Uranium Mineralogical and Chemical Features of the Na-Metasomatic Type Uranium Deposit in the Longshoushan Metallogenic Belt, Northwestern China
Abstract
1. Introduction
2. Geological Setting
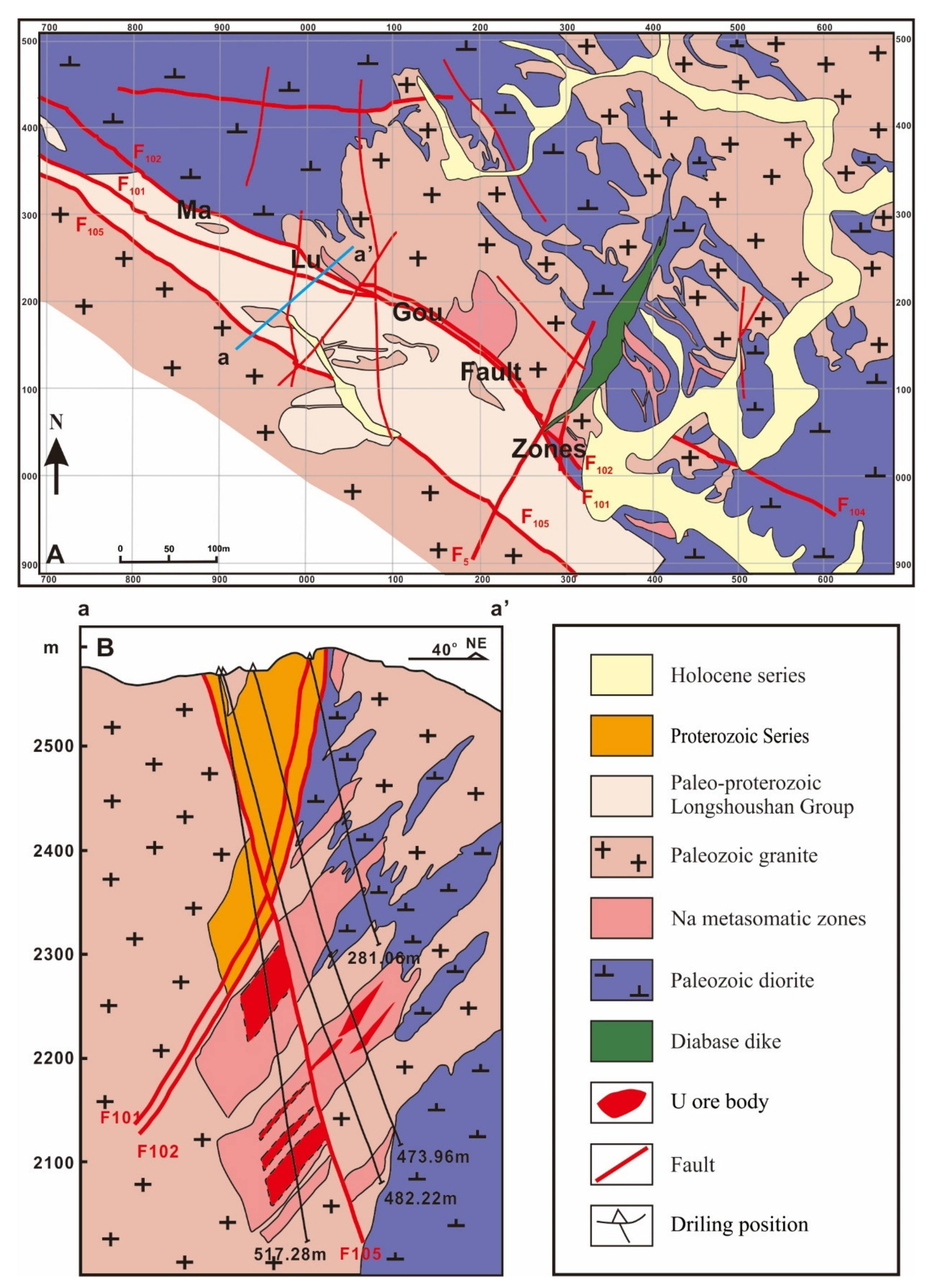
2.1. The Longshoushan Uranium Metallogenetic Belt (LMB)
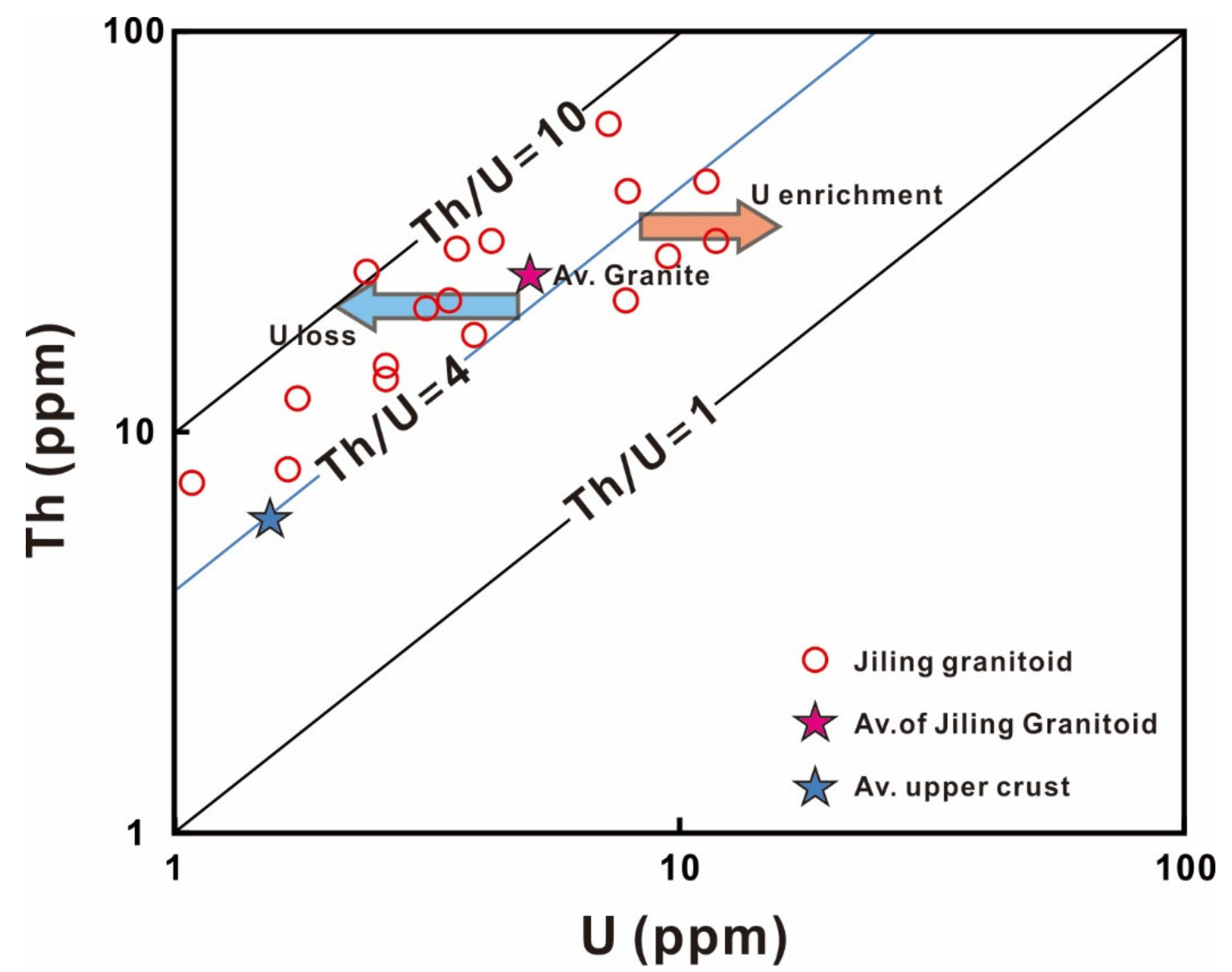
2.2. The Jiling Uranium Deposit
3. Analytical Methods
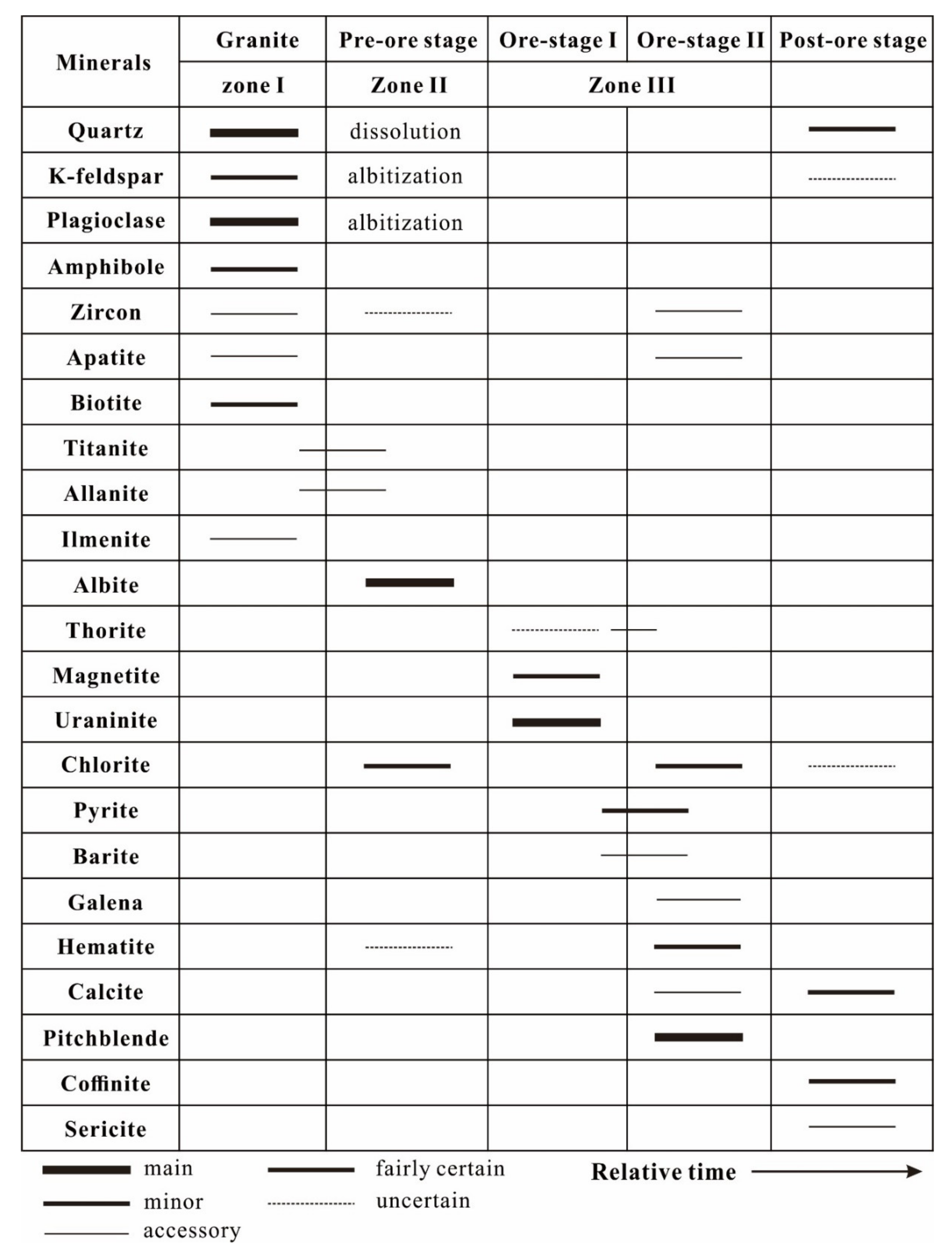
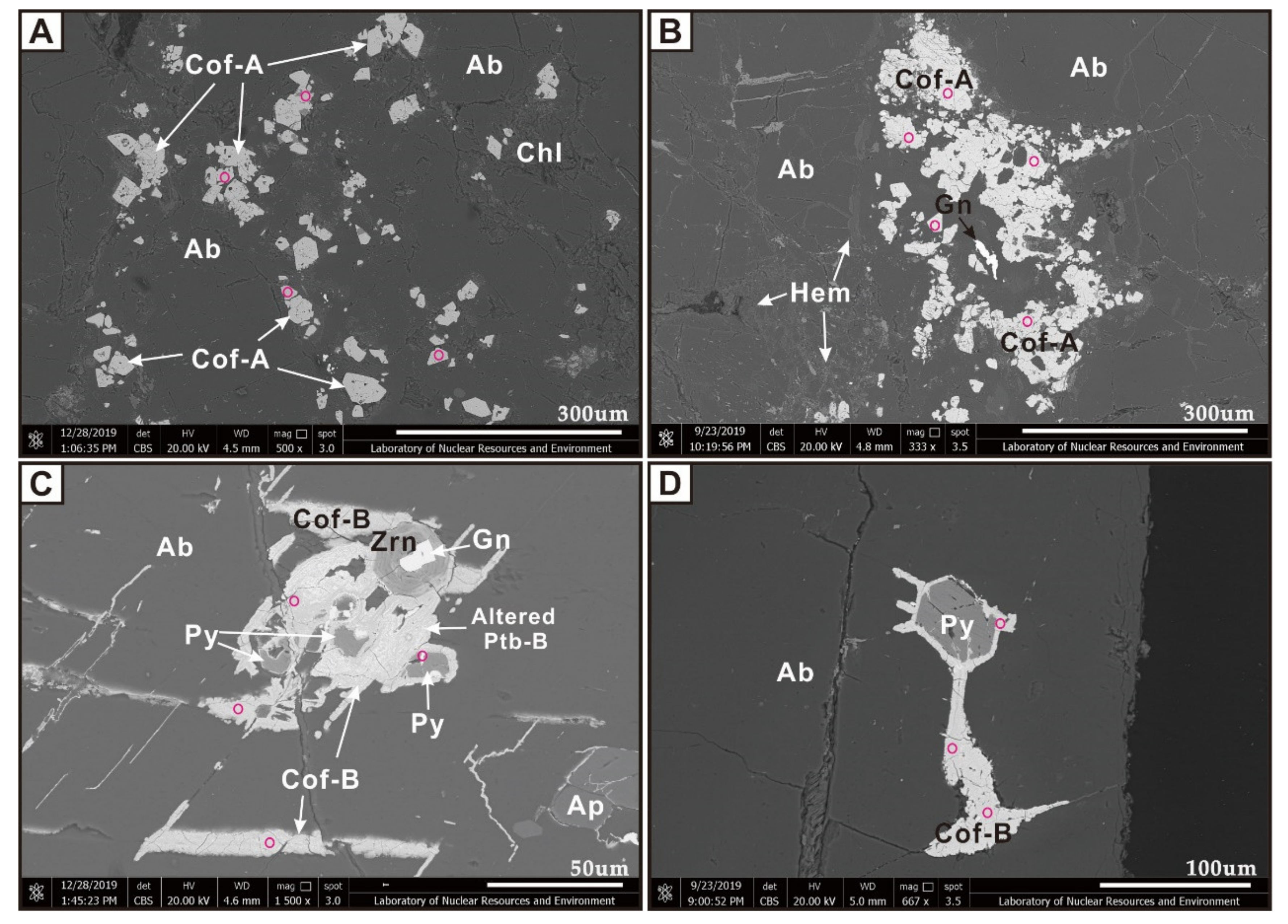
4. Petrography and Mineral Assemblage of Uranium Mineralization
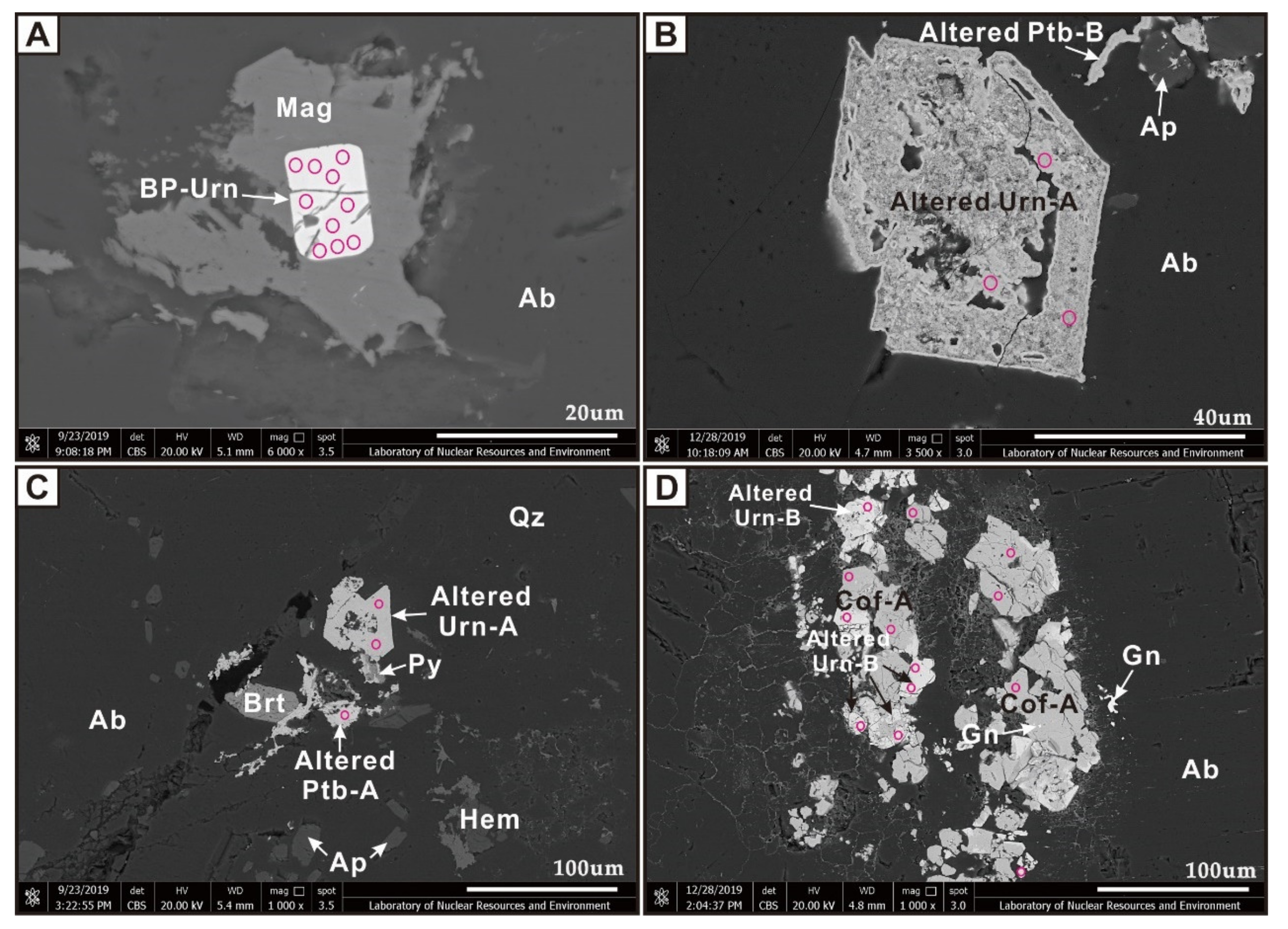
4.1. Uraninite
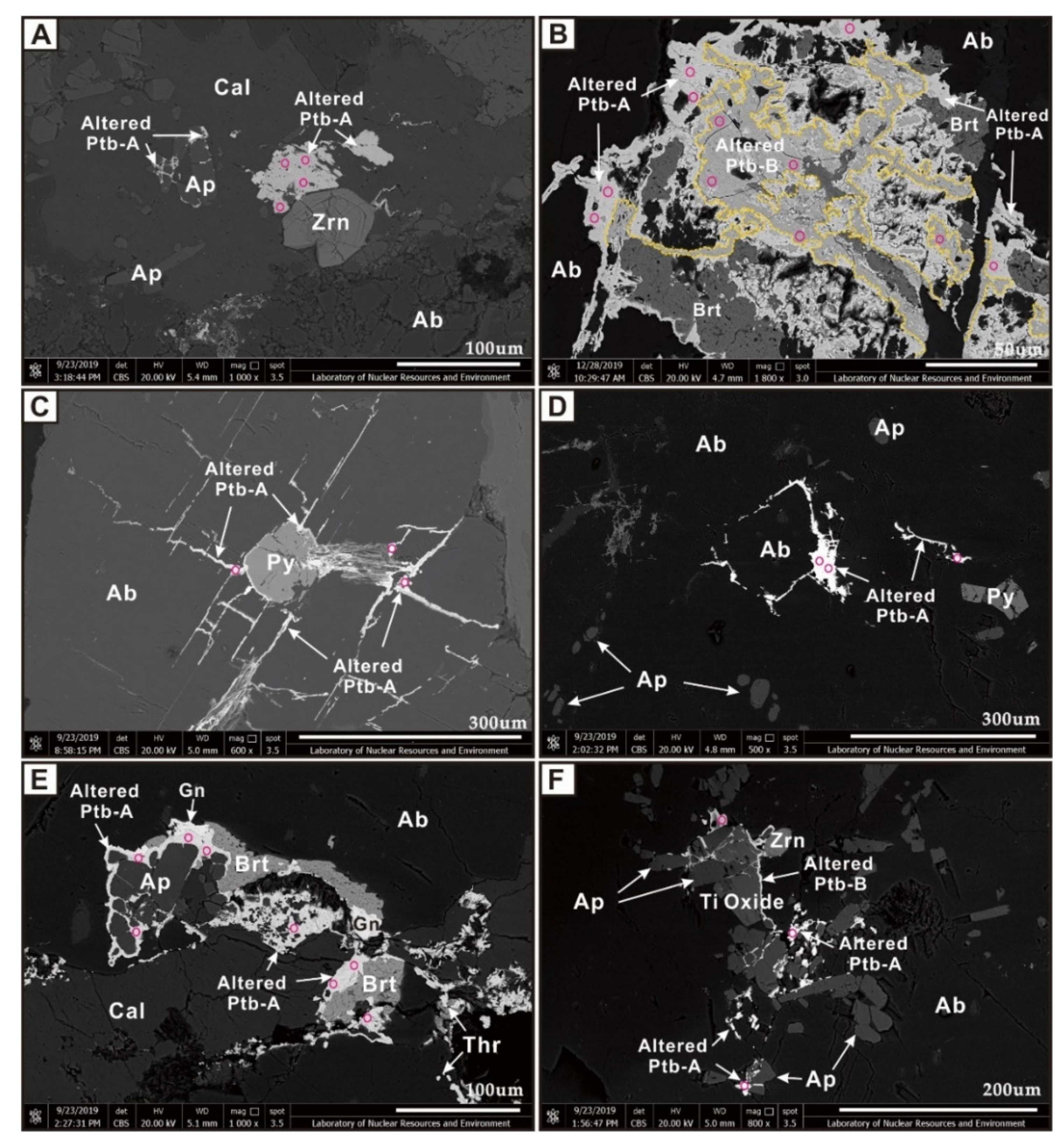
4.2. Pitchblende
4.3. Coffinite
5. Results
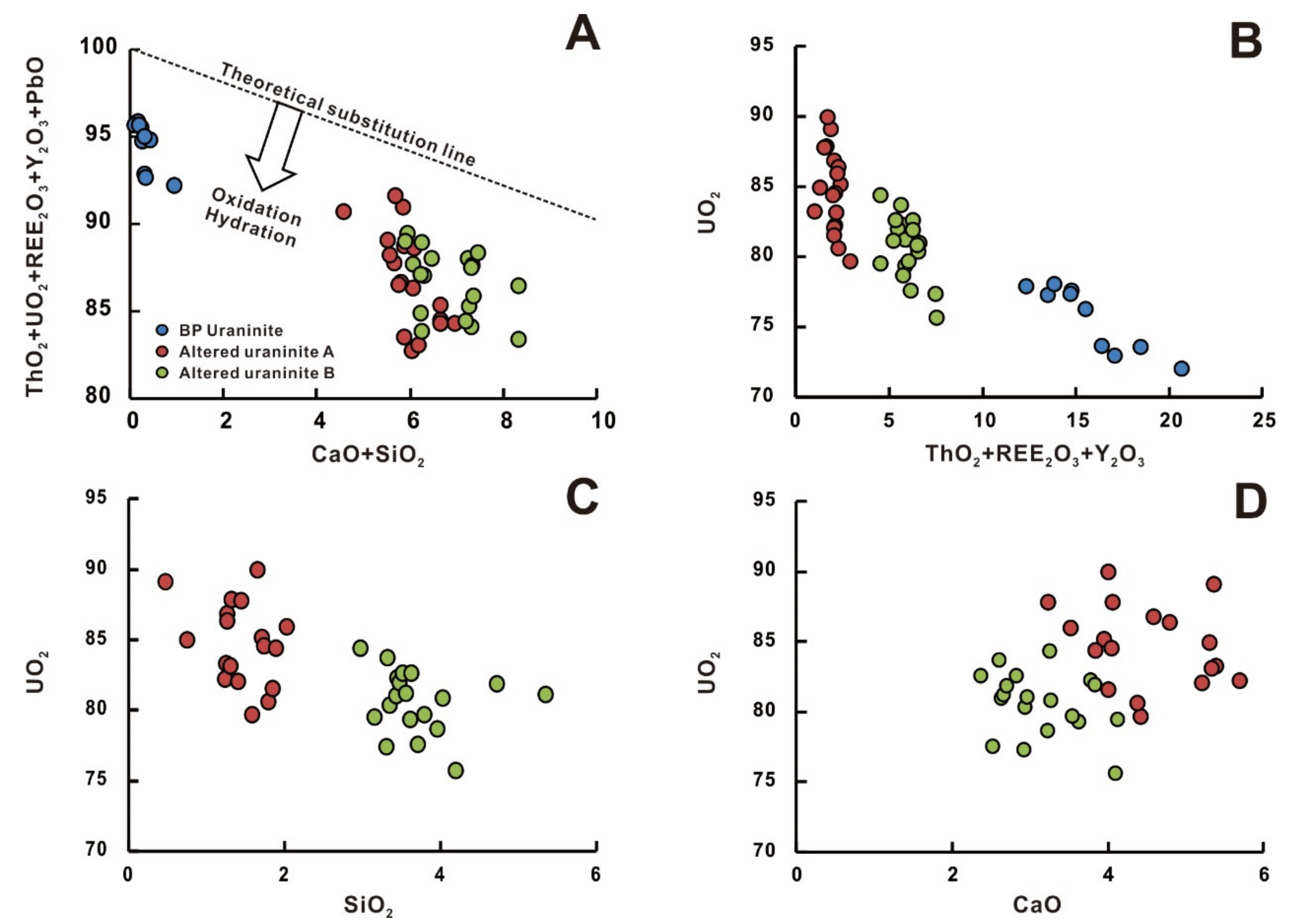
5.1. Composition of Uraninite
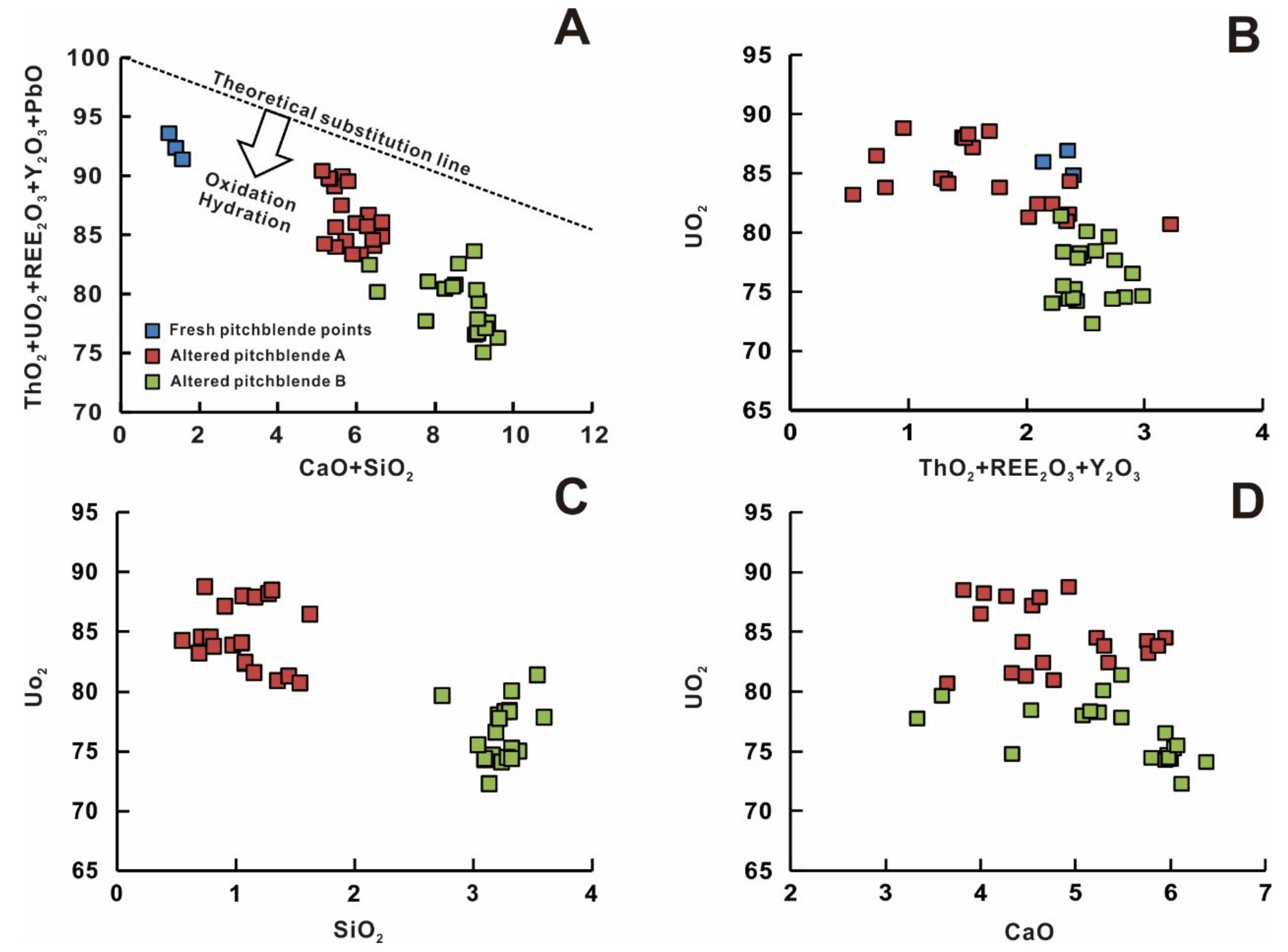
5.2. Composition of Pitchblende
5.3. Composition of Coffinite
6. Discussion
6.1. Geochemical Evolution of Uranium Mineralization
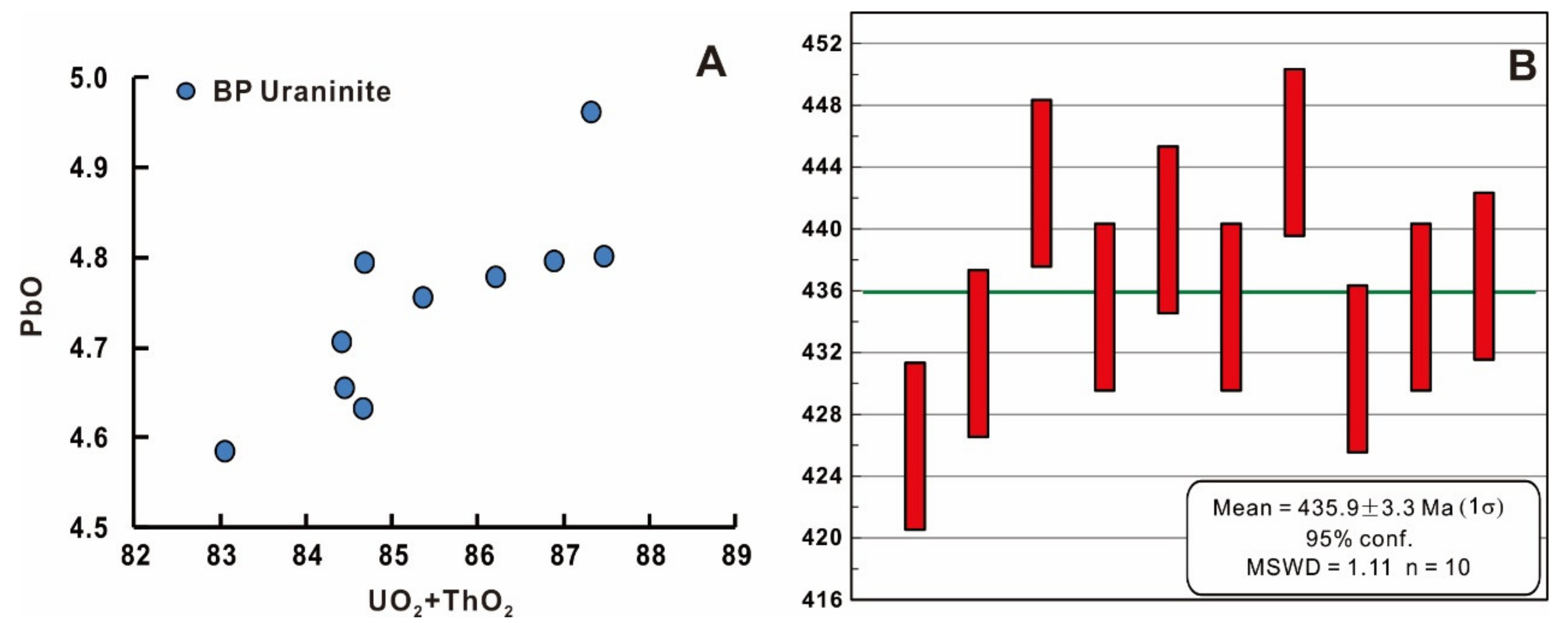
6.2. U-Th-Pb Chemical Dating of Uraninite and Pitchblende
6.3. Origin of Uranium Mineralization
6.4. Uranium Mineralization Process
6.5. Hydrothermal Alteration of Primary Uraninite
6.6. Comparison with Other Na-Metasomatic Uranium Deposit
- (a)
- The Jiling uranium deposit is hosted only in the Jiling intrusive granitoidic complex, whereas uranium mineralization in Central Ukraine also can be hosted in iron formations, this may be because the iron formation can provide a reduction condition for U precipitation in the ore-forming fluids in Central Ukraine. The iron formation also makes a difference in the mineralogy;
- (b)
- Due to the extensive U sources there is a thermal plume in the central part of the Ukrainian Shield, the uranium mineralization is spread over a belt of about 150 km in central Ukraine. Due to the limitations of magmatic activity, the uranium mineralization occurrences extend only over several tens kilometers in the Jiling area;
- (c)
- Due to banded iron formations, the metasomatic alteration episodes are more complex in the Central Ukraine uranium deposits. The Na-metasomatic episode, which is largely predominant in the Jiling deposit, is followed in the Ukraine by an important Ca-metasomatic episode with a new formation of Ca-rich pyroxene and garnet, only represented by minor amounts of carbonates in Jiling, and a late K-potassic event with a new formation of biotite, absent in Jiling [5];
- (d)
- In central Ukraine and many other deposits there is a time gap between granite emplacement and ore deposit formation; granites were emplaced at 2050 Ma and the mineralization was formed at 1800 Ma, whereas in Jiling, the granitoids and the uranium mineralization were formed around 430 Ma. There was no significant time gap between the first uranium mineralization and host granite emplacement. This is a complicated issue that is affected by manyfactors, such as different metallogenic geological backgrounds, different metallogenic evolution processes, and different properties of ore-forming fluids between these two places [5];
- (e)
- Due to the different ore-forming temperatures and host-rock geochemical characteristics between these two places, the uranium minerals predominantly consists of dominantly brannerite, with lesser amounts of low Th-uraninite, U-ferropseudobrookite, and pitchblende in central Ukraine. However, in the Jiling uranium deposit, high Th-REE-Y-uraninite, pitchblende, and coffinite are the main minerals; U-Ti minerals are absent;
- (f)
- The ore fluids in the Ukraine correspond to waters of surficial or basinal origin, whereas, in the Jiling uranium deposit ore fluids for the primary mineralization are of magmatic origin.
7. Conclusions
- (1)
- Three kinds of uranium minerals, high Th-REE-Y-uraninite, pitchblende, and coffinite, are present in the Jiling uranium deposit, and a great deal of the uraninite and pitchblende were subjected to different degrees of hydrothermal alteration. The pitchblende was the predominant uranium mineral among these three uranium minerals.
- (2)
- The similarity of the chemical age of the primary uraninite in the Jiling deposit at 435.9 ± 3.3 Ma, with that of the enclosing intrusions, and the high Th and REEY contents of primary uraninite suggest its crystallization at a high temperature from a magmatic-hydrothermal fluid. The primary uraninite was subjected to at least two alteration events to form altered uraninite A, mainly enriched in calcium, and B, mainly enriched in silica, and coffinite A.
- (3)
- Limited data on the chemical age of fresh pitchblende, suggested a formation age at about 361 Ma. The low Th and REEY contents of fresh pitchblende both suggested its crystallization at a low temperature and possibly from a meteoric-dominated ore fluid. According to the different chemical composition of fresh and altered pitchblende, the fresh pitchblende was also subjected to at least two alteration events to form altered pitchblende A and B, where B had more silica, but with a similar amount of calcium to A.
- (4)
- In summary, after the Jiling pluton and syenite emplacement, Na-metasomatic alteration was developed extensively along the Malugou fault and secondary structures. This Na-metasomatic alteration was associated with the uranium mineralization. Uraninite was formed at high temperature and possibly from a magmatic-hydrothermal fluid during ore stage I. It would be interesting to determine whether intrusions similar to the Jiling granitoid exist in the area, as they could represent interesting targets for further exploration. Pitchblende was formed at a low temperature, possibly of meteoric origin, essentially from the remobilization of the primary uraninite mineralization during ore stage II. Uraninite and pitchblende were finally partly altered into two types of coffinite during a late alteration episode.
- (5)
- The relatively high content of LREE in the uranium minerals formed by hydrothermal alteration enriched in Si, suggested that the Si-rich fluid have relatively high content of LREE.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- IAEA Tecdoc Series. Geological Classification of Uranium Deposit and Description of Selected Examples; IAEA Tecdoc 1842; IAEA: Vienna, Austria, 2018. [Google Scholar]
- Bruneton, P.; Cuney, M.; Dahlkamp, F.J.; Zaluski, G. “IAEA geological classification of uranium deposits”, Uranium Raw Material for the Nuclear Fuel Cycle: Exploration, Mining, Production, Supply and Demand, Economics and Environmental issues. In URAM 2014 Book of Abstracts; Abstract No. 187; IAEA: Vienna, Austria, 2014. [Google Scholar]
- Bruneton, P.; Cuney, M. “Geology of Uranium Deposits”, Uranium for Nuclear Power; Woodhead Publishing Series in Energy No. 93; Abstract No. 187; Hore-lacy, I., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 11–52. [Google Scholar]
- IAEA. OECD Nuclear Energy Agency Uranium 2018: Resources, Production and Demand (The Red Book); IAEA: Vienna, Austria, 2018. [Google Scholar]
- Cuney, M.; Emetz, A.; Mercadier, J.; Mykchaylov, V.; Shunko, V.; Yuslenko, A. Uranium deposits associated with Na-metasomatism from central Ukraine: A review of some of the major deposits and genetic constraints. Ore Geol. Rev. 2012, 44, 82–106. [Google Scholar] [CrossRef]
- Wilde, A. Towards a model for albitite-type uranium. Minerals 2013, 3, 36–48. [Google Scholar] [CrossRef]
- René, M. Alteration of granitoids and crystalline rocks and uranium mineralisation in the Bor pluton area, Bohemian Massif, Czech Republic. Ore Geol. Rev. 2017, 81, 188–200. [Google Scholar] [CrossRef]
- Chen, Y.J.; Zhao, R.Y.; Wu, B. Discovery of cryptoexplosive breccias in the Jiling uranium deposit of the Longshoushan area, Gansu Province and their genesis. Geol. Explor. 2012, 48, 1101–1108. (In Chinese) [Google Scholar]
- Chen, Y.J.; Fu, C.M.; Wang, G.; Zhao, R.Y.; Wang, W.; Miao, H.J. Carbon and oxygen isotopes in granite-type hydrothermal uranium deposits: A case study of the Jiling uranium ore field in Longshoushan, Gansu Province. Geol. Explor. 2014, 50, 0641–0648. (In Chinese) [Google Scholar]
- Chen, Y.J.; Wang, W.; Wang, G.; Zhao, R.Y. Characteristics of alteration in Jiling uranium mining area in Longshoushan area of Gansu. Miner. Resour. Geol. 2015, 29, 144–151. (In Chinese) [Google Scholar]
- Wei, Z.Y.; Zhang, S.M.; Liu, J.Z.; Chen, Y.J.; Fu, C.M.; Zhang, L. Characteristics and significance of chlorite in the Longshoushan alkali-metasomatic type uranium deposit. Acta Petrol. Et Mineral. 2014, 33, 517–526. [Google Scholar]
- Zhang, J.M.; Zhao, R.Y.; Wang, G.; Nie, L. The geological characteristics and significances of A-type porphyritic granite in the Jiling uranium deposit in the Longshou Mountains, Gansu Province. Bulletin of Mineralogy. Petrol. Geochem. 2017, 36, 813–823. (In Chinese) [Google Scholar]
- Gou, X.M.; Quan, J.P.; Liu, Z.G.; Feng, W.; Song, X.S.; Chen, Y.J. Characteristics of fluid inclusions and their geological significance: A case study of the Jiling uranium deposit in Gansu Province. Geol. Bull. China 2017, 36, 534–540. (In Chinese) [Google Scholar]
- Zhao, R.Y.; Chen, Y.C.; Chen, Y.J.; Rong, X.; Wang, G.; Li, T. The characteristics of geochemistry and alteration of the Jiling uranium deposit in the Longshou Mountains, Gansu Province: A case study from drill ZKJ29-3. Earth Sci. 2018, 45, 434–450. [Google Scholar]
- Mercadier, J.; Cuney, M.; Lach, P.; Boiron, M.C.; Bonhoure, J.; Richard, A.; Leisen, M.; Kister, P. Origin of uranium deposits revealed by their rare earth element signature. Terra Nova 2011, 23, 264–269. [Google Scholar] [CrossRef]
- Spano, T.L.; Simonetti, A.; Wheeler, T.; Carpenter, G.; Freet, D.; Balboni, E.; Dorais, C.; Burns, P.C. A novel nuclear forensic tool involving deposit type normalized rare earth element signatures. Terra Nova 2017, 29, 294–305. [Google Scholar] [CrossRef]
- Eglinger, A.; André-Mayer, A.S.; Vanderhaeghe, O.; Mercadier, J.; Cuney, M.; Decrée, S.; Feybesse, J.L.; Milesi, J.P. Geochemical signatures of uranium oxides in the Lufilian belt: From unconformity-related to syn-metamorphic uranium deposits during the Pan-African orogenic cycle. Ore Geol. Rev. 2013, 54, 197–213. [Google Scholar] [CrossRef]
- Alexandre, P.; Kyser, K.; Layton-Matthews, D.; Joy, B.; Uvarova, Y. Chemical compositions of natural uraninite. Can. Miner. 2015, 53, 595–622. [Google Scholar] [CrossRef]
- Frimmel, H.E.; Schedel, S.; Brätz, H. Uraninite chemistry as forensic tool for provenance analysis. Appl. Geochem. 2014, 48, 104–121. [Google Scholar] [CrossRef]
- MacMillan, E.; Cook, N.J.; Ehrig, K.; Ciobanu, C.L.; Pring, A. Uraninite from the Olympic Dam IOCG-U-Ag deposit: Linking textural and compositional variation to temporal evolution. Am. Miner. 2016, 101, 1295–1320. [Google Scholar] [CrossRef]
- Mercadier, J.; Annesley, I.R.; Mckechnie, C.L.; Bogdan, T.S.; Creighton, S. Magmatic and metamorphic uraninite mineralization in the western margin of the Trans-Hudson Orogen (Saskatchewan, Canada): A uranium source for unconformity-related uranium deposits? Econ. Geol. 2013, 108, 1037–1065. [Google Scholar] [CrossRef]
- Depiné, M.; Frimmel, H.E.; Emsbo, P.; Koenig, A.E.; Kern, M. Trace element distribution in uraninite from Mesoarchaean Witwatersrand conglomerates (South Africa) supports placer model and magmatogenic source. Miner. Depos. 2013, 48, 423–435. [Google Scholar] [CrossRef]
- Pal, D.C.; Rhede, D. Geochemistry and chemical dating of uraninite in the Jaduguda uranium deposit, Singhbhum shear zone, India-implications for uranium mineralization and geochemical evolution of uraninite. Econ. Geol. 2013, 108, 1499–1515. [Google Scholar] [CrossRef]
- Ozha, M.K.; Pal, D.C.; Mishra, B.; Desapati, T.; Shaji, T.S. Geochemistry and chemical dating of uraninite in the Samarkiya area, central Rajasthan, northwestern India—Implication for geochemical and temporal evolution of uranium mineralization. Ore Geol. Rev. 2017, 88, 23–42. [Google Scholar] [CrossRef]
- Yuan, F.; Jiang, S.Y.; Liu, J.; Zhang, S.; Xiao, Z.; Liu, G.; Hu, X. Geochronology and geochemistry of uraninite and coffinite: Insights into ore-forming process in the pegmatite-hosted uraniferous province, north qinling, central China. Minerals 2019, 9, 552. [Google Scholar] [CrossRef]
- Song, S.; Niu, Y.; Su, L.; Xia, X. Tectonics of the North Qilian orogen, NW China. Gondwana Res. 2013, 23, 1378–1401. [Google Scholar] [CrossRef]
- Zhao, R.Y.; Chen, Y.J.; Wu, B.; Wang, G. A metallogenic model of the sodic-metasomatic type uranium deposit in the Jiling area of Longshoushan, Gansu Province. Geol. Explor. 2013, 49, 0067–0074. (In Chinese) [Google Scholar]
- Li, S.Z.; Li, T.; Zhao, S.J.; Li, X.Y.; Liu, X.; Guo, L.L.; Yu, S.Y.; Li, S.J. Proto-Tethys Ocean in East Asia (IV): Deformation and evolution of microcontinents in the west segment of the northern Proto-Tethys Tectonic Domain. Acta Petrol. Sin. 2017, 33, 1615–1632. [Google Scholar]
- Zhang, Z.Q.; Wang, K.X.; Wang, G.; Liu, X.D.; Liu, W.H.; Wu, B. Petrogenesis and tectonic significances of the Paleozoic Jiling syenite in the mountain Longshou area, Gansu Province. Geol. Rev. 2018, 64, 1017–1029. (In Chinese) [Google Scholar]
- Wang, K.X.; Yu, C.D.a.; Yan, J.; Liu, X.D.; Liu, W.H.; Pan, J.Y. Petrogenesis of Early Silurian granitoids in the Longshoushan area and their implications for the extensional environment of the North Qilian Orogenic Belt, China. Lithos 2019, 342–343, 152–174. [Google Scholar] [CrossRef]
- Zhang, J.D.; Li, Z.Y.; Cai, Y.Q.; Guo, Q.Y.; Li, Y.L.; Han, C.Q. The main advance and achievements in the Potential Evaluation of Uranium Resource in China. Uranium Geol. 2012, 28, 321–326. (In Chinese) [Google Scholar]
- Zhang, C.; Jin, J.F. A tentative discussion on the synchronous enrichment of biotite and uraninite in the Hongshiquan uranium deposit. Acta Petrol. Et Mineral. 1989, 3, 242–251. (In Chinese) [Google Scholar]
- Xin, C.L.; Ma, W.Y.; An, G.B.; Yang, G.L.; Sun, X.H. Geological characteristics and mineralization mechanism of the No. 207 uranium deposit in Longshoushan, Gansu Province. Acta Geol. Sin. 2013, 87, 577–590. (In Chinese) [Google Scholar]
- Liu, Z.Y.; Wang, S.Y.; Gu, D.Z.; Liu, H.G.; Du, L.T.; Gao, H.L. Discussion on the characteristics of ore-bearing rock and the genesis of the Jiling uranium deposit. Northwestern Geol. 2018, 51, 160–170. (In Chinese) [Google Scholar]
- Gang, C.; Naldrett, A.J. Characteristics of Ni-Cu-PGE mineralization and genesis of the Jinchuan deposit, northwest China. Econ. Geol. 1992, 87, 1475–1495. [Google Scholar]
- Li, X.H.; Su, L.; Chung, S.L.; Li, Z.X.; Liu, Y.; Song, B.; Liu, D.Y. Formation of the Jinchuan ultramafic intrusion and the world’s third largest Ni-Cu sulfide deposit: Associated with the ∼825 Ma south China mantle plume? Geochem. Geophys. Geosystems. 2005, 6, 11. [Google Scholar] [CrossRef]
- Lu, Y.G.; Lesher, C.M.; Deng, J. Geochemistry and genesis of magmatic Ni-Cu-(PGE) and PGE-(Cu)-(Ni) deposit in China. Ore Geol. Rev. 2019, 107, 863–887. [Google Scholar] [CrossRef]
- Zhao, R.Y.; Jiang, C.Y.; Chen, X.; Wang, G.; Chen, Y.J.; Nie, L. Geological features of albitite veins and their relationship with uranium mineralization in the middle Longshou Mountains of Gansu Province. Geol. Explor. 2015, 51, 1069–1078. (In Chinese) [Google Scholar]
- Zhao, R.Y. The Study on Geological Characteristics and Metallogeny of Jiling Sodium-Metasomatic Uranium Deposit in Longshou Mountain Metallogenic Belt in Gansu Province. Ph.D. Thesis, Chang An University, Xi’an, China, 2016. (In Chinese). [Google Scholar]
- Zhao, Y.Y.; Zhang, S.M.; Tang, L.; Yao, H.F.; Yang, C.S. Sr-Nd-Pb isotopic characteristics and its geological significance of the Jiling grantic pluton in the middle Longshou mountains. Earth Sci. 2016, 41, 1016–1030. (In Chinese) [Google Scholar]
- Zhao, Y.Y.; Zhang, S.M.; Tang, L.; Yao, H.F. Caledonian granitic magmatism and U mineralization in middle Longshoushan. Gansu Geol. 2015, 24, 71–78. (In Chinese) [Google Scholar]
- Lan, D.C.; Zhang, S.M.; Qin, Y.; Yang, C.S.; Wang, L.L.; Qi, J.W. Characteristics and Ar-Ar dating of mafic dykes in Hongshiquan area, Gansu Province. Earth Sci. 2019, 44, 3469–3483. (In Chinese) [Google Scholar]
- Tang, L. Granites’ Characteristics and Zircon LA-ICP-MS U-Pb Dating of Jiling Area in Longshoushan, Gansu Province. Master’s Thesis, East China University of Technology, Nanchang, China, 2015. (In Chinese). [Google Scholar]
- Zhong, J.; Wang, S.Y.; Gu, D.Z.; Cai, Y.Q.; Fan, H.H.; Shi, C.H.; Hu, C.N. Geology and fluid geochemistry of the Na-metasomatism U deposits in the Longshoushan uranium metallogenic belt, NW China: Constraints on the ore-forming process. Ore Geol. Rev. 2020, 116, 103214. [Google Scholar] [CrossRef]
- Kempe, U. Precise electron micropobe age determination in altered uraninite: Consequences on the intrusion age and the metallogenic significance of the Kirchberg granite (Erzgebirge, Germany). Contrib. Mineral. Petrol. 2003, 145, 107–118. [Google Scholar] [CrossRef]
- Holmes, A. The Association of Lead with Uranium in Rock-Minerals, and Its Application to the Measurement of Geological Time. Proc. R. Soc. A Math. Phys. Eng. Sci. 1911, 85, 248–256. [Google Scholar]
- Alexandre, P.; Kyser, T.K. Effects of cationic substitutions and alteration in uraninite, and implications for the dating of uranium deposits. Can. Mineral. 2005, 43, 1005–1017. [Google Scholar] [CrossRef]
- Bowles, J.F.W. Age dating of individual grains of uraninite in rocks from electron microprobe analyses. Chem. Geol. 1990, 83, 47–53. [Google Scholar] [CrossRef]
- Jaffey, A.H.; Flynn, K.F.; Glendenin, L.E.; Bentley, W.C.; Essling, A.M. Precision Measurement of Half-Lives and Specific Activities of 235U and 238U. Phys. Rev. C. 1971, 4, 1889–1906. [Google Scholar] [CrossRef]
- Janeczek, J.; Ewing, R.C. Structural formula of uraninite. J. Nucl. Mater. 1992, 190, 128–132. [Google Scholar] [CrossRef]
- Kotzer, T.G.; Kyser, T.K. O, U, and Pb isotopic and chemical variations in uraninite: Implications for determining the temporal and fluid history of ancient terrains. Am. Mineral. 1993, 78, 1262–1274. [Google Scholar]
- Evins, L.Z.; Jensen, K.A.; Ewing, R.C. Uraninite recrystallization and Pb loss in the Oklo and Bangombé natural fission reactors, Gabon. Geochim. Cosmochim. Acta. 2005, 69, 1589–1606. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Mukhopadhyay, J.; Mishra, B.; Chakrabarti, K.; De, S.; Ghosh, G. Uraniferous paleoplacers of the Mesoarchean Mahagiri Quartzite, Singhbhum craton, India: Depositional controls, nature and source of >3.0 Ga detrital uraninites. Ore Geol. Rev. 2016, 72, 1290–1306. [Google Scholar] [CrossRef]
- Fryer, B.J.; Taylor, R.P. Rare-earth element distributions in uraninites: Implications for ore genesis. Chem. Geol. 1987, 63, 101–108. [Google Scholar] [CrossRef]
- Sun, S.S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. Geol. Soc. Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Tao, J.; Li, W.; Wyman, D.A.; Wang, A.; Xu, Z. Petrogenesis of Triassic granite from the Jintan pluton in Central Jiangxi Province, South China: Implication for uranium enrichment. Lithos 2018, 320–321, 62–74. [Google Scholar] [CrossRef]
- Sun, L.Q.; Ling, H.F.; Shen, W.Z.; Wang, K.X.; Huang, G.L. Petrogenesis of two Triassic A-type intrusions in the interior of South China and their implications for tectonic transition. Lithos 2017, 284, 642–653. [Google Scholar] [CrossRef]
- Cuney, M.; Friedrich, M. Physicochemical and crystal-chemical controls on accessory mineral paragenesis in granitoids: Implications on uranium metallogenesis. Bull. Miner. 1987, 110, 235–247. [Google Scholar] [CrossRef]
- Cuney, M. Felsic magmatism and uranium deposits. BSGF Earth Sci. Bull. 2014, 2, 75–92. [Google Scholar] [CrossRef]
- Tullis, J.; Yund, R.A. Dynamic recrystallisation of feldspar: A mechanism for ductile shear zone formation. Geology 1985, 13, 238–241. [Google Scholar] [CrossRef]
- Zhong, J.; Fan, H.H.; Gu, D.Z.; Wang, S.Y.; Chen, J.Y.; Shi, C.H.; Li, H.Q. Major and trace element migration and metallogenic process of the Xinshuijing U-Th deposit in the Longshoushan metallogenic belt, Gansu Province. Geol. China 2016, 43, 1393–1408. (In Chinese) [Google Scholar]
- Zhao, Y.Y. Petrology, Geochemistry Characteristics and Geological Significance of Paleozoic Granites in Middle Longshou Moutains, Gansu Provonce; East China University of Technology: Nanchang, China, 2016. (In Chinese) [Google Scholar]
- Robie, R.A.; Hemingway, B.S. Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar (105Pascals) pressure and at higher temperatures. US Geol. Surv. Bull. 1995, 2131, 16. [Google Scholar]
- Fayek, M.; Janeczek, J.; Ewing, R.C. Mineral chemistry and oxygen isotopic analyses of uraninite, pitchblende and uranium alteration minerals from the Cigar Lake deposit, Saskatchewan, Canada. Appl. Geochem. 1997, 12, 549–565. [Google Scholar] [CrossRef]
- Macmillan, E.; Cook, N.J.; Ehrig, K.; Pring, A. Chemical and textural interpretation of late-stage coffinite and brannerite from the Olympic Dam IOCG-Ag-U deposit. Mineral. Mag. 2017, 81, 1323–1366. [Google Scholar] [CrossRef]
- Deditius, A.P.; Utsunomiya, S.; Wall, M.A.; Pointeau, V.; Ewing, R.C. Crystal chemistry and radiation-induced amorphization of p-coffinite from the natural fission reactor at Bangombe, Gabon. Am. Mineral. 2009, 94, 827–837. [Google Scholar] [CrossRef]
- Johannesson, K.H.; Lyons, W.B.; Stetzenbach, K.J.; Byrne, R.H. The solubility control of rare earth elements in natural terrestrial waters and the significance of PO43− and CO32− in limiting dissolved rare earth concentrations: A review of recent information. Aquat. Geochem. 1995, 157–173. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Migdisov, A.A.; Samson, I.M. Hydrothermal mobilisation of the rare earth elements-a tale of “ceria” and “yttria” . Elements 2012, 8, 355–360. [Google Scholar] [CrossRef]
- Zhong, J.R.; Guo, Z.T. The geological characteristics and metallogenetic control factors of the Lianshanguan uranium deposit, northeast China. Precambrian Res. 1988, 39, 51–64. [Google Scholar] [CrossRef]
- Lobato, L.M.; Fyfe, W.S. Metamorphism, metasomatism, and mineralization at Lagoa Real, Bahia, Brazil. Econ. Geol. 1990, 85, 968–989. [Google Scholar] [CrossRef]
- Polito, P.A.; Kyser, T.K.; Stanley, C. The Proterozoic, albitite-hosted, Valhalla uranium deposit, Queensland, Australia: A description of the alteration assemblage associated with uranium mineralisation in diamond drill hole V39. Miner. Depos. 2009, 44, 11–40. [Google Scholar] [CrossRef]
- Alexandre, P. Mineralogy and geochemistry of the sodium metasomatism-related uranium occurrence of Aricheng South, Guyana. Miner. Depos. 2010, 45, 351–367. [Google Scholar] [CrossRef]

| Sample | SiO2 | CaO | UO2 | PbO | ThO2 | SrO | FeO | Al2O3 | La2O3 | Ce2O3 | Pr2O3 | Nd2O3 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BP Uraninite A (n = 10) | Min | n.d. | 0.05 | 72.07 | 4.58 | 6.51 | n.d. | 1.23 | n.d. | 0.13 | 0.74 | 0.03 | 0.80 | |
| Max | 0.79 | 0.23 | 78.08 | 4.96 | 12.59 | 0.04 | 2.06 | n.d. | 0.13 | 1.34 | 0.46 | 1.29 | ||
| Median | 0.17 | 0.12 | 76.82 | 4.77 | 9.81 | 0.02 | 1.62 | n.d. | 0.13 | 1.08 | 0.16 | 0.93 | ||
| std. Dev | 0.21 | 0.05 | 2.21 | 0.10 | 1.89 | 0.02 | 0.23 | n.d. | n.d. | 0.20 | 0.12 | 0.13 | ||
| Altered Uraninite A (n = 18) | Min | 0.47 | 3.24 | 79.69 | 0.04 | n.d. | n.d. | 0.81 | 0.03 | 0.10 | 0.42 | 0.02 | 0.11 | |
| Max | 2.02 | 5.69 | 89.96 | 1.18 | 1.28 | 0.21 | 1.51 | 0.13 | 0.24 | 1.00 | 0.43 | 0.48 | ||
| Median | 1.42 | 4.40 | 84.77 | 0.16 | 0.39 | 0.03 | 1.14 | 0.06 | 0.13 | 0.70 | 0.20 | 0.25 | ||
| std. Dev | 0.38 | 0.71 | 2.84 | 0.25 | 0.40 | 0.06 | 0.18 | 0.03 | 0.05 | 0.15 | 0.09 | 0.10 | ||
| Altered Uraninite B (n = 19) | Min | 2.97 | 2.37 | 75.71 | 0.05 | 1.33 | 0.06 | 1.13 | 0.03 | 0.20 | 1.33 | 0.00 | 0.13 | |
| Max | 5.34 | 4.11 | 84.37 | 0.13 | 4.35 | 0.16 | 2.06 | 0.24 | 0.62 | 2.35 | 0.58 | 0.29 | ||
| Median | 3.55 | 2.95 | 81.03 | 0.07 | 3.28 | 0.11 | 1.55 | 0.12 | 0.42 | 1.80 | 0.23 | 0.23 | ||
| std. Dev | 0.55 | 0.53 | 2.17 | 0.02 | 0.72 | 0.03 | 0.29 | 0.05 | 0.13 | 0.30 | 0.13 | 0.04 | ||
| Fresh Pitchblende (n = 3) | Min | 0.18 | 1.04 | 84.85 | 4.19 | 0.75 | n.d. | 0.19 | 0.04 | n.d. | 0.32 | 0.12 | 0.21 | |
| Max | 0.37 | 1.20 | 86.91 | 4.28 | 0.83 | n.d. | 0.98 | 0.09 | n.d. | 0.64 | 0.28 | 0.25 | ||
| Median | 0.31 | 1.09 | 85.93 | 4.26 | 0.82 | n.d. | 0.92 | 0.06 | n.d. | 0.47 | 0.22 | 0.23 | ||
| std. Dev | 0.08 | 0.07 | 0.84 | 0.04 | 0.03 | n.d. | 0.36 | 0.03 | n.d. | 0.13 | 0.07 | 0.02 | ||
| Altered Pitchblende A (n = 20) | Min | 0.55 | 3.65 | 80.73 | 0.04 | 0.04 | 0.04 | 0.82 | 0.02 | 0.10 | 0.37 | 0.11 | 0.16 | |
| Max | 1.62 | 5.94 | 88.80 | 0.45 | 1.31 | 0.14 | 1.48 | 0.16 | 0.24 | 0.72 | 0.37 | 0.46 | ||
| Median | 1.06 | 4.64 | 84.21 | 0.26 | 0.14 | 0.09 | 1.18 | 0.05 | 0.15 | 0.62 | 0.20 | 0.20 | ||
| std. Dev | 0.29 | 0.68 | 2.65 | 0.09 | 0.50 | 0.04 | 0.19 | 0.04 | 0.05 | 0.10 | 0.07 | 0.10 | ||
| Altered Pitchblende B (n = 20) | Min | 2.74 | 3.33 | 69.90 | 0.03 | 0.71 | 0.01 | 0.92 | 0.05 | 0.11 | 0.44 | 0.11 | 0.14 | |
| Max | 3.60 | 6.37 | 81.40 | 6.93 | 1.36 | 0.26 | 2.03 | 0.47 | 0.28 | 1.10 | 0.39 | 0.43 | ||
| Median | 3.25 | 5.64 | 76.05 | 0.09 | 0.92 | 0.14 | 1.03 | 0.17 | 0.18 | 0.67 | 0.15 | 0.28 | ||
| std. Dev | 0.18 | 0.83 | 2.76 | 1.49 | 0.13 | 0.07 | 0.24 | 0.08 | 0.06 | 0.15 | 0.08 | 0.08 | ||
| Coffinite A (n = 39) | Min | 10.01 | 2.01 | 49.64 | 0.03 | 2.31 | 0.05 | 0.29 | 0.18 | 0.14 | 1.36 | 0.11 | 0.28 | |
| Max | 17.11 | 3.91 | 66.35 | 0.20 | 8.97 | 0.07 | 1.06 | 0.58 | 0.51 | 2.72 | 0.65 | 1.56 | ||
| Median | 14.58 | 2.64 | 58.73 | 0.07 | 4.55 | 0.06 | 0.53 | 0.37 | 0.26 | 1.89 | 0.38 | 0.88 | ||
| std. Dev | 1.44 | 0.38 | 4.14 | 0.05 | 1.42 | 0.01 | 0.18 | 0.10 | 0.10 | 0.30 | 0.14 | 0.29 | ||
| Coffinite B (n = 29) | Min | 10.31 | 1.87 | 58.45 | 0.01 | 0.03 | 0.06 | 0.15 | 0.38 | 0.15 | 1.44 | 0.26 | 0.49 | |
| Max | 20.30 | 3.24 | 71.22 | 1.98 | 0.11 | 0.11 | 1.09 | 1.05 | 0.36 | 2.60 | 0.61 | 1.61 | ||
| Median | 15.00 | 2.45 | 63.03 | 0.07 | 0.04 | 0.06 | 0.33 | 0.65 | 0.23 | 1.97 | 0.42 | 1.15 | ||
| std. Dev | 1.71 | 0.36 | 2.66 | 0.54 | 0.03 | 0.02 | 0.28 | 0.20 | 0.05 | 0.27 | 0.10 | 0.27 | ||
| Sample | Sm2O3 | Eu2O3 | Gd2O3 | Tb2O3 | Dy2O3 | Ho2O3 | Er2O3 | Tm2O3 | Yb2O3 | Lu2O3 | Y2O3 | Total | ΣREE2O3 | |
| BP Uraninite A (n = 10) | Min | 0.23 | n.d. | 0.31 | n.d. | 0.20 | 0.21 | 0.21 | n.d. | 0.16 | 0.21 | 0.95 | 96.84 | 3.01 |
| Max | 0.74 | n.d. | 0.87 | n.d. | 1.35 | 0.49 | 0.43 | n.d. | 0.63 | 0.30 | 2.51 | 99.39 | 6.32 | |
| Median | 0.40 | n.d. | 0.49 | n.d. | 0.56 | 0.27 | 0.28 | n.d. | 0.31 | 0.26 | 1.71 | 98.51 | 3.73 | |
| std. Dev | 0.15 | n.d. | 0.18 | n.d. | 0.35 | 0.10 | 0.09 | n.d. | 0.19 | 0.04 | 0.48 | 0.96 | 1.09 | |
| Altered Uraninite A (n = 18) | Min | 0.11 | n.d. | 0.11 | n.d. | 0.21 | n.d. | n.d. | 0.22 | 0.16 | 0.22 | 0.12 | 90.08 | 0.94 |
| Max | 0.24 | n.d. | 0.21 | n.d. | 0.56 | n.d. | n.d. | 0.22 | 0.21 | 0.22 | 0.44 | 98.68 | 2.00 | |
| Median | 0.15 | n.d. | 0.16 | n.d. | 0.37 | n.d. | n.d. | 0.22 | 0.18 | 0.22 | 0.19 | 94.03 | 1.34 | |
| std. Dev | 0.05 | n.d. | 0.03 | n.d. | 0.12 | n.d. | n.d. | n.d. | 0.02 | n.d. | 0.10 | 2.42 | 0.33 | |
| Altered Uraninite B (n = 19) | Min | n.d. | n.d. | 0.13 | n.d. | 0.17 | n.d. | n.d. | n.d. | 0.16 | 0.23 | n.d. | 91.88 | 2.13 |
| Max | n.d. | n.d. | 0.35 | n.d. | 0.20 | n.d. | n.d. | n.d. | 0.23 | 0.38 | n.d. | 97.77 | 3.33 | |
| Median | n.d. | n.d. | 0.18 | n.d. | 0.19 | n.d. | n.d. | n.d. | 0.20 | 0.28 | n.d. | 95.18 | 2.91 | |
| std. Dev | n.d. | n.d. | 0.08 | n.d. | 0.02 | n.d. | n.d. | n.d. | 0.03 | 0.06 | n.d. | 1.66 | 0.39 | |
| Fresh Pitchblende (n = 3) | Min | 0.21 | n.d. | 0.13 | n.d. | 0.20 | n.d. | 0.24 | n.d. | 0.28 | n.d. | 0.13 | 94.13 | 1.31 |
| Max | 0.21 | n.d. | 0.31 | n.d. | 0.20 | n.d. | 0.32 | n.d. | 0.28 | n.d. | 0.13 | 94.96 | 1.53 | |
| Median | 0.21 | n.d. | 0.17 | n.d. | 0.20 | n.d. | 0.28 | n.d. | 0.28 | n.d. | 0.13 | 94.62 | 1.51 | |
| std. Dev | n.d. | n.d. | 0.08 | n.d. | n.d. | n.d. | 0.04 | n.d. | 0.00 | n.d. | n.d. | 0.34 | 0.10 | |
| Altered Pitchblende A (n = 20) | Min | 0.14 | n.d. | 0.16 | n.d. | 0.17 | 0.24 | 0.24 | n.d. | n.d. | n.d. | 0.10 | 90.38 | 0.42 |
| Max | 0.23 | n.d. | 0.31 | n.d. | 0.47 | 0.24 | 0.24 | n.d. | n.d. | n.d. | 0.28 | 97.07 | 2.21 | |
| Median | 0.15 | n.d. | 0.17 | n.d. | 0.26 | 0.24 | 0.24 | n.d. | n.d. | n.d. | 0.15 | 93.16 | 1.26 | |
| std. Dev | 0.04 | n.d. | 0.05 | n.d. | 0.09 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.05 | 2.22 | 0.42 | |
| Altered Pitchblende B (n = 20) | Min | 0.11 | n.d. | 0.12 | n.d. | 0.19 | 0.19 | 0.21 | 0.22 | 0.17 | 0.14 | 0.08 | 85.88 | 1.10 |
| Max | 0.28 | n.d. | 0.23 | n.d. | 0.35 | 0.19 | 0.23 | 0.24 | 0.17 | 0.33 | 0.28 | 94.37 | 2.46 | |
| Median | 0.21 | n.d. | 0.15 | n.d. | 0.27 | 0.19 | 0.22 | 0.23 | 0.17 | 0.24 | 0.15 | 88.79 | 1.43 | |
| std. Dev | 0.05 | n.d. | 0.04 | n.d. | 0.06 | n.d. | 0.01 | 0.01 | n.d. | 0.07 | 0.04 | 2.06 | 0.33 | |
| Coffinite A (n = 39) | Min | 0.11 | n.d. | 0.12 | n.d. | 0.19 | n.d. | 0.21 | 0.22 | 0.18 | 0.25 | 0.42 | 78.41 | 2.79 |
| Max | 0.33 | n.d. | 0.68 | n.d. | 0.48 | n.d. | 0.50 | 0.33 | 0.64 | 0.33 | 1.73 | 93.90 | 5.62 | |
| Median | 0.21 | n.d. | 0.32 | n.d. | 0.33 | n.d. | 0.29 | 0.26 | 0.37 | 0.27 | 0.77 | 86.63 | 4.73 | |
| std. Dev | 0.06 | n.d. | 0.14 | n.d. | 0.09 | n.d. | 0.07 | 0.04 | 0.12 | 0.03 | 0.28 | 3.88 | 0.74 | |
| Coffinite B (n = 29) | Min | 0.13 | n.d. | 0.14 | n.d. | 0.18 | n.d. | 0.22 | 0.23 | 0.18 | 0.29 | 0.27 | 85.32 | 2.89 |
| Max | 0.51 | n.d. | 0.67 | n.d. | 0.49 | n.d. | 0.51 | 0.28 | 0.69 | 0.29 | 1.74 | 93.66 | 6.39 | |
| Median | 0.21 | n.d. | 0.40 | n.d. | 0.29 | n.d. | 0.27 | 0.25 | 0.39 | 0.29 | 1.18 | 88.38 | 5.23 | |
| std. Dev | 0.08 | n.d. | 0.14 | n.d. | 0.10 | n.d. | 0.09 | 0.02 | 0.15 | n.d. | 0.44 | 1.83 | 1..02 |
| BP Uraninite | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sample | SiO2 | CaO | UO2 | PbO | ThO2 | FeO | U/Th | Age |
| 11-1.9-1 | 0.17 | 0.14 | 77.91 | 4.71 | 6.51 | 2.06 | 11.70 | 426.00 |
| 11-1.9-2 | 0.16 | 0.11 | 77.35 | 4.80 | 9.54 | 1.54 | 7.92 | 432.00 |
| 11-1.9-3 | 0.14 | 0.05 | 78.08 | 4.96 | 9.25 | 1.50 | 8.25 | 443.00 |
| 11-1.9-4 | 0.01 | 0.23 | 77.61 | 4.80 | 7.07 | 1.99 | 10.73 | 435.00 |
| 11-1.9-5 | 0.00 | 0.11 | 72.07 | 4.63 | 12.59 | 1.81 | 5.60 | 440.00 |
| 11-1.9-6 | 0.79 | 0.16 | 72.98 | 4.58 | 10.07 | 1.23 | 7.09 | 435.00 |
| 11-1.9-7 | 0.31 | 0.12 | 73.66 | 4.78 | 12.55 | 1.60 | 5.74 | 445.00 |
| 11-1.9.8 | 0.12 | 0.08 | 77.39 | 4.80 | 10.08 | 1.71 | 7.51 | 431.00 |
| 11-1.9-9 | 0.21 | 0.10 | 76.29 | 4.76 | 9.07 | 1.55 | 8.23 | 435.00 |
| 11-1.9-10 | 0.19 | 0.16 | 73.67 | 4.66 | 10.77 | 1.64 | 6.69 | 437.00 |
| Mean | 0.21 | 0.13 | 75.70 | 4.75 | 9.75 | 1.66 | 7.94 | 435.90 |
| Std. Dev | 0.21 | 0.05 | 2.21 | 0.10 | 1.89 | 0.23 | 1.87 | 5.39 |
| Fresh Pitchblende Data Points | ||||||||
| 10-1.11-2 | 0.18 | 1.04 | 86.91 | 4.28 | 0.82 | 0.19 | 104.14 | 358.00 |
| 10-1.11-8 | 0.31 | 1.09 | 85.93 | 4.19 | 0.83 | 0.92 | 101.24 | 357.00 |
| 10-1.11-9 | 0.37 | 1.20 | 84.85 | 4.26 | 0.75 | 0.98 | 110.62 | 368.00 |
| Mean | 0.29 | 1.11 | 85.90 | 4.24 | 0.80 | 0.69 | 105.33 | 361.00 |
| Std. Dev | 0.08 | 0.07 | 0.84 | 0.04 | 0.03 | 0.36 | 3.92 | 4.97 |
| Sample | U | Th | Th/U |
|---|---|---|---|
| LSS12-02 * | 7.80 | 21.50 | 2.76 |
| LSS12-03 * | 3.48 | 21.50 | 6.18 |
| GS-16-28 | 4.22 | 30.30 | 7.18 |
| GS-16-32 | 2.61 | 13.70 | 5.25 |
| GS-16-50 | 1.75 | 12.15 | 6.94 |
| ZKN9-2-2 | 3.90 | 17.60 | 4.51 |
| LSS12-10 * | 7.23 | 58.90 | 8.15 |
| LSS-26 * | 9.47 | 27.70 | 2.93 |
| GS-16-34 | 1.08 | 7.49 | 6.94 |
| GS-16-31 | 1.67 | 8.09 | 4.84 |
| GS-16-45 | 3.15 | 20.40 | 6.48 |
| GS-16-46 | 3.62 | 28.80 | 7.96 |
| GS-16-29 | 2.62 | 14.65 | 5.59 |
| D13-086 * | 7.90 | 40.13 | 5.08 |
| 04LTW01 * | 11.80 | 30.20 | 2.56 |
| LSS12-05 * | 11.30 | 42.60 | 3.77 |
| GS-16-36 | 2.39 | 25.40 | 10.63 |
| Min | 1.08 | 7.49 | 2.56 |
| Max | 11.80 | 58.90 | 10.63 |
| Mean | 5.06 | 24.77 | 5.75 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.-D.; Wang, K.-X.; Liu, X.-D.; Cuney, M.; Pan, J.-Y.; Wang, G.; Zhang, L.; Zhang, J. Uranium Mineralogical and Chemical Features of the Na-Metasomatic Type Uranium Deposit in the Longshoushan Metallogenic Belt, Northwestern China. Minerals 2020, 10, 335. https://doi.org/10.3390/min10040335
Yu C-D, Wang K-X, Liu X-D, Cuney M, Pan J-Y, Wang G, Zhang L, Zhang J. Uranium Mineralogical and Chemical Features of the Na-Metasomatic Type Uranium Deposit in the Longshoushan Metallogenic Belt, Northwestern China. Minerals. 2020; 10(4):335. https://doi.org/10.3390/min10040335
Chicago/Turabian StyleYu, Chi-Da, Kai-Xing Wang, Xiao-Dong Liu, Michel Cuney, Jia-Yong Pan, Gang Wang, Lin Zhang, and Jian Zhang. 2020. "Uranium Mineralogical and Chemical Features of the Na-Metasomatic Type Uranium Deposit in the Longshoushan Metallogenic Belt, Northwestern China" Minerals 10, no. 4: 335. https://doi.org/10.3390/min10040335
APA StyleYu, C.-D., Wang, K.-X., Liu, X.-D., Cuney, M., Pan, J.-Y., Wang, G., Zhang, L., & Zhang, J. (2020). Uranium Mineralogical and Chemical Features of the Na-Metasomatic Type Uranium Deposit in the Longshoushan Metallogenic Belt, Northwestern China. Minerals, 10(4), 335. https://doi.org/10.3390/min10040335






