef]
- Frías, M.; Sanchez de Rojas, M.I.; García, R.; Valdés, A.J.; Medina, C. Effect of activated coal mining wastes on the properties of blended cement. Cem. Concr. Compos. 2012, 34, 678–683. [Google Scholar] [CrossRef]
- Frías, M.; García, R.; Vigil de la Villa, R.; Martínez-Ramírez, S. Coal mining waste as a future eco-efficient supplementary cementing material: Scientific aspects. Recycling 2016, 1, 232–241. [Google Scholar] [CrossRef] [Green Version]
- Hemalatha, M.S.; Santhanam, M. Characterizing supplementary cementing materials in blended mortars. Constr. Build. Mater. 2018, 191, 440–459. [Google Scholar] [CrossRef]
- Terzić, A.; Pezo, L.; Mijatović, N.; Stojanović, J.; Kragović, M.; Miličić, L.; Andrić, L. The effect of alternations in mineral additives (zeolite, bentonite, fly ash) on physico-chemical behavior of Portland cement based binders. Constr. Build. Mater. 2018, 180, 199–210. [Google Scholar] [CrossRef]
- Terzić, A.; Pezo, L.; Miličić, L.; Mijatović, N.; Radojević, Z.; Radulović, D.; Andrić, L. Thermal and mechanical behavior of composite mortars containing natural sorptive clays and fly ash. Sci. Sinter. 2019, 51, 39–56. [Google Scholar] [CrossRef] [Green Version]
- Berdoudi, S.; Hebhoub, H.; Djebien, R. Valorization and recycling of quarries waste as an addition cement. J. Appl. Eng. Sci. 2017, 15, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Abdelaziz, M.A.; Abd El-Aleem, S.; Menshawy, W.M. Effect of fine materials in local quarry dusts of limestone and basalt on the properties of Portland cement pastes and mortars. Int. J. Eng. Res. Technol. 2014, 3, 1038–1056. [Google Scholar]
- Owsiak, Z.; Mazur, A. Analysing the pozzolanic reactivity of chalcedony dust in cement paste. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Busan, Korea, 25–27 August 2017; Volume 245, p. 022002. [Google Scholar]
- Wang, Q.; Yao, G.; Zhu, X.; Wang, J.; Wu, P.; Lyu, X. Preparation of Portland cement with gold ore tailings. Adv. Mater. Sci. Eng. 2019, 2019, 1324065. [Google Scholar] [CrossRef] [Green Version]
- Yao, G.; Liu, Q.; Wang, J.; Wu, P.; Lyu, X. Effect of mechanical grinding on pozzolanic activity and hydration properties of siliceous gold ore tailings. J. Clean. Prod. 2019, 217, 12–21. [Google Scholar] [CrossRef]
- Rakhimov, R.Z.; Rakhimova, N.R.; Gayfullin, A.R.; Morozov, V.P. Effect of the addition of thermally activated heavy loam to Portland cement on the properties of cement stone. Inorg. Mater. Appl. Res. 2018, 9, 679–686. [Google Scholar] [CrossRef]
- Mendes, T.M.; Guerra, L.; Morales, G. Basalt waste added to Portland cement. Acta Sci. Technol. 2016, 38, 431–436. [Google Scholar] [CrossRef]
- Dobiszewska, M.; Beycioğlu, A. Investigating the influence of waste basalt powder on selected properties of cement paste and mortar. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Busan, Korea, 25–27 August 2017; Volume 245, p. 022027. [Google Scholar]
- El-Didamony, H.; Helmy, I.M.; Osman, R.M.; Habboud, A.M. Basalt as pozzolana and filler in ordinary portland cement. American J. Eng. Appl. Sci. 2015, 8, 263. [Google Scholar] [CrossRef] [Green Version]
- Khan, K.; Amin, M.N.; Saleem, M.U.; Qureshi, H.J.; Al-Faiad, M.A.; Qadir, M.G. Effect of fineness of basaltic volcanic ash on pozzolanic reactivity, ASR epansion and drying shrinkage of blended cement mortars. Materials 2019, 12, 2603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, F.R.D.; Pecchio, M.; Bendoraitis, D.P.; Montanheiro, T.J.; Kihara, Y. Basalt mine-tailings as raw-materials for Portland clinker. Cerâmica 2010, 56, 39–43. [Google Scholar] [CrossRef]
- Dobiszewska, M.; Pichór, W.; Szołdra, P. Effect of basalt powder addition on properties of mortar. Matec Web Conf. 2019, 262, 06002. [Google Scholar] [CrossRef]
- Sabouang, C.J.N.; Mbey, J.A.; Liboum Thomas, F.; Njopwouo, D. Talc as raw material for cementitious products formulation. J. Asian Ceram. Soc. 2014, 2, 263–267. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-K.; Soh, J.-S. Performance of magnesia cement using MgCO3 and serpentine. J. Korean Ceram. Soc. 2016, 53, 116–121. [Google Scholar] [CrossRef]
- Khudyakova, L.I.; Paleev, P.L. Application of magnesium silicate rocks in the production of cements with mineral additives. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Moscow, Russia, 27 May–6 June 2019; Volume 320, p. 012035. [Google Scholar]
- Khudyakova, L.I.; Kotova, I.Y.; Timofeeva, S.S. Composite binders from mining waste. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Nanchang, China, 4–5 January 2020; Volume 408, p. 012053. [Google Scholar]
- Kislov, E.V. Russian nephrite resources and related genesis study. In Proceedings of the China International Gems & Jewelry Academic Conference. Abstract volume, Beijing, China, 19–23 October 2019; pp. 211–213. [Google Scholar]
- Harlow, G.E.; Sorenson, S.S. Jade (nephrite and jadeitite) and serpentinite: Metasomatic connections. Int. Geol. Rev. 2005, 47, 114–128. [Google Scholar] [CrossRef]
- Gomboev, D.M.; Androsov, V.P.; Kislov, E.V. Kavokta deposit of light-colored nephrite: Occurrence and characteristics of the composition. Geol. Miner. Explor. 2017, 9, 44–50. (In Russian) [Google Scholar]
- Kislov, E.V.; Popov, M.P.; Erokhin, Y.V.; Mikheeva, A.V. Nephrite of Bazhenov Deposit of Chrysotile Asbestos, Middle Urals: Geology and Mineralogy. Gemology: Collection of Articles; Publishing House of Tomsk CSTI: Tomsk, Russia, 2019; pp. 104–112. (In Russian) [Google Scholar]
- Kislov, E.V. The Development of the Mineral Resource Base of Nephrite of Buryatia. Gemology: Collection of Articles; Publishing House of Tomsk CSTI: Tomsk, Russia, 2017; pp. 82–89. (In Russian) [Google Scholar]
- Malyshev, A.V.; Kislov, E.V. Geology of the Ulan Khoda Nephrite Deposit in the Southeastern Part of East Sayan. Metallogeny of Ancient and Modern Oceans–2015. Mineral Deposits of Oceanic Structures: Geology, Mineralogy, Geochemistry, and Formation Conditions; IMin UB RAS: Miass, Russia, 2015; pp. 220–223. (In Russian) [Google Scholar]
- Suturin, A.N.; Zamaletdinov, R.S.; Sekerina, N.V. Nephrite Deposits; Publishing House of ISU: Irkutsk, Russia, 2015; 377p. (In Russian) [Google Scholar]
- Suturin, A.N.; Zamaletdinov, R.S. The Nephrites; Publishing House of Science: Novosibirsk, Russia, 1984; 155p. (In Russian) [Google Scholar]
- Yurgenson, G.A. Jewelry and Ornamental Stones of Transbaikalia; Publishing House of Science: Novosibirsk, Russia, 2001; 390p. (In Russian) [Google Scholar]
- GOST 31108-2016. Common Cements. Specifications. Interstate Standard. MKS 91.100.10; GostPerevod: Moscow, Russia, 2017. [Google Scholar]
- GOST 4013-82. Gypsum and Gypsum-Anhydrite Rock for the Manufacture of Binders. Specification. Interstate Standard. MKS 91.100.10. OKP 57 4322; GostPerevod: Moscow, Russia, 1983. [Google Scholar]
- GOST 23732-2011 Water for Concrete and Mortars. Specifications. Interstate Standard. MKS 91.100.30; GostPerevod: Moscow, Russia, 2012. [Google Scholar]
- Zyryanov, M.S.; Ahmetzhanov, A.M.; Manushina, A.S.; Potapova, E.N. Determination of puzzolanic activity of metakaolins. Adv. Chem. Chem. Technol. 2016, 30, 44–46. [Google Scholar]
- Ferraz, E. Pozzolanic activity of metakaolins by the French Standard of the modified Chapelle Test: A direct methodology. Acta Geodyn. Geometerialia Asp. 2015, 12, 289–298. [Google Scholar] [CrossRef] [Green Version]
| Basic Oxides | ||||||||
|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | FeO | MgO | CaO | Na2O | K2O | LOI |
| 56.20 | 0.94 | 0.21 | 3.28 | 22.37 | 13.48 | 0.04 | 0.03 | 3.41 |
| Basic Minerals | ||||
|---|---|---|---|---|
| C3S | C2S | C3A | C4AF | Different |
| 60.0 | 17.0 | 6.0 | 13.0 | 4.0 |
| Basic Oxides | |||||||
|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | MgO | CaO | Fe2O3 | Na2O + K2O | SO3 | LOI |
| 21.60 | 4.92 | 0.91 | 64.88 | 4.31 | 1.17 | 0.89 | 1.04 |
| Basic Oxides | ||||||||
|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | SO3 | LOI |
| 1.76 | 0.03 | 0.07 | 4.27 | 31.17 | 0.07 | 0.03 | 40.92 | 17.25 |
| Properties | Cement with Nephrite Additive | Portland Cement 400 D0 |
|---|---|---|
| Start of setting | 4 h 51 min | 3 h 20 min |
| End of setting | 7 h 45 min | 5 h 20 min |
| Dissolving of cone | 117 | 114 |
| Compression strength, MPa | 84.2 | 66.7 |
| Compression strength after HMT, MPa | 34.4 | 19.4 |
| Mean density, kg/m3 | 2256 | 2315 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khudyakova, L.I.; Kislov, E.V.; Paleev, P.L.; Kotova, I.Y. Nephrite-Bearing Mining Waste As a Promising Mineral Additive in the Production of New Cement Types. Minerals 2020, 10, 394. https://doi.org/10.3390/min10050394
Khudyakova LI, Kislov EV, Paleev PL, Kotova IY. Nephrite-Bearing Mining Waste As a Promising Mineral Additive in the Production of New Cement Types. Minerals. 2020; 10(5):394. https://doi.org/10.3390/min10050394
Chicago/Turabian StyleKhudyakova, Liudmila I., Evgeniy V. Kislov, Pavel L. Paleev, and Irina Yu. Kotova. 2020. "Nephrite-Bearing Mining Waste As a Promising Mineral Additive in the Production of New Cement Types" Minerals 10, no. 5: 394. https://doi.org/10.3390/min10050394