Extra-Framework Content in Sodalite-Group Minerals: Complexity and New Aspects of Its Study Using Infrared and Raman Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Chemical Composition
3.2. Infrared Spectroscopy
3.3. Raman Spectroscopy
3.4. X-ray Diffraction Data and Crystal Structure
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Taylor, D. The sodalite group of minerals. Contrib. Mineral. Petrol. 1967, 16, 172–188. [Google Scholar] [CrossRef]
- Sahl, K.; Chatterjee, N.D. The crystal structure of bicchulite, Ca2[Al2SiO6](OH)2. Zeits. Krist. 1977, 146, 35–41. [Google Scholar] [CrossRef]
- Sahl, K. Refinement of the crystal structure of bicchulite, Ca2[Al2SiO6](OH)2. Zeits. Krist. 1980, 152, 13–21. [Google Scholar] [CrossRef]
- Uchida, E.; Iiyama, J.T. On kamaishilite, Ca2Al2SiO6(OH)2; a new mineral (tetragonal), dimorphous with bicchulite, from the Kamaishi mine, Japan. Proc. Jpn. Acad. 1981, 57B, 239–243. (In English) [Google Scholar] [CrossRef][Green Version]
- Peterson, R.C. The structure of hackmanite, a variety of sodalite, from Mont St-Hilaire, Quebec. Can. Mineral. 1983, 21, 549–552. [Google Scholar]
- Hassan, I.; Grundy, H.D. The crystal structures of sodalite-group minerals. Acta Cryst. 1984, B40, 6–13. [Google Scholar] [CrossRef]
- Hassan, I.; Buseck, P. Cluster ordering and antiphase domain boundaries in hauyne. Can. Mineral. 1989, 27, 173–180. [Google Scholar]
- Hassan, I.; Grundy, H.D. The structure of nosean, ideally Na8[Al6Si6O24]SO4·H2O. Can. Mineral. 1989, 27, 165–172. [Google Scholar]
- Hassan, I.; Peterson, R.C.; Grundy, H.D. The structure of lazurite, ideally Na6Ca2(Al6Si6O24)S2, a member of the sodalite group. Acta Cryst. C 1985, 41, 827–832. [Google Scholar] [CrossRef]
- Gobeltz-Hautecoeur, N.; Demortier, A.; Lede, B.; Lelieur, J.P.; Duhayon, C. Occupancy of the sodalite cages in the blue ultramarine pigments. Inorg. Chem. 2002, 41, 2848–2854. [Google Scholar] [CrossRef]
- Bellatreccia, F.; Della Ventura, G.; Piccinini, M.; Cavallo, A.; Brilli, M. H2O and CO2 in minerals of the hauyne-sodalite group: A FTIR spectroscopy study. Mineral. Mag. 2009, 73, 399–413. [Google Scholar] [CrossRef]
- Fechtelkord, M. Structural study of Na8[AlSiO4]6(CO3)x(HCOO)2-2x(H2O)4x, 0.2 ≤ x ≤ 1, synthesized in organic solvents: Order and disorder of carbonate and formate anions in sodalite. Micropor. Mesopor. Mater. 1999, 28, 335–351. [Google Scholar] [CrossRef]
- Gesing, T.M.; Buhl, J.C. Crystal structure of a carbonate-nosean Na8[AlSiO4]6CO3. Eur. J. Miner. 1998, 10, 71–77. [Google Scholar] [CrossRef]
- Yakubovich, O.V.; Kotel’nikov, A.R.; Shchekina, T.I.; Gramenitskiy, E.N.; Zubkov, E.S. New representative in the sodalite structure type with extraframework anions [AlF6]3–. Crystallogr. Rep. 2011, 56, 190–197. [Google Scholar] [CrossRef]
- Gramenitskii, E.N.; Kotel’nikov, A.R.; Shchekina, T.I.; Yakubovich, O.V.; Devyatova, V.N.; Zubkov, E.S.; Suk, N.I.; Vigasina, M.F.; Kotel’nikova, Z.A. Composition, structure and conditions of formation of fluorine-containing sodalite (experimental data). Geochem. Int. 2018, 56, 521–534. [Google Scholar] [CrossRef]
- Hassan, I.; Grundy, H.D. The crystal structure of basic cancrinite, ideally Na8[Al6Si6O24](OH)2·3H2O. Can. Mineral. 1991, 29, 123–130. [Google Scholar]
- Nishanbaev, T.P.; Rassomahin, M.A.; Blinov, I.A.; Popova, V.I. Minerals of sodalite-cancrinite pegmatites from the Vishnevogorsk miaskite massif, South Urals. Mineralogy 2016, 3, 40–52. (In Russian) [Google Scholar]
- Schmitt, A.K.; Wetzel, F.; Cooper, K.M.; Zou, H.; Wörner, G. Magmatic longevity of Laacher See volcano (Eifel, Germany) indicated by U–Th dating of intrusive carbonatites. J. Petrol. 2010, 51, 1053–1085. [Google Scholar] [CrossRef]
- Frechen, J. Vorgänge der Sanidinit-Bildung im Laacher Seegebiet. Fortschr. Mineral. 1947, 26, 147–166. (In German) [Google Scholar]
- Frechen, J. Siebengebirge am Rhein, Laacher Vulkangebiet, Maargebiet der Westeifel. Sammlung geologischer Führer, 3. Auflage; Schweitzerbart: Stuttgart, Germany, 1976; p. 209. (In German) [Google Scholar]
- Schmitt, A.K. Laacher See revisited: High-spatial-resolution zircon dating indicates rapid formation of a zoned magma chamber. Geology 2006, 34, 597–600. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Krivovichev, S.V.; Pakhomova, A.S.; Pekov, I.V.; Schäfer, C.; Vigasina, M.F.; Van, K.V. Laachite, (Ca,Mn)2Zr2Nb2TiFeO14, a new zirconolite-related mineral from the Eifel volcanic region, Germany. Eur. J. Mineral. 2014, 26, 103–111. [Google Scholar] [CrossRef]
- Evsyunin, V.G.; Sapozhnikov, A.N.; Kashaev, A.A.; Rastsvetaeva, R.K. Crystal Structure of Triclinic Lazurite. Crystallogr. Rep. 1997, 42, 938–945. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlisPro Software System, version 1.171.39.46; Rigaku Oxford Diffraction: Oxford, UK, 2018. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Chukanov, N.V.; Chervonnyi, A.D. Infrared Spectroscopy of Minerals and Related Compounds; Springer: London, UK, 2016; p. 1109. [Google Scholar] [CrossRef]
- Clark, R.J.H.; Cobbold, D.G. Characterization of sulfur radical-ions in solutions of alkalipolysulfides in dimethylformamide and hexamethylphosphoramide and in solid-state in ultramarine blue, green, and red. Inorg. Chem. 1978, 17, 3169–3174. [Google Scholar] [CrossRef]
- Li, S.; Liu, M.; Sun, L. Preparation of acid-resisting ultramarine blue by novel two-step silica coating process. Indust. Eng. Chem. Res. 2011, 50, 7326–7331. [Google Scholar] [CrossRef]
- Chivers, T.; Elder, P.J.W. Ubiquitous trisulfur radical anion: Fundamentals and applications in materials science, electrochemistry, analytical chemistry and geochemistry. Chem. Soc. Rev. 2013, 42, 5996–6005. [Google Scholar] [CrossRef]
- Chukanov, N.V. Infrared Spectra of Mineral Species: Extended Library; Springer-Verlag GmbH: Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA; London, UK, 2014; p. 1716. ISBN 9400771274. [Google Scholar]
- Buhl, J.-C. Kinetic investigations of the formation of carbonate sodalite. React. Kinet. Catal. Lett. 1992, 48, 641–648. [Google Scholar] [CrossRef]
- Balassone, G.; Bellatreccia, F.; Mormone, A.; Biagioni, C.; Pasero, M.; Petti, C.; Mondillo, N.; Fameli, G. Sodalite-group minerals from the Somma-Vesuvius volcanic complex, Italy: A case study of K-feldspar-rich xenoliths. Mineral. Mag. 2012, 76, 191–212. [Google Scholar] [CrossRef]
- Armbruster, T.; Schreyer, W.; Hoefs, J. Very high CO2 cordierite from Norwegian Lapland: Mineralogy, petrology, and carbon isotopes. Contrib. Mineral. Petrol. 1982, 81, 262–267. [Google Scholar] [CrossRef]
- Sheft, I.; Perkins, A.J. Anhydrous hydrogen fluoride: Raman spectrum of the liquid. J. Inorg. Nucl. Chem. 1976, 38, 665–668. [Google Scholar] [CrossRef]
- IMA List of Minerals. Available online: http://cnmnc.main.jp/ (accessed on 16 April 2020).
- Cámara, F.; Bellatreccia, F.; Della Ventura, G.; Mottana, A. Farneseite, a new mineral of the cancrinite-sodalite group with a 14 layer stacking sequence. Eur. J. Mineral. 2005, 17, 839–846. [Google Scholar] [CrossRef]
- Della Ventura, G.; Bellatreccia, F.; Bonaccorsi, E. CO2 molecules in pitiglianoite, a mineral of the cancrinite-sodalite group. Eur. J. Mineral. 2005, 17, 847–851. [Google Scholar] [CrossRef]
- Della Ventura, G.; Bellatreccia, F.; Parodi, G.C.; Cámara, F.; Piccinini, M. Single-crystal FTIR and X-ray study of vishnevite, ideally [Na6(SO4)][Na2(H2O)2](Si6Al6O24). Amer. Mineral. 2007, 92, 713–721. [Google Scholar] [CrossRef]
- Della Ventura, G.; Bellatreccia, F.; Piccinini, M. Channel CO2 infeldspathoids: A review of existing data and new perspectives. Rend. Accad. Lincei 2008, 19, 141–159. [Google Scholar] [CrossRef]
- Cámara, F.; Bellatreccia, F.; Della Ventura, G.; Gunter, M.E.; Sebactiani, M.; Cavallo, A. Kircherite, a new mineral of the cancrinite-sodalite group with a 36-layer stacking sequence: Occurrence and crystal structure. Am. Mineral. 2012, 97, 1494–1504. [Google Scholar] [CrossRef][Green Version]
- Armbruster, T.; Bloss, F.D. Channel CO2 in cordierites. Nature 1980, 286, 140–141. [Google Scholar] [CrossRef]
- Le Breton, N. Infrared investigation of CO2-bearing cordierites. Contrib. Mineral. Petrol. 1989, 103, 387–396. [Google Scholar] [CrossRef]
- Kolesov, B.A.; Geiger, C.A. Cordierite II: The role of CO2 and H2O. Am. Mineral. 2000, 85, 1265–1274. [Google Scholar] [CrossRef]
- Khomenko, V.M.; Langer, K. Carbon oxides in cordierite channels: Determination of CO2 isotopic species and CO by single crystal IR spectroscopy. Am. Mineral. 2005, 90, 1913–1917. [Google Scholar] [CrossRef]
- Aines, R.D.; Rossman, G.R. The high temperature behaviour of water and carbon dioxide in cordierite and beryl. Am. Mineral. 1984, 69, 319–327. [Google Scholar]
- Charoy, B.; de Donato, P.; Barres, O.; Pintho-Choelo, C. Channel occupancy in an alkali-poor beryl from Serra Blanca (Goias, Brazil): Spectroscopic characterization. Am. Mineral. 1996, 81, 395–403. [Google Scholar] [CrossRef]
- Bellatreccia, F.; Della Ventura, G.; Gatta, G.D.; Guidi, M.C.; Harley, S. Carbon dioxide in pollucite, a feldspathoid with the ideal composition (Cs,Na)16Al16Si32O96×nH2O. Mineral. Mag. 2012, 76, 903–911. [Google Scholar] [CrossRef]
- Kolesov, B.A.; Geiger, C.A. Molecules in the SiO2-clathrate melanophlogite: A single-crystal Raman study. Am. Mineral. 2003, 88, 1364–1368. [Google Scholar] [CrossRef]
- McLaughlan, S.D.; Marshall, D.J. Paramagnetic resonance of sulfur radicals in synthetic sodalites. J. Phys. Chem. 1970, 74, 1359–1363. [Google Scholar] [CrossRef]
- Platonov, A.N.; Tarashchan, A.N.; Belichenko, V.P.; Povarennikh, A.S. Spectroscopic study of sulfide sulfur in some framework aluminosilicates. Const. Prop. Miner. 1971, 5, 61–72. (In Russian) [Google Scholar]
- Samoilovich, M.I. An ESR study of sulfur-bearing radical ions in minerals. Geokhimiya 1971, 4, 477–483. (In Russian) [Google Scholar]
- Clark, R.J.H.; Dines, T.J.; Kurmoo, M. On the nature of the sulphur chromophores in ultramarine blue, green, violet, and pink and of the selenium chromophore in ultramarine selenium: Characterization of radical anions by electronic and resonance Raman spectroscopy and the determination of their excited-state geometries. Inorg. Chem. 1983, 22, 2766–2772. [Google Scholar]
- Reinen, D.; Lindner, G.-G. The nature of the chalcogen colour centres in ultramarine-type solids. Chem. Soc. Rev. 1999, 28, 75–84. [Google Scholar] [CrossRef]
- Reshetnyak, N.B.; Tretyakova, L.I.; Vokhmentsev, A.Y. Investigation of colour centrums in natural lazurite by means of Raman spectroscopy. Mineral. Zhurnal 1986, 8, 49–60. (In Russian) [Google Scholar]
- Tauson, V.L.; Sapozhnikov, A.N. On the nature of lazurite colouring. Zap. Ross. Mineral. Obs. 2003, 132, 102–107. (In Russian) [Google Scholar]
- Fleet, M.E. XANES spectroscopy of fulfur in earth minerals. Canad. Mineral. 2005, 43, 1811–1838. [Google Scholar] [CrossRef]
- Steudel, R.; Chivers, T. Correction for ‘The role of polysulfide dianions and radical anions in the chemical, physical and biological sciences, including sulfur-based batteries’. Chem. Soc. Rev. 2019, 48, 3279–3319. [Google Scholar] [CrossRef] [PubMed]
- Tauson, V.L.; Sapozhnikov, A.N.; Shinkareva, S.N.; Lustenberg, E.E. Indicative properties of lazurite as a member of clathrasil mineral family. Dokl. Earth Sci. 2011, 441, 1732–1737. [Google Scholar] [CrossRef]







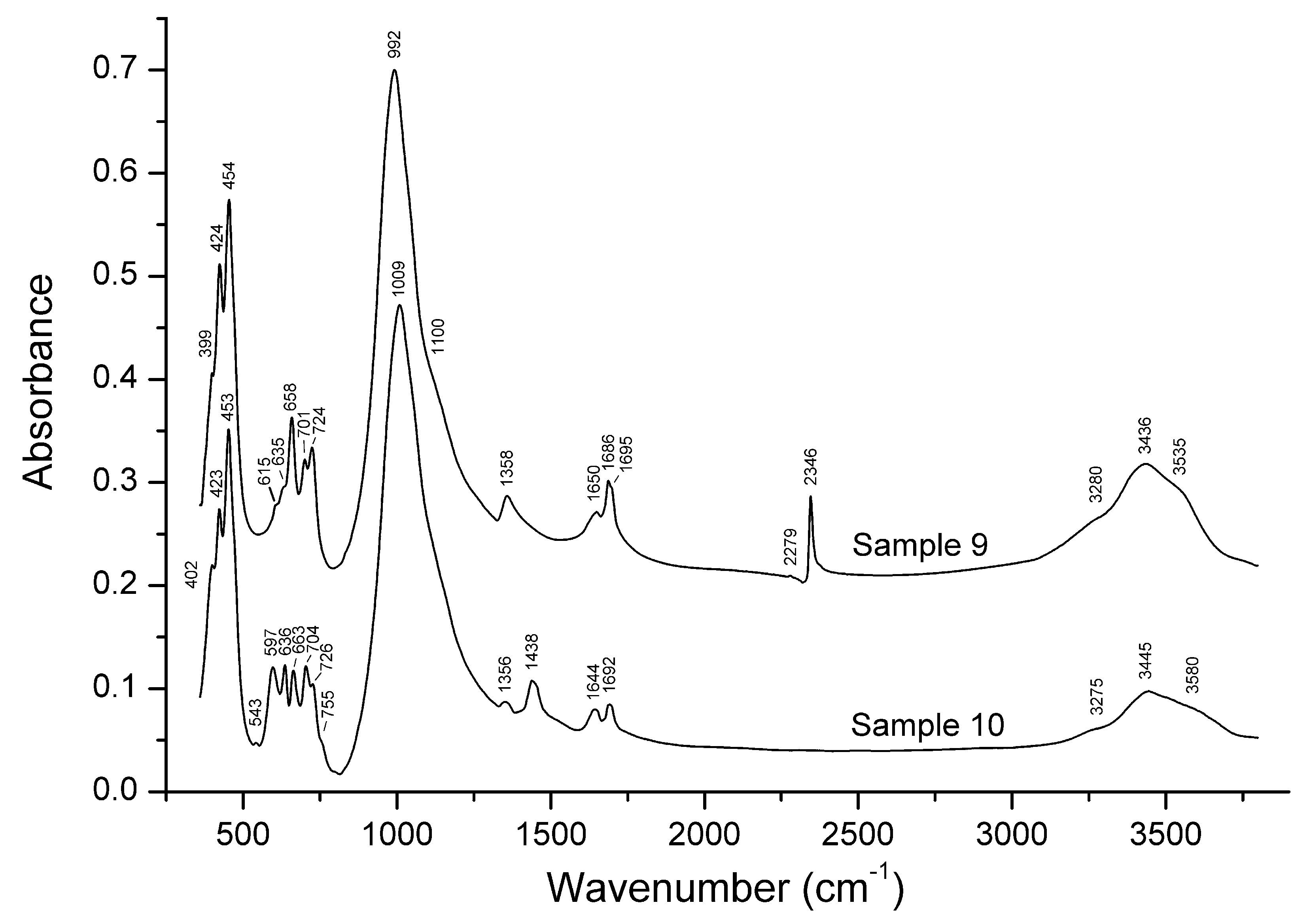


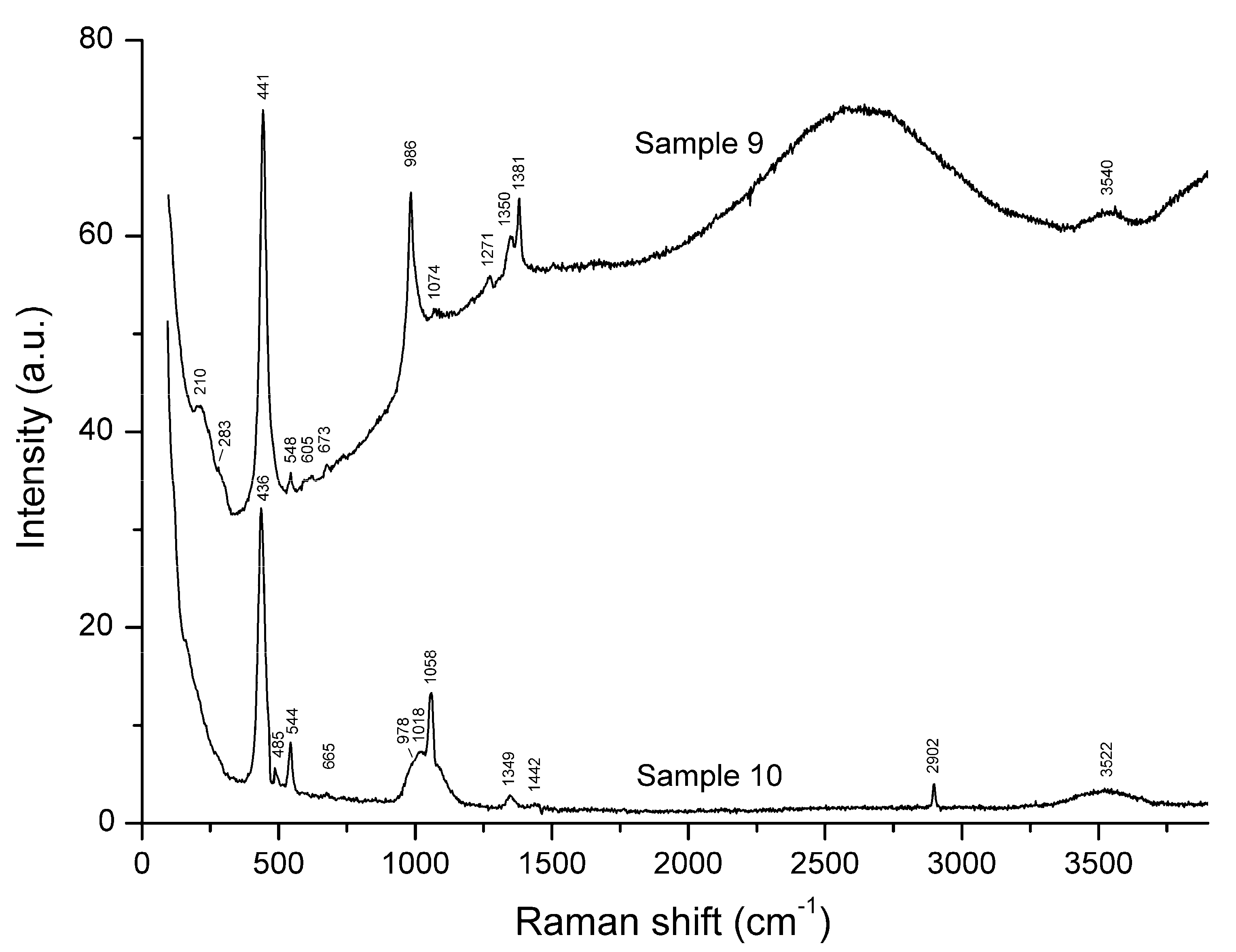
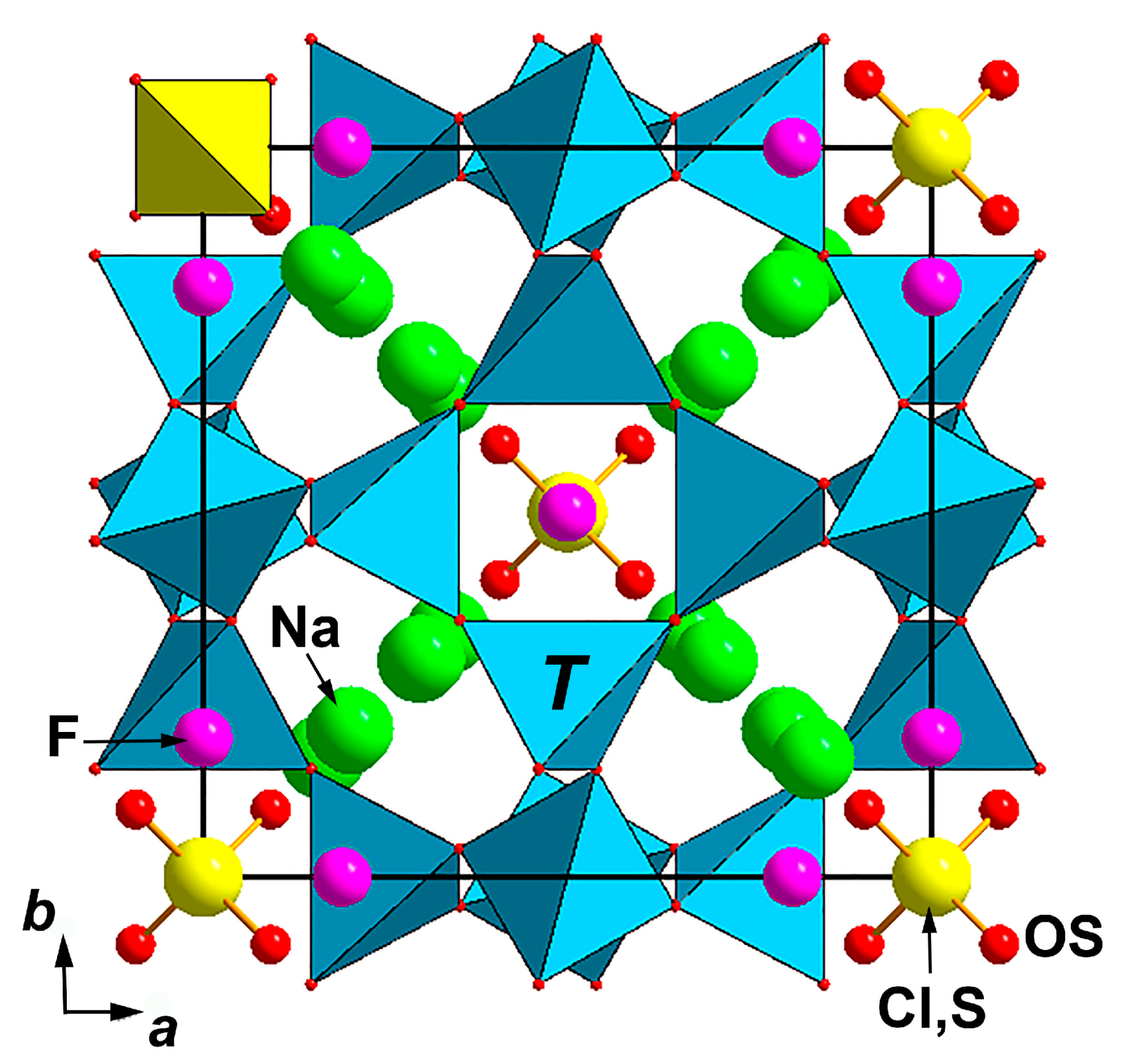
| Sample No. | EDS | CO2 Sorption | IR Spectroscopy | Raman Spectroscopy | Single-Crystal XRD |
|---|---|---|---|---|---|
| 1 | [17] | + | + | − | − |
| 2 | + | + | + | − | − |
| 3 | + | + | + | + | − |
| 4 | + | + | + | − | − |
| 5 | + | + | + | − | − |
| 6 | + | + | + | + | − |
| 7 | + | + | + | + | − |
| 8 | + | + | + | − | − |
| 9 | + | − | + | + | + |
| 10 | [14] | − | + | + | − |
| Crystal sizes, mm | 0.07 × 0.11 × 0.13 |
| Temperature, K | 293 |
| Radiation and wavelength, Å | MoKα; 0.71073 |
| F000* | 461 |
| Diffractometer | Xcalibur S CCD |
| θ range for data collection, ° | 5.514–30.359 |
| h, k, l ranges | −12 ≤ h ≤ 12, −12 ≤ k ≤ 12, −12 ≤ l ≤ 12 |
| Reflections used in the refinement: total/with I > 2σ(I) | 728/725 |
| Data reduction | CrysAlisPro Version 1.171.39.46 |
| Absorption correction | multi-scan |
| Refinement method | Full–matrix least–squares on F2 |
| Number of refined parameters | 21 |
| Final R indices (with I > 2σ (I)) R1/wR2 | 0.0430/0.1050 |
| R indices (with all data) | 0.0433/0.1052 |
| GoF | 1.161 |
| Largest diffraction peak and hole, e/Å3 | 0.71 and −0.33 |
| Sample No. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compo-nent | 1 a | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 b |
| Na2O | 24.97 | 16.96 | 18.60 | 18.33 | 19.02 | 20.66 | 17.88 | 18.08 | 19.00 | 21.5 |
| K2O | bdl | 2.90 | bdl | 0.13 | bdl | 0.66 | 1.06 | 0.53 | 4.04 | bdl |
| CaO | bdl | 1.77 | 4.80 | 6.14 | 6.54 | 2.17 | 0.77 | 6.25 | 0.59 | bdl |
| Al2O3 | 31.86 | 26.85 | 25.75 | 26.86 | 28.00 | 26.28 | 24.44 | 27.33 | 30.21 | 32.2 |
| Fe2O3 | bdl | 0.47 | 0.31 | 0.52 | 0.23 | 0.58 | 0.15 | 0.31 | bdl | bdl |
| SiO2 | 35.20 | 36.97 | 30.83 | 33.41 | 33.80 | 33.28 | 33.52 | 33.52 | 39.83 | 42.7 |
| CO2 | bdl | 0.48 | 1.35 | 0.78 | 0.24 | 1.81 | 0.82 | 0.65 | 1.66 | - |
| SO3 | bdl | 9.27 | 14.97 | 10.41 | 11.56 | 11.88 | 19.01 | 13.07 | 1.42 | bdl |
| F | bdl | 0.17 | bdl | 0.21 | 0.70 | bdl | bdl | 0.27 | 1.65 | 6.3 |
| Cl | 7.97 | 0.37 | bdl | 0.27 | 0.45 | 0.20 | 0.56 | 0.40 | 0.62 | bdl |
| –O=(F,Cl) | −1.80 | −0.15 | 0 | −0.15 | −0.40 | −0.05 | −0.13 | −0.20 | −0.83 | −2.7 |
| Total | 98.20 | 96.06 | 96.61 | 96.91 | 100.14 | 97.47 | 98.96 c | 100.21 | 98.19 | 100.0 |
| Formula coefficients calculated on Al + Si + Fe = 12 atoms per formula unit | ||||||||||
| Na | 7.98 | 5.72 | 7.05 | 6.52 | 6.61 | 7.43 | 6.66 | 6.38 | 5.86 | 6.21 |
| K | 0 | 0.64 | 0 | 0.03 | 0 | 0.16 | 0.26 | 0.12 | 0.82 | 0 |
| Ca | 0 | 0.33 | 1.00 | 1.21 | 1.26 | 0.43 | 0.16 | 1.22 | 0.10 | 0 |
| Al | 6.20 | 5.51 | 5.93 | 5.80 | 5.91 | 5.75 | 5.54 | 5.86 | 5.66 | 5.65 |
| Fe | 0 | 0.06 | 0.05 | 0.07 | 0.03 | 0.08 | 0.02 | 0.04 | 0 | 0 |
| Si | 5.80 | 6.43 | 6.02 | 6.13 | 6.06 | 6.17 | 6.44 | 6.10 | 6.34 | 6.35 |
| C | 0 | 0.11 | 0.36 | 0.20 | 0.06 | 0.46 | 0.22 | 0.16 | 0.36 | 0 |
| S | 0 | 1.21 | 2.20 | 1.43 | 1.55 | 1.66 | 2.75 | 1.78 | 0.17 | 0 |
| F | 0 | 0.09 | 0 | 0.12 | 0.05 | 0 | 0 | 0.16 | 0.83 | 2.99 |
| Cl | 2.22 | 0.11 | 0 | 0.08 | 0.32 | 0.06 | 0.18 | 0.12 | 0.17 | 0 |
| Sample 3 | Sample 6 | Sample 7 | Sample 9 | Sample 10 | Assignment |
|---|---|---|---|---|---|
| Raman Shift (cm−1) | |||||
| 220w | 220w | 210 | Combination of low-frequency lattice modes | ||
| 254 | 265 | 262 | S3− bending (ν2) | ||
| 287w | 290sh | 283sh | Combination of low-frequency lattice modes | ||
| 331w | 327w | S4− symmetric stretching (ν1–A1) | |||
| 396 | Anhydrite admixture | ||||
| 437w | 441s | 436s | SO4 bending and/or δ[O–Si(Al)–O] | ||
| 485 | AlF6 stretching. | ||||
| 505w | Anhydrite admixture | ||||
| 544s | 550s | 544s | 548w | 544 | S3− symmetric stretching (ν1) and/or AlF6 stretching |
| 578sh | 582sh | S3− antisymmetric stretching (ν3) | |||
| 605w | HF translational mode | ||||
| 673w | 665w | HF translational mode? | |||
| 680w | 667w | S4− stretching (ν3–E) | |||
| 802 | 808 | 806 | S3− combination mode (ν1 + ν2) | ||
| 988 | 987 | 986s | 978sh | SO4 symmetric stretching and/or stretching vibrations of the framework | |
| 1012w | Anhydrite admixture | ||||
| 1018 br | Admixture of aluminosilicate glass | ||||
| 1058s | CO3 symmetric stretching | ||||
| 1074w | HF librational mode | ||||
| 1088s | 1098s | 1094s | S3− overtone (2 × ν1) | ||
| 1271w | CO2 Fermi resonance | ||||
| 1350 | 1349 | H+ | |||
| 1351 | 1355 | 1362 | S3− combination mode (2 ν1 + ν2) | ||
| 1381 | CO2 Fermi resonance | ||||
| 1442w | CO3 asymmetric stretching | ||||
| 1632 | 1642 | 1642 | S3− overtone (3 × ν1) | ||
| 1894 | 1907w | 1908w | S3− combination mode (3 × ν2 + ν1) | ||
| 2178 | 2188 | 2179 | S3− overtone (4 × ν1) | ||
| 2420w | 2450w | 2450w | S3− combination mode (4 × ν2 + ν1) | ||
| 2712 | 2730w | 2730w | S3− overtone (5 × ν1) | ||
| 2902 | CH4 | ||||
| 3256 | 3257w | S3− overtone (6 × ν1) | |||
| 3511w | 3540 | 3522 | H2O stretching vibrations | ||
| Site | x | y | z | Ueq | s.o.f * | Q |
|---|---|---|---|---|---|---|
| T | 0.25 | 0.5 | 0.0 | 0.0102(5) | Si0.528Al0.472 | 12 |
| O | 0.1480(4) | 0.1480(4) | 0.4605(5) | 0.0275(12) | O1.00 | 24 |
| Na1 | 0.297(4) | 0.297(4) | 0.297(4) | 0.060(8) | Na0.159K0.024 | 8 |
| Na2 | 0.164(3) | 0.164(3) | 0.164(3) | 0.060(8) | Na0.288K0.045 | 8 |
| Na3 | 0.198(3) | 0.198(3) | 0.198(3) | 0.060(8) | Na0.288K0.045 | 8 |
| Cl, S | 0.0 | 0.0 | 0.0 | 0.024(4) | Cl0.096S0.078 | 2 |
| OS | 0.4074(16) | 0.4074(16) | 0.4074(16) | 0.024(4) | O0.078 | 8 |
| F | 0.0 | 0.0 | 0.191(4) | 0.034(10) | F0.128 | 12 |
| Na1–O | 2.415(19) × 3 | Na3–O | 2.462(16) × 3 |
| –F | 2.61(5) × 3 | –F | 2.54(3) × 3 |
| –O | 3.13(2) × 3 | –O | 2.919(7) × 3 |
| –Cl | 3.19(6) | –OS | 2.96(4) × 3 |
| –Cl | 3.11(4) | ||
| Na2*–OS | 2.50(4) × 3 | T–O | 1.6671(14) × 4 |
| –O | 2.70(2) × 3 | S–OS | 1.45(2) × 4 |
| –O | 3.035(13) × 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chukanov, N.V.; Vigasina, M.F.; Zubkova, N.V.; Pekov, I.V.; Schäfer, C.; Kasatkin, A.V.; Yapaskurt, V.O.; Pushcharovsky, D.Y. Extra-Framework Content in Sodalite-Group Minerals: Complexity and New Aspects of Its Study Using Infrared and Raman Spectroscopy. Minerals 2020, 10, 363. https://doi.org/10.3390/min10040363
Chukanov NV, Vigasina MF, Zubkova NV, Pekov IV, Schäfer C, Kasatkin AV, Yapaskurt VO, Pushcharovsky DY. Extra-Framework Content in Sodalite-Group Minerals: Complexity and New Aspects of Its Study Using Infrared and Raman Spectroscopy. Minerals. 2020; 10(4):363. https://doi.org/10.3390/min10040363
Chicago/Turabian StyleChukanov, Nikita V., Marina F. Vigasina, Natalia V. Zubkova, Igor V. Pekov, Christof Schäfer, Anatoly V. Kasatkin, Vasiliy O. Yapaskurt, and Dmitry Yu. Pushcharovsky. 2020. "Extra-Framework Content in Sodalite-Group Minerals: Complexity and New Aspects of Its Study Using Infrared and Raman Spectroscopy" Minerals 10, no. 4: 363. https://doi.org/10.3390/min10040363
APA StyleChukanov, N. V., Vigasina, M. F., Zubkova, N. V., Pekov, I. V., Schäfer, C., Kasatkin, A. V., Yapaskurt, V. O., & Pushcharovsky, D. Y. (2020). Extra-Framework Content in Sodalite-Group Minerals: Complexity and New Aspects of Its Study Using Infrared and Raman Spectroscopy. Minerals, 10(4), 363. https://doi.org/10.3390/min10040363






