Structure and Phase Changes of Nickel Slag in Oxidation Treatment
Abstract
1. Introduction
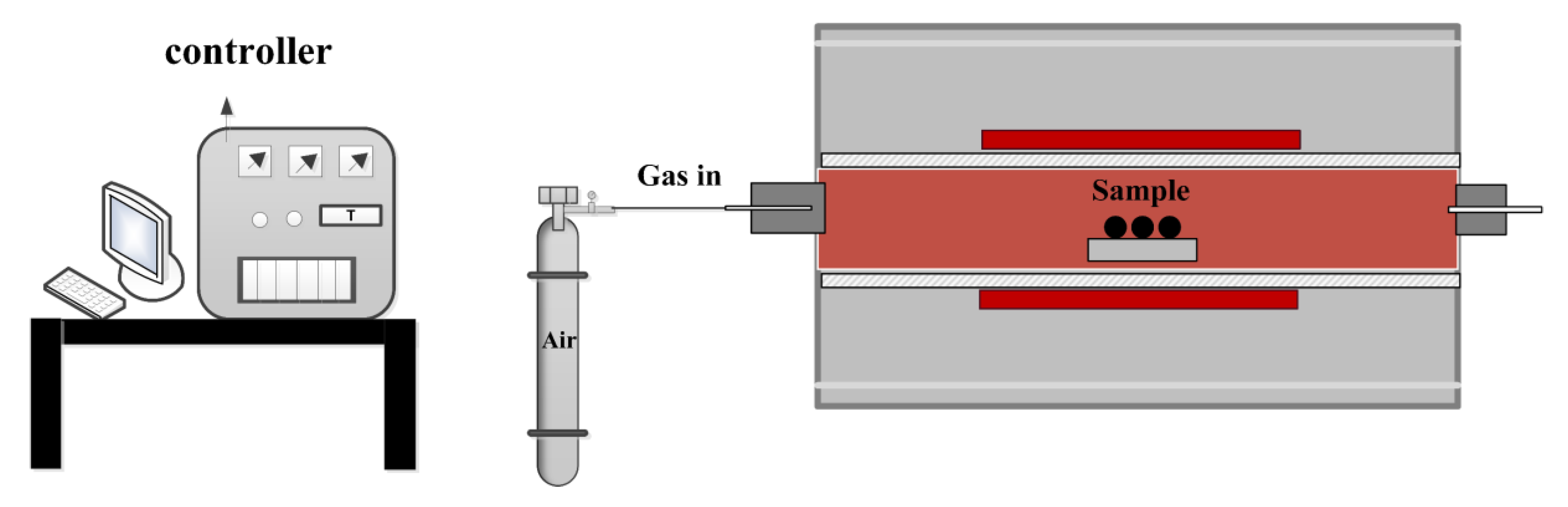
2. Materials and Methods
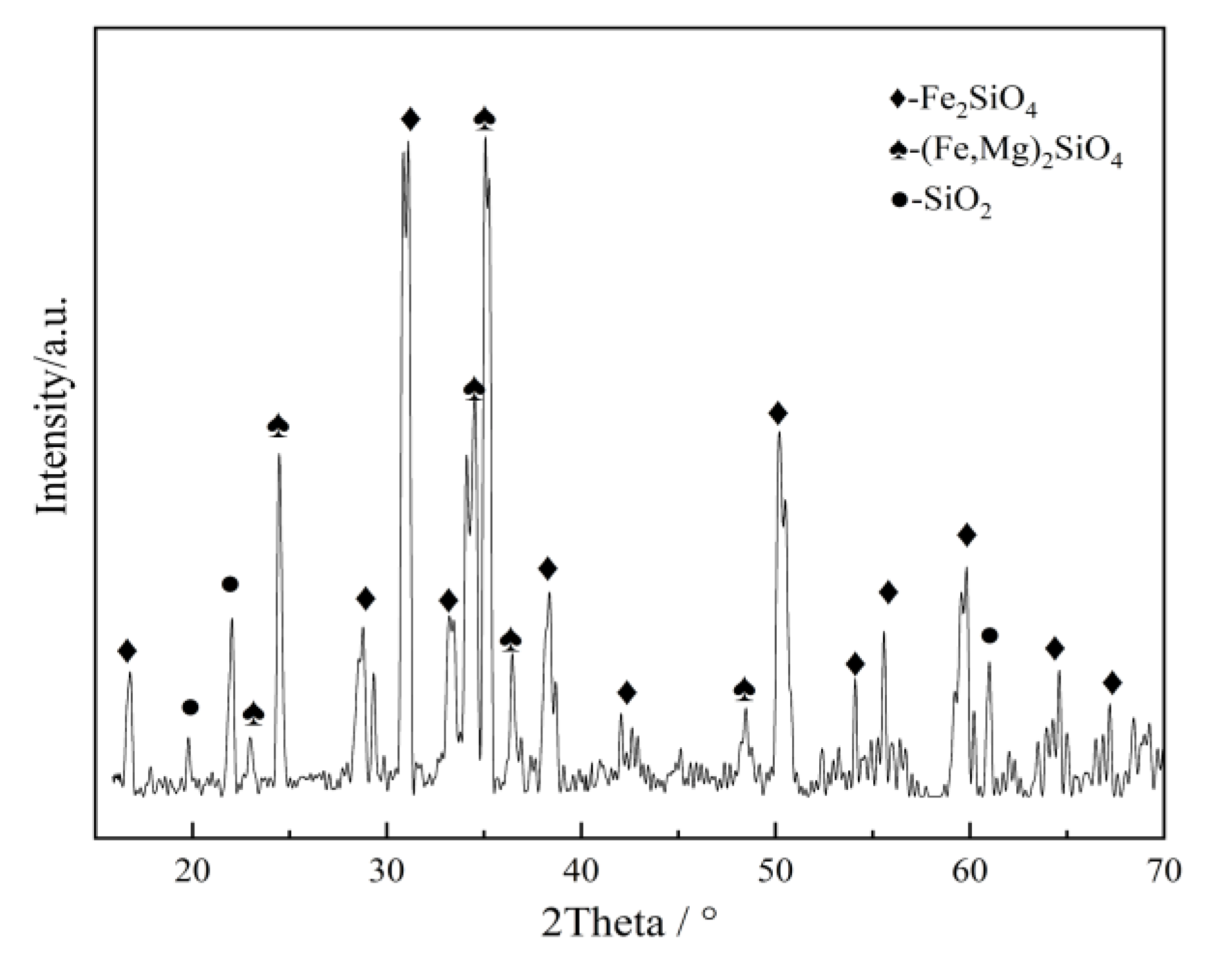
2.1. Experimental Materials
2.2. Phase Structure Characterization
3. Results
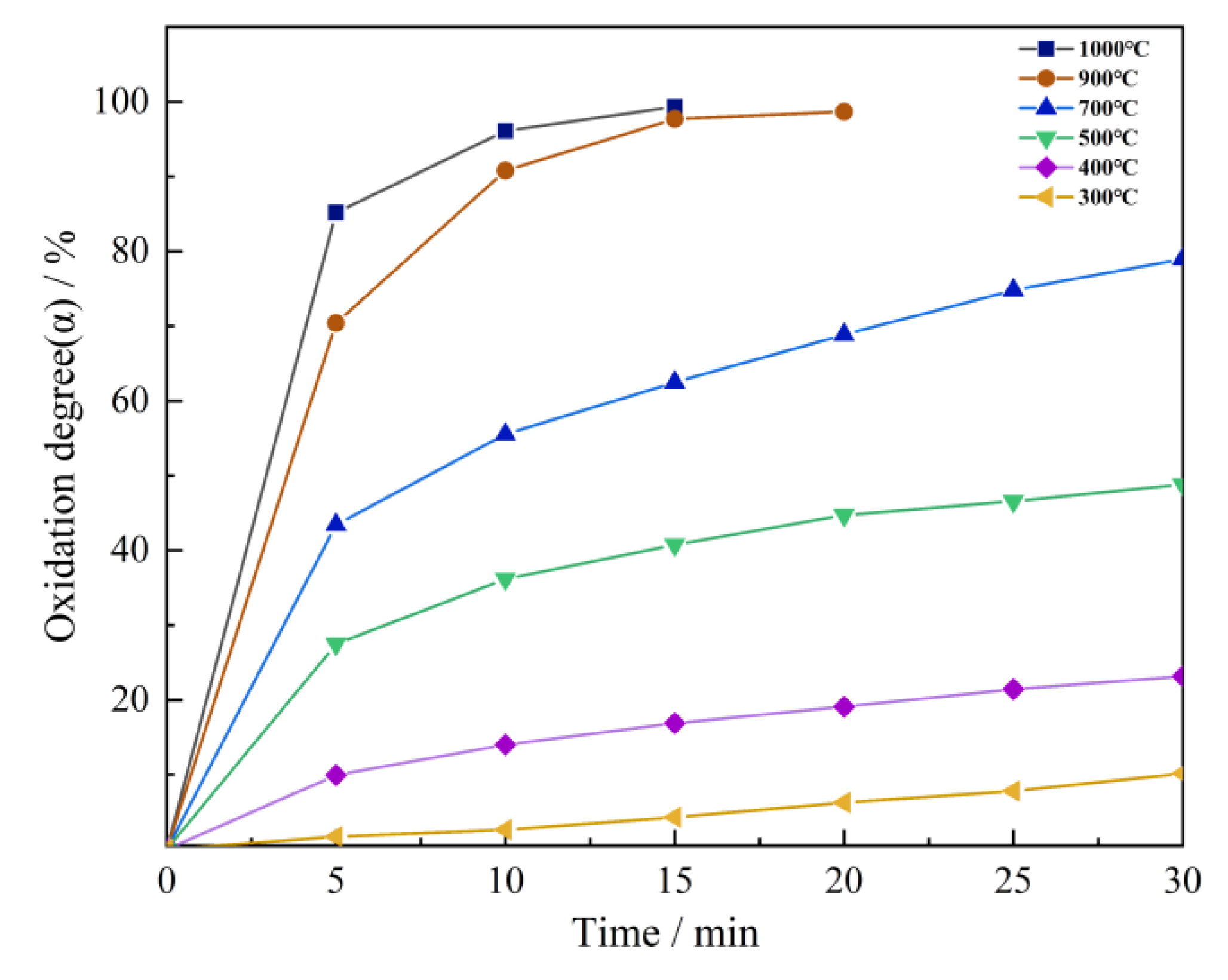
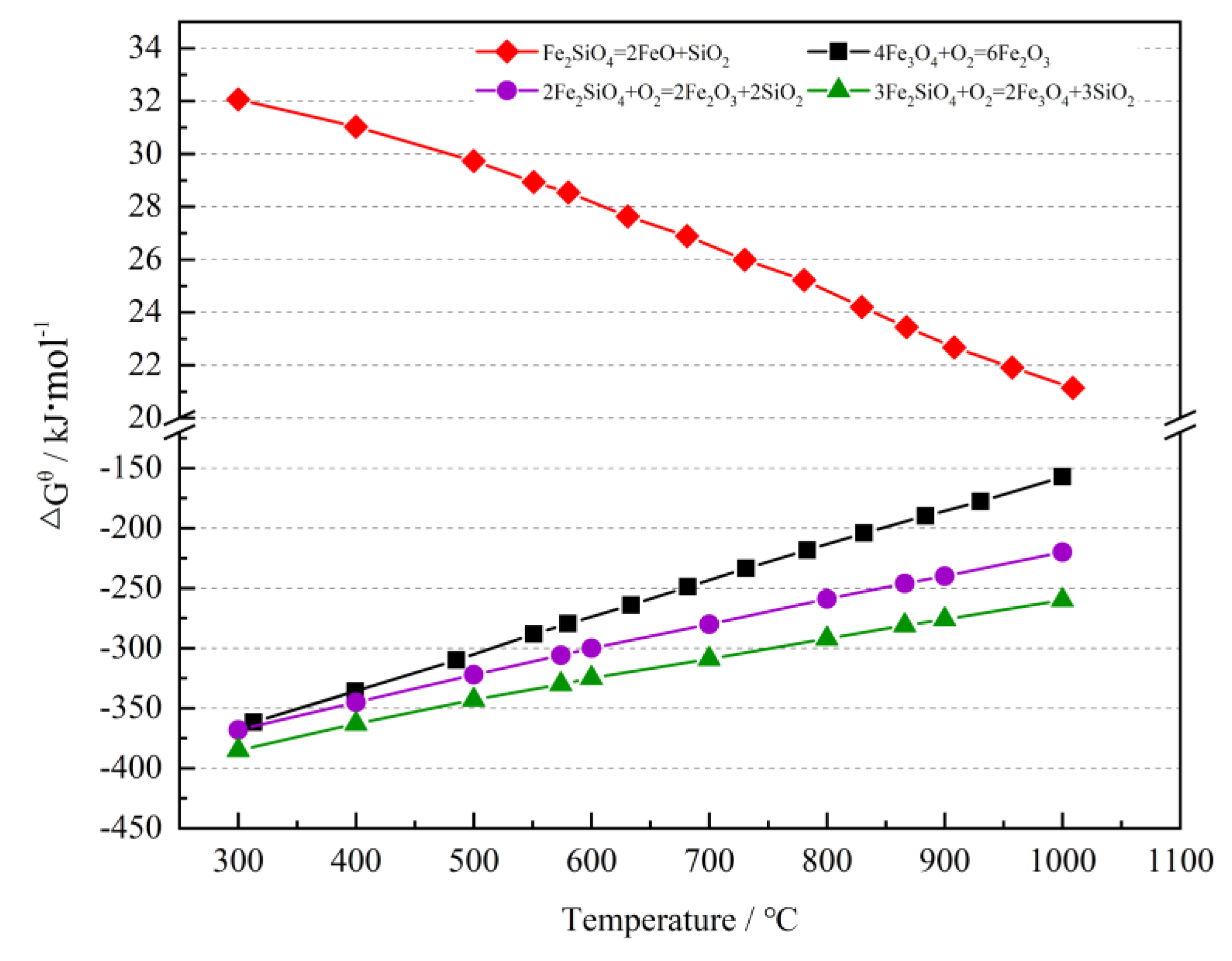
3.1. Change in Fe Oxidation Rate in Oxidation
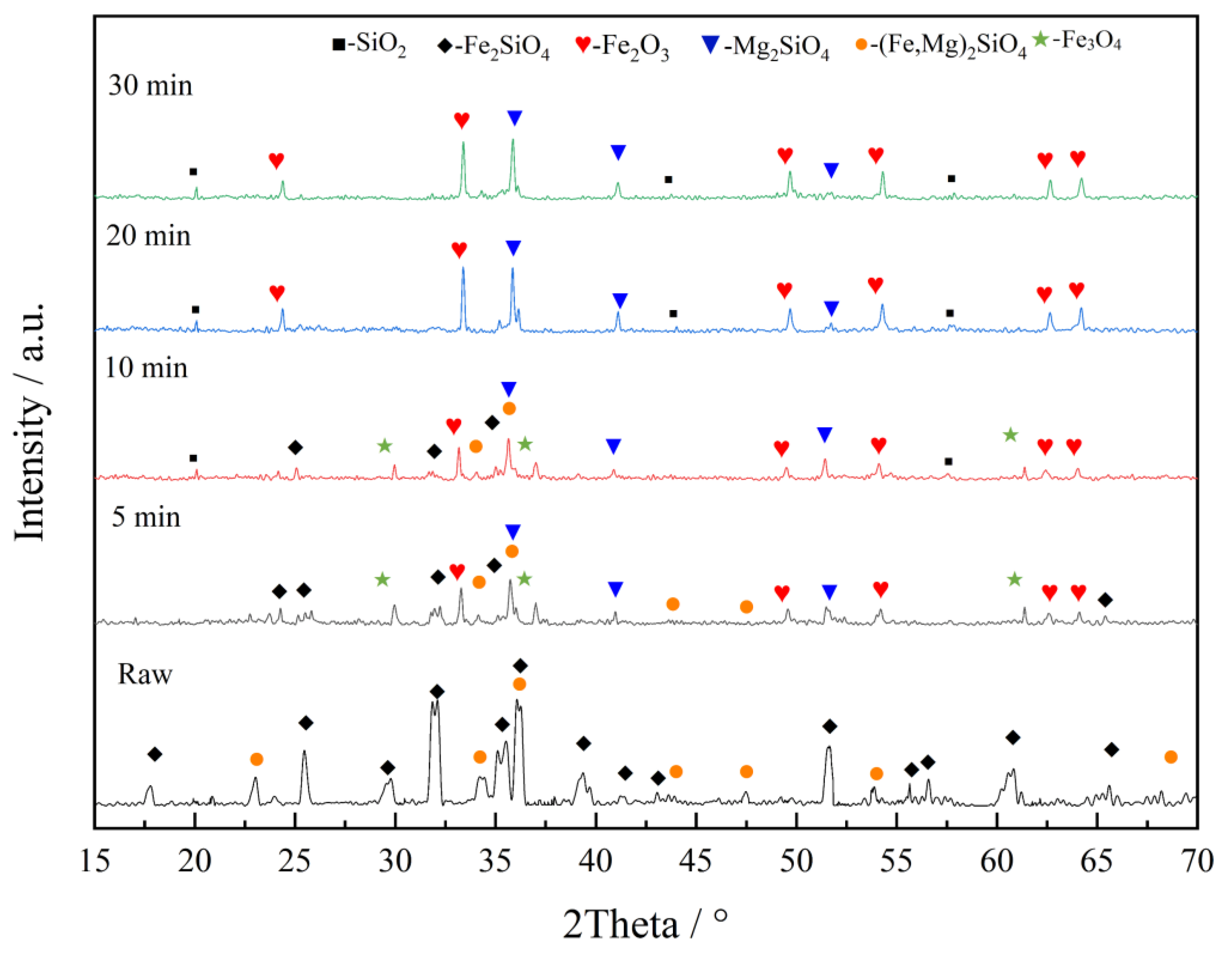
3.2. Phase Change in Oxidation
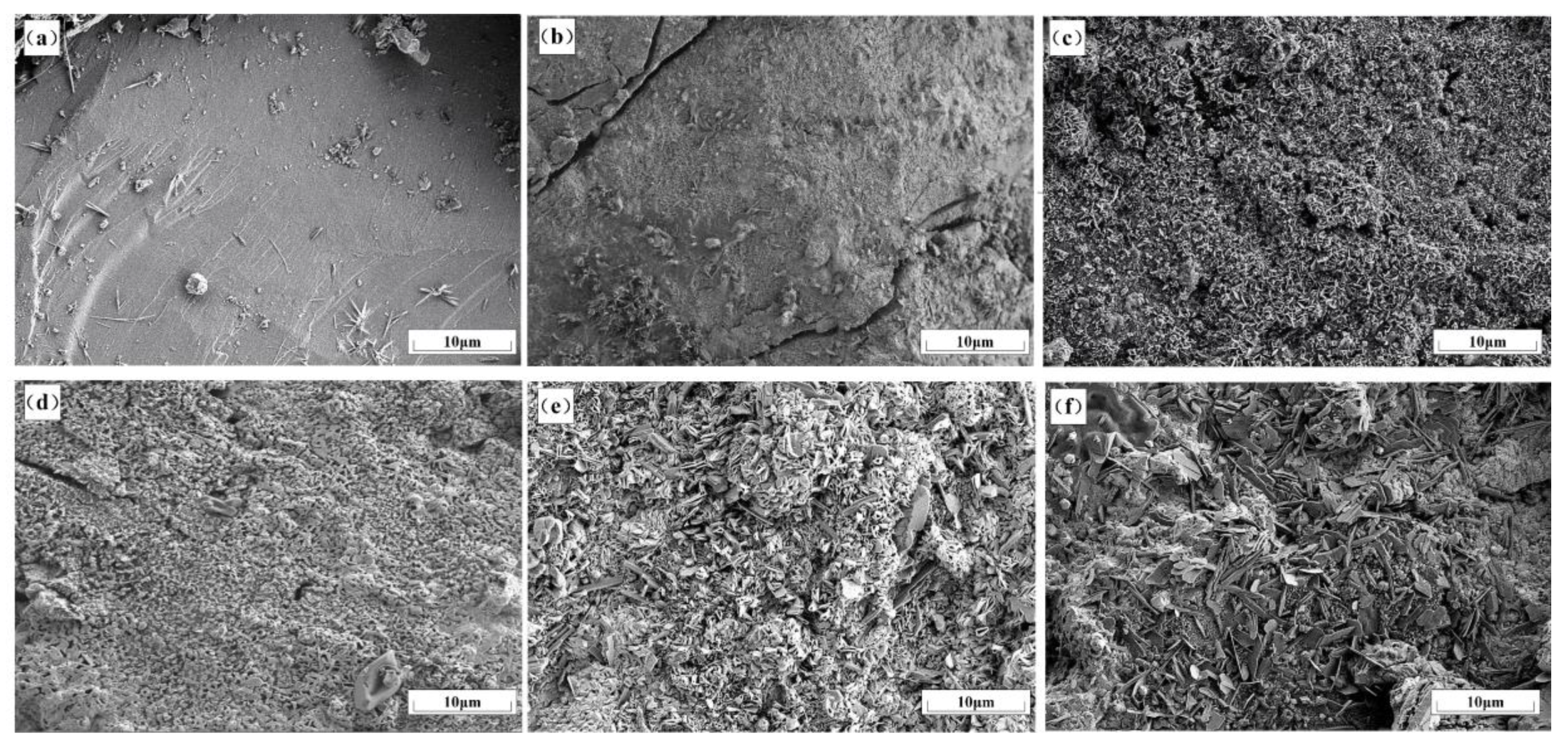
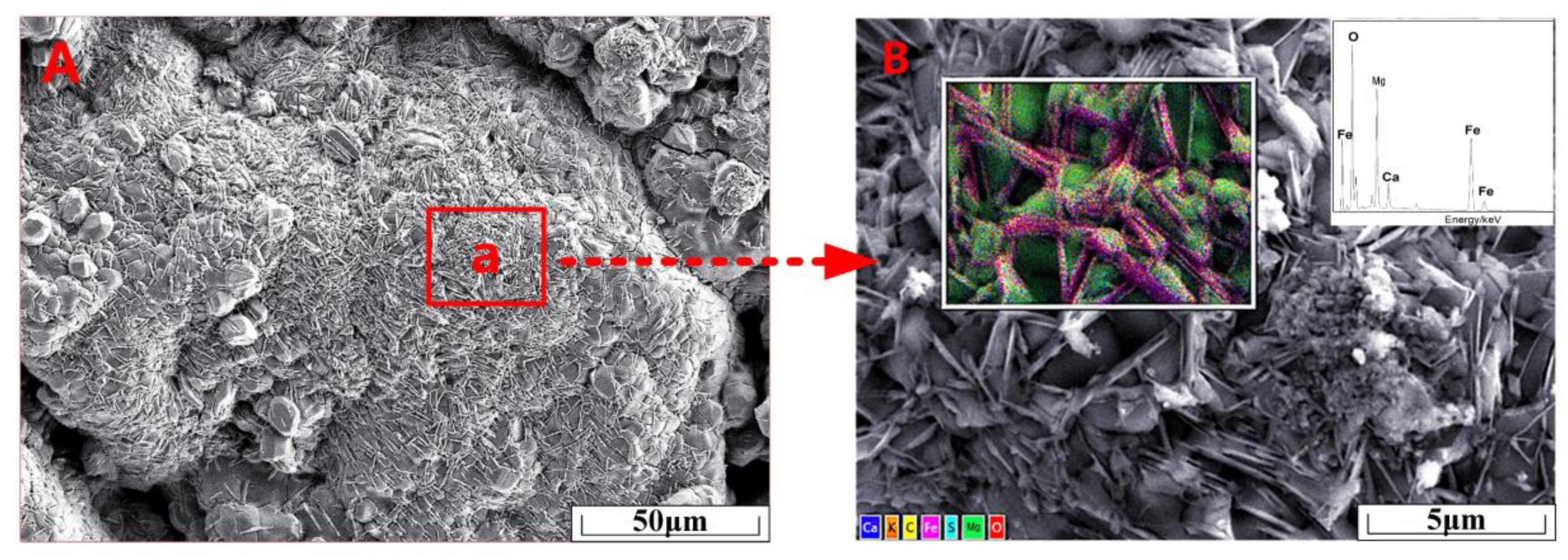
3.3. Microstructure Change in Nickel Slag in Oxidation
3.4. Crystal Structure Behavior during Oxidation in Nickel Slag
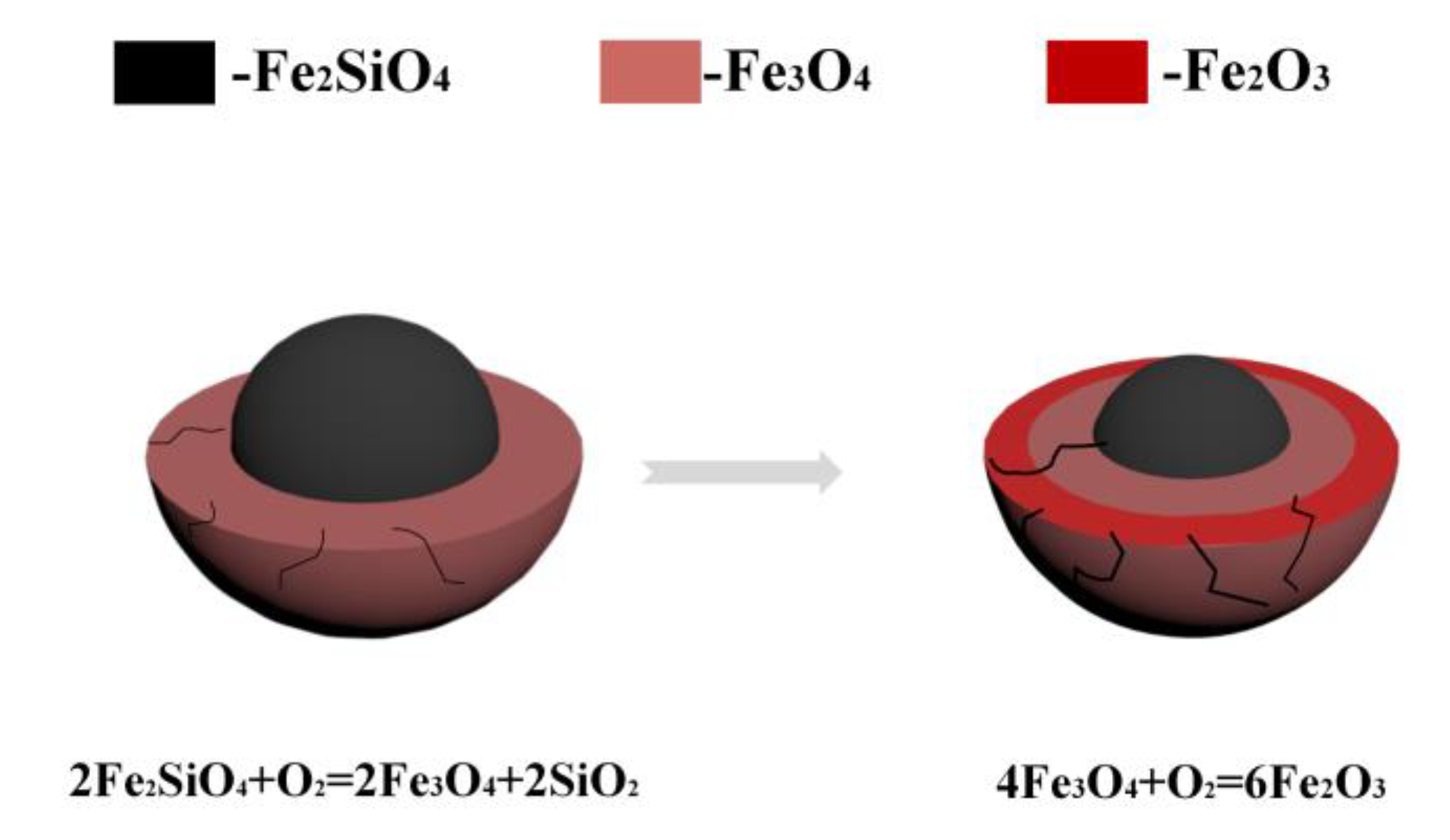
3.5. Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, X.M.; Shen, M.; Wang, C.; Cui, Y.R.; Zhao, J.X. Current Situation and Development of Comprehensive Utilization of Nickel Slag. Int. Mater. Rev. 2017, 31, 100–105. [Google Scholar] [CrossRef]
- Piatak, N.M.; Parsons, M.B.; Seal, R.R. Characteristics and environmental aspects of slag: A review. Appl. Geochem. 2015, 57, 236–266. [Google Scholar] [CrossRef]
- Wang, Y.F. Industrial Study and Application on Recycling Valuable Metals in Nickel Leaching Slag. Copp. Eng. 2010, 2, 44–47. [Google Scholar]
- Guo, Y.G.; Zhu, R.; Wang, Y. The fundamental research to extract iron from the nickel slag by smelting reduction process. Ind. Heat. 2015, 44, 40–43. (In Chinese) [Google Scholar]
- Ni, W.; Ma, M.S.; Wang, Y.L. Thermodynamic and Kinetic in Recovery of Iron from Nickel Residue. J. Univ. Sci. Technol. Beijing 2009, 31, 163–168. [Google Scholar] [CrossRef]
- Li, X.M.; Li, Y.; Zhang, X.Y.; Wen, Z.Y.; Xing, X.D. Growth characteristics of metallic iron particles in the direct reduction of nickel slag. MMTB 2020. [Google Scholar] [CrossRef]
- Pan, J.; Zheng, G.L.; Zhu, D.Q.; Zhou, X.L. Utilization of nickel slag using selective reduction followed by magnetic separation. Trans. Nonferrous Met. Soc. China 2013, 23, 3421–3427. [Google Scholar] [CrossRef]
- Li, X.M.; Wen, Z.Y.; Li, Y.; Wang, W.A.; Xing, X.D. Catalytic effect of sodium carbonate on the carbothermic reduction of nickel slag. Chin. J. Process Eng. 2020, 20, 182–188. (In Chinese) [Google Scholar] [CrossRef]
- Li, X.M.; Wen, Z.Y.; Li, Y.; Yang, H.B.; Xing, X.D. Improvement of carbothermic reduction of nickel slag by addition of CaCO3. Trans. Nonferrous Met. Soc. China 2019, 29, 2658–2666. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zhang, J.L.; Xing, X.D.; Liu, Z.J.; Zhang, Y.P.; Liu, X.L.; Liu, Y.R. Phase Transitions and Atomic-Scale Migration during the Oxidation of a Titania/Ferrous Oxide Solution. JOM 2015, 68, 656–667. [Google Scholar] [CrossRef]
- Park, E.; Ostrovski, O. Effects of Oxidation of Titania Ferrous Ore on the Ore Structure and Reduction Behavior. ISIJ Int. 2004, 44, 74–81. [Google Scholar] [CrossRef]
- Guo, Y.F.; Lv, Y.N.; Jiang, T.; Qiu, G.Z. Effect of pre-oxidation on Panzhihua ilmenite in solid state reduction process. J. Univ. Sci. Technol. Beijing 2010, 32, 413–419. [Google Scholar] [CrossRef]
- Liu, S.S.; Guo, Y.F.; Qiu, G.Z.; Jiang, T.; Chen, F. Solid-state reduction kinetics and mechanism of pre-oxidized vanadium-titanium magnetite concentrate. Trans. Nonferrous Met. Soc. China 2014, 24, 3372–3377. [Google Scholar] [CrossRef]
- Xing, X.D.; Pang, Z.G.; Mo, C.; Wang, S.; Ju, J.T. Effect of MgO and BaO on viscosity and structure of blast furnace slag. J. Non-Cryst. Solids 2020, 530, 119801. [Google Scholar] [CrossRef]
- Gou, H.P.; Zhang, G.H.; Chou, K.C. Influence of Pre-oxidation on Carbothermic Reduction Process of Ilmenite Concentrate. ISIJ Int. 2015, 55, 928–933. [Google Scholar] [CrossRef]




| Components | TFe | FeO | SiO2 | MgO | Al2O3 | CaO | Ni | Cu | Co | S |
| wt. % | 39.40 | 49.68 | 32.50 | 9.70 | 2.30 | 1.20 | 0.455 | 0.338 | 0.144 | 0.868 |
| Temperature | a/Å | Error/± | b/Å | Error/± | c/Å | Error/± | Volume/Å3 |
|---|---|---|---|---|---|---|---|
| 400 °C | 5.0342 | 0.0055 | 5.0342 | 0.0036 | 13.7483 | 0.0012 | 301.75 |
| 500 °C | 5.0330 | 0.0046 | 5.0330 | 0.0056 | 13.7396 | 0.0034 | 301.41 |
| 700 °C | 5.0206 | 0.0082 | 5.0206 | 0.0062 | 13.7196 | 0.0011 | 299.49 |
| 900 °C | 5.0065 | 0.0043 | 5.0065 | 0.0026 | 13.6411 | 0.0026 | 296.11 |
| 1000 °C | 5.0142 | 0.0085 | 5.0142 | 0.0024 | 13.6733 | 0.0016 | 297.72 |
| ideal unit cell size | 5.0160 | - | 5.0160 | - | 13.6520 | - | 297.52 |
| Temperature | a/Å | Error/± | b/Å | Error/± | c/Å | Error/± | Volume/Å3 |
|---|---|---|---|---|---|---|---|
| Raw | 5.7012 | 0.0030 | 10.3312 | 0.0028 | 4.7628 | 0.0046 | 302.59 |
| 300 °C | 6.0797 | 0.0018 | 10.4601 | 0.0028 | 4.8152 | 0.0011 | 306.23 |
| 400 °C | 6.0830 | 0.0012 | 10.4583 | 0.0018 | 4.8162 | 0.0007 | 306.40 |
| 500 °C | 6.1147 | 0.0047 | 10.4716 | 0.0055 | 4.8265 | 0.0025 | 309.03 |
| ideal unit cell size | 5.7060 | - | 10.3120 | - | 4.7620 | - | 301.70 |
| Temperature | a/Å | Error/± | b/Å | Error/± | c/Å | Error/± | Volume/Å3 |
|---|---|---|---|---|---|---|---|
| 700 °C | 5.6972 | 0.0042 | 11.5185 | 0.0082 | 8.2440 | 0.0084 | 540.99 |
| 900 °C | 5.6939 | 0.0032 | 11.4198 | 0.0268 | 8.2973 | 0.0142 | 539.53 |
| 1000 °C | 5.6816 | 0.0056 | 11.4031 | 0.0198 | 8.2068 | 0.0204 | 538.72 |
| ideal unit cell size | 5.6960 | - | 11.4440 | - | 8.2481 | - | 537.65 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhang, X.; Zang, X.; Xing, X. Structure and Phase Changes of Nickel Slag in Oxidation Treatment. Minerals 2020, 10, 313. https://doi.org/10.3390/min10040313
Li X, Zhang X, Zang X, Xing X. Structure and Phase Changes of Nickel Slag in Oxidation Treatment. Minerals. 2020; 10(4):313. https://doi.org/10.3390/min10040313
Chicago/Turabian StyleLi, Xiaoming, Xinyi Zhang, Xuyuan Zang, and Xiangdong Xing. 2020. "Structure and Phase Changes of Nickel Slag in Oxidation Treatment" Minerals 10, no. 4: 313. https://doi.org/10.3390/min10040313
APA StyleLi, X., Zhang, X., Zang, X., & Xing, X. (2020). Structure and Phase Changes of Nickel Slag in Oxidation Treatment. Minerals, 10(4), 313. https://doi.org/10.3390/min10040313




