Transformation of Pb, Cd, and Zn Minerals Using Phosphates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrolytic Acidity
2.3. Immobilization of Heavy Metals with Phosphorus Compounds
- The experimental system (ES)—the amount of potassium phosphate (KH2PO4) was calculated according to the expected reaction product, metal phosphate (Me3(PO4)2). Thus, the molar ratio of metal to phosphate (Me:PO4) was 3:2. The content of Zn, Cd, and Pb determined by Szrek et al., (2011) was used as the amount of metal [53]. Total concentrations of Zn, Pb and Cd were obtained by digesting the solid samples using aqua regia (a mixture of 38% hydrochloric acid and 65% nitric acid in ratio 3:1 of HCl to HNO3, by volume). The digestion lasted for 16 h at 25 °C and 2 h at 120 °C. The digests were analyzed for metals using atomic absorption spectrometer. Throughout the experiment, the moisture level was kept at 25% (by adding a volume of water equivalent to the loss of evaporated water).
- A control system (CS)—The soil samples were allowed to react with double-distilled water. The moisture level was kept at 25%.
2.4. Solid-Association of Zn, Cd and Pb
2.5. Methods of Analysis
3. Results and Discussion
3.1. Mineral Composition of Soil
3.2. Hydrolytic Acidity Parameters for Soil Samples
3.3. Immobilization of Heavy Metals with Phosphorus Compounds
3.4. Solid-Association of Zn, Cd and Pb
3.5. SEM-EDS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xiao, R.; Wang, S.; Li, R.H.; Wang, J.J.; Zhang, Z.Q. Soil heavy metal contamination and health risks associated with artisanal gold mining in Tongguan, Shaanxi, China. Ecotoxicol. Environ. Saf. 2017, 141, 17–24. [Google Scholar] [CrossRef]
- Perez-Esteban, J.; Escolastico, C.; Masaguer, A.; Vargas, C.; Moliner, A. Soluble organic carbon and pH of organic amendments affect metal mobility and chemical speciation in mine soils. Chemosphere 2014, 103, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Forjan, R.; Asensio, V.; Rodriguez-Vila, A.; Covelo, E.F. Contribution of waste and biochar amendment to the sorption of metals in a copper mine tailing. Catena 2016, 137, 120–125. [Google Scholar] [CrossRef]
- Liu, J.W.; Mwamulima, T.; Wang, Y.M.; Fang, Y.; Song, S.X.; Peng, C.S. Removal of Pb(II) and Cr(VI) from aqueous solutions using the fly ash-based adsorbent material-supported zero-valent iron. J. Mol. Liq. 2017, 243, 205–211. [Google Scholar] [CrossRef]
- Hasani, T.; Eisazadeh, H. Removal of Cd (II) by using polypyrrole and its nanocomposites. Synth. Met. 2013, 175, 15–20. [Google Scholar] [CrossRef]
- Saif, M.M.S.; Kumar, N.S.; Prasad, M.N.V. Binding of cadmium to Strychnos potatorum seed proteins in aqueous solution: Adsorption kinetics and relevance to water purification. Colloids Surf. B Biointerfaces 2012, 94, 73–79. [Google Scholar] [CrossRef]
- Padmavathiamma, P.K.; Li, L.Y. Phytoremediation technology: Hyper-accumulation metals in plants. Water Air Soil Pollut. 2007, 184, 105–126. [Google Scholar] [CrossRef]
- Omraei, M.; Esfandian, H.; Katal, R.; Ghorbani, M. Study of the removal of Zn(II) from aqueous solution using polypyrrole nanocomposite. Desalination 2011, 271, 248–256. [Google Scholar] [CrossRef]
- Sanderson, P.; Thangavadivel, K.; Ranganathan, S.; Chadalavada, S.; Naidu, R.; Bowmanc, M. Effectiveness of gravity based particle separation and soil washing for reduction of Pb in a clay loam shooting range soil. Environ. Technol. Innov. 2019, 16, 7. [Google Scholar] [CrossRef]
- Xia, W.Y.; Feng, Y.S.; Jin, F.; Zhang, L.M.; Du, Y.J. Stabilization and solidification of a heavy metal contaminated site soil using a hydroxyapatite based binder. Constr. Build. Mater. 2017, 156, 199–207. [Google Scholar] [CrossRef]
- Ballesteros, S.; Rincon, J.M.; Rincon-Mora, B.; Jordan, M.M. Vitrification of urban soil contamination by hexavalent chromium. J. Geochem. Explor. 2017, 174, 132–139. [Google Scholar] [CrossRef]
- Marques, A.; Rangel, A.; Castro, P.M.L. Remediation of heavy metal contaminated soils: Phytoremediation as a potentially promising clean-up technology. Crit. Rev. Environ. Sci. Technol. 2009, 39, 622–654. [Google Scholar] [CrossRef]
- Wu, H.P.; Lai, C.; Zeng, G.M.; Liang, J.; Chen, J.; Xu, J.J.; Dai, J.; Li, X.D.; Liu, J.F.; Chen, M.; et al. The interactions of composting and biochar and their implications for soil amendment and pollution remediation: A review. Crit. Rev. Biotechnol. 2017, 37, 754–764. [Google Scholar] [CrossRef]
- Sprocati, A.R.; Alisi, C.; Tasso, F.; Marconi, P.; Sciullo, A.; Pinto, V.; Chiavarini, S.; Ubaldi, C.; Cremisini, C. Effectiveness of a microbial formula, as a bioaugmentation agent, tailored for bioremediation of diesel oil and heavy metal co-contaminated soil. Process. Biochem. 2012, 47, 1649–1655. [Google Scholar] [CrossRef]
- Ye, S.J.; Zeng, G.M.; Wu, H.P.; Zhang, C.; Dai, J.; Liang, J.; Yu, J.F.; Ren, X.Y.; Yi, H.; Cheng, M. Biological technologies for the remediation of co-contaminated soil. Crit. Rev. Biotechnol. 2017, 37, 1062–1076. [Google Scholar] [CrossRef] [PubMed]
- Ucaroglu, S.; Talinli, I. Recovery and safer disposal of phosphate coating sludge by solidification/stabilization. J. Environ. Manag. 2012, 105, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Rosestolato, D.; Bagatin, R.; Ferro, S. Electrokinetic remediation of soils polluted by heavy metals (mercury in particular). Chem. Eng. J. 2015, 264, 16–23. [Google Scholar] [CrossRef]
- Yun, S.M.; Kang, C.S.; Kim, J.; Kim, H.S. Evaluation of soil flushing of complex contaminated soil: An experimental and modeling simulation study. J. Hazard. Mater. 2015, 287, 429–437. [Google Scholar] [CrossRef]
- Basta, N.T.; Gradwohl, R.; Snethen, K.L.; Schroder, J.L. Chemical immobilization of lead, zinc, and cadmium in smelter-contaminated soils using biosolids and rock phosphate. J. Environ. Qual. 2001, 30, 1222–1230. [Google Scholar] [CrossRef]
- Gray, C.W.; Dunham, S.J.; Dennis, P.G.; Zhao, F.J.; McGrath, S.P. Field evaluation of in situ remediation of a heavy metal contaminated soil using lime and red-mud. Environ. Pollut. 2006, 142, 530–539. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Ragone, P.; Fiore, S. Immobilization of Ni by synthesising zeolite at low temperatures in a polluted soil. Chemosphere 2010, 78, 1172–1176. [Google Scholar] [CrossRef] [PubMed]
- Farrell, M.; Jones, D.L. Use of composts in the remediation of heavy metal contaminated soil. J. Hazard. Mater. 2010, 175, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Xu, J.H.; Lv, Z.Y.; Xie, R.J.; Huang, L.M.; Jiang, J.P. Impacts of biochar and oyster shells waste on the immobilization of arsenic in highly contaminated soils. J. Environ. Manag. 2018, 217, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—A review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef]
- Ryan, J.A.; Zhang, P.C.; Hesterberg, D.; Chou, J.; Sayers, D.E. Formation of chloropyromorphite in a lead-contaminated soil amended with hydroxyapatite. Environ. Sci. Technol. 2001, 35, 3798–3803. [Google Scholar] [CrossRef]
- Cao, X. Immobilization of heavy metals in contaminated soils amended by phosphate-, carbonate-, and silicate-based materials: From lab to field. In Twenty Years of Research and Development on Soil Pollution and Remediation in China; Luo, Y., Tu, C., Eds.; Springer: Singapore, 2018; pp. 535–543. [Google Scholar]
- Seshadri, B.; Bolan, N.S.; Choppala, G.; Kunhikrishnan, A.; Sanderson, P.; Wang, H.; Currie, L.D.; Tsang, D.C.W.; Ok, Y.S.; Kim, G. Potential value of phosphate compounds in enhancing immobilization and reducing bioavailability of mixed heavy metal contaminants in shooting range soil. Chemosphere 2017, 184, 197–206. [Google Scholar] [CrossRef]
- Cao, X.D.; Wahbi, A.; Ma, L.N.; Li, B.; Yang, Y.L. Immobilization of Zn, Cu, and Pb in contaminated soils using phosphate rock and phosphoric acid. J. Hazard. Mater. 2009, 164, 555–564. [Google Scholar] [CrossRef]
- Bolan, N.S.; Adriano, D.C.; Duraisamy, P.; Mani, A. Immobilization and phytoavailability of cadmium in variable charge soils. III. Effect of biosolid compost addition. Plant. Soil 2003, 256, 231–241. [Google Scholar] [CrossRef]
- Ruby, M.V.; Davis, A.; Nicholson, A. In-situ formation of lead phosphates in soils as a method to immobilize lead. Environ. Sci. Technol. 1994, 28, 646–654. [Google Scholar] [CrossRef]
- Hettiarachchi, G.M.; Pierzynski, G.M.; Ransom, M.D. In situ stabilization of soil lead using phosphorus and manganese oxide. Environ. Sci. Technol. 2000, 34, 4614–4619. [Google Scholar] [CrossRef]
- Cao, X.D.; Ma, L.Q.; Chen, M.; Singh, S.P.; Harris, W.G. Impacts of phosphate amendments on lead biogeochemistry at a contaminated site. Environ. Sci. Technol. 2002, 36, 5296–5304. [Google Scholar] [CrossRef] [PubMed]
- Zwonitzer, J.C.; Pierzynski, G.M.; Hettiarachchi, G.M. Effects of phosphorus additions on lead, cadmium, and zinc bioavailabilities in a metal-contaminated soil. Water Air Soil Pollut. 2003, 143, 193–209. [Google Scholar] [CrossRef]
- Mahar, A.; Wang, P.; Li, R.; Zhang, Z. Immobilization of lead and cadmium in contaminated soil using amendments: A review. Pedosphere 2015, 25, 555–568. [Google Scholar] [CrossRef]
- Maenpaa, K.A.; Kukkonen, J.V.K.; Lydy, M.J. Remediation of heavy metal-contaminated soils using phosphorus: Evaluation of bioavailability using an earthworm bioassay. Arch. Environ. Contam. Toxicol. 2002, 43, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Mosby, D.E.; Casteel, S.W.; Blanchar, R.W. Lead immobilization using phosphoric acid in a smelter-contaminated urban soil. Environ. Sci. Technol. 2001, 35, 3553–3559. [Google Scholar] [CrossRef]
- Tang, X.Y.; Zhu, Y.G.; Chen, S.B.; Tang, L.L.; Chen, X.P. Assessment of the effectiveness of different phosphorus fertilizers to remediate Pb-contaminated soil using in vitro test. Environ. Int. 2004, 30, 531–537. [Google Scholar] [CrossRef]
- Huang, G.Y.; Su, X.J.; Rizwan, M.S.; Zhu, Y.F.; Hu, H.Q. Chemical immobilization of Pb, Cu, and Cd by phosphate materials and calcium carbonate in contaminated soils. Environ. Sci. Pollut. Res. 2016, 23, 16845–16856. [Google Scholar] [CrossRef]
- Falamaki, A.; Tavallali, H.; Eskandari, M.; Farahmand, S.R. Immobilizing some heavy metals by mixing contaminated soils with phosphate admixtures. Int. J. Civ. Eng. 2016, 14, 75–81. [Google Scholar] [CrossRef]
- Abbaspour, A.; Golchin, A. Immobilization of heavy metals in a contaminated soil in Iran using di-ammonium phosphate, vermicompost and zeolite. Environ. Earth Sci. 2011, 63, 935–943. [Google Scholar] [CrossRef]
- Basta, N.T.; McGowen, S.L. Evaluation of chemical immobilization treatments for reducing heavy metal transport in a smelter-contaminated soil. Environ. Pollut. 2004, 127, 73–82. [Google Scholar] [CrossRef]
- Melamed, R.; Cao, X.D.; Chen, M.; Ma, L.Q. Field assessment of lead immobilization in a contaminated soil after phosphate application. Sci. Total Environ. 2003, 305, 117–127. [Google Scholar] [CrossRef]
- Austruy, A.; Shahid, M.; Xiong, T.T.; Castrec, M.; Payre, V.; Niazi, N.K.; Sabir, M.; Dumat, C. Mechanisms of metal-phosphates formation in the rhizosphere soils of pea and tomato: Environmental and sanitary consequences. J. Soils Sediments 2014, 14, 666–678. [Google Scholar] [CrossRef]
- Cao, X.D.; Liang, Y.; Zhao, L.; Le, H.Y. Mobility of Pb, Cu, and Zn in the phosphorus-amended contaminated soils under simulated landfill and rainfall conditions. Environ. Sci. Pollut. Res. 2013, 20, 5913–5921. [Google Scholar] [CrossRef]
- Su, X.J.; Zhu, J.; Fu, Q.L.; Zuo, J.C.; Liu, Y.H.; Hu, H.Q. Immobilization of lead in anthropogenic contaminated soils using phosphates with/without oxalic acid. J. Environ. Sci. 2015, 28, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Almaroai, Y.A.; Usman, A.R.A.; Ahmad, M.; Moon, D.H.; Cho, J.S.; Joo, Y.K.; Jeon, C.; Lee, S.S.; Ok, Y. Effects of biochar, cow bone, and eggshell on Pb availability to maize in contaminated soil irrigated with saline water. Environ. Earth Sci. 2014, 71, 1289–1296. [Google Scholar] [CrossRef]
- Guo, F.Y.; Ding, C.F.; Zhou, Z.G.; Huang, G.X.; Wang, X.X. Stability of immobilization remediation of several amendments on cadmium contaminated soils as affected by simulated soil acidification. Ecotoxicol. Environ. Saf. 2018, 161, 164–172. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Takaoka, M.; Oshita, K.; Tanida, H. Incomplete transformations of Pb to pyromorphite by phosphate-induced immobilization investigated by X-ray absorption fine structure (XAFS) spectroscopy. Chemosphere 2009, 76, 616–622. [Google Scholar] [CrossRef]
- Huang, G.Y.; Gao, R.L.; You, J.W.; Zhu, J.; Fu, Q.L.; Hu, H.Q. Oxalic acid activated phosphate rock and bone meal to immobilize Cu and Pb in mine soils. Ecotoxicol. Environ. Saf. 2019, 174, 401–407. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Yang, J.E.; Ro, H.M.; Lee, Y.H.; Ok, Y.S. Effects of soil dilution and amendments (mussel shell, cow bone, and biochar) on Pb availability and phytotoxicity in military shooting range soil. Ecotoxicol. Environ. Saf. 2012, 79, 225–231. [Google Scholar] [CrossRef]
- Darwish, M.; Aris, A.; Puteh, M.H.; Jusoh, M.N.H.; Kadir, A.A. Waste bones ash as an alternative source of P for struvite precipitation. J. Environ. Manag. 2017, 203, 861–866. [Google Scholar] [CrossRef]
- Chen, L.; Kivela, J.; Helenius, J.; Kangas, A. Meat bone meal as fertiliser for barley and oat. Agric. Food Sci. 2011, 20, 235–244. [Google Scholar] [CrossRef]
- Szrek, D.; Bajda, T.; Manecki, M. A comparative study of the most effective amendment for Pb, Zn and Cd immobilization in contaminated soils. J. Environ. Sci. Health Part A Toxic Hazard. Subst. Environ. Eng. 2011, 46, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency EPA. Method 1311: Toxicity Characteristic Leaching Procedure; Waste, O.O.S., Ed.; EPA: Washington, DC, USA, 1992.
- Parkhurst, D. User’s Guide to PHREEQC, A Computer Program for Speciation, Reaction-Path, Advective-Transport, and Inverse Geochemical Calculations; Water-Resources Investigations Report 95-4227; U.S. Geological Survey: Reston, VA, USA, 1995; p. 143.
- Allison, J.D.; Brown, D.S.; Novo-Gradac, K.J. MINTEQA2/PRODEFA2, A Geochemical Assessment Model for Environmental Systems: Version 3.0 User’s Manual EPA/600/3-91/021; US Environmental Protection Agency, Environmental Research Laboratory: Athens, GA, USA, 1991.
- Ayati, M.; Madsen, H.E.L. Solubility product of the cadmium phosphate Cd5H2(PO4)(4)center dot 4H(2)O at 37 degrees C. J. Chem. Eng. Data 2001, 46, 113–116. [Google Scholar] [CrossRef]
- Zhu, Y.N.; Zhu, Z.Q.; Zhao, X.; Liang, Y.P.; Dai, L.Q.; Huang, Y.H. Characterization, dissolution and solubility of synthetic cadmium hydroxylapatite Cd5(PO4)3OH at 25–45 degrees C. Geochem. Trans. 2015, 16, 11. [Google Scholar] [CrossRef]
- Nriagu, J.O. Formation and Stability of Base Metal Phosphates in Soils and Sediments. In Phosphate Minerals; Nriagu, J.O., Moore, P.B., Eds.; Springer: Berlin/Heidelberg, Germany, 1984. [Google Scholar]
- Calmano, W.; Forstner, U. Chemical-extraction of heavy-metals in polluted river sediments in central-Europe. Sci. Total Environ. 1983, 28, 77–90. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace-metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Shuman, L.M. Separating soil iron-oxide and manganese-oxide fractions for micro-element analysis. Soil Sci. Soc. Am. J. 1982, 46, 1099–1102. [Google Scholar] [CrossRef]
- Mehra, O.P.; Jackson, M.L. Iron oxide removal from soils and clays by a dithionite-citrate system buffered with sodium bicarbonate. Clays Clay Miner. 1958, 7, 317–327. [Google Scholar] [CrossRef]
- Filgueiras, A.V.; Lavilla, I.; Bendicho, C. Chemical sequential extraction for metal partitioning in environmental solid samples. J. Environ. Monit. 2002, 4, 823–857. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, Z.; Wang, M.; Guo, G.L.; Du, P.; Li, F.S. Phosphate-induced differences in stabilization efficiency for soils contaminated with lead, zinc, and cadmium. Front. Environ. Sci. Eng. 2018, 12, 9. [Google Scholar] [CrossRef]
- Edwards, A.C. Phosphate in soils: Interactions with Micronutrients, Radionuclides and heavy metals. Eur. J. Soil Sci. 2015, 66, 962. [Google Scholar] [CrossRef]
- Zeng, G.M.; Wan, J.; Huang, D.L.; Hu, L.; Huang, C.; Cheng, M.; Xue, W.J.; Gong, X.M.; Wang, R.Z.; Jiang, D.N. Precipitation, adsorption and rhizosphere effect: The mechanisms for Phosphate-induced Pb immobilization in soils—A review. J. Hazard. Mater. 2017, 339, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhou, S.; Chen, S. Remediation of Heavy Metal-Contaminated Soils by Phosphate Fertilizers. In Twenty Years of Research and Development on Soil Pollution and Remediation in China; Luo, Y., Tu, C., Eds.; Springer: Singapore, 2018. [Google Scholar]
- Xu, S.H.; Boyd, S.A. Cation-Exchange Chemistry of Hexadecyltrimethylammonium in a Subsoil Containing Vermiculite. Soil Sci. Soc. Am. J. 1994, 58, 1382–1391. [Google Scholar] [CrossRef]
- Sun, L.; Chen, S.; Chao, L.; Sun, T.H. Effects of flooding on changes in Eh, pH and speciation of cadmium and lead in contaminated soil. Bull. Environ. Contam. Toxicol. 2007, 79, 514–518. [Google Scholar] [CrossRef]
- Ma, Q.Y.; Logan, T.J.; Traina, S.J. Lead immobilization from aqueous-solutions and contaminated soils using phosphate rocks. Environ. Sci. Technol. 1995, 29, 1118–1126. [Google Scholar] [CrossRef]
- Cotter-Howells, J.; Caporn, S. Remediation of contaminated land by formation of heavy metal phosphates. Appl. Geochem. 1996, 11, 335–342. [Google Scholar] [CrossRef]
- Laperche, V.; Logan, T.J.; Gaddam, P.; Traina, S.J. Effect of apatite amendments on plant uptake of lead from contaminated soil. Environ. Sci. Technol. 1997, 31, 2745–2753. [Google Scholar] [CrossRef]
- Cao, X.D.; Harris, W. Carbonate and magnesium interactive effect on calcium phosphate precipitation. Environ. Sci. Technol. 2008, 42, 436–442. [Google Scholar] [CrossRef]
- Cao, X.; Harris, W.G.; Josan, M.S.; Nair, V.D. Inhibition of calcium phosphate precipitation under environmentally-relevant conditions. Sci. Total Environ. 2007, 383, 205–215. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, T.C.; Huang, Y.H.; Dahab, M.F.; Surampalli, R. Effects of long-term wastewater application on chemical properties and phosphorus adsorption capacity in soils of a wastewater land treatment system. Environ. Sci. Technol. 2005, 39, 7240–7245. [Google Scholar] [CrossRef]
- Chen, S.B.; Zhu, Y.G.; Ma, Y.B. The effect of grain size of rock phosphate amendment on metal immobilization in contaminated soils. J. Hazard. Mater. 2006, 134, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Theodoratos, P.; Papassiopi, N.; Xenidis, A. Evaluation of monobasic calcium phosphate for the immobilization of heavy metals in contaminated soils from Lavrion. J. Hazard. Mater. 2002, 94, 135–146. [Google Scholar] [CrossRef]
- Brown, S.; Christensen, B.; Lombi, E.; McLaughlin, M.; McGrath, S.; Colpaert, J.; Vangronsveld, J. An inter-laboratory study to test the ability of amendments to reduce the availability of Cd, Pb, and Zn in situ. Environ. Pollut. 2005, 138, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Q.; Rao, G.N. Effects of phosphate rock on sequential chemical extraction of lead in contaminated soils. J. Environ. Qual. 1997, 26, 788–794. [Google Scholar] [CrossRef][Green Version]
- Wójcik, A.J.; Chmura, J. Deposits of natural resources and changes in the environment caused by mining in the area of Bukowno (Upper Silesia Region, Poland). Górnictwo I Geoinżynieria 2005, 29, 219–236. [Google Scholar]
- Berti, W.R.; Cunningham, S.D. In-place inactivation of Pb in Pb contaminated soils. Environ. Sci. Technol. 1997, 31, 1359–1364. [Google Scholar] [CrossRef]
- McKenzie, R.M. Adsorption of Lead and Other Heavy-Metals on Oxides of Manganese and Iron. Aust. J. Soil Res. 1980, 18, 61–73. [Google Scholar] [CrossRef]
- Trivedi, P.; Axe, L. Modeling Cd and Zn sorption to hydrous metal oxides. Environ. Sci. Technol. 2000, 34, 2215–2223. [Google Scholar] [CrossRef]
- Dong, D.M.; Nelson, Y.M.; Lion, L.W.; Shuler, M.L.; Ghiorse, W.C. Adsorption of Pb and Cd onto metal oxides and organic material in natural surface coatings as determined by selective extractions: New evidence for the importance of Mn and Fe oxides. Water Res. 2000, 34, 427–436. [Google Scholar] [CrossRef]
- Manecki, M.; Bogucka, A.; Bajda, T.; Borkiewicz, O. Decrease of Pb bioavailability in soils by addition of phosphate ions. Environ. Chem. Lett. 2006, 3, 178–181. [Google Scholar] [CrossRef]
- Cabrera, F.; Dearambarri, P.; Madrid, L.; Toca, C.G. Desorption of phosphate from iron-oxides in relation to equilibrium ph and porosity. Geoderma 1981, 26, 203–216. [Google Scholar] [CrossRef]
- Beveridge, T.J.; Meloche, J.D.; Fyfe, W.S.; Murray, R.G.E. Diagenesis of metals chemically complexed to bacteria—Laboratory formation of metal phosphates, sulfides, and organic condensates in artificial sediments. Appl. Environ. Microbiol. 1983, 45, 1094–1108. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Bolan, N.; Megharaj, M.; Naidu, R. Isolation of phosphate solubilizing bacteria and their potential for lead immobilization in soil. J. Hazard. Mater. 2011, 185, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Topolska, J.; Latowski, D.; Kaschabek, S.; Manecki, M.; Merkel, B.J.; Rakovan, J. Pb remobilization by bacterially mediated dissolution of pyromorphite Pb5(PO4)3Cl in presence of phosphate-solubilizing Pseudomonas putida. Environ. Sci. Pollut. Res. 2014, 21, 1079–1089. [Google Scholar] [CrossRef]
- Drewniak, L.; Sklodowska, A.; Manecki, M.; Bajda, T. Solubilization of Pb-bearing apatite Pb-5(PO4)(3)Cl by bacteria isolated from polluted environment. Chemosphere 2017, 171, 302–307. [Google Scholar] [CrossRef]
- Rodriguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Chen, X.B.; Wright, J.V.; Conca, J.L.; Peurrung, L.M. Effects of pH on heavy metal sorption on mineral apatite. Environ. Sci. Technol. 1997, 31, 624–631. [Google Scholar] [CrossRef]
- Cabala, J.; Krupa, P.; Misz-Kennan, M. Heavy Metals in Mycorrhizal Rhizospheres Contaminated By Zn–Pb Mining and Smelting Around Olkusz in Southern Poland. Water Air Soil Pollut. 2008, 199, 139–149. [Google Scholar] [CrossRef]
- Bolewski, A. Mineralogia Szczegółowa; Wydawnictwa Geologiczne: Warszawa, Poland, 1975; p. 528. [Google Scholar]
- Letcher, T. Thermodynamics, Solubility and Environmental Issues; Letcher, T., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2007. [Google Scholar]
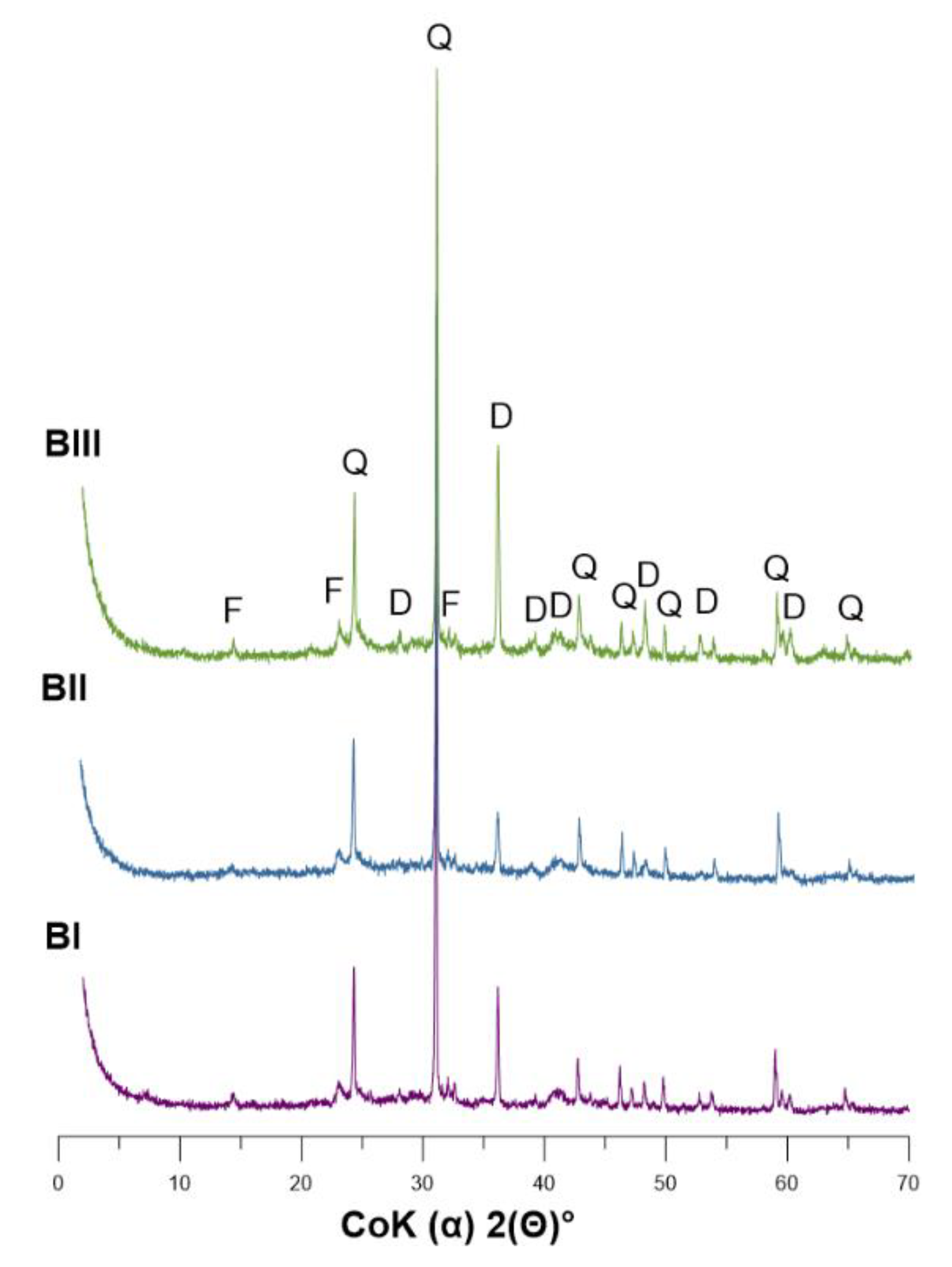
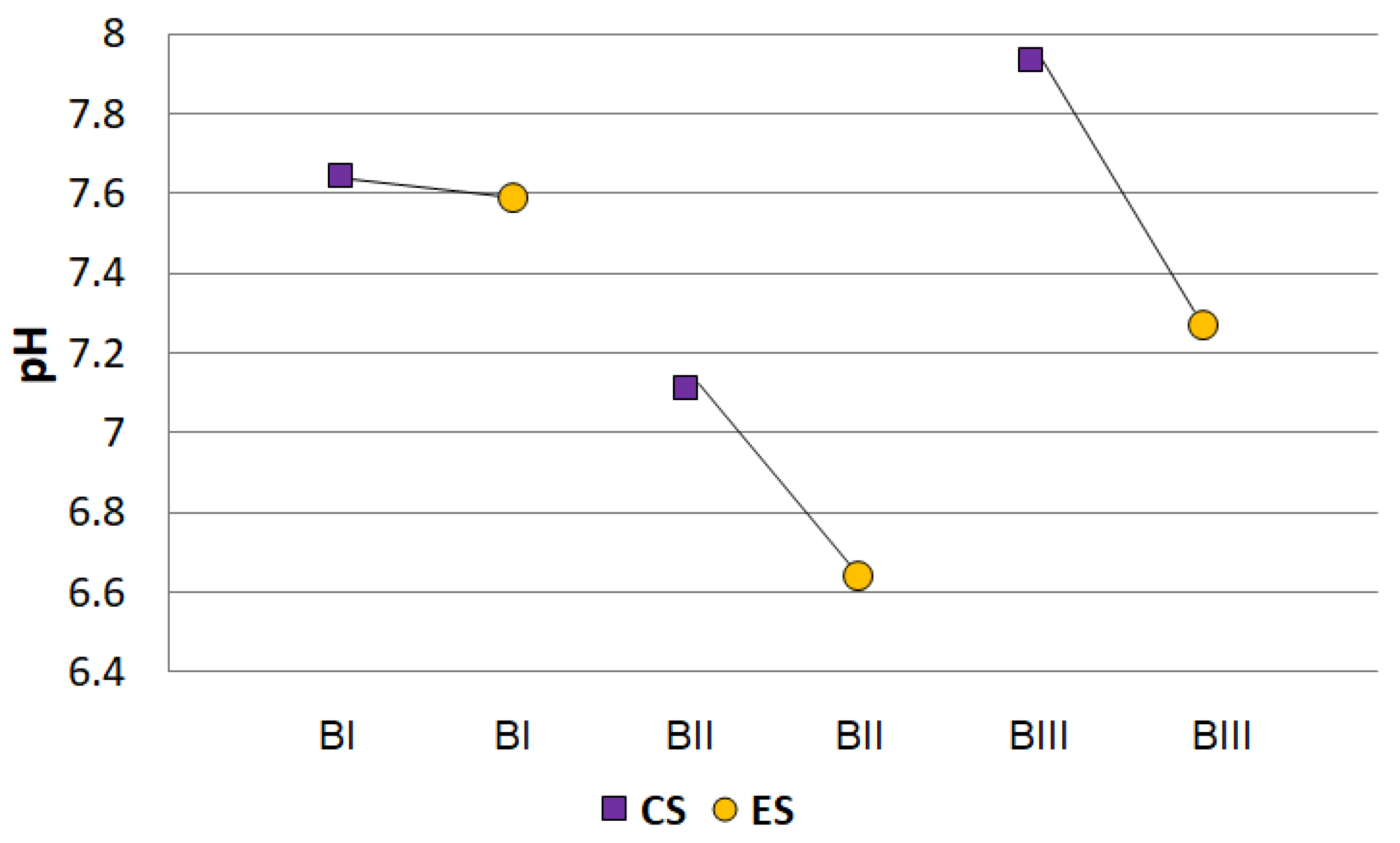
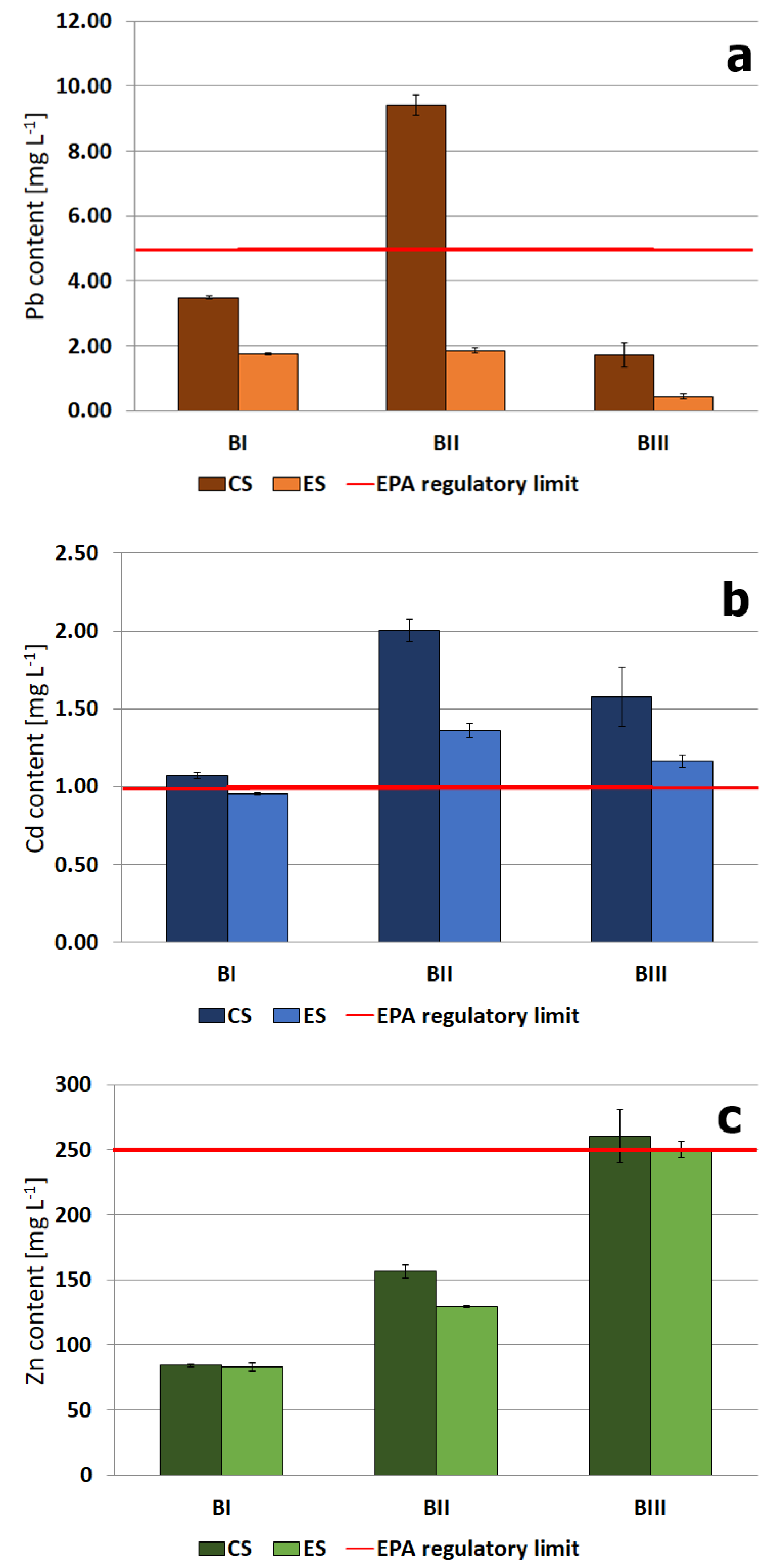


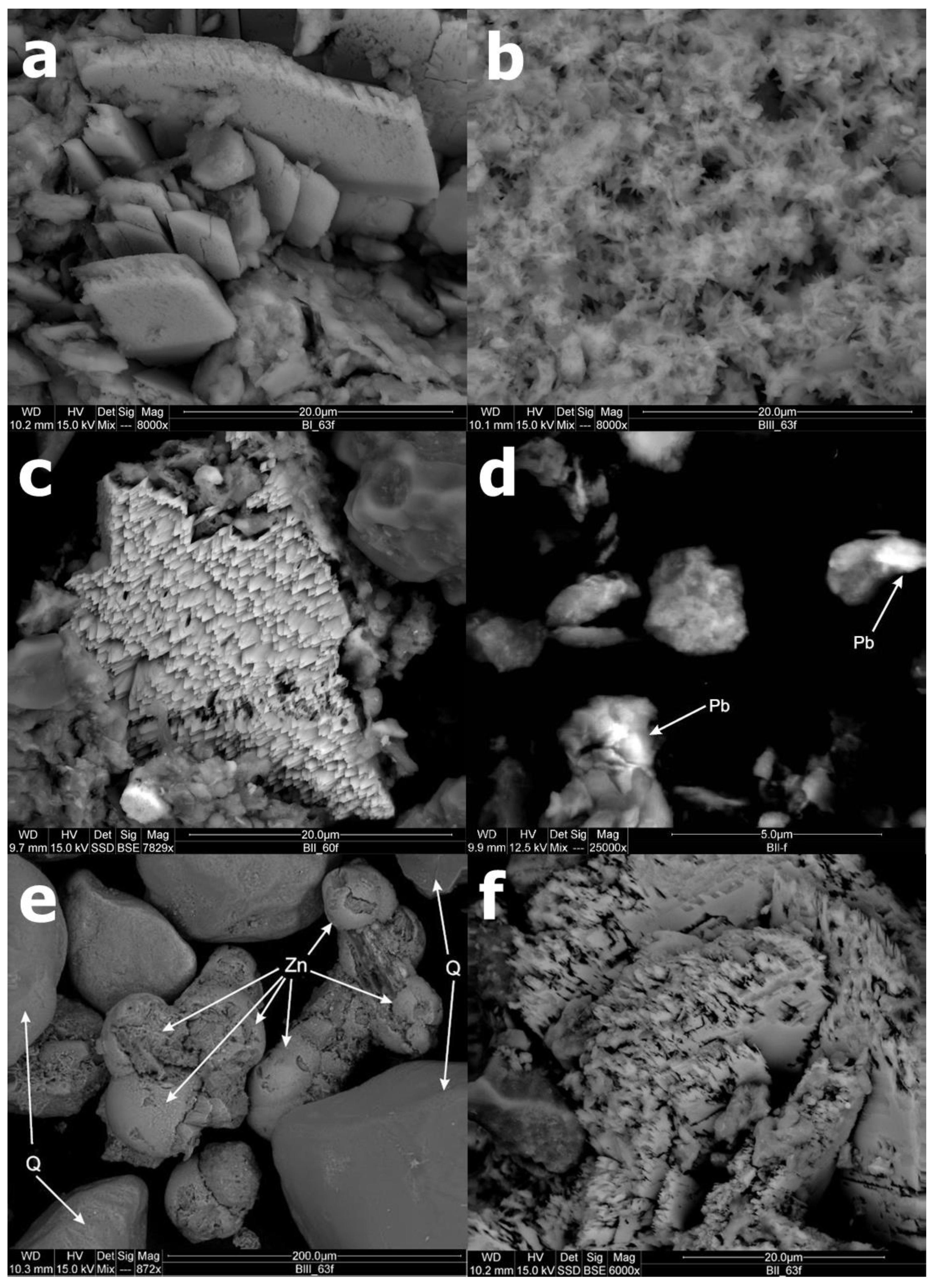
| Sample | Particle Size Analysis (%) 1 | OM (%) 2 | LOI (%) 3 | Total Content (mg/kg) 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Sand | Silt | Clay | Cd | Zn | Pb | |||
| BI | 69 | 12 | 19 | 2.51 | 0.23 | 34 ± 1.7 | 3078 ± 137 | 699 ± 6 |
| BII | 63 | 17 | 20 | 6.75 | 0.35 | 78 ± 1.4 | 8103 ± 78 | 2479 ± 83 |
| BIII | 71 | 18 | 11 | 4.17 | 0.38 | 53 ± 0.4 | 19173 ± 4 | 910 ± 15 |
| Element | BI (%) | BII (%) | BIII (%) |
|---|---|---|---|
| Na2O | 0.28 | 0.40 | 0.36 |
| MgO | 2.15 | 1.91 | 2.48 |
| Al2O3 | 3.87 | 4.99 | 4.59 |
| SiO2 | 79.46 | 68.52 | 63.07 |
| P2O5 | 0.14 | 0.22 | 0.97 |
| SO3 | 0.21 | 1.63 | 1.13 |
| K2O | 0.83 | 0.89 | 0.59 |
| CaO | 2.98 | 3.18 | 5.98 |
| TiO2 | 0.25 | 0.31 | 0.27 |
| Cr2O3 | 0.01 | 0.10 | 0.05 |
| MnO | 0.18 | 0.37 | 0.05 |
| Fe2O3 | 2.06 | 4.18 | 2.95 |
| NiO | 0.01 | 0.01 | 0.01 |
| CuO | 0.01 | 0.01 | 0.01 |
| ZnO | 0.54 | 1.14 | 0.54 |
| Rb2O | 0.00 | 0.01 | 0.00 |
| SrO | 0.00 | 0.01 | 0.01 |
| ZrO2 | 0.82 | 0.08 | 0.05 |
| PbO | 0.10 | 0.27 | 0.09 |
| As2O3 | <0.01 | 0.02 | 0.01 |
| CdO | <0.01 | 0.01 | 0.01 |
| BaO | <0.01 | 0.04 | 0.03 |
| V2O5 | 0.01 | 0.01 | <0.01 |
| SnO2 | <0.01 | <0.01 | 0.04 |
| Cl | <0.01 | <0.01 | 0.01 |
| Co2O3 | <0.01 | <0.01 | 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrunik, M.; Wołowiec, M.; Wojnarski, D.; Zelek-Pogudz, S.; Bajda, T. Transformation of Pb, Cd, and Zn Minerals Using Phosphates. Minerals 2020, 10, 342. https://doi.org/10.3390/min10040342
Andrunik M, Wołowiec M, Wojnarski D, Zelek-Pogudz S, Bajda T. Transformation of Pb, Cd, and Zn Minerals Using Phosphates. Minerals. 2020; 10(4):342. https://doi.org/10.3390/min10040342
Chicago/Turabian StyleAndrunik, Magdalena, Magdalena Wołowiec, Daniel Wojnarski, Sylwia Zelek-Pogudz, and Tomasz Bajda. 2020. "Transformation of Pb, Cd, and Zn Minerals Using Phosphates" Minerals 10, no. 4: 342. https://doi.org/10.3390/min10040342
APA StyleAndrunik, M., Wołowiec, M., Wojnarski, D., Zelek-Pogudz, S., & Bajda, T. (2020). Transformation of Pb, Cd, and Zn Minerals Using Phosphates. Minerals, 10(4), 342. https://doi.org/10.3390/min10040342






