Experimental Study on Strengthening Carbothermic Reduction of Vanadium-Titanium-Magnetite by Adding CaF2
Abstract
1. Introduction
2. Experiments
2.1. Experimental Materials
2.2. Experimental Process and Characterization Method
3. Results and Discussion
3.1. The Thermodynamic Analysis of Carbothermic Reduction
3.2. Influence of Additive Amounts of CaF2
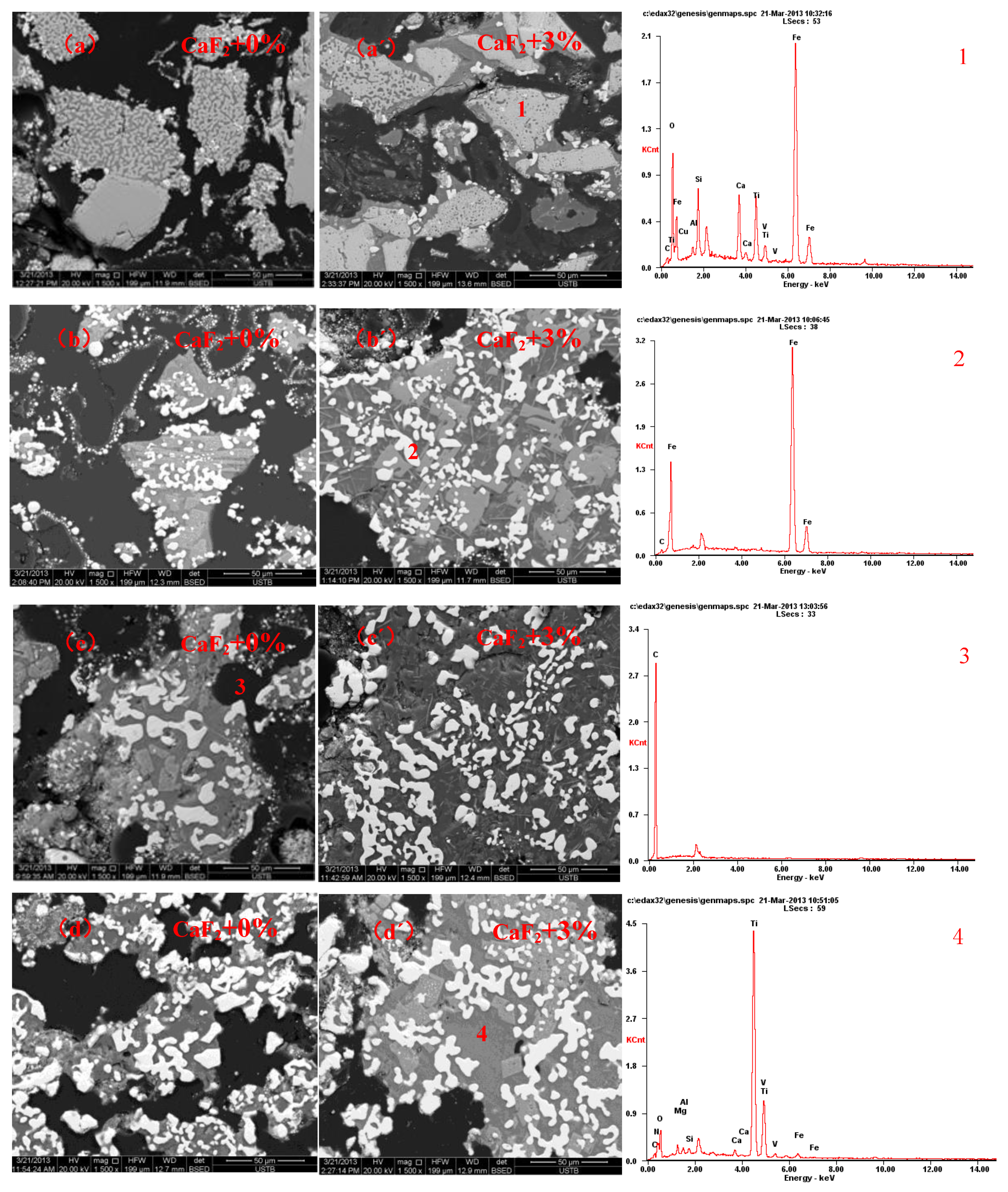
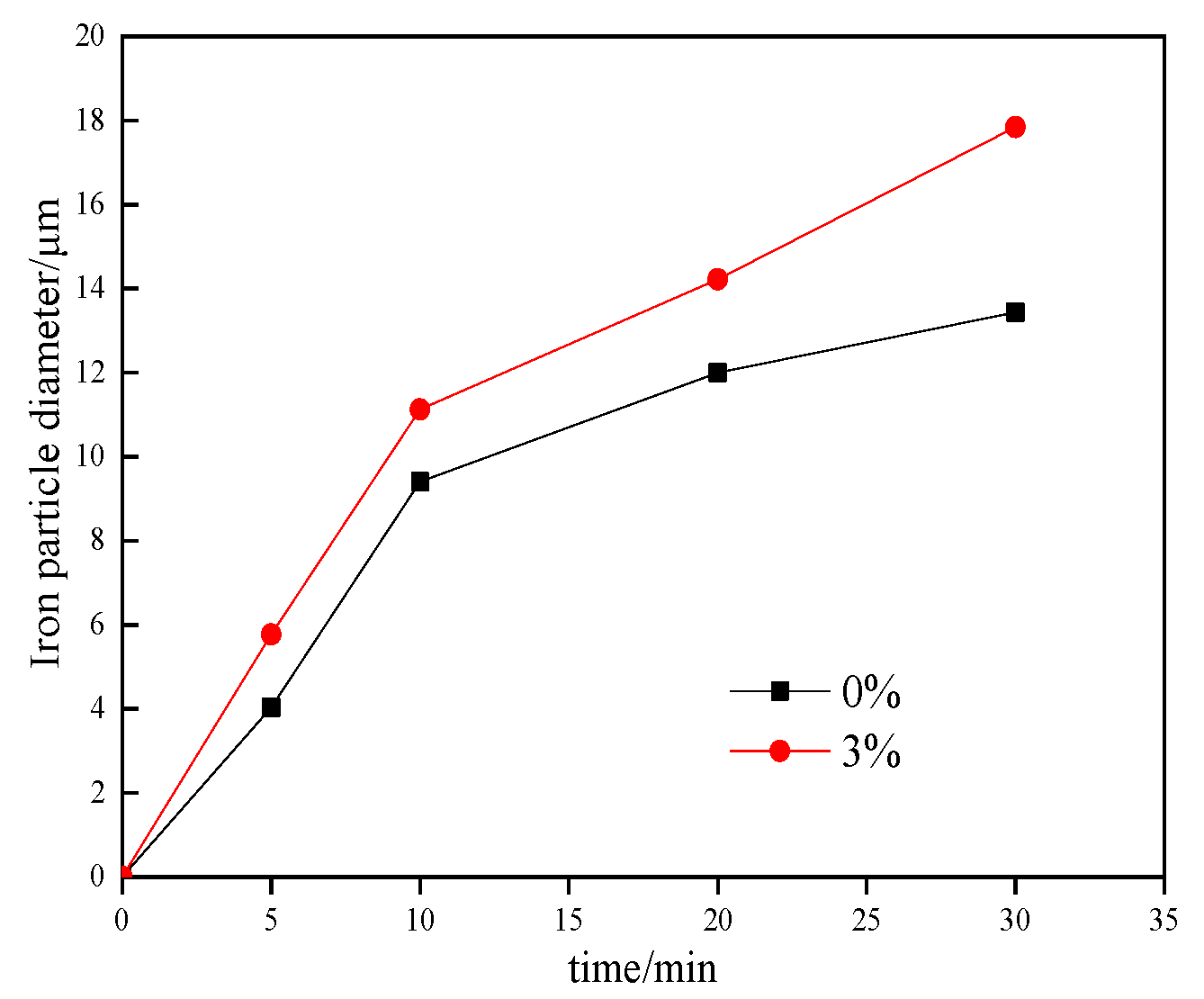
3.3. Morphological Analysis
3.4. XRD Analysis
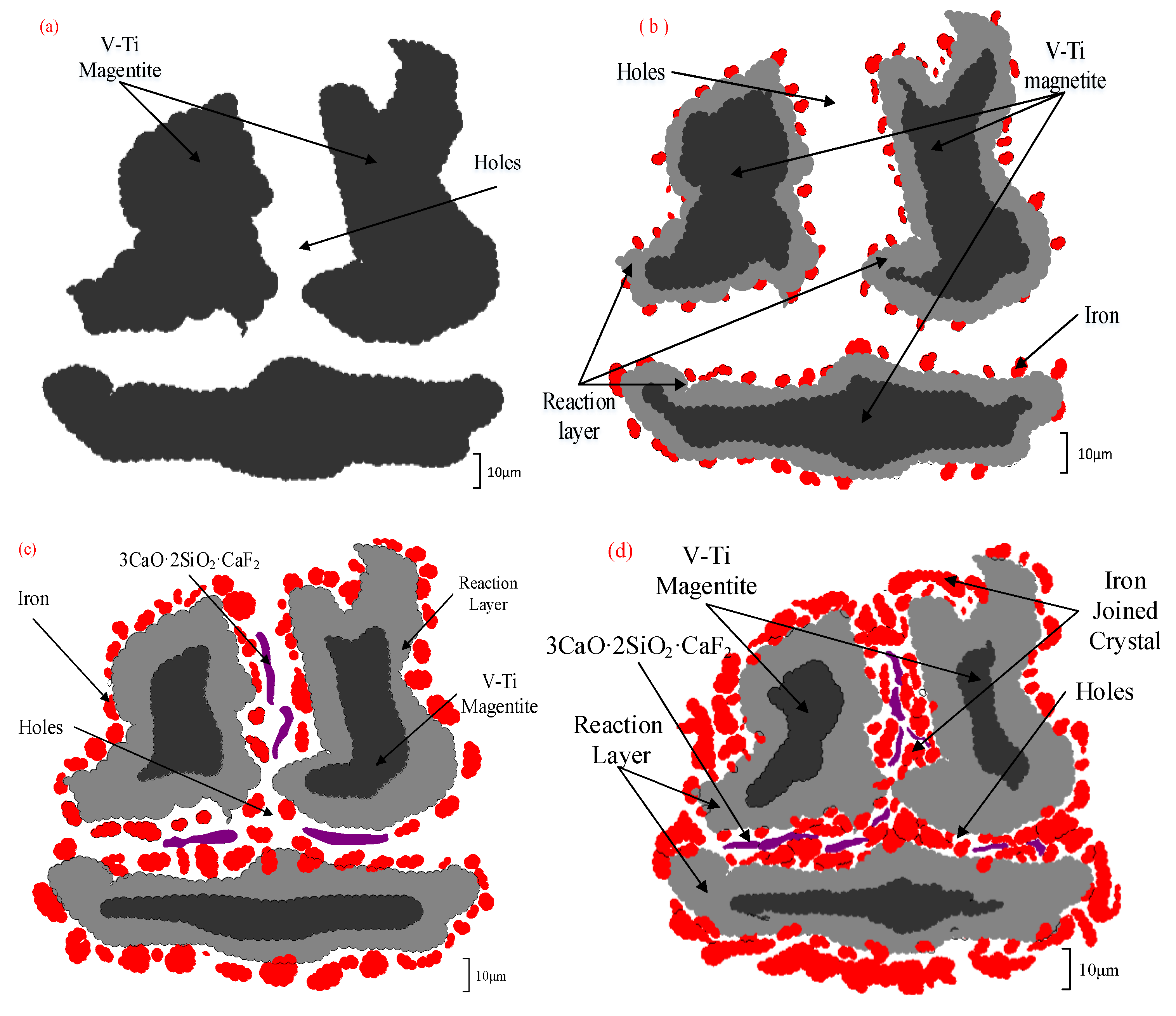
3.5. Mechanism Analysis of CaF2
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, R.; Lv, X.; Bai, C.; Zhang, K.; Qiu, G.B. Enhancement reduction of Panzhihua ilmenite concentrate with coke and conglomeration of metal with ferrosilicon. Steel Res. Int. 2013, 84, 892–898. [Google Scholar] [CrossRef]
- Pesl, J.; Eric, R.H. High temperature carbothermic reduction of Fe2O3-TiO2-MxOy oxide mixtures. Miner. Eng. 2002, 15, 971–984. [Google Scholar] [CrossRef]
- Xing, X.D.; Wang, S.; Pang, Z.G.; Zhang, Q.L. Effect of B2O3 on the carbothermal reduction of vanadium titanium magnetite. Metall. Res. Technol. 2019, 116, 630. [Google Scholar]
- Jiang, T.; Xu, J.; Guan, S.F.; Xue, X.-X. Study on coal-based direct reduction of high-chromium vanadium-titanium magnetite. Northeast. Univ. 2015, 85, 77–80. [Google Scholar]
- Cheng, G.J.; Xue, X.X.; Gao, Z.X.; Jiang, T.; Yang, H.; Duan, P.N. Effect of Cr2O3 on the reduction and smelting mechanism of high-chromium vanadium-titanium magnetite pellets. ISIJ Int. 2016, 56, 1938–1947. [Google Scholar] [CrossRef]
- Lv, W.; Lv, X.W.; Xiang, J.Y.; Zhang, Y.Y.; Li, S.P.; Bai, C.G.; Song, B.; Han, K.X. A novel process to prepare high-titanium slag by carbothermic reduction of pre-oxidized ilmenite concentrate with the addition of Na2SO4. Int. J. Miner. Process. 2019, 167, 68–78. [Google Scholar] [CrossRef]
- Xing, X.D.; Chen, Y.F.; Liu, Y.R. Study of the reduction mechanism of ironsands with addition of blast furnace bag dust. Metall. Res. Technol. 2018, 115, 214. [Google Scholar] [CrossRef]
- Chen, D.S.; Song, B.; Wang, L.N.; Qi, T.; Wang, W.J. Direct reduction and enhanced reduction of vanadium-bearing titanomagnetite concentrates. J. Univ. Sci.Technol. B 2011, 33, 1331–1336. [Google Scholar]
- Lv, X.W.; Lun, Z.G.; Yin, J.Q.; Bai, C.G. Carbothermic reduction of vanadium titanomagnetite by microwave irradiation and smelting behavior. ISIJ Int. 2013, 53, 1115–1119. [Google Scholar] [CrossRef]
- Xing, X.D.; Pang, Z.G.; Mo, C.; Wang, S.; Ju, J.T. Effect of MgO and BaO on viscosity and structure of blast furnace slag. J. Non-Cryst. Solids 2020, 530, 119801. [Google Scholar] [CrossRef]
- El-Tawil, S.Z.; Morsi, J.M.; Abdalla, F.H.A. Reductive roasting of pre-oxidized titanomagnetite ore. Rare Metal 1990, 9, 170–178. [Google Scholar]
- Shi, L.Y.; Zhen, Y.L.; Chen, D.S.; Wang, L.N.; Qi, T. Carbothermic reduction of vanadium-titanium magnetite in molten NaOH. ISIJ Int. 2018, 58, 627–632. [Google Scholar] [CrossRef]
- Xing, X.D.; Liu, Y.R.; Ju, J.T.; Liu, Z.J.; Liu, X.L.; Li, N.Y.; Shen, Y.S. Synthesis and non-isothermal carbothermic reduction of FeTiO3-Fe2O3 solid solution systems. Rare Metal Mater. Eng. 2018, 47, 2775–2781. [Google Scholar]
- Xing, X.D.; Wang, S.; Zhang, Q.L. Thermogravimetric analysis and kinetics of mixed combustion of waste plastics and semicoke. J. Chem. 2019, 2019, 8675986. [Google Scholar] [CrossRef]
- Song, B.; Lv, X.W.; Miao, H.H.; Han, K.X.; Zhang, K.; Huang, R. Effect of Na2B4O7 addition on carbothermic reduction of ilmenite concentrate. ISIJ Int. 2016, 56, 2140–2146. [Google Scholar] [CrossRef]
- Liu, S.S.; Guo, Y.F.; Qiu, G.; Jiang, Z.; Chen, F. Solid-state reduction kinetics and mechanism of pre-oxidized vanadium-titanium magnetite concentrate. Trans. Nonferrous Metals Soc. 2014, 24, 3372–3377. [Google Scholar] [CrossRef]
- Wu, J.H.; Sun, K.; Ma, Y.Y.; Liu, M.Y.; Zhang, Y. Catalytic effect of alkali chlorides on carbothermic reduction of pre oxidized ilmenite. Trans. Nonferrous Metals Soc. 2000, 10, 813–816. [Google Scholar]
- Paktunc, D.; Thibault, Y.; Sokhanvaran, S.; Yu, D. Influences of alkali fluxes on direct reduction of chromite for ferrochrome production. SAIMM 2018, 118, 1305–1314. [Google Scholar] [CrossRef]
- Chen, S.Y.; Fu, X.J.; Chu, M.S.; Li, X.Z.; Liu, Z.G.; Tang, J. Carbothermic reduction mechanism of vanadium-titanium magnetite. J. Iron Steel Res. Int. 2016, 23, 409–414. [Google Scholar] [CrossRef]
- Cui, S.Q.; Lu, Q.; Feng, S.; Sun, Y.Q.; Chen, S.J.; Li, F.M.; Wan, X.Y.; Kang, Z.P. Research on effect of additives on viscosity of titanium-bearing blast furnace slag of Chengde steel. Iron Steel Van. Titanium 2014, 35, 68–72. [Google Scholar]
- Yu, D.W.; Paktunc, D. Carbothermic reduction of chromite fluxed with aluminum spent potlining. Trans. Nonferrous Metals Soc. China 2019, 29, 200–212. [Google Scholar] [CrossRef]
- Sun, H.Y.; Adetoro, A.A.; Wang, Z.; Pan, F.; Li, L. Direct reduction behaviors of titanomagnetite ore by carbon in fluidized bed. ISIJ Int. 2016, 56, 936–943. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zhang, J.L.; Jiao, K.X.; Liu, Z.J.; Barati, M. Effect of pre-oxidation on the kinetics of reduction of ironsand. J. Alloy Compd. 2017, 729, 874–883. [Google Scholar] [CrossRef]
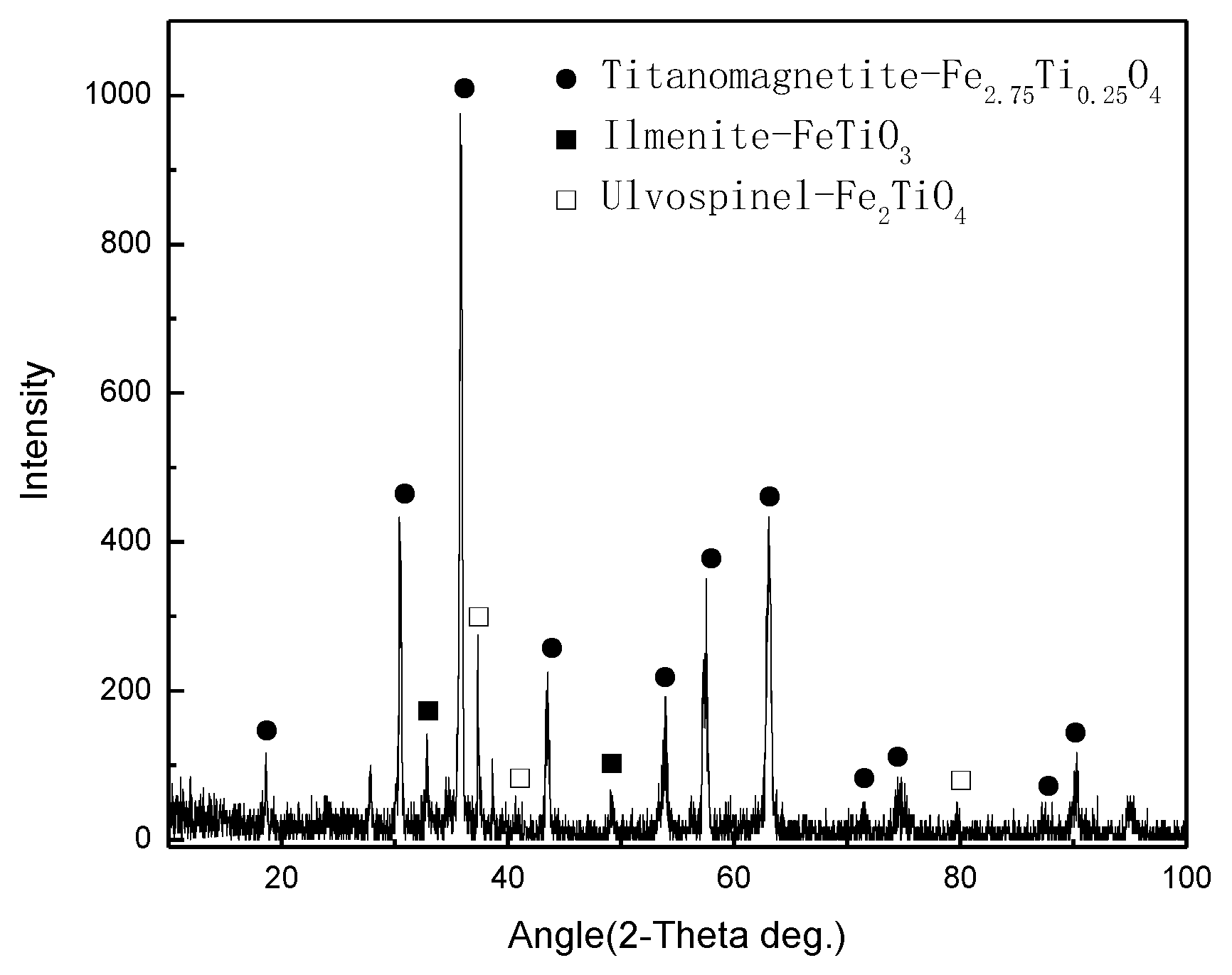
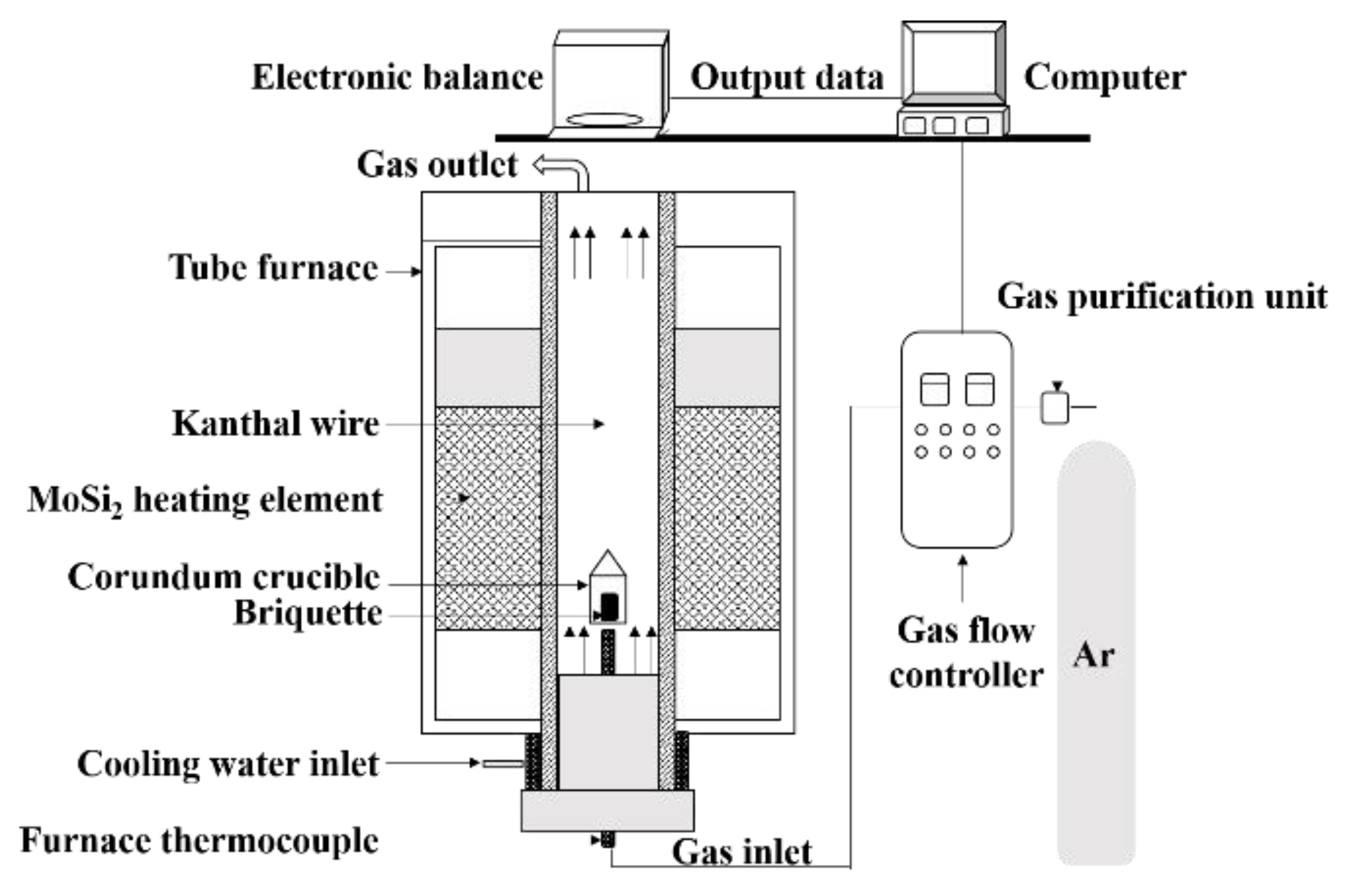
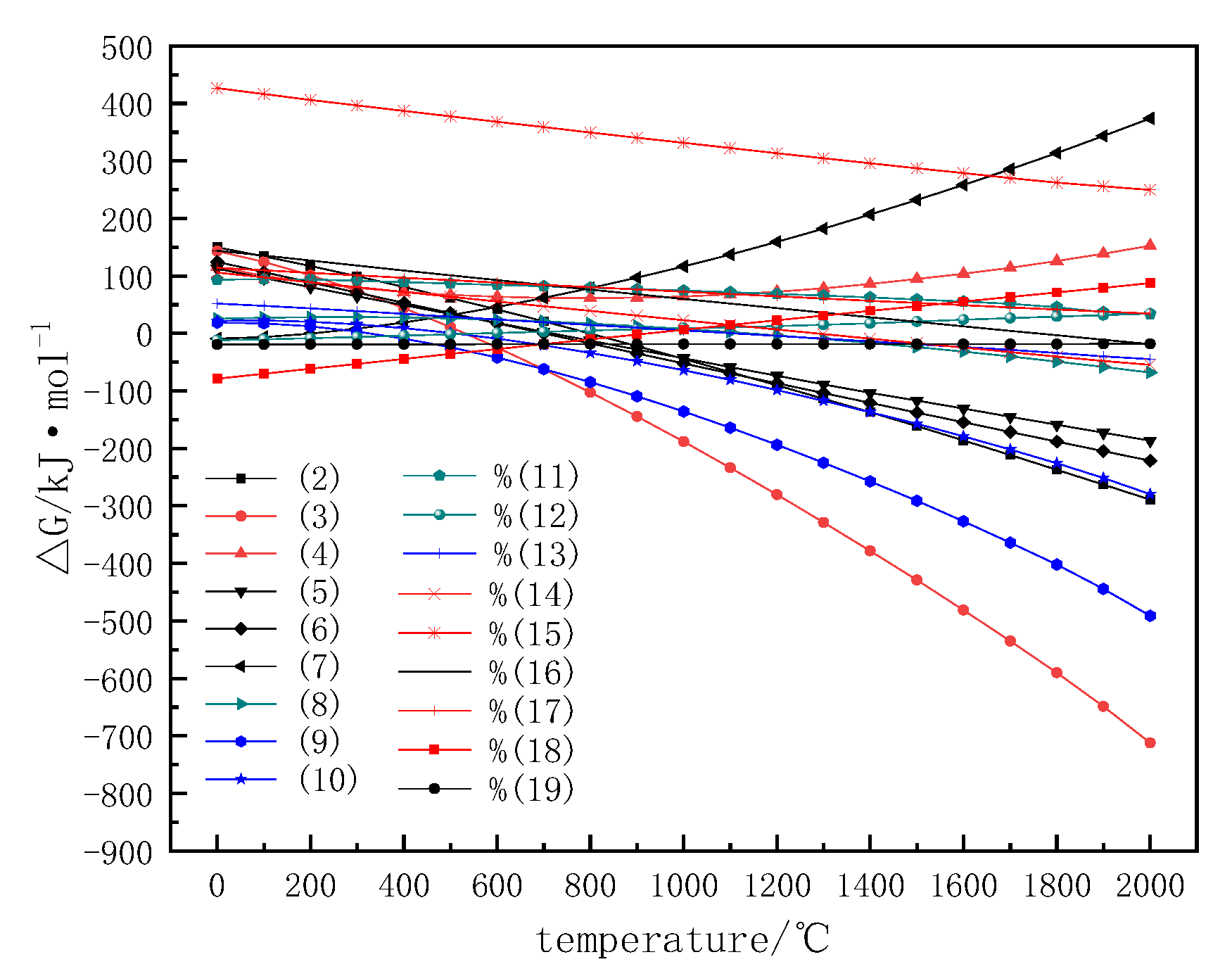
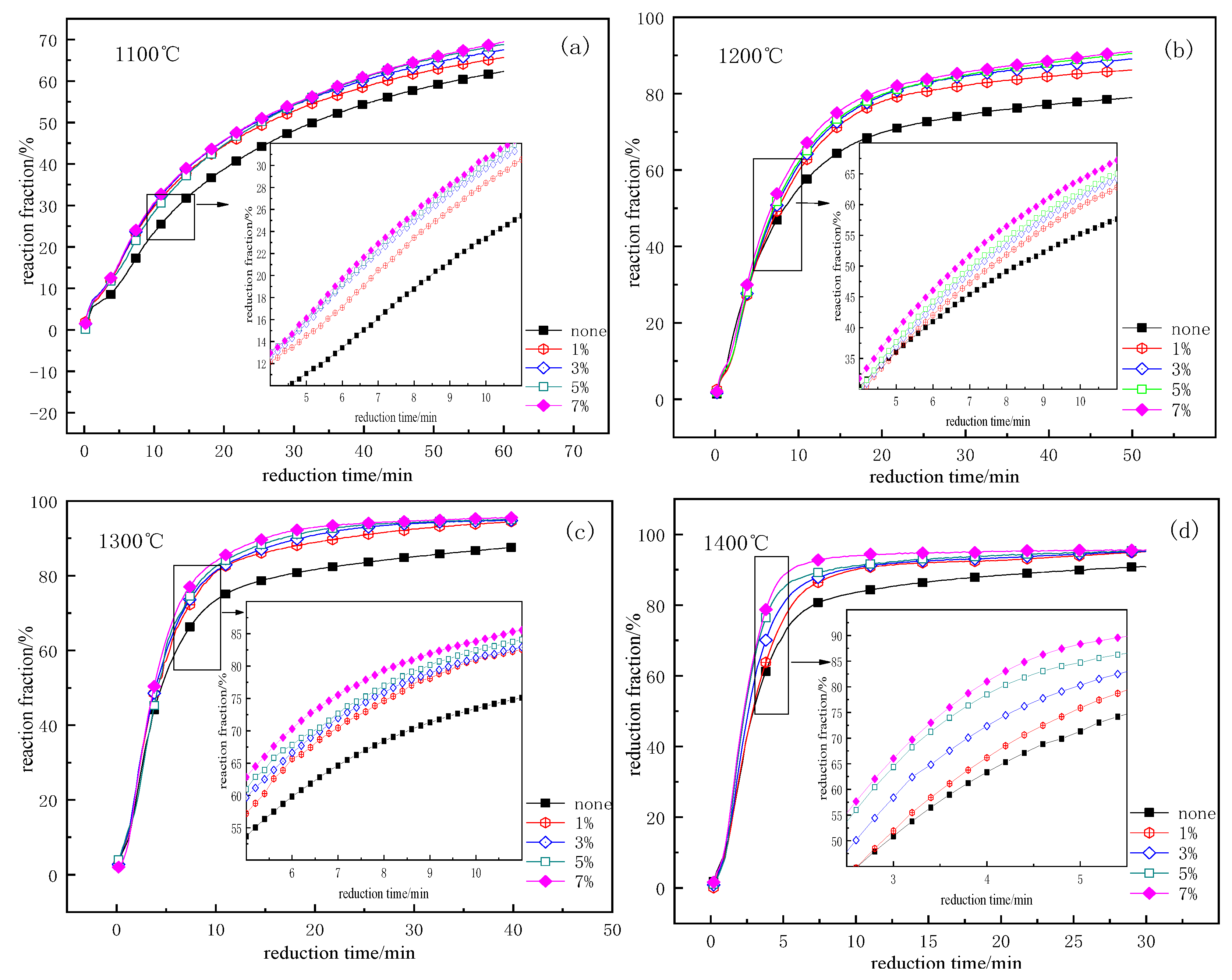


| FeO | Fe2O3 | V2O5 | TiO2 | SiO2 | Al2O3 | CaO | MgO |
|---|---|---|---|---|---|---|---|
| 20.60 | 51.27 | 1.85 | 12.96 | 6.28 | 1.74 | 3.72 | 0.29 |
| Reaction Equation | Gibbs Free Energy (△G)/kJ bs fre | Temperature Range /°C | Equation |
|---|---|---|---|
| 2FeTiO3 + C = FeTi2O5 + Fe + CO | 106.59 − 21.864T | 400–1400 | (2) |
| FeTi2O5 + C = 2TiO2 + Fe + CO | 101.06 − 42.408T | 400–1400 | (3) |
| Fe2TiO4 + C = FeTiO3 + Fe + CO | 88.607 − 0.0268T 3.8712 + 0.0478T | 400–800 800–1400 | (4) |
| FeO + C = Fe + CO | 151.78 − 0.1529T | 400–1400 | (5) |
| C + CO2 = 2CO | 169.39 − 0.1738T | 400–1400 | (6) |
| Fe2TiO4 + CO = FeTiO3 + Fe + CO2 | −9.5332 + 18.816T | 400–1400 | (7) |
| 2FeTiO3 + CO = FeTi2O5 + Fe + CO2 | 43.199 − 0.0225T 48.709 − 0.0563T | 400–700 700–1400 | (8) |
| FeTi2O5 + CO = 2TiO2 + Fe + CO2 | 174.74 − 0.2503T | 400–1400 | (9) |
| FeTiO3 + CO = TiO2 + Fe + CO2 | 118.63 − 0.1476T | 400–1400 | (10) |
| 3TiO2 + CO = Ti3O5 + CO2 | 107.4 − 0.0263T | 400–1400 | (11) |
| FeO + CO = Fe + CO2 | −5.6599 + 2.0844T | 400–1400 | (12) |
| 3TiO2 + C = Ti3O5 + CO | 273.595 − 0.1981T | 400–1400 | (13) |
| TiO2 + 3C = TiC + 2CO | 527.685 − 0.3367T | 400–1400 | (14) |
| Ti3O5 + 3C = 3TiC + 2.5O2 | 590.396 − 0.1914T | 400–700 | (15) |
| SiO2 + C = SiO + CO | 668.07 − 0.3288T | 400–1400 | (16) |
| SiO2 + CO = SiO + CO2 | 505.76 − 0.1598T | 400–800 | (17) |
| SiO + 3CO = SiC + 2CO2 | −403.51 + 0.339T | 400–1400 | (18) |
| SiO + 2C = SiC + CO | −78.89 + 0.0010T | 400–1400 | (19) |
| Crystal Lattice Parameters | TTM | FeTiO3 | FeTi2O5 | Fe2TiO4 | ||||
|---|---|---|---|---|---|---|---|---|
| 0%CaF2 | 3%CaF2 | 0%CaF2 | 3%CaF2 | 0%CaF2 | 3%CaF2 | 0%CaF2 | 3%CaF2 | |
| a/Å | 5.07824 | 5.07623 | 5.07934 | 5.07951 | 3.76023 | 3.75751 | 8.49324 | 8.50731 |
| ± | 0.0001 | 0.0002 | 0.0002 | 0.0001 | 0.0002 | 0.0001 | 0.0002 | 0.0003 |
| b/Å | - | - | - | - | 9.65635 | 9.65635 | - | - |
| ± | 0.0001 | 0.0003 | ||||||
| c/Å | 13.43312 | 13.45201 | - | - | 9.98301 | 9.98190 | - | - |
| ± | 0.0002 | 0.0001 | 0.0002 | 0.0001 | ||||
| Volume/Å3 | 346.42 | 346.63 | 131.04 | 131.06 | 362.27 | 363.36 | 612.66 | 615.71 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, X.; Du, Y.; Zheng, J.; Chen, Y.; Ren, S.; Ju, J. Experimental Study on Strengthening Carbothermic Reduction of Vanadium-Titanium-Magnetite by Adding CaF2. Minerals 2020, 10, 219. https://doi.org/10.3390/min10030219
Xing X, Du Y, Zheng J, Chen Y, Ren S, Ju J. Experimental Study on Strengthening Carbothermic Reduction of Vanadium-Titanium-Magnetite by Adding CaF2. Minerals. 2020; 10(3):219. https://doi.org/10.3390/min10030219
Chicago/Turabian StyleXing, Xiangdong, Yueli Du, Jianlu Zheng, Yunfei Chen, Shan Ren, and Jiantao Ju. 2020. "Experimental Study on Strengthening Carbothermic Reduction of Vanadium-Titanium-Magnetite by Adding CaF2" Minerals 10, no. 3: 219. https://doi.org/10.3390/min10030219
APA StyleXing, X., Du, Y., Zheng, J., Chen, Y., Ren, S., & Ju, J. (2020). Experimental Study on Strengthening Carbothermic Reduction of Vanadium-Titanium-Magnetite by Adding CaF2. Minerals, 10(3), 219. https://doi.org/10.3390/min10030219





